Abstract
Background
Hot flashes are a complication of androgen deprivation therapy given to men with prostate cancer. A previous clinical study indicated that use of low dose gabapentin (900mg/day) was well-tolerated and decreased hot flash frequency to a moderate degree for 4 weeks. The purpose of this current study was to examine the efficacy and toxicity of gabapentin over a longer term.
Methods
In this open-label continuation phase study, men in a previous phase III trial of gabapentin for hot flashes, were studied. These patients were allowed to start, or continue, gabapentin and to titrate the dose to maximum efficacy, up to 900mg/day. They were to complete a hot flash diary daily and keep weekly logs of toxicity, satisfaction with hot flash control, and effect on QoL, for eight additional weeks.
Results
The moderate reduction in hot flash frequency and severity appeared to be maintained during the continuation phase. Those originally on the placebo or lowest dose of 300mg/day had improved hot flash control. Minimal toxicities reported.
Conclusions
Low dose gabapentin appears to provide moderate efficacy for long term treatment of hot flashes in men undergoing androgen deprivation therapy for prostate cancer and seems to be well tolerated.
Keywords: hot flashes, men, prostate cancer, gabapentin
Background
Hot flashes are a problematic issue for many men diagnosed with prostate cancer, affecting up to 75% of those on medical or surgical androgen deprivation therapy1,2. Along with the physical symptoms of hot flashes, men describe accompanying feelings of anxiety, irritability, and being out of control, significantly affecting their perception of quality of life3.
At present, there are few therapeutic options for treating men who suffer from hot flashes. Clonidine, which demonstrated some effectiveness in treating hot flashes in women, failed to show significant benefit in men when subjected to a randomized, placebo-controlled, double blind trial4. Estrogen and progesterone analog treatments have shown significant reductions of hot flashes in placebo-controlled trials in men, with approximately a 75% reduction, as compared to a 25% reduction with a placebo5,6. However, these hormonal therapies have undesirable side effects, including breast enlargement and tenderness with estrogen agents and increasing prostate-specific antigen (PSA) levels in some men taking progesterone analogs7.
Pilot data suggest smaller benefits for hot flash reductions from low dose selective serotonin reuptake inhibitors/selective serotonin-norepinephrine reuptake inhibitors, but these data have yet to be confirmed in larger Phase III trials8,9.
A recent phase III clinical study demonstrated that gabapentin (Neurontin), a non-hormonal antiepileptic often used to treat neuropathic pain, reduced hot flash frequency and severity to a moderate degree, as compared to a placebo, in men undergoing androgen ablation therapy over a four-week period, with mild side effects10. The study also demonstrated significantly improved quality of life measures and relief from self-reported hot flash distress in the patients who received a target dose of 900mg/day gabapentin. This current manuscript reports results for the efficacy and toxicity of gabapentin in an open-label, continuation study that extended eight weeks beyond the initial four-week randomized treatment period in this patient population.
Methods
Patient eligibility characteristics
This study population consisted of a subset of patients who completed an original randomized, double blind phase III trial comparing gabapentin with placebo for the treatment of hot flashes in men10. Eligible men for the original study had a history of prostate cancer and troublesome hot flashes, and were on stable androgen deprivation therapy with a good performance status. They had no significant history of renal insufficiency, had not previously used gabapentin, and were not concurrently using or planning to use any of the following agents while participating in this study: chemotherapy, androgens, estrogens, or progesterone analogs. Antidepressant use was allowed if the patients had been on a stable course for at least one month and did not plan to modify treatment during the study. Informed written consent was obtained and local Internal Review Boards monitored the study.
The previous randomized gabapentin trial lasted five weeks. After one week, used for baseline documentation of hot flash frequency and severity, patients received one of four oral treatment regimens: 300mg gabapentin daily for 28 days, versus 300 mg gabapentin daily for 7 days and then twice daily for 21 days, versus 300mg gabapentin daily for 7 days then twice daily for 7 days and then thrice daily for 14 days, versus placebo × 28 days. Gabapentin was provided by Pfizer Corporation, New York. Patients recorded hot flash frequency and severity daily (in hot flash diaries), answered symptom/side effect/quality of life questionnaires weekly, and completed the Profile of Mood States-Brief (POMS-B)11 after the baseline week and again after the four weeks of treatment. Descriptions of hot flash severity rankings were given at protocol initiation12.
