Abstract
Rationale
Previous studies in rodents show that early exposure to methylphenidate alters later responsiveness to drugs of abuse. An interesting feature of these studies is that early methylphenidate treatment decreases the rewarding value of cocaine when measured by conditioned place preference (CPP), but the same treatment increases cocaine self-administration.
Objective
The goal of the present study was to examine the effects of early methylphenidate exposure on cocaine-induced responding using both reward paradigms.
Methods
Rats were treated with methylphenidate (0, 2, or 5 mg/kg) from postnatal day (PD) 11 to PD 20 and then cocaine-induced CPP or cocaine self-administration was measured in separate groups of rats in adulthood. The CPP procedure included eight days of acquisition training, eight days of extinction training, and a reinstatement test. Rats were conditioned with 0, 10 or 20 mg/kg cocaine. Reinstatement was assessed after a priming dose of cocaine (10 mg/kg). For the self-administration experiment, a jugular catheter was implanted and rats were trained to press a lever reinforced with cocaine (0.25 or 0.75 mg/kg/infusion) on a fixed ratio (FR) 1 schedule. Rats were gradually moved from an FR1 to an FR10 schedule and, after criterion was reached, rats were placed on a progressive ratio schedule for five days.
Results
Cocaine produced robust rewarding effects as determined by both the CPP and self-administration experiments; however, early methylphenidate exposure only enhanced the reinforcing effects of cocaine on the self-administration paradigm. Interestingly, this methylphenidate enhancement was only seen in male rats.
Conclusions
These data suggest that in males methylphenidate enhances the reinforcing value of cocaine, but not cocaine-associated cues.
Introduction
Attention deficit/hyperactivity disorder (ADHD) is the most commonly diagnosed psychiatric disorder of childhood, with prevalence estimates ranging from 5-12% (Biederman and Farone 2005; Bloom et al. 2009). Psychostimulant compounds, such as methylphenidate, are the treatment of choice for this disorder, because numerous studies have demonstrated that methylphenidate increases overall academic performance and reduces the core symptoms of ADHD, including inattention, hyperactivity, and impulsivity (Swanson et al. 2004; Biderman and Farone 2005; Dopheide and Pliszka 2009; Scheffler et al. 2009). Despite the acknowledged efficacy of methylphenidate for the treatment of ADHD, the consequences of extended drug use are of concern because few long-term developmental studies have been conducted. Of particular significance is the lack of data on the effects of long-term methylphenidate use in “atypical” patient populations such as preschool-aged children.
Understanding the consequences of early methylphenidate treatment is important because adult animals studies have shown that repeated exposure to psychostimulants, including methylphenidate, produces persistent changes in behavior and neuronal functioning (Robinson and Berridge 1993, 2000; Gaytan et al. 1997; Pierce and Kalivas 1997; Dafny and Yang 2006). For example, repeated psychostimulant exposure induces behavioral sensitization in adult rodents, a phenomenon that models aspects of psychostimulant addiction (Robinson and Berridge 1993, 2000; Pierce and Kalivas 1997; Dafny and Yang 2006). In addition, repeated methylphenidate treatment alters gene expression, dopamine release, and cAMP signal transduction in the mesolimbic and nigrostriatal dopamine systems, i.e., brain areas linked with reward and motivated behavior (Crawford et al. 1998; Sproson et al. 2001; Yano and Steiner 2007; Souza et al. 2008).
While it is unknown whether early methylphenidate exposure produces similar changes in the neuronal functioning of juvenile rodents, a few ontogentic studies have shown that early methylphenidate treatment alters the rewarding values of drugs of abuse when tested in adulthood (Brandon et al. 2001; Andersen et al. 2002; Achat-Mendes 2003; Carlezon et al. 2003; Crawford et al. 2007). For example, all of the available research suggest that early methylphenidate exposure alters cocaine's reinforcing potential; however, the reported effects were in opposing directions. Specifically, one study found that early methylphenidate exposure increased cocaine self-administration (Brandon et al. 2001), while other studies reported that early methylphenidate exposure depressed cocaine-induced CPP (Andersen et al. 2002; Carlezon et al. 2003). Differences in outcome could have resulted from procedural differences, such as age of onset of drug treatment (adolescent versus preadolescent drug treatment) or drug administration protocol (1 versus 2 injections per day). Alternatively, the differences in outcome could have resulted from the different reward paradigms being used (i.e., self-administration versus CPP). While it is generally reported that various drug classes produce concordant results when assessed using self-administration and CPP paradigms (Bardo and Bevins 2000), a few studies have found that the pattern of results produced by the two paradigms differs substantially. For instance, pretreatment with dopamine D2 receptor antagonists blocks cocaine self-administration but does not block the expression of cocaine-induced CPP (Cervo and Samanin 1995; Baker et al. 1998). Thus, while early methylphenidate exposure alters later responsiveness to drugs of abuse, it is still uncertain whether methylphenidate increases or decreases these rewarding effects.
The goal of the present study was to further investigate the effects of early methylphenidate treatment on cocaine-rewarded behavior using both the CPP and self-administration paradigms. To this end, separate groups of rats were treated with methylphenidate from postnatal day (PD) 11 to PD 20, with testing for cocaine-induced CPP or cocaine self-administration beginning on PD 60. It was predicted that early methylphenidate treatment would enhance the rewarding effects of cocaine using both the CPP and self-administration procedures.
