Abstract
Chemokine receptor 4 (CXCR4) is expressed in a variety of cancers including breast, brain, ovarian and prostate. CXCR4/CXCL12 interactions are critical for tumor development, growth and metastasis. Neoplastic tissue including metastases express high levels of CXCR4 compared to normal tissue. Previous clinical and preclinical observations suggest that CXCR4 levels could be used as a predictive marker of metastatic potential. Here we report single photon emission computed tomography (SPECT)/CT imaging of CXCR4 expression levels in experimental brain tumors using 125I-labeled anti-CXCR4 monoclonal antibodies (rmAbs). hCXCR4 antibody 12G5 and control IgG2A antibody were radiolabeled by the Iodogen method. rmAbs were obtained in 40 – 60% yield, with 1.4 – 1.9 MBq/µg specific radioactivities and >95% purity. SCID mice harboring U87 xenografts were used for ex vivo biodistribution and imaging studies. Surface CXCR4 expression levels on U87 tumor derived cells (U87-TMD) were analyzed by flow cytometry. Biodistribution and imaging studies showed specific accumulation of [125I]12G5 in U87 tumors with tumor/muscle uptake ratios reaching 15 ± 3 at 48 h postinjection. The tumor/tumor uptake ratio for [125I]12G5/[125I]IgG2A was 2.5 at 48 h postinjection. Flow cytometry analysis of tumor derived cells showed 2–7 fold increase in CXCR4 expression relative to inoculums accounting for the high rmAB uptake observed in the tumors. Our data demonstrate the feasibility of imaging CXCR4 expression in experimental brain tumors. The elevated CXCR4 levels observed may have been, in part, due to hypoxic tumor microenvironment.
Keywords: hypoxia, tumor microenvironment, molecular imaging, xenograft, chemokine
Introduction
Interactions between chemokines and their receptors are emerging as an important group of mediators within the tumor microenvironment. Of the known chemokines stromal derived factor 1, or CXCL12, and its receptor CXCR4, have gained attention due to their role in promoting cancer metastasis and stem cell homing and engraftment. CXCR4 expression has also been proposed as a prognostic factor in several cancers including brain, breast, colon, prostate, melanoma, and osteosarcoma (1).
Several brain tumor cell lines, primary tumors and metastases demonstrate high concentrations of CXCR4 receptors compared to normal brain parenchyma (2, 3). CXCR4 levels were elevated with respect to tumor grade, demonstrating the highest levels in grade IV tumors (glioblastomas) (2). Rempel et al. showed that the CXCR4/SDF1 axis modulates VEGF expression and angiogenesis as well as the immune response (2). Low molecular weight inhibitors targeting CXCR4 not only inhibit the growth of primary brain tumors but also synergize with standard, cytotoxic chemotherapy (4, 5). Those observations suggest that CXCR4 could be an important target for imaging primary tumors and metastases within the neuraxis, possibly providing important prognostic information or for the purpose of therapeutic monitoring.
Recent improvements in diagnostic imaging have enabled early detection and management of cancer (6). For example, mammograms are the current clinical mainstay of breast cancer imaging – serving as an example of a successful image-based screening test for cancer. Magnetic resonance (MR) imaging is frequently used to delineate the extent of disease in breast, central nervous system (CNS) and other cancers (7). Highly sensitive functional imaging techniques such as positron emission tomography (PET) with [18F]fluorodeoxyglucose (FDG), for glucose metabolism, and [18F]fluorothymidine (FLT)-PET, for tumor proliferation, are used for diagnosis, staging and therapeutic monitoring of cancer (8, 9). However, these metabolic imaging techniques cannot detect specific receptor expression in the tumor, such as estrogen receptor (ER), HER2 or CXCR4, which have important prognostic implications (10, 11). Noninvasive imaging studies of ER and HER2 expression by nuclear imaging techniques are under way (12–14). Of these receptors only CXCR4 is directly involved in the metastatic process and is a well characterized biomarker for direct imaging of tumor metastatic potential (15–17).
Because of their target-binding specificity, monoclonal antibodies (mAbs) provide an attractive choice as a molecular scaffold for radiopharmaceutical-based imaging. That potential has largely gone unrealized due to the relatively poor pharmacokinetic properties of mAbs, such as long circulation time and large size, mitigating solid tumor penetration. However, radiolabeled mAbs generated recently are providing viable clinical imaging agents (18). Due to the importance of CXCR4 in brain tumor development, growth and metastasis we generated a radiolabeled version of a mouse anti-human CXCR4 antibody (12G5) (19) and used it to evaluate CXCR4 expression in U87 human glioblastoma xenografts using single photon emission computed tomography (SPECT).
Materials and Methods
Cell lines
The human glioblastoma cell line U87 was purchased from American Type Culture Collection (Rockville, MD) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 mg/mL of streptomycin. A U87 cell line stably transfected with human CD4 and CXCR4 (U87-stb-CXCR4) was obtained from the NIH AIDS Research and Reference Reagent Program. The U87-stb-CXCR4 cell line was maintained in DMEM supplemented with 15%FBS, 1µg/mL puromycin, 300 µg/mL G418, 100 U/mL of penicillin, and 100 mg/mL of streptomycin. Both the cell lines were maintained in a humidified incubator with 5% CO2. All cell culture reagents were purchased from Invitrogen (Gibco, Invitrogen, Carlsbad, CA).
