Abstract
Objective
To measure the change in quality-of-life (QoL) after endoscopic sinus surgery (ESS) in patients with medically recalcitrant chronic rhinosinusitis (CRS) and minimally affected computed tomography (CT) scans of the paranasal sinuses.
Study Design
Prospective, multi-center cohort study.
Setting
Three academic, tertiary care centers.
Subjects and Methods
A total of 778 patients with CRS were enrolled between January, 2001 and April, 2009 after electing ESS. For the purposes of this analysis, patients with nasal polyposis, history of prior sinus surgery, or follow up less than 6 months were excluded. Final study patients were categorized as low-stage CT CRS (Lund-Mackay ≤ 3; n=17) and high-stage CT CRS (Lund-MacKay > 3; n=207). Primary outcome measures included two disease-specific QoL instruments: the Rhinosinusitis Disability Index (RSDI) and the Chronic Sinusitis Survey (CSS).
Results
In patients with low-stage CT CRS, a statistically significant improvement was found across all disease-specific QoL scores (all p ≤ 0.012), with the exception of the CSS medication usage subscale (p=0.073). These QoL improvements were comparable to those in patients with high-stage CT CRS.
Conclusion
Some patients will present with CRS that is refractory to medical therapy even though their CT demonstrates relatively minimal disease. Based on the results of this study, ESS is associated with improved QoL in patients with low-stage CT CRS and can provide significant benefit to carefully selected patients with minimally affected CT scans.
Level of Evidence
2c
Keywords: Computed tomography, low-stage, quality-of-life, endoscopic, surgery, chronic rhinosinusitis, sinusitis, disease severity
Introduction
Chronic Rhinosinusitis (CRS) is a disease characterized by persistent sinonasal mucosal inflammation producing both symptomatic and objective evidence of inflammation. In 2007, Rosenfeld et al. published Clinical Guidelines for Adult Sinusitis,1 which updated and refined the diagnostic criteria for CRS. An important component to the CRS diagnostic criteria was the requirement of clinically observed empirical evidence of inflammation. These findings include one or more of the following: middle meatal mucopurulence or edema, nasal polyps, or radiographic imaging of the paranasal sinuses showing inflammation.
Using the latest clinical guidelines, it is possible to diagnose CRS in the presence of a normal or minimally irregular CT scan. Due to the fact that recalcitrant CRS with a minimally irregular CT is a relatively small subset, surgical outcomes for this subgroup have not been sufficiently evaluated. Etiologies of CRS with minimally irregular CT may include: low imaging sensitivity to detect symptomatic mucosal inflammation, variable patient-related symptom thresholds, and non-rhinosinusitis pathology mimicking CRS symptoms. Furthermore, investigations into the associations between CT stage and clinical outcomes have been inconsistent. A study by Stewart et al.2 demonstrated that CT severity correlated with symptom improvement following treatment, while other studies have demonstrated a poor correlation between CRS CT staging and both symptom severity and clinical outcomes after ESS.3–8 Although the correlation was small, a study by Bhattacharyya et al. evaluated three separate CT staging systems and demonstrated that the Lund-Mackay staging system correlated most with sinonasal symptoms.4
Most otolaryngologists have encountered patients with symptoms and endoscopic findings suggestive of CRS, however CT evaluation demonstrates minimal mucosal disease. Despite failure of medical therapy and elimination of non-rhinologic etiologies, there is often a reluctance to perform ESS in the setting of a low-stage CT scan. The primary objective of this study was to evaluate quality-of-life (QoL) outcomes of CRS patients with low-stage CT scans who underwent ESS after failed medical therapy. Our hypothesis was that CRS patients with failed medical therapy and low-stage CT scans will experience improved QoL after ESS similar to that of patients with high-stage CT scores.
