Abstract
Nucleotide reverse transcriptase inhibitors, such as zidovudine (azidothymidine, AZT) and stavudine, represent a class of approved antiretroviral agents for highly active antiretroviral therapy, which prolongs the life expectancy of patients infected with human-immunodeficiency virus. Unfortunately, the use of these drugs is associated with known toxicities in the liver, skeletal muscle, heart and other organs, which may involve increased reactive oxygen species (ROS) generation, among other mechanisms. Resveratrol is a polyphenolic plant-derived antioxidant abundantly found in certain grapes, roots, berries, peanuts and red wine. This study, using primary human cardiomyocytes, evaluated the effects of AZT and pre-treatment with resveratrol on mitochondrial ROS generation and the cell death pathways. AZT induced concentration-dependent cell death, involving both caspase-3 and -7 and poly(ADP-ribose) polymerase activation, coupled with increased mitochondrial ROS generation in human cardiomyocytes. These effects of AZT on mitochondrial ROS generation and cell death may be attenuated by resveratrol pre-treatment. The results demonstrate that mitochondrial ROS generation plays a pivotal role in the cardiotoxicity of AZT in human cardiomyocytes, and resveratrol may provide a potential strategy to attenuate these pathological alterations, which are associated with widely used antiretroviral therapy.
Keywords: resveratrol, azidothymidine, cell death, cardiomyocytes, reactive oxygen species
Introduction
Nucleotide reverse transcriptase inhibitors, such as zidovudine (azidothymidine, AZT) and stavudine, represent a class of approved antiretroviral agents for highly active antiretroviral therapy, which prolongs the life expectancy of patients infected with human-immunodeficiency virus (HIV). Unfortunately, the use of these drugs is associated with known toxicities in various organs, including the liver, skeletal muscle and heart. HIV-positive patients are at risk of developing end-organ damage, including cardiomyopathy, which is seemingly becoming increasingly prevalent (1). Cardiomyopathy manifests as dilated cardiomyopathy, or as isolated left or right ventricular dysfunction; both are associated with a poor prognosis, resulting in symptomatic heart failure in up to 5% of HIV patients (1). The precise mechanism of HIV-associated cardiomyopathy has yet to be elucidated. It may involve direct myocardial infection with HIV, post-infective cardiac autoimmunity, nutritional deficiencies, or the cardiotoxic effects of antiretroviral drugs, such as AZT (1). Indeed, numerous preclinical reports implicate the direct cardiotoxic effects of AZT in various rodent models. This may involve multiple mechanisms, including the generation of reactive oxygen species (ROS) in cardiomyocytes and endothelial cells (2-12).
Resveratrol (3,4',5-trihydroxystilbene), a plant-derived polyphenolic compound belonging to a class of stilbenes (abundantly found in certain grapes, roots, berries and peanuts) was recently shown to exert various cardiovascular protective effects in pre-clinical models of myocardial ischemic reperfusion injury and atherosclerosis (13,14), metabolic diseases (15-17), and in aged mice (18-21). In this study, we explored the effects of AZT on mitochondrial ROS generation and associated cell death (both apoptotic and necrotic) using human primary cardiomyocytes, and evaluated the effects of resveratrol on these pathological processes.
Materials and methods
Reagents and cell culture
AZT and resveratrol were purchased from Sigma Chemicals (St. Louis, MO, USA). Human cardiomyocytes (HCM) were obtained from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured in polylysine-coated plates using complete cardiac myocyte medium according to the manufacturer's recommendation as previously described (22). HCM were treated with AZT as indicated in cardiomyocyte growth medium for 48 h.
