Abstract
This multi-center study examined prevalence of cognitive and academic delays in children following liver transplant (LT). 144 patients ages 5–7 and 2 years post-LT were recruited through the SPLIT consortium and administered the Wechsler Preschool and Primary Scale of Intelligence, 3rd Edition (WPPSI-III), the Bracken Basic Concept Scale, Revised (BBCS-R), and the Wide Range Achievement Test, 4th edition (WRAT-4). Parents and teachers completed the Behavior Rating Inventory of Executive Function (BRIEF). Participants performed significantly below test norms on intelligence quotient (IQ) and achievement measures (Mean WPPSI-III Full Scale IQ = 94.7± 13.5; WRAT-4 Reading = 92.7± 17.2; WRAT-4 Math = 93.1± 15.4; p<0001). 26% of patients (14% expected) had “mild to moderate” IQ delays (Full Scale IQ=71–85) and 4% (2% expected) had “serious” delays (Full Scale IQ ≤70; p<0.0001). Reading and/or math scores were weaker than IQ in 25%, suggesting learning disability, compared to 7% expected by CDC(1) statistics (p<0.0001). Executive deficits were noted on the BRIEF, especially by teacher report (Global Executive Composite = 58; p<0.001). Results suggest a higher prevalence of cognitive and academic delays and learning problems in pediatric LT recipients compared to the normal population.
Keywords: liver transplant, pediatric liver disease, psychological aspects of organ transplantation, cognition disorders, neuropsychological tests, learning disorders
INTRODUCTION
Neurological injury early in life due to conditions such as perinatal complications, traumatic brain injury, and cancer has the potential to inflict significant, long-lasting developmental consequences (2–5). Hepatic encephalopathy and other neurological insults associated with end stage liver disease may have a similar potential. The majority of pediatric liver transplant recipients experience end-stage liver disease early in life, and in most large series, the median age at transplantation is less than 2 years (6, 7). In the decades since the first pediatric liver transplantation (LT), major strides have been made in managing morbidity and limiting mortality in this patient group, but significant concerns remain regarding functional outcomes in children following LT, especially in the areas of cognitive function and school performance (8, 9). Few studies have examined neurocognitive outcomes in pediatric patients with liver disease and transplantation. Nevertheless, evidence is mounting to suggest pediatric recipients of LT experience significant cognitive deficits ranging from a depression in overall intelligence quotient (IQ) to less obvious neuropsychological dysfunction (10–14). Mean IQ scores typically fall in the low average to average range, with over-representation at the lower end of the IQ spectrum. Early disease onset, poor nutritional status and growth deficits, and longer duration of illness prior to transplant have been implicated as factors associated with poorer outcomes (15–17). A handful of studies have reported an increased prevalence of learning problems including below average academic achievement (10), IQ/achievement discrepancy (12), and parent report of learning disability and special education services (18). Although problems with attention and executive function (EF) have also been noted anecdotally, these domains have yet to be systematically examined in pediatric LT recipients. Experience in other pediatric disease groups would suggest these areas of function may be compromised as well (19–22).
Previous neurocognitive studies in pediatric LT recipients have been limited by significant methodological flaws. Primarily, data were derived from small, single center samples that may result in site-specific findings and consequently limit generalizability to the larger population of patients. The only multi-site study of pediatric LT patients reports on school outcomes based on parent report, not direct testing (18). Further, because single center designs significantly limit sample size, samples have generally included patients who ranged widely in age. This necessitated combining scores from different measures of the same construct (e.g., IQ) which may or may not be psychometrically similar. The primary purpose of the present study was to assess the prevalence of cognitive and academic delays following pediatric LT in a large, multi-center cohort of pediatric LT recipients utilizing the same standardized instruments at each testing time point. The age range of 5 up to 7 years was selected because school entry represents a time of significant new cognitive and learning challenges and this age range limited participants to a select group who had received transplants very early in life (under age 5). We chose to focus on children who had experienced advanced liver disease in early childhood since we hypothesized they would be particularly vulnerable to neurocognitive insult (23). A further advantage of the tight age range was participants were all transplanted in a contemporary time period between 1999–2007, limiting possible effects due to changes in standard of care over time.
MATERIALS AND METHODS
Study population
FOG is an independently funded ancillary study of the SPLIT registry. Twenty medical centers elected to participate in the FOG study and patients at these centers were identified and recruited through the infrastructure of the SPLIT registry between 6/1/05 and 12/31/09. Eligible patients were pediatric single organ LT recipients who were 1) age 5 years, 0 months through 6 years, 11 months, 29 days at testing, 2) maintaining active follow-up in the SPLIT registry as defined by entry of follow-up data within one year either before or after the age eligibility window, 3) fluent in English, both patient and primary caregiver, and 4) at least two years from their most recent LT. By definition, patients meeting these criteria all received LT prior to age 5 years. Patients with combined organ transplant or known uncorrectable vision or hearing impairment were excluded. Prior to testing, all patients underwent a hearing screen and those with uncorrected hearing loss between 500 – 4000 HZ were excluded (24). Patients with serious neurological injury that would preclude participation in testing (e.g., no speech, severe motor deficits) or that could significantly affect validity (i.e., uncontrolled seizures, current evidence of hepatic encephalopathy) were also excluded.
Study design
This study was designed as a longitudinal assessment of neurocognitive function beginning in the earliest primary school years, and continuing with follow-up testing two years later. This report includes data from the first testing time point. The study was approved by the Institutional Review Boards at participating centers and written informed consent was obtained prior to participation. Participants were recruited, consented, and tested at the transplant center where they received medical follow-up. Tests were performed or supervised by licensed psychologists. Results of Surveys of Health Related Quality of Life (HRQOL) were the focus of a separate report (25). Data pertaining to demographic and medical variables and school outcomes were obtained from the SPLIT registry (18).
Instruments and Testing Procedure
The standardized testing battery included the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) (26), the Word Reading and Math Computation subtests of the Wide Range Achievement Test, Fourth Edition (WRAT-4) (27, 28), and the School Readiness Composite of the Bracken Basic Concept Scale, Revised (BBCS-R/ SRC) (29). Of note, the first eight patients in the study completed the previous edition of the WRAT and data from the two versions were pooled since item content is highly similar. Composite scores for the WPPSI-III including Full Scale IQ (FSIQ), Verbal IQ (VIQ), Performance IQ (PIQ), and Processing Speed (PS) were generated from the eight core subtests. Two optional subtests assessing vocabulary knowledge were also administered in order to obtain the General Language Composite (GLC). The normative population mean is 100 and the standard deviation is 15 for all scores.
Examiners at the individual centers completed a Validity Rating Form (VRF) following testing to provide information regarding factors that might have interfered with test administration. Data from cases where the examiner indicated serious concerns and data from cases in which a VRF was not completed (2 cases) were not included in the final analysis. No more than 5 cases had serious concerns on each measure. Additional missing IQ and achievement data (no more than 6 cases on each measure) was due to examiner error or logistical problems resulting in incomplete test administration.
Parents and teachers completed the Behavior Rating Inventory of Executive Function (BRIEF) (30). The BRIEF validated for children age 5 to 18 provides ratings of EF using questions that are clearly tied to real life situations. Sample questions include: “Underestimates time needed to finish tasks,” “leaves a trail of belongings wherever he/she goes,” “forgets what he/she was doing,” “forgets to hand in homework, even when completed,” “acts too wild or out of control.” This measure includes 8 sub-scales and yields an overall Global Executive Composite (GEC), and two summary indices; Metacognition (MI) and Behavioral Regulation (BRI). The BRI is composed of the Inhibit, Shift, and Emotional Control subscales, while the MI is composed of Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor subscales. This measure yields T scores, with higher scores indicating more concerns. The normative population mean is 50 and the standard deviation is 10.
In order to highlight the prevalence of intellectual delays in the sample, patients were divided into subgroups based on FSIQ scores. We labeled patients with an IQ score falling between one to two standard deviations below the published mean of 100 as “mild to moderately” delayed (FSIQ = 71–85), and those with IQ scores more than two standard deviations below the mean as “seriously” delayed (FSIQ ≤70). Patients in the “seriously” delayed category had IQ scores typically indicative of mental retardation (31) while those in the “mild to moderately” delayed category had IQ scores falling in the range of borderline intellectual functioning to the lower end of the low average range (26). We operationally defined “Learning Disability” (LD) as a discrepancy of 15 points or more (one standard deviation) between intellectual ability (WPPSI-III FSIQ) and achievement (WRAT-4 Reading or Math Computation) (12, 31). Data from the previously reported HRQOL measurements in this population, specifically the School Function Sub-scale of the PedsQL™4.0 Generic core scale were also included in the analysis comparing indicators of school function between FSIQ subgroups.
Statistical analysis
Eligible-enrolled and eligible–but not enrolled patients were compared by chi square statistics. Of note, non-participants who were eligible for less than 30 days due to an intervening birthday were not included in the participant versus eligible non-participant comparison. Patients’ age-normed standard scores on the WPPSI-III, BBCS-R/SRC, and WRAT-4 were converted into z scores for each subject by comparing them to the normal population mean and standard deviation. Comparisons were made using the Student’s t-test. The Type I error rate was maintained at 0.05 by the Hochberg adjustment for multiple comparisons; an adjustment was made separately for each instrument (32). To determine the magnitude of the differences, effect sizes were calculated. Effect sizes for differences in means are designated as small (0.20), medium (0.50), and large (0.80) in magnitude (33). Categorical comparisons of IQ group, learning disability, BRIEF and education variables were made using chi square goodness of fit statistics or Fisher’s exact test as appropriate.
RESULTS
Participant Characteristics
Of the 456 SPLIT patients eligible for enrollment during the study period, 144 (32 %) participated. Reasons for failure to participate were available for 51% of non-participants and included time burden (57 patients), distance from the medical center (25), no clinic visit during eligibility window (44), refused consent (12), and other/unknown (22). Reasons for non-participation in the remaining 49% of eligible subjects were not gathered since they were still eligible for enrollment at the time of this analysis.
Participants and non-participants did not differ on basic demographic or medical variables including age at transplant, gender, race, primary diagnosis, donor type, primary insurance at transplant, or primary caregiver’s education. Participants were more often transplanted prior to 2002 than non-participants (p=0.04). Median age at transplant for participants was 1.19 years (range 0.07–4.75) and median time since transplant was 4.87 years (range 2.03–6.68). Table 1 provides descriptive statistics for participants on select transplant variables and Table 2 details information regarding school attendance and resource utilization available for 107 (74%) of the 144 patients. 26 (25%) patients had missed 11 or more school days due to illness or doctor visits within the past 12 months. Of note, one child was home schooled, 70 (67%) were in kindergarten, 31 (30%) were in first grade, 1 patient was in second grade and 4 were either in preschool or parents did not specify a grade on the data collection form. Eight patients (8%) had been held back or repeated a grade, and 33 (31%) had received special education services during the past 12 months.
Table 1.
Patient Characteristics
| Total (n = 144) | ||
|---|---|---|
| Median | Range | |
| Age at testing (years) | 6.26 | 5.01 – 6.99 |
| Age at LT (years) | 1.19 | 0.07 – 4.75 |
| Interval from LT (years) | 4.87 | 2.03 – 6.68 |
| PELD score at LT (9% missing) | 16.30 | −9.69 – 46.58 |
| Height Z score at LT | −1.71 | −7.80 – 6.08 |
| Weight Z score at LT | −1.28 | −8.94 – 2.19 |
| INR at LT | 1.3 | 0.8 – 6.1 |
| Albumin at LT (g/dL) | 3.1 | 1.5 – 4.8 |
| Total Bilirubin at LT (mg/dL) | 11.1 | 0.0 – 58.0 |
| Total Bilirubin at testing (mg/dL)** | 0.5 | 0.1 – 5.6 |
| Albumin at testing (g/dL)** | 4.2 | 2.8 – 5.1 |
| N | % | |
| Female | 83 | 58% |
| Race | ||
| White | 84 | 58% |
| Black | 21 | 15% |
| Hispanic | 21 | 15% |
| Other or missing (n=1) | 18 | 12% |
| Primary Diagnosis | ||
| Biliary Atresia | 84 | 58% |
| Acute Liver Failure | 14 | 10% |
| Other cholestatic | 20 | 14% |
| Metabolic* | 14 | 10% |
| Other | 12 | 8% |
| Status at transplant | ||
| ICU | 30 | 21% |
| Hospitalized | 21 | 15% |
| Home | 93 | 65% |
| Number of Liver transplants | ||
| 1 | 137 | 95% |
| 2 | 6 | 4% |
| 3 | 1 | 1% |
| Initial immunotherapy*** | ||
| Cyclosporine | 31 | 22% |
| Tacrolimus | 94 | 65% |
| Induction therapy | ||
| None | 100 | 69% |
| Yes | 44 | 31% |
| Total number of rejection episodes | ||
| 0 | 60 | 42% |
| 1 | 52 | 36% |
| 2 – 7 | 32 | 22% |
Metabolic diagnosis included Urea cycle defects (n=2), Wilson’s disease (n=0), alpha-1-anti-trypsin deficiency (n=3), Tyrosinemia (n=2), Neonatal Hemachromatosis (n=1), Inborn error in bile acid metabolism (n=1), other metabolic (n=5)
Values missing on 5% of cohort
Values missing on 13% of cohort
Table 2.
Attendance and Special Educational Resource Utilization
| Total | ||
|---|---|---|
| N | % | |
| Total | 107 | 100 |
| History of Head Start or Early Intervention Program | 50 | 47 |
| History of special education or resource educational services as recommended by Individual Educational Plan (IEP) | 30 | 28 |
| History of 504 Plan | 7 | 6 |
| Full days of school missed during past 12 months due to illness or doctor visits | ||
| 0–4 days | 48 | 46 |
| 5–10 days | 31 | 30 |
| 11–20 days | 15 | 14 |
| 21–30 days | 7 | 7 |
| 31+ days | 4 | 4 |
| Special education support received during the past 12 months | 33 | 31 |
| Speech/Language | 28 | 85 |
| Reading/Language Arts | 18 | 55 |
| Physical Occupational Therapy | 12 | 36 |
| Math | 8 | 24 |
| Other (social skills/sensory diet) | 3 | 9 |
| Special education <50% of time | 16 | 48 |
| Special education >50% of time | 10 | 30 |
| Special education timing not specified | 7 | 21 |
| Attention Deficit/Hyperactivity Disorder Diagnosis | 5 | 5 |
| Taking medication for ADHD | 2 | |
| Learning Disability Diagnosis | 5 | 5 |
| Mental Retardation Diagnosis | 3 | 3 |
Prevalence of Cognitive Delays
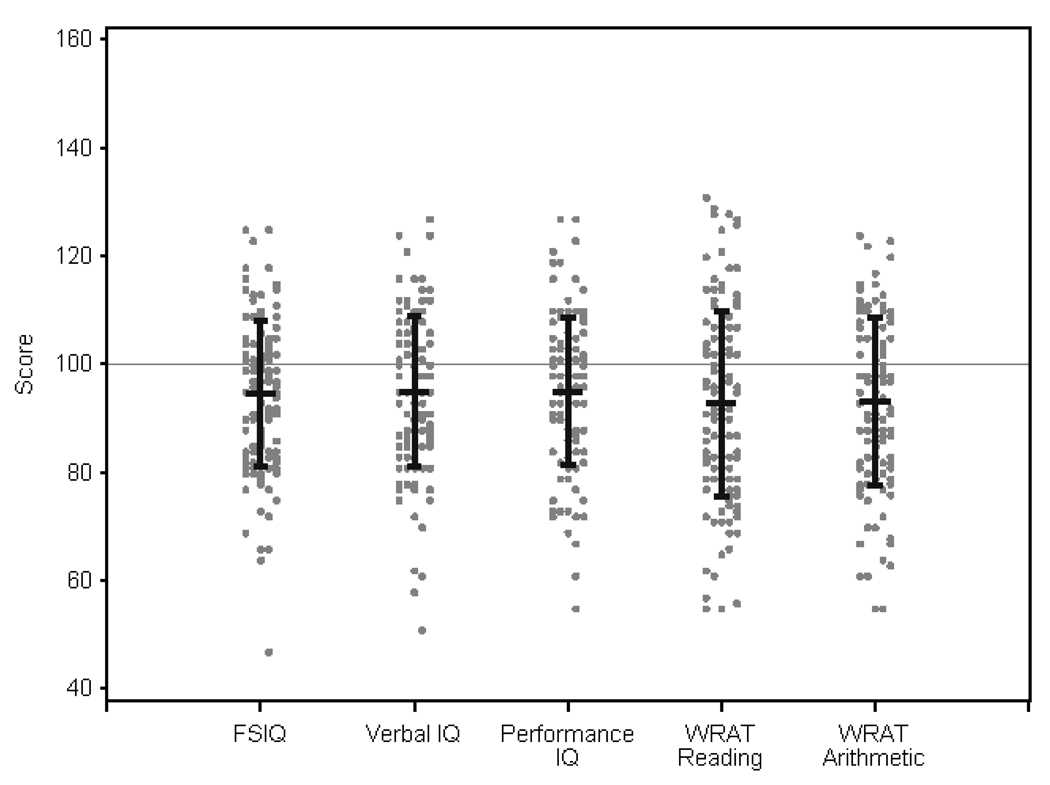
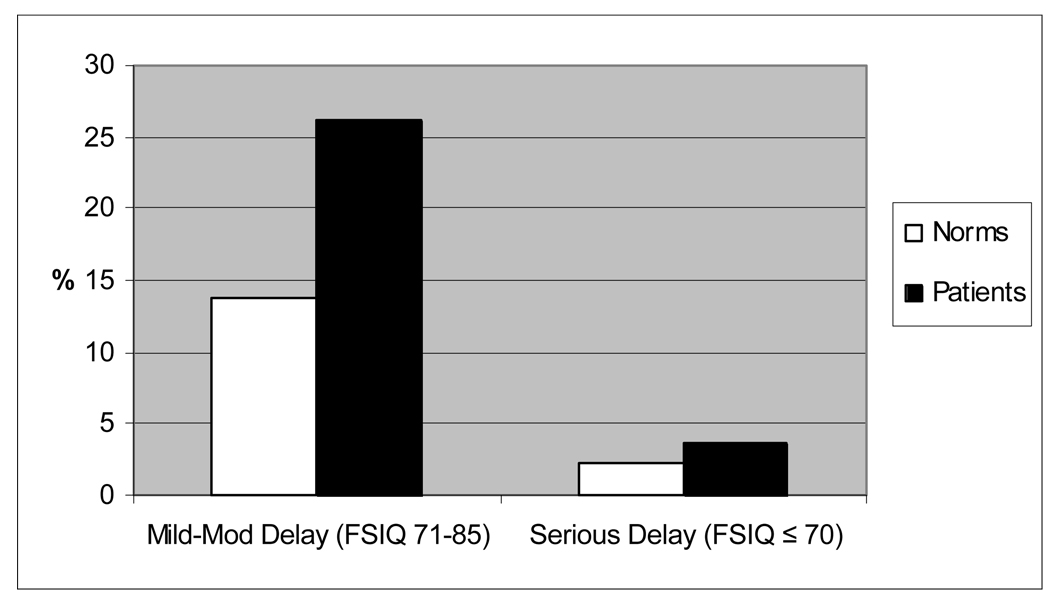
Table 3 summarizes intelligence and academic achievement scores and Figure 1 displays the distribution of these scores. Patients scored significantly lower than test norms on the WPPSI-III FSIQ, VIQ, and PIQ composites (p≤.0001). PSQ and GLC did not differ significantly from norms. As seen in Figure 2, significantly more patients fell in the “mild to moderately” or “seriously” delayed groups than expected (p < 0.0001). 26% of our sample were “mild to moderately” delayed versus 14% expected, and 4% of our sample were “seriously” delayed (FSIQ ≤70) versus 2% expected. Most patients (76%) had similar VIQ and PIQ scores (≤15 point discrepancy), and of the remaining patients, equal numbers had higher scores on each scale.
Table 3.
IQ and Achievement Scores
| Variable | Sample Size | Mean ± SD | Adjusted Significance Level |
Effect Size |
|---|---|---|---|---|
| WPPSI-III | ||||
| Full Scale IQ | 134 | 94.7 ± 13.5 | <0.0001 | −0.35 |
| Verbal IQ | 134 | 95.0 ± 13.8 | 0.0001 | −0.33 |
| Performance IQ | 134 | 94.9 ± 13.5 | 0.0001 | −0.34 |
| Processing Speed | 132 | 98.3 ± 15.7 | NS | −0.11 |
| General Language Composite | 135 | 98.7 ± 14.8 | NS | −0.09 |
| WRAT-4 | ||||
| Reading | 140 | 92.7 ± 17.2 | <0.0001 | −0.49 |
| Math Computation | 139 | 93.1 ± 15.4 | <0.0001 | −0.46 |
| BBCS-R | ||||
| School Readiness Composite | 138 | 98.2 ± 16.5 | NS | −0.12 |
Note: All measures have a mean=100 and SD=15 for the normal population
Figure 1. Distribution of IQ and Achievement Scores.
The figure shows the distribution of patient scores on the WPPSI-III and WRAT-4. Test norms for each measure are 100±15.
Figure 2. Intellectual Delay on WPPSI-III: Patients vs. Test Norms.
The proportion of patients categorized based on WPPSI-III Full Scale IQ falling 1–2 standard deviations below the mean (“Mild to Moderate Delay”) and 2 or more standard deviations below the mean (“Serious Delay”) was compared to expected rates based on the normal sample (p< 0.0001)
Prevalence of Academic Delays and Learning Disability
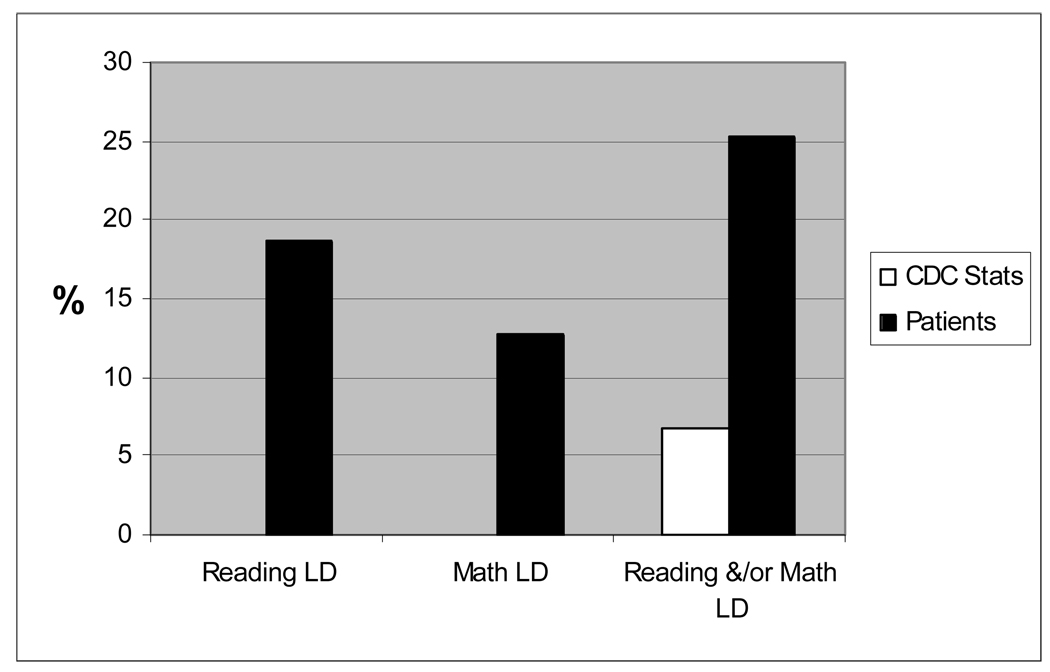
As seen in Table 3, both Reading and Math Computation subtests on the WRAT-4 were significantly below test norms (p<0.0001), although the mean School Readiness Composite of the BBCS-R was not. There were 25 patients (19%) with a pattern of Reading LD, 17 patients (13%) with Math LD, and 8 patients (6%) with both Reading and Math LD. Figure 3 shows that significantly more patients (N=34, 25.3%) had profiles suggesting Reading and/or Math LD in contrast to the expected rate of 6.7% in the general population based on Centers for Disease Control and Prevention (CDC) statistics for ages 5–11 (34) (p <0.0001).
Figure 3. Prevalence of Learning Disability (LD) profile.