Materials and methods
Subjects
A total of 209 male and female rats of Sprague-Dawley descent (Charles River, Hollister, CA, USA) were used. The rats were raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups on PD 3 (day of parturition = PD 0) and were kept with the dam until weaning on PD 25. After weaning, rats were group housed in maternity cages, with same-sex litter mates. Rats undergoing food deprivation were housed individually. The colony room was climate controlled (21-24 °C) and kept on a 12:12 light/dark cycle. Except for rats in the self-administration study, food and water was freely available. The self-administration rats were kept at approximately 90% of free feed weight to increase motivation to press the lever for a sucrose pellet or cocaine administration. All animals were treated in accordance with the “Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003) and a research protocol approved by the Institutional Animal Care and Use Committee at CSUSB.
Apparatus
Cocaine CPP conditioning and testing were conducted in rectangular wooden chambers that had three compartments: two large compartments and one small placement chamber, arranged in a truncated T-shape. The two large compartments (37 × 30 × 45 cm) were adjacent to each other and separated by a removable partition. The smaller placement chamber (18 × 18 × 45 cm) was located at the side of the junction between the two larger compartments and was connected to them by a removable partition. When opened, rats were able to enter either large compartment from the placement chamber. The color, flooring, and odor of the large compartments varied, with one having white walls, wire mesh flooring, and pine bedding; while the other compartment had black walls, metal rod flooring, and cedar bedding. The small placement chamber had gray walls and a solid wood floor.
Cocaine self-administration was conducted in standard operant-conditioning chambers (Coulbourn Instruments, Allentown, PA) measuring 29 × 26 × 33 cm. Each operant chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Two stainless steel response levers were located on the front wall 2 cm above the chamber floor on either side of a food aperture that was also located 2 cm above the floor. A stimulus light was located above one of the stainless steel levers (active lever) while the second lever (inactive lever) did not have a stimulus light. A press on the active lever was followed by reinforcement, while presses on the inactive lever had no scheduled consequences. On the wall opposite the levers was a house light which remained on throughout the duration of the testing session. A counterbalanced arm holding a liquid swivel was located near the ceiling of the chamber and was attached by Tygon tubing to a 20-ml syringe pump located outside the cubicle.
Drugs
Methylphenidate hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO). For the CPP experiment, cocaine hydrochloride was also obtained from Sigma-Aldrich, whereas the cocaine hydrochloride used in the self-administration experiment was supplied by the National Institute of Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). Methylphenidate was dissolved in saline and injected intraperitoneally (IP) at a volume of 5 ml/kg. Cocaine for the CPP experiment was dissolved in saline and injected subcutaneously (IP) at a volume of 1 ml/kg, while cocaine for the self-administration experiment was dissolved in sterile saline.
Procedures
In Vivo Drug Treatment
Beginning on PD 11, rats were weighed and injected with methylphenidate (0, 2, or 5 mg/kg IP) for 10 consecutive days. The 2.0 mg/kg dose of methylphenidate was selected as this dose produces clinically relevant levels of methylphenidate in the plasma (Gerasimov et al. 2000). We also chose a higher dose as preschool aged children are exposed to greater doses than school aged children because of age-related differences in absorption and metabolism (Wigal et al. 2007). The PD 11-PD 20 injection period was chosen because this age span is comparable to early childhood in humans (Andersen 2003, 2005). After methylphenidate pretreatment, rats were left undisturbed until testing.
Cocaine-Induced CPP Procedure
Following acclimation to handling from PD 55-PD 59, 113 male and female young adult rats (n = 6-7) from the three pretreatment conditions were randomly assigned to groups. The CPP procedure was identical to that previously described (McDougall et al 2008). Briefly, a 20-day biased designed CPP procedure was used, which consisted of one habituation day, eight conditioning days, one acquisition test day, eight extinction days, one extinction test day, and one reinstatement test. A biased design (i.e., all rats received drug in the white compartment) was used as the majority of rats showed a preference for the black compartment on the habituation day. On the habituation day (PD 60), rats were allowed free access to the black and white compartments of the CPP chamber for 15 min. Conditioning was conducted on PD 61-PD 68, with all rats receiving alternating daily placements into the white and black compartments. During conditioning rats were injected with cocaine (0, 10, or 20 mg/kg) and restricted to the white compartment or injected with saline and restricted to the black compartment for 30 min. On the acquisition test day (PD 69), rats were left uninjected and given free access to the black and white compartments for 15 min. Extinction occurred on the following eight days (PD 70-PD 77) and consisted of daily injections of saline and alternating 30 min placements in the black and white compartments. On the extinction test day (PD 78), rats were put into the placement chamber and allowing free access to the black and white compartments for 15 min. On the reinstatement test day (PD 79), all rats received a priming injection of cocaine (10 mg/kg IP) followed by free access to the black and white compartments for 15 min.
Self-Administration Procedure
Lever Press Training
On PD 60, 96 male and female rats (n = 7-9) were housed individually and trained to lever-press for sucrose pellets. At the beginning of each session, a sucrose pellet was placed on the active lever. The lever designated as active remained constant for each rat throughout the experiment. Training continued until rats received at least 50 pellets on an FR1 schedule for two days. Rats received 30-min handshaping sessions if lever training was not acquired within four days and they continued to receive training sessions until criterion was met. On the completion of lever training, indwelling jugular catheters were surgically implanted.
