Abstract
Purpose
To compare various meibum collection methods and extraction techniques.
Methods
Sixty subjects, all successful contact lens wearers, were seen on two visits. Meibum was collected from the lower lid of the right eye with a glass microcapillary tube, and with a Dacron swab, cytology microbrush or spatula from the left eye. Extraction with 2:1 chloroform:methanol was done either immediately or after data collection was complete. Individual samples were divided into four equal aliquots for analysis of total lipids, cholesterol and inorganic phosphates via assay based techniques. Effects of collection method, extraction, and dry eye status were examined using repeated measures ANOVA and logistic regression.
Results
Total lipids showed significance of collection device (p<0.0001), but not for extraction technique (p=0.13) or dry eye status (p=0.97). Dacron swab collection was associated with more total lipid on average than each other collection device (p<0.0001). The cholesterol assay showed significance of collection device (p<0.0001) and extraction technique (p=0.0002), but not dry eye status (p=0.55). Spatula collection was associated with more cholesterol on average than each other collection device (p<0.0001). For inorganic phosphates, immediate extraction (p<0.0001), cytology microbrush collection (p<0.0001), and non-dry eye status (p=0.03) were associated with the greater likelihood of detection.
Conclusions
Dacron swab collection was associated with the highest average amount of total lipid detected, whereas spatula collection and immediate extraction was associated with the highest average amount of cholesterol detected. Cytology microbrush collection with immediate extraction on non-dry eye subjects was associated with the highest probability of detection of inorganic phosphates.
Keywords: lipids, dry eye, meibomian gland, collection devices, evaluation methods
Controversy exists related to the collection of meibomian gland secretions. The literature describes a variety of methods for meibomian gland secretion collection.1–10 A significant concern when collecting samples from human subjects is to efficiently obtain adequate sample quantity for subsequent analysis. It is possible to obtain large quantities through rigorous methods of collection or multiple collection sessions, allowing for methods of analysis such as nuclear magnetic resonance (NMR) spectroscopy11 which require a significant amount of sample. Our goal in this study was to optimize single sample collection for analysis methods capable of using small volume samples.
Linton et al reported collection of a total of 200mg from 539 subjects with a solid glass rod and meibomian cyst curette.1 Sullivan et al describes use of a chalazion curette for collection.9–10 Nichols et al describes use of a 0.5µL glass microcapillary tube, successfully used as a collection device to obtain adequate individual sample quantity for analysis by mass spectrometry.2 Use of a spatula, frequently described as platinum, has been reported in several studies.3–8 While the spatula has been widely used, it is often used after a lid conformer and cotton swab in combination to express the meibomian gland secretions. This is reportedly uncomfortable of even painful for some, with limited improvement in comfort when accompanied by topical anesthetic.12
Lipid collection is a challenge and many collection devices should be considered. Devices used in this study were selected as they are small, compatible with meibum collection, and commonly used in biological sample collection, delivery of substances, or surgical procedures in living human applications. The microcapillary tube has been used for tear collection, meibum collection and for transfer of micro amounts of human fluids in laboratory settings.2, 13–14 The Dacron swab has been utilized for obtaining cell and mucous samples for bacterial culture as well as other laboratory uses such as polymerase chain reaction (PCR) experiments.15 Common uses of the cytology microbrush include collection of human cell samples and in dental applications.16–18 Stainless steel spatulas have been used for ocular surgical procedures19–21 and for collection of human meibum.3–8
Contamination of meibum samples including tears, lid margin epithelial cells, conjunctival cells, and the thin layer of epithelium overlying a portion of currently inactive meibomian glands22 is of considerable concern. Butovich et al suggested meibomian gland cells are an additional potential source of “pure” meibum contamination.12 Freshly expressed meibum may differ from resident meibum, and even the most careful and precise collection of meibum may result in the inclusion of micro quantities of tears and/or cells. The simple act of digital expression may alter the normal state of the meibomian secretions, with progressively less “normal” conditions when expressions are repeated and/or with the addition of anesthetic.
Lipid extraction solvents employed in human specimens are varied and include chloroform, methanol, hexane, toluene, and ether.23–26 Selection of the most appropriate solvent is dependent upon several factors including type of sample and intended downstream analysis. Chloroform, methanol, or a mixture of chloroform and methanol (concentration ratio of 2 parts chloroform to 1 part methanol) are commonly reported for extraction of human meibum in both dry eye and non-dry eye subjects.2–8 Paradoxically, subsequent analyses as reported by different investigators utilizing the same extraction solvent may lead to conflicting results.4, 6–10
The purpose of this study was to compare four varied methods of collection of human meibomian gland secretions using assay-based techniques. Ultimately this will facilitate analysis of large numbers of subjects with multiple meibum collections in a timely and cost-effective manner. In addition, effects of immediate extraction were compared with frozen samples followed by later extraction, as well as comparisons of subjects without dry eye symptoms and those with dry eye symptoms.