Methods
Study Population and Data Collection
Study subjects were recruited from three tertiary rhinology clinics during enrollment for a prospective, multi-institutional cohort study between January, 2001 and April, 2009. Overall findings from this cohort have been previously reported.9,10 Inclusion criteria consisted of: 1) age > 18 years, 2) CRS defined by the Task Force Criteria11, and 3) sinonasal symptoms failed to resolve after medical therapy including, but not limited to, three or more weeks of culture-directed or broad-spectrum antibiotics and at least one trial of systemic corticosteroid therapy. Exclusion criteria included: 1) Follow-up < 6 months, 2) nasal polyposis, and 3) history of prior sinus surgery. Nasal polyposis and history of prior surgery were excluded since inclusion would potentially create a bias in that patients with polyposis would likely be high-stage CT and history of prior surgery confounds the CT staging system since the patency of the osteomeatal complex would have been affected by prior surgery.
Low-stage CT was defined as having a Lund-Mackay score of 3 or less. High-stage CT was defined as having a CT score of 4 or more.12 Where warranted, patients with low-stage CT were referred for evaluation to a headache/ pain specialist to rule out non-rhinologic etiologies for symptoms. We have previously found that improvements in postoperative QoL scores are stable between 6 and 20 months, therefore a minimum 6-month follow-up was necessary for inclusion.13 Informed consent and study protocol documents were approved by Institutional Review Boards at each enrollment site.
Preoperative data collection was documented by the enrolling physician at each enrollment site from patient history/medical chart review and included patient characteristics such as age, gender, preoperative CT and endoscopic exam findings, and CRS cofactors such as history of prior sinus surgery, asthma, acetylsalicylic acid (ASA) intolerance, allergy (confirmed by skin-prick testing or mRAST), depression, current tobacco usage, and nasal polyposis.
The enrolling physician at each enrollment site performed all pre- and post-operative patient assessments. Computed tomography and endoscopy were scored using the Lund-Mackay and Lund-Kennedy scoring methods, respectively14,15. The Lund Mackay staging system is a measure of the degree of opacification in the maxillary, sphenoid, ethmoid, osteomeatal complex, and frontal sinus regions (score range: 0–24). The Lund-Kennedy scoring method quantifies pathologic states within the paranasal sinuses including nasal polyposis, mucosal discharge, edema, crusting, and scarring (score range: 0–20).
Quality of Life Evaluation
All study patients were asked to complete two disease-specific QoL surveys pre-operatively and at each post-operative visit for the duration of the study. The Rhinosinusitis Disability Index (RSDI) is a 30-question survey comprised of three individual subscales to measure the impact of sinus disease on the physical, functional, and emotional domains on a continuum (score range: 0–120).16 Higher RSDI total and subscale scores represent a higher impact of disease. The Chronic Sinusitis Survey (CSS) is a 6-question survey designed to measure sinusitis-specific symptoms and medication use within the preceding 8-week period (score range: 0–100).17 Lower total and subscale scores indicate a greater impact of CRS. A trained research coordinator assisted each patient in the completion of both QoL surveys and the enrolling physician was blinded to QoL responses for the study entirety. The main outcome of interest was operationalized by the change in QoL scores as measured by the RSDI and CSS total and subscale scores (last post-operative score minus pre-operative score).
Statistical Analysis
Data were collected, transcribed, and manually scored after each clinic visit by the research coordinator on standardized clinical research forms. All data was deidentified and securely stored in a relational database during the collection period (Microsoft FoxPro; Microsoft Corp., Redmond, WA.). Statistical analysis was accomplished using SPSS statistical software (version 17.0; SPSS Inc., Chicago, IL.). Descriptive statistics (means, standard deviations, 95% confidence interals, and frequencies) and distributions were assessed for all patient cofactors, surgical procedures, and QoL outcome variables. Paired t-tests were used to test for significant improvement in mean QoL between preoperative scores and follow-up responses over time. Independent sample t-tests were used to assess differences in mean QoL improvement between cohorts low and high-stage CT patients. Independent sample t-tests and chi-square tests were also used to assess significant differences in the frequency of baseline patient cofactors between low and high-stage CT groups.
Results
Baseline Characteristics
A total of 778 patients with medically recalcitrant CRS were initially enrolled. After the appropriate exclusion criteria were applied, a total of 17 patients with low-stage CT CRS and 207 patients with high-grade CT CRS were included in this study for final analysis (Figure 1). Baseline characteristics for the cohorts are outlined in Table 1. Characteristics between the low and high-stage CT CRS groups were similar with the exception of the mean Lund-Kennedy endoscopy scores, which were lower in the low-stage CT cohort (1.7 vs. 3.4; p=0.008).
Figure 1.
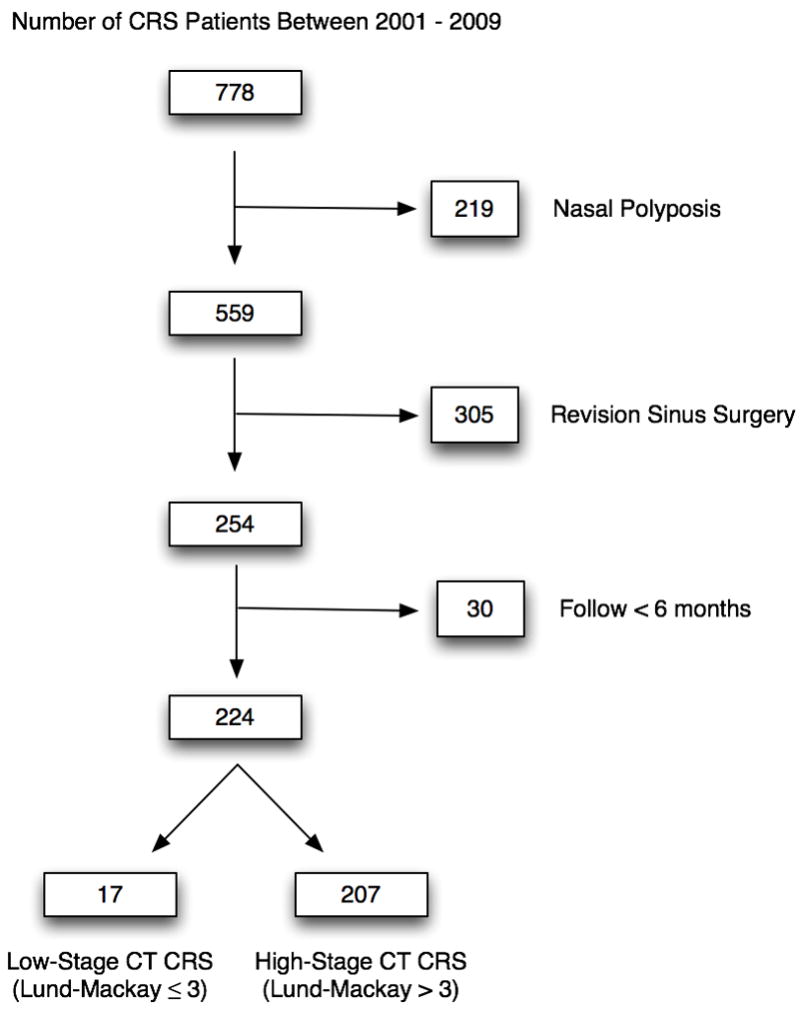
Total baseline enrollment and exclusion criteria for low- and high-stage CT CRS
Table 1.
Baseline characteristics for low-stage CT cohort (n=17) vs. high stage CT (n=207)
| Characteristics | Low-stage CT | High-stage CT | p-value | ||
|---|---|---|---|---|---|
| Mean (SD) | n(%) | Mean (SD) | n(%) | ||
| Follow-up (mo.) | 16.0 (7.7) | 17.4 (7.3) | 0.312 | ||
| Age | 45.8 (143.5) | 44.5 (13.3) | 0.687 | ||
| Gender | |||||
| Male | 4 (23.5) | 84 (40.6) | |||
| Female | 13 (76.5) | 123 (59.4) | 0.203 | ||
| Asthma | 6 (35.3) | 50 (24.2) | 0.308 | ||
| ASA Intolerance | 2 (11.8) | 4 (1.9) | 0.068 | ||
| Allergy | 7 (41.2) | 54 (26.1) | 0.179 | ||
| Depression | 5 (29.4) | 33 (15.9) | 0.155 | ||
| Current Smoker | 2 (11.8) | 20 (9.7) | 0.677 | ||
| Lund-MacKay CT Score | 1.8 (1.0) | 9.5 (5.3) | <0.001 | ||
| Lund-Kennedy Endoscopy | |||||
| Score | 1.7 (1.9) | 3.4 (2.7) | 0.008 | ||
CT = computed tomography; SD = standard deviation; mo. = months; ASA = acetylsalicylic acid. A p-value <0.05 indicates statistically significant differences.
Surgical Procedures
Patients in the low-stage CT CRS cohort commonly underwent bilateral surgery (64.7%), and maxillary antrostomy and partial ethmoidectomy were the most common procedures. For the high-stage CT CRS cohort, the majority underwent bilateral surgery (76.3%), and maxillary antrostomy and total ethmoidectomy were the most common procedures.
Quality-of-Life Outcomes
Low-stage CT CRS pre-operative mean scores for the total RSDI and CSS were 41.7(17.5) and 38.5(19.9), respectively. These pre-operative QoL values are comparable to previously published CRS cohorts10,18. Following ESS for low-stage CT CRS, a statistically significant improvement was found across all disease-specific QoL total and subscale mean scores (all p ≤ 0.012; Table 2), with the exception of CSS medication use (p=0.073).
Table 2.
Mean pre-operative and post-operative quality-of-life scores for low-stage CT CRS (n=17)
| Outcome measure: | Pre-operative | Post-operative | p-value | ||
|---|---|---|---|---|---|
| Mean (SD) | [range] | Mean (SD) | [range] | ||
| RSDI physical | 16.5 (7.0) | [6, 27] | 9.2 (6.7) | [0, 22] | 0.002 |
| RSDI functional | 13.3 (6.0) | [4, 23] | 8.5 (6.7) | [0, 24] | 0.001 |
| RSDI emotional | 11.8 (7.4) | [0, 26] | 6.5 (6.9) | [0, 25] | 0.004 |
| RSDI total | 41.7 (17.5) | [11, 75] | 24.1 (19.0) | [0, 64] | <0.001 |
| CSS symptom | 33.3 (20.4) | [0, 75] | 57.8 (29.2) | [0, 91.7] | 0.011 |
| CSS medication | 43.6 (25.6) | [0, 83.3] | 56.4 (31.3) | [0, 100] | 0.073 |
| CSS total | 38.5 (19.9) | [4.2, 75] | 57.1 (25.3) | [0, 96] | 0.012 |
SD = standard deviation; RSDI = Rhinosinusitis Disability Index; CSS = Chronic Sinusitis Survey; A p-value < 0.05 indicates statistically significant improvement over time.
When evaluating both the low and high-stage CT cohorts, both groups demonstrated QoL improvement after ESS. Comparing mean QoL differences in post-operative improvement for both RSDI and CSS total scores, there was no difference between low and high-stage CT cohorts (p=0.201 and p=0.178, respectively; Table 3). This suggests that patients with low-stage CT CRS experience similar overall QoL improvements as patients with high-stage CT CRS.
Table 3.
Mean improvements in quality-of-life scores between low-stage and high-stage CT CRS
| Outcome measure: | Low-stage CT (n=17) | High-stage CT (n=207) | p-value | ||
|---|---|---|---|---|---|
| Mean (SD) | [range: LL, UL] | Mean (SD) | [range: LL, UL] | ||
| RSDI physical | −7.4 (7.0) | [−19, 4] | −8.8 (8.3) | [−27, 19] | 0.406 |
| RSDI functional | −4.8 (4.6) | [−15, 3] | −8.2 (7.7) | [−30, 17] | 0.046 |
| RSDI emotional | −5.4 (6.6) | [−18, 5] | −6.0 (7.5) | [−27, 14] | 0.605 |
| RSDI total | −17.5 (15.4) | [−49, 2] | −22.9 (21.0) | [−78, 33] | 0.201 |
| CSS symptom | 24.5 (30.5) | [−17, 83] | 35.1 (32.6) | [−67, 100] | 0.170 |
| CSS medication | 12.7 (26.4) | [−25, 75] | 19.0 (32.3) | [−92, 100] | 0.399 |
| CSS total | 18.6 (26.1) | [−17, 71] | 27.0 (25.6) | [−45, 96] | 0.178 |
SD = standard deviation; CT=computed tomography; LL = 95% confidence interval lower limit; UL=95% confidence interval upper limit; RSDI = Rhinosinusitis Disability Index; CSS = Chronic Sinusitis Survey.
Discussion
In this study, we have shown that patients with chronic rhinosinusitis (CRS) and low-stage CT are a small subset of patients undergoing primary endoscopic sinus surgery (ESS) in a large multi-institutional cohort. Baseline characteristics are similar to their high-stage CT counterparts with the exception of a worse endoscopy score in patients with high-stage CT CRS. Interestingly, baseline disease-specific QoL is not different between the cohorts and patients with low-stage CT CRS improve in disease specific QoL after ESS nearly to the same degree as patients with high-stage CT scores.
Chronic rhinosinusitis is commonly associated with abnormal CT findings, including mucosal thickening, bony changes, and sinus opacification. Treatment is primarily with medical therapy while ESS is reserved for persistent symptoms despite medical efforts. While several recent studies have demonstrated improvement in QoL following ESS, none of these studies specifically evaluated the small subgroup of patients with CRS and minimally affected CT scans.10,19–22
Using the 1997 Task Force definition of CRS, Hwang et al.7 demonstrated that 35% of patients fulfilling the diagnosis for CRS had normal CT scans. In 2007, a study by Hopkins et al. demonstrated that 21% of patients undergoing ESS for medically refractory CRS had Lund-Mackay scores within the normal range.3 Our study has identified a 6% frequency of low-stage CT CRS undergoing primary ESS in a large multi-institutional cohort, which is a lower prevalence than the previously published findings. However, we used a lower Lund-Mackay score cut-off than other studies, as scores of four were not included into our low-stage CT group. Despite some differences in the prevalence of low stage CT CRS, this patient population can present a challenging clinical dilemma since there is often a reluctance to perform surgery for CRS with low-stage CT.
Overall, patients suffering from CRS experience QoL improvement following ESS.9,10,17,19–23 The majority of patients in prior studies had elevated Lund-Mackay scores; therefore it would not have been appropriate to conclude that patients with low-stage CT CRS experience similar improvements. The results from this study suggest that, similar to patients with high-stage CT CRS, carefully selected patients with low-stage CT CRS experience a significant QoL benefit from ESS.
An important component in the evaluation of patients with low-stage CT is the consideration of possible non-rhinologic etiologies of symptoms. The symptoms of CRS can mimic several other facial pain etiologies, such as migraine and neuralgia. In the 2007 Sinus, Allergy, and Migraine Study (SAMS)24, 97% of self diagnosed “sinus headaches” were actually some form of headache syndrome. Migraine or probable migraine constituted 85% of “sinus headaches.” Another facial pain etiology to consider is Sluder syndrome or Sphenopalatine Neuralgia. It commonly presents with facial pain in the territory of the paranasal sinuses and produces some degree of nasal autonomic dysfunction inducing nasal congestion and rhinorrhea.25 A simple diagnostic technique that may be employed in the clinic involves topically anesthetizing the sphenopalatine region to see if an improvement in pain occurs. A positive test suggests a neuropathic pain etiology.26 A diligent work-up with both nasal endoscopy and consultation with facial pain experts are usually necessary to ensure a confounding etiology is not mimicking CRS.