Surgical procedure
Rats were pretreated with atropine sulfate (10 mg/kg) to prevent bronchial secretions and to facilitate respiration, and then immediately anesthetized with a ketamine/ xylazine solution (80:6 mg/kg). If necessary, isoflourane anesthesia was used to complete the surgery. The catheters were constructed from silastic tubing connected to a bent 22 gauge metal cannula (Plastics One, Roanoke, VA). Catheters were inserted into a small incision in the jugular vein and secured with sutures. The other end of the catheter ran subcutaneously along the neck and exited through an incision across the skull. The metal cannula end was secured to the top of the skull using dental acrylic cement and small anchor screws drilled into the skull. After surgery, animals were treated with a commercially available solution of ketoprofen to control pain. Throughout the experiment, catheter patency was maintained by daily flushing with 0.1 ml bacteriostatic saline containing heparin sodium (70 USP U/ml, iv) and ticarcillin disodium (20 mg/ml, iv). For the first five days after surgery, streptokinase (0.67 mg/ml, iv) was also administered. Catheter patency was confirmed periodically by infusing the rapid, short-acting anesthetic methohexital sodium (16.7 mg/ml, iv).
Cocaine Self-Administration
After a five day recovery period, rats were randomly assigned to groups allowed to lever press for cocaine infusions (0.25 or 0.75 mg/kg, iv) on an FR1 schedule for 2-h. The 0.75 mg/kg dose was chosen as it produces robust responding on both fixed and progressive ratio schedules while the 0.25 mg/kg dose was used to assess possible shifts in the dose response curve (Lu et al. 2005; Liu et al. 2005, Zavala et al. 2007, 2008a, 2008b). Lever presses on the active lever resulted in simultaneous presentation of the stimulus light and a sound cue (500 Hz, 10 dB above background), followed 1 s later by an infusion of cocaine. The stimulus complex and infusion remained on for an additional 6 s. After each infusion, the active lever became inactive for 27 s, which was indicated by the house light turning off. After the 27 s inactivation, the active lever was reactivated and the house light was illuminated. When rats reached criterion (7 infusions per session for at least two days), they were moved to an FR3 schedule and then an FR10 schedule. Rats were tested on each schedule for a minimum of five days. After reaching criterion on the FR 10 schedule, rats were placed on a progressive ratio (PR) schedule where the number of responses necessary to receive the cocaine infusion increased exponentially through the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 693, 737, 901. Each PR session was terminated when the rat failed to complete the ratio for a particular reinforcer within 1 h after delivery of the previous reinforcer. Rats received five consecutive daily sessions of progressive ratio training. The number of reinforcements received, the last ratio successfully completed, the number of responses made on the active and inactive levers, and the number of trials necessary to reach criterion were recorded.
Data analysis
Body weights during the drug pretreatment phase for each experiment were analyzed using separate repeated measures analysis of variance (ANOVA; pretreatment dose × sex × day). Data for the CPP experiment were analyzed as preference scores (time spent in the drug paired compartment on the test day minus time spent on the habituation day) using ANOVA (sex × pretreament drug × conditioning drug). Data for the self-administration experiemnt (e.g., number of cocaine infusions and days to criteria,) were analyzed by separate ANOVAs (pretreament drug × conditioning drug × sex). When preliminary analyses found sex-dependent differences in responding, data were analyzed by separate ANOVAs (pretreament drug × conditioning drug) for each sex. Dunnett tests and Tukey tests were used for making post hoc comparisons (p < 0.05). In all cases, litter effects were controlled by assigning no more than one subject from a litter to a particular group.
Results
Cocaine-Induced CPP
Body Weight
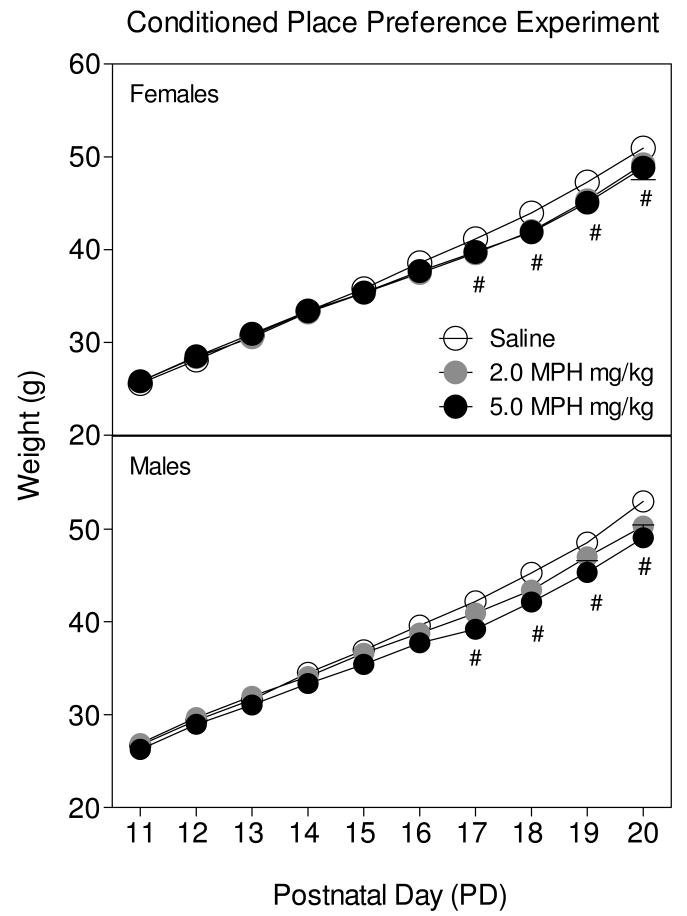
Body weight for male and female rats (Fig. 1) progressively increased across PD 11-PD 20 [day main effect, F9,954 = 5401.86, p < 0.001]. Rats pretreated with 5.0 mg/kg MPH exhibited reduced body weights compared to saline controls on PD 17 and 20 [day × pretreatment dose interaction, F18, 954 = 11.48, p < 0.001, Dunnett tests]. Body weight was not affected by sex. By PD 60 body weights of the methylphenidate- and saline-pretreated rats did not differ, although male rats (M= 372.2 g, SEM ± 4.8) weighed significantly more than females (M= 230.7 g, SEM ± 2.5) [Sex main effect: F1,107 = 702.91; p < 0.001].