METHODS
Subject Enrollment
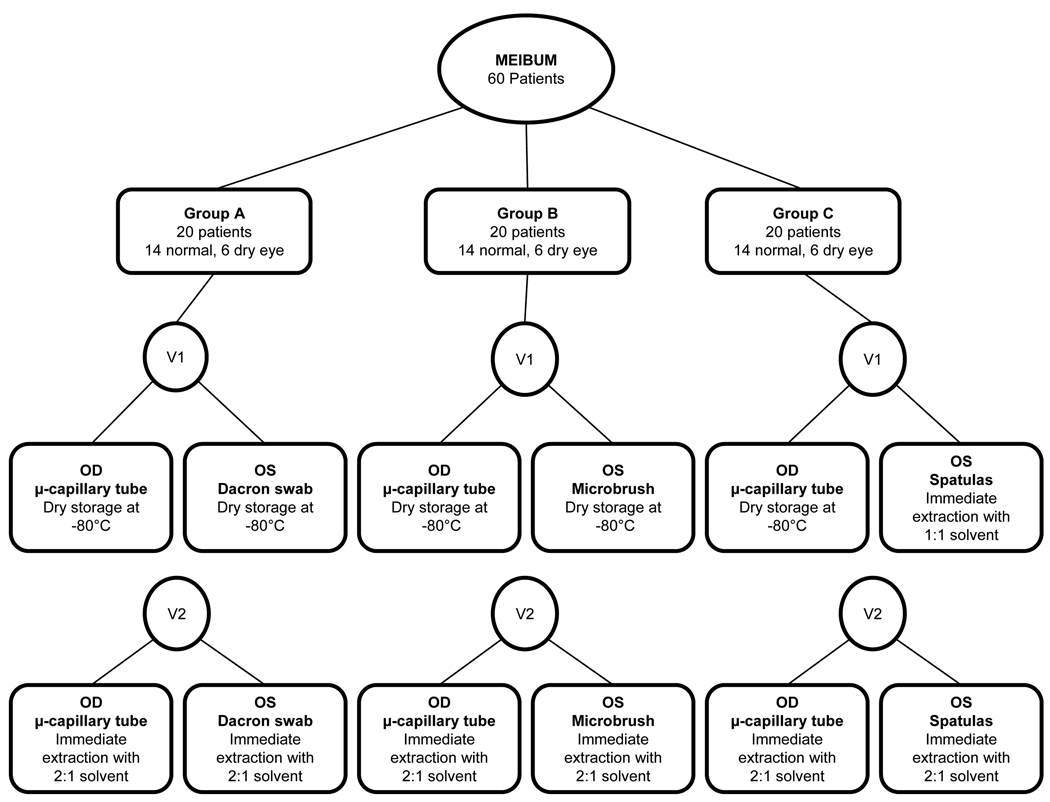
This research was conducted in accordance with the Declaration of Helsinki with approval of the Biomedical Institutional Review Board at The Ohio State University. Informed consent was obtained from subjects after explanation of the nature and possible consequences of the study. A total of 60 contact lens wearers were enrolled in the study (42 non-dry eye subjects and 18 dry eye subjects) and were seen on two visits separated by 14 days. Classification of dry eye status was determined by subject completion of the Contact Lens Dry Eye Questionnaire (CLDEQ)27 at the first visit. Subjects were grouped into one of three groups, Group A, Group B and Group C, based on collection technique assignments as described below. Continuous rolling enrollment into one of the three groups was done as subjects presented and qualified as dry eye or non dry eye. Once a category for dry eye status was filled in a group, the subject was placed in the next group until all groups were filled. The flowchart in Figure 1 illustrates the study design for this project.
Figure 1.
Study design flowchart. Separation of the sixty subjects into three groups of twenty based on type of meibum collection is illustrated in this diagram. Sample collection was done with glass microcapillary tube from the right eye, and either Dacron swab (Group A), cytology microbrush (Group B), or spatulas (Group C) from the left eye. Differences in extraction technique are also shown, with visit one using frozen as collected samples with later extraction (with the exception of Group C, left eye), and visit two samples being extracted immediately.
Collection Techniques
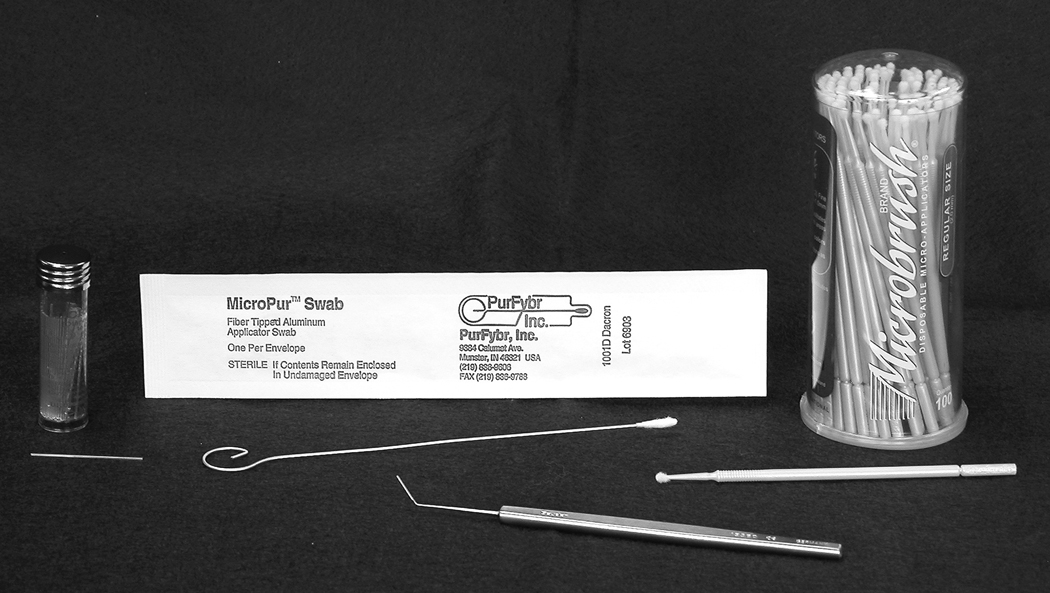
Four meibum collection techniques were evaluated and compared (Figure 2). The microcapillary tube (Drummond Microcap 0.5 µL, Drummond Scientific Company, Broomall, PA, USA) was used to collect meibum from the right eye of each subject at both visits. All microcapillary tubes were weighed (UMX2 Ultra-microbalance, Mettler Toledo, Columbus, OH, USA) pre and post meibum collection. The three meibum techniques used for the left eye at both visits were as follows: For Group A, Dacron swab (MicroPur™ Swab, PurFybr, Inc., Munster, IN, USA); for Group B, cytology microbrush (Microbrush®, Microbrush® International, Grafton, WI, USA); and for Group C, three stainless steel spatulas (Bangerter Iris Spatula, Geuder, Heidelberg, Germany).
Figure 2.
The collection devices used in the study.
All samples were obtained by gentle digital expression of the meibomian glands in the lower lids of each eye. Our goal was to find a more patient friendly alternative to the hard press method that would be tolerable for subjects with repeated collection of meibum. During collection, the lid margin was rolled away from the globe to minimize contamination of the sample with tears and conjunctival cells. Effort was made to collect newly expressed visible meibum. It was desired to collect approximately 1mm of measured meibum in the microcapillary tube, with a range achieved of approximately 0.25mm to 1.5mm. For the Dacron swab and cytology microbrush, collections over the lid margin were made by rotating the device to expose a new area on the device after each stroke across the lid margin. The long sides of the angled portion and the rounded tip of the spatula were used to collect meibum. This process was repeated with each of the three spatulas.
Contact Lenses and Care Solution
New contact lenses (O2 Optix (lotrafilcon B),CIBA VISION Corporation, Duluth, GA, USA) were fit and dispensed with a supply of contact lens care solution (OPTI-FREE RepleniSH, Alcon, Inc., Hünenberg, Switzerland) at visit one. Instruction on proper contact lens care and cleaning was provided. There is currently no evidence to show that contact lens type and cleaning method will or will not alter meibum quality or properties. Subjects were instructed to wear only the contact lenses and to use only the care solution they were given at the first visit during the study period. Contact lenses were collected from subjects using metal tweezers on visit two, and stored at −80°C for future analysis.
