Abstract
Objective
To determine if socioemotional disinhibition and executive dysfunction are related to dissociable patterns of brain atrophy in neurodegenerative disease. Previous studies have indicated that behavioral and cognitive dysfunction in neurodegenerative disease are linked to atrophy in different parts of the frontal lobe, but these prior studies did not establish that these relationships were specific, which would best be demonstrated by a double dissociation.
Method
Subjects included 157 patients with neurodegenerative disease. A semi-automated parcellation program (Freesurfer) was used to generate regional cortical volumes from structural MRI scans. Regions of interest (ROIs) included anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), middle frontal gyrus (MFG) and inferior frontal gyrus (IFG). Socioemotional disinhibition was measured using the Neuropsychiatric Inventory. Principal component analysis including three tasks of executive function (EF; verbal fluency, Stroop Interference, modified Trails) was used to generate a single factor score to represent EF.
Results
Partial correlations between ROIs, disinhibition, and EF were computed after controlling for total intracranial volume, MMSE, diagnosis, age, and education. Brain regions significantly correlated with disinhibition (ACC, OFC, IFG, and temporal lobes) and EF (MFG) were entered into separate hierarchical regressions to determine which brain regions predicted disinhibition and EF. OFC was the only brain region to significantly predict disinhibition and MFG significantly predicted executive functioning performance. A multivariate general linear model demonstrated a significant interaction between ROIs and cognitive-behavioral functions.
Conclusions
These results support a specific association between orbitofrontal areas and behavioral management as compared to dorsolateral areas and EF.
Keywords: Brain behaviour and relationships, prefrontal, emotion regulation, executive control
Introduction
Dysfunction in the prefrontal cortex (PFC) is associated with a variety of neurocognitive and behavioral syndromes ranging from difficulty with working memory, concentration, and multitasking to depression, obsessive-compulsive behavior, and impaired social behavior. A large body of evidence suggests that the diversity of impairments seen with frontal lobe disease is due to the fact that various subregions within the PFC mediate different aspects of cognitive and emotional function (Adolphs, 2003; Dolan, 2002). Because brain pathology often affects the frontal lobes in a relatively focal pattern, a better understanding of the division of labor within the frontal lobe is important for improved counseling of patients and can be valuable for early diagnosis and differential diagnosis, particularly in emerging neurodegenerative diseases (Gregory & Hodges, 1996; Nedjam et al., 2004).
Although our knowledge of the functional topography in the frontal lobes is incomplete, one commonly held view holds that the dorsal and lateral prefrontal regions are most closely associated with cognitive control, problem solving, and planning abilities (Duncan & Owen, 2000; Jamadar et al., 2010), while the medial (particularly the ventromedial) and orbital frontal regions are more closely associated with emotional and social processing, including tracking the hedonic value of environmental stimuli, registering the consequences of actions for the organism's well-being, and tracking social and emotional cues in the internal and external environments (Kouneiher et al., 2009; Ridderinkof et al., 2004). The dorsal-ventral dichotomy was introduced into behavioral analysis after being observed in patients with focal lesions (Blumer & Benson, 1975) and schizophrenia (Goldberg, 1985). Among the many studies that have contributed to this view are studies of patients with focal brain lesions, which have linked damage in the ventromedial prefrontal and orbitofrontal region to impaired modulation of social behavior (Beer, John, Scabini, & Knight, 2006; Eslinger & Damasio, 1985; Hornak et al., 2003; Hornak, Rolls, & Wade, 1996) and linked dorsolateral prefrontal injury with impaired cognitive regulation, including problems with error monitoring and set shifting (B. Yochim, Baldo, Nelson, & Delis, 2007; B. P. Yochim, Baldo, Kane, & Delis, 2008).
Similar brain-behavior relationships have been found in neurodegenerative diseases where impaired socioemotional behavior has been associated with cortical volume loss or hypometabolism in the ventromedial prefrontal cortex (Peters et al., 2006; Rosen et al., 2005; Zamboni et al., 2008) and problems with executive functioning have been liked with changes in the dorsolateral prefrontal cortex (Aizenstein et al., 2009; Ohrmann et al., 2008; Watari et al., 2008). In most cases, however, studies in neurodegenerative disease have examined brain-behavior relationships using whole brain, voxel-based methods, which identify regions that relate to the behavior of interest, but these studies do not typically account for variation in other potentially relevant regions and thus they do not establish that the regions identified make independent contributions to the function of interest after accounting for other relevant regions. This is particularly the case for behaviors dependent on the frontal lobes, because these functions may be based on several component processes and thus become impaired from damage to more than one region (O'Doherty, 2004). Conceivably, impaired regulation of socioemotional behavior in neurodegenerative disease could be due to failed processing in dorsolateral regions mediating classical executive functions. This potential overlap between cognitive and socioemotional functions is even reflected in terminology used to describe frontal lobe functions. For example, the term inhibition, usually used to refer to the ability to suppress prepotent responses in the service of task performance, is an executive function, and usually linked to dorsal and lateral frontal regions (Levine, Stuss, & Milberg, 1997); however, the term disinhibition is often used to refer to the social and emotional impairments seen in patients with ventromedial frontal lobe disease, who show disregard for social conventions, impulsivity, and poor risk assessment-deficits that appear to result from a lack of ability to suppress one's impulses (Wittenberg et al., 2008). This overlap in potential mechanisms leading on the one hand to poor performance on tasks such as the Stroop task and on the other hand to socioemotional impairments seen in some neurodegenerative diseases raises the general question of whether the socioemotional impairments are caused by a failure of executive functions or whether they represent independent processes. Indeed, many theories and empirical studies of emotion highlight the role of classical “cognitive” processing in mediating emotional functioning (Olsson & Ochsner, 2008; Phelps, 2006). Dissociating the anatomical regions associated with socioemotional disinhibition from those associated with classical executive dysfunction would provide support for the idea that the socioemotional problems seen in neurodegenerative disease arise from processes that are at least partially independent of classical executive functions.
The current study examined whether the brain regions contributing to socioemotional disinhibition do so independently of executive functioning, and whether conversely, executive functioning has brain relationships that are independent of those associated with socioemotional disinhibition. To accomplish this, we examined the neural correlates of socioemotional disinhibition and executive functioning (EF) in a mixed sample of elderly patients with neurodegenerative disease. Our hypotheses were based on models of prefrontal function suggesting that medial frontal areas mediate socioemotional control, and dorsolateral structures are more heavily involved in executive functioning.