Fig 1.
Mean body weight in grams (±SEM) of male and female rats (n =16-21) treated with methylphenidate (0, 2, or 5 mg/kg) from PD 11-PD 20. Rats were assessed for cocaine-induced CPP as adults. #Indicates a significant difference between rats pretreated with 5.0 mg/kg MPH from rats pretreated with saline.
CPP
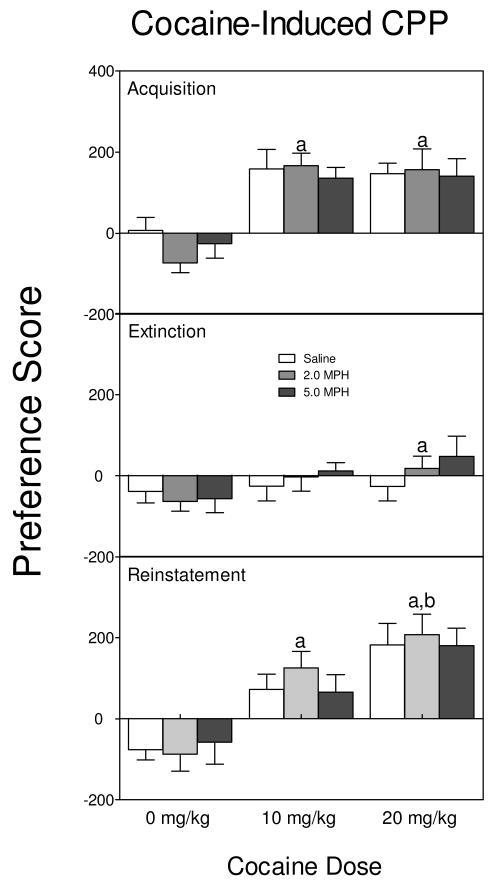
On the acquisition day, rats given cocaine (10 or 20 mg/kg) showed a preference for the drug-paired compartment (Fig. 2, upper graph) when compared to rats given saline [cocaine main effect, F2, 95 = 27.22, p < 0.001, Tukey tests]. This preference for the drug-paired compartment was not affected by sex or methylphenidate treatment. After eight days of extinction training (Fig. 2, middle graph), rats conditioned with 20 mg/kg cocaine spent more time in the drug-paired compartment than rats given saline [cocaine main effect, F2, 95 = 3.85, p < 0.05, Tukey tests]. After a priming dose of cocaine (10 mg/kg), the preference for the drug-paired compartment was robustly reinstated in rats previously conditioned with either the 10 or 20 mg/kg cocaine (Fig. 2, lower graph) [cocaine main effect, F2, 95 = 27.73, p < 0.001, Tukey tests]. In addition, rats conditioned with the higher dose of cocaine (20 mg/kg) spent more time in the drug-paired compartment when compared to rats conditioned with the lower dose (10 mg/kg). There was no evidence that methylphenidate affected the acquisition, extinction or reinstatement of cocaine-induced CPP.
Fig 2.
Mean Preference score (±SEM) on the acquisition, extinction, and reinstatement test day. Male and female rats (n = 6-7) were given alternating daily injections of cocaine (0, 10, or 20 mg/kg) and saline from PD 61-PD 68 and a test day on PD 69. Acquisition training was followed by eight extinction days where rats were given saline each day, with another test day on PD 78. On PD 79, all rats were injected with 10 mg/kg cocaine and given a final reinstatement test day. Rats were previously exposed to methylphenidate (0, 2, or 5 mg/kg) from PD 11-PD 20. aIndicates a significant difference from rats conditioned with 0 mg/kg cocaine. bIndicates a significant difference from rats conditioned with 10 mg/kg cocaine.
