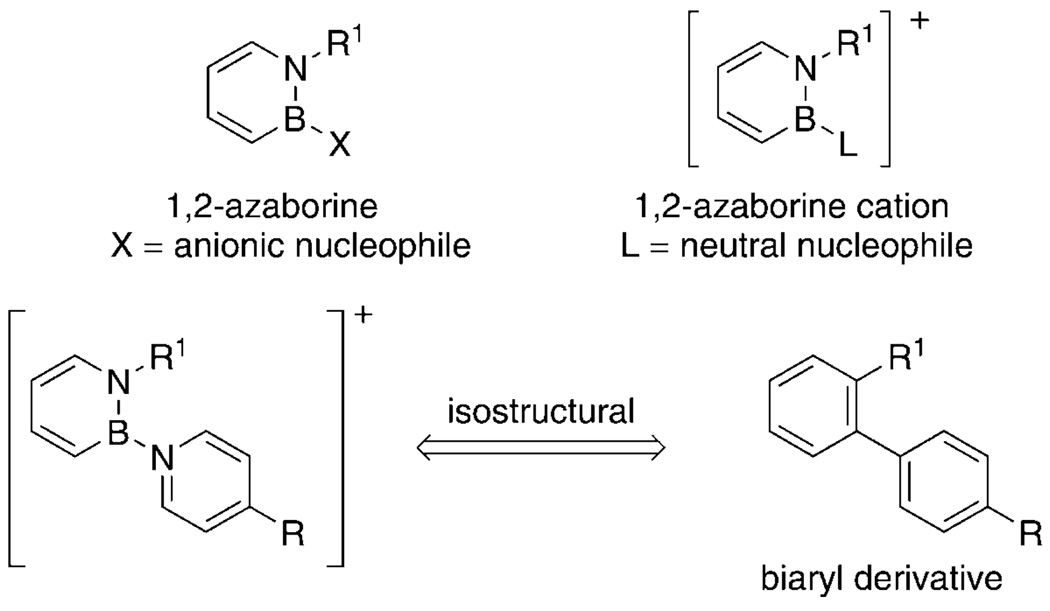
1,2-Dihydro-1,2-azaborine is a six-membered aromatic heterocycle that is isoelectronic with benzene through the replacement of a C=C unit in benzene with an isoelectronic B–N unit.[1,2] Since the pioneering work by Dewar et al.,[3,4] significant advances have been made in the synthesis and reactivity studies of this family of heterocycles.[5–7] Our continued exploration of the 1,2-azaborine motif[8–15] has led us to consider the synthesis of cationic 1,2-azaborines, for which no examples have been reported. In particular, we envisioned that substitution of 1,2-azaborine on the boron atom with pyridine derivatives would furnish cationic biaryl-type structures[16] having the potential for use in materials applications (Scheme 1). Herein we report the synthesis, structural characterization, and optoelectronic properties of pyridine-substituted 1,2-azaborine cations, including a cationic heterocyclic analogue of para-terphenyl.
Scheme 1.
1,2-Azaborine cations.
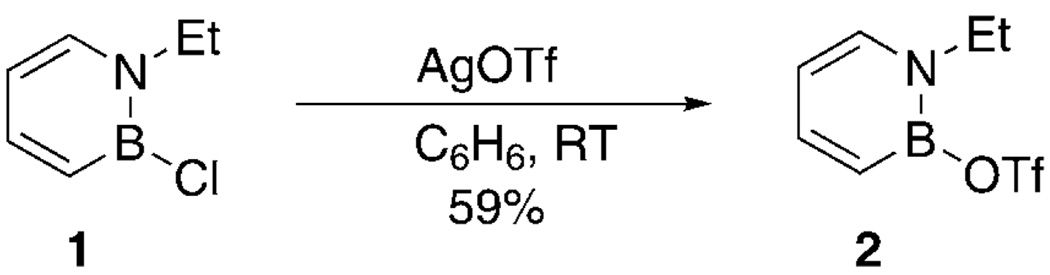
We have previously established nucleophilic substitution of the B–Cl bond in 1,2-azaborines by anionic nucleophiles (with Cl− serving as the leaving group).[8] Less reactive neutral nucleophiles did not displace the chloride from the boron atom. We hypothesized that a better leaving group on the boron atom (e.g., OTf) could render it susceptible to nucleophilic attack by weaker neutral nucleophiles. In the course of our studies, we discovered that silver reagents facilitate the ligand exchange at the boron position in 1,2-azaborines.[13] We were thus pleased to discover that treatment of 1,2-azaborine 1 with AgOTf produced the substituted 1,2-azaborine 2 in 59% yield as an extremely moisture-sensitive liquid (Scheme 2). The 1,2-azaborine 2 was characterized by 1H, 11B, and 13C NMR spectroscopy as well as IR spectroscopy.
Scheme 2.
Synthesis of 2. Tf = trifluoromethanesulfonyl.
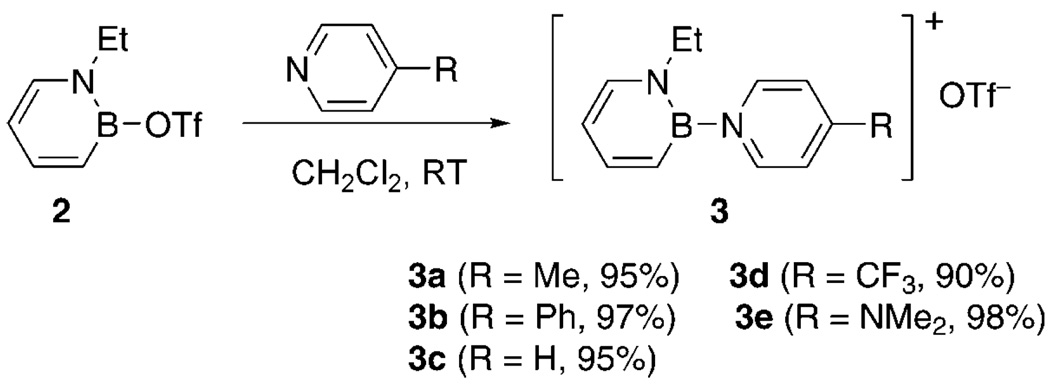
Heterocycle 2 readily reacts with para-substituted pyridines to form the desired cationic 1,2-azaborines 3. As can be seen from Scheme 3, the substitution reaction is independent of the electronic nature of the nucleophile. Excellent yields have been obtained with both electron-rich and electron-poor pyridines.
Scheme 3.
Synthesis of 1,2-azaborine cations 3.
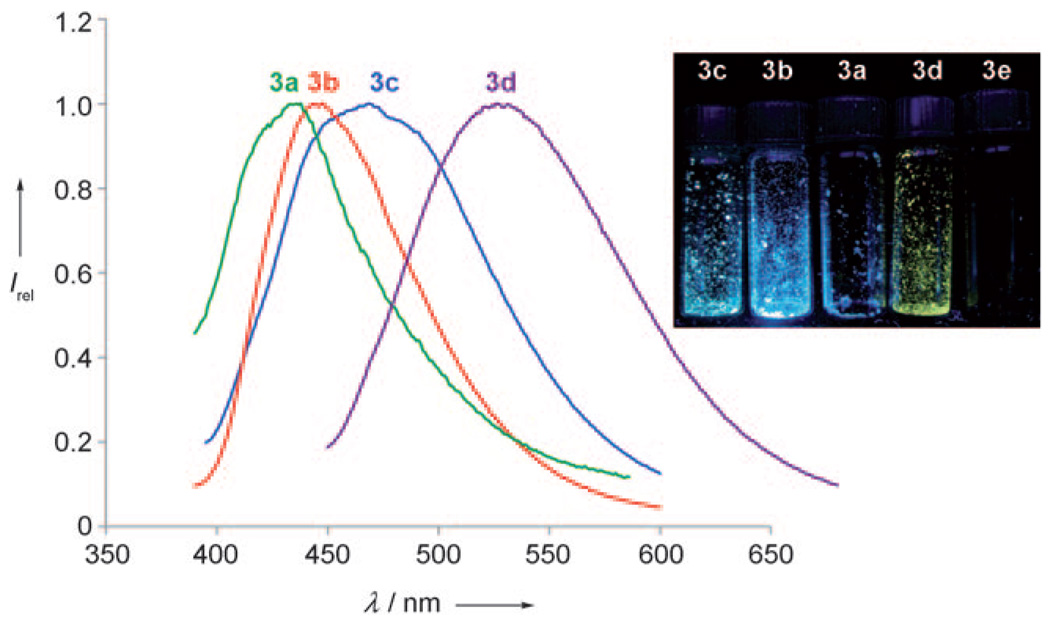
The 1,2-azaborine cations 3 are highly crystalline solids that fluoresce under UV light. We thus explored the solid-state fluorescence of the pyridine-substituted 1,2-azaborine cations 3. The solid-state fluorescence and quantum yields of aromatic hydrocarbons, including para-terphenyl, have been reported using an integrating sphere.[17] We have recorded the fluorescence spectra of crystalline samples of 3a–e (Figure 1), all of which were freshly recrystallized prior to making the fluorescence measurements. The solid-state fluorescence of 3a (R = Me) shows a peak at λem = 436 nm (ΦPL = 0.03) and is visibly less fluorescent than samples of 3b and 3c under a UV lamp (λ = 365 nm; see Figure 1, right). The solid-state emission spectrum of 3b (R = Ph) showed a relatively narrow band at λem = 448 nm (ΦPL = 0.86). The high quantum yield observed for 3b is quite similar to the values obtained for para-terphenyl, though the emission maximum of the cationic 3b is bathochromically shifted relative to the all-carbon para-terphenyl by approximately 75 nm.[17] We found that solid samples of 3c (R = H) were blue-green fluorescent (see Figure 1) with a relatively broad emission maximum at λem = 469 nm (ΦPL = 0.30). Compound 3d (R = CF3) exhibits yellow-green emission at λem = 527 nm (ΦPL = 0.11), which contrasts the blue emission observed for geometrically similar 3a. The data illustrated in Figure 1 suggest that the para substituent on pyridine has a substantial effect upon the emissive properties of 1,2-azaborine cations. DMAP-substituted 3e (R = NMe2;DMAP = 4-dimethylaminopyridine) does not fluoresce in the solid state (Figure 1, right), which is consistent with the reported fluorescence quenching by the presence of a nitrogen lone pair of electrons.[18]
Figure 1.
Normalized solid-state fluorescence spectra and images (under UV irradiation) of 1,2-azaborine cations 3.
We also determined the absorption properties of 1,2-azaborine cations 3 in solution. The absorption maximum of 3a (R = Me) in CH2Cl2 was found at λ = 287 nm with an extinction coefficient of ε = 12 713 m−1 cm−1. The absorption spectrum of 3b showed a broad, featureless peak at λ = 292 nm (ε = 21 869 m−1 cm−1), which is close to that observed for 3a, but is slightly bathochromically shifted from the absorption maximum of para-terphenyl (observed at λ = 280 nm in CH2Cl2).[19] The absorption spectrum of 3c (R = H) also showed a broad peak at λ = 286 nm (ε = 8624 m−1 cm−1). The observed absorption peaks of 3d at λ = 285 nm (ε = 8126 m−1 cm−1) and 3e at λ = 283 nm (ε = 21 303 m−1 cm−1) are relatively unchanged from the other derivatives of 3.
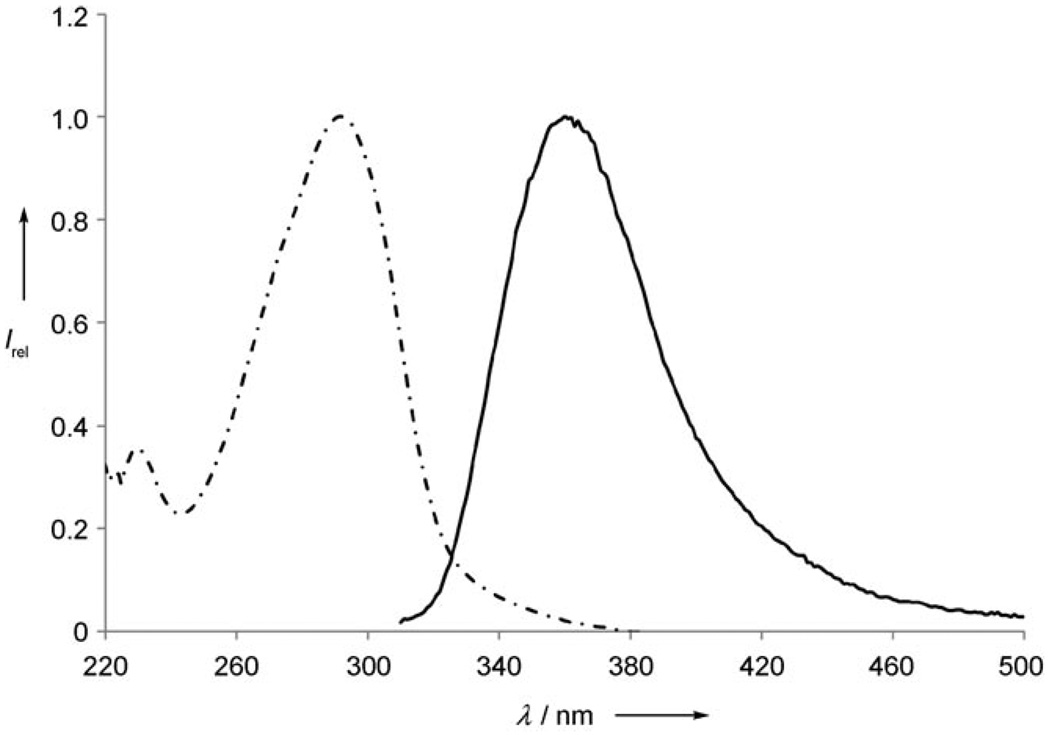
Interestingly, of the prepared cationic derivatives, only terphenyl analogue 3b was found to be fluorescent in solution. The fluorescence spectrum of 3b in CH2Cl2 showed an emission peak at λem = 360 nm (ΦPL = 0.06), and the absorption maximum was found at λ = 292 nm in CH2Cl2 (Figure 2). The large Stokes shift of 68 nm for 3b is indicative of considerable reorganization between the ground and the excited state. We also observed a bathochromic shift in the emission peak when MeCN was used as the solvent (λem = 382 nm, ΦPL = 0.17) instead of CH2Cl2. Furthermore, the fluorescence of 3b in MeCN was quenched upon the addition of NaI,[20] highlighting the potential of these materials in sensing applications.
Figure 2.
Normalized absorption (dashed line) and emission (solid line) spectra of 3b in CH2Cl2.
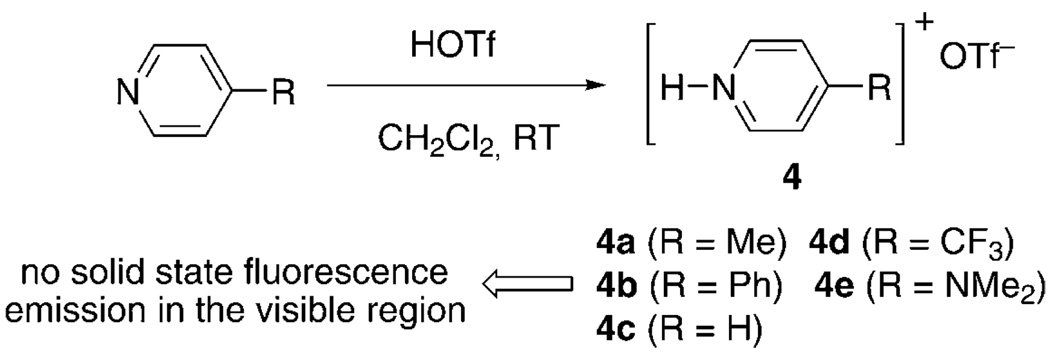
Table 1 summarizes the photophysical properties of pyridine-substituted 1,2-azaborine cations 3a–e. To assess whether the extended conjugation provided by the 1,2-azaborine ring is critical for the observed optoelectronic properties of the 1,2-azaborine cations 3, we prepared the corresponding protonated pyridinium species 4a–e (Scheme 4). We determined that under the same conditions for 3, pyridinium triflate salts 4a–e do not exhibit solid-state fluorescence emission in the visible region. This is consistent with a critical role of the 1,2-azaborine moiety in the observed emission properties of 1,2-azaborine cations 3.
Table 1.
Photophysical data for 3a–3e.
| Compound | Absorbance [nm][a] |
ε [m−1cm−1][a] |
Emission [nm] |
Φpl |
|---|---|---|---|---|
| 3a | 287 | 8126 | 436[b] | 0.03[b] |
| 3b | 292 | 21 869 | 448[b] | 0.86[b] |
| 360[a] | 0.06[a] | |||
| 382[c] | 0.17[c] | |||
| 3c | 286 | 8624 | 469 | 0.30[b] |
| 3d | 287 | 12713 | 527 | 0.11[b] |
| 3e | 283 | 21 303 | N/A | N/A |
In CH2Cl2 (10−5m).
Solid-state emission.
In MeCN (10−5m).
N/A = not applicable.
Scheme 4.
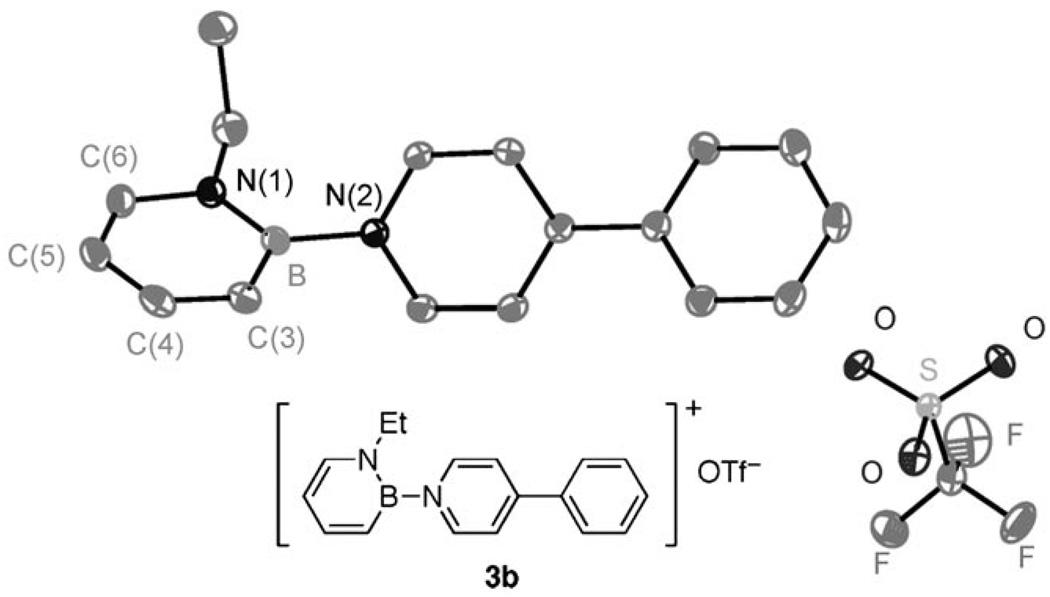
We have obtained the X-ray crystal structures of 1,2-azaborine cations 3 (except for 3c), thus unambiguously establishing their structural identity. As a representative example, the solid-state structure of 3b is illustrated in Figure 3 and reveals that the pyridine nitrogen atom is bound to the boron atom with the triflate group serving as a noncoordinating anion.[21] As expected, the dative exocyclic B–N bond (B-N(2) = 1.531(2) Å) in 3b is significantly longer than the covalent exocyclic B–NPh2 bond in a 1,2-azaborine recently reported in our group (B-N(2) = 1.486(2) Å).[9] The exocyclic B–N bond in cationic 3b is slightly shorter than the B–N bond in the charge-neutral borabenzene-4-phenylpyridine adduct (B-N = 1.551(3) Å) reported by Fu and coworkers.[22] The 1,2-azaborine ring in 3b is completely planar and is twisted by approximately 50° relative to the pyridine ring. In contrast, the phenyl ring of 3b is only slightly twisted relative to the pyridine ring (18°).
Figure 3.
ORTEP illustration of 3b, with thermal ellipsoids drawn at the 35% probability level (hydrogen atoms have been omitted for clarity). Bond distances (in Å): B-N(1) 1.413(2), B-N(2) 1.531(2), B-C(3) 1.496(2), C(3)-C(4) 1.369(2), C(4)-C(5) 1.408(3), C(5)-C(6) 1.358(2), C(6)-N(1) 1.3736(19); Torsion angles: 1,2-azaborine-pyridine = 50.5°; pyridine-phenyl = 18.0°.
We were also interested in examining the structural features of the 1,2-azaborine ring in 3b. The intra-ring B–N bond is short (B-N(1) = 1.413(2) Å), as is the intra-ring B–C bond (B-C(3) = 1.496(2) Å), which is consistent with bond distances observed for electron-deficient 1,2-azaborines.[13]
Selected bond parameters for 1,2-azaborine cations 3 are given in Table 2. The para substituent in the pyridine ring has little influence on the observed bond lengths, which are virtually identical for all derivatives. The torsion angles between the pyridine and 1,2-azaborine ring are similar for derivatives 3a, 3b, and 3d, although in 3e (R = NMe2) the 1,2-azaborine ring is nearly perpendicular to the pyridine ring. It noteworthy that the 1,2-azaborine cations 3 represent a new family of borenium cations for which only a few members have been structurally characterized by single-crystal diffraction.[23]
Table 2.
Selected bond distances [in Å] and angles [°] for 1,2-azaborine cations 3.
| Compound | B-N(1) [Å] | B-C(3) [Å] | B-N(2) [Å] | Ring torsion[a] |
|---|---|---|---|---|
| 3a | 1.418(5) | 1.481(5) | 1.528(5) | 57.1° |
| 3b | 1.413(2) | 1.496(2) | 1.531(2) | 50.5° |
| 3d | 1.416(4) | 1.489(4) | 1.526(4) | 58.0° |
| 3e | 1.423(5) | 1.489(6) | 1.527(5) | 77.8° |
Torsion angle between the 1,2-azaborine and pyridine ring.
In summary, we prepared the first examples of 1,2-azaborine cations through a nucleophilic substitution reaction between pyridine nucleophiles and the highly Lewis acidic 1,2-azaborine 2. 1,2-Azaborine cations 3a–3d exhibit solid-state fluorescence that is distinct from the neutral all-carbon analogues. Furthermore, 4-phenylpyridine-substituted 1,2- azaborine cation 3b, an analogue of terphenyl, displays solution-phase fluorescence in addition to solid-state emission. Control experiments establish the 1,2-azaborine ring as an essential component for the observed optoelectronic properties. This study highlights the unique properties of 1,2-azaborine cations and underscores the potential utility of these complexes in materials applications.
Experimental Section
3b: In a glove box, a solution of 4-phenylpyridine (0.073 g, 0.47 mmol in 1.0 mL CH2Cl2) was added to a stirred solution of 2 (0.100 g, 0.392 mmol in 1.0 mL CH2Cl2). The mixture was stirred for 1 h at room temperature. At the conclusion of the reaction, the solution was cooled to −20°C and left at that temperature for 24 h. The desired product precipitated out of the solution as a crystalline solid. The supernatant was decanted and the crystallized product was washed with n-pentane (3 × 5 mL). Residual solvents were removed under reduced pressure to provide 3b as clear, colorless crystals (0.155 g, 97%). 1H NMR (600 MHz, CD2Cl2): δ = 8.82 (d, 3JHH = 6.9 Hz, 2H), 8.44 (d, 3JHH = 6.9 Hz, 2H), 8.02 (dd, 3JHH = 9.8, 6.6 Hz, 1H), 7.97 (dd, 3JHH = 8.1 Hz, 4JHH = 1.7 Hz, 2H), 7.68 (m, 3H), 7.55 (d, 3JHH = 6.6, 1H), 6.85 (app t, 3JHH = 7.5 Hz, 2H), 3.83 (q, 3JHH = 7.3 Hz, 2H), 1.38 ppm (t, 3JHH = 7.3 Hz, 3H). 13C NMR (75 MHz, CD2Cl2): δ = 158.1, 149.5, 145.8, 139.6, 134.3, 133.2, 130.5, 128.7, 125.7, 124 (br), 115.7, 47.7, 18.2 ppm. 11B NMR (192.5 MHz, CD2Cl2): δ = 31.0 ppm. FTIR (thin film) 3220, 3138, 3078, 2915, 1638, 1612, 1513, 1488, 1474, 1442, 1412, 1377, 1349, 1292, 1233, 1218, 1174, 1151, 1029, 833, 765, 736, 693 cm−1. HRMS (EI) calcd for C7H9BNO3SF3 [M+] 255.03484, found 255.03528.
Supplementary Material
Footnotes
Support for this research has been provided by the University of Oregon and the National Institutes of Health (National Institute of General Medical Sciences, Grant R01-GM094541). Funding for the University of Oregon Chemistry Research and Instrumentation Services has been furnished in part by the National Science Foundation (CHE-0234965).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201004084.