Antibody radiolabeling
The mouse antihuman CXCR4 antibody (clone 12G5) was obtained from the NIH AIDS Research and Reference Reagent Program as well as purchased from R&D systems, Inc. (Cat. no. MAB170). Isotype matched control mouse IgG2A antibody was purchased from R&D systems, Inc. (Clone 20102, Cat. No. IC003P). [125I]NaI was purchased from MP Biomedicals (Costa Mesa, CA). Antibodies were labeled using a modified version of the Iodogen method (1). Briefly, 37 – 74 MBq (1 – 2 mCi) of [125I]NaI was incubated with 25 µg of purified antibody in 50–100 µL of phosphate buffered saline (PBS) in an Iodogen coated reaction vial. Reaction times varied between 5 – 10 min. Radiolabeled antibody was purified from low molecular weight impurities using size exclusion chromatography on a Sephadex G-25 desalting column (Amersham Biosciences, Piscataway, NJ) preconditioned with PBS, pH 7.4. Protein purities were determined by instant thin layer chromatography.
Receptor binding assays
U87 and U87-stb-CXCR4 cells seeded in 6 well plates at 60 – 80% confluence were used for receptor binding assays. Cells were incubated with 37 kBq (1µCi)/mL of either [125I]IgG2A or [125I]12G5 in PBS supplemented with 0.5% BSA and 2 mM EDTA for 30 and 60 min at 4°C. After incubation, cells were washed quickly four times with 4°C binding buffer, trypsinized using non-enzymatic buffer and cell associated activity was determined in a gamma spectrometer (1282 Compugamma CS: Pharmacia/LKB Nuclear, Inc., Gaithersburg, MD). Radioactivity values were converted into percentage of incubated dose per µg of total protein. Experiments were performed in triplicate and repeated three times. Immunoreactive fraction (IF) of [125I]12G5 was determined using U87-stb-CXCR4 cells as described previously (20). Data were fitted according to linear regression analysis using PRIZM software and the extrapolation to the ordinate was used to calculate the immunoreactive fraction.
Animal models
Male Balb/C SCID mice, six to eight weeks old, weighing between 25 – 30 g were purchased from the National Cancer Institute (Frederick, MD). All of the experimental procedures using the animals were conducted according to protocols approved by the Johns Hopkins Animal Care and Use Committee. Mice were implanted subcutaneously with U87 cells (4 × 106 cells/100 µL) in the right upper flank. After two to three weeks, when the tumor size was 600 – 800 mm3, the animals were used for biodistribution and SPECT/CT imaging experiments.
SPECT-CT imaging
An X-SPECT small animal SPECT/CT system (Gamma Medica, Northridge, CA) was used for image acquisition. Three mice for each radiolabeled antibody were used for imaging studies. After an intravenous injection of 37 MBq (1 mCi) of radiolabeled antibody, images were acquired at 24, 48 and 72 h postinjection. The SPECT projection data were acquired using two low energy, high resolution parallel-hole collimators with a radius-of-rotation of 4.65 cm (spatial resolution 1.6 mm). The tomographic data were acquired in 64 projections over 360 degrees at 40 sec/projection. Following tomography, CT imaging was acquired in 512 projections to allow anatomic coregistration. Data were reconstructed using the ordered subsets-expectation maximization algorithm and analyzed using AMIDE software (free software provided by SourceForge). Maximum intensity projection images were generated using Amira 5.2.0 software (Visage Imaging Inc., Carlsbad, CA).
Ex vivo biodistribution
Animals were injected with 74 kBq (2 µCi) of either [125I]IgG2A or [125I]12G5 in 200 µL of saline via tail vein injection. At 24, 48, 72, 96 and 120 h following injection, blood, lung, muscle, heart, spleen, stomach, small intestine, kidney, liver, and tumor samples were collected, weighed, and their radioactivity content was determined in an automated gamma spectrometer. Aliquots of the injected tracer were counted along with the samples and served as standards for the calculation of the percentage injected dose per gram of tissue (%ID/g). Three animals per time point were used. Ratios [125I]12G5 uptake to control [125I]IgG2A antibody uptake as well as tumor-to-muscle and tumor-to-blood ratios were calculated. One set of mice with tumor size <200 mm3 were also used for biodistribution studies.
Tumor cell extraction and flow cytometry
Tumors from an additional group of five mice were extracted and CXCR4 expression levels were analyzed by flow cytometry and immunoblot analysis. Briefly, U87 tumors were cut into multiple small cubes and dissociated at 37°C for 30 min with 0.5 mg/mL of collagenase II in DMEM medium. The cell suspension was filtered through a cell strainer with 70 µm nylon mesh (Becton Dickinson Biosciences, Franklin Lakes, NJ). The dissociation procedure was repeated three times to maximize the cell yield. Cells collected by centrifugation were washed three times with DMEM fortified with 10% fetal bovine serum and cultured in DMEM medium as described above. The tumor extracted cells were seeded at 2 × 106 cells/mL in 100 mm plates and used for flow cytometry analysis after one or two passages. At 50 – 70% confluence, cells were washed twice with PBS buffer and plates were placed on ice to prevent receptor internalization. Cells were detached using a nonenzymatic cocktail (Cat. no. C5914, Sigma). To induce hypoxic conditions in vitro, U87 cells were incubated with an hypoxia mimetic (0.1 mM of CoCl2) for 48 hours prior to processing. The expression levels of CXCR4 in the normoxic and hypoxic conditions were determined by immunostaining with CXCR4 12G5 mAb conjugated to PE (Cat. no. FAB170P, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Cells were analyzed for CXCR4 expression on a FACSCalibur flow cytometer. Ten thousand events were acquired in list mode and data were analyzed using Cellquest software.
Protein lysates and immunoblot analysis
U87 and tumor derived U87 (U87 – TMD) cells in 100 mm plates were washed twice with PBS and homogenized with TB Buffer [100 mM Tris (pH 6.7), 2 % SDS, 12 % glycerol] containing protease inhibitor (PI) cocktail (Sigma, St. Louis, MO). Protein concentrations were determined using a Biorad protein assay kit (Biorad laboratories, Hercules, CA). Standard concentrations of bovine serum albumin were used to obtain a calibration curve. Aliquots containing 50 µg of the total protein along with a molecular weight standard were loaded and electrophoretically separated on a 10% polyacrylamide gel. Separated proteins were transferred onto nitrocellulose membranes and blocked with 5% nonfat milk with 0.05% Tween-20 for one hour at room temperature. Membranes were then incubated with the rabbit anti-CXCR4 polyclonal antibody (1:500 dilution) raised against a peptide corresponding to amino acids 1 to 14 of human CXCR4 (ProSci Inc., Poway, CA) followed by mouse anti-rabbit secondary antibody conjugated to horseradish peroxidase (1: 2,500 dilution). After three washes bands were visualized using the Supersignal West Pico chemiluminescent substrate kit (Pierce Biotechnology Inc., Rockford, IL). β-Actin served as a control.
Data analysis
Statistical analysis was performed using an unpaired two tailed t-test. P-values of less than 0.05 for the comparison between CXCR4 specific and control antibody uptake were considered to be statistically significant.
Results
Antibody radiolabeling
Yields for radioiodination were typically 40 – 60 % with purities greater than 95%. Specific radioactivities of the iodinated antibody were 1.85 and 1.48 MBq/µg for [125I]12G5 and [125I]IgG2A, respectively.
Antibody specificity
As demonstrated in Figure 1, [125I]12G5 showed > 98 % specificity towards U87-stb-CXCR4 cells. The levels of [125I]12G5 binding to U87 were similar to but significantly different (P = 0.04) from that of control [125I]IgG2A antibody. These results were in agreement with the low surface CXCR4 expression levels observed in U87 cells with flow cytometry (Figures 1 & 5). The immunoreactive fraction of the radiolabeled antibody was found to be 0.87 as indicated in Figure 1B.
Figure 1. [125I]12G5 binding specificity to CXCR4.
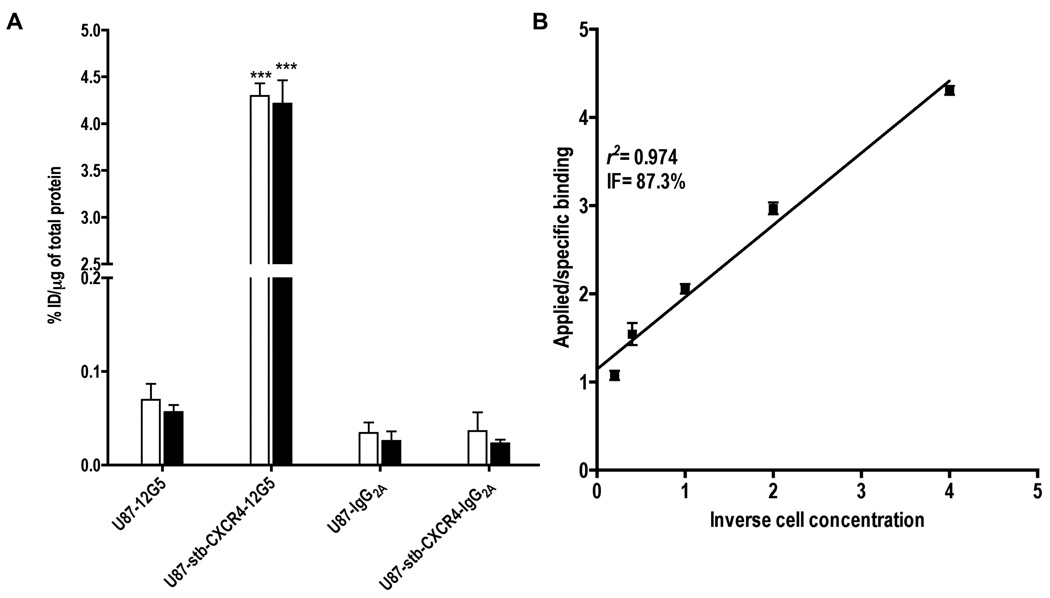
U87 and U87-stb-CXCR4 cells at 60 – 70% confluency in 6 well plates were incubated with 37kBq (1µCi)/mL of rmAbs at 4°C for 30 (clear bars) and 60 (solid bars) min. Data are represented as percentage of incubated dose per microgram of protein (%ID/µg) and represent mean values of three experiments ± the standard error of the mean (SEM) (Panel A). The significance of the value is indicated by asterisks (*) and the comparative reference is the control cell line for the respective rmAb. *P < 0.05, **P < 0.01, ***P < 0.001. Immunoreactivity plot of [125I]12G5 for U87-stb-CXCR4 cells (Panel B).
Figure 5. Flow cytometry and immunoblot analysis of CXCR4 expression.
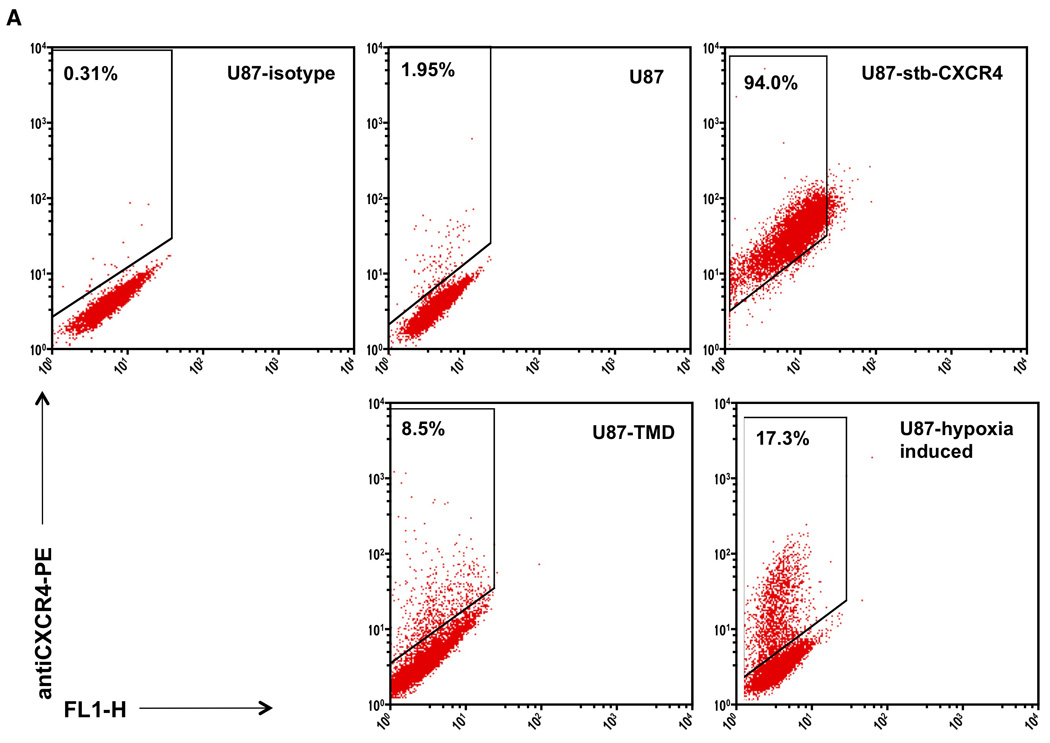
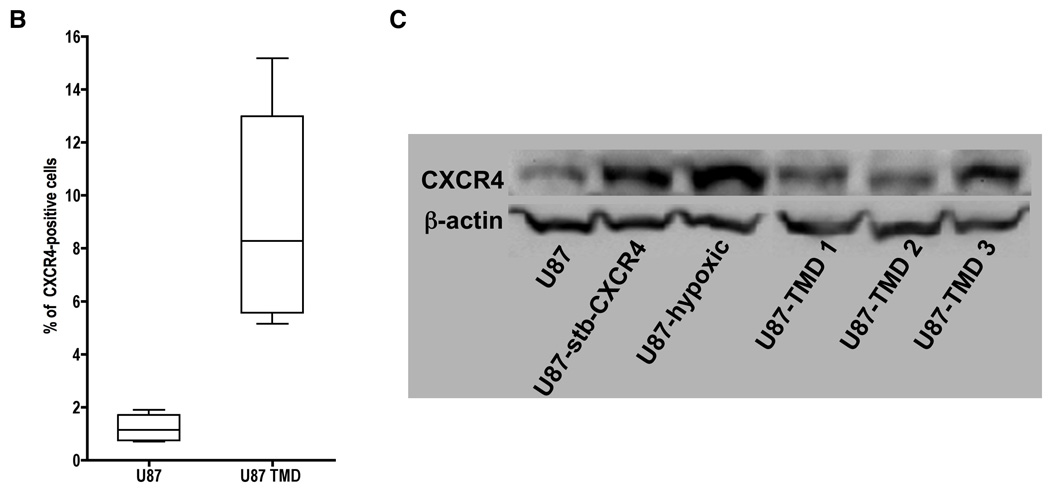
U87 cells stained with matched isotype control IgG2A-PE as well as U87, U87-stb-CXCR4 representative U87 TMD and U87-hypoxia induced cell lines stained with 12G5-PE were analyzed on a BD FACScan instrument (Panel A). Whisker graph showing the CXCR4 levels in U87-TMD cells. U87 cells from different passages were used as control (Panel B). Immunoblot analysis of CXCR4 expression in U87, U87-hypoxic and U87-TMD cells (Panel C).
SPECT/CT imaging
SPECT/CT imaging of U87 tumors with [125I]12G5 demonstrated a clear and specific accumulation of radioactivity in the U87 tumors by 24 hrs and the radioactivity was slowly clearing by 72 h (Figure 2A). The tumor/nontumor contrast was maximum at 48 h after injection. The control [125I]IgG2A antibody also showed accumulation of radioactivity, although less than [125I]12G5 (Figure 2B). Maximum intensity projection of [125I]12G5 showed highest uptake in the spleen (Figure 2C).
Figure 2. SPECT/CT imaging of CXCR4 expression in subcutaneous U87 xenografts with rmAbs.
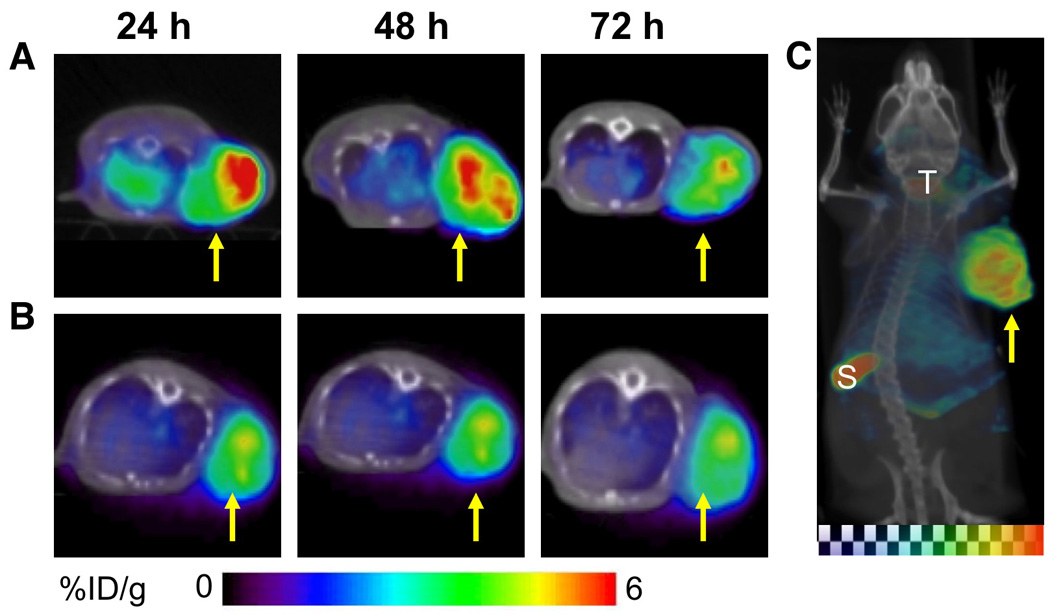
SCID mice bearing U87 glioblastoma xenografts were given 37 MBq (1 mCi) of either [125I]12G5 (Panel A) or [125I]IgG2A (Panel B) via tail vein injection. SPECT/CT images were acquired at 24, 48 and 72 h postinjection of the rMAbs. All the images were adjusted to the same maximum value to show the relative active concentrations of the tracers. Arrow depicts tumor in the coronal sections. Maximum intensity projection image of [125I]12G5 injected mouse at 48 hrs post injection (Panel C); arrow, tumor; S, spleen; T, thyroid.
Ex vivo biodistribution
To quantify the degree of radiopharmaceutical uptake on a per organ basis, U87 tumor bearing animals were injected with either [125I]12G5 or [125I]IgG2A antibody and were followed for five days. Figure 3 shows the combined experiments with both antibodies at 24, 48 and 72 h postinjection. The animals injected with [125I]12G5 animals showed consistently higher tumor uptake relative to animals that received [125I]IgG2A. The [125I]12G5/[125I]IgG2A ratios were 2.1, 2.5 and 1.7 at 24, 48 and 72 h postinjection, respectively. In the case of [125I]12G5 antibody, the amount of radioactivity increased in tumors until 48 h and then decreased where as the radioactivity decreased with time after 24 h in normal organs. The [125I]IgG2A antibody showed monotonic decrease in radioactivity both in tumors and normal organs. The tissue/muscle ratios of [125I]12G5 reached a maximum at 48 h postinjection but those of [125I]IgG2A remained uniform until 72 hrs (Figure 4). Both radiolabeled antibodies demonstrated uniform uptake in all non-tumor organs. Rapid clearance of activity from the blood was observed perhaps due to deiodination of the antibody. Spleen was noted to have the highest accumulation of radioactivity at all times.
Figure 3. Ex vivo biodistribution data for rmAbs in tumor bearing SCID mice.
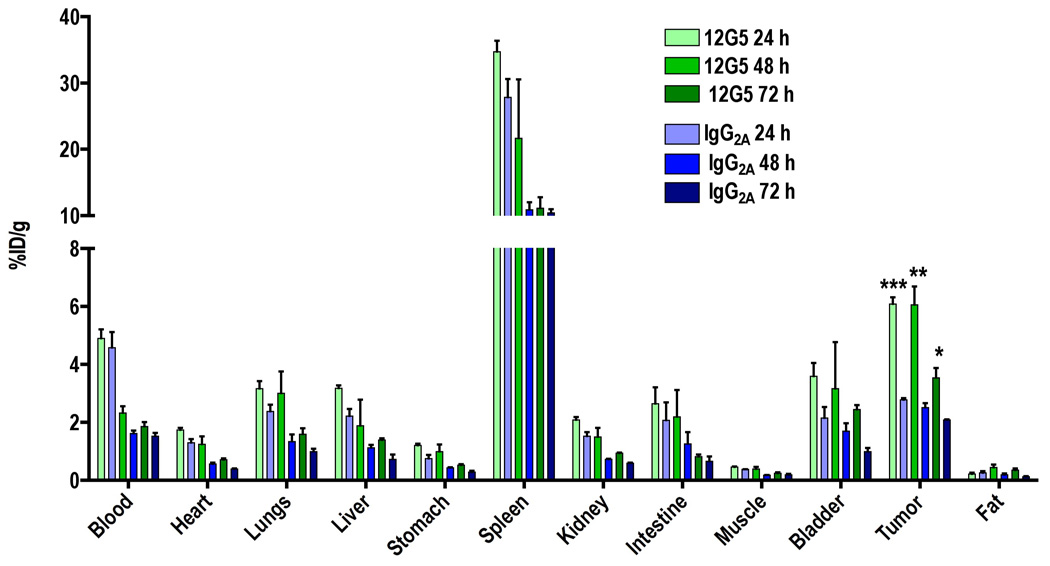
SCID mice harboring U87 glioblastoma xenografts were given 74 kBq (2 µCi) of either [125I]12G5 or [125I]IgG2A via tail vein injection. At 24, 48 and 72 h postinjection selected tissues and tumors were harvested, weighed and radioactivity was counted in gamma spectrometer. All the values were converted into percentage of injected dose per gram of tissue (%ID/g). Data are means ± SEM of three animals. The significance of the value is indicated by asterisks (*) and the comparative reference is the [125I]IgG2A uptake. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4. Time-activity curves for rmAbs in U87 xenografts.
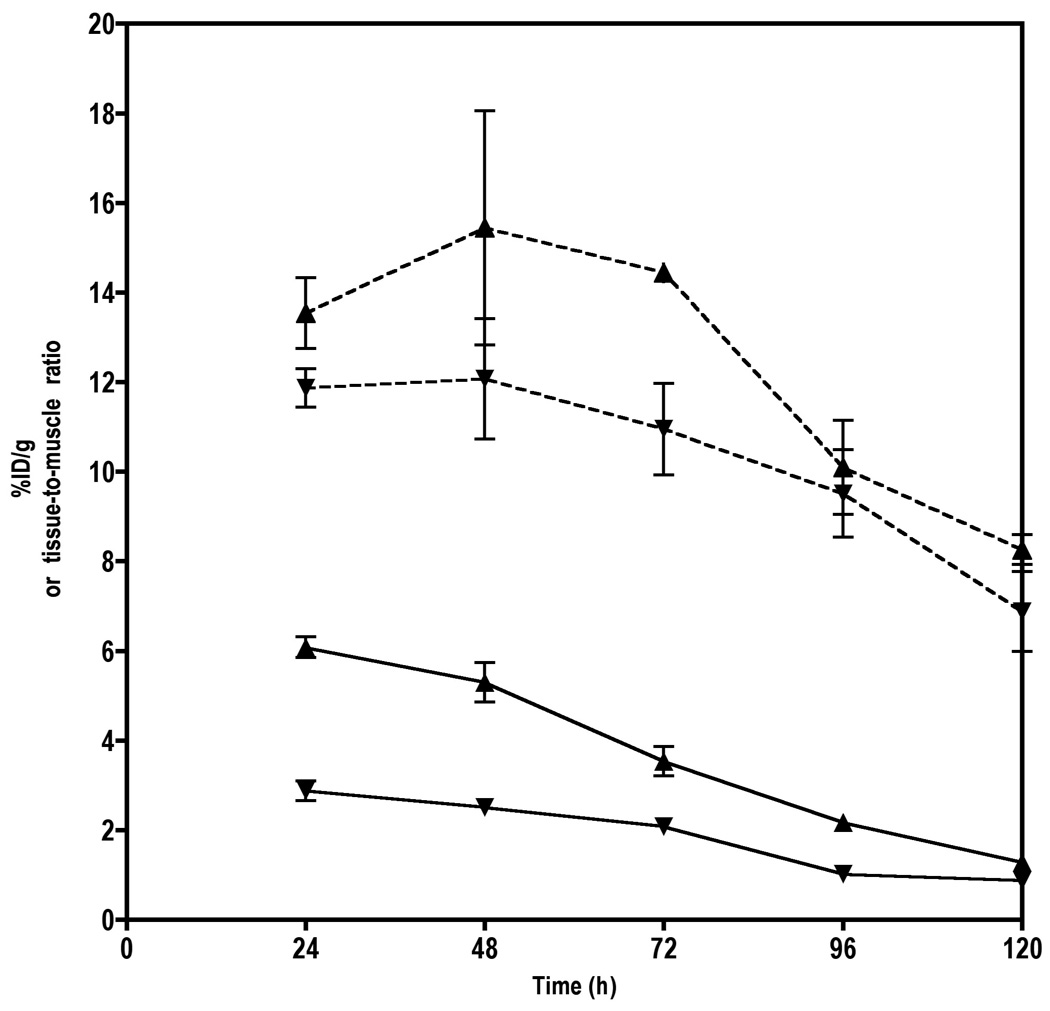
Tumor time activity curves (solid lines) and tumor-to-muscle ratios (dashed lines) of either [125I]12G5 (▲) and [125I]IgG2A (▼) in U87 tumor bearing SCID mice. Data are means ± SEM of three animals.
Flow cytometry of tumor derived cells and immunoblotting
To assess the percentage of CXCR4-positive cells within the tumors, cells were extracted and analyzed by flow cytometry after one or two passages. The tumor derived cells showed a two- to seven-fold increase in surface CXCR4 expression levels (Figure 5A – G). Because most of the radioactivity observed was within the center of the tumors, we were prompted to investigate further the origin of this result. Because CXCR4 is known to have a hypoxia responsive element (HRE) in the promoter region (21), we suspected that hypoxic upregulation of CXCR4 expression occurred within the tumors, and was more pronounced near the core of the tumors, where oxygen tension was expected to be lowest (22). U87 cells maintained under hypoxic conditions showed an increase in CXCR4 levels suggesting the role of hypoxia in driving CXCR4 expression. The total protein expression levels also indicated a similar pattern (Figure 5G).
Discussion
To monitor CXCR4 expression noninvasively, we radioiodinated and evaluated a CXCR4 mAb in mice harboring subcutaneous U87 brain tumor xenografts using SPECT/CT. We also associated the observed tumor uptake and retention of radioactivity with the surface and total CXCR4 protein expression levels that may have been upregulated by clonal expansion of high CXCR4 expressing cells.
As mentioned at the outset, CXCR4 expression has been proposed as a prognostic marker in several cancers (1). In addition, metastases often have elevated levels of CXCR4 expression (17, 23). Therefore, imaging agents for noninvasive detection of CXCR4 would be beneficial not only for the assessment of metastatic potential of the primary tumor but also for detection of systemic metastases. CXCR4 is expressed in several organs including spleen, thymus, lung and bone marrow, therefore therapeutic agents targeted toward CXCR4 may result in unwanted toxicity (24). Imaging patients prior to therapy with a CXCR4-based imaging agent may enable avoidance of toxicity through quantification of normal organ radiopharmaceutical distribution.
Noninvasive detection of CXCR4 levels could be achieved using suitably functionalized, specifically targeted low molecular weight agents or mAbs. Currently, there is only one reported attempt to image CXCR4 expression in cancer using nuclear imaging techniques. Hanaoka et al. developed and evaluated 111In labeled DTPA-Ac-TZ14011, a peptide analog of CXCL12. The authors found that conjugation with DTPA and labeling with 111In reduced the specificity of the analog to CXCR4 by 6 fold (25). To investigate the feasibility of CXCR4-based imaging, we radiolabeled a well characterized CXCR4 antibody, 12G5, with [125I]NaI. 12G5 recognizes a determinant in the first and second extracellular loops of CXCR4 and its specificity to CXCR4 is well established (26). Direct antibody radiolabeling with the Iodogen method, did not impair the specificity of [125I]12G5 antibody to CXCR4, as indicated by the in vitro binding studies. Imaging and biodistribution studies demonstrated a significant uptake of [125I]12G5 in the U87 tumors in spite of some nonspecific uptake observed with [125I]IgG2A antibody. The uptake observed by the control antibody suggests that at least a portion of the uptake seen by [125I]12G5 was also not due to receptor-mediated binding, but rather to the enhanced permeability and retention (EPR) effect, commonly observed with macromolecular agents (27). The highest uptake of the radiolabeled mAbs was seen in spleen followed by the tumor. Similar non-specific uptake of rmAbs in liver and spleen has been observed due to a) cross-reactivity with antigen-positive circulating cells, normal B-cells in the blood or spleen; and b) nonantigenic binding of antibody, such as Fc binding (28, 29). Methods have been developed to reduce the non-specific uptake based on predosing strategies (30, 31) that could be applied in the future studies.
