Abstract
Objectives/Hypothesis
The inner ear is at risk for sensorineural hearing loss in both acute and chronic otitis media (OM), but the underlying mechanisms underlying sensorineural hearing loss are unknown. Previous gene expression array studies showed cytokine genes might be upregulated in the cochleas of mice with acute and chronic otitis media. This implies that the inner ear could manifest a direct inflammatory response to OM that may cause sensorineural damage. Therefore, to better understand inner ear cytokine gene expression during OM, quantitative RT-PCR and immunohistochemistry were performed on mouse models to evaluate middle and inner ear inflammatory and remodeling cytokines.
Study Design
Basic science experiment.
Methods
An acute OM model was created in Balb/c mice by a transtympanic injection of S. pneumoniae in one ear; the other ear used as a control. C3H/HeJ mice were screened for unilateral chronic OM with the non-infected ear serving as control.
Results
Both acute and chronic OM caused both the middle ear and inner tissues in these two mouse models to over express numerous cytokine genes related to tissue remodeling (TNFα, FGF, BMP) and angiogenesis (VEGF), as well as inflammatory cell proliferation (IL-1α,β, IL-2, IL-6). Immunohistochemistry confirmed that both the middle ear and inner ear tissues expressed these cytokines.
Conclusion
Cochlear tissues are capable of expressing cytokine mRNA that contributes to the inflammation and remodeling that occur in association with middle ear disease. This provides a potential molecular basis for the transient and permanent sensorineural hearing loss often reported with acute and chronic OM.
Level of Evidence
N/A
Keywords: cytokines, acute otitis media, chronic otitis media, mouse, middle ear, inner ear
INTRODUCTION
Otitis media (OM) is a common childhood disorder that results from bacterial or viral infection of the middle ear. Nearly all children experience at least one episode of acute otitis media (AOM), while some children experience recurrent or chronic infections 1. Episodes of AOM are usually transient and the middle ear inflammation clears within a few days. Inflammatory cytokines expressed during this phase are primarily TNF-α, Il-1β, and IL-8 2, 3. However, when chronic OM (COM) occurs, prolonged inflammation places the middle ear and inner ear at risk for tissue destruction and remodeling. Temporal bones of patients with chronic otitis media show middle ear changes (mucosal edema, fibrosis, increased goblet cells), as well as significant inner ear pathology, including loss of hair cells and spiral ganglion cells, inflammatory cells in the perilymphatic spaces, and reduction/fibrosis of the stria vascularis and spiral ligament 4, 5, 6. Permanent inner ear pathology can result, leading to sensorineural hearing loss, delayed speech development, and social and scholastic difficulties. The ability to control these inner ear sequelae is critical to protect children’s hearing, but the immune processes elicited in the inner ear are poorly understood. It is known that inflammatory factors move through the round window7 and that cultured spiral ligament fibrocytes produce inflammatory cytokines upon exposure to middle ear pathogens8, with both mechanisms likely leading to destruction of cochlear tissues.
Our preliminary gene expression array screening studies of mouse AOM and COM mouse showed cochlear tissues express cytokine genes in response to bacterial components or cytokines, or both9–10. This direct induction of a local inflammatory response may underlie the sensorineural hearing loss observed in acute and chronic middle ear infection. The purpose of this investigation was to investigate further middle ear and cochlear cytokine gene expression in our established mouse models for AOM11 and COM12 and to test the hypothesis that a destructive intracochlear immune response to otitis media may have a destructive impact on the cochlear itself. By evaluating these cochlear inflammatory responses in both AOM and COM, we were able to quantify their respective inner ear impact and their potential for causing sensorineural hearing loss. Previous work with the AOM and COM mouse model has established the presence of inflammatory cells in the inner ear spaces, hair cell abnormalities, and impact of histologically-proven inner ear inflammation on mouse auditory brainstem response (ABR) thresholds11, 13. Knowing that the inner ear is affected histologically and physiologically (ABR) by the presence of inflammatory cells and bacteria, we sought to define the cytokine profile in the inner ear in AOM and COM. We also examined a broader profile of middle and inner ear cytokines by quantitative RT-PCR to include not only inflammatory cytokines, but also those involved in tissue remodeling and angiogenesis.
MATERIALS AND METHODS
Our established mouse models for AOM11 and COM12 were used for gene expression studies. Female BALB/c and C3H/HeJ mice were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained under normal housing conditions until used. AOM was generated in the BALB/c mouse by transtympanic administration of heat-killed Streptococcus pneumonia (S pneumo). The C3H/HeJ mouse was used as a model for COM because it cannot mount an effective immune response to gram-negative bacteria due to a nonfunctional toll-like receptor 4 (TLR4). It develops a spontaneous and chronic colonization in the middle ear by Klebsiella oxytoca14. As suggested by previous work, analyses included inflammatory cytokines of the interleukin family (IL), the tissue remodeling cytokines that include bone morphogenetic proteins (BMP), fibroblast growth factors (FGF), transforming growth factor (TGF), and tumor necrosis factor (TNF), and angiogenic cytokines, such as vascular endothelial growth factor (VEGF) and certain FGFs. All animal protocols were approved by OHSU IACUC to assure compliance with federal animal welfare guidelines.
AOM Mouse Model
Balb/c mice (10–12 weeks of age) were sedated with a subcutaneous injection of ketamine (100 mg/ml; 0.067 mg/gm) and xylazine (20 mg/ml; 0.013 mg/gm), and examined under an operating microscope to confirm absence of otitis media. Acute inflammation was established in the mice by unilaterally injecting 5 µl of 109 heat-killed S pneumo transtympanically in 18 mice. The contralateral ear served as a non-inflamed control. After 24 hours, mice were killed and the two sides harvested. For the inner ear assessment, the inner ear was removed in 12 mice and 4 inner ears were pooled for each sample, giving 3 samples ipsilateral and 3 samples contralateral to the inoculated side. To avoid contamination from middle ear contents, the inner ear was carefully removed from the bulla and the inner ear contents were scraped off the cochlea and washed in 0.1 M PO4 buffer prior to storage in RNA later (Ambion, Inc., Austin, TX). For the middle ear tissue processing, the middle and inner ear tissues (6 mice) were processed together in order to keep all middle ear inflammatory contents contained and not destroy mucosal material by separating. Two middle ears were combined for each sample, providing three samples of inoculated middle ears and three samples of control middle ears. Tissues were placed in RNAlater (Ambion, Inc., Austin, TX) at −20°C until RNA was extracted. Additional acute OM mice were killed at 24 hours for immunohistochemistry.
