Abstract
Objectives:
Advanced tumor disease and metastatic spinal cord compression (MSCC) are two entities with a high impact on patients’ quality of life. However, prognostic factors on the outcome after primary decompressive surgery are less well-defined and not yet standardized. The aim of this review was to identify prognostic variables that predict functional or ambulatory outcomes in surgically treated patients with symptomatic MSCC.
Materials and Methods:
We conducted MEDLINE database searches using relevant keywords in order to identify abstracts referring to prognostic factors on ambulatory outcomes in surgically treated MSCC patients. Details of all selected articles were assembled and the rates of ambulation were stratified.
Results:
Evidence from five retrospective comparative trials and one observational prospective study summarizes different prognostic factors with a positive or negative influence on postoperative ambulatory status. Ambulatory patients maintaining ambulation status after decompression of the spinal cord constituted 62.1%. The overall rate of MSCC patients losing the ability to ambulate was 7.5% compared to 23.5 % who regained ambulation. Preoperative ambulation status, time to surgery, compression fracture and individual health status seem to be the most relevant prognostic factors for ambulatory outcome.
Conclusions:
There is a lack of standardized prognostic tools which allow predicting outcome in surgically treated patients. A quantitative score consisting of reliable prognostic tools is essential to predict loss and/or regain of ambulation and requires validation in future prospective clinical trials.
Keywords: Functional outcome, metastatic spinal cord compression, prognostic factors
INTRODUCTION
Advanced tumor disease and metastatic spinal cord compression (MSCC) are two entities with a high impact on patients’ quality of life. As the life expectancy of patients with treatable malignancy and treatment options have improved considerably in the last decade,[1–4] orthopedic and spinal surgeons are nowadays frequently confronted with MSCC. Cord compression occurs in approximately 5% of all cancer patients[5] and requires emergent decompressive surgery which is considered to be the “gold standard” in tumors which are not specifically radiosensitive.[6,7] This is also supported by the evidence of regained ambulation after primary radiation therapy which ranged from 18[8] to 51%[9] and primary surgical treatment of MSCC (50[10] –100%).[11] Carefully selected patients in acceptable health conditions with a single site of cord compression, who have not been paraplegic for more than 48 hours, are considered to receive decompressive surgery before radiotherapy.[6]
Ambulation constitutes a primary outcome measure in evaluating different treatment outcomes in MSCC. Postoperative ambulation is a functional parameter contributing to quality of life and to a decline of spinal cord injury related complications[12] in MSCC patients, with a positive prognostic influence on survival outcome.[13,14] Improvement of functional outcome is of clinical relevance in MSCC patients as it contributes directly to quality of life, health care, nursing costs and long-term consequences such as pressure sores, neurogenic bowel and bladder dysfunction. In many instances, oncologic spine surgery intends to prolong survival, preventing neurological decline and improving functional outcomes. Preoperative neurological status and motor function are suggested to have a positive impact on postoperative ambulation.[12] Considering ambulatory rates, the proportion of patients maintaining ambulation and the proportion of patients regaining ambulation need to be distinguished.[6]
Ambulatory recovery in patients with metastatic spinal cancer treated by ventral and dorsal stabilization has been reported to range between 40 and 100%.[15,16] Furthermore, laminectomy alone has been considered to improve ambulatory function only in 23–47%.[17,18] The range of functional improvement gives reason to question the role of possible predictive factors in patients with MSCC.
As most studies include patients with vertebral metastases as well as symptomatic MSCC, essential factors with a positive or negative influence on postoperative functional outcome can be easily missed.[19,20] Proper patient selection is a prerequisite to construct reasonable guidelines for managing MSCC and to analyze the influence of preoperative prognostic tools on outcome measures. The primary aim of this review was to identify the prognostic variables that predict the ambulatory outcome in surgically treated patients with symptomatic MSCC.