Cocaine self administration
Body Weight
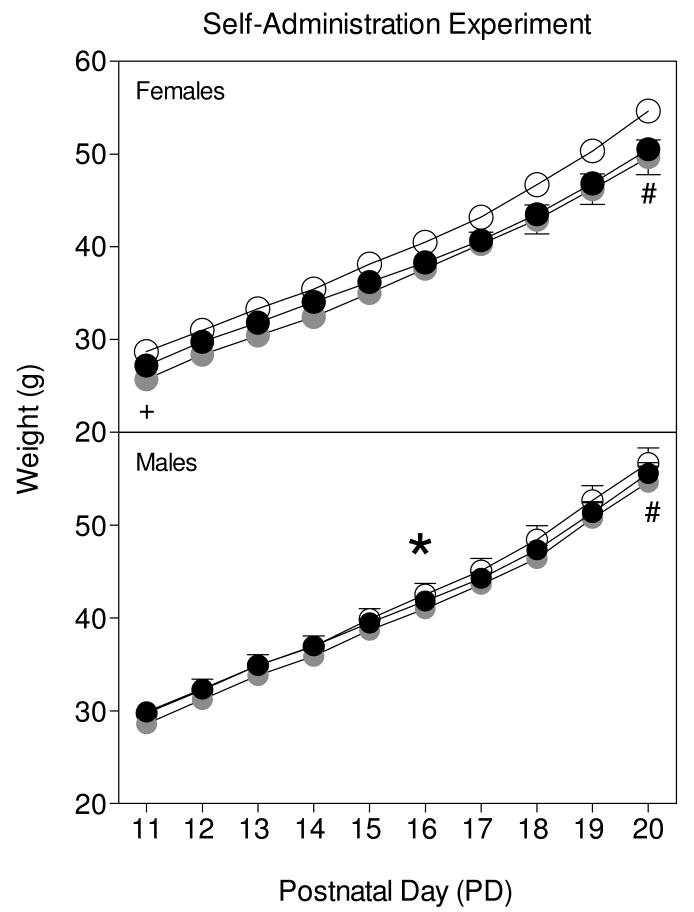
Body weight for male and female rats (Fig. 3) progressively increased across PD 11- PD 20 [day main effect, F9,810 = 3946.33, p < 0.001]. Rats pretreated with 2.0 mg/kg MPH exhibited reduced body weights compared to saline controls on PD 20 [day × pretreatment dose interaction, F18, 810 = 3.25, p < 0.001, Dunnett tests]. In addition, male rat pups had significantly greater body weights as compared to females pups [sex main effect, F1,90 = 11.40, p <0.001] Adult body weights of the methylphenidate- and saline-pretreated rats did not differ, although male rats (M = 387.5 g, SEM ± 5.4) weighed significantly more than females (M= 241.63 g, SEM ± 3.1) [Sex main effect: F1,85 = 567.29; p < 0.001].
Fig 3.
Mean body weight in grams (±SEM) of male and female rats (n =14-17) treated with methylphenidate (0, 2, or 5 mg/kg) from PD 11-PD 20. Rats were assessed for cocaine self-administration as adults. #Indicates a significant difference between rats pretreated with 2.0 mg/kg MPH from rats pretreated with saline. *Indicates a significant difference from female rats.
Fixed Ratio Schedule
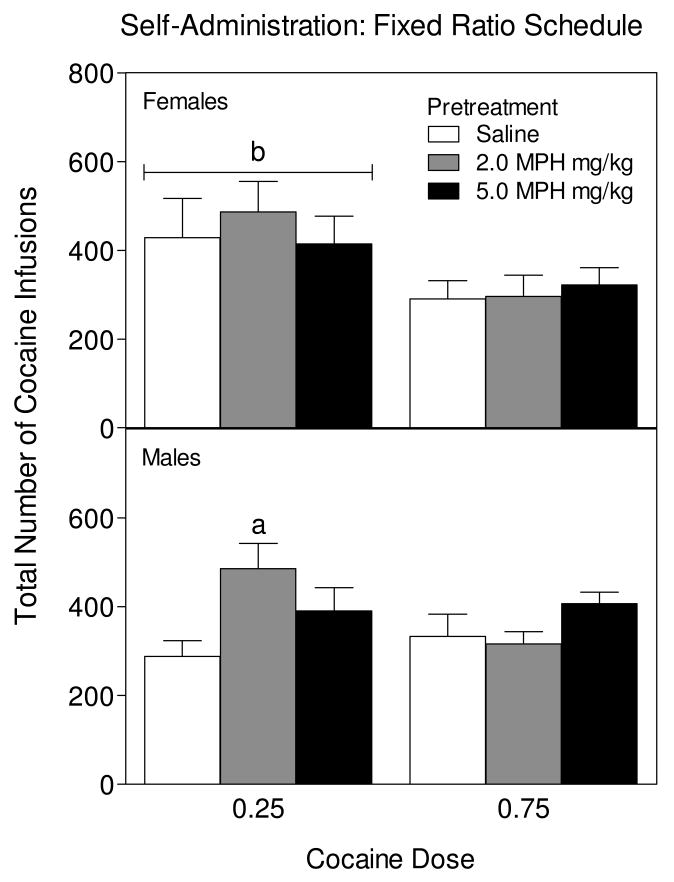
In female rats, the number of days to criterion on each FR schedule was not affected by cocaine dose or methylphenidate treatment (FR1, M = 5 days to criterion, ±0.0 SEM; FR3, M = 5.85 days, ±0.37 SEM; FR10, M = 5.76 days, ±0.33 SEM). The total number of infusions earned varied depending on the dose of cocaine (Fig. 4, upper graph), because female rats trained with the lower dose of cocaine (0.25 mg/kg) received more infusions than female rats given the higher dose of cocaine [cocaine main effect, F1, 40 = 7.95, p < 0.05]. In female rats, early exposure to methylphenidate did not alter the number of cocaine infusions earned.
Fig 4.
Mean total number of cocaine infusions (0.25 or 0.75 mg/kg/infusion) earned by 46 adult female rats (n = 7-8) and 50 adult male rats (n = 8-9) during self-administration training and trained on a FR schedule of reinforcement. Rats were previously exposed to methylphenidate (0, 2, or 5 mg/kg) from PD 11-PD 20. aIndicates a significant difference from rats pretreated with saline and trained with 0.25 mg/kg cocaine. bIndicates a significant difference from rats trained with 0.75 mg/kg cocaine.