The mAb used in this study is known to recognize only certain conformations of CXCR4 receptor (26, 32). Consequently, it is possible that we are underestimating the actual concentration of CXCR4 expression on the cell surface, which may vary between cell types. If they could be generated, other radiolabeled antibodies that detect all conformations of CXCR4 may improve the specificity of radioimmunoimaging of CXCR4, and may also lower the nonspecific binding observed (32).
Biodistribution studies with small tumors (<200 mm3) showed no significant difference between the [125I]12G5 and [125I]IgG2A uptake at 48 h post injection (P=0.08). To further investigate, if the significant retention (P=0.009) of radioactivity observed in larger tumors (600–800 mm3) is due to [125I]12G5 binding to CXCR4 and not merely EPR, we extracted the tumor cells and analyzed for surface CXCR4 expression by flow cytometry after one or two passages. The tumor derived cells showed a two- to seven-fold increase in surface CXCR4 expression. Based on literature evidence, we postulated that the increased CXCR4 expression was either due to tumor hypoxia or clonal selection of high CXCR4 expressing cells. U87 cells grown under conditions simulating hypoxia demonstrated a considerable increase (five- to ten-fold) in surface CXCR4 expression, which could account for the high uptake observed in the tumors. To confirm that the high radio-mAb uptake seen was not just because of changes in surface expression levels but due to an overall increase in protein expression, we also analyzed the total CXCR4 protein levels in U87 wild type and tumor derived cells by immunoblot analysis. The immunoblot results demonstrated a considerable increase in total CXCR4 protein expression levels in both the hypoxia-induced U87 cells and the tumor derived cell lines. Those observed CXCR4 expression levels indicate that the high [125I]12G5 uptake seen in larger tumors could, in part, be due to hypoxia induced CXCR4 expression or tumor microenvironment mediated clonal selection of high CXCR4 expressing cells. Because of the extraction and passage of cells, the HIF-1α levels in the U87-TMD cells could not be assessed. On the other hand, elevated CXCR4 expression levels are known to persist even after 10 passages in culture, perhaps due to clonal selection (33). Even though there is evidence that HIF-1α upregulates CXCR4 expression in glioblastomas (34) it is not clear from our studies if the uptake observed is due to hypoxia or clonal selection.
Even though we used a subcutaneous brain xenograft model to demonstrate the principle, the observed results have translational potential to intracranial models. Due to compromised blood brain barrier, antibodies have been used as therapeutic and imaging agents for brain tumors and other lesions within the central nervous system (35–37). The imaging of CXCR4 expression may be particularly useful in detecting infiltrative brain tumors that are known to have elevated CXCR4 expression levels (38). Those tumors often do not enhance with standard magnetic resonance-based contrast agents, even though they tend to be high-grade.
In summary, these results demonstrate the feasibility of radioimmunoimaging of CXCR4 expression with an antihuman mAb. Also, these results indicate the possibility of imaging tumor microenvironment induced CXCR4 expression, thereby providing an indirect readout on the heterogeneity of tumors.
Acknowledgements
We thank James Fox and Gilbert Green for assistance with imaging, Lee Blosser and Ada Tam for assistance with flow cytometry and Dr. Catherine Foss for helpful discussions. This work was partially supported by grants from the National Cancer Institute, U24 CA92871, and the Education and Research Foundation of the SNM.
References
- 1.Wong D, Korz W. Translating an Antagonist of Chemokine Receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008 Dec 15;14(24):7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 2.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000 Jan;6(1):102–111. [PubMed] [Google Scholar]
- 3.Sehgal A, Keener C, Boynton AL, Warrick J, Murphy GP. CXCR-4, a chemokine receptor, is overexpressed in and required for proliferation of glioblastoma tumor cells. J Surg Oncol. 1998 Oct;69(2):99–104. doi: 10.1002/(sici)1096-9098(199810)69:2<99::aid-jso10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redjal N, Chan JA, Segal RA, Kung AL. CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin Cancer Res. 2006 Nov 15;12(22):6765–6771. doi: 10.1158/1078-0432.CCR-06-1372. [DOI] [PubMed] [Google Scholar]
- 6.Berry DA, Cronin KA, Plevritis SK, et al. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. N Engl J Med %R 101056/NEJMoa050518. 2005 October 27;353(17):1784–1792. doi: 10.1056/NEJMoa050518. 2005. [DOI] [PubMed] [Google Scholar]
- 7.Lehman C, Schnall M. Imaging in breast cancer: Magnetic resonance imaging. Breast Cancer Research. 2005;7(5):215–219. doi: 10.1186/bcr1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benard F, Turcotte E. Imaging in breast cancer: Single-photon computed tomography and positron-emission tomography. Breast Cancer Research. 2005;7(4):153–162. doi: 10.1186/bcr1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenny L, Coombes R, Vigushin D, Al-Nahhas A, Shousha S, Aboagye E. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. European Journal of Nuclear Medicine and Molecular Imaging. 2007;34(9):1339–1347. doi: 10.1007/s00259-007-0379-4. [DOI] [PubMed] [Google Scholar]