MATERIALS AND METHODS
To identify relevant studies referring to prognostic tools before surgery with a possible impact on ambulatory outcome as primary endpoint in patients with MSCC, an electronic search of the MEDLINE (PubMed) database, EMBASE and Cochrane Collaboration Library was conducted. The search strategy used both key words and the following medical subject heading (MeSH) terms: metastatic compression of the spinal cord, metastatic spinal cord compression (MSCC), malignant cord compression (MCC) outcome, operation, laminectomy, anterior approach, posterolateral approach, vertebrectomy, prognosis, functional recovery and ambulation. Abstracts and references of all identified articles were also examined for importance, relevance, and overlap.
We limited our search to clinical studies in adults published from 1966 to March 2010 in English language and excluded all experimental, animal studies, expert opinion, case reports and case series. Clinical studies including a surgical approach were regarded as eligible for this review and graded for their level of evidence (I–III). All clinical trials including surgery in combination to other treatment options (e.g., radiotherapy, chemotherapy or corticosteroids) were excluded. Combined therapies were regarded as confounders influencing primary surgical outcome. Studies were also excluded if surgical treatment was repeated because of a relapse.
Two independently working reviewers (C.P. and C.H.F.) reviewed the abstracts of all articles including ambulation outcome and determined their relevance for the current study. Details of all selected articles including only patients with MSCC were assembled and the rates of ambulation were stratified.
RESULTS
The search strategy for metastatic spinal cord compression resulted in 2224 potentially relevant articles from MEDLINE considering the study question, but only 12 surgical clinical articles[12,18,21–30] were eligible for further text review. Six articles[18,21,24,26,27,29] which did not include any statement on prognostic factors influencing ambulation outcome were excluded after a second review [Figure 1]. Finally, six articles[12,22,23,25,28,30] and their references were analyzed for relevant prognostic tools influencing ambulatory function. The search in other relevant literature databases yielded no additional articles.
Figure 1.
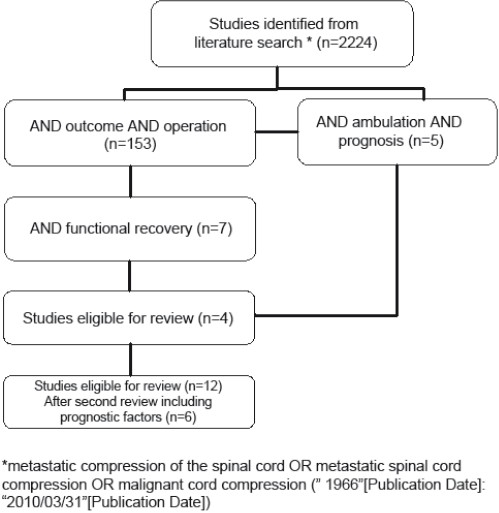
Outline of the literature search on metastatic spinal cord compression
Prognostic factors were found to be related to mechanical factors, biological factors, patients’ general status and the duration of neurological symptoms [Table 1].
Table 1.
An overview of prognostic factors in the included surgical prospective and retrospective studies
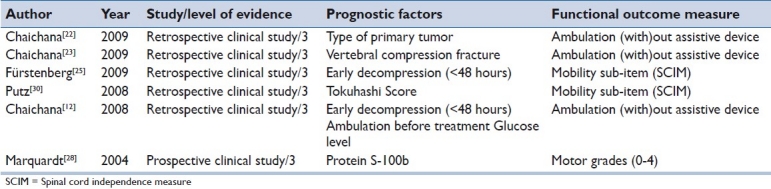
The number of different primary tumors causing symptomatic MSCC evaluated in the included studies ranged from 4 to 11.[12,22,23,25,28,30]
Mechanical factor
Preoperative vertebral compression fracture resulting in spinal instability was associated with decreased postoperative ambulatory status. Therefore, patients with MSCC and a pathological vertebral compression fracture should be identified as soon as possible due to a diminished possibility of walking postoperatively and/or maintaining of the ability to walk.[12,23] Further, compression fractures were assumed to be a negative predictor of functional outcome in five studies[12,23,23,25,30] (statistically significant within two studies).[12,23]
Chaichana et al.[23] pointed out that an initial compression fracture due to spinal instability has a poor functional outcome compared to progressive spinal stenosis. The authors analyzed only those patients who maintained ambulation. Controversially, the authors noted a much higher functional recovery rate in non-ambulatory patients due to compression fractures compared to those with epidural metastatic compression of the spinal cord. Neither the extent of myelopathy in the fracture group and the comparison group nor the location of MSCC was discussed. Further, the degree of metastatic infiltration of vertebrae as well as the timing between onset of neurological deficit and decompression of the spinal cord was not taken into account.
Patient related factors
All six studies[12,22,23,25,28,30] confirmed that the preoperative ambulation status constitutes a prognostic variable that predicts ambulatory outcome. However, only two[12,23] studies reported that this correlation was statistically significant.
After surgical intervention, incomplete MSCC patients with an average Tokuhashi score[31] of 10 showed not only neurological improvement in the ASIA Impairment Scale (AIS), but also the best prognosis to regain ambulatory function. Thirty-one patients with a high Tokuhashi Score and a survival prognosis of more than 12 months benefited from an early surgical treatment with moderate improvement in sensorimotor function.[30]
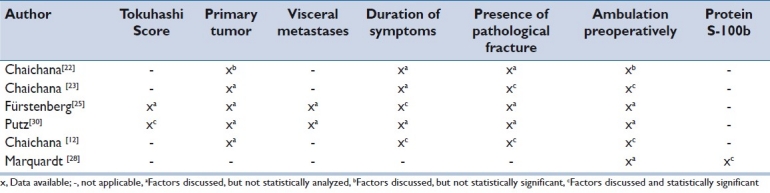
Table 2 shows an overview of prognostic variables discussed in all six studies that predict ambulation outcome in MSCC patients. As the type of tumor and the presence of visceral metastases are included in the Tokuhashi Score, these parameters were regarded as equivalent and related to patients’ health conditions. One study[30] found that the Tokuhashi Score was significantly related to ambulatory outcome. Further, visceral metastases included as sub-items in the Tokuhashi Score were assessed by two studies.[25,30]
Table 2.
Overview of prognostic variables that predict ambulatory outcome in metastatic spinal cord compression patients
Biological factors
The type of primary tumor is not associated with ambulatory outcome.[22] The type of primary cancer was mentioned in five studies,[12,22,23,25,30] but statistical analysis assessing the influence on ambulation outcome after surgery [Table 2] was applied in one study.[22] The prognosis to regain ambulation depending on the type of cancer was analyzed in a retrospective clinical trial limited to six types of primary cancer (lung, prostate, mamma, kidney, gastrointestinal tumors and melanoma), but without statistical significance.
Marquardt et al.[28] found a significant positive correlation between preoperative values of protein S-100b and initial degree of paresis.
Duration of neurological symptoms
Prompt surgical intervention within 48 hours after the appearance of sensorimotor dysfunction results in an increased likelihood of recovering ambulation.[12,25] The factor time as a prognostic factor was cited by five studies,[12,22,23,25,30] but only two studies[12,25] confirmed this hypothesis.
Clinical data and ambulation outcome
Overall, a pooled number of 423 patients from five retrospective comparative trials and one observational prospective study were included in this review and screened for prognostic factors having an impact on functional outcome after decompression of the spinal cord.
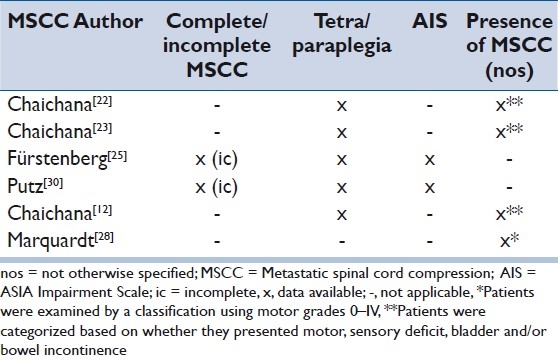
Table 3 presents the reported clinical data of MSCC before surgical intervention. Five studies[12,22,23,25,30] differentiated between tetra- and paraplegia, but only two studies[25,30] used the ASIA classification and included patients with incomplete MSCC. As the neurological and functional recovery potential is higher in incomplete compared to complete spinal cord injury (SCI),[32] possible confounding factors within the four remaining studies[12,22,23,28] have to be considered. As mentioned above, one study[28] failed to indicate any clinical or neurological information for MSCC.
Table 3.
Reported descriptive clinical data of MSCC before surgical intervention
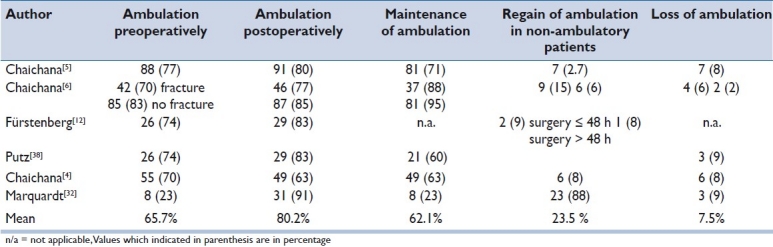
Ambulation was assessed using different outcome measures including ambulation with and without assistive devices,[12,22,23] evaluation of motor grades 0–IV[28] and the sub-item mobility of the SCIM[25,30] as a more precise method to assess functional outcome. We stratified in all six studies parameters reflecting ambulation before and after surgery [Table 4]. The mean recovery of ambulation in preoperatively non-ambulatory patients was 23.5%, ranging from 2.7 to 88%, whereas 7.5% (range 2–9%) lost the ability to ambulate. In 62.1% (range 23–95%) of all cases, ambulatory patients maintained ambulation after decompression of the spinal cord. Overall, 80.2% of the patients across all studies were able to walk postoperatively, compared to 65.7% preoperatively.
Table 4.
Ambulation outcome pre- and postoperatively
DISCUSSION
As stated by the National Cancer Institute (www.cancer.gov), a prognostic factor is a condition or a characteristic of a patient that can be used to estimate the chance of recovery from disease. The current literature in spine oncology is mainly based on retrospective studies defining important prognostic factors affecting local recurrence and survival.[33] These indices were developed from historical data and included not all prognostic factors or relevant information. The principle purpose of a prognostic factor is to guide treatment decision-making in individual patients.
The biological, patient related, mechanical and time-dependent aspects influencing the prognosis of functional outcome have been discussed in this review. Evidence from five retrospective comparative trials and one observational prospective study summarizes four different prognostic factors with a significantly positive or negative impact on postoperative ambulatory status: (1) vertebral compression fracture,[23] (2) early decompression (<48 hours),[12,25] (3) Tokuhashi Score[30] and (4) ambulation before treatment.[12] On the other side, the discrepancies in the analysis of the factors, primary tumor[22] and protein S-100b,[28] might have resulted from limited patient-related confounding factors and different assessment tools.
With regard to the influence of the type of primary tumor on ambulatory outcome,[12,22] it is suggested to use the Tokuhashi Score.[31] The parameter “primary site of the cancer” is one of the six parameters of the Tokuhashi Score and is rated from 0 to 5 to differentiate the tumor biology and aggressiveness. The true prognostic value of histopathologic findings remains unclear as different types of tumors were grouped in the same study population. Of note, the location of MSCC at cervical, thoracic or lumbar level results in different deficits of sensorimotor function. The thoracic spine is the most common site for vertebral metastases, with an occurrence of 70% of all metastases to the spine.[34] This consequence has already been highlighted by previous studies, including traumatic injury and degenerative disease of the thoracic spine.[20,35]
Until standardized outcome measures have been introduced, results of studies included in the current review should be evaluated critically. In our own studies,[25,30] we stated that patient related factors are important and need to be considered in prognosis. For this purpose, the Tokuhashi Score is an excellent tool which represents not only the general health status but also tumor related indices, and takes the severity of the neurological deficit into account.
We found a mean of 62.1% (range 23–95%) of patients maintaining ambulation after surgical treatment. This confirms the hypothesis that ambulatory status before surgical treatment is a strong prognostic factor for postoperative ambulation.[12] Delay in diagnosis and treatment can lead to functional decline. In contrast to our study, Chaichana et al. reported on a lower postoperative ambulation status (63%) compared to ambulatory function preoperatively (70%). It remains unclear if these patients who lost ambulation had disease progression leading to pain and incapacity to walk. In this review, the loss of ambulation rate was 7.5% (range 2–9%), whereas the recovery rate of ambulation was 23.5% (range 2.7–88%). The wide range of recovery of ambulation is due to the results of Marquardt et al.[28] (88%), whereas the other included studies[12,22,23,25,30] showed recovery rates of ambulation between 2.7 and 15%. The timing of surgery is an important prognostic factor influencing the therapeutic effect of the operation and should be performed within 48 hours if the patient is a candidate for surgery.[12,25] The influence of early surgical decompression (<48 hours) was supported by two studies,[12,25] with similar distribution of patients regaining ambulation (8–9%).
The definition of ambulation differs in the literature and has not yet been standardized.[21,12,22–30,36] Patchell et al.[37] defined ambulation as the ability to take at least two steps with each foot either unassisted or using an assistive device after radiotherapy. Maranzano et al.[38] considered a patient ambulatory when walking with or without support at 1 month was possible. In addition, several studies measured ambulation at different time points.[39,11,40] For this reason, we suggest to use the mobility sub-item of the Spinal Cord Independence Measure[41] preoperatively, postoperatively and at properly defined time points in order to standardize ambulation or functional outcome more properly.
Progress in spine surgery provides an opportunity for direct decompression of the spinal cord and stabilization. In fact, early decompressive surgery has become the standard treatment in metastatic lesions that are not radiosensitive. Patchell et al.[37] justified the use of surgery as the first line of therapy in ambulant patients because 20% of the patients randomized to the radiotherapy arm crossed over to surgery due to the occurrence of neurological deterioration and the loss of the ability to walk. Only 30% of them regained the ability to walk. This could be due to a primary unstable spine which constitutes a predictor for poor outcome.[23] In this randomized prospective controlled trial, overall, 63% of non-ambulant patients regained the ability to walk with surgery and radiotherapy as compared with only 19% in those receiving radiotherapy alone.
The assessment of spinal instability in MSCC is described by different scores.[33,42–44] The direct relation between the resulting pathological fractures and the neurological deficit remains unclear. Actually, there are two types of MSCC causing sudden or slow compression of the spinal cord. Sudden compression can be explained by disruption of arterial blood flow to the spinal cord with fast-growing tumors or pathologic fracture leading to spinal cord infarction, whereas slowly growing tumors with a low proliferation rate cause slow compression through venous congestion and edema.[45]
In this review, we selected only studies that included MSCC patients who received primary surgical treatment and in whom the parameter ambulation was analyzed pre- and postoperatively. By applying this strict approach, we were able to include only six studies (five retrospective trials and one observational prospective study) with a level of evidence graded 3–4. However, as there is no consensus on a common set of prognostic factors, which allow predicting outcome in surgically treated patients in the current MSCC literature, we consider this approach to be justified. To this point, no classification system has been established and validated in the framework of prospective clinical trials. Our intention was to use the best available literature to develop the basis for a quantitative score based on a ranking scheme of prognostic factors to predict quality of life, loss and/or regain of ambulation in the MSCC patient population.