Simultaneous determination of cell death and mitochondrial superoxide/ROS generation
Mitochondrial superoxide/ROS generation and cell death were determined as previously described (23,24). Briefly, the cells were loaded with MitoSOX Red (Invitrogen, Carlsbad, CA, USA) for 30 min, followed by trypsinization as well as staining with APC-Annexin-V and Sytox Green (Invitrogen). The samples were run on a flow cytometer with 488 nm excitation to measure oxidized MitoSOX Red in the FL2 and FL3 channels, APC-Annexin V (FL4) and Sytox Green (FL1). Data were also collected from the FSC (forward scatter), SSC (side scatter) and FL1 and FL4 channels. The cells were plotted for FSC and SSC. Cell debris with low FSC and SSC were excluded from the analysis. The cells were then analyzed for APC-Annexin V (FL4) and Sytox Green (FL1). Cells that exhibited apoptosis (FL-4-positive) or were dead (FL-1-positive) were excluded from the analysis, and MitoSOX Red staining was analyzed for each population as a histogram of mean intensity (FL2). Thus, MitoSOX Red of the cells analyzed excluded any non-specific interferences from apoptotic and dead cells (23).
Caspase-3 and -7 activity
The activity of caspase-3 and -7 was measured using the Apo-one Homogeneous Caspase-3/7 assay kit (Promega), according to the manufacturer's instructions (25).
Poly(ADP-ribose) polymerase activity
Poly(ADP-ribose) polymerase (PARP) activity was assayed by colorimetry according to the manufacturer's instructions, as previously described (Trevigen) (25).
Statistical analysis
Results are reported as the means ± SEM. P-values <0.05 were considered significant. Statistical analysis between two measurements were determined using the two-tailed unpaired Student's t-test.
Results
Resveratrol attenuates azidothymidine-induced cell death in human cardiomyocytes
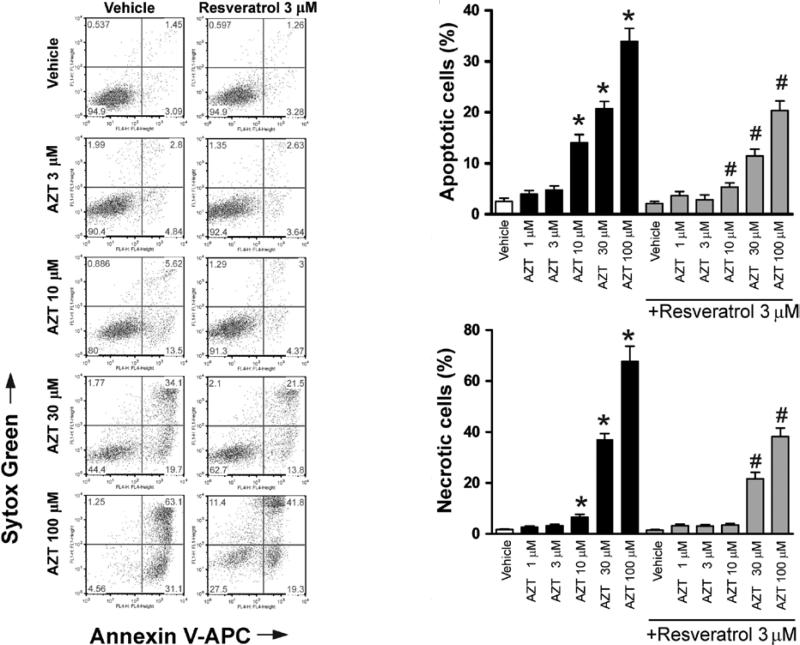
Primary cardiomyocytes were treated with various concentrations of AZT for 48 h. Cell apoptosis and necrosis were then determined by flow cytometric analysis. AZT treatment increased cell apoptosis and necrosis in human primary cardiomyocytes in a concentration-dependent manner. Pre-treatment with 3 μM resveratrol attenuated the AZT-induced cell apoptosis and necrosis (Fig. 1).
Figure 1.
