Abstract
Phospholipases A2 (PLA2s) belong to a superfamily of enzymes responsible for hydrolyzing the sn-2 fatty acids of membrane phospholipids. These enzymes are known to play multiple roles for maintenance of membrane phospholipid homeostasis and for production of a variety of lipid mediators. Over 20 different types of PLA2s are present in the mammalian cells, and in snake and bee venom. Despite their common function in hydrolyzing fatty acids of phospholipids, they are diversely encoded by a number of genes and express proteins that are regulated by different mechanisms. Recent studies have focused on the group IV calcium-dependent cytosolic cPLA2, the group VI calcium-independent iPLA2, and the group II small molecule secretory sPLA2. In the central nervous system (CNS), these PLA2s are distributed among neurons and glial cells. Although the physiological role of these PLA2s in regulating neural cell function has not yet been clearly elucidated, there is increasing evