Abstract
Substantial evidence has emerged over the past decades for a role of genetics in the development of human refractive error. There also is an emmetropization mechanism that uses visual signals to match the axial length to the focal plane. There has been little discussion of how these two important factors might interact. We explore here ways in which genetic factors driving axial growth may interact with the emmetropization mechanism, mostly to produce emmetropic eyes but often to produce myopia. An important factor may be a normal, yet reduced ability of juvenile eyes to use myopia to restrain genetically driven axial elongation. Reduced ability to respond to myopia by slowing axial elongation may contribute to the development of myopia in cases where genetics alone would make the axial length longer than the focal plane.
Keywords: emmetropization, focal plane, axial length, myopia, gene-environment interaction
Refractive error occurs when the axial length of the eye does not match the focal plane, which is produced by the cornea, the lens and the anterior chamber. If the axial length is too short, and therefore the retinal photoreceptors are in front of the location of the focal plane, the eye is hyperopic. If the axial length places the retina behind the focal plane, images are in focus in front of the retina and the eye is myopic. Thus, for most eyes it is the relationship between focal plane and axial length that is important, and not the specifics of either.1
Data from several types of studies suggest a genetic component to human juvenile-onset myopia. For instance, it has been found that the odds of a child becoming myopic rise with the number of myopic parents.2 Even non-myopic children with myopic parents tend to have longer eyes than non-myopic children of non-myopic parents.3 Heritability values from twin studies are generally high (0.5 to 0.90).4–6 Numerous loci associated with myopia have been reported from association studies using microsatellite markers and single nucleotide polymorphisms (SNPs).7,8 However, it appears so far that no single gene and, in particular, no mutation in the coding region of a gene, directly causes juvenile-onset myopia. The search for myopia-related genes continues, but the genetic contribution to juvenile-onset myopia is multifactorial and complex.
Over the past 35 years, studies also have shown the existence of an emmetropization mechanism in both animals and humans.9–12 The details of how this mechanism operates are emerging, but much remains to be discovered. For the present purposes it is sufficient to note that this mechanism uses a hyperopic refractive state as a stimulus for increased axial elongation. In a hyperopic eye, the retina is located in front of (closer to the cornea than) the focal plane. The hyperopia is detected by the retina and produces an increase in the axial elongation rate of the growing eye by altering the biochemistry and biomechanical properties of the scleral shell.13,14 This moves the retina away from the cornea and toward the focal plane, reducing the hyperopia. Conversely, in young animals and humans, a myopic refractive state produces slowing of the elongation rate of the developing eye.15–19 As the optics of the eye mature (cornea flattens, anterior chamber deepens, lens power decreases) the focal plane moves away from the cornea and “catches up” with the photoreceptors. Note that the alterations in axial elongation produced by the emmetropization mechanism are modulations of the normal postnatal growth, not a cessation or initiation of growth.
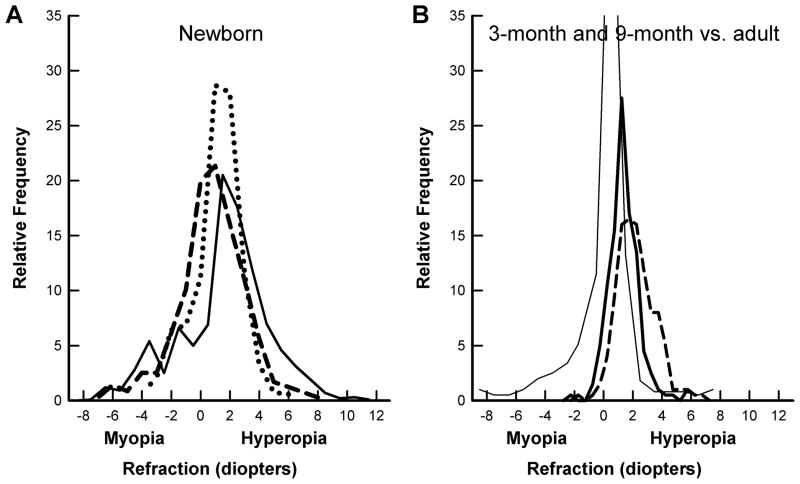
The emmetropization mechanism is found in many species of animals, from fish to macaque monkeys20,21 making it highly unlikely that a mechanism of fundamental importance for clear vision, and survival, would not exist humans. As illustrated in Fig. 1, it has been known for many years that infants and young children emmetropize1,22,23 producing a leptokurtic refractive distribution in adults in which there are many eyes that are emmetropic or, more typically, slightly hyperopic. The animal studies simply provide direct evidence of the mechanism by which this occurs. In all of the animal models, it is the axial elongation that is modulated to the refractive target, not the reverse as was often supposed before studies in animal models began in the 1970s. How this emmetropization mechanism interacts with the developing focal plane throughout the infantile and juvenile periods is the topic of this article.
Figure 1.
A. Refractive error distribution at birth from three studies; dashed line, Cook & Glasscock 24, solid line: Goldschmidt25 and dotted line, Zonis & Miller.26 B. Refractive distribution at 3 months (dashed line) and 9 months of age (dark solid line) from Mutti et al.10 compared with an adult distribution (thin solid line)23). At birth, the refractive distribution is broad. This narrows rapidly so that by nine months, nearly all children are emmetropic or slightly hyperopic. In adults, the distribution is narrower, but myopia has become more prevalent.
Development of the Focal Plane – the Genetic Target
It appears that the focal plane, both at birth and throughout childhood, is controlled by genetics. It has been found23,27 that the powers of the cornea and lens and anterior chamber depth are normally distributed, suggesting that they, like other body dimensions (height, finger length, etc.) are inherited as quantitative traits controlled by many genes. How the many genes interact to produce the structural proteins, growth factors, enzymes, etc. that produce a particular focal plane at birth and how they guide postnatal changes in the focal plane is unknown. It has been estimated that height in humans involves hundreds of genetic variants in at least 180 loci that together account for only about 10% of the phenotypic variance in height.28 There is no reason to assume that corneal and lenticular power during pre- and postnatal development involves fewer genes. Something as simple as gender affects the focal plane; males have flatter corneas and less powerful lenses than females29,30 making the focal plane longer.
In addition to evidence of genetic control of the focal plane, there is little evidence that the visual environment affects corneal or lenticular power. Animal studies using mammals (monkeys and tree shrews) in which negative or positive lens wear produces substantial effects on axial elongation have found little effect on corneal or lens parameters.13,31 There is evidence of changes in corneal astigmatism in macaque monkeys in studies where the refractive development is affected by positive or negative lens wear,32 but the changes in overall power of the cornea, when compared with the changes in axial elongation, are modest. In a study of a large population of monkeys that participated in many studies using lenses, Qiao-Grider et al.31 found no consistent environmental effect on any crystalline lens parameter.
During early postnatal development and also during the slower juvenile growth period, in children and in animals, the focal plane moves away from the cornea due to a flattening of the cornea and a reduction in lens power.10,29,33,34 Between 3 and 9 months postnatally, Mutti et al.10 found reductions in the average corneal (−1.07 ± 1.09 D) and lens power (−3.62 ± 2.13 D). The corneal power reaches nearly adult levels at a younger age than does lens power, so that Zadnik et al.30 found only a small (~0.4 D) reduction in corneal power between ages 6 and 14. Calculated lens power continues to decrease to older ages, decreasing by about 2.5 D during this same time period while anterior chamber depth increases slightly. Thus, focal plane location (distance from the corneal surface) clearly increases over time, more rapidly during the early infantile postnatal period and more slowly thereafter. Without knowing its exact form, it is reasonable to represent focal plane development, like other growth-related parameters, as increasing with the log of age. This is the basis for the “genetic focal plane” growth curve in Fig. 2.
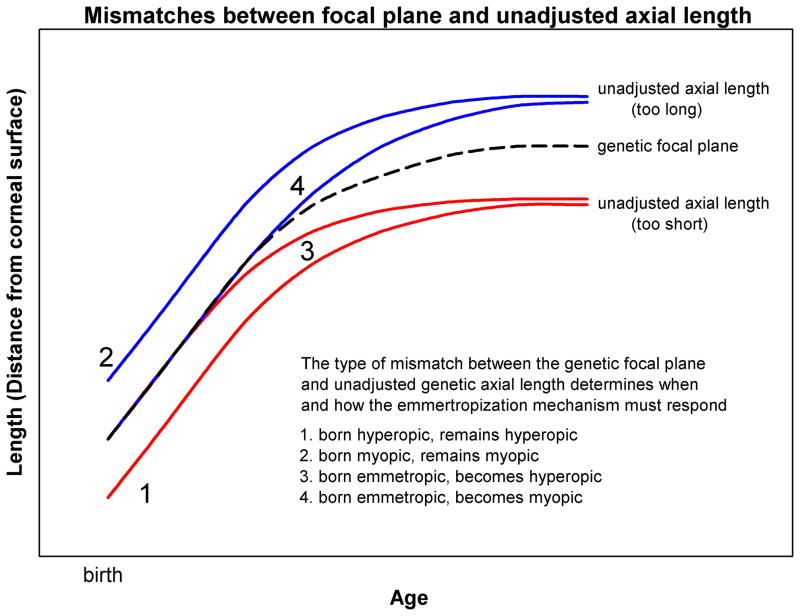
Figure 2.