COM Mouse Model
C3H/HeJ mice were raised until they developed middle ear infections (4–6 months of age). These mice were not cultured for type of bacteria in the observed purulent middle ear effusion, but prior studies with this animal model have uniformly revealed infection with the Gram-negative, rod-shaped bacterium typical of the Klebsiella family14. They were sedated as above and 18 were selected that had one ear infected (visual confirmation by microscopic otoscopy) and one ear clear of inflammation as a control. Ears were harvested, middle and inner ears processed as above, and stored in RNAlater as above. Additional unilateral COM mice were killed for immunohistochemistry.
mRNA Extraction
To obtain sufficient RNA for processing, 2 middle ears or 4 inner ears were combined for each analysis, providing three samples for each treatment. RNA was extracted using the Qiagen® (Valencia, CA) RNeasy Mini Kit. Tissue was transferred to a tube with 600 µl of extraction buffer and homogenized using a PowerGen 125 per the Mini Kit protocol. RNA was quantified using a NanoDrop and all samples were made to have a concentration of at least 25 ng/µl.
qRT-PCR
To quantify the inflammatory response in acute and chronic OM mouse models, real-time qRT-PCR studies were conducted of cytokines identified from our previous gene expression array studies9–10. Primers were obtained for a subset of inflammatory and remodeling cytokines that were deemed likely to be important based upon our preliminary studies: IL-1α, IL-1β, IL-2, IL-6, TNFα, TGFβ, BMP6, BMP7, FGF1, FGF3 and VEGFα. All primers/probe sets were obtained from Applied Biosystems (Carlsbad, California) and samples were run in triplicate on an Applied Biosystems 7300 RT-PCR according to the manufacturer’s protocols. The analytical program made the statistical comparisons between control and inflamed ears and the fold change was calculated through the diagnostic programs within the PCR analysis protocols. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The cytokine response indicated in fold-change is a ratio of test RNA to GAPDH. GAPDH normalizes for the number of cells because as a housekeeping gene it is considered to be a constant. Student’s t-test was used to compare control ears to inflamed ears for both the AOM and COM mouse models and a statistical value with a probability < 0.05 was judged as significant.
Immunohistochemistry
Immunohistochemistry of the inner and middle ear was conducted using antibodies against the products of genes identified in the RT-PCR studies as most likely being important. This was to confirm that the IE and ME tissues expressed the specific genes in question and to localize that expression. Acute and chronic OM mice were sedated as above and perfused intracardially with 3% paraformaldehyde in 0.1 M phosphate buffer. Ear tissues were removed, decalcified in EDTA, and embedded in paraffin. Serial 5 µm sections were mounted on glass slides, heated at 50 °C for 30 minutes, and deparaffinized. Sections were stained with primary antibodies against IL-1α, IL-1β, IL-6 and TNF-α (Santa Cruz Biotechnology, Santa Cruz, CA). These antibodies were selected because they showed the most significant expression in both the acute and chronic ears by RT-PCR. Primary antibodies were detected with secondary antibodies conjugated to AlexaFluor 488 (Invitrogen/Molecular Probes, Eugene, OR). Sections were observed with a Leica DM2500 fluorescence microscope and photographed with Leica FC420C digital camera driven by Application Suite v3 software. Control sections were run with buffer only and no primary antibody.