After all documentation from the initial phase III study was submitted for each individual patient, the study code was broken and each patient was given the option to continue gabapentin therapy, or start active drug therapy if he was on the placebo, as part of this eight-week open-label continuation phase study. Patients who had been randomized to receive gabapentin were advised to continue with their current dosing regimen if hot flashes were well controlled with minimal toxicities. However, patients were allowed to titrate their dose to the next lower dose if desired or, alternatively, to the next higher dose if hot-flash control was less than ideal and toxicities were not very appreciable. These modifications were to be made per clinical discretion and at one step (300 mg/day) intervals per week, not exceeding a total dose of 900mg/day.
Patients were to be called every other week by study nurses to confirm their current dosing, answer questions, and encourage compliance. As in the initial study, patients were given a questionnaire booklet with instructions, a set of daily hot flash diaries, weekly symptom experience diaries, and a POMS-B, the latter for completion at the end of the eight-week period. The symptom experience questionnaires addressed the following possible side effects of gabapentin: increased appetite, nausea, dry mouth, dizziness, fatigue, trouble walking or balance problems, muscle pain, difficulty with concentration, constipation, sleepiness/sleep problems, blurred vision, anxiety, and mood changes. Also included on the weekly questionnaires were queries about quality of life and satisfaction with hot flash control. These questions were scaled from 0–10, with 10 being most severe.
Data analysis
Efficacy measures calculated from the hot flash diaries included hot flash frequency, as well as a hot flash score. Hot flash frequency was simply the number of hot flashes reported in each 24-hour period. The total hot flash score was a combination of the frequency and severity of the hot flashes. A number was assigned for each level of hot flash severity: 1 for mild, 2 for moderate, 3 for severe, and 4 for very severe. The hot flash score was determined by multiplying the patient’s mean hot flash severity for the day times the hot flash frequency. The Symptom Experience Diary was used to assess the side effects, using a numeric analogue scale to rate each item where 0 indicated ‘no incidence’ and 10 indicated ‘as bad as it can be.’ The results of all patient-reported outcomes were transformed onto a 0–100 scale with 100 indicating the most positive outcomes and 0 the most negative. Summary statistics were compiled for baseline characteristics of this population and Kruskal-Wallis methodology was employed to compare study endpoints between the study arms assigned during the double-blind portion. Change from baseline in patient reported side effects were calculated and ANOVA and Bonferoni techniques employed to identify any significant differences. Frequency counts and chi-square tests were conducted to determine relationships in study endpoints over time.
Results
Participant Profile
In the double-blind portion of the study, a total of 223 patients were randomized. Eight patients canceled and one patient was ineligible during this phase, resulting in 214 evaluable patients. The results of the double-blind portion of the study have been published10. In summary, the randomized portion of this trial demonstrated that low dose gabapentin decreased the hot flash score to a moderate degree, without significant toxicities, in men receiving androgen ablation therapy for prostate cancer. Mean hot flash scores decreased by 4.1 units in the placebo group and by 3.2, 4.6, and 7.0 units in the gabapentin 300mg, 600mg, and 900mg per day treatment groups, respectively. The drug was well tolerated and patients reported increased quality of life and satisfaction with hot flash control.
Of the 214 evaluable patients, 147 (67%) registered to remain on the protocol-continuation phase following the first 4 weeks of gabapentin/placebo therapy. Data were provided by 117 (80%) of these patients – 34, 32, 21, and 30 from the placebo, 300 mg/d, 600 mg/d, and 900 mg/d arms, respectively. The demographic characteristics of the men who participated in the continuation phase were representative of the patients in the double blind portion of the study (Table 1), although there were better performance scores (p =0.02) in the men opting to continue treatment on this open label portion of the protocol.
Table 1.