Hypothetical postnatal growth of the focal plane modeled as the log of age (dashed line) and four of the many possible growth patterns for the genetically-guided, unadjusted axial length. “1” and “2” depict unadjusted axial growth that is parallel to the focal plane, but either shorter or longer starting at birth so that, without an emmetropization mechanism, the eyes would remain either hyperopic (“1”) or myopic (“2”). “3” and “4” depict unadjusted axial growth that initially is the same as the focal plane, but diverges later in the postnatal period, creating eyes that would become either hyperopic (“3) or myopic (“4”).
Genetically-guided (Unadjusted) Growth of Axial Length
Axial length is broadly distributed at birth and, at birth, is not closely related to refractive state.35,36 Refraction also is broadly distributed over a wide range at birth24–26 as are both corneal and lenticular powers, it thus seems reasonable to assume that axial length is normally distributed at that time and represents the read-out of many genes.
Throughout development, it is the scleral shell of the eye that controls retinal location. Genetic factors involved in determining growth of the axial length must include those that control cell division of the scleral fibroblasts, the amount of collagen made by the fibroblasts, the number and thickness of the scleral lamellae, the composition of the many other components of the sclera such as glycosaminoglycans, integrin-mediated cell adhesions, etc. Much of the growth of axial length during the infantile and juvenile periods presumably is driven by these same genetic factors, as is the case with other body parts and presumably follows a time-course that increases in relationship to the log of age as indicated in Fig. 2.
Although the focal plane and the unadjusted (genetic) axial length both may increase with the log of age, there is no reason to expect that these two functions would be parallel throughout development. For instance, in a child born with hyperopia, the unadjusted axial length is shorter than the focal plane. If there were no emmetropization mechanism, the axial length might remain shorter throughout infancy and childhood (“1” in Fig. 2). A similar situation might occur for a child born myopic, with an axial length longer than the focal plane (“2” in Fig. 2). However, the unadjusted axial length might be guided by genetics to increase at a slower, or a faster, rate than the focal plane, so that, in the absence of an emmetropization mechanism, an eye born with a match between the axial length and focal plane would eventually become either hyperopic (“3” in Fig. 2) or myopic (“4” in Fig. 2). All such scenarios, and many others, are possible during postnatal development; different parts of the body grow at different rates. For instance, the head grows less than do the arms and legs so that the overall shape of a baby is different from that of a teenager.
There is an important reason we do not have data on how axial length would change based solely on genetic factors: the genetically-guided axial elongation is modulated by the visually-guided emmetropization mechanism. In order to achieve and then maintain emmetropia, the emmetropization mechanism adjusts the axial elongation rate of the growing eye to move the photoreceptors to the focal plane and then keep them there as the focal plane continues to change. There may be naturally-occurring situations in which the emmetropization mechanism fails to function37 and could provide data on the shape of the genetically-guided, unadjusted, growth of axial length, but these have not been systematically examined.