To optimize MSCC care, we must face the challenging search for factors contributing to neurologic and functional outcome by using appropriate classification systems, e.g., standards of American Spinal Injury Association Impairment Scale[46] or mobility sub-item of the Spinal Cord Independence Measure.[41] Since there are several known predictive factors influencing functional outcomes after surgical decompression in MSSC patients, it is unlikely that a single prognostic factor or marker will predict functional outcomes. The introduction of a valid and reliable spinal oncology specific measure including prognostic factors may provide a more robust and standardized measure in future studies. A well-designed prospective trial taking into account most relevant prognostic factors may provide an answer to this problem and should be supported by collaboration of centers specialized in oncologic spine surgery and spinal cord medicine.
CONCLUSION
Due to a lack of standardized prognostic tools, prediction of ambulatory outcome after primary surgery in MSCC patients is currently limited. Preoperative ambulation status, time to surgery, compression fracture and individual health status seem to be the most relevant and statistically significant prognostic factors for ambulatory outcome. The evaluation and integration of identified prognostic factors in preoperative assessment protocols of future prospective clinical trials is important to evaluate the quality of life and factors predicting loss and/or regain of ambulation.
Acknowledgments
The authors thank Mrs. Gassmann for her help in the extraction of relevant articles for this review.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chu DZ. Improved survival with adjuvant external-beam radiation therapy in lymph node-negative pancreatic cancer: a United States population-based assessment. Cancer. 2008;113:1110–1. doi: 10.1002/cncr.23602. [DOI] [PubMed] [Google Scholar]
- 2.Kachroo S, Tong L, Spitz MR, Xing Y, Merriman K, Zhu DK, et al. Trends in prevalence of prognostic factors and survival in lung cancer patients from 1985 to 2004 at a tertiary care center. Cancer Detect Prev. 2008;32:101–8. doi: 10.1016/j.cdp.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo JH, Jalaludin B, Wong SK, Kneebone A, Connor SJ, Leong RW. Improved survival in young women with colorectal cancer. Am J Gastroenterol. 2008;103:1488–95. doi: 10.1111/j.1572-0241.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 4.Winter H, Meimarakis G, Hoffmann G, Hummel M, Rüttinger D, Zilbauer A, et al. Does Surgical Resection of Pulmonary Metastases of Head and Neck Cancer Improve Survival? Ann Surg Oncol. 2008;15:2915–26. doi: 10.1245/s10434-008-0001-4. [DOI] [PubMed] [Google Scholar]
- 5.Rades D, Blach M, Nerreter V, Bremer M, Karstens JH. Metastatic Spinal Cord Compression.Influence of time between onset of motoric deficits and start of irradiation on therapeutic effect. Strahlenther Onkol. 1999;175:378–81. doi: 10.1007/s000660050024. [DOI] [PubMed] [Google Scholar]
- 6.George R, Jeba J, Ramkumar G, Chacko AG, Leng M, Tharyan P. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev. 2008;8:CD006716. doi: 10.1002/14651858.CD006716.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Klimo P, Kestle JRW, Schmidt MH. (2005) A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–75. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podd TJ, Carpenter DS, Baughan CA, et al. Spinal cord compression: prognosis and implications for treatment fractionation. Clin Oncol (R Coll Radiol) 1992;4:341–4. doi: 10.1016/s0936-6555(05)81121-5. [DOI] [PubMed] [Google Scholar]
- 9.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–67. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 10.Mannion RJ, Wilby M, Godward S, Lyratzopoulos G, Laing RJ. The surgical management of metastatic spinal disease: prospective assessment and long-term follow-up. Br J Neurosurg. 2007;21:593–8. doi: 10.1080/02688690701593579. [DOI] [PubMed] [Google Scholar]