Methods
Subjects
We studied 157 well-characterized patients (mean age 64.5 years, standard deviation = 8.7) with neurodegenerative disease recruited through the University of California San Francisco Memory and Aging Center. Criteria for inclusion were a diagnosis of a neurodegenerative disease, the availability of valid Neuropsychiatric Inventory (NPI) data (see below), the availability of valid neuropsychological data (see below), a total Mini-Mental State Examination (MMSE) score > 15, and a high-quality research MRI scan within 6 months of the NPI and neuropsychological assessment. Diagnoses included Alzheimer's disease (AD, n = 35), behavioral-variant frontotemporal dementia (bvFTD, n = 32), semantic dementia (SD, n = 18), progressive non-fluent aphasia (PNFA, n = 6), progressive supranuclear palsy (PSP, n = 8), corticobasal degeneration (CBD, n = 10), and mild cognitive impairment (MCI, n = 48). A heterogeneous group of patients was chosen in order to mitigate the contribution of disease specific regional atrophy to the analysis. Patients were diagnosed using published diagnostic criteria (Boeve, Lang, & Litvan, 2003; Litvan et al., 1996; McKhann et al., 1984; Neary et al., 1998; Petersen et al., 2001) after a comprehensive evaluation including neurological history and examination, a nursing evaluation, laboratory evaluation, and neuropsychological testing of memory, executive function, language, visuospatial skills and mood using a previously described standard protocol (Kramer et al., 2003). The neurologist recorded all co-morbid medical conditions during the clinical history, and the diagnostic determination included consideration of whether there were any co-morbid conditions that would cast doubt on the determination that the cognitive or behavioral impairment is due to a neurodegenerative disease. Patients with such conditions resulting in diagnostic uncertainty were not included in this analysis. See Table 1 for demographic, neurobehavioral, neuropsychological, and functional information for all patients and by diagnosis. Premorbid intellectual functioning was in the average range overall, as estimated by the Information subscale from the WAIS-IV, a measure of general information acquired from culture (Mean scaled score=9.0, SD=3.7). Current intellectual functioning was in the low average range overall, as estimated by the Block Design subscale from the WAIS-IV, a measure of fluid abstract reasoning and problem solving (Mean scaled score=7.6, SD=4.0). Sixteen subjects had Clinical Dementia Rating (CDR) (Morris, 1997) scores of 0, 78 had scores of 0.5, 51 had scores of 1.0, 10 had scores of 2.0, and 2 had unknown scores. 125 patients were right handed while 10 were left handed, 3 ambidextrous, and 19 of unknown handedness. The sample's ethnic breakdown was as follows: 143 Caucasian, 1 African American, 4 Asian American, 3 of mixed or other ethnicity, and 6 unknown.
Table 1.
General demographic, neurobehavioral, neuropsychological, and functional information for all subjects and by diagnosis.
| Variable | All | AD | FTD | SD | PNFA | PSP | CBD | MCI |
|---|---|---|---|---|---|---|---|---|
| Age | 64.5 (8.7) |
61.5 (8.5) |
61.0 (7.5) |
63.6 (7.4) |
58.0 (7.0) |
68.0 (7.2) |
69.3 (6.2) |
68. (9.0) |
| Education | 16.3 (2.6) |
15.0 (2.9) |
16.6 (2.5) |
15.9 (2.7) |
17.2 (1.0) |
17.3 (3.0) |
15.6 (2.1) |
17.1 (2.2) |
| MMSE | 25.8 (3.9) |
22.0 (3.7) |
25.9 (3.5) |
25.3 (4.2) |
26.3 (4.4) |
26.6 (2.2) |
25.9 (2.5) |
28.5 (1.6) |
| CDR | 0.7 (0.5) |
.92 (.40) |
1.1 (.47) |
.53 (.32) |
.42 (.20) |
.88 (.52) |
.50 (.62) |
.42 (.19) |
| CDR box score | 3.7 (3.0) |
5.3 (1.8) |
6.7 (2.7) |
2.7 (1.8) |
2.1 (1.5) |
5.8 (2.4) |
2.8 (3.6) |
1.1 (0.9) |
| NPI Disinhibition | 2.0 (3.5) |
0.2 (0.5) |
6.7 (3.9) |
3.8 (4.2) |
0.7 (1.6) |
1.4 (2.1) |
0.9 (1.5) |
0.0 (0.2) |
| D-words | 10.3 (6.1) |
8.9 (5.1) |
9.5 (7.2) |
7.1 (3.8) |
6.0 (7.1) |
6.5 (1.8) |
7.4 (5.9) |
15.3 (3.6) |
| Stroop Interference | 31.4 (19.3) |
15.0 (11.3) |
30.8 (18.0) |
32.0 (16.1) |
28.6 (18.4) |
23.0 (9.2) |
18.1 (10.2) |
47.7 (15.8) |
| Modified Trails | 68.8 (41.1) |
99.6 (32.3) |
76.2 (38.9) |
56.3 (37.4) |
74.4 (43.0) |
90.2 (36.0) |
98.2 (30.9) |
35.5 (24.6) |
| EF factor score | 0.0 (1.0) |
.07 (.7) |
.01 (1.0) |
.01 (.8) |
.02 (1.1) |
.06 (.6) |
.08 (.6) |
0.0 (.6) |
| Gender (M:F) | 80:77 | 16:19 | 23:9 | 10:8 | 1:5 | 4:4 | 4:6 | 22:26 |
Abbreviations: AD=Alzheimer's disease, bvFTD= behavioral-variant frontotemporal dementia, SD=semantic dementia, PNFA=progressive non-fluent aphasia, PSP=progressive supranuclear palsy, CBD=corticobasal degeneration, MCI=mild cognitive impairment, MMSE=Mini-Mental State Examination, CDR=Clinical Dementia Rating, NPI=Neuropsychiatric Inventory
This study was approved by the UCSF committee on human research, with consent being obtained by the patient, or an authorized surrogate if the patient was deemed by the staff obtaining consent not to have the capacity to consist. All patients were asked to assent to participation, even if they could not formally consent.