RESULTS
qRT-PCR
Acute OM
Significant middle ear inflammation occurred following S pneumo inoculation of BALB/c mice. Several cytokines were upregulated and only a few were downregulated in the inner ear. A survey of expression results (Table 1) showed multiple cytokines impacted in both the middle ear and inner ear 24 hours following inoculation.
Table 1.
| A AOM Middle Ear Cytokines | |||
|---|---|---|---|
| Cytokine | Fold Change |
t-test value |
Probability |
| Bone Morphogenetic Protein 6 | 1.12 | 0.645 | 0.530 |
| Bone Morphogenetic Protein 7 | 1.29 | 4.040 | 0.002 |
| Fibroblast Growth Factor 1 | 0.96 | −0.651 | 0.525 |
| Fibroblast Growth Factor 3 | 0.69 | −0.878 | 0.399 |
| Interleukin -1α | 2.73 | 8.760 | < 0.0001 |
| Interleukin -1β | 4.19 | 10.639 | < 0.0001 |
| Interleukin -2 | 0.92 | −0.189 | 0.854 |
| Interleukin -6 | 7.87 | 8.959 | < 0.0001 |
| Transforming Growth Factor β3 | 0.93 | −0.517 | 0.617 |
| Tumor Necrosis Factor α | 1.60 | 6.252 | < 0.0001 |
| Vascular Endothelial Growth Factor α | 0.92 | −0.587 | 0.568 |
| B AOM Inner Ear Cytokines | |||
|---|---|---|---|
| Cytokine | Fold Change |
t-test value |
Probability |
| Bone Morphogenetic Protein 6 | 1.23 | 1.083 | 0.296 |
| Bone Morphogenetic Protein 7 | 0.86 | −2.832 | 0.015 |
| Fibroblast Growth Factor 1 | 1.18 | 2.614 | 0.020 |
| Fibroblast Growth Factor 3 | 1.31 | 1.152 | 0.270 |
| Interleukin -1α | 1.44 | 3.165 | 0.008 |
| Interleukin -1β | 3.19 | 14.645 | < 0.0001 |
| Interleukin -2 | 1.19 | 0.498 | 0.629 |
| Interleukin -6 | 0.16 | −4.546 | 0.001 |
| Transforming Growth Factor β3 | 1.84 | 4.957 | 0.0001 |
| Tumor Necrosis Factor α | 1.90 | 7.254 | < 0.0001 |
| Vascular Endothelial Growth Factor α | 1.67 | 4.173 | 0.0007 |
Values in bold type represent fold change with p<0.05
Middle Ear
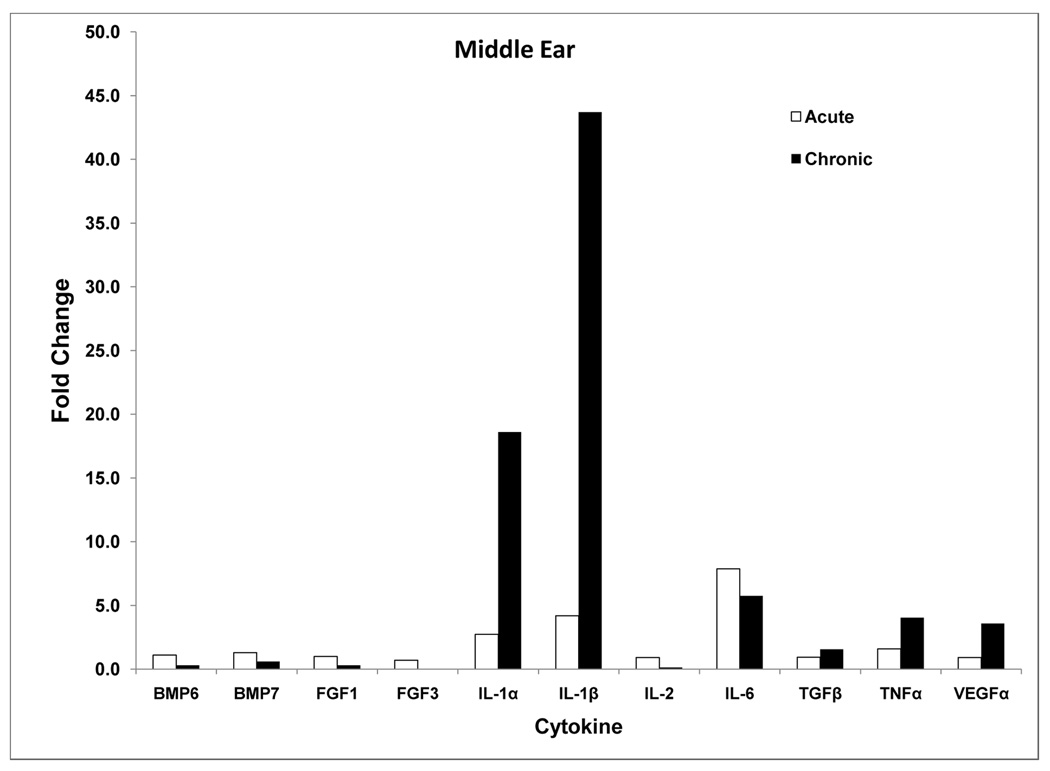
There was significant elevation of middle ear mRNA for the inflammatory cytokines IL-1α, IL-1β, IL-6, TNFα, and BMP7 (Table 1A, Fig. 1A). The strongest response was seen for the interleukins with IL-1α expressed 2.7 fold, IL-1β upregulated greater than 4 fold, and IL-6 showing nearly 8 fold change. Expression of TNFα and BMP7 was significantly increased, although not to the extent of the interleukins.
Figure 1.
A: qRT-PCR of middle ear cytokines comparing acute and chronic OM. Chronic OM caused greater expression of IL-1α and IL-1β, while most other cytokines were similar in their expression in the two OM models. See Tables 1A and 2A for significant probability values.
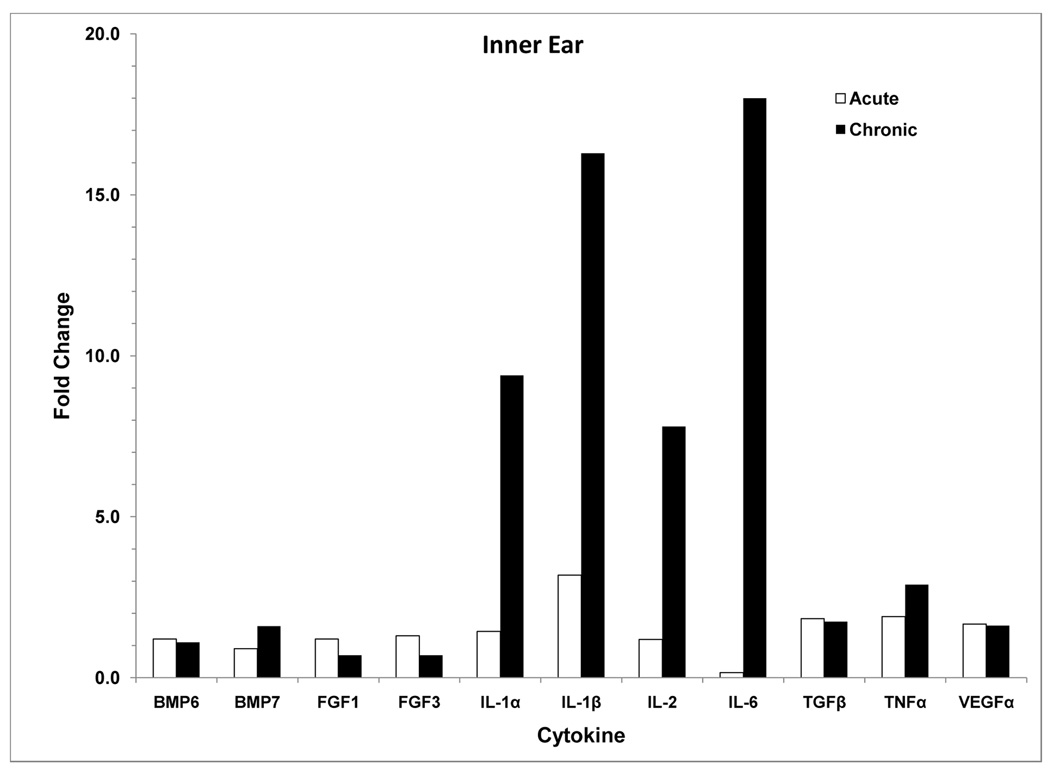
B: qRT-PCR of inner ear cytokines comparing acute and chronic OM. Several of the cytokine genes were expressed in greater amounts in the chronic condition. The interleukins in particular were significantly higher in the cochlea with the long standing infection. See Tables 2A and 2B for significant probability values.
Inner Ear
Cochlear tissues showed increased expression of these same cytokines, as well as FGF1, TGFβ, and VEGFα (Table 1B, Fig. 1B). The strongest response was seen in IL-1β with more than 3 fold increased activity. However, TGFb, TNFα, and VEGFα also showed greater than 1.5-fold fold-change. Some cytokine genes showed less activity in the inner ear during acute middle ear inflammation. BMP7 and IL-6 appeared to be expressed at significantly lower levels than normal.
Chronic OM
RT-PCR results show multiple cytokines upregulated or downregulated in both the inner and middle ear in these chronically infected mice (Table 2).
Table 2.
| A COM Middle Ear Cytokines | |||
|---|---|---|---|
| Cytokine | Fold Change |
t-test value |
Probability |
| Bone Morphogenetic Protein 6 | 0.28 | −3.353 | 0.007 |
| Bone Morphogenetic Protein 7 | 0.63 | 2.265 | 0.047 |
| Fibroblast Growth Factor 1 | 0.28 | 5.304 | 0.0007 |
| Fibroblast Growth Factor 3 | n.d. | n.d. | n.d. |
| Interleukin -1α | 18.61 | −6.269 | 0.0001 |
| Interleukin -1β | 43.71 | −7.076 | < 0.0001 |
| Interleukin -2 | 0.14 | −1.033 | 0.336 |
| Interleukin -6 | 5.75 | −1.618 | 0.137 |
| Transforming Growth Factor β3 | 1.55 | 4 | 0.088 |
| Tumor Necrosis Factor α | 4.04 | −3.613 | 0.006 |
| Vascular Endothelial Growth Factor α | 3.58 | 4.899 | 0.0008 |
| B COM Inner Ear Cytokines | |||
|---|---|---|---|
| Cytokine | Fold Change |
t-test value |
Probability |
| Bone Morphogenetic Protein 6 | 1.06 | 0.134 | 0.896 |
| Bone Morphogenetic Protein 7 | 1.60 | −4.393 | 0.0005 |
| Fibroblast Growth Factor 1 | 0.67 | 4.271 | 0.0006 |
| Fibroblast Growth Factor 3 | 0.68 | 1.450 | 0.168 |
| Interleukin -1α | 9.39 | −13.464 | < 0.0001 |
| Interleukin -1β | 16.29 | −15.173 | <0.0001 |
| Interleukin -2 | 7.80 | 6.737 | < 0.0001 |
| Interleukin -6 | 18.00 | −10.019 | < 0.0001 |
| Transforming Growth Factor β3 | 1.74 | 3.101 | 0.008 |
| Tumor Necrosis Factor α | 2.89 | −12.155 | < 0.0001 |
| Vascular Endothelial Growth Factor α | 1.62 | 6.000 | < 0.0001 |
Values in bold type represent fold change with p<0.05
n.d. = below detection thresholds
Middle Ear
RT-PCR results for the chronic OM mouse model (Table 2A, Fig. 1A) showed significant change in middle ear mRNA expression of BMP6, BMP7, FGF1, IL-1α, β, TNFα, and VEGFα. Inflammatory processes appeared robust as indicated by greater expression of IL-1α, IL-1β, TNFα, and VEGFα. The greatest change was seen in IL-1α and IL-1β (18 and 44-fold respectively). TNFα and VEGF genes were expressed 3–4 fold greater than normal. On the other hand, the remodeling cytokines genes appeared to be suppressed by the chronic disease, as indicated by the significant reduction in expression of BMP6, BMP7, FGF1, and FGF3. FGF3 was reduced to levels not measureable by the technique, indicating it was significantly suppressed by the chronic infection.
Inner Ear
Inner ear cytokine mRNA in the chronic OM mouse was significantly changed in a pattern similar to the middle ear. The greatest changes were seen in the interleukins (IL-1α, -1β, -2, -6), but also more than 1.5-fold increased expression in BMP7, TGFβ, TNFα and VEGFα (Table 2B, Fig. 1B). Interestingly BMP6 and the FGFs were generally unchanged or even downregulated, while BMP7 was upregulated only 1.6 fold. However, overall genes in the inner ear tissues were substantially upregulated during the chronic middle ear inflammatory activities. Comparison of inner ear cytokine expression between acute and chronic OM showed significantly greater expression in the chronically inflamed condition (Figure 1B). In general, the pro-inflammatory interleukins were significantly upregulated in the acute middle ear inflammation, but they were elevated several fold more in the chronic OM condition.
Immunohistochemistry
The greatest impact on cytokine expression was seen for IL-1α, IL-1 β, IL-6, and TNF-α. Therefore, the local staining for these cytokines was examined by immunohistochemistry to determine where they were predominantly expressed.
The antibody to IL-1α in the AOM mice labeled middle ear epithelium, tympanic membrane and inner ear lateral wall (Fig. 2A). The entire lining of the middle ear mucosa was labeled, suggesting the cytokine was present throughout the epithelium. This extended to the lining of the tympanic membrane and ossicles. The inner ear also showed considerable staining of this cytokine in the lateral wall, including both the stria vascularis and underlying spiral ligament (Fig. 2B). Staining also was seen in the organ of Corti and spiral ganglion, suggesting the cytokine was quite pervasive once it was produced. In the chronic OM mouse, similar staining patterns were seen in the middle ear and inner ear. The content of the middle ear was strongly stained and indicated the IL-1α was present throughout the inflammatory contents (Fig. 2C). It also was present in the lateral wall structures of the inner ear, similar to the pattern seen in the acute OM mice. The stria vascularis stained particularly strong and the area of antibody uptake in the lateral wall corresponded to areas occupied by fibrocyte Types II and IV.