Resveratrol attenuates AZT-induced cell death in human cardiomyocytes. Human cardiomyocytes were pre-treated with or without various concentrations of resveratrol for 2 h, followed by treatment with various doses of AZT with or without resveratrol. After 48 h, the cells were harvested for flow cytometric analysis with Annexin V for apoptosis and Sytox Green for necrosis. Left panel, representative flow cytometry charts demonstrating the effects of AZT with or without resveratrol pre-treatment on cell death (apoptotic and necrotic) in human primary cardiomyocytes; right panel, the quantification of apoptotic and necrotic cell death from the left panel. Values represent the means ± SEM (n=4). *P<0.05, vehicle vs. AZT; #P<0.05, AZT with or without resveratrol, respectively.
Resveratrol attenuates azidothymidine-induced caspase-3 and -7 activity in human cardiomyocytes
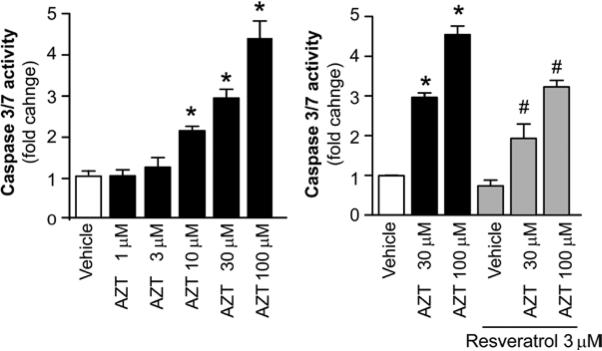
To further understand the underlying mechanisms of AZT-induced cardiomyocyte apoptosis and the prevention of such cell death by resveratrol, caspase-3 and -7 activity, which is indicative of cell apoptosis was measured. AZT treatment induced the elevation of caspase-3 and -7 activity in human primary cardiomyocytes in a concentration-dependent manner. Pre-treatment with resveratrol prevented AZT-induced caspase-3 and -7 activity (Fig. 2).
Figure 2.
Resveratrol attenuates the AZT induction of caspase-3 and -7 activity in human cardiomyocytes. Human cardiomyocytes were treated with resveratrol and AZT, as described in Fig. 1. The cells were harvested and caspase-3 and -7 activity was measured. Left panel, the concentration-dependent effect of AZT on caspase-3 and -7 activity in human cardiomyocytes; right panel, the effects of AZT with or without resveratrol pre-treatment. Values represent the means ± SEM (n=3-6). *P<0.05, vehicle vs. AZT; #P<0.05, AZT with or without resveratrol, respectively.
Resveratrol attenuates azidothymidine-induced PARP activity in human cardiomyocytes
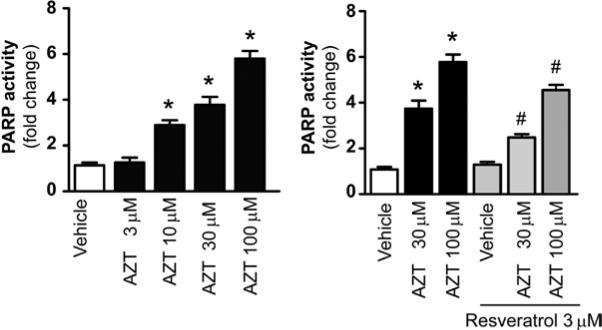
The activation of PARP has been shown to play an important role in inducing necrotic cell death. We investigated whether AZT-induced cell death is mediated via the activation of PARP. AZT treatment increased PARP activity in human primary cardiomyocytes in a concentration-dependent manner. Resveratrol pre-treatment significantly attenuated the AZT-induced increase in PARP activity (Fig. 3).
Figure 3.
Resveratrol attenuates the AZT induction of PARP activity in human cardiomyocytes. Human cardiomyocytes were treated as described in Fig. 1. The cells were harvested and PARP activity was measured. Left panel, the concentration-dependent effect of AZT on PARP activity in human cardiomyocytes; right panel, the effects of AZT with or without resveratrol pre-treatment. Values represent the means ± SEM (n=8). *P<0.05, vehicle vs. AZT; #P<0.05, AZT with or without resveratrol, respectively.