Measure of socioemotional disinhibition
For the purposes of this paper, we will refer to the socioemotional impairments that appear to reflect a lack of restraint and social decorum as simply disinhibition. Disinhibition was measured using the Neuropsychiatric Inventory (NPI; (Cummings, 1997). The NPI is a validated, caregiver-based behavioral rating system developed for the assessment of dementia that evaluates the presence or absence, severity (rated 1-3, 3 being most severe) and frequency (rated 1-4, 4 being more frequent) of twelve major behavior disorders, including disinhibition. An index of severity is created for each behavioral variable by multiplying the frequency and severity scores, creating a frequency by severity product (F X Sprod – see (Levy, Miller, Cummings, Fairbanks, & Craig, 1996). Between-rater reliability across NPI domains varies between 93.6 and 100%, while test-retest reliability correlations are 0.79 for frequency and 0.86 for severity (Cummings, 1997). Between rater-reliability for the disinhibition scale was reported as 100%, while test-retest reliability correlations were 0.97 for frequency and 0.87 for severity (Cummings et al., 1994).
The NPI begins with one probe question for each scale, and if this is endorsed, additional test items are administered to more fully characterize the patient's behavioral symptoms. The initial probe question for the NPI Disinhibition scale is, “Does the patient act impulsively, or without thinking? Do they do or say things that are usual?” Ratings were obtained from caregivers, usually immediate family members, by a geriatric specialized nurse trained in its administration who made the judgment about the consistency and reliability of the data. NPI scores from suspect raters were not used.
Measure of executive function
All subjects were administered three tasks to measure executive functioning, including verbal fluency (d-word generation: number correctly generated in one minute), response inhibition (Stroop Interference Test: total number correct when identifying color of words printed in a dissonant color of ink), and set shifting (modified Trails (Kramer et al., 2003): completion time). The neuropsychological evaluation was always collected by a clinician trained in its administration. A principal component analysis using a varimax rotation showed that these three tasks reflected a unitary construct explaining more than 76% of the variance. This analysis was used to generate a single factor score to represent executive function (EF). Correlation coefficients between the three EF tests are as follows: Modified Trails and Stroop Interference r=-.78, Verbal fluency and Modified Trails r=-.58, Verbal fluency and Stroop Interference r=.58 (all p values <.001). Correlation coefficients between the disinhibition score and each of the three EF tests as well as the factor score are as follows: Verbal fluency r= -.20, p<.05; Modified Trails r=.08, p>.05; Stroop Interference r=-.06, p>.05, EF factor score r=-.14, p>.05. Correlations between disinhibition and the EF tests were low and significantly smaller than the moderate to high correlations between the three EF tests. These results give credence to the distinction between behavioral disinhibition and EF.
Acquisition and analysis of MRI data
MR Image Acquisition
MRI scans were acquired on a 1.5T Magnetom VISION system (Siemens Inc., Iselin, NJ, USA) using a standard quadrature head coil. Volumetric magnetization-prepared rapid gradient echo (MP-RAGE) MRI (TR/TE/inversion time [TI] = 10/4/300 msec) imaging was used to obtain T1-weighted structural images of the entire brain. The T1 images were in a coronal orientation, with a 15° flip angle, with 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness.
Image Analysis
The T1 MPRAGE structural MR images were analyzed using Freesurfer (FS), a surface-based structural MRI analysis tool that is freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Previous publications have described and validated the software (Dale, Fischl, & Sereno, 1999; Fischl, Liu, & Dale, 2001; Fischl, Sereno, & Dale, 1999; Segonne et al., 2004). FS processing was performed according to standard protocols, and described in the FS documentation (http://surfer.nmr.mgh.harvard.edu/) and prior publications. The procedure, in brief, involves the removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004) and intensity normalization to correct for MR field bias artifacts (Sled, Zijdenbos, & Evans, 1998), followed by automated Talairach-aligned transformation and volumetric segmentation of cortical grey and white matter (Fischl et al., 2002; Fischl, Salat et al., 2004). Estimated TIV is calculated via an atlas normalization procedure (Buckner et al., 2004). Inaccuracies in the automated skull-stripping procedure were manually corrected when dura and other non-brain tissue interfered with the accuracy of cortical surface generation. The surfacing algorithm analyzes intensity and continuity data, and corrects topological defects to generate a continuous cortical ribbon (Fischl & Dale, 2000; Fischl et al., 2001; Segonne et al., 2004; Segonne, Pacheco, & Fischl, 2007). The cortical surface is then inflated and registered to a spherical atlas and parcellated into regions of interest (ROI) based on gyral and sulcal structure (Desikan et al., 2006; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999; Fischl, van der Kouwe et al., 2004), from which regional cortical thicknesses and volumes can be calculated. The resulting thickness estimates that generate volume have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). Cortical grey matter volumes, the sum of the thickness over the regional surface area, were used for this study. However, the contributions of cortical thickness and surface area to volume may differ; therefore, we examined the data using all three types of variables (volume, thickness, area).
Cortical regions in the frontal lobe delineated by Desikan et al. (2006) were combined for the purposes of this study to reduce the number of multiple comparisons and included three medial/ventral frontal regions, the rostral and caudal anterior cingulate (ACC) and orbitofrontal cortex (OFC), and two lateral frontal regions, the middle frontal gyrus (MFG) and inferior frontal gyrus (IFG). It is important to differentiate between rostral and caudal ACC because neuroimaging studies have shown that separate areas of the ACC are involved in cognition and emotion (Bush, Luu, & Posner, 2000). Because the prefrontal cortex is not solely responsible for executive functioning and may not be solely responsible for socioemotional disinhibition, we also examined potential contributions from other candidate regions including the parietal lobes, temporal lobes, and cerebellum. The ACC was bounded by the cingulate sulcus anteriorly and superiorly, and caudally by the mamillary bodies; the OFC included the medial and lateral orbital gyri up to the lateral orbital sulcus, and extended medially until it bordered the ACC and superior frontal gyrus. The MFG was bounded medially and laterally by the superior and inferior frontal sulci respectively, and bounded caudally by the precentral sulcus. The rostral extent of the MFG was the rostral extent of the superior frontal sulcus. The IFG was bounded rostrally by the rostral extent of the inferior frontal sulcus, caudally by the precentral gyrus, medially by the lateral bank of the inferior frontal sulcus, and laterally by the lateral orbital sulcus. The parietal lobe aggregated the postcentral gyrus, supramarginal gyrus, superior parietal cortex, inferior parietal cortex, and precuneus cortex. The temporal lobe was composed of entorhinal cortex, parahippocampal gyrus, temporal pole, fusiform gyrus, superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, transverse temporal cortex, and banks of the superior temporal sulcus. Cerebellar cortex was included as well.