Lipid Extraction Protocols
With the exception of Group C, all samples collected at visit one were stored frozen at −80°C for later extraction. Frozen samples were thawed and extracted with 2:1 chloroform:methanol solvent after subject visits were complete and analyzed as a group. All samples collected at visit two were immediately extracted with 2:1 chloroform: methanol solvent. Samples collected from the left eye with stainless steel spatulas (Group C) were immediately extracted with 1:1 chloroform:methanol at visit one, and with 2:1 chloroform:methanol at visit two. All extractions were performed in amber glass vials capped with a polytetrafluoroethylene (PTFE) liner.
The same protocol was used for immediate and later extraction from microcapillary tube, Dacron swab, and cytology microbrush collection devices. First, 600µL 2:1 chloroform:methanol solvent was pipetted into an amber glass vial containing the collection device and sample. After 15 minutes of extraction time, an additional 400µL of solvent was pipetted into the vial. The extract was split into four aliquots of 150µL each and stored at −80°C for later analysis.
All samples collected with stainless steel spatulas were immediately extracted. Three glass vials were used, one for placing each of the three metal spatulas in immediately after sample collection. Chloroform:methanol solvent in the amount of 2000µL was pipetted into glass vial #1 containing spatula #1 for 5 minutes, and this process was repeated for the remaining spatulas. The combined extract was split into four aliquots by pipetting 300µL into amber glass vials and stored at −80°C for later analysis.
Laboratory Assays
Assays performed were for total lipid, cholesterol, and inorganic phosphate and are described in further detail below28–32 Aliquot one was assigned to total lipid assay, aliquot two to cholesterol assay and aliquot three to the inorganic phosphate assay. All aliquots were thawed to room temperature and air-dried overnight just prior to the assays. The total lipid assay title is somewhat misleading, as it does not assess the “total lipid” content, rather the unsaturated lipid content. Cholesterol is not an unsaturated lipid, thus the need for a separate assay. Analytical blanks were run with each assay, and values for blanks subtracted from sample values. Standard curves were produced using common laboratory methods for each of the three assays.
Total Lipid Assay
For the total lipid assay, lyophilized oleamide (Sigma-Aldrich, St Louis, MO) was used to generate a standard curve. To standards and samples, 20µL 34N sulfuric acid was added, with subsequent incubation at 100°C for 10 minutes. After cooling to room temperature in a water bath, 80µL of assay reagent (1.2mg/ml vanillin in 68% phosphoric acid) was added to each vial. Following incubation at room temperature for 45 minutes, absorbance was measured at 530nm (Tecan i-control Infinite 200 plate reader, Tecan Group Ltd, Switzerland).32
Total Cholesterol and Cholesterol Esters Assay
Cholesterol oleate (Sigma-Aldrich, St Louis, MO) standards were prepared from known concentrations in 0.1M phosphate buffer pH 7.0 and a reaction mixture was prepared using phosphate buffer (Na2HPO4, NaH2PO4, Sodium cholate, 4-Aminoantipyrine, Phenol, and PEG 6000) peroxidase, cholesterol oxidase and cholesterol esterase. To each standard and sample 50µL of 0.1M phosphate buffer was added. Next, 207.5µL of the reaction mixture was added to each standard and sample vial. After incubating vials in a 37°C water bath for 60 minutes, absorbance was measured at 500nm.30–31, 33
Inorganic Phosphate Assay
Inorganic phosphates may be precursors to phospholipids in the tear film. Phosphatidylcholine (Sigma-Aldrich, St Louis, MO) standards were prepared in amber vials for use in the inorganic phosphate assay. To each standard and sample, 10µL of 10N sulfuric acid and 30µL of 70% perchloric acid were added. All vials were incubated at 140°C for 2.5 hours. After cooling the hydrolyzed standards and samples to room temperature, they were resuspended in 50µL of HPLC water. To each vial, 75µL of assay reagent one (0.2% malachite green in HPLC water, 4.2% ammonium molybdate in 4N HCl) and 10µL of assay reagent two (1.5% Tween20, HPLC water) were added. After 20 minutes of room temperature incubation, the absorbance was measured at 645nm.28–29
Statistical Analyses
Repeated measures analysis of variance (ANOVA) was used to analyze the dependent variables total lipid and cholesterol. Means, standard deviations, ranges, and medians of the observed data are reported, as well as the p values of the effect of the three independent variables (dry eye status, collection device, and extraction technique). In addition, 95% confidence intervals (95% CI) are provided for parameter estimates. Each dependent variable was fitted in a separate multivariate model with all independent variables. Data from the assays of total lipid and cholesterol were skewed. The skew was moderated by using the square root of values to make the ANOVA assumption of normality more tenable. The dependent variable inorganic phosphate data were transformed to presence or absence and modeled using repeated measures logistic regression. The transformation was required due to a large number of samples (62.1%) with zero values for this assay. Statistics describing proportions and probabilities are presented as odds ratio estimates with corresponding p-values.
RESULTS
The subject sample (n=60) was predominantly female (78%) and without dry eye (70%), with a mean age of 25.7 ± 5.7 years. A total of 240 meibum samples were collected, four from each subject, and each sample was separated into four aliquots. Each assay was done on a different aliquot so direct comparisons between assays are not possible. Table 1 shows summary statistics of observed data for each outcome measure as a function of collection device. Of the 240 samples, assay detected no total lipid in seven; six were collected by microcapillary tube, one was collected by cytology microbrush. Assay detected no cholesterol in sixteen samples; all were collected by microcapillary tube. Sample loss during the extraction, aliquot and assay process may have reduced the quantity of lipid present to undetectable quantities by these methods.
Table 1.
Outcome measures as a function of collection device. Means (µg) and standard deviations are shown for each assay: total lipid, cholesterol, and inorganic phosphate. Ranges and medians are displayed in the adjacent column for each assay. Collection devices are identified across the horizontal dimension.