In male rats, the number of days to reach criterion on each FR schedule was unaffected by cocaine dose or methylphenidate exposure (FR1, M = 5.76 days to criterion, ±0.37 SEM; FR3, M = 5.72 days, ±0.36 SEM; FR10, M = 6.08 days, ±0.54 SEM). Male rats did differ from females, however, because the number of cocaine infusions received by male rats varied according to methylphenidate exposure condition and cocaine dose. Specifically, male rats pretreated with 2 mg/kg methylphenidate and trained with the 0.25 mg/kg cocaine dose earned more cocaine infusions than similarly trained saline-pretreated rats (Fig. 4, lower graph) [pretreatment × cocaine dose interaction, F2, 44 = 3.66, p < 0.05, Tukey tests]. In contrast, the number of cocaine infusions were not significantly affected in male rats pretreated with 5 mg/kg methylphenidate and trained with the 0.25 mg/kg cocaine dose or in both methylphenidate pretreated groups trained with the 0.75 mg/kg cocaine dose.
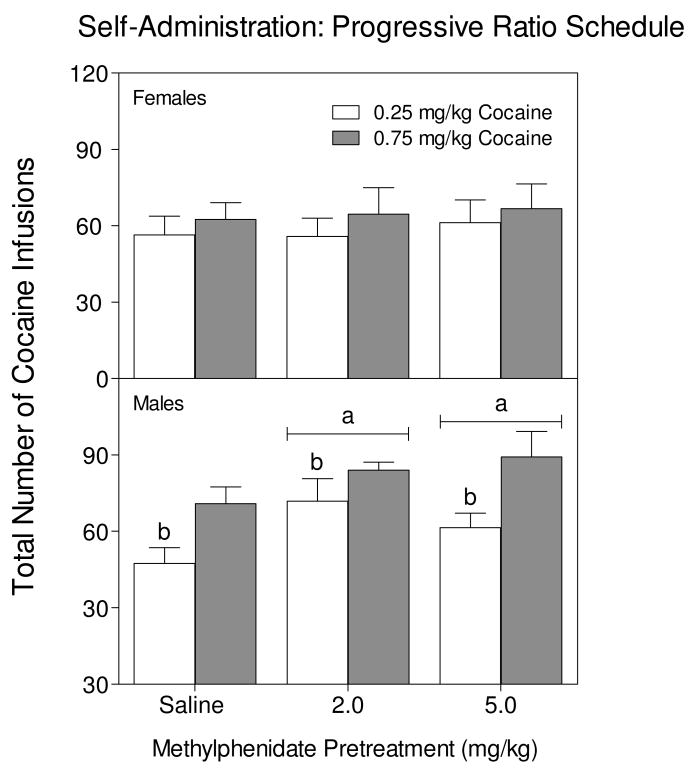
Progressive Ratio Schedule
Responding on the progressive ratio schedule differed according to sex because female rats earned fewer cocaine infusions than male rats (females, M=62.91±3.29 SEM; males, M =73.04, ±3.49 SEM) [sex main effect, F1,91 = 4.82, p <0.05]. In female rats, responding for cocaine on a PR schedule was not affected by methylphenidate exposure or cocaine dose, because female rats obtained similar amounts of cocaine regardless of group (Fig. 5, upper graph). In male rats, the reverse was true because both cocaine dose and methylphenidate exposure affected the number of cocaine infusions earned (Fig. 5, lower graph). Specifically, male rats trained with the higher cocaine dose (0.75 mg/kg), regardless of methylphenidate pretreatment condition, had higher break points (i.e., more cocaine infusions) than male rats trained with the lower dose of cocaine [cocaine main effect, F 1, 44= 12.34, p < 0.001]. Methylphenidate exposure also increased the break point, because rats pretreated with either dose of methylphenidate (2.0 or 5.0 mg/kg) had higher breakpoints when compared to saline-pretreated rats [pretreatment main effect, F 2, 44 = 3.76, p < 0.05, Dunnett tests].
Fig 5.
Mean total number of cocaine infusions (0.25 or 0.75 mg/kg) earned by adult male and female rats during the PR schedule of reinforcement. Rats were previously exposed to methylphenidate (0, 2, or 5 mg/kg) from PD 11-PD 20. These are the same rats as in Fig. 4. aIndicates a significant difference from saline-pretreated rats. bIndicates a significant difference from rats trained with 0.75 mg/kg cocaine.
Discussion
Early treatment with methylphenidate alters the rewarding value of cocaine in adult rats (Brandon et al. 2001; Andersen et al. 2002; Carlezon et al. 2003). However, the direction of methylphenidate's effects on cocaine-rewarded behavior is unclear, because a self-administration study indicated that early methylphenidate exposure increased the rewarding effects of cocaine (Brandon et al. 2003), while CPP studies show that methylphenidate decreased cocaine reward effectiveness (Andersen et al. 2002; Carlezon et al. 2003). Results from the present study suggest that methylphenidate's ability to alter the rewarding properties of cocaine varies according to the type of reward paradigm utilized. Specifically, we found that methylphenidate increased the rewarding strength of cocaine when measured using a self-administration procedure, but methylphenidate did not alter the magnitude of cocaine-induced CPP.
In the current investigation, we used both FR and PR schedules to assess methylphenidate-induced changes in cocaine reward strength in male and female young adult rats. In female rats, training on an FR schedule of reinforcement produced reliable lever press responding that was sensitive to cocaine dose. Specifically, female rats received more cocaine-infusions when trained with the lower dose of cocaine (0.25 mg/kg) than the 0.75 mg/kg dose. A similar dose response effect has been reported for female rats on an FR3 schedule, in which peak responding occurred at 0.125 mg/kg/infusion (Kosten et al. 2006). A dose-dependent effect was not apparent on the PR schedule, because females received a similar number of infusions using both the low and high dose. Methylphenidate treatment did not alter the number of cocaine infusions received by female rats on any of the FR or PR schedules.