To implement FS processing, our study utilized the LONI Pipeline environment (Rex, Ma, & Toga, 2003) (http://pipeline.loni.ucla.edu), which was developed by LONI and used to distribute FS processing tasks to an offsite CPU cluster located at UCLA - LONI.
Statistical analysis
Statistical analyses were computed using SPSS software package (version 16.0 for Windows, SPSS Inc., Chicago, IL). Demographic and neuropsychological characteristics were compared across patient groups using a one-way ANOVA and follow-up post-hoc testing. Initial correlations were computed between demographic/clinical variables and the dependent variables (EF and disinhibition), and significant variables were controlled for in subsequent analyses.
In order to first identify which of the regions of interests were candidate regions to explain either of the dependent variables, partial correlations were computed to examine relationships between regions of interest (cortical volume measurements), NPI disinhibition, and the executive function factor score, controlling for total intracranial volume (TIV), MMSE, and other demographic/clinical variables that correlated significantly with the dependent variables. Once candidate regions were identified that were correlated with the dependent variable, these relationships were further refined by taking all regions that potentially explained EF and disinhibition and entering them into hierarchical regressions controlling for covariates in order to identify which of these regions independently predicts disinhibition and EF after accounting for the volumes of other candidate regions. Specifically, brain regions significantly correlated with disinhibition were entered into one hierarchical regression and brain regions significantly correlated with executive function were entered into a separate hierarchical regression. Two follow-up hierarchical regressions were used to examine the amount of variance explained by any significant brain regions above and beyond all other regions of interest and covariates. Brain regions that significantly predicted disinhibition and EF were entered in the second step, while all other brain regions correlating with these dependent variables and covariates were entered in the first step.
Finally, in order to specifically test whether the regions independently predicting socioemotional disinhibition and EF in the separate behavior-specific linear regressions still predict these behaviors independently of each other, a multivariate general linear model was created including those regions independently predicting EF and disinhibition, along with TIV, MMSE, diagnosis, age, and education to look for region-by-behavior interactions and to examine the potential contributions of each region to both behaviors.
To examine potentially hemisphere-specific effects, analyses were first conducted with left and right hemispheres summed for each brain region. However, the differential contribution of each hemisphere in predicting disinhibition and EF was also examined with an interaction test. If the hemisphere interaction effect was significant, then all analyses were repeated for the left and right hemisphere separately for that function.
Cortical volumes are a product of thickness and area. Therefore, after first analyzing the data using volume measurements, we then conducted analyses using cortical surface area and mean cortical thickness measurements to assess whether these two parameters have different relationships to the targeted behaviors.
Results
Group Demographics and Cognitive and Behavioral Characteristics
Demographic and neuropsychological characteristics were compared across patient groups. Differences in sample sizes across groups and associated Type II error may have impacted results; however, samples were combined in main analyses. One-way ANOVA detected significant group effects for several variables, which were investigated further with post-hoc testing. Age did not significantly differ across diagnostic groups, with the exception of patients with MCI who were significantly older than patients with AD and bvFTD. Education was comparable, except that patients with MCI were more educated that patients with AD. Patients with AD showed significantly lower MMSE scores than all other diagnostic cohorts whereas patients with MCI scored higher on the MMSE than patients with bvFTD and SD. The executive function factor did not significantly differ by disease, with the exception of the MCI group who performed better on all cognitive tasks, as would be expected. Patients with bvFTD were significantly more disinhibited than all other patients and patients with SD were more disinhibited than patients with AD and MCI, as measured by the NPI.
Regions associated with EF and disinhibition, and behavior-specific regressions
Initial correlations computed between demographic/clinical variables and the dependent variables revealed that age (r=-.25, p<.01) and working diagnosis correlated with disinhibition (r=-.35, p<.001) while education (r=.22, p<.05) and diagnosis (r=.55, p<.001) correlated with executive functioning. These variables, along with TIV and MMSE, were controlled for in all subsequent analyses. Partial correlations between regions of interest, disinhibition, and EF were computed after controlling for covariates (see Table 2). Rostral ACC, OFC, IFG, and temporal lobes significantly correlated with disinhibition. MFG only correlated with EF.
Table 2.
Partial correlations between regions of interest, disinhibition, and EF controlling for MMSE, TIV, diagnosis, age, and education for whole brain and by hemisphere.
| Brain Region | Disinhibition r |
Executive Functioning r |
||||
|---|---|---|---|---|---|---|
| All | Right | Left | All | Right | Left | |
| Orbitofrontal cortex | -.43*** | -.41*** | -.37*** | -.02 | -.04 | -.06 |
| Caudal anterior cingulate | -.16 | -.17 | -.04 | -.06 | -.10 | -.04 |
| Rostral anterior cingulate | -.31*** | -.30** | -.19* | -.09 | -.12 | -.09 |
| Middle frontal gyrus | -.15 | -.17 | -.04 | .24** | .21* | .19* |
| Inferior frontal gyrus | -.23* | -.27** | -.06 | .11 | .10 | .04 |
| Parietal lobes | .10 | .04 | .18 | .14 | .08 | .18 |
| Temporal lobes | -.35*** | -.39*** | -.19* | -.14 | -.10 | .-.14 |
| Cerebellum | -.08 | -.04 | -.11 | .02 | -.02 | .06 |
Note:
p<.05.
p<.01.
p<.001
Brain regions significantly correlated with disinhibition (Rostral ACC, OFC, IFG, and temporal lobes) were entered into a hierarchical regression to determine which brain regions predicted disinhibition (See Table 3). After controlling for covariates in the first step, all of the brain regions taken together contributed significantly to the model in the second step. These four brain regions taken together explained an additional 15.6% of the variance in disinhibition, with only the OFC making a significant individual contribution. A follow-up hierarchical regression was used to examine the specific incremental contribution of OFC in predicting disinhibition, over and above the contribution of the other brain regions. Results showed that OFC made a unique and incremental contribution to predicting disinhibition above and beyond all other brain regions correlated with disinhibition as well as covariates. OFC alone explained 4.7% additional variance in disinhibition after taking other brain regions into account.