Demographic Baseline Characteristic of Evaluable Patients
| Continued on Study (N=117) |
Did Not Continue (N=97) |
P Value |
|
|---|---|---|---|
| Treatment Arm | 0.065 | ||
| 300 mg/d | 32 (27%) | 22 (23%) | |
| 600 mg/d | 21 (18%) | 32 (33%) | |
| 900 mg/d | 30 (26%) | 24 (25%) | |
| Placebo | 34 (29%) | 19 (20%) | |
| No. of Hot Flashes per Day | 0.477 | ||
| 2–3 | 13 (11%) | 13 (13%) | |
| 4–9 | 51 (44%) | 48 (50%) | |
| >=10 | 53 (45%) | 36 (37%) | |
| Hot Flashes Duration (Month) | 0.160 | ||
| <9 | 75 (64%) | 53 (55%) | |
| >=9 | 42 (36%) | 44 (45%) | |
| Performance Score | 0.017 | ||
| 0 | 100 (86%) | 70 (72%) | |
| 1 | 17 (15%) | 27 (28%) | |
| Race | 0.328 | ||
| White | 110 (94%) | 87 (90%) | |
| Black or African American | 6 (5%) | 9 (9%) | |
| Asian | 0 (0%) | 1 (1%) | |
| Not reported: patient refused or not available | 1 (1%) | 0 (0%) | |
| Age | 0.0694 | ||
| N | 117 | 97 | |
| Mean (SD) | 69.4 (7.64) | 69.6 (8.50) |
Dose Levels
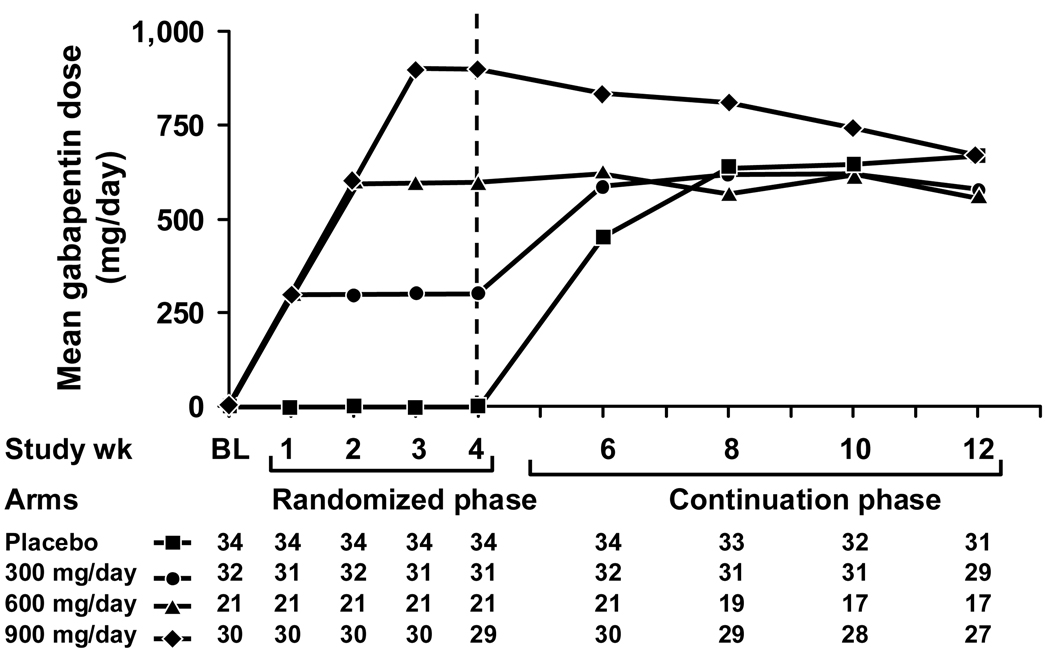
The mean weekly gabapentin dose levels during the eight continuation study weeks are depicted in Figure 1 for the four initial study arms. Throughout the eight-week continuation phase, regardless of their starting dose regimens, patients from each of the original treatment or placebo arms gravitated towards a mean gabapentin dose of about 600 mg/d, a trend displayed in Figure 1.
Figure 1.
Mean gabapentin doses over time, segregated by the initial 4 study arms.