Modulation of Axial Elongation by the Emmetropization Mechanism
Early Emmetropization
During early postnatal development, in the infantile rapid growth period,29 both animals and humans emmetropize and use the eye’s refractive state to adjust its axial growth so that the axial length comes to match the focal plane. In normal human infants, the visual environment has an impact on refractive state shortly after birth. As seen in Fig. 1A & 1B, during the first postnatal weeks and months, the distribution of refractive error narrows.10,22 Some, but not all, of this reduction occurs simply as a function of increasing eye size (described as “passive proportional growth” by Wallman and Adams38). However, infant eyes that are hyperopic at 3 months of age emmetropize by elongating more rapidly than average, so that by nine months the retina moves toward the focal plane. In contrast, eyes that are emmetropic at 3 months elongate more slowly.10 These adjustments appear to be guided by visual signals; if the signals are absent, as in corneal opacification,39 congenital cataract40 and ptosis,41 eyes elongate faster than normal as do animal eyes exposed to form deprivation.42,43
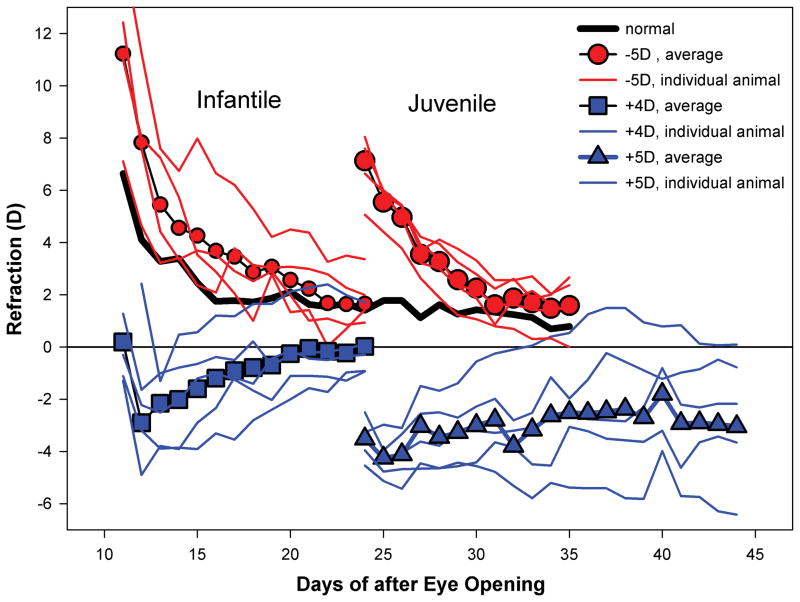
The same early emmetropization is also seen in animals. Newborn, or newly hatched animals also have a broad distribution of refractive errors.44–47 Typically, but not universally, they are hyperopic. Over a relatively short time, the refractive errors diminish. In tree shrews, this is seen as a rapid decrease from hyperopia as shown in Fig. 3.16 The emmetropization in young animals is visually guided. Making young animal eyes artificially hyperopic with negative lenses during this period consistently produces increased axial elongation (above what would occur normally as measured by untreated fellow control eyes or in normal eyes) so that the eyes become emmetropic with the lens in place (Fig. 3).16,19,48,49 Without the lens, the eyes are then myopic but, from the “eye’s point of view,” the elongation simply re-establishes emmetropia. Similarly, making young animal eyes myopic with positive lenses causes a slowing of the axial elongation rate and the eyes become emmetropic while wearing the positive lens (Fig. 3).16,17 This “re-emmetropization” occurs across a number of species.15,50,51
Figure 3.
Tree shrew eyes are extremely hyperopic (~25 D) at the time of eye opening. Ten days later, (thick solid line) the hyperopia is decreasing rapidly and then declines more slowly as the eyes approach emmetropia.52 Small circles show the average effect of binocular minus lens wear in infantile animals along with individual variability. The plots show refraction measured with the lens in place (average of right and left eyes). Minus-lens compensation is the return to emmetropic refractions after initial hyperopia. In young (infantile) tree shrews a similar re-emmetropization occurs when plus lenses are used (squares and individual plots). Older (juvenile) animals (large circles) continue to re-emmetropize to minus lenses. However, older animals (triangles) are less able to use myopia, produced by the plus lenses, to slow elongation so many eyes remain myopic wearing the lens. Data shown here are condensed from a recent paper.16
It is worth noting that even though the two eyes generally begin life with similar refractions, each eye contains an emmetropization mechanism that works to achieve emmetropia independent of the other eye. This is shown by the many instances in which monocular lens treatments have been applied. To be sure, there are (mostly unknown) central mechanisms that affect both eyes, and the untreated control eye often is slightly affected by treatment of its fellow eye.53 However, when lenses are used monocularly and sufficient time is allowed, the treated eye compensates for lenses and the control eye remains mostly unaffected. Of course, if binocular lenses are used, both eyes respond.
Later Emmetropization
After eyes have achieved emmetropia, or a nearly emmetropic refractive state, the emmetropization mechanism remains active in maintaining emmetropia as the focal plane continues to move away from the cornea. In children, this occurs as the eye elongates 2–4 mm.54,55 That maintenance of emmetropia is visually guided is shown by two examples. In tree shrews, if negative-lens treatment (making the eye optically hyperopic) is begun any time during the juvenile, slower-growth period, the eyes elongate and compensate for the lens, as shown in Fig. 3. This occurs even in young adult tree shrews, though the rate at which the eyes compensate is slower than in younger animals.48 As another example, if visual input is removed from tree shrews that have emmetropized in the light by placing them in continuous darkness, the eyes begin to elongate more rapidly than normal and the eyes become myopic.46 Thus, visual input (images on the retina) is needed to modulate and control the elongation rate of the eye.