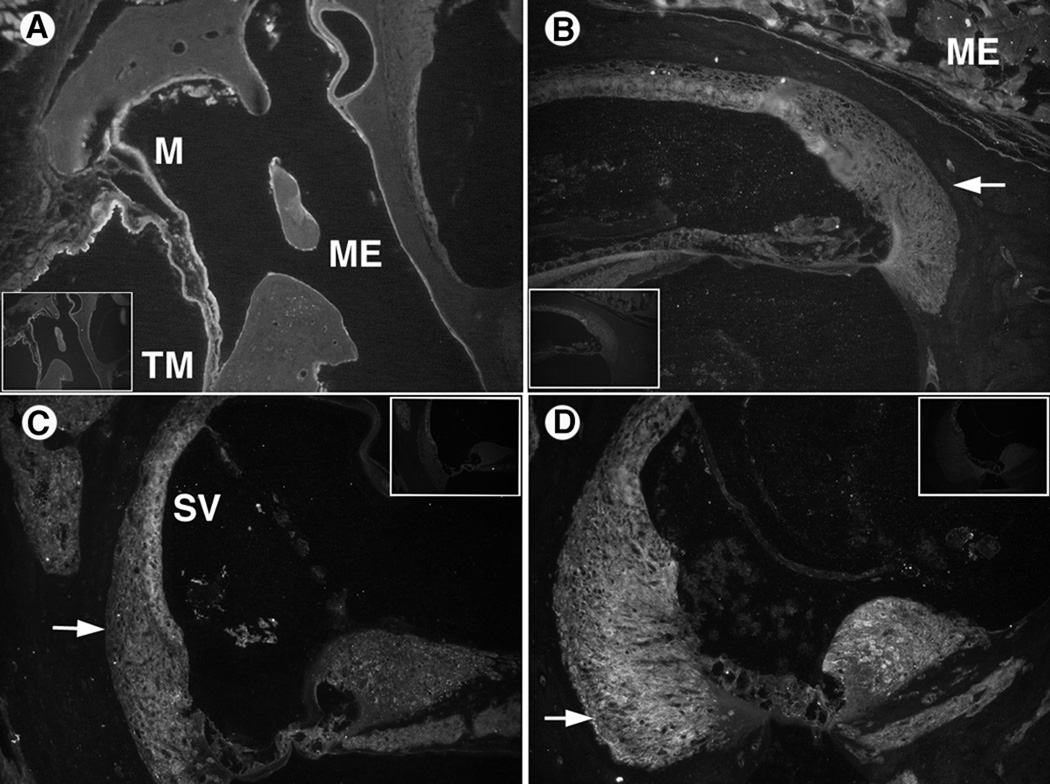
Figure 2.
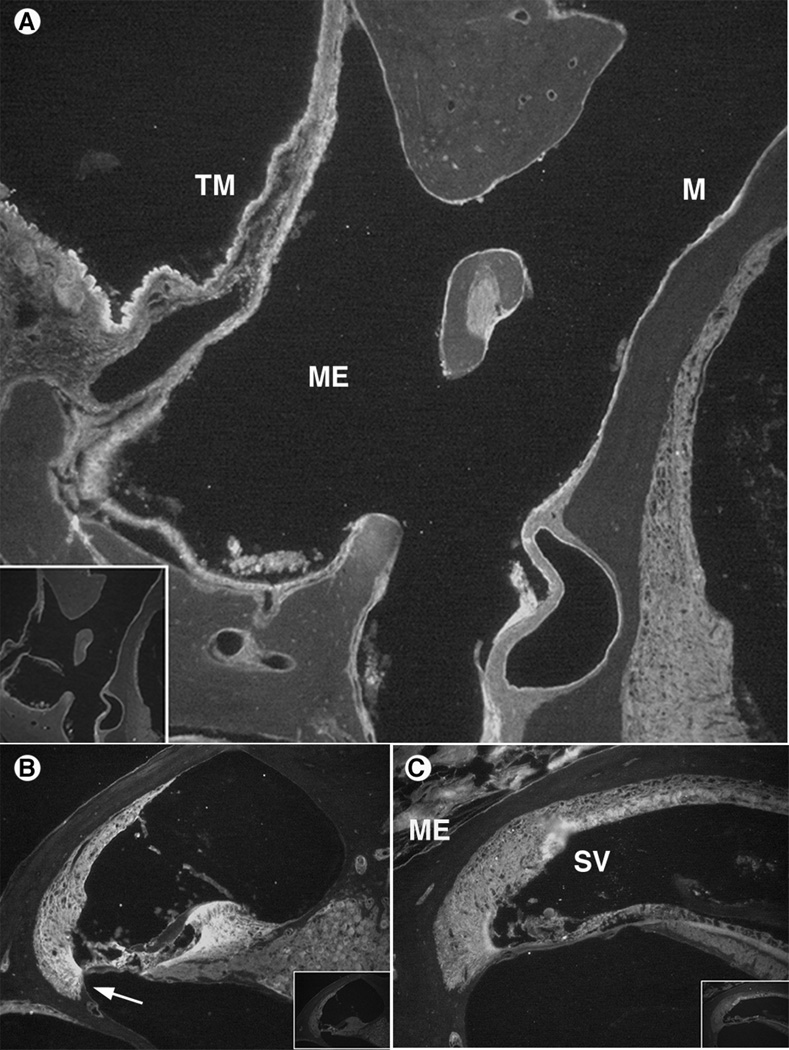
IL-1α immunohistochemistry results. Control sections (no primary antibody) are inset in corner of antibody stained sections.
A. AOM model demonstrated significant IL-1α antibody staining in the middle ear (ME) mucosa (M) and tympanic membrane (TM). Even the outer epithelial layer of the TM was labeled, suggesting a widespread inflammatory response.
B. AOM inner ear with staining in the lateral wall spiral ligament (arrow) in areas of fibrocyte Type II and IV. Significant labeling also was seen in the organ of Corti and moderate staining was observed in the spiral ganglion neurons medially.
C. COM inner ear section with staining in the stria vascularis (SV) and spiral ligament. The middle ear (ME) contents also stained external to the cochlear bony capsule.
The IL-1β antibody stained in a pattern similar to the IL-1α in the acute OM mouse. It occurred in the middle ear mucosa and inner ear throughout the scala media (Fig. 3A, B). The entire mucosal lining along the cochlear wall showed the cytokine presence, suggesting it was produced by tissues there. It also stained heavily in the cochlear spiral ligament and stria vascularis (Fig. 3A, B). The organ of Corti and spiral ganglion stained significantly with this antibody, indicating its presence virtually throughout the cochlea. In the chronic OM mouse, IL-1β antibody was also noted in the inner ear lateral wall, stria vascularis, organ of Corti, and middle ear mucosa (Fig. 3C, D.).
Figure 3.