Resveratrol attenuates azidothymidine-induced mitochondrial reactive oxygen species/superoxide generation in human cardiomyocytes
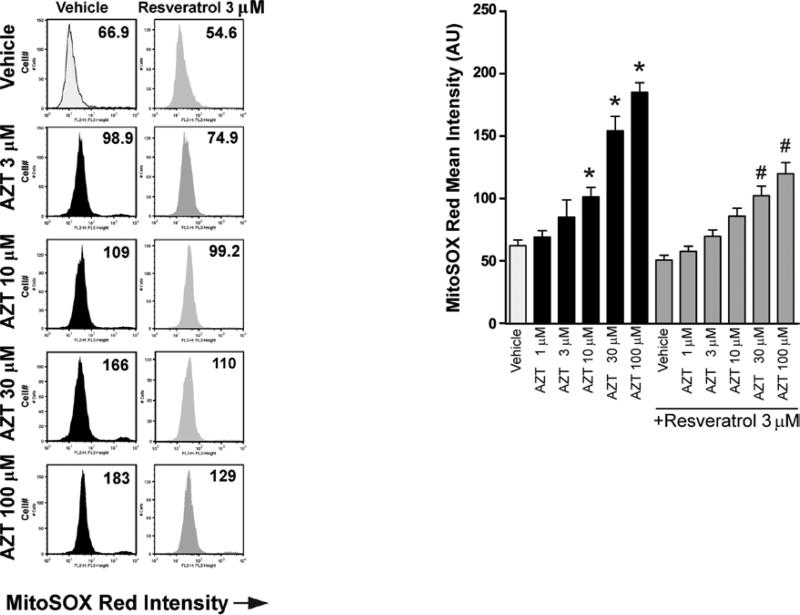
Mitochondria are one of the major cellular producers of ROS, and increased ROS generation promotes the activation of various cell death pathways. Thus, AZT-induced cell death in human cardiomyocytes was examined to determine whether it involves increased mitochondrial ROS generation. AZT treatment significantly increased mitochondrial ROS generation in human primary cardiomyocytes in a concentration-dependent manner, which was attenuated by resveratrol pre-treatment (Fig. 4).
Figure 4.
Resveratrol attenuates AZT-induced mitochondrial ROS generation in human cardiomyocytes. Human cardiomyocytes were treated as described in Fig. 1. The cells were harvested and analyzed by flow cytometric analysis using MitoSOX Red dye. Left panel, representative flow cytometry charts demonstrating the effects of AZT with or without resveratrol pre-treatment on mitochondrial ROS generation in human primary cardiomyocytes; right panel, the quantification of MitoSOX Red mean intensity from the left panel (3 experiments). Values represent the means ± SEM (n=4). *P<0.05, vehicle vs. AZT; #P<0.05, AZT with or without resveratrol, respectively.
Discussion
In this study, using primary human cardiomyocytes, we demonstrated that mitochondrial ROS generation and the consequent activation of various cell death pathways contribute to the cardiotoxicity of the retroviral agent AZT. We also demonstrated that resveratrol pre-treatment was capable of attenuating AZT-induced mitochondrial ROS generation and the activation of interrelated cell death pathways.
The cardiotoxic effects of antiretroviral drugs such as AZT have been implicated in the development of HIV-associated cardiomyopathy in humans (1). Indeed, AZT induces cardiomyopathy in various rodent model systems. The cardiotoxicity of AZT is complex and likely involves multiple interrelated mechanisms, such as the inhibition of cardiac mitochondrial DNA polymerase-γ (4), alterations in pyrimidine deoxyribonucleotide pools by the inhibition of thymidine kinase 2 (7), increased myocardial oxidative stress and attenuated antioxidant defense (5,6,9,26,27), oxidative damage in cardiac mitochondria (2,3), increased endothelial cell oxidative stress and mitochondrial dysfunction (8,11), and activation of the nuclear enzyme PARP (9). Supporting the role of oxidative stress in the development of AZT-induced cardiomyopathy, transgenic mitochondrial superoxide dismutase and mitochondrially-targeted catalase prevent antiretroviral drug-induced oxidative stress and cardiomyopathy in mice (12).