Asymmetry: Diminished Response to Positive Lenses in Older Animals
In older, juvenile tree shrews that have achieved a nearly-emmetropic refractive state, the response to myopia produced by positive lenses differs from that found in young animals that are still early in the emmetropization process.16 As shown in Fig. 3, plus lens wear in older animals has less of an effect; some eyes respond with slowed elongation and their refraction moves toward emmetropia, but most eyes remain myopic while wearing the lens. The diminished response to positive lenses has two possible explanations: the emmetropization mechanism might become insensitive to myopia, or the ability of the mechanism to use myopia to control and axial elongation might diminish. It does not appear that there is a loss of sensitivity to myopia. Tree shrews, of the same age as the positive lens-treated animals, that have compensated for a negative lens are myopic if the lens is removed. The myopic, elongated eyes generally recover quickly from the induced myopia, so they must be able to detect the myopic refractive state.16 These results suggest that normal eyes in older animals that are made myopic with plus lenses have a reduced ability to use that myopia to slow their axial elongation rate to restore emmetropia.
Extrapolation to Humans
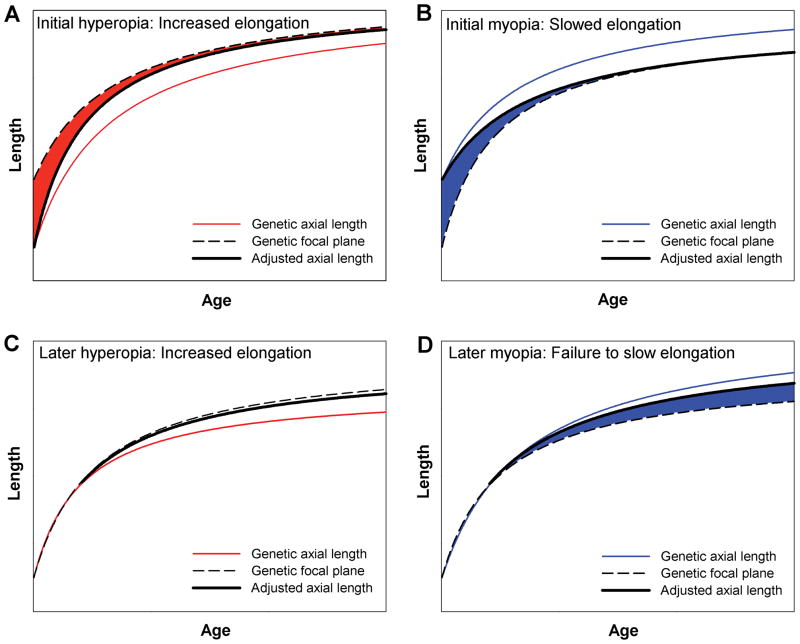
Most young children, including those who subsequently become myopic in later childhood, succeed in becoming emmetropic or nearly so. The prevalence of myopia is low in children ages 1 to 5 years of age.10,29,56,57 As in the young animals, the emmetropization mechanism adjusts the genetically-guided growth of the eye as needed to establish and maintain emmetropia, regardless of whether the eye began myopic or hyperopic (Fig. 4A & 4B).58,59 Given the continued ability of hyperopia to produce an adjustment in axial growth in older animals, it is likely in humans that hyperopia continues to be effective in altering axial elongation in cases where a mismatch develops at older ages that would make the eye hyperopic. These eyes emmetropize by elongating the axial length beyond the unadjusted length (Fig. 4C).
Figure 4.
Examples of emmetropization from hyperopia (A) or myopia (B) at birth or early in life. The emmetropization mechanism produces an adjusted axial length (thick line) that is longer or shorter than the genetic axial length and matches the adjusted axial length to the focal plane (dashed line) so that refractive error (shaded area) is reduced; Later-developing hyperopia (C) also engages the emmetropization mechanism so eyes elongate and remain emmetropic. To the extent that children are like tree shrews, later developing myopia (D) may not produce slowed elongation, allowing myopia (shaded area) to persist or increase.
A potential source of human myopia could arise from a combination of two events, a later-developing mismatch (4 in Figs. 2) in which the unadjusted axial length would become longer than the focal plane coupled with a loss of the ability to use myopia to slow the growth of the eye. In this case, the eyes would gradually become more myopic (Fig. 4D). How much myopia would develop would depend on two factors: the difference between the focal plane and the unadjusted, genetically-guided axial length and any retained ability of the emmetropization mechanism to use myopia to slow the unadjusted axial growth (Fig. 3).
The asymmetry between the continued ability of hyperopia in juvenile animals to adjust the axial length so as to make it longer, coupled with an inability in older, juvenile animals, and possibly children, to use myopia to slow axial elongation, also has implications for eyes in which there is close agreement between the focal plane and the genetically-guided axial length. Under-accommodation (a “lag” of accommodation) to near targets produces an eye that is functionally hyperopic while looking at the near target, such as a book. Although there is disagreement about when it begins, progressing myopic children tend to have a larger accommodative lag than do emmetropic children.60,61 If a child doing nearwork has an accommodative lag that creates hyperopic defocus on the retina and hyperopia remains a stimulus for axial elongation, this would bias the child’s eye toward axial elongation. A concern about this scenario has been that, in animals, removal of the hyperopia for 2 hours per day prevents the eyes from elongating.62–64 However, if eyes that are unable to use myopia to slow elongation are, in addition, exposed to hyperopia through nearwork and underaccommodation, this might lead to myopia development.
Caveats and Implications for Future Studies
The foregoing suggestions about the ways the emmetropization mechanism interacts with the genetically-guided, unadjusted axial growth of the eye and, in particular, the diminished ability to use myopia to slow axial growth, emerged from observations in tree shrews. Although tree shrews (mammals closely related to primates) have proven to be excellent models for the study of refractive development and refractive error, it is important to learn if an asymmetry in the ability of the emmetropization mechanism to alter axial growth is a general phenomenon. It has been pointed out that most studies in animal models use animals soon after birth (or hatching)65 at a point when the emmetropization mechanism seems able to adjust axial elongation using either hyperopia or myopia. It would be important to learn with positive-lens studies if the inability of older, juvenile, animals to use myopia is a general phenomenon across species by testing older monkeys and chicks. Although the focus of this essay has been on interactions that might produce myopia, there also are implications for persistent hyperopia. If the unadjusted genetic axial length would be far shorter than the focal plane, it might not be possible for the emmetropization mechanism to increase axial elongation sufficiently to reduce and remove the hyperopia.
As was stated earlier, there is evidence for both genetic and environmental factors in the development of human myopia but there has been little discussion of how this interaction might occur without something being wrong with either the emmetropization mechanism or the genetics. The animal data on which this speculative essay is based suggest that there may be a normal decrease in the ability of the emmetropization mechanism to use myopia to slow axial elongation and that this may impart a natural bias toward developing myopia. Throughout evolution, it presumably was rare for juvenile or adult vertebrate eyes to encounter significant periods of hyperopia, so that once eyes had achieved emmetropia it was not an issue if myopia became less effective in controlling eye growth. However, when humans developed environments with extensive, and long-duration nearwork demands, if the accommodative mechanism did not sufficiently remove the hyperopia associated with near work and if myopia, except in infants, is unable to slow the axial elongation rate, it would be natural that at least some eyes develop myopia for distant objects.
Acknowledgments
Support for the research on tree shrews that forms the basis for this article has come from the National Institutes of Health, RO1 EY-005922 and P30 EY-003909.
References
- 1.Sorsby A, Benjamin B, Davey JB, Sheridan M, Tanner JM. Medical Research Council Special Report Series No. 293. London: Her Majesty’s Stationary Office; 1957. Emmetropia and its aberrations. [PubMed] [Google Scholar]
- 2.Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–40. [PubMed] [Google Scholar]
- 3.Zadnik K, Satariano WA, Mutti DO, Sholtz RI, Adams AJ. The effect of parental history of myopia on children’s eye size. JAMA. 1994;271:1323–7. [PubMed] [Google Scholar]
- 4.Dirani M, Chamberlain M, Garoufalis P, Chen C, Guymer RH, Baird PN. Refractive errors in twin studies. Twin Res Hum Genet. 2006;9:566–72. doi: 10.1375/183242706778024955. [DOI] [PubMed] [Google Scholar]
- 5.Hornbeak DM, Young TL. Myopia genetics: a review of current research and emerging trends. Curr Opin Ophthalmol. 2009;20:356–62. doi: 10.1097/ICU.0b013e32832f8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guggenheim JA, Kirov G, Hodson SA. The heritability of high myopia: a reanalysis of Goldschmidt’s data. J Med Genet. 2000;37:227–31. doi: 10.1136/jmg.37.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton TT, Metlapally R, Young TL. Myopia. In: Klintworth GK, Garner A, editors. Garner and Klintworth’s Pathobiology of Ocular Disease. 3. New York: Taylor & Francis; 2008. pp. 537–56. [Google Scholar]