There are a few limitations of this study to consider when evaluating these findings: First, the dichotomization of low-stage CT (score range: 0 – 3) vs. high-stage CT (score range: 4 – 24) is somewhat arbitrary but based upon current evidence which suggests that low Lund-Mackay scores can be considered an incidental finding within the normal, non-CRS population.12 Secondly, the opacification of a single sinus resulting in a low-stage CT score may represent a different pathological entity as compared to non-opacifying inflammation in two or three sinuses also resulting in a low-stage CT score. Further evaluation of our low-stage CT cohort found that only 2/17 cases were isolated unilateral sinus pathology (sphenoid (n=1); frontal (n=1)). Elimination of these cases in the analyses did not change the results. Despite these possible study limitations, we feel this study is strengthened through the use of stringent CRS diagnostic criteria, a multi-institutional design, and use of validated survey instruments.
In a time of changing health care delivery, clinicians must take the lead in managing finite clinical resources. The clinical benefit of ESS in patients with low-stage CT CRS has been questioned and there may be a reluctance to perform ESS in such patients. This reluctance may stem from the assumption that patients with recalcitrant symptoms should have objective CT evidence of severe inflammation. However, the published literature would refute this assumption since symptoms and QoL do not correlate well with CT stage.3,5–8 Furthermore, some clinicians may fear medicolegal fallout from intra- or post-operative complications arising in patients with low-stage CT CRS since others, with the benefit of hindsight, might suggest that surgery was unnecessary based on the low-stage pre-operative CT findings. Careful documentation of a diligent workup and discussion with the patient regarding their clinical dilemma is important.
Although this study has demonstrated that ESS can improve disease-specific QoL in recalcitrant low-stage CT CRS, patient selection is imperative. Overall, this unique subgroup represented a small fraction of patients presenting for ESS. An appropriate trial of medical therapy is warranted and a diligent preoperative work-up to exclude non-rhinologic disease processes that mimic CRS should be considered. Proper patient selection will maximize both post-operative clinical success and health care resource utilization.
Conclusion
This study evaluates a relatively small subset of CRS patients with low-stage CT undergoing ESS. After failed medical therapy and exclusion of other potential etiologies, patients with low-stage CT CRS can present a challenging dilemma given the minimal findings on CT and general reluctance to perform ESS since the potential post-operative clinical benefit is commonly questioned. Based on the results of this study, ESS is associated with improved QoL in carefully evaluated and selected patients with low-stage CT CRS.
Acknowledgments
Supported by grant funding from the NIH/NIDCD R01 DC005805 (PI/PD: Smith, TL).
Footnotes
The Institutional Review Board at Oregon Health & Science University provided approval and oversight for all investigational protocols and annual review.
Public clinical trial registration (http://www.clinicaltrials.gov) ID: NCT00799097
Conflict of Interest: There is no conflict of interest for Luke Rudmik, MD.
Potential conflicts of interest exist as Timothy L. Smith, MD, MPH and Jess Mace, MPH, were funded by grant support from the NIH/NIDCD. Timothy L. Smith is also a consultant for Intersect and Entrigue which provided no financial support for this investigation.