Changes in Hot Flashes
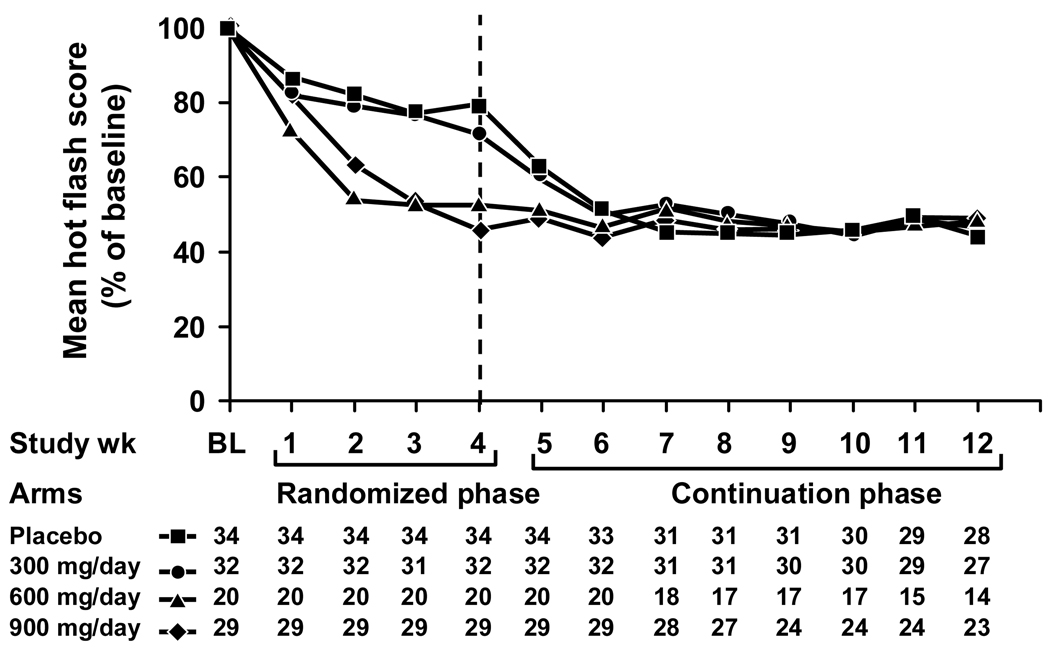
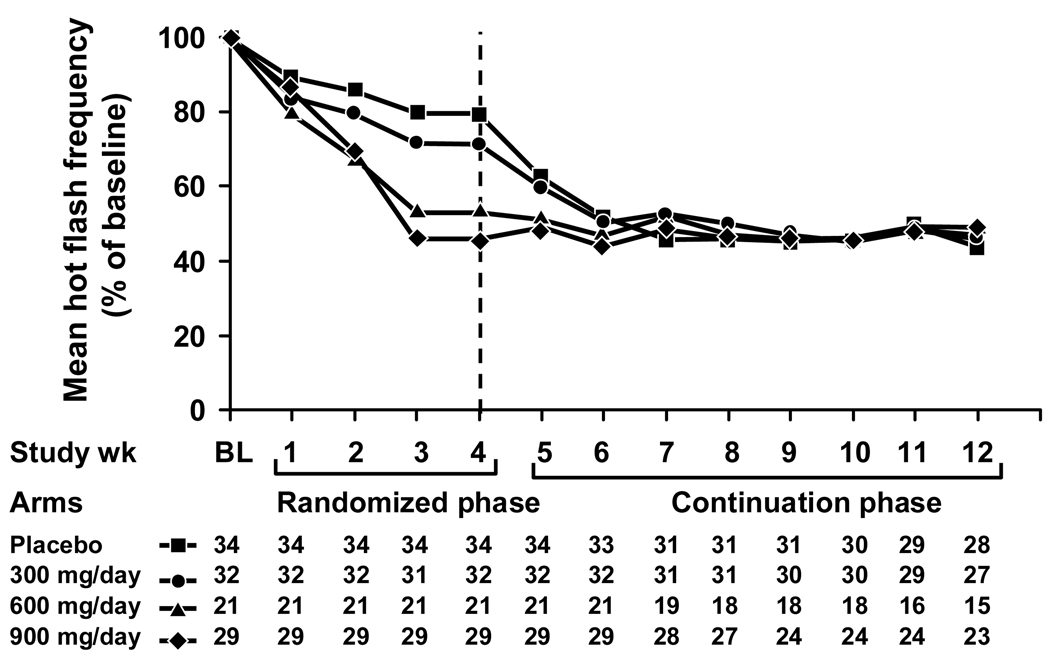
Hot flash changes in score and frequency from baseline in the four study arms during the initial 4-week double blinded treatment period and the subsequent 8 week open-label continuation period are illustrated in Figures 2 and 3. These Figures demonstrate that hot flashes decreased in the initial placebo and 300 mg/d gabapentin arms when they began treatment or titrated to higher doses. By the end of the continuation phase, patients in all original placebo and treatment groups had achieved similar levels of hot flash frequency and score reductions. Comparing the data in the 12th treatment week (at the conclusion of the initial 4 weeks of the randomized trial plus the 8 week continuation trial period) to the baseline week, mean hot flash scores decreased by 44%, 46%, 48%, and 49% in the original placebo and the 300 mg/d, 600 mg/d, and 900 mg/d gabapentin groups, respectively. Correspondingly, hot flash frequencies decreased by 49%, 57%, 51%, and 51% from the baseline week in the placebo, 300 mg, 600 mg, and 900 mg/day arms, respectively.