- 8.Hysi PG, Young TL, Mackey DA, Andrew T, Fernandez-Medarde A, Solouki AM, Hewitt AW, Macgregor S, Vingerling JR, Li YJ, Ikram MK, Fai LY, Sham PC, Manyes L, Porteros A, Lopes MC, Carbonaro F, Fahy SJ, Martin NG, van Duijn CM, Spector TD, Rahi JS, Santos E, Klaver CC, Hammond CJ. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42:902–5. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–80. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 11.Wildsoet CF. Active emmetropization—evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–90. [PubMed] [Google Scholar]
- 12.Norton TT. Animal models of myopia: learning how vision controls the size of the eye. ILAR J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 13.Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 14.Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484–92. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optom Vis Sci. 1991;68:364–8. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Siegwart JT, Jr, Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–9. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8:1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- 18.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 20.Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol Vis Sci. 2007;48:4829–37. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- 21.Smith EL, 3rd, Hung LF, Harwerth RS. Effects of optically induced blur on the refractive status of young monkeys. Vision Res. 1994;34:293–301. doi: 10.1016/0042-6989(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 22.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 23.Stenstrom S. Investigation of the variation and the correlation of the optical elements of human eyes. Am J Optom Arch Am Acad Optom. 1948;25:496–504. doi: 10.1097/00006324-194810000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–13. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- 25.Goldschmidt E. Refraction in the newborn. Acta Ophthalmol (Copenh) 1969;47:570–8. doi: 10.1111/j.1755-3768.1969.tb08143.x. [DOI] [PubMed] [Google Scholar]
- 26.Zonis S, Miller B. Refractions in the Israeli newborn. J Pediatr Ophthalmol. 1974;11:77–81. [Google Scholar]
- 27.Sorsby A, Leary GA, Fraser GR. Family studies on ocular refraction and its components. J Med Genet. 1966;3:269–73. doi: 10.1136/jmg.3.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, Willer CJ, Jackson AU, Vedantam S, Raychaudhuri S, Ferreira T, Wood AR, Weyant RJ, Segre AV, Speliotes EK, Wheeler E, Soranzo N, Park JH, Yang J, Gudbjartsson D, Heard-Costa NL, Randall JC, Qi L, Vernon Smith A, Magi R, Pastinen T, Liang L, Heid IM, Luan J, Thorleifsson G, Winkler TW, Goddard ME, Sin Lo K, Palmer C, Workalemahu T, Aulchenko YS, Johansson A, Carola Zillikens M, Feitosa MF, Esko T, Johnson T, Ketkar S, Kraft P, Mangino M, Prokopenko I, Absher D, Albrecht E, Ernst F, Glazer NL, Hayward C, Hottenga JJ, Jacobs KB, Knowles JW, Kutalik Z, Monda KL, Polasek O, Preuss M, Rayner NW, Robertson NR, Steinthorsdottir V, Tyrer JP, Voight BF, Wiklund F, Xu J, Hua Zhao J, Nyholt DR, Pellikka N, Perola M, Perry JR, Surakka I, Tammesoo ML, Altmaier EL, Amin N, Aspelund T, Bhangale T, Boucher G, Chasman DI, Chen C, Coin L, Cooper MN, Dixon AL, Gibson Q, Grundberg E, Hao K, Juhani Junttila M, Kaplan LM, Kettunen J, Konig IR, Kwan T, Lawrence RW, Levinson DF, Lorentzon M, McKnight B, Morris AP, Muller M, Suh Ngwa J, Purcell S, Rafelt S, Salem RM, Salvi E, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–8. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA. Refraction and its components during the growth of the eye from the age of three. Med Res Counc Annu Rep. 1961;301(Special):1–67. [PubMed] [Google Scholar]
- 30.Zadnik K, Manny RE, Yu JA, Mitchell GL, Cotter SA, Quiralte JC, Shipp M, Friedman NE, Kleinstein RN, Walker TW, Jones LA, Moeschberger ML, Mutti DO. Ocular component data in schoolchildren as a function of age and gender. Optom Vis Sci. 2003;80:226–36. doi: 10.1097/00006324-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd Nature of the refractive errors in rhesus monkeys (Macaca mulatta) with experimentally induced ametropias. Vision Res. 2010;50:1867–81. doi: 10.1016/j.visres.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Smith EL., 3rd Astigmatism in monkeys with experimentally induced myopia or hyperopia. Optom Vis Sci. 2005;82:248–60. doi: 10.1097/01.opx.0000159357.61498.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–42. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 34.Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40:214–29. [PubMed] [Google Scholar]