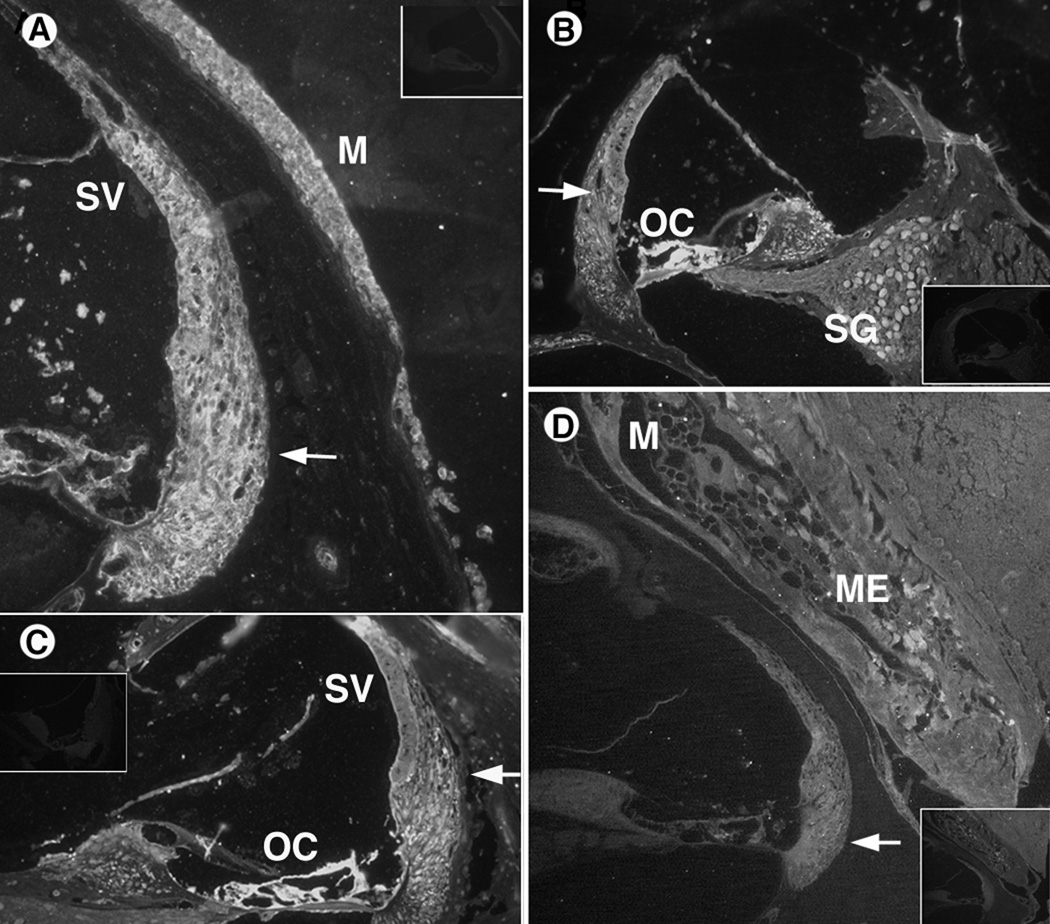
IL-1β immunohistochemistry results. Control sections (no primary antibody) are inset in corner of antibody stained sections.
A. AOM inner section emphasizing cochlear lateral wall. Positive staining is seen in the spiral ligament (arrow) and stria vascularis. The middle ear mucosa (M) also showed significant staining for IL-1β.
B. AOM inner section with cytokine staining in the lateral wall (arrow), as well as the organ of Corti and spiral ganglion neurons medially.
C. COM section of inner ear with IL-1 β immunostaining in spiral ligament of the lateral wall (arrow), while less staining is seen in the stria vascularis (SV). The organ of Corti (OC) also appears heavily labeled with immunostaining, suggesting cytokine continuity with the perilymphatic space of the spiral ligament.
D. COM inner ear section with positive staining of the inflammatory debris in middle ear (ME), middle ear mucosa (M), and inner ear lateral wall (arrow).
The IL-6 antibody stain was noted in the middle ear epithelium and tympanic membrane in the acute OM mouse (Fig. 4). The entire mucosal lining of the middle ear space was heavily labeled, as was the lining of the tympanic membrane (Fig 4A). In the chronic OM mouse, IL-6 antibody was noted in the lateral wall and middle ear mucosa (Fig. 4B). The cochlear staining appeared less distinct, although the stria vascularis appeared to have the cytokine present.
Figure 4.
IL-6 (A, B) and TNFα (C, D) immunohistochemistry results. Control sections (no primary antibody) are inset in corner of antibody stained sections.
A: AOM middle ear section shows IL-6 antibody staining in the middle ear (ME) mucosa (M) and both external and internal layers of the tympanic membrane (TM).
B: COM inner ear section with IL-6 antibody label in the lateral wall (arrow).
C: AOM inner ear section stained with TNF-α antibody showing its presence in the lateral wall (arrow) and stria vascularis (SV).
D: COM inner ear section with significant TNF-α antibody label in the lateral wall (arrow).
In the acute OM mouse, TNFα antibody was noted in the inner ear lateral wall, including both spiral ligament and stria vascularis (Fig 4C). The stria vascularis appeared more heavily labeled than the spiral ligament, although the latter still showed significant staining. The chronic OM mouse also showed strong TNFα in the cochlear lateral wall (Fig. 4D), particularly the spiral ligament area of fibrocyte Types II and IV.
DISCUSSION
Middle ear inflammation
The results confirm significant cytokine gene expression in the middle ear 24 hours postinoculation. The strongest response in the AOM model was the interleukins IL-1α, IL-1β and IL-6, with lesser, but still significant, expression for TNFα. The quantification of cytokine mRNA shows their relative expression parallels the major cytokines in the inflammatory profile of acute OM outlined by Juhn et al.15. Previous studies have shown that inflammation peaks at 3–5 days and is largely cleared by day 711. The present study looked at the 1 day time point after inflammation induction in the AOM model. Thus, this assessment reflects the key cytokines involved at the onset of inflammation.
The present study also provides an assessment of cytokine profiles within a chronically infected middle ear. The C3H/HeJ mice are unable to mount an effective immune response against gram-negative bacteria because of their TLR4 defect11, leading to chronic infection with Klebsiella oxytoca bacteria14. Toll-like receptors (TLRs) are a part of the innate immune response, recognizing the molecular signatures of microbial pathogens through cell-surface receptors16. Each TLR recognizes a specific pathogen-associated molecular pattern. TLR activation initiates intracellular NF-kappa β signaling and cytokine gene expression that ultimately leads to cellular immune responses. The C3H/HeJ mouse has a single amino acid substitution which renders the TLR4 gene response to infectious challenges diminished, not absent. This diminished response then leads to a chronic infection as the animal is unable to eradicate the bacteria effectively. It is also postulated that with the resulting chronic infection, the animal mounts a response through other innate immunity pathways, such as the toll-like receptor 2 (TLR2) pathway, since TLR2 is known to recognize a broader range of microbes than other TLRs8. Toll-like receptor 9 (TLR9) could also represent another pathway for this mouse model to respond to infection, as TLR 9 responds to the CpG motifs in bacterial DNA16. The chronic middle ear infection seen in the C3H/HeJ mice caused nearly every inflammatory cytokine to be expressed at significantly higher levels than normal. The cytokine IL-1α is upregulated nearly 19 fold and IL-1β more than 43 fold. Increased VEGF RNA supports the significant vascular permeability and neovascularization seen in chronic disease11. Interestingly, the tissue remodeling cytokines, such as the BMPs and FGFs were dramatically downregulated, suggesting they are not directly involved in the middle ear changes during this prolonged infection. Thus, the two models for middle ear inflammation show similar inflammatory profiles, while differing mainly in degree of expression. Immunohistochemistry confirmed cytokine production in the middle ear mucosa and tympanic membrane epithelium in both the acute and chronic models in response to bacterial stimulation.