Consistent with these reports, we demonstrated increased concentration-dependent mitochondrial ROS generation by AZT in human cardiomyocytes. Increased ROS generation in cardiomyocytes may trigger the activation of various mitochondrial-dependent and -independent cell death pathways involved in apoptotic and necrotic cell death (e.g., activation of caspases and PARP-1) (28). Furthermore, superoxide in the mitochondria may react with nitric oxide to generate a highly reactive oxidant, peroxynitrite (29), which may impair cellular function and lead to cell death (25,30) and/or dysfunction (31) in cardiomyocytes via multiple interrelated mechanisms. Indeed, AZT induced caspase-3 and -7 and PARP-dependent cell death in cardiomyocytes.
Numerous recent studies provide evidence that resveratrol treatment attenuates myocardial ischemic reperfusion injury and atherosclerosis (13), and improves endothelial function in rodent models of type 2 diabetes (21,32,33), as well as in aged mice and rats (21,34). The multiple synergistic effects of resveratrol are likely to be responsible for its cardiovascular protective potential, which may include up-regulation of eNOS, increased NO bioavailability (32,35,36), promotion of mitochondrial biogenesis (37), down-regulation of TNF-α and inhibition of NADPH oxidases (32), inhibition of NF-κB (38-40) and attenuation of mitochondrial ROS generation (33), among others. Consistent with its antioxidant properties, resveratrol treatment attenuated the AZT-induced mitochondrial ROS generation and the activation of caspase-3 and -7 and the PARP-dependent cell death pathways in human cardiomyocytes.
Collectively, these results demonstrate that AZT induces increased mitochondrial ROS generation in human cardiomyocytes, triggering the activation of caspase-3 and -7 and the PARP-1-dependent cell death pathways. AZT-induced cell death is attenuated by resveratrol pre-treatment, which decreases mitochondrial roS generation, providing a potential strategy for the attenuation of these antiretroviral-therapy associated pathological alterations.
Acknowledgements
This study was supported by the Intramural Research Program of the National Institutes of Health/NIAAA (to P.P.). B.H. is a recipient of a Fellowship from the Hungarian Scientific Research Foundation: NKTH-OTKA-EU (MB08A-80238).
References
- 1.Currie PF, Boon NA. Immunopathogenesis of HIV-related heart muscle disease: current perspectives. AIDS. 2003;17(Suppl 1):21–28. doi: 10.1097/00002030-200304001-00004. [DOI] [PubMed] [Google Scholar]
- 2.De la Asuncion JG, Del Olmo ML, Gomez-Cambronero LG, Sastre J, Pallardo FV, Vina J. AZT induces oxidative damage to cardiac mitochondria: protective effect of vitamins C and E. Life Sci. 2004;76:47–56. doi: 10.1016/j.lfs.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Lewis W, Papoian T, Gonzalez B, et al. Mitochondrial ultra-structural and molecular changes induced by zidovudine in rat hearts. Lab Invest. 1991;65:228–236. [PubMed] [Google Scholar]
- 4.Lewis W, Simpson JF, Meyer RR. Cardiac mitochondrial DNA polymerase-gamma is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circ Res. 1994;74:344–348. doi: 10.1161/01.res.74.2.344. [DOI] [PubMed] [Google Scholar]
- 5.Mak IT, Goldfarb MG, Weglicki WB, Haudenschild CC. Cardiac pathologic effects of azidothymidine (AZT) in Mg-deficient mice. Cardiovasc Toxicol. 2004;4:169–177. doi: 10.1385/ct:4:2:169. [DOI] [PubMed] [Google Scholar]
- 6.Mak IT, Nedelec LF, Weglicki WB. Pro-oxidant properties and cytotoxicity of AZT-monophosphate and AZT. Cardiovasc Toxicol. 2004;4:109–115. doi: 10.1385/ct:4:2:109. [DOI] [PubMed] [Google Scholar]