Patients with scores on WRAT Reading and/or Math falling 1 or more standard deviations (15 or more points) below WPPSI-III Full Scale IQ are described as having a Learning Disability profile (LD). The proportion of patients with LD is compared to published CDC statistics (p< 0.0001 for both comparisons).
Deficits in Executive Function
Parent BRIEF
The BRIEF parent report was completed on 139 patients, but data from 6 patients were removed from the analysis due to elevated inconsistency. Pediatric LT patients demonstrated significantly lower working memory abilities compared to the standard normative population (p <0.005, effect size = 0.40). However, GEC, MI, and BRI were not significantly different from the normative population, see Table 4.
Table 4.
Parent BRIEF T Scores and Comparison to Normative Population
| N | Mean | Standard Deviation |
Effect Size |
Adjusted Significance Level |
|
|---|---|---|---|---|---|
| Behavioral Regulation Index | 133 | 51.50 | 11.37 | 0.150 | NS |
| Metacognition Index | 130 | 52.31 | 11.23 | 0.231 | NS |
| Global Executive Composite | 130 | 52.39 | 11.40 | 0.239 | NS |
| Inhibit score | 133 | 52.51 | 11.03 | 0.251 | NS |
| Shift score | 133 | 50.33 | 10.70 | 0.033 | NS |
| Emotional Control score | 133 | 51.04 | 11.39 | 0.104 | NS |
| Initiate score | 133 | 50.08 | 10.91 | 0.008 | NS |
| Working Memory score | 133 | 54.00 | 11.83 | 0.400 | <0.005 |
| Plan/Organize score | 130 | 52.17 | 11.55 | 0.217 | NS |
| Organization of Materials score | 133 | 51.78 | 9.60 | 0.178 | NS |
| Monitor score | 132 | 51.33 | 12.54 | 0.133 | NS |
Note: The normative population for the BRIEF has a mean T score of 50 and a standard deviation of 10.
Higher scores signify lower function. The Hochberg adjustment was used to control for multiple comparisons.
Effect sizes designated as small (.20), medium (.50), and large (.80).
NS= not significant.
Teacher BRIEF
The Teacher BRIEF was completed for 77 patients. Reasons for missing forms were as follows: not administered due to summer break (40), forms not returned (18), not attending school (2), patient home schooled (4), parent refused (1), and unknown (2). An additional 5 cases were excluded from analysis due to inconsistent scoring. All Teacher BRIEF T scores for the pediatric liver transplant sample were significantly different from the normative population (p < 0.005), see Table 5. The majority of the effect sizes were large in magnitude. Similar to the Parent BRIEF scores, the largest effect size is evidenced on the Working Memory subscale (effect size = 0.94).
Table 5.
Teacher BRIEF T Scores and Comparison to Normative Population
| N | Mean | Standard Deviation |
Effect size | Adjusted significance level |
|
|---|---|---|---|---|---|
| Behavioral Regulation Index | 72 | 56.71 | 15.24 | 0.671 | <0.005 |
| Metacognition Index | 70 | 58.21 | 14.75 | 0.821 | <0.001 |
| Global Executive Composite | 70 | 58.14 | 15.01 | 0.814 | <0.001 |
| Inhibit score | 72 | 56.46 | 15.03 | 0.646 | <0.005 |
| Shift score | 72 | 54.07 | 11.55 | 0.407 | <0.005 |
| Emotional Control score | 72 | 56.72 | 17.23 | 0.672 | <0.005 |
| Initiate score | 72 | 58.40 | 13.97 | 0.840 | <0.0001 |
| Working Memory score | 72 | 59.38 | 15.16 | 0.938 | <0.0001 |
| Plan/Organize score | 70 | 55.59 | 13.96 | 0.559 | <0.005 |
| Organization of Materials score | 72 | 56.56 | 12.61 | 0.656 | <0.001 |
| Monitor score | 72 | 57.54 | 15.45 | 0.754 | <0.001 |
Note: The normative population for the BRIEF has a mean T score of 50 and a standard deviation of 10. The Hochberg adjustment was used to control for multiple comparisons.
Effect sizes designated as small (.20), medium (.50), and large (.80).
NS= not significant.
FSIQ and Indicators of School Function
We also examined how EF, as measured by the GEC score of the BRIEF, the PedsQL™ 4.0 School Function sub-scale, and attendance varied by FSIQ sub-group, see Table 6. Among children with a FSIQ ≥ 86, 9.3% by parent report and 18.8% by teacher report had abnormal GEC scores consistent with clinically relevant EF deficits. Executive function deficits were more prevalent in the lower IQ groups. Likewise, PedsQL™ 4.0 School Function scores and special educational resource utilization varied by FSIQ group. There was no relationship between FSIQ and school attendance.
Table 6.
Distribution of Full-Scale IQ Scores by Selected Indicators of Lower School Function
| Sample Size |
Standard FSIQ Score ≥ 86 |
Standard FSIQ Score 71–85 |
Standard FSIQ Score ≤ 70 |
P Value |
|
|---|---|---|---|---|---|
| Executive Function | |||||
| BRIEF Parent Report | 122 | 86 | 31 | 5 | 0.008 |
| Parent GEC Elevated (T score ≥ 65) | 17 | 9.3% | 19.4% | 60.0% | |
| Parent GEC WNL (T score <65) | 105 | 90.7% | 80.6% | 40.0% | |
| BRIEF Teacher Report | 64 | 48 | 14 | 2 | 0.110 |
| Teacher GEC Elevated (T score ≥ 65) | 16 | 18.8% | 42.9% | 50.0% | |
| Teacher GEC WNL (T score <65) | 48 | 81.2% | 57.1% | 50.0% | |
| PedsQL™ 4.0 Generic Core Scale | 124 | 88 | 31 | 5 | 0.005 |
| School Function Subscale ≤ 25th percentile | 29 | 15.9% | 38.7% | 60.0% | |
| School Function Subscale > 25th percentile | 95 | 84.1% | 61.3% | 40.0% | |
| Education form available | 134 | 94 | 35 | 5 | 0.400 |
| Yes | 98 | 73.4% | 68.6% | 100.0% | |
| No | 36 | 26.6% | 31.4% | 0.0% | |
| Attended school in past 12 months | 98 | 67 | 24 | 5 | 0.494 |
| Yes | 96 | 97.1% | 100.0% | 100.0% | |
| No | 2 | 2.9% | 0.0% | 0.0% | |
| Special education support during the past 12 months | 96 | 67 | 24 | 5 | <0.001 |
| Support received | 29 | 22.4% | 37.5% | 100.0% | |
| No support received | 67 | 77.6% | 62.5% | 0.0% | |
| School Attendance during past 12 months | 96 | 67 | 24 | 5 | 0.591 |
| Missed ≤ 10 School Days | 73 | 74.6% | 75.0% | 100.0% | |
| Missed > 10 School Days | 23 | 25.4% | 25.0% | 0.0% |
Post hoc Analysis of Outcomes by Center Participation Rate
Considering the participation rate was lower than 50% overall, a post hoc analysis comparing primary outcomes between patients at high participation rate centers (≥50%) and all other centers was conducted. As seen in Table 7, there were no significant differences between FSIQ or WRAT-4 scores based on center participation, thus making ascertainment bias by center recruitment strategies or logistics unlikely.
Table 7.
IQ and Achievement Scores by Site Participation Rate
| Site Participation Rate |
WPPSI-III FSIQ | WRAT- Reading | WRAT- Math | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | Std Err | Mean | Std Err | Mean | Std Err | |
| ≥50%* | 60 | 92.5 | 14.1 | 94.4 | 18.2 | 94.3 | 18.6 |
| <50% | 84 | 96.2 | 12.9 | 91.6 | 16.5 | 92.2 | 12.8 |
| T-test p-values | 144 | p=0.1144 | p=0.4080 | p=0.1927 | |||
DISCUSSION
The findings indicate that not only did participants show significantly lower intellectual ability overall compared to the normal population, but twice as many patients as expected evidenced IQ delays of one or more standard deviations. Twenty-six percent of our sample had mild to moderate cognitive delay (WPPSI-III FSIQ between 71–85) and 4% had serious delay (FSIQ ≤ 70). The observed intellectual deficits would be expected to hinder academic performance as well as independent functioning long-term. Reading and math scores were significantly below test norms, and comparison of IQ and academic achievement revealed that even at this early age, 25% of patients had profiles suggesting reading and/or math LD. In addition, nearly 19% of patients in the higher IQ group and 43–50% of those with IQ <86 had clinically relevant EF deficits that were apparent in the classroom. The high prevalence of academic delays and EF deficits in the sample is all the more striking given participants were just starting school.
The rate of LD in this sample is much higher than CDC reports in the general population (7%), but consistent with previous, smaller reports of cognitive outcomes in pediatric LT recipients (12). Although the patients demonstrated adequate mastery of simple school readiness concepts such as recognition of colors, numbers, and shapes as measured by the BBCS-R and single word vocabulary on the WPPSI-III GLC, the downward shift of IQ, the discordance between IQ and academic achievement, and the prevalence of EF deficits suggest this group is already significantly delayed in early academic skill building and cognitive development. An established diagnosis of learning disability or mental retardation was only reported for 5 and 3 percent of the group, respectively; however, 31% had received special education support during the previous 12 months. This suggests learning problems had already been recognized by the school system in many cases.
This study provides the first evidence for EF deficits in pediatric LT patients with deficits reported by multiple informants (parents and teachers). Executive skills are vital to the learning process. Teacher report on the BRIEF highlighted the most dramatic concerns. This suggests teachers’ input, which is often overlooked in neuropsychological research due to logistical challenges in obtaining it, is critical in defining the scope of the problem. The reason for elevated concerns among teachers as compared to parents is unclear. This finding may reflect the young age of participants, with about 2/3 in kindergarten. At this early elementary level, EF demands are likely to be greater in the classroom than at home (e.g., limited homework requirements). Problems with EF can have a significant impact on academic functioning in later childhood and on job performance and independent living skills in adulthood. In children with various neurological insults (TBI, cancer treatment), EF deficits often emerge as a function of age, in a pattern labeled “growth into deficits” (35). In this scenario, a neurological insult adversely impacts EF, but the deficit does not become apparent until the demands are sufficiently high, increasing as the child ages.
Several single center studies have suggested developmental delay is prevalent in children who have survived LT, especially among patients with advanced liver disease early in infancy (13, 14, 17, 36). This multi-site design allowed us to determine the prevalence of cognitive delay in a large patient group that we believe is representative of the general population of children who receive LT at this age across North America. The distribution of parental education in our group was similar to or higher than that of the WPPSI-III standardization sample (26) with 70% of our sample completing at least some college or beyond compared to 60% in the WPPSI-III standardization sample. This suggests the IQ deficits found cannot be attributed to lower socioeconomic status. Cognitive testing was performed at least two years after LT to minimize the impact of incomplete rehabilitation following the procedure and patients with uncontrolled seizures or current evidence of hepatic encephalopathy were excluded. Language factors were controlled by limiting participants to those who were fluent in English and had passed a hearing screen. Another important methodological advantage of the current study was the use of a small age range allowing use of a consistent test battery for all patients, which resulted in less variability in time since transplant, era of transplant and age at transplant. Our findings confirm developmental delay is a common problem for patients that receive LT in early childhood even in contemporary experience.
This cohort was selected to include patients who had all received LT prior to five years of age, with 57 (40%) being transplanted prior to 12 months of age. Hepatic encephalopathy, chronic malnutrition and other aspects of advanced liver failure are hypothesized to have a greater impact on the developing brain of an infant as compared to an older child. This report provides strong evidence that children who have received LT in infancy and early childhood are at high risk for cognitive delays and learning problems, well after the initial post-transplant period. Deficits in these areas, first recognized in pediatric LT patients more than twenty years ago, persist as important limitations to optimal long-term outcomes. These findings suggest early screening for cognitive delay at or before the age of school entry can help identify the sub-population of patients that will require educational interventions and special support services. Such services are most effective when delivered early in a child’s school experience, when there is more potential for neural plasticity (37, 38). Considering the large percentage of LT patients with adverse cognitive outcomes, it would be reasonable to support policies that fund intervention services at an even younger age, similar to the approach that has been taken for pre-term infants (39).
We accurately predicted less than 60% of eligible patients would participate and thus chose to recruit patients from a large number of centers in order to yield the projected sample size. Despite the relatively low recruitment rate, comparisons suggest our sample was not biased by important demographic or medical factors, or by center-specific recruitment practices and logistics. Although parents of LT recipients expressed sincere interest in issues surrounding school performance, many chose not to participate, citing problems related to travel, time off from work and school absence as a deterrent. Nevertheless, comparison between participants and non-participants did not suggest bias on the basis of demographic factors, and our sample appears to be similar to the full SPLIT cohort and the IQ standardization sample in important respects. Comparison of special education resource utilization of this sample with the full SPLIT cohort reveals 31% of these participants versus 34% of the full SPLIT cohort (ages 6–11 years) were receiving special educational support (IEP). (18) Likewise 36% of the participants had a history of evaluation for an Individualized Educational Plan (IEP) versus 35% of patients 6–11 years of age in the overall SPLIT cohort. Thus, it would seem that this patient group does not have an over-representation of LT recipients with academic problems.
This report includes the preliminary findings of a longitudinal study that will include testing at a second time point (two years later) to help establish whether deficits observed at this early age are static or progressive. Also, future multi-variant modeling of risk factors may help determine if pre or post transplant variables have the largest impact. Since one fourth of the sample missed more than 10 school days in the prior 12 months, it will be important to consider the potential contribution of missed school days to cognitive and academic outcomes. Confirming the prevalence of neurocognitive deficits in older recipients and determining predictors of these functional outcomes will be key in driving modifications in medical care and public policy to optimize post-LT quality of life.
In summary, we report the results of the first multi-center study to examine neurocognitive functioning in children following LT. Early school-age children who had received LT prior to 5 years of age displayed twice the rate of intellectual delay and three times the rate of learning disability compared to the general population. Classroom performance suggested EF deficits are prevalent even at this early age. Analyses are in progress in this longitudinal study to assess the developmental course of these delays over time and to identify risk factors that predict adverse cognitive and academic outcomes.
Acknowledgments
This study was supported by R01 HD045694 of the National Institute of Child Health and Human Development and U01 DK061693 of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
Studies of Pediatric Liver Transplantation Functional Outcomes Group:
Medical Center (Principle Investigator [sample size])
University of California, Los Angeles (Sue McDiarmid, MD [15])
Cincinnati Children’s Hospital Medical Center (John Bucuvalas, MD [11])
The Children’s Hospital, Denver (Ronald Sokol, MD [10])
Children’s Medical Center, Dallas (Naveen Mittal, MD [4])
Hospital for Sick Children, Toronto (Vicky Ng, MD [17])
University of Nebraska (Alan Langnas, DO [6])
Mount Sinai Medical Center (Nanda, Kerkar, MD [2])
University of Alberta, Edmonton (Susan Gilmour, MD [2])
Children's Memorial Hospital (Estella Alonso, MD [27])
University of Miami/Jackson Memorial (Tomoaki Kato, MD [3])
University of California, San Francisco (Philip Rosenthal, MD [1])
Children’s Mercy Hospital, Kansas City (James F. Daniel, MD [6])
St. Louis Children’s Hospital (Ross Shepherd, MD [11])
Texas Children’s Hospital (Saul Karpen, MD, PhD [2])
University of Minnesota (Abhi Humar, MD [1])
Children's Hospital of Pittsburgh (George Mazariegos, MD [12])
University of California, San Diego (Joel E. Lavine, MD [1])
Alfred I. DuPont Hospital for Children (Stephen Dunn, MD [4])
Boston Children’s Hospital (Maureen Jonas, MD [4])
University of Michigan (Emily Fredricks, PhD [5])
(Principle Investigator [Number of patients tested])
REFERENCES
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children National Health Interview Survey, 2007. Vital & Health Statistics - Series 10 ata From the National Health Survey. 2009;(239):1–80. [PubMed] [Google Scholar]
- 2.Kolb B, Fantie B. Development fo the Child's Brain and Behavior. In: Reynolds C, Fletcher-Janzen E, editors. Handbook of Clinical Child Neuropsychology. New York: Springer Science and Businesss Media, LLC; 2009. pp. 19–46. [Google Scholar]
- 3.Nicholson H, Fennell L, Butler R. Childhood Brain Tumors. In: Meyers C, Perry J, editors. Cognition and Cancer. Cambridge: Cambridge University Press; 2009. pp. 198–210. [Google Scholar]
- 4.Stanford L, Dorflinger J. Pediatric Brain Injury:Mechanisms and Amelioration. In: Reynolds C, Fletcher-Janzen E, editors. Handbook of Clinical Child Neuropsychology. New York: Springer Science and Business Media, LLC; 2009. pp. 169–186. [Google Scholar]
- 5.Taylor H. Low Birth Weight. In: Morgan J, Ricker J, editors. Textbook of Clinical Neuropsychology. New York: Taylor and Francis; 2008. pp. 308–332. [Google Scholar]
- 6.Group SR. Studies of Pediatric Liver Transplantation (SPLIT) Year 2000 Outcomes. Transplantation. 2001;72:463–476. doi: 10.1097/00007890-200108150-00018. [DOI] [PubMed] [Google Scholar]
- 7.Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. Journal of the American College of Surgeons. 2009;208(5):682–689. doi: 10.1016/j.jamcollsurg.2009.01.023. discusion 9-91. [DOI] [PubMed] [Google Scholar]
- 8.Alonso EM, Shepherd R, Martz KL, Yin W, Anand R, Group SR. Linear growth patterns in prepubertal children following liver transplantation. American Journal of Transplantation. 2009;9(6):1389–1397. doi: 10.1111/j.1600-6143.2009.02634.x. [DOI] [PubMed] [Google Scholar]