|
Collection Device |
||||||||
|---|---|---|---|---|---|---|---|---|
| MICROCAPILLAR N = 120 |
DACRON SWAB N = 40 |
CYTOLOGY MICROBRUSH N = 40 |
SPATULAS N = 40 |
|||||
| Mean (µg) | Range & Median |
Mean (µg) | Range & Median |
Mean (µg) | Range & Median |
Mean (µg) | Range & Median |
|
| Total Lipid | 0.63 ±0.81 | 0.00-6.44 0.41 |
5.14 ±2.07 | 1.37-10.08 4.91 |
1.80 ±1.85 | 0.00-10.99 1.37 |
2.12 ±1.90 | 0.10-8.95 1.67 |
| Cholesterol | 0.33 ±0.40 | 0.00-3.83 0.28 |
0.70 ±0.31 | 0.11-1.49 0.71 |
0.68 ±0.43 | 0.06-2.60 0.59 |
1.42 ±0.66 | 0.60-3.44 1.37 |
| Inorganic Phosphates | 0.05 ±0.11 | 0.00-0.57 0.00 |
0.06 ±0.18 | 0.00-0.97 0.00 |
0.07 ±0.09 | 0.00-0.39 0.01 |
0.03 ±0.04 | 0.00-0.14 0.00 |
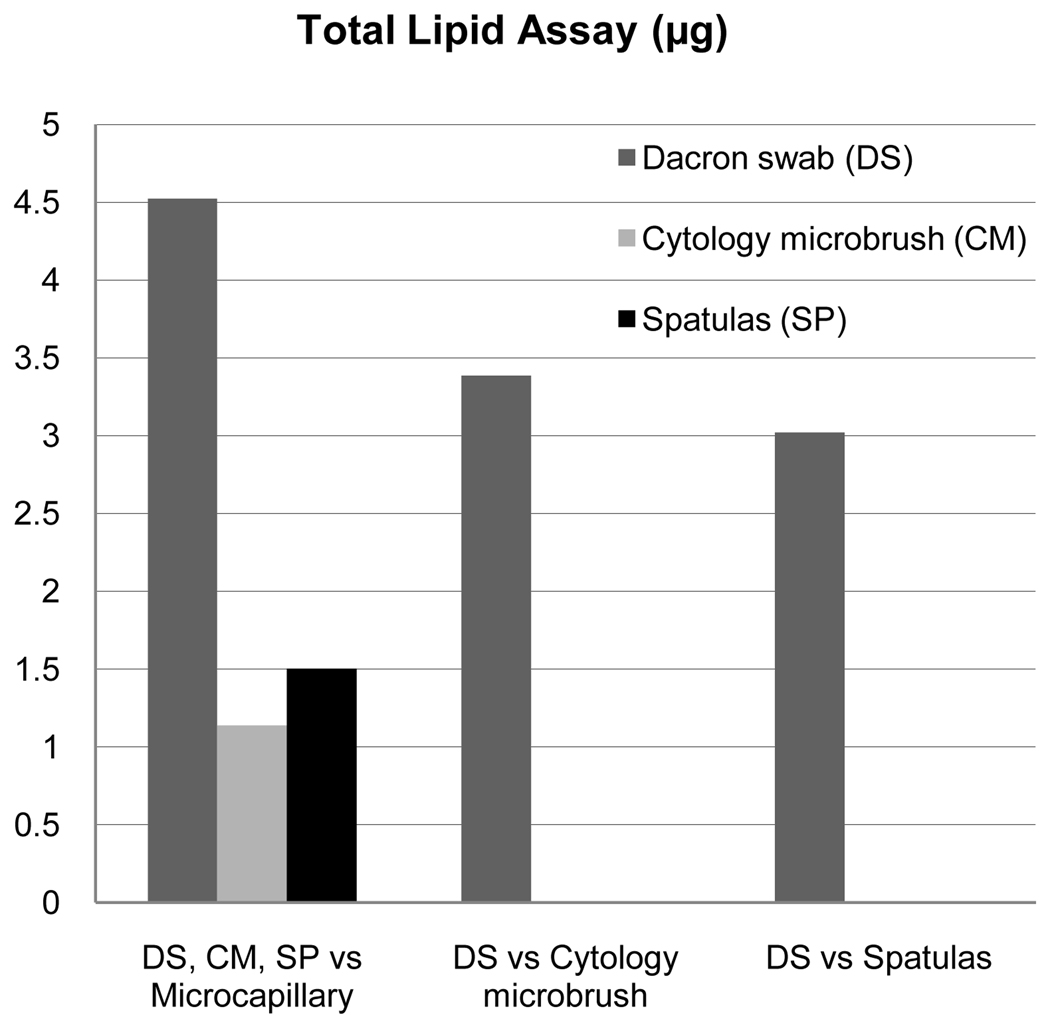
Three predictors were evaluated for outcome measures of total lipid, cholesterol, and inorganic phosphate assays: dry eye, collection device and extraction technique. Analysis of total lipid showed statistical significance only for the collection device (p<0.0001), but not for extraction technique (p=0.13) or dry eye status (p=0.97). Figure 3 illustrates relative quantities associated for each statistically significant collection device comparison. The Dacron swab was associated with the largest collection of total lipids when compared with the other devices; the on average yield for Dacron swab was 4.52 µg (95% CI 3.99–5.05) more than glass microcapillary tube, 3.39 µg (95% CI 2.70–4.07) more than cytology microbrush, and 3.02 µg (95% CI 2.30–3.74) more than spatula, with each difference demonstrating statistical significance (p<0.0001). The cytology microbrush was associated with 1.14 µg (95% CI 0.61–1.67) more total lipid when compared to the glass microcapillary tube and the spatula was associated with 1.50 µg (95% CI 0.93–2.07) more total lipid when compared to cytology microbrush (both p<0.0001). There was no difference in total lipid collection when comparing the spatula to cytology microbrush (0.37 µg, 95% CI −0.35–1.08, p=0.32).
Figure 3.
Relative Effectiveness of Detection by Total Lipid Assay (µg).32 Total lipid assay was only significant for collection device. Displayed are comparative effectiveness values by collection device only for statistically significant devices.