Table 3.
Hierarchical regression results for whole brain and by hemisphere using total volume and surface area measurements controlling for TIV, MMSE, age, and diagnosis in Step 1, examining which brain regions predicted disinhibition in Step 2, and determining the contribution of the OFC above and beyond all other regions of interest in Step 3.
| Whole Brain Volume | Right Hemisphere Volume | Left Hemisphere Volume | Whole Brain Area | |
|---|---|---|---|---|
| Step 1 | F(4,152)=8.2; p<.001; R2=.177 |
F(4,152)=8.2; p<.001; R2=.177 |
F(4,152)=8.2; p<.001; R2=.177 |
F(4,152)=8.2; p<.001; R2=.177 |
| Step 2 | ΔF(4,148)=8.6; p<.001; ΔR2=.156 |
ΔF(4,148)=9.0; p<.001; ΔR2=.162 |
ΔF(3,149)=8.0; p<.001; ΔR2=.114 |
ΔF(2,150)=3.0; p<.05; ΔR2=.031 |
| Step 3 | ΔF(1,148)=10.4; p=.002; ΔR2=.047 |
ΔF(2,148)=7.0; p=.001; ΔR2=.062 |
ΔF(1,149)=14.2; p<.001; ΔR2=.067 |
ΔF(1,150)=5.3; p<.05; ΔR2=.028 |
| OFC; β= -0.48 |
Right OFC; p<.05; β= -0.28 |
Left OFC; β= -0.38 |
OFC; β= -0.27 |
|
| Right Temporal lobe; p<.05; β= -0.23 |
The only brain region significantly correlated with EF (MFG) was entered into a hierarchical regression to determine whether it predicted EF after controlling for TIV, MMSE, and clinical/demographic variables significantly correlated with EF (See Table 4). After controlling for covariates in the first step, MFG contributed significantly to the model in the second step. MFG alone explained 3.5% additional variance in EF after taking other covariates into account.
Table 4.
Hierarchical regression results using total volume and surface area measurements controlling for TIV, MMSE, education, and diagnosis in Step 1, and examining the specific and incremental contribution of MFG in predicting EF in Step 2.
| Volume | Area | |
|---|---|---|
| Step 1 | F(4,121)=25.2; p<.001; R2=.455 |
F(4,121)=25.2; p<.001; R2=.455 |
| Step 2 | ΔF(1,120)=8.4; p=.005; ΔR2=.035 |
ΔF(1,120)=9.0; p=.003; ΔR2=.038 |
| MFG; β= 0.24 | MFG; β= 0.28 |
Dissociations between EF and disinhibition-related regions
A multivariate general linear model was then used to explicitly test whether these brain-behavior relationships (MFG with EF and OFC with disinhibition) were dissociable. The multivariate general linear model examined the strength of these relationships independent of one another's interrelationships by taking into account associations among the two brain regions, TIV, MMSE, diagnosis, age, and education. There was a significant interaction effect between brain regions and functions (F(2,117)= 8.5, p<.001, power=.96), illustrating that the different brain regions contributed differently to cognitive and socioemotional functions. Examination of main effects showed that OFC independently predicted disinhibition (F(1,118)= 24.2, p<.001, power=1.0) while MFG independently predicted executive functioning (F(1,118)= 10.5, p=002, power=.90) (see Figure 1). Independent contributions of OFC to predicting EF and MFG to predicting disinhibition were not significant (p>.05).
Figure 1. Lateral View of the Brain.
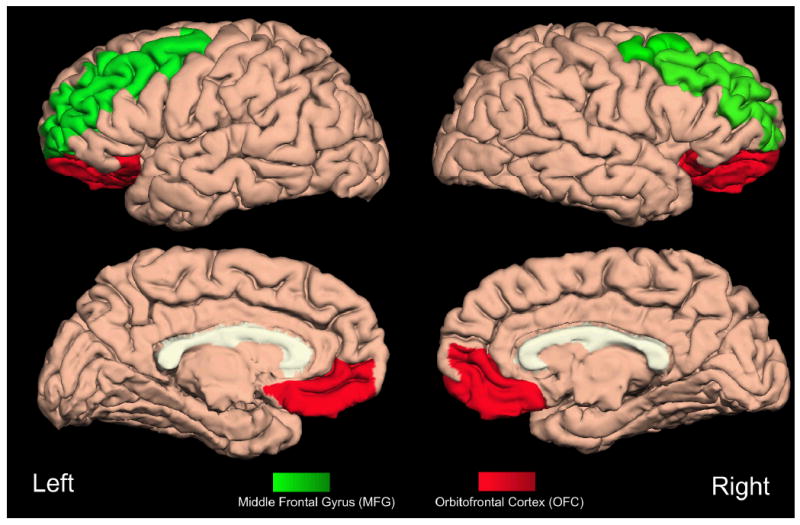
A lateral view of the brain depicts orbitofrontal cortex (OFC) and middle frontal gyrus (MFG) brain regions.
Laterality
The differential contributions of left and right hemisphere brain regions in predicting disinhibition and EF were examined. A mixed model ANOVA revealed a significant interaction effect between hemisphere and disinhibition (F(1,153)= 12.2, p<.01, power=.93), but the hemisphere by EF interaction effect was not significant (p>.05). Therefore, analyses were repeated for brain regions in the right and left hemisphere separately to examine laterality effects in the predicting of disinhibition.