Interestingly, early methylphenidate exposure did enhance cocaine reward strength on the FR schedule in male rats. Specifically, male rats pretreated with methylphenidate (2 mg/kg) received more cocaine infusions than saline-pretreated males. Cocaine dose and methylphenidate pretreatment also produced differences in the number of cocaine infusions earned by males rats on the PR schedule. Regardless of methylphenidate treatment condition, male rats worked harder (i.e., had greater breakpoints) for 0.75 mg/kg cocaine when compared to the 0.25 mg/kg dose. This preference for the higher dose of cocaine is consistent with results from other progressive ratio studies (Mandt et al. 2008). Early methylphenidate treatment also increased the breakpoints of male rats, because males pretreated with either 2 or 5 mg/kg methylphenidate received significantly more cocaine infusions than saline-pretreated rats. When results from relevant studies are considered together, it appears that reinforcing strength of self-administered cocaine is enhanced when methylphenidate exposure occurs on PD 11-PD 20 (present study) or PD 35-PD 45 (Brandon et al. 2001).
Few studies using early methylphenidate treatment have included female subjects, but it now appears that female rats are generally less sensitive to the effects of methylphenidate pretreatment. In our previous studies, methylphenidate's ability to enhance morphine-induced CPP did not vary according to sex; however, methylphenidate did cause a greater potentiation of morphine-induced hyperthermia and antinociception in males than females (Crawford et al. 2007; Halladay et al. 2009). The reason for this sex difference is unclear but it is possible that variations in gonadal hormones are responsible because estrogens are neuroprotective against a variety of neurotoxic agents and female rat pups have larger serum levels of estrodial than male rats on PD 1-21 (Banu et al. 2002; Brann et al. 2007; Arnold and Beyer 2009). In any case these findings suggest that: (1) early methylphenidate exposure can affect male and female rats differently and (2) male rats may be more sensitive to the effects of methylphenidate than females.
As expected, cocaine administration enhanced the preference for a previously non-preferred environment (i.e., cocaine-induced CPP). We also found that preference for the drug-paired compartment was no longer apparent after extinction training and that it was robustly reinstated after a cocaine priming injection. The initial acquisition of cocaine-induced CPP was similar after both a moderate (10 mg/kg) and a high (20 mg/kg) dose of cocaine. This was not surprising because the acquisition of cocaine-induced CPP in rodents is often not sensitive to dose (Bardo et al. 1995; Bardo and Bevins 2000). In contrast to the acquisition results, dose effects were evident after an extended extinction phase and after a cocaine prime (10 mg/kg). Specifically, rats treated with the higher dose of cocaine (20 mg/kg) extinguished slower (i.e., spent more time in the formerly drug paired room) after eight extinction sessions than saline-treated. Moreover, we found that the higher dose of cocaine reinstated a greater preference for the formerly drug-paired compartment than 10 mg/kg cocaine. The acquisition of cocaine-induced CPP was similar for both male and female rats. This result again was not surprising because sex effects are only inconsistently found, with some studies showing greater CPP in females (Russo et al. 2003; Balda et al. 2006; Zakharova et al. 2009) and others studies showing no sex differences (Crawford et al. 1995; Campbell and Spear 1999; McDougall et al. 2008). The studies showing sex differences reveal that female rats acquire cocaine-induced CPP at lower doses and with fewer drug pairings than male rats, but both sexes respond similarly when a larger number of drug pairings and higher doses of cocaine are used (Russo et al. 2003; Zakharova et al. 2009). Thus, sex differences were probably not apparent in our study because of the number of drug-environment pairings (4 exposures to each compartment) and the relatively high doses of cocaine (10 and 20 mg/kg) used.
Interestingly, results from our CPP experiment showed that early methylphenidate exposure on PD 11-PD 20 did not affect cocaine-induced reward. In contrast, rats exposed to methylphenidate during the preadolescent period (PD 20-PD 35) are reported to show a significantly reduced preference for the cocaine-paired compartment (Andersen et al. 2002; Carlezon et al. 2003). Likewise, mice exposed to methylphenidate from PD 26-PD 32 exhibit a weaker cocaine-induced CPP than control mice, but these methylphenidate-treated animals also show enhanced reinstatement after both extinction training and a cocaine priming injection (Achat-Mendes et al. 2003). These discrepant results may indicate that methylphenidate exposure during the preweanling period (PD 11-PD 20) is insufficient to induce long-term changes in neuronal reward system functioning that are detectable using the CPP procedure. This hypothesis is probably incorrect because methylphenidate exposure during the same time period does enhance morphine-induced CPP, sucrose reinforced lever pressing, and cocaine self-administration (see Fig. 4 and 5; also Crawford et al. 2007). Alternatively, it is possible that neural changes produced by early methylphenidate exposure during the preweanling period selectively impact sensitivity to cocaine, but not cocaine-associated environmental cues. This dissociation between the neural substrates for cocaine-conditioned cues and cocaine has been previously demonstrated, because Brown et al. (2008) showed that the NMDA antagonist, MK-801, blocks reinstatement of cocaine-induced CPP but not cocaine self-administration.
Another possibility is the differences in self-administration and CPP found in the current study may have been modulated by variations in housing and food deprivation conditions. In the self-administration experiment rats were individually housed and food deprived while rats in the CPP experiment were group housed with food available ad libitum. While housing condition seems to have a fairly limited effect on cocaine reward, several studies have demonstrated that food restriction or deprivation enhances the rewarding effects of cocaine in both self-administration and CPP paradigms (see Lu et al. 2003 and Bardo et al. 1995 for review). Thus, it is possible that the effects of methylphenidate on reward are small and were potentiated by the food deprivation in the self-administration experiment. Food deprivation, however, is not necessary to find enhanced CPP following methylphenidate pretreatment because we previously reported increased morphine-induced CPP using nearly identical housing and CPP procedures (Crawford et al. 2007).