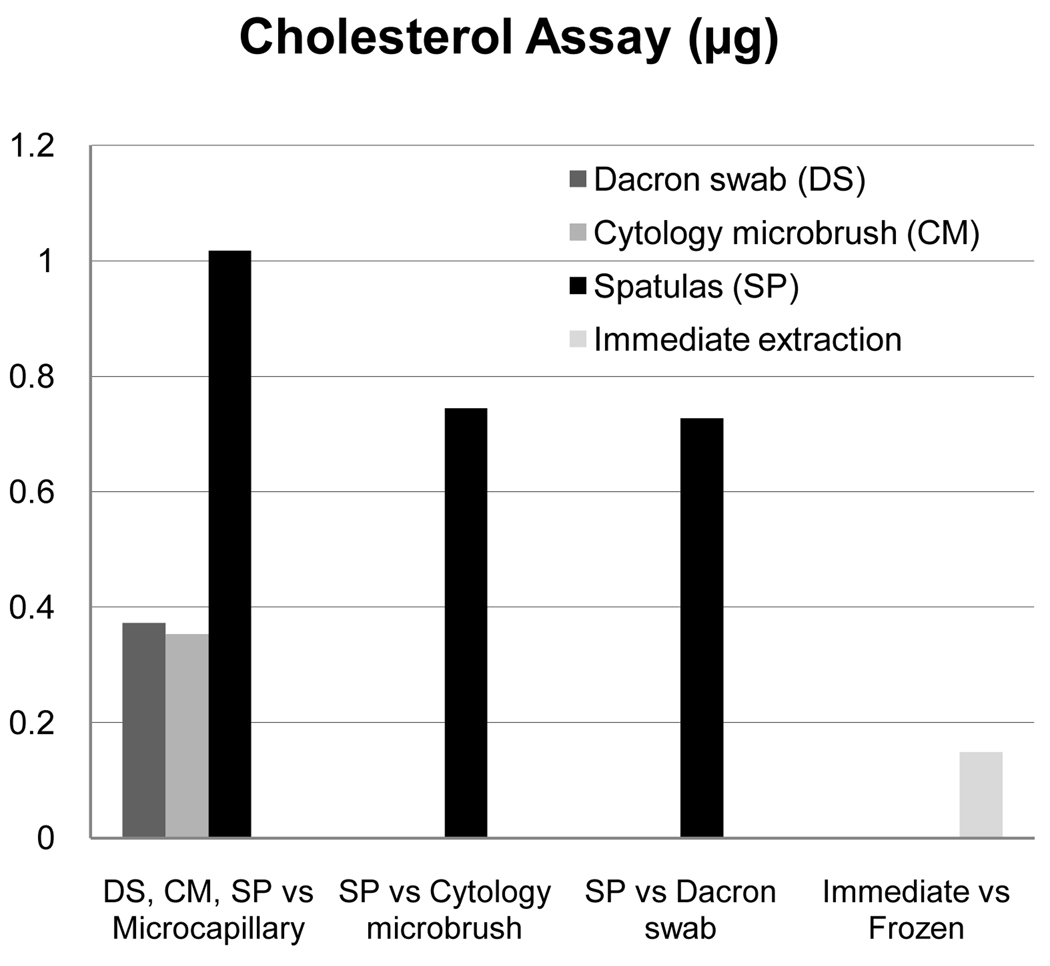
For total cholesterol and cholesterol esters, there were differences based on collection device (p<0.0001) and extraction technique (p=0.0002), but not for dry eye status (p=0.55). Relative quantities are illustrated in Figure 4. There was a difference between cytology microbrush compared to glass microcapillary tube (0.35 µg, 95% CI 0.19–0.51, p<0.0001), the Dacron swab compared to glass microcapillary tube (0.37 µg, 95% CI 0.21–0.53, p<0.0001), spatula compared to Dacron swab (0.65 µg, 95% CI 0.44–0.85, p<0.0001), and spatula compared to cytology microbrush (0.67 µg, 95% CI 0.46–0.87, p<0.0001). For spatula compared to glass microcapillary tube, there was greater than 1µg difference (1.02 µg, 95% CI 0.85–1.19, p<0.0001). Dacron swab compared to cytology microbrush was not significantly different (0.020 µg, 95% CI −0.18–0.22 p=0.84). Immediate extraction yielded only a small advantage in cholesterol (0.15 µg, 95% CI 0.03–0.27) over frozen with later extraction, yet was statistically significant (p=0.02).
Figure 4.
Relative Effectiveness of Detection by Cholesterol Assay (µg).30–31, 33 Cholesterol assay was significant for collection device and extraction technique. Displayed are comparative effectiveness values by collection device and extraction technique only for statistically significant values.
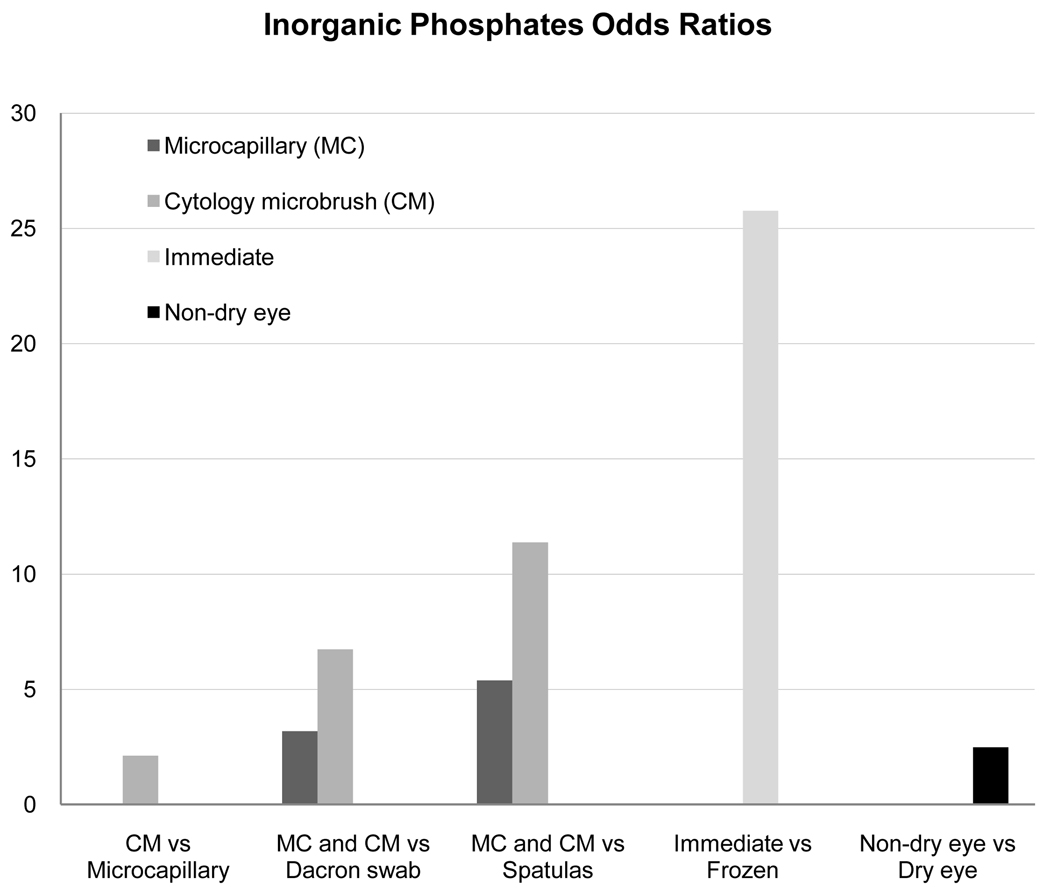
The inorganic phosphate analysis demonstrated statistical significance for collection device (p<0.0001), extraction technique (p<0.0001), and for dry eye status (p=0.02). The proportion of samples with presence of inorganic phosphates by collection device in descending order was as follows: cytology microbrush (50%), spatula (40%), microcapillary tube (38%), and Dacron swab (23%). Odds ratio estimates showed improved detection of inorganic phosphates with glass microcapillary tube over spatula (p<0.0001), and Dacron swab (p=0.0021). The cytology microbrush was associated with more collection of inorganic phosphates over glass microcapillary tube (p=0.044), Dacron swab (p=0.0001), and spatula (p<0.0001). The odds of detection of inorganic phosphates with the cytology microbrush were 11 times higher (95% CI 4.65–27.84) than with the spatula while Dacron swab comparison to spatula collection did not significantly improve the odds of detection (1.69, 95% CI 0.64–4.46, p=0.29). Immediate extraction yielded a 26 times higher (95% CI 10.01–66.26) likelihood of inorganic phosphate detection (p<0.0001) over delayed extraction (Figure 5). Non-dry eye subjects (normals) revealed a higher probability of observing inorganic phosphates than dry eye subjects (p=0.03).Table 2 provides, by dry eye status, the distribution of the proportion of collected samples from a subject that contained inorganic phosphates, where 64% of non-dry eye subjects had inorganic phosphates in at least half of their samples. In comparison, only 28% of the dry eye subjects had inorganic phosphates in at least half of their samples.