Partial correlations between regions of interest for both hemispheres and disinhibition were computed after controlling for covariates (see Table 2). Left OFC, left rostral ACC, left temporal lobe, right OFC, right rostral ACC, right IFG, and right temporal lobe correlated with disinhibition. These regions were entered into separate hierarchical regressions to determine which brain regions predicted disinhibition. After controlling for TIV, MMSE, age, and diagnosis in the first step, all of the right hemisphere brain regions taken together contributed significantly to the model in the second step (See Table 3). These four right hemisphere brain regions taken together explained an additional 16.2% of the variance in disinhibition, with only the right OFC and the right temporal lobe making significant individual contributions. A follow-up hierarchical regression was used to examine the specific incremental contribution of right OFC and right temporal lobe in predicting disinhibition, over and above the contribution of the other right hemisphere brain regions (rostral ACC and IFG). Results showed that right OFC and right temporal lobe made a unique and incremental contribution to predicting disinhibition above and beyond all other right hemisphere brain regions correlated with disinhibition as well as covariates. Right OFC and right temporal lobe taken together explained 6.2% additional variance in disinhibition after taking other brain regions into account. After controlling for TIV, MMSE, age, and diagnosis in the first step, all of the left hemisphere brain regions taken together contributed significantly to the model in the second step (See Table 3). These three left hemisphere brain regions taken together explained an additional 11.4% of the variance in disinhibition, with only the left OFC making a significant individual contribution. A follow-up hierarchical regression was used to examine the specific incremental contribution of left OFC in predicting disinhibition, over and above the contribution of the other left hemisphere brain regions (rostral ACC and temporal lobe). Results showed that left OFC made a unique and incremental contribution to predicting disinhibition above and beyond the other left hemisphere brain region correlated with disinhibition as well as covariates. Left OFC alone explained 6.7% additional variance in disinhibition after taking other brain regions into account. Taken together, results show that disinhibition is strongly associated with tissue loss in the right hemisphere, and that OFC is a particularly important brain region mediating behavioral disinhibition.
Area and Thickness Measurements
We repeated all analyses with thickness and surface area separately. Results were replicated using cortical surface area data. OFC independently predicted disinhibition while MFG independently predicted EF (See Tables 3 and 4). The interaction effect between brain regions and functions was significant as well (F(2,117)= 7.2, p=.001, power=.93). Examination of main effects showed that OFC independently predicted disinhibition (F(1,118)= 9.8, p<.01, power=.87) while MFG independently predicted executive functioning (F(1,118)= 8.8, p<01, power=.84). Mean cortical thickness measurements of OFC (r= -.20, p<.05) and temporal lobes(r= -.21, p<.05) significantly correlated with disinhibition; however, taken together they did not significantly predict disinhibition after controlling for TIV, MMSE, diagnosis, and age (p>.05). No brain regions significantly correlated with EF when using mean cortical thickness measurements. This finding suggests that the relationships between regional volumes and behavior are more closely related to the area measurements than the cortical thickness.
Discussion
These results support a specific association between orbitofrontal areas and socioemotional disinhibition as compared to dorsolateral prefrontal areas and classical executive functions. OFC predicted disinhibition, but not executive functioning; whereas MFG predicted executive functioning, but not disinhibition. Disinhibition was strongly associated with tissue loss in the right hemisphere and with the OFC in particular, while differential contributions of left vs. right MFG in predicting EF could not be identified. Results were replicated using cortical surface area measurements, but mean cortical thickness measurements did not predict behavior similarly to volume.
Findings correspond with previous research linking the dorsolateral prefrontal cortex (DLPFC) to EF. Focal lesions in the DLPFC result in decreased performance on tasks of abstraction, set shifting, and error monitoring (Moore, Schettler, Killiany, Rosene, & Moss, 2009; B. Yochim et al., 2007; B. P. Yochim et al., 2008). In neurodegenerative disease, decreases in lateral prefrontal cortex volume are associated with an increased tendency toward rule violation errors during EF tasks (Possin et al., 2009), and left DLPFC disease has been associated with EF as measured by a sorting task specifically in patients with FTD (Huey et al., 2009). Patients with subcortical dementias, such as Huntington's disease and Parkinson's disease, who have dysfunction in dorsolateral prefrontal circuits at the level of basal ganglia, also show executive dysfunction (Tekin & Cummings, 2002). These findings, and similar ones in the literature on neurodegenerative disease, were generated using techniques such as voxel-based morphometry (VBM), which is typically used to identify brain behavior relationships at every location in the brain independently, based on statistical relationships exceeding a particular threshold (usually p<0.05 corrected for multiple comparisons). None of these studies used methodology that would establish whether the brain regions identified relate to the behavior of interest independent from other brain regions that may relate to the behavior but fall below the statistical threshold used for the analysis. There are several approaches that can be used to deal with this issue. The approach taken here of entering volumes for several frontal ROIs previously identified as having roles in EF and socioemotional behavior incorporates a-priori knowledge to test the specificity of the brain-behavior relationships and was able to establish a specific relationship between MFG volume and EF, independent of brain volumes in other regions. This finding reinforces previous studies linking dorsolateral frontal regions with classical EF, including inhibitory processes that are considered integral to control of cognitive performance. Despite the association between OFC and behavior described as disinhibited, our data indicate that OFC does not contribute significantly to classical EF.
The neural mechanisms underlying EF is a topic of ongoing debate; however, Miller and Cohen (2001) argue that cognitive control is the primary function of the prefrontal cortex (PFC). They propose a model by which cognitive control is accomplished by increasing the gain of sensory or motor neurons that are engaged by task or goal relevant elements of the external environment (Miller & Cohen, 2001). The cognitive control construct that they describe is mediated by neural connections between the PFC and sensory and motor cortices, and is crucial in selective attention, error monitoring, and other aspects of EF.
Findings are also consistent with previous research examining neural correlates of socioemotional disinhibition. Patients with neurodegenerative disease or lesions involving the right frontal lobe are more disinhibited and show decreased ability to behave in a socially appropriate manner as compared to patients with left frontal lobe damage (Mychack, Kramer, Boone, & Miller, 2001; Tranel, Bechara, & Denburg, 2002). Mychack and colleagues (2001) found that eleven out of twelve patients with predominantly right-sided FTD displayed socially undesirable behavior as an early symptom whereas only two of nineteen patients with left-sided FTD exhibited these behaviors. Several studies have linked disinhibition as measured by the NPI with tissue loss (Rosen et al., 2005; Massimo et al., 2009) and hypometabolism (Peters et al., 2006) in the ventromedial portion of the OFC. The current study extends these prior findings by establishing the specificity of the relationship between OFC and socioemotional disinhibition, and supporting the idea that these behavioral deficits are due to mechanisms that are at least partially independent of those supporting EF.