- 9.Weissberg-Benchell J, Zielinski T, Rodgers S, Neff Greenley R, Askenazi D, Goldstein S, et al. Health-Related Quality of Life: Feasibility, Reliability and Validity of the PedsQL™ Transplant Module. American Journal of Transplantation. American Journal of Transplantation. 2010 doi: 10.1111/j.1600-6143.2010.03149.x. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Gilmour S, Adkins R, Liddell GA, Jhangri G, Robertson CM. Assessment of psychoeducational outcomes after pediatric liver transplant. American Journal of Transplantation. 2009;9(2):294–300. doi: 10.1111/j.1600-6143.2008.02480.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaller T, Schulz K, Sander K, Boeck A, Rogiers X, Burdelski M. Cognitive abilities in children after liver transplantation. Transplantation. 2005;79:1252–1256. doi: 10.1097/01.tp.0000161251.20520.42. [DOI] [PubMed] [Google Scholar]
- 12.Kennard B, Stewart S, Phelan-McAuliffe D, Waller D, Bannister M, Fioravani V, et al. Academic outcome in long-term survivors of pediatric liver transplantation. J Dev Behav Pediatr. 1999;20:17–23. doi: 10.1097/00004703-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Krull K, Fuchs C, Yurk H, Boone P, Alonso E. Neurocognitive outcome in pediatric liver transplant recipients. Pediatr Transplant. 2003 Apr;7(2):111–118. doi: 10.1034/j.1399-3046.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart SM, Uauy R, Waller DA, Kennard BD, Benser M, Andrews WS. Mental and motor development, social competence, and growth one year after successful pediatric liver transplantation. J Pediatr. 1989;114(4 Pt 1):574–581. doi: 10.1016/s0022-3476(89)80696-1. [DOI] [PubMed] [Google Scholar]
- 15.Schultz K, Wein C, Boeck A, Rogers X, Burdelski M. Transplantation, 75(8), pgs 1236–1240. Cognitive performance of children who have undergone liver transplantation. Transplantation. 2003;75:1236–1240. doi: 10.1097/01.TP.0000062843.10397.32. [DOI] [PubMed] [Google Scholar]
- 16.Stewart S, Campbell R, McCallon D, Waller D, Andrews W. Cognitive patterns in school-age children with end-stage liver disease. J Dev Behav Pediatr. 1992;13:331–338. [PubMed] [Google Scholar]
- 17.Wayman K, Cox K, Esquivel C. Neurodevelopmental outcome of young children with extrahepatic biliary atresia 1 year after liver transplantation. J Pediatr. 1997;131:894–898. doi: 10.1016/s0022-3476(97)70039-8. [DOI] [PubMed] [Google Scholar]
- 18.Gilmour SM, Sorensen LG, Anand R, Yin W, Alonso E. School outcomes in children registered in the Studies of Pediatric Liver Transplantation (SPLIT) consortium. Liver Transpl. 2010;16:1041–1048. doi: 10.1002/lt.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buizer AI, de Sonneville LM, Veerman AJ. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: a critical review of the literature. Pediatric Blood & Cancer. 2009;52(4):447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- 20.Gerson AC, Butler R, Moxey-Mims M, Wentz A, Shinnar S, Lande MB, et al. Neurocognitive outcomes in children with chronic kidney disease: Current findings and contemporary endeavors. Mental Retardation & Developmental Disabilities Research Reviews. 2006;12(3):208–215. doi: 10.1002/mrdd.20116. [DOI] [PubMed] [Google Scholar]
- 21.McNally K, Rohan J, Pendley J, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with Type 1 Diabetes. Diabetes Care. 2010;33:1159–1162. doi: 10.2337/dc09-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taras H, Potts-Datema W. Chronic health conditions and student performance at school. Journal of School Health. 2005;75(7):255–266. doi: 10.1111/j.1746-1561.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 23.Fine RN, Alonso EM, Fischel JE, Bucuvalas JC, Enos RA, Gore-Langton RE. Pediatric transplantation of the kidney, liver and heart: summary report. Pediatric Transplantation. 2004;8(1):75–86. doi: 10.1111/j.1399-3046.2004.2s050.x. [DOI] [PubMed] [Google Scholar]
- 24.Bucuvalas JC, O'Connor A, Buschle K, Krug S, Ryckman FC, Atherton H, et al. Risk of hearing impairment in pediatric liver transplant recipients: a single center study. Pediatr Transplant. 2003 Aug;7(4):265–269. doi: 10.1034/j.1399-3046.2003.02054.x. [DOI] [PubMed] [Google Scholar]
- 25.Alonso EM, Limbers C, Neighbors K, Martz K, Bucuvalas JC, Webb T, et al. Cross-sectional analysis of health-related quality of life in pediatric liver transplant recipients. J of Pediatr. 2010;156:270–276. doi: 10.1016/j.jpeds.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler preschool and primary scale of intelligence-third edition. San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- 27.Wilkinson G. Wide range acheivement test-third edition. Wilmington: Wide Range Inc.; 1993. [Google Scholar]
- 28.Wilkinson G, Robertson G, editors. Wide range achievment test-fourth edition. Lutz, FL: Psychological Assessment Resources, Inc.; 2006. [Google Scholar]
- 29.Bracken B. Bracken basic concept scale-revised. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 30.Gioia G, Isquith P, Guy S, Kenworthy L. Behavior rating inventory of executive function. Odessa, FL: Psychological Assessment Services, Inc.; 2000. [Google Scholar]
- 31.Association D-IAP. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, D.C.: American Psychiatric Association; 1994. (DSM-IVTM) [Google Scholar]
- 32.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in Medicine. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 34.Bloom B, Cohen R. Summary Health. 2009 [Google Scholar]
- 35.Mahone E, Slomine B. Managing dysexecutive disorders. In: Hunter S, Donders J, editors. Pediatric Neuropsychological Intervention. New York: Cambridge University Press; 2007. [Google Scholar]
- 36.Adeback A, Nemeth A, Fischler B. Cognitive and emotional outcome after pediatric liver transplantation. Pediatr Transplantation. 2003;7:385–389. doi: 10.1034/j.1399-3046.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 37.Spevack T. A developmental approach to pediatric neuropsychological intervention. In: Hunter S, CDonders J, editors. Pediatric Neuropsychological Intervention. Cambridge: Cambridge University Press; 2007. pp. 6–29. [Google Scholar]
- 38.Zigmond N. Searching for the most effective service delivery model for students with learning disabilities. In: Swanson H, Harris K, Graham S, editors. Handbook of Learning Disabilities. New York: The Guilford Press; 2003. pp. 110–122. [Google Scholar]
- 39.McCormick MC, Brooks-Gunn J, Buka SL, Goldman J, Yu J, Salganik M, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117(3):771–780. doi: 10.1542/peds.2005-1316. [DOI] [PubMed] [Google Scholar]