Inner ear inflammation
Another key finding was the significant expression of cytokine genes by the inner ear tissues. This provides for the first time evidence for direct inflammatory gene expression by cochlear tissues during otitis media, which could explain the permanent damage seen in the cochlea with chronic otitis media. Preliminary findings of inner ear gene expression were reported previously for acute and chronic otitis media models with inflammatory gene arrays9–10. However, those studies used expression array techniques that did not permit actual quantification of RNA levels as in the present study. Thus the current findings expand significantly on these earlier studies. The profile of inner ear cytokines in the acute OM model showed that within 24 hours a variety of key inflammatory cytokines are being expressed by inner ear tissues. This included not only the interleukins, but also TNFα, TGFβ, and VEGFα. Thus, the response by inner ear tissues is rapid, extensive, and broad based. Furthermore, a number of tissue remodeling and repair genes are expressed, in the inner ear, adding to the potentially pathologic response during the transient inflammation in the middle ear.
There also was widespread inner ear gene expression during the ongoing infection in chronic otitis media. Nearly every gene examined was significantly upregulated in the inner ear tissues in the C3H/HeJ mouse, with the interleukins the most active. Their expression was elevated in AOM, but dramatically more so in the chronic condition. This demonstrated the significant inflammatory environment to which the inner ear is exposed with a chronically infected middle ear. This suggests a pattern of local gene expression that could have an impact on inner ear integrity. Significant gene expression also was seen for those cytokines that are involved with tissue remodeling (TNFα, TGFβ) and angiogenesis (VEGFα), potentially explaining the proliferation of new bone, connective tissue, and neovascularization seen in chronic OM. This reflects the extent of exposure that inner ear tissues experience with a chronically infected middle ear. These ongoing inflammatory patterns reflect the underlying mechanisms for cochlear pathology and hearing loss in this mouse model12 and potentially at work in human chronic middle ear infections.
Parallels between the RNA increase in the middle and inner ears not only explain the cochlear inflammation and remodeling seen with prolonged middle ear disease, but also implies similar underlying mechanisms. Either the bacterial components are eliciting the same response in both locations, or the cytokines produced in the middle ear are causing parallel expression by inner ear tissues. Inner ear inflammation in otitis media is presumably due largely to the easy passage of molecules through the round window membrane. The round window is a semi-permeable membrane that is lined on the middle ear side with epithelial cells joined by tight junctions4. However, there is little barrier to movement for the small inflammatory cytokines (10–50 kD), lipopolysaccharide endotoxin (10 kD), and other bacterial components that result from middle ear infection17. Immunohistochemistry results confirmed IL-1α and TNFα staining was strongest in areas of fibrocyte Types II and IV. The fibrocytes in the lateral wall of the cochlea are known to be important for K+ recycling via gap junctions, as well as potential autocrine and paracrine roles via connective tissue growth factor18. There is a possibility that the inner ear fibrocytes also have an inflammatory role in the inner ear. Immunohistochemistry of the present and previous studies9–10 confirms the presence of these cytokines in the spiral ligament where the predominant cell type is the fibrocyte. TLR2 receptors have been shown to exist on fibrocytes and mediate their production of cytokines upon stimulation with bacterial components8. TLR2 recognizes lipid-based molecules, specifically lipoproteins and lipotechoic acid of gram-positive bacteria, and plays a broader role in the recognition of a variety of bacteria and bacterial antigens. The observation of TLR2 receptors on fibrocytes, coupled with the present findings, implicates them as one of the major effectors of inner ear inflammatory cytokine production.
Permanent hearing loss
The extensive upregulation of cochlear cytokine genes with middle ear inflammation provides the potential mechanism underlying the sensorineural hearing loss that accompanies OM. This acute inflammation in the mouse subsides after 7 days 11, so it is assumed that the inflammatory gene expression would return to normal as well. However, permanent hearing loss resulting from AOM may be due to the cytokine expression reaching sufficient levels to cause hair cell damage. While the cochlear effects of acute disease are usually transient, there is a subset of patients who suffer repeated and persistent middle ear inflammation and are at increased risk for cochlear damage and sensorineural hearing loss1. Prevalence of children with significant sensorineural hearing loss ranges from 9–21%19, increasing to 40–55% 6 if they had multiple infections in their younger years. The incidence of permanent threshold shifts increases with chronic otitis media20–21 as 20–67% of such cases show inner ear pathology and inflammatory cells in the perilymphatic spaces6, probably entering via the round window 22–23.
There is limited evidence for the mechanism of sensorineural hearing loss seen in OM and whether or not it is from the cytokines or from bacterial products that reside in the purulent effusion that accompanies OM. However, it known that both bacteria and exotoxin do move through the round window membrane24–25 enabling them to potentially damage inner ear tissues. Evidence for spiral ligament fibrocyte chemokine production in response to bacterial challenge, also exists, thus providing a mechanism for inner ear inflammatory cell recruitment26. It has also been shown that the hearing loss that accompanies the chronic OM seen in the C3H/HeJ mice can be prevented by clearing the middle ear infection13. The pronounced expression of cochlear cytokines and their gene products in the chronic OM mouse offers a potential explanation for the sensorineural hearing loss in the chronically inflamed human middle ear.
Conclusion
The findings of our study provide a molecular basis for the transient and permanent sensorineural hearing loss often reported with acute and chronic OM. Consistent patterns of inner ear inflammatory factors were seen in the two OM models, suggesting that common processes may be involved in the sensorineural aspects of otitis media. Identification of these inflammatory processes involved may be beneficial in the design of therapies to protect the inner ear during both acute and chronic middle ear inflammation.
Acknowledgments
Supported by NIH NIDCD R01 DC 09455 and NIDCD P30 DC005983
Footnotes
Presented at Association for Research in Otolaryngology, Phoenix, AZ, February 6–10, 2008
Conflict of Interest: None.