The underlying mechanisms linking OFC and behavioral disinhibition are poorly understood, although impaired processing of reinforcers may be central to this process (Rolls & Grabenhorst, 2008). OFC projects to the amygdala, cingulate cortex, ventral striatum and head of the caudate nucleus (Rolls & Grabenhorst, 2008), brain regions that are involved with the processing and response to the reward value of stimuli. Similarly, it projects to entorhinal and perirhinal cortex, providing a route for reward information to reach the hippocampus for remembering (Rolls & Xiang, 2005). It also connects to the preoptic region and lateral hypothalamus, where neurons alter their firing rates in response to food and show sensory-specific satiety (Rolls, Burton, & Mora, 1976). These connections provide several routes via which the OFC can influence behavior (Rolls, 2005). The OFC reacts to the reward and punishment values of stimuli in the environment and guides adaptation of behaviors based upon the changing nature of these reinforcers (Kringelbach & Rolls, 2004). For example, OFC activity increases when smelling a desirable food, but with satiety the activation found with the same food dampens (O'Doherty et al., 2000). Similarly, the current pleasantness or reward value of odiferous somatosensory, and visual inputs are determined within the OFC (Rolls & Grabenhorst, 2008). These patterns of activity are linked to emotions, because emotions can be viewed as states elicited by instrumental reinforcers (Rolls, 2005). The firing patterns in the OFC can be seen as providing specific contexts for potential rewards and punishments so that their current value can be calculated based on the specific context and bodily state. It is easy to understand how failure to update reward and punishment values to the current context can lead to socially inappropriate behaviors. For instance, flirtatious comments might be appropriate for a college student at a fraternity party, but not for a married man visiting the mall with his family.
PFC contributions to EF and disinhibition, while significant and unique, only explained small proportions of the variance in the two dependent variables. Of course, DLPFC is not exclusively responsible for executive functioning. Other brain regions, as well as demographic variables contribute to EF. A larger proportion of the variance in EF was explained by control demographic/clinical variables, TIV, and diagnosis in particular. Furthermore, research suggests that EF is not anatomically restricted to the frontal lobes, but also depends upon the integrity of subcortical white matter and the basal ganglia, and neural networks that rely on input from posterior structures (S. W. Anderson, Damasio, Jones, & Tranel, 1991; Ravizza & Ciranni, 2002). After controlling for global cognition, TIV, diagnosis, and education the MFG uniquely accounted for only 3.5% of the variance in EF in the current study, but this is to be expected given that EF is a complex and multifaceted function that utilizes regions extending throughout the brain. Deficits in EF may be mediated by dysfunction in bilateral frontostriatal and frontoparietal networks that involve the ACC, DLPFC, and parietal cortex in the region of the intraparietal sulcus (Wang et al., 2009; Wolf et al., 2008). Similarly, the OFC uniquely accounted for only 4.7% of the variance in disinhibition. As is the case with MFG and EF, OFC is likely part of a network of regions relevant to behavioral regulation. Disinhibition in patients with bvFTD is also correlated with atrophy in the right nucleus accumbens, right superior temporal sulcus, and right mediotemporal limbic structures, reflecting the connections of the temporal lobe with orbitofrontal regions (Zamboni, Huey, Krueger, Nichelli, & Grafman, 2008). Future research should examine brain networks related to disinhibition and EF.
Our findings are also notable in light of evidence that the right hemisphere plays an important role in the processing of emotion (Anderson et al., 2000; Rosen et al., 2006; Tranel et al.). Hemispheric specialization appears to have led to asymmetric elaborations of neural pathways (Tucker, Luu, & Pribram, 1995). Understanding the inherent asymmetries of these networks may be important in interpreting the growing evidence that the left and right frontal lobes contribute differently to normal and pathological forms of socioemotional behavior (Tucker et al., 1995).
Although volume measurements are most commonly used, our data analysis procedure allowed us a unique opportunity to analyze area and thickness data separately. Cortical surface area predicted behavior similarly to volume, but that mean cortical thickness did not. This suggests that atrophy in neurodegenerative disease may reflect multiple processes that affect surface area and cortical thickness differently. Other studies have also found that these two parameters are differentially affected by the disease. Dickerson et. al. (2009) showed differential effects on thickness and area in healthy elderly and Alzheimer patients. Panizzon et al. (2009) showed that although both thickness and area are highly heritable, the overlap in the genetic contributions to them is very low, indicating that cortical volume measures combine at least two distinct sources of genetic influences. The factors influencing the relative effects on cortical thickness versus surface area of aging and neurodegenerative disease have not been explored in great detail, but our results suggest that the different effects on these two parameters, and how these separate effects relate to behavior, should be a subject for future studies.
Our approach, which was designed to test whether our regions of interest accounted for specific behaviors independent of other relevant brain regions in the frontal lobes, constitutes a relative strength of the study. The current study provided a unique perspective on brain-behavior relationships because most studies do not specifically examine independent contributions of brain regions in predicting behaviors. A specific association was found between orbitofrontal areas and socioemotional behavior as compared to dorsolateral areas and EF. In addition to enhancing our understanding of the relationships between brain regions and specific cognitive-behavioral functions, a better understanding of this division of labor within the frontal lobe is important for early diagnosis, differential diagnosis, and improved counseling of patients. Our findings differ from some prior findings, such as the association of disinhibition with lesions in the ventromedial prefrontal cortex, including the area above the medial OFC. Discordant findings may be a result of different measures of disinhibition or EF, MRI acquisition and analytic techniques, or other methodological differences. Although human lesion-mapping supports the localization of response inhibition to the right inferior frontal cortex (Aron, Robbins, & Poldrack, 2004), this study examined cognitive inhibition as measured by Go/NoGo or Stop-signal tasks as opposed to socioemotional disinhibition. The difference in quantitative methods used to measure our constructs (neuropsychological tests assessing EF vs. questionnaire evaluating socioemotional disinhibition) is a limitation of the study and may have influenced results. Relative weaknesses of the current study also include a lack of autopsy confirmed diagnoses and only using one measure of socioemotional disinhibition. Although the NPI is a validated measure of disinhibition commonly used in research, future studies should consider including other measures as well to explore the possible independent contributions of brain regions within the PFC in predicting socioemotional disinhibition.