Figure 5.
Relative Odds Ratios for Detection by Inorganic Phosphate Assay.28–29 Inorganic phosphate assay was significant for collection device, extraction technique, and dry eye status. Displayed are comparative odds ratio values by collection device, extraction technique, and dry eye status only for statistically significant values.
Table 2.
Proportion of 4 samples from a subject containing inorganic phosphate. By dry eye status, the table shows the number and percent of subjects with a given proportion. It can be seen there is a trend for more of the subject’s samples to contain inorganic phosphates if the subject does not have dry eye.\
|
Dry Eye Status |
||||
|---|---|---|---|---|
| Proportion | Non Dry-Eye | Dry Eye | ||
| N | % | N | % | |
| 0.00 (0/4) | 4 | 10 | 4 | 22 |
| 0.25 (1/4) | 11 | 26 | 9 | 50 |
| 0.50 (2/4) | 22 | 52 | 4 | 22 |
| 0.75 (3/4) | 4 | 10 | 1 | 6 |
| 1.00 (4/4) | 1 | 2 | 0 | 0 |
| Total | 42 | 100 | 18 | 100 |
The probability of detecting inorganic phosphates was highest for subjects without dry eye using cytology microbrush collection with immediate extraction (probability 0.88, 95% CI 0.77–0.94). Detection of inorganic phosphates was least likely with dry eye subjects using Dacron swab collection and later extraction (probability 0.02, 95% CI 0.00–0.06).
DISCUSSION
Sample collection with the spatula was associated with the largest mean quantity of cholesterol detected, while the cytology microbrush was associated with the largest mean quantity of inorganic phosphate detected, and collection with the Dacron swab was associated with the largest mean quantity of total lipid detected. This evidence may lead one to conclude the best way to choose a collection device is by first determining which type of lipid is to be studied, and then to choose the appropriate device for the greatest yield. While this argument may be valid, there are other important considerations regarding the devices including downstream analysis and solvent use as well as patient comfort and tolerability. All subjects completed the study, suggesting good tolerability of all methods of meibum collection used in this study.
The expression of meibum is challenging and quite varied among individual subjects. Clinical judgment is required to determine the specific combination of techniques required to obtain sufficient sample quantity. Anatomic variables, such as size and position of meibomian glands, lid laxity, palpebral fissure width, and blink rate all have an impact on technique. Comparison of “hard expression” and “soft expression” techniques for individual subjects would provide valuable information. We did not conduct this comparative analysis for subjects in this study and only used “soft expression.” It will be of interest to see the results of such evaluation in the future.
The glass microcapillary tube has the distinct advantage of the ability to visualize the meibum sample during the collection process. The sample can be directly evaluated for obvious contamination with tears and the quantity collected can be measured in terms of length of sample in the tube by a slit lamp reticule as well as weighed. Removal of the sample from the tube is somewhat challenging. Another issue is solidification of the meibum sample during the collection process. It is highly probable the solidification process is a limiting factor on collection quantities, particularly for more viscous and difficult to express meibum. Results from this study (Table 1) showed the glass microcapillary tube to yield the lowest mean quantities for both total lipid and cholesterol outcome measures, although the sample collected with a microcapillary is likely composed of fresh rather than resident meibum. Single capillary collected meibum samples are adequate in size for lipid analysis using mass spectrometry with direct infusion.2
The cytology microbrush was developed for use in dental applications, and is formulated from purposefully non-absorbent materials. To our knowledge there are no previously published reports of the use of a cytology microbrush for the collection of meibomian gland secretions. A study of collection of endocervical cell samples using a cytology brush yielded more cells than a Dacron swab, yet there is evidence the variation may be technique dependent rather than device dependent.34 Design of the cytology microbrush used in the present study includes a cluster of very fine, distinct and rigid bristles. The bristles are a potential source of irritation of the subjects’ lid margin, possibly causing reflex tearing and a subsequent increase in the likelihood of collecting tears with meibum. In addition, the stiff nature of the bristles appears capable of effectively increasing collection of cellular material from the lid margin.
Although similarly designed as the cytology microbrush to be non-absorbent, the Dacron swab as a collection device presents another set of issues to consider. Dacron is a trade name for polyethylene terephthalate (C10H8O4) known industrially as PET, and commonly referred to as polyester. During the processing of the synthetic fiber, copolymers and stabilizers may be used. Antimony trioxide (Sb2O3) is a catalyst frequently used during PET manufacturing (90%). Although we did not evaluate directly for antimony in this study, if present, random effects during extraction and on the results of laboratory assays conducted may have occurred due to interaction with laboratory solvents, possibly inducing unreliable results and contamination of samples. Analytical blanks were run with the assays to minimize the impact of device contamination on results.
Stainless steel spatulas possess the distinct disadvantage of required immediate extraction, as freezing or storing spatulas would be cumbersome. Thus, samples in this study were immediately extracted upon collection. Each surface of three spatulas was used only once for each subject; consequently, three spatulas were used for one lid to increase the quantity of lipid collected. This method was effective for sample collection, but less convenient compared with the other three methods. Sample contamination with stainless steel spatula collection is not expected as organic solvents do not react with stainless steel, and removal of lipid from stainless steel is effective with organic solvents.