References
- 1.Pichichero ME. Recurrent and persistent otitis media. Pediatric Infectious Disease Journal. 2000;19:911–916. doi: 10.1097/00006454-200009000-00034. [DOI] [PubMed] [Google Scholar]
- 2.Juhn SK, Jung TT, Lin J, Rhee CK. Effects of inflammatory mediators on middle ear pathology and on inner ear function. Annals of the New York Academy of Sciences. 1997;830:130–142. doi: 10.1111/j.1749-6632.1997.tb51885.x. [DOI] [PubMed] [Google Scholar]
- 3.Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur Cytokine Netw. 2002;13:161–172. [PubMed] [Google Scholar]
- 4.Cureoglu S, Schachern P, Rinaldo A, Tsuprun V, Ferlito A, Paparella M. Round window membrane and labyrinthine pathological changes: an overview. Acta Oto-Laryngologica. 2005;125:9–15. doi: 10.1080/00016480410022534. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhoff WL, Kim CS, Paparella MM. Pathology of chronic otitis media. The Annals of Otology, Rhinology & Laryngology. 1978;87:749–760. doi: 10.1177/000348947808700602. [DOI] [PubMed] [Google Scholar]
- 6.Paparella MM, Morizono T, Le CT, et al. Sensorineural hearing loss in otitis media. The Annals of Otology, Rhinology & Laryngology. 1984;93:623–629. doi: 10.1177/000348948409300616. [DOI] [PubMed] [Google Scholar]
- 7.Schachern P, Tsuprun V, Cureoglu S, et al. The round window membrane in otitis media: effect of pneumococcal proteins. Arch Otolaryngol Head Neck Surg. 2008;134:658–662. doi: 10.1001/archotol.134.6.658. [DOI] [PubMed] [Google Scholar]
- 8.Moon S, Woo J-I, Lee H-Y, et al. Toll-like receptor 2-dependent NF-kappaB activation is involved in nontypeable Haemophilus influenzae-induced monocyte chemotactic protein 1 up-regulation in the spiral ligament fibrocytes of the inner ear. Infection and immunity. 2007;75:3361–3372. doi: 10.1128/IAI.01886-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine acute otitis media. Laryngoscope. 2007;117:22–29. doi: 10.1097/01.mlg.0000240170.48584.73. [DOI] [PubMed] [Google Scholar]
- 10.Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine chronic otitis media. Otolaryngol Head Neck Surg. 2007;137:332–337. doi: 10.1016/j.otohns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 11.MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear Res. 2006;219:12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 12.MacArthur CJ, Hefeneider SH, Kempton JB, Trune DR. C3H/HeJ mouse model for spontaneous chronic otitis media. Laryngoscope. 2006;116:1071–1079. doi: 10.1097/01.mlg.0000224527.41288.c4. [DOI] [PubMed] [Google Scholar]
- 13.MacArthur CJ, Kempton JB, DeGagne J, Trune DR. Control of chronic otitis media and sensorineural hearing loss in C3H/HeJ mice: glucocorticoids vs mineralocorticoids. Otolaryngol Head Neck Surg. 2008;139:646–653. doi: 10.1016/j.otohns.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacArthur C, Pillers D-A, Pang J, Degagne J, Kempton JB, Trune D. Gram-negative pathogen Klebsiella oxytoca is associated with spontaneous chronic otitis media in Toll-like receptor 4-deficient C3H/HeJ mice. Acta Oto-Laryngologica. 2008;128:132–138. doi: 10.1080/00016480701387124. [DOI] [PubMed] [Google Scholar]
- 15.Juhn SK, Jung MK, Hoffman MD, et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008;1:117–138. doi: 10.3342/ceo.2008.1.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.DeMaria TF. Localization of Nontypeable Haemophilus influenzae Endotoxin in the Middle and Inner Ear During Experimental Otitis Media. Acta Oto-Laryngologica. 1999;119:583–587. doi: 10.1080/00016489950180838. [DOI] [PubMed] [Google Scholar]
- 18.Adams JC. Immunocytochemical traits of type IV fibrocytes and their possible relations to cochlear function and pathology. J Assoc Res Otolaryngol. 2009;10:369–382. doi: 10.1007/s10162-009-0165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly KA, Hunter LL, Levine SC, Lindgren BR, Giebink GS. Relationships between otitis media sequelae and age. The Laryngoscope. 1998;108:1306–1310. doi: 10.1097/00005537-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Cureoglu S, PA S, Paparella M, Lindgren B. Cochlear changes in chronic otitis media. Laryngoscope. 2004;114:622–626. doi: 10.1097/00005537-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Papp Z, Rezes S, Jokay I, et al. Sensorineural hearing loss in chronic otitis media. Otology & Neurotology. 2003;24:141–144. doi: 10.1097/00129492-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Djeric DR, Schachern PA, Paparella MM, Jaramillo M, Haruna S, Bassioni M. Otitis media (silent): A potential cause of childhood meningitis. The Laryngoscope. 1994;104:1453–1460. doi: 10.1288/00005537-7990632. [DOI] [PubMed] [Google Scholar]
- 23.Goycoolea MV. Oval and round window membrane changes in otitis media in the human An ultrastructural study. Acta Otolaryngol. 1995;115:282–285. doi: 10.3109/00016489509139310. [DOI] [PubMed] [Google Scholar]
- 24.Schachern P, Paparella M, Hybertson R, Sano S, Duvall A. Bacterial tympanogenic labyrinthitis, meningitis and sensorineural damage. Arch Otolaryngol Head Neck Surg. 1992;118:53–57. doi: 10.1001/archotol.1992.01880010057016. [DOI] [PubMed] [Google Scholar]
- 25.Lundman L, Santi PA, Morizono T, Harada T, Juhn SK, Bagger-Sjoback D. Inner ear damage and passage through the round window membrane of Pseudomonas aeruginosa exotoxin A in a chinchilla model. Ann Otol Rhinol Laryngol. 1992;101:437–444. doi: 10.1177/000348949210100511. [DOI] [PubMed] [Google Scholar]
- 26.Moon SK, Park R, Lee HY, et al. Spiral ligament fibrocytes release chemokines in response to otitis media pathogens. Acta Otolaryngol. 2006;126:564–569. doi: 10.1080/00016480500452525. [DOI] [PubMed] [Google Scholar]