References
- 1.Bosdet MJD, Piers WE. Can. J. Chem. 2009;87:8–29. [Google Scholar]
- 2.Liu Z, Marder TB. Angew. Chem. 2008;120:248–250. Angew. Chem. Int. Ed. 2008, 47, 242 – 244. [Google Scholar]
- 3.Dewar MJS, Marr PA. J. Am. Chem. Soc. 1962;84:3782. [Google Scholar]
- 4.Davies KM, Dewar MJS, Rona P. J. Am. Chem. Soc. 1967;89:6294–6297. [Google Scholar]
- 5.Ashe AJ, III, Fang X. Org. Lett. 2000;2:2089–2091. doi: 10.1021/ol0001113. [DOI] [PubMed] [Google Scholar]
- 6.Ashe AJ, III, Fang X, Fang X, Kampf JW. Organometallics. 2001;20:5413–5418. [Google Scholar]
- 7.Ashe AJ., III Organometallics. 2009;28:4236–4248. [Google Scholar]
- 8.Marwitz AJV, Abbey ER, Jenkins JT, Zakharov LN, Liu S-Y. Org. Lett. 2007;9:4905–4908. doi: 10.1021/ol702383u. [DOI] [PubMed] [Google Scholar]
- 9.Abbey ER, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2008;130:7250–7252. doi: 10.1021/ja8024966. [DOI] [PubMed] [Google Scholar]
- 10.Marwitz AJV, Matus MH, Zakharov LN, Dixon DA, Liu S-Y. Angew. Chem. 2009;121:991–995. doi: 10.1002/anie.200805554. Angew. Chem. Int. Ed. 2009, 48, 973 – 977. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Marwitz AJV, Matthews BW, Liu S-Y. Angew. Chem. 2009;121:6949–6951. Angew. Chem. Int. Ed. 2009, 48, 6817 – 6819. [Google Scholar]
- 12.Lamm AN, Liu S-Y. Mol. BioSyst. 2009;5:1303–1305. doi: 10.1039/b904120f. [DOI] [PubMed] [Google Scholar]
- 13.Marwitz AJV, McClintock SP, Zakharov LN, Liu S-Y. Chem. Commun. 2010;46:779–781. doi: 10.1039/b919632c. [DOI] [PubMed] [Google Scholar]
- 14.Campbell PG, Zakharov LN, Grant DJ, Dixon DA, Liu S-Y. J. Am. Chem. Soc. 2010;132:3289–3291. doi: 10.1021/ja9106622. [DOI] [PubMed] [Google Scholar]
- 15.Daly AM, Tanjaroon C, Marwitz AJV, Liu S-Y, Kukolich SG. J. Am. Chem. Soc. 2010;132:5501–5506. doi: 10.1021/ja1005338. [DOI] [PubMed] [Google Scholar]
- 16.For a neutral boron-containing biphenyl analogue, see: Boese R, Finke N, Henkelmann J, Maier G, Paetzold P, Reisenauer HP, Schmid G. Chem. Ber. 1985;118:1644–1654.
- 17.Katoh R, Suzuki K, Furube A, Kotani M, Tokumaru K. J. Phys. Chem. C. 2009;113:2961–2965. [Google Scholar]
- 18.Van S-P, Hammond GS. J. Am. Chem. Soc. 1978;100:3895–3902. [Google Scholar]
- 19.See the Supporting Information for details.
- 20.Fluorescence quenching by iodide has been reported in paraterphenyl, see: Watkins AR. J. Phys. Chem. 1974;78:2555–2558.
- 21.Crystallographic data for 3b: C18H18BF3N2O3S, Mr=410.21, crystal size 0.36 × 0.31 × 0.24 mm3, monoclinic, space group P21/n, a=9.3812(9), b=14.3671(13), c=14.2217(13) Å, β=110.940(1)°, V=1882.0(3) Å3, Z=4, ρcalc=1.448 g cm−3, µ=0.223 mm−1, F(000)=848, MoKα-radiation λ=0.71073 Å, T=173(2) K, 2Θmax=54.00°, 17178 reflections measured [Rint=0.0255], 4101 reflections observed, 325 refined parameters, R1=0.0376, wR2=0.1050 for reflections with I > 2σ(I), R1=0.0451, wR2=0.1131, and GOF=1.024 for all data, max/min residual electron density +0.439/−0.201 eÅ−3. CCDC 782514 (3b) 782515 (3a), 782517 (3d), and 782516 (3e) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 22.Qiao S, Hoic DA, Fu GC. Organometallics. 1997;16:1501–1502. [Google Scholar]
- 23.Piers WE, Bourke SC, Conroy KD. Angew. Chem. 2005;117:5142–5163. doi: 10.1002/anie.200500402. Angew. Chem. Int. Ed. 2005, 44, 5016 – 5036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.