Figure 2.
Mean hot flash scores over time, segregated by the initial 4 study arms.
Figure 3.
Mean hot flash frequencies over time, segregated by the initial 4 study arms.
Using the 4th week (the last week of the initial randomized study period) as a new baseline for the continuation period, median hot flash scores decreased by 57%, 39%, 19%, and only 4% in the original placebo and gabapentin 300mg/d, 600mg/d, and 900mg/d arms respectively.
Toxicity
From the randomized, placebo-controlled portion of the study, the only statistically significant differences between the combined gabapentin arms and the placebo arm were appetite loss and constipation, with the significant differences favoring the gabapentin arms (i.e., patients on the placebo arm reported more trouble with both appetite loss and constipation). Among patients who were evaluable for the continuation phase analysis, there were no suggestions of increased toxicity from the baseline week as assessed by the Symptom Experience Diaries (including items listed in the Methods Section).
Discussion
Management of hot flashes
Prior to the longitudinal data presented here, there was no readily available information describing the long-term efficacy and potential toxicities of low dose gabapentin for treating hot flashes in men. The results from this continuation phase evaluation support that gabapentin moderately decreases hot flash scores and frequencies without significant toxicities, consistent with the results seen in the placebo-controlled portion of this trial10.
The data suggest that a dose of 600–900 mg/d of gabapentin is better for treating hot flashes than is 300 mg/d. This is demonstrated in Figure 2, which shows that the 300 mg/d arm had further reductions of hot flash scores when patients were allowed to titrate their gabapentin dose upward, to a maximum of 900 mg/d. This is also supported by Figure 1, which shows that patients tended to end up at higher doses than 300 mg/d when allowed to modify their gabapentin regimen, changing daily dosing to achieve maximal efficacy.
It is interesting that a majority of the patients on the open label continuation phase migrated toward a dose of 300 mg twice daily. This may speak to the possibility that thrice daily dosing is inconvenient for patients and less likely to be adhered to.
Additionally, the results corroborate that gabapentin effectively decreases hot flashes for eight weeks beyond the initial four weeks, which were tested in the randomized portion of this protocol. This is illustrated in Figures 2 and 3, which show sustained reductions in hot flash scores and frequencies, respectively, during the continuation phase. These figures also show a relative plateau in hot flash reduction achieved for each study arm.
This sustained effect of gabapentin therapy is consistent with what has been observed in women, where randomized, placebo-controlled trials demonstrated that gabapentin, at a dose of 900mg/d, performs better than placebo out to 12 weeks duration, with a 54% reduction in hot flash score, compared to a 29% reduction with placebo13.
With regard to toxicities, the continuation phase portion of the study did not suggest any toxicity on average, which was consistent with the relative lack of significant side effects reported in the randomized portion of this particular trial. While this is somewhat surprising, as gabapentin is known to cause a variety of side effects, one explanation for this lack of toxicity may be that the beneficial effects of the therapy on hot flashes decreased some patient-experienced symptoms. This may have countered-balanced the toxicities that patients would have otherwise attributed to gabapentin.
The words “support,” “suggest,” and “corroborate” are purposely utilized in the preceding paragraphs, as opposed to more definitive words such as “demonstrating,” “prove,” and/or “confirm.” This is because this was an open-label, observational study, as opposed to the first part of this study, which was a double blinded, placebo-controlled clinical trial.
Based on results from the randomized trial and the supporting evidence gained through the open-label continuation phase of this study, gabapentin appears to be a relatively well tolerated non-hormonal option for the management of hot flashes in men undergoing androgen ablation therapy for the treatment of prostate cancer.