- 11.Sundaresan N, Digiacinto GV, Hughes JE, Cafferty M, Vallejo A. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645–50. doi: 10.1097/00006123-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62:683–92. doi: 10.1227/01.neu.0000317317.33365.15. [DOI] [PubMed] [Google Scholar]
- 13.Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003;97:476–84. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 14.Weigel B, Maghsudi M, Neumann C, Kretschmer R, Müller FJ, Nerlich M. Surgical management of symptomatic spinal metastases.Postoperative outcome and quality of life. Spine. 1999;24:2240–6. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Livingston KE, Perrin RG. The neurosurgical management of spinal metastases causing cord and cauda equina compression. J Neurosurg. 1978;49:839–43. doi: 10.3171/jns.1978.49.6.0839. [DOI] [PubMed] [Google Scholar]
- 16.Villavicencio AT, Oskouian RJ, Roberson C, Stokes J, Park J, Shaffrey CI, et al. Thoracolumbar vertebral reconstruction after surgery for metastatic spinal tumors: long-term outcomes. Neurosurg Focus. 2005;19:E8. doi: 10.3171/foc.2005.19.3.9. [DOI] [PubMed] [Google Scholar]
- 17.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien) 1998;140:957–67. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 18.Siegal T, Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: a prospective study. Neurosurgery. 1985;17:424–32. doi: 10.1227/00006123-198509000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hammerberg KW. Surgical treatment of metastatic spine disease. Spine (Phila Pa 1976) 1992;17:1148–53. doi: 10.1097/00007632-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Onimus M, Papin P, Gangloff S. Results of surgical treatment of spinal thoracic and lumbar metastases. Eur Spine J. 1996;5:407–11. doi: 10.1007/BF00301969. [DOI] [PubMed] [Google Scholar]
- 21.Abel R, Keil M, Schläger E, Akbar M. Posterior decompression and stabilization for metastatic compression of the thoracic spinal cord: is this procedure still state of the art? Spinal Cord. 2008;46:595–602. doi: 10.1038/sc.2008.11. [DOI] [PubMed] [Google Scholar]
- 22.Chaichana KL, Pendleton C, Sciubba DM, Wolinsky JP, Gokaslan ZL. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression.Clinical article. J Neurosurg Spine. 2009;11:56–63. doi: 10.3171/2009.1.SPINE08657. [DOI] [PubMed] [Google Scholar]
- 23.Chaichana KL, Pendleton C, Wolinsky JP, Gokaslan ZL, Sciubba DM. Vertebral compression fractures in patients presenting with metastatic epidural spinal cord compression. Neurosurgery. 2009;65:267–74. doi: 10.1227/01.NEU.0000349919.31636.05. [DOI] [PubMed] [Google Scholar]
- 24.Chen YJ, Chang GC, Chen HT, Yang TY, Kuo BI, Hsu HC, et al. Surgical results of metastatic spinal cord compression secondary to non-small cell lung cancer. Spine (Phila Pa 1976) 2007;32:E413–8. doi: 10.1097/BRS.0b013e318074d6c7. [DOI] [PubMed] [Google Scholar]
- 25.Fürstenberg CH, Wiedenhöfer B, Gerner HJ, Putz C. The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J Bone Joint Surg Br. 2009;91:240–4. doi: 10.1302/0301-620X.91B2.20894. [DOI] [PubMed] [Google Scholar]
- 26.Harris JK, Sutcliffe JC, Robinson NE. The role of emergency surgery in malignant spinal extradural compression: assessment of functional outcome. Br J Neurosurg. 1996;10:27–33. [PubMed] [Google Scholar]
- 27.Jansson KA, Bauer HC. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15:196–202. doi: 10.1007/s00586-004-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquardt G, Setzer M, Seifert V. Protein S-100b as serum marker for prediction of functional outcome in metastatic spinal cord compression. Acta Neurochir (Wien) 2004;146:449–52. doi: 10.1007/s00701-004-0242-3. [DOI] [PubMed] [Google Scholar]