- 7.Morris GW, Iams TA, Slepchenko KG, Mckee EE. Origin of pyrimidine deoxyribonucleotide pools in perfused rat heart: implications for 3'-azido-3'-deoxythymidine-dependent cardiotoxicity. Biochem J. 2009;422:513–520. doi: 10.1042/BJ20082427. [DOI] [PubMed] [Google Scholar]
- 8.Sutliff RL, Dikalov S, Weiss D, et al. nucleoside reverse transcriptase inhibitors impair endothelium-dependent relaxation by increasing superoxide. Am J Physiol Heart Circ Physiol. 2002;283:H2363–H2370. doi: 10.1152/ajpheart.00151.2002. [DOI] [PubMed] [Google Scholar]
- 9.Szabados E, Fischer GM, Toth K, et al. Role of reactive oxygen species and poly-ADP-ribose polymerase in the development of AZT-induced cardiomyopathy in rat. Free Radic Biol Med. 1999;26:309–317. doi: 10.1016/s0891-5849(98)00199-3. [DOI] [PubMed] [Google Scholar]
- 10.Freyssenet D, DiCarlo M, Escobar P, Grey J, Schneider J, Hood DA. Zidovudine (AZT) induced alterations in mitochondrial biogenesis in rat striated muscles. Can J Physiol Pharmacol. 1999;77:29–35. doi: 10.1139/cjpp-77-1-29. [DOI] [PubMed] [Google Scholar]
- 11.Kline ER, Bassit L, Hernandez-Santiago BI, et al. Long-term exposure to AZT, but not d4T, increases endothelial cell oxidative stress and mitochondrial dysfunction. Cardiovasc Toxicol. 2009;9:1–12. doi: 10.1007/s12012-008-9029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler JJ, Cucoranu I, Fields E, et al. Transgenic mitochondrial superoxide dismutase and mitochondrially targeted catalase prevent antiretroviral-induced oxidative stress and cardiomyopathy. Lab Invest. 2009;89:782–790. doi: 10.1038/labinvest.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature Reviews. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology. 2006;76:69–75. doi: 10.1159/000089720. [DOI] [PubMed] [Google Scholar]
- 16.Thirunavukkarasu M, Penumathsa SV, Koneru S, et al. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–729. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zang M, Xu S, Maitland-Toolan KA, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 18.Barger JL, Kayo T, Vann JM, et al. a low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay P, Rajesh M, Batkai S, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358:203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay P, Rajesh M, Batkai S, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–H1483. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weglicki WB, Chmielinska JJ, Tejero-Taldo I, et al. Neutral endopeptidase inhibition enhances substance P mediated inflammation due to hypomagnesemia. Magnes Res. 2009;22:S167–S173. [PMC free article] [PubMed] [Google Scholar]
- 27.Papparella I, Ceolotto G, Berto L, et al. Vitamin C prevents zidovudine-induced NAD(P)H oxidase activation and hypertension in the rat. Cardiovasc Res. 2007;73:432–438. doi: 10.1016/j.cardiores.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levrand S, Vannay-Bouchiche C, Pesse B, et al. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med. 2006;41:886–895. doi: 10.1016/j.freeradbiomed.2006.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–310. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ungvari Z, Labinskyy N, Mukhopadhyay P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungvari Z, Orosz Z, Rivera A, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 35.Bradamante S, Barenghi L, Piccinini F, et al. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol. 2003;465:115–123. doi: 10.1016/s0014-2999(03)01441-9. [DOI] [PubMed] [Google Scholar]
- 36.Taubert D, Berkels R. Upregulation and activation of eNOS by resveratrol. Circulation. 2003;107:e78–79. doi: 10.1161/01.cir.0000060819.46705.ee. [DOI] [PubMed] [Google Scholar]
- 37.Csiszar A, Labinskyy N, Pinto JT, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 40.Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]