- 10.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science %R 101126/science3798106. 1987 January 9;235(4785):177–182. doi: 10.1126/science.3798106. 1987. [DOI] [PubMed] [Google Scholar]
- 11.Woo S, Bae J, Kim C, Lee J, Koo B. A Significant Correlation between Nuclear CXCR4 Expression and Axillary Lymph Node Metastasis in Hormonal Receptor Negative Breast Cancer. Annals of Surgical Oncology. 2008;15(1):281–285. doi: 10.1245/s10434-007-9595-1. [DOI] [PubMed] [Google Scholar]
- 12.Perik PJ, Lub-De Hooge MN, Gietema JA, et al. Indium-111-Labeled Trastuzumab Scintigraphy in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol %R 101200/JCO2005038448. 2006 May 20;24(15):2276–2282. doi: 10.1200/JCO.2005.03.8448. 2006. [DOI] [PubMed] [Google Scholar]
- 13.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006 Jun 20;24(18):2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 14.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988 Oct;169(1):45–48. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 15.Bian XW, Yang SX, Chen JH, et al. Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery. 2007 Sep;61(3):570–578. doi: 10.1227/01.NEU.0000290905.53685.A2. discussion 578–579. [DOI] [PubMed] [Google Scholar]
- 16.Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5(5):R144–R150. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvucci O, Bouchard A, Baccarelli A, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006 Jun;97(3):275–283. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 18.Wu AM. Antibodies and Antimatter: The Resurgence of Immuno-PET. J Nucl Med. 2009 Jan;50(1):2–5. doi: 10.2967/jnumed.108.056887. [DOI] [PubMed] [Google Scholar]
- 19.McKnight A, Wilkinson D, Simmons G, et al. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997 Feb;71(2):1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984 Aug 3;72(1):77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 21.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003 Nov 3;198(9):1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wachsberger P, Burd R, Dicker AP. Tumor Response to Ionizing Radiation Combined with Antiangiogenesis or Vascular Targeting Agents: Exploring Mechanisms of Interaction. Clin Cancer Res. 2003 June 1;9(6):1957–1971. 2003. [PubMed] [Google Scholar]
- 23.Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003 Jun 1;89(3):462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SK, Pillarisetti K. Cutting edge: CXCR4-Lo: molecular cloning and functional expression of a novel human CXCR4 splice variant. J Immunol. 1999 Sep 1;163(5):2368–2372. [PubMed] [Google Scholar]
- 25.Hanaoka H, Mukai T, Tamamura H, et al. Development of a 111In-labeled peptide derivative targeting a chemokine receptor, CXCR4, for imaging tumors. Nucl Med Biol. 2006 May;33(4):489–494. doi: 10.1016/j.nucmedbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Baribaud F, Edwards TG, Sharron M, et al. Antigenically distinct conformations of CXCR4. J Virol. 2001 Oct;75(19):8957–8967. doi: 10.1128/JVI.75.19.8957-8967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 28.Beatty JD, Beatty BG, O'Conner-Tressel M, Do T, Paxton RJ. Mechanisms of tissue uptake and metabolism of radiolabeled antibody--role of antigen: antibody complex formation. Cancer Res. 1990 Feb 1;50(3 Suppl):840s–845s. [PubMed] [Google Scholar]
- 29.Jones PL, Brown BA, Sands H. Uptake and metabolism of 111In-labeled monoclonal antibody B6.2 by the rat liver. Cancer Res. 1990 Feb 1;50(3 Suppl):852s–856s. [PubMed] [Google Scholar]
- 30.Buchsbaum DJ, Wahl RL, Glenn SD, Normolle DP, Kaminski MS. Improved Delivery of Radiolabeled Anti-B1 Monoclonal Antibody to Raji Lymphoma Xenografts by Predosing with Unlabeled Anti-B1 Monoclonal Antibody. Cancer Res. 1992 February 1;52(3):637–642. 1992. [PubMed] [Google Scholar]
- 31.Beatty BG, O'Conner-Tressel M, Do T, Paxton RJ, Beatty JD. Mechanism of decreasing liver uptake of 111In-labeled anti-carcinoembryonic antigen monoclonal antibody by specific antibody pretreatment in tumor bearing mice. Cancer Res. 1990 Feb 1;50(3 Suppl):846s–851s. [PubMed] [Google Scholar]
- 32.Carnec X, Quan L, Olson WC, Hazan U, Dragic T. Anti-CXCR4 monoclonal antibodies recognizing overlapping epitopes differ significantly in their ability to inhibit entry of human immunodeficiency virus type 1. J Virol. 2005 Feb;79(3):1930–1933. doi: 10.1128/JVI.79.3.1930-1933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003 Jun 13;278(24):21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 34.Zagzag D, Lukyanov Y, Lan L, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006 Dec;86(12):1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 35.Kim KJ, Wang L, Su YC, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006 Feb 15;12(4):1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 36.Zalutsky MR, Moseley RP, Coakham HB, Coleman RE, Bigner DD. Pharmacokinetics and Tumor Localization of 131I-Labeled Anti-Tenascin Monoclonal Antibody 81C6 in Patients with Gliomas and Other Intracranial Malignancies. Cancer Res. 1989 May 15;49(10):2807–2813. 1989. [PubMed] [Google Scholar]
- 37.Takasu S, Takahashi T, Okamoto S, et al. Radioimmunoscintigraphy of intracranial glioma xenograft with a technetium-99m-labeled mouse monoclonal antibody specifically recognizing type III mutant epidermal growth factor receptor. J Neurooncol. 2003 Jul;63(3):247–256. doi: 10.1023/a:1024320516341. [DOI] [PubMed] [Google Scholar]
- 38.Ehtesham M, Winston JA, Kabos P, Thompson RC. CXCR4 expression mediates glioma cell invasiveness. Oncogene. 2006 May 4;25(19):2801–2806. doi: 10.1038/sj.onc.1209302. [DOI] [PubMed] [Google Scholar]