Acknowledgments
Funding: This trial was funded by a National Cancer Institute grant. Gabapentin and matching placebo tablets were provided by Pfizer Corporation, New York, NY, USA.
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35103, CA-63849, CA-63848, CA-35195, CA-35272, CA-35269, CA-35101, CA-60276, CA-52352, CA-37417, CA-35448. This work was also supported by US National Institutes of Health Grant – CA-124477 (PI Charles Loprinzi, MD). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Additional participating institutions include: Iowa Oncology Research Association CCOP, Des Moines, IA 50314 (Robert Behrens, M.D.); Rapid City Regional Oncology Group, Rapid City, SD 59709 (Richard C. Tenglin, M.D.); CentraCare Clinic, St. Cloud, MN 56301 (Donald Jurgens, M.D.); Quain and Ramstad Clinic, Bismarck, ND 58506 (Edward J. Wos, D.O.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, M.D.); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, M.D.); Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (Donald B. Wender, M.D.); Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882 (Shaker R. Dakhil, M.D.); Illinois Oncology Research Assn. CCOP, Peoria, IL 61615-7828 (John W. Kugler, M.D.); Montana Cancer Consortium, Billings, MT 59101 (Benjamin T. Marchello, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Columbus CCOP, Columbus, OH 53215 (J. Philip Kuebler, M.D., Ph.D.); Hawaii Minority-Based CCOP (William S. Loui, M.D.)
References
- 1.Schow DA, Renfer LG, Rozanski TA, et al. Prevalence of hot flushes during and after neoadjuvant hormonal therapy for localized prostate cancer. South Med J. 1998;91:855–857. doi: 10.1097/00007611-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Charig CR, Rundle JS. Flushing. Long-term side effect of orchiectomy in treatment of prostatic carcinoma. Urology. 1989;33:175–178. doi: 10.1016/0090-4295(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 3.Clark JA, Wray N, Brody B, et al. Dimensions of quality of life expressed by men treated for metastatic prostate cancer. Soc Sci Med. 1997;45:1299–1309. doi: 10.1016/s0277-9536(97)00058-0. [DOI] [PubMed] [Google Scholar]
- 4.Loprinzi C, Goldberg RM, O'Fallon JR, et al. Transdermal clonidine for ameliorating postorchiectomy hot flashes. J Urol. 1993;151:634–636. doi: 10.1016/s0022-5347(17)35034-6. [DOI] [PubMed] [Google Scholar]
- 5.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 6.Atala A, Amin M, Harty JI. Diethylstilbestrol in treatment of postorchiectomy vasomotor symptoms and its relationship with serum follicle- stimulating hormone, luteinizing hormone, and testosterone. Urology. 1992;39:108–110. doi: 10.1016/0090-4295(92)90264-w. [DOI] [PubMed] [Google Scholar]
- 7.Burch PA, Loprinzi CL. Prostate-specific antigen decline after withdrawal of low-dose megestrol acetate. J Clin Oncol. 1999;17:1087–1088. [PubMed] [Google Scholar]
- 8.Quella SK, Loprinzi CL, Sloan J, et al. Pilot evaluation of venlafaxine for the treatment of hot flashes in men undergoing androgen ablation therapy for prostate cancer. J Urol. 1999;162:98–102. doi: 10.1097/00005392-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Loprinzi CL, Barton DL, Carpenter LA, et al. Pilot evaluation of paroxetine for treating hot flashes in men. Mayo Clin Proc. 2004;79:1247–1251. doi: 10.4065/79.10.1247. [DOI] [PubMed] [Google Scholar]
- 10.Loprinzi CL, Dueck AC, Khoyratty BS, et al. A phase III randomized, double-blind, placebo-controlled trial of gabapentin in the management of hot flashes in men (N00CB) Ann Oncol. 2009;20:542–549. doi: 10.1093/annonc/mdn644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNair DM, Loor M, Droppleman LF. PRofile of mood states manual. New York, NY: Multi-Health Systems; 2003. [Google Scholar]
- 12.Quella S, Loprinzi CL, Dose AM. A qualitative approach to defining "hot flashes" in men. Urol Nurs. 1994;14:155–158. [PubMed] [Google Scholar]
- 13.Guttuso T, Jr, Kurlan R, McDermott MP, et al. Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337–345. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]