Contamination of samples remains to be considered. Alteration of the natural physiological status of the subject’s ocular surface and eyelid would occur if the lid margin is cleaned with a cotton swab prior to collection with a spatula or other device, including reflex tearing. To consider the composition of pure lipid from the meibomian gland in an absolute sense is quite different than considering the actual composition of meibum collected from the lid margin in living subjects, which has interacted with the ocular surface. It is likely the structure and function of meibum as part of the lipid layer of the tear film is somewhat different than freshly expressed meibum. In addition, with such small quantities collected, the choice of collection method can influence the analysis outcome. Although it has been suggested meibomian gland cellular products from the secretion process may be a source of contamination12, they are an integral part of meibum as collected from human subjects, and may contribute to the function of the lipid in the tear film.
Controversy exists over the presence of inorganic phosphates (for example, phospholipids) in human meibomian gland secretions. Although the holocrine secretion from the meibomian glands supports the presence of cellular membranes containing polar lipids in meibum, scientific evidence has been presented in support of both perspectives. Shine and McCulley reported finding three types of inorganic phosphates (phospholipids) in both normal and chronic blepharitis human subjects.4, 6 The relative composition of polar lipids (phospholipids were found to be approximately 70% of polar lipids in normals) was compared between normals and those with chronic blepharitis revealing significant identifiable differences between groups. Sullivan et al also reported on identification of polar lipids in human meibum, with notable differences between those with complete androgen insensitivity and normal controls, as well as identifiable variation with age.9–10 Other reports indicative of phospholipids in meibum come from Greiner’s work with rabbits11, 35 and the possible implication through a contribution to the tear film of meibomian gland disease patients by Yamada.29 Butovich and coworkers propose that contamination of the human meibum sample with tears and/or cells is necessary for detection of inorganic phosphates.12 This position is supported by results from studies completed by Butovich and others reported in two papers on mass spectrometry analysis of human meibum.7–8 Utilizing capillary collected tears and lipid samples collected by spatula, Butovich et al. showed that lipid composition differs for meibomian gland secretions and aqueous tears. Methods of detection and analysis are a possible cause of these differences. Sample preparation protocols and solvent choices are one potential source of detection variability. Mass spectrometric analysis involves many variables; one of significance may be the choice of prior chromatographic separation versus direct injection of the sample.
If one holds to the opinion inorganic phosphates indicate sample contamination, the results from this study suggest collection of meibum samples should be accomplished by spatulas and not via cytology microbrush. Given the fact that the present results show an increased probability of detecting inorganic phosphates in subjects without dry eye, decreased levels may be present in those with dry eye disease, suggesting a possible bioindicator of disease. Literature suggests low phospholipid levels may exist in the presence of inflammatory disease due to increased levels of phospholipases.36–38 The increased detection of inorganic phosphates cannot be attributed to the collection of larger overall sample quantities in the normal subjects, as the results indicated no difference in mean quantity of total lipids detected based on dry eye status.
Immediate extraction yields significant improvement for detection of both cholesterol and inorganic phosphates over frozen samples with later extraction. Utilizing this method requires careful planning prior to sample collection as one must be prepared to process samples immediately after collection, which is a time intensive process. Nonetheless, the controversy surrounding inorganic phosphates in human meibum may only be resolved if immediate extraction is employed in future research in this field.
CONCLUSIONS
The spatula method of collection has the most balanced benefits of those in the present study if yield is important in the analysis approach. The highest mean quantity of cholesterol detection was associated with spatula collection, as well as the lowest mean quantity of inorganic phosphates. Although inorganic phosphate was detected in 40% of samples collected with this technique, the lowest mean quantity of inorganic phosphate was associated with spatula collection of all four techniques in the current study. Spatula collection was second only to the Dacron swab in terms of association with mean quantity of total lipid detected, without the substantial prospective sources of device contamination. Of additional benefit will be results from planned analysis of samples by mass spectrometry to determine with higher specificity the composition of the samples as well as the collection devices.
Extensive review of the literature fails to reveal studies comparing the same human meibum collection and extraction methods. Statistical significance for subjects’ dry eye status was established only for detection of inorganic phosphates in this study with the unanticipated result of higher rates of detection for non-dry eye subjects. Immediate extraction of lipid from samples appears to be of general benefit and should be carefully considered for studies in this field. While it is difficult to ascertain the practical implications of statistical significance in this study, given the small sample quantities available, it is important to maximize detection using the most successful methods. Of considerable importance, variable results may occur with different laboratory assay-based or other protocols, and care should be taken to match the collection device with appropriate analyses. Additional research in various laboratory methods of meibum analysis is indicated to further clarify the significance of meibum composition and its application for clinical conditions. It is outside the scope of this manuscript to discuss the contact lens findings from those collected in this study.
ACKNOWLEDGMENTS
The authors would like to thank Heather L. Chandler for contributions to the initial study design. Funding was provided for this study through NIH T32EY013359 (K Haworth), and NIH R01EY015519 (K Nichols PI). Kelly K. Nichols is a consultant for Inspire Pharmaceuticals, Alcon, Allergan, and Pfizer. Data included in this manuscript was presented orally on May 5, 2009 during the Association for Research in Vision and Ophthalmology (ARVO) 2009 annual meeting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Linton RG, Curnow DH, Riley WJ. The Meibomian Glands: An Investigation into the Secretion and Some Aspects of the Physiology. Br J Ophthalmol. 1961;45:718–723. doi: 10.1136/bjo.45.11.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols KK, Ham BM, Nichols JJ, Ziegler C, Green-Church KB. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48:34–39. doi: 10.1167/iovs.06-0753. [DOI] [PubMed] [Google Scholar]