- 29.Martenson JA, Jr, Evans RG, Lie MR, Ilstrup DM, Dinapoli RP, Ebersold MJ, et al. Treatment outcome and complications in patients treated for malignant epidural spinal cord compression (SCC) J Neurooncol. 1985;3:77–84. doi: 10.1007/BF00165175. [DOI] [PubMed] [Google Scholar]
- 30.Putz C, Wiedenhöfer B, Gerner HJ, Fürstenberg CH. Tokuhashi prognosis score: an important tool in prediction of the neurological outcome in metastatic spinal cord compression: a retrospective clinical study. Spine (Phila Pa 1976) 2008;33:2669–74. doi: 10.1097/BRS.0b013e318188b98f. [DOI] [PubMed] [Google Scholar]
- 31.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–91. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 32.Curt A, Van Hedel HJ, Klaus D, Dietz V. Recovery from a spinal cord injury: signifi cance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25:677–85. doi: 10.1089/neu.2007.0468. [DOI] [PubMed] [Google Scholar]
- 33.Sundaresan N, Boriani S, Okuno S. State of the Art Management in Spine Oncology.A Worldwide Perspective on Its Evolution, Current State, and Future. Spine. 2009;34:7–20. doi: 10.1097/BRS.0b013e3181bac476. [DOI] [PubMed] [Google Scholar]
- 34.Klimo P, Jr, Kestle JR, Schmidt MH. Treatment of metastatic spinal epidural disease: a review of the literature. Neurosurg Focus. 2003;15:E1. doi: 10.3171/foc.2003.15.5.1. [DOI] [PubMed] [Google Scholar]
- 35.Ronen J, Goldin D, Itzkovich M, Bluvshtein V, Gelernter I, Gepstein R, et al. Outcomes in patients admitted for rehabilitation with spinal neurological lesions following intervertebral disc herniation. Spinal Cord. 2004;42:621–6. doi: 10.1038/sj.sc.3101642. [DOI] [PubMed] [Google Scholar]
- 36.Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317:18–21. doi: 10.1136/bmj.317.7150.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–8. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 38.Maranzano E, Bellavita R, Rossi R, De Angelis V, Frattegiani A, Bagnoli R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23:3358–65. doi: 10.1200/JCO.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 39.Graham PH, Capp A, Delaney G, Goozee G, Hickey B, Turner S, et al. A pilot randomised comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal-cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clin Oncol (R Coll Radiol) 2006;18:70–6. doi: 10.1016/j.clon.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Vecht CJ, Haaxma-Reiche H, van Putten WL, de Visser M, Vries EP, Twijnstra A. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39:1255–7. doi: 10.1212/wnl.39.9.1255. [DOI] [PubMed] [Google Scholar]
- 41.Catz A, Itzkovich M. Spinal cord independence measure: comprehensive ability rating scale for the spinal cord lesion patient. J Rehabil Res Dev. 2007;44:65–8. doi: 10.1682/jrrd.2005.07.0123. [DOI] [PubMed] [Google Scholar]
- 42.Cybulski GR. Methods of surgical stabilization for metastatic disease of the spine. Neurosurgery. 1989;25:240–52. doi: 10.1097/00006123-198908000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Harrington KD. Metastatic disease of the spine. J Bone Joint Surg Am. 1986;68:1110–15. [PubMed] [Google Scholar]
- 44.Kostuik JP, Weinstein JN. Differential diagnosis and surgical treatment of metastatic spine tumors. In: Frymoyer JW, editor. The adult spine. New York: Raven Press; 1991. pp. 861–88. [Google Scholar]
- 45.Prewett S, Venkitaraman R. Metastatic spinal cord compression: Review of the evidence for a radiotherapy dose fractionation schedule. Clin Oncol (R Coll Radiol) 2010;22:222–30. doi: 10.1016/j.clon.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Marino RJ. International Standards for Neurological and Functional Classification of Spinal Cord Injury. Chicago, IL: American Spinal Injury Association. 2000 doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]