References
- 1.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 2.Stewart MG, Donovan DT, Parke RB, Jr, et al. Does the severity of sinus computed tomography findings predict outcome in chronic sinusitis? Otolaryngol Head Neck Surg. 2000;123:81–4. doi: 10.1067/mhn.2000.105922. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins C, Browne JP, Slack R, et al. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007;137:555–61. doi: 10.1016/j.otohns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya N. A comparison of symptom scores and radiographic staging systems in chronic rhinosinusitis. Am J Rhinol. 2005;19:175–9. [PubMed] [Google Scholar]
- 5.Basu S, Georgalas C, Kumar BN, et al. Correlation between symptoms and radiological findings in patients with chronic rhinosinusitis: an evaluation study using the Sinonasal Assessment Questionnaire and Lund-Mackay grading system. Eur Arch Otorhinolaryngol. 2005;262:751–4. doi: 10.1007/s00405-004-0891-0. [DOI] [PubMed] [Google Scholar]
- 6.Stewart MG, Johnson RF. Chronic sinusitis: symptoms versus CT scan findings. Curr Opin Otolaryngol Head Neck Surg. 2004;12:27–9. doi: 10.1097/00020840-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hwang PH, Irwin SB, Griest SE, et al. Radiologic correlates of symptom-based diagnostic criteria for chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2003;128:489–96. doi: 10.1016/S0194-59980223295-7. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya N. Radiographic stage fails to predict symptom outcomes after endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2006;116:18–22. doi: 10.1097/01.mlg.0000192284.22703.04. [DOI] [PubMed] [Google Scholar]
- 9.Smith TL, Mendolia-Loffredo S, Loehrl TA, et al. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005;115:2199–205. doi: 10.1097/01.mlg.0000182825.82910.80. [DOI] [PubMed] [Google Scholar]
- 10.Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: a multi-institutional prospective cohort study. Otolaryngol Head Neck Surg. 2010;142:55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 12.Ashraf N, Bhattacharyya N. Determination of the “incidental” Lund score for the staging of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2001;125:483–6. doi: 10.1067/mhn.2001.119324. [DOI] [PubMed] [Google Scholar]
- 13.Soler ZM, Smith TL. Quality-of-life outcomes after endoscopic sinus surgery. How long is enough? Otolaryngol Head Neck Surg. 2010 doi: 10.1016/j.otohns.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 15.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–4. [PubMed] [Google Scholar]
- 16.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997;123:1175–9. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 17.Gliklich RE, Metson R. Effect of sinus surgery on quality of life. Otolaryngol Head Neck Surg. 1997;117:12–7. doi: 10.1016/S0194-59989770199-2. [DOI] [PubMed] [Google Scholar]
- 18.Birch DS, Saleh HA, Wodehouse T, et al. Assessing the quality of life for patients with chronic rhinosinusitis using the “Rhinosinusitis Disability Index”. Rhinology. 2001;39:191–6. [PubMed] [Google Scholar]
- 19.Macdonald KI, McNally JD, Massoud E. Quality of life and impact of surgery on patients with chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2009;3(8):286–93. [PubMed] [Google Scholar]
- 20.Chester AC. Symptom outcomes following endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2009;17:50–8. doi: 10.1097/MOO.0b013e32831b9e2a. [DOI] [PubMed] [Google Scholar]
- 21.Mace J, Michael YL, Carlson NE, et al. Effects of depression on quality of life improvement after endoscopic sinus surgery. Laryngoscope. 2008;118:528–34. doi: 10.1097/MLG.0b013e31815d74bb. [DOI] [PubMed] [Google Scholar]
- 22.Litvack JR, Griest S, James KE, et al. Endoscopic and quality-of-life outcomes after revision endoscopic sinus surgery. Laryngoscope. 2007;117:2233–8. doi: 10.1097/MLG.0b013e31814539e8. [DOI] [PubMed] [Google Scholar]
- 23.Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. Am J Rhinol. 2008;22:297–301. doi: 10.2500/ajr.2008.22.3172. [DOI] [PubMed] [Google Scholar]
- 24.Eross E, Dodick D, Eross M. The Sinus, Allergy and Migraine Study (SAMS) Headache. 2007;47:213–24. doi: 10.1111/j.1526-4610.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 25.Ahamed SH, Jones NS. What is Sluder’s neuralgia? J Laryngol Otol. 2003;117:437–43. doi: 10.1258/002221503321892253. [DOI] [PubMed] [Google Scholar]
- 26.Kanai A, Suzuki A, Kobayashi M, et al. Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br J Anaesth. 2006;97:559–63. doi: 10.1093/bja/ael180. [DOI] [PubMed] [Google Scholar]