Acknowledgments
Funding:This work was supported by the National Institute on Aging (NIA) [grant numbers 1 RO1-AG022983-01 to J.K., 5 P01-AG019724-02 to B.M.], National Institute of Neurological Disorders and Stroke (NINDS) [grant number HHSN-271200623661C to J.K.], and the State of California Alzheimer's Disease Research Center of California (ARCC) [grant number 3 P50-AG23501 to B.M.]. This study utilized the LONI Pipeline environment, which was partially funded by National Institutes of Health [grant numbers P41 RR013642, R01 MH71940 and U54 RR021813]. In the past Dr. Miller has spoken with support from Pfizer, Novartis, and Lundbeck Pharmaceuticals and has consulted with Lundbeck. All other authors have nothing to disclose.
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14(4):526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. J Clin Exp Neuropsychol. 1991;13(6):909–922. doi: 10.1080/01688639108405107. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18(6):871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Blumer D, Benson D. Personality changes with frontal and temporal lesions. In: Benson DF, Blumer D, editors. Psychiatric Aspects of Neurologic Disease. New York: Grune & Stratton; 1975. pp. 151–170. [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54 5:S15–19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30(3):432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298(5596):1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35(12):1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23 1:S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Akinesia, tardive dysmentia, and frontal lobe disorder in schizophrenia. Schizophr Bull. 1985;11(2):255–263. doi: 10.1093/schbul/11.2.255. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer's disease. J Neural Transm. 1996;47:103–123. doi: 10.1007/978-3-7091-6892-9_6. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126(Pt 7):1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34(4):247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Huey ED, Goveia EN, Paviol S, Pardini M, Krueger F, Zamboni G, et al. Executive dysfunction in frontotemporal dementia and corticobasal syndrome. Neurology. 2009;72(5):453–459. doi: 10.1212/01.wnl.0000341781.39164.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar S, Hughes M, Fulham WR, Michie PT, Karayanidis F. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.01.090. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12(7):939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Levine B, Stuss DT, Milberg WP. Effects of aging on conditional associative learning: process analyses and comparison with focal frontal lesions. Neuropsychology. 1997;11(3):367–381. doi: 10.1037//0894-4105.11.3.367. [DOI] [PubMed] [Google Scholar]
- Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioral distinctions. Arch Neurol. 1996;53(7):687–690. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, et al. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2009;27(1):96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Rosene DL, Moss MB. Effects on executive function following damage to the prefrontal cortex in the rhesus monkey (Macaca mulatta) Behav Neurosci. 2009;123(2):231–241. doi: 10.1037/a0014723. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9 1:173–176. doi: 10.1017/s1041610297004870. discussion 177-178. [DOI] [PubMed] [Google Scholar]
- Mychack P, Kramer JH, Boone KB, Miller BL. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56(11 Suppl 4):S11–15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- Nedjam Z, Devouche E, Dalla Barba G. Confabulation, but not executive dysfunction discriminate AD from frontotemporal dementia. Eur J Neurol. 2004;11(11):728–733. doi: 10.1111/j.1468-1331.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, et al. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11(4):893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Kugel H, Bauer J, Siegmund A, Kolkebeck K, Suslow T, et al. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr Res. 2008;106(2-3):156–163. doi: 10.1016/j.schres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12(2):65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. 2006;21(5-6):373–379. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Possin KL, Brambati SM, Rosen HJ, Johnson JK, Pa J, Weiner MW, et al. Rule violation errors are associated with right lateral prefrontal cortex atrophy in neurodegenerative disease. J Int Neuropsychol Soc. 2009;15(3):354–364. doi: 10.1017/S135561770909050X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Ciranni MA. Contributions of the prefrontal cortex and basal ganglia to set shifting. J Cogn Neurosci. 2002;14(3):472–483. doi: 10.1162/089892902317361985. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19(3):1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control; the role of the prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Burton MJ, Mora F. Hypothalamic neuronal responses associated with the sight of food. Brain Res. 1976;111(1):53–66. doi: 10.1016/0006-8993(76)91048-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86(3):216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Xiang JZ. Reward-spatial view representations and learning in the primate hippocampus. J Neurosci. 2005;25(26):6167–6174. doi: 10.1523/JNEUROSCI.1481-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128(Pt 11):2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Wilson MR, Schauer GF, Allison S, Gorno-Tempini ML, Pace-Savitsky C, et al. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44(3):365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38(4):589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Pribram KH. Social and emotional self-regulation. Ann N Y Acad Sci. 1995;769:213–239. doi: 10.1111/j.1749-6632.1995.tb38141.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J. Effective Connectivity of the Fronto-parietal Network during Attentional Control. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Watari K, Elderkin-Thompson V, Ajilore O, Haroon E, Darwin C, Pham D, et al. Neuroanatomical correlates of executive functioning in depressed adults with type 2 diabetes. J Clin Exp Neuropsychol. 2008;30(4):389–397. doi: 10.1080/13803390701440486. [DOI] [PubMed] [Google Scholar]
- Wittenberg D, Possin KL, Rascovsky K, Rankin KP, Miller BL, Kramer JH. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychol Rev. 2008;18(1):91–102. doi: 10.1007/s11065-008-9056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schonfeldt-Lecuona C, Ecker D, Landwehrmeyer B. Aberrant connectivity of lateral prefrontal networks in presymptomatic Huntington's disease. Exp Neurol. 2008;213(1):137–144. doi: 10.1016/j.expneurol.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Yochim B, Baldo J, Nelson A, Delis DC. D-KEFS Trail Making Test performance in patients with lateral prefrontal cortex lesions. J Int Neuropsychol Soc. 2007;13(4):704–709. doi: 10.1017/S1355617707070907. [DOI] [PubMed] [Google Scholar]
- Yochim BP, Baldo JV, Kane KD, Delis DC. D-KEFS Tower Test performance in patients with lateral prefrontal cortex lesions: The importance of error monitoring. J Clin Exp Neuropsychol. 2008:1–6. doi: 10.1080/13803390802448669. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology. 2008;71(10):736–742. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]