In summary, exposure to methylphenidate during the preweanling period increases the strength of cocaine as a reinforcer when measured by self-administration but not CPP. The effects of methylphenidate exposure are sex-dependent, because only males showed enhanced cocaine self-administration. When considered together, these data suggest that early exposure to methylphenidate may enhance the effects of cocaine in males, but may not increase susceptibility to relapse or long-term use.
Acknowledgments
We gratefully acknowledge Dr. Janet Neisewander for her substantial assistance with the self-administration procedure. We also thank Dr. Sanders McDougall for his meticulous proofreading of the manuscript. This work was supported by PHS grant GM073842.
References
- Achat-Mendes C, Anderson KL, Itzhak Y. Methylphenidate and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropharmacology. 2003;45:106–115. doi: 10.1016/s0028-3908(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends in Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;111:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Baker DA, Fuchs RA, Specio SE, Khroyan TV, Neisewander JL. Effects of intraaccumbens administration of SCH-23390 on cocaine-induced locomotion and conditioned place preference. Synapse. 1998;30:181–193. doi: 10.1002/(SICI)1098-2396(199810)30:2<181::AID-SYN8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Banu SK, Govindarajulu P, Aruldhas MM. Developmetnal profiles of TSH, sex steroids, and their receptors in the thyroid and their relevance to thyroid growth in immature rats. Steroids. 2002;67:137–144. doi: 10.1016/s0039-128x(01)00144-1. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Biederman J, Farone S. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National health interview survey, 2007. Vital Health Stat. 2009;239:1–88. [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh V, Khan MM. Neurotropic and neuroprotective actions of estrogen: basic mechanism and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Villafranca SW, Cyr MC, Farley CM, Reichel CM, Gheorghe SL, Krall CM, McDougall SA. Effects of early methylphenidate exposure on morphine- and sucrose-reinforced behaviors in adult rats: Relationship to dopamine D2 receptors. Brain Res. 2007;1139:245–253. doi: 10.1016/j.brainres.2006.12.079. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2000;48:131–137. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: A review of its locomotor effects. Brain Res Bull. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Dopheide JA, Pliszka SR. Attention-deficit-hyperactivity disorder: an update. Pharmacotherapy. 2009;29:658–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- Gaytan O, al-Rahim S, Swann A, Dafny N. Sensitization to locomotor effects of methylphenidate in the rat. Life Sci. 1997;61:PL101–PL107. doi: 10.1016/s0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- Halladay LR, Iñiguez SD, Furqan F, Previte MC, Chisum AM, Crawford CA. Methylphenidate potentiates morphine-induced antinociception, hyperthermia, and locomotor activity in young adult rats. Pharmacol Biochem Behav. 2009;92:190–196. doi: 10.1016/j.pbb.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology. 2006;31:70–76. doi: 10.1038/sj.npp.1300779. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DCS, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Shaham Y, Hope BT. Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J Neurochem. 2005;94:161–168. doi: 10.1111/j.1471-4159.2005.03178.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:497–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. Individual differences in cocaine-induced locomotor activity in male Sprague-Dawley rats and their acquisition of and motivation to self administer cocaine. Psychopharmacology (Berl) 2008;201:195–202. doi: 10.1007/s00213-008-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Reichel CM, Farley CM, Flesher MM, Der-Ghazarian T, Cortez AM, Wacan JJ, Martinez CE, Varela FA, Butt AE, Crawford CA. Postnatal manganese exposure alters dopamine transporter function in adult rats: potential impact on nonassociative and associative process. Neuroscience. 2008;154:846–860. doi: 10.1016/j.neuroscience.2008.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millichap JG. Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e1358–e1365. doi: 10.1542/peds.2007-1332. [DOI] [PubMed] [Google Scholar]
- Motoyo Y, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas RW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:193–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;38:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Scheffler RM, Brown TT, Fulton BD, Hinshaw SP, Levine P, Stone S. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123:1273–1279. doi: 10.1542/peds.2008-1597. [DOI] [PubMed] [Google Scholar]
- Souza RP, Soares EC, Rosa DVF, Souza BR, Réus GZ, Barichello T, Gomes KM, Gomez MV, Quevedo J, Romano-Silva MA. Methylphenidate alter NCS-1 expression in rat brain. Neurochem Int. 2008;53:12–16. doi: 10.1016/j.neuint.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Sproson EJ, Chantrey J, Hollis C, Marsden CA, Fone KCF. Effect of repeated methylphenidate administration on presynaptic dopamine and behavior in young adult rats. J Psychopharmacol. 2001;152:67–75. doi: 10.1177/026988110101500202. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, Kollins S, Nguyen As, DeCory HH, Dirksen SJH, Hatch S, Comacs Study Group A comparison of once-daily exstended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (The Comacs Study) Pediatrics. 2004;113:e206–e216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Gupta S, Greenhill L, Posner K, Lerner M, Steinhoff K, Wigal T, Kapelinski A, Martinez J, Modi NB, Stehli A, Swanson J. Pharmacokinetics of methylphenidate in preschoolers with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmcol. 2007;17:153–164. doi: 10.1089/cap.2007.0043. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and Glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Euro Neuropsychopharmacol. 2008a;18:600–611. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008b;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]