- 35.Larsen JS. The sagittal growth of the eye. IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol. 1971;49:873–86. doi: 10.1111/j.1755-3768.1971.tb05939.x. [DOI] [PubMed] [Google Scholar]
- 36.Gernet H, Hollwich F. Oculometry of infantile glaucoma. Ber Zusammenkunft Dtsch Ophthalmol Ges. 1969;69:341–8. [PubMed] [Google Scholar]
- 37.Cregg M, Woodhouse JM, Stewart RE, Pakeman VH, Bromham NR, Gunter HL, Trojanowska L, Parker M, Fraser WI. Development of refractive error and strabismus in children with Down syndrome. Invest Ophthalmol Vis Sci. 2003;44:1023–30. doi: 10.1167/iovs.01-0131. [DOI] [PubMed] [Google Scholar]
- 38.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–63. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 39.Meyer C, Mueller MF, Duncker GI, Meyer HJ. Experimental animal myopia models are applicable to human juvenile-onset myopia. Surv Ophthalmol. 1999;44 (Suppl 1):S93–102. doi: 10.1016/s0039-6257(99)00091-0. [DOI] [PubMed] [Google Scholar]
- 40.Rabin J, Van Sluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Invest Ophthalmol Vis Sci. 1981;20:561–4. [PubMed] [Google Scholar]
- 41.von Noorden GK, Lewis RA. Ocular axial length in unilateral congenital cataracts and blepharoptosis. Invest Ophthalmol Vis Sci. 1987;28:750–2. [PubMed] [Google Scholar]
- 42.Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–7. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- 43.Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–8. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- 44.Pickett-Seltner RL, Sivak JG, Pasternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28:323–8. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- 45.Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. J Comp Physiol (A) 1992;170:565–74. doi: 10.1007/BF00199333. [DOI] [PubMed] [Google Scholar]
- 46.Norton TT, Amedo AO, Siegwart JT., Jr Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006;47:4700–7. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ofri R, Millodot S, Shimoni R, Horowitz IH, Ashash E, Millodot M. Development of the refractive state in eyes of ostrich chicks (Struthio camelus) Am J Vet Res. 2001;62:812–5. doi: 10.2460/ajvr.2001.62.812. [DOI] [PubMed] [Google Scholar]
- 48.Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–76. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 50.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Troilo D, Quinn N, Baker K. Accommodation and induced myopia in marmosets. Vision Res. 2007;47:1228–44. doi: 10.1016/j.visres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80:623–31. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rucker FJ, Zhu X, Bitzer M, Schaeffel F, Wallman J. Inter-ocular interactions in lens compensation: yoking and anti-yoking. Invest Ophthalmol Vis Sci. 2009;50 E-Abstract 3931. [Google Scholar]
- 54.Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci. 2005;46:2317–27. doi: 10.1167/iovs.04-0945. [DOI] [PubMed] [Google Scholar]
- 55.Fledelius HC, Christensen AC. Reappraisal of the human ocular growth curve in fetal life, infancy, and early childhood. Br J Ophthalmol. 1996;80:918–21. doi: 10.1136/bjo.80.10.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zadnik K, Mutti DO, Friedman NE, Adams AJ. Initial cross-sectional results from the Orinda Longitudinal Study of Myopia. Optom Vis Sci. 1993;70:750–8. doi: 10.1097/00006324-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, Manny RE, Mutti DO, Yu JA, Zadnik K. Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121:1141–7. doi: 10.1001/archopht.121.8.1141. [DOI] [PubMed] [Google Scholar]
- 58.Howland HC, Waite S, Peck L. Noninvasive Assessment of the Visual System, 1993 Technical Digest Series. Vol. 3. Washington, DC: Optical Society of America; 1993. Early focusing history predicts later refractive state: a longitudinal photorefractive study; pp. 210–3. [Google Scholar]
- 59.Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vision Sci. 1993;8:337–44. [Google Scholar]
- 60.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–4. [PubMed] [Google Scholar]
- 61.Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837–46. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- 62.Shaikh AW, Siegwart JT, Jr, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–15. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 63.Schmid KL, Wildsoet CF, Pettigrew JD. The effect of daily periods of normal vision on refractive adaptation in chicks. Invest Ophthalmol Vis Sci. 2011:52. in press. [Google Scholar]
- 64.Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, Smith EL., 3rd Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48:957–62. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zadnik K, Mutti DO. How applicable are animal myopia models to human juvenile onset myopia? Vision Res. 1995;35:1283–8. doi: 10.1016/0042-6989(94)00234-d. [DOI] [PubMed] [Google Scholar]