- 3.Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102. doi: 10.1016/j.chemphyslip.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26:89–94. doi: 10.1076/ceyr.26.2.89.14515. [DOI] [PubMed] [Google Scholar]
- 5.Shine WE, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res. 2003;76:417–420. doi: 10.1016/s0014-4835(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852. doi: 10.1001/archopht.116.7.849. [DOI] [PubMed] [Google Scholar]
- 7.Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776. doi: 10.1007/s11745-007-3080-2. [DOI] [PubMed] [Google Scholar]
- 8.Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J Lipid Res. 2007;48:2220–2235. doi: 10.1194/jlr.M700237-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–1699. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- 11.Greiner JV, Glonek T, Korb DR, Leahy CD. Meibomian gland phospholipids. Curr Eye Res. 1996;15:371–375. doi: 10.3109/02713689608995827. [DOI] [PubMed] [Google Scholar]
- 12.Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids: a review. Curr Eye Res. 2008;33:405–420. doi: 10.1080/02713680802018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sack R, Conradi L, Beaton A, Sathe S, McNamara N, Leonardi A. Antibody array characterization of inflammatory mediators in allergic and normal tears in the open and closed eye environments. Exp Eye Res. 2007;85:528–538. doi: 10.1016/j.exer.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Green-Church KB, Nichols KK, Kleinholz NM, Zhang L, Nichols JJ. Investigation of the human tear film proteome using multiple proteomic approaches. Mol Vis. 2008;14:56–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfel R, Pfeffer M, Essbauer S, Nerkelun S, Dobler G. Evaluation of sampling technique and transport media for the diagnostics of adenoviral eye infections. Adenovirus sampling and transport. Graefes Arch Clin Exp Ophthalmol. 2006;244:1497–1504. doi: 10.1007/s00417-006-0283-9. [DOI] [PubMed] [Google Scholar]
- 16.Goncalves PF, Lima LL, Sallum EA, Casati MZ, Nociti FH., Jr. Root cementum may modulate gene expression during periodontal regeneration: a preliminary study in humans. J Periodontol. 2008;79:323–331. doi: 10.1902/jop.2008.070327. [DOI] [PubMed] [Google Scholar]
- 17.Magne P, Cascione D. Influence of post-etching cleaning and connecting porcelain on the microtensile bond strength of composite resin to feldspathic porcelain. J Prosthet Dent. 2006;96:354–361. doi: 10.1016/j.prosdent.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Mehrotra R, Hullmann M, Smeets R, Reichert TE, Driemel O. Oral cytology revisited. J Oral Pathol Med. 2009;38:161–166. doi: 10.1111/j.1600-0714.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi A, Yokogawa H, Sugiyama K. Descemet stripping with automated endothelial keratoplasty for bullous keratopathies secondary to argon laser iridotomy--preliminary results and usefulness of double-glide donor insertion technique. Cornea. 2008;27 Suppl 1:S62–S69. doi: 10.1097/ICO.0b013e31817f38e9. [DOI] [PubMed] [Google Scholar]
- 20.Sekundo W. New forceps and spatula for easy retropupillary implantation of iris claw lenses in aphakia: experience in 4 years of use. Eur J Ophthalmol. 2008;18:442–444. doi: 10.1177/112067210801800320. [DOI] [PubMed] [Google Scholar]
- 21.Bayramlar H, Cekic O, Totan Y. Manual tunnel incision extracapsular cataract extraction using the sandwich technique. J Cataract Refract Surg. 1999;25:312–315. doi: 10.1016/s0886-3350(99)80077-7. [DOI] [PubMed] [Google Scholar]
- 22.Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62. doi: 10.1016/b978-0-12-024922-0.50005-9. [DOI] [PubMed] [Google Scholar]
- 23.Lemaire R, Wisztorski M, Desmons A, Tabet JC, Day R, Salzet M, Fournier I. MALDI-MS direct tissue analysis of proteins: Improving signal sensitivity using organic treatments. Anal Chem. 2006;78:7145–7153. doi: 10.1021/ac060565z. [DOI] [PubMed] [Google Scholar]
- 24.Stark M, Jornvall H, Johansson J. Isolation and characterization of hydrophobic polypeptides in human bile. Eur J Biochem. 1999;266:209–214. doi: 10.1046/j.1432-1327.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Vassar V, Hagen C, Ludwig J, Thomas R, Zhou J. One-step method of phosphatidylcholine extraction and separation. Biotechniques. 2007;42:442. doi: 10.2144/000112435. 44. [DOI] [PubMed] [Google Scholar]
- 26.Knust U, Erben G, Spiegelhalder B, Bartsch H, Owen RW. Identification and quantitation of phenolic compounds in faecal matrix by capillary gas chromatography and nano-electrospray mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3119–3129. doi: 10.1002/rcm.2702. [DOI] [PubMed] [Google Scholar]
- 27.Nichols JJ, Mitchell GL, Nichols KK, Chalmers R, Begley C. The performance of the contact lens dry eye questionnaire as a screening survey for contact lens-related dry eye. Cornea. 2002;21:469–475. doi: 10.1097/00003226-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Hohenwallner W, Wimmer E. The Malachite green micromethod for the determination of inorganic phosphate. Clin Chim Acta. 1973;45:169–175. doi: 10.1016/0009-8981(73)90406-3. [DOI] [PubMed] [Google Scholar]
- 29.Yamada M, Mochizuki H, Kawashima M, Hata S. Phospholipids and their degrading enzyme in the tears of soft contact lens wearers. Cornea. 2006;25:S68–S72. doi: 10.1097/01.ico.0000247217.16510.f2. [DOI] [PubMed] [Google Scholar]
- 30.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 31.Moshides JS. Enzymatic determination of high density lipoprotein phospholipid using a sensitive reagent and a Cobas Bio Centrifugal Analyzer. Scand J Clin Lab Invest. 1988;48:59–64. doi: 10.3109/00365518809085394. [DOI] [PubMed] [Google Scholar]
- 32.Neumann PTM, Gessner MO. Total lipids. In: Graça MASGF, Gessner MO, editors. Methods to Study Litter Decomposition: A Practical Guide. New York: Springer; 2005. pp. 91–95. [Google Scholar]
- 33.Pucker AD, Thangavelu M, Nichols JJ. Enzymatic quantification of cholesterol and cholesterol esters from silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010;51:2949–2954. doi: 10.1167/iovs.08-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiland TL, Noller KL, Smith TF, Ory SJ. Comparison of Dacron-tipped applicator and cytobrush for detection of chlamydial infections. J Clin Microbiol. 1988;26:2437–2438. doi: 10.1128/jcm.26.11.2437-2438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greiner JV, Glonek T, Korb DR, Booth R, Leahy CD. Phospholipids in meibomian gland secretion. Ophthalmic Res. 1996;28:44–49. doi: 10.1159/000267872. [DOI] [PubMed] [Google Scholar]
- 36.Bazan HE, Birkle DL, Beuerman R, Bazan NG. Cryogenic lesion alters the metabolism of arachidonic acid in rabbit cornea layers. Invest Ophthalmol Vis Sci. 1985;26:474–480. [PubMed] [Google Scholar]
- 37.Ronkko S, Rekonen P, Kaarniranta K, Puustjarvi T, Terasvirta M, Uusitalo H. Phospholipase A2 in chamber angle of normal eyes and patients with primary open angle glaucoma and exfoliation glaucoma. Mol Vis. 2007;13:408–417. [PMC free article] [PubMed] [Google Scholar]
- 38.Weerheim AM, Kolb AM, Sturk A, Nieuwland R. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal Biochem. 2002;302:191–198. doi: 10.1006/abio.2001.5552. [DOI] [PubMed] [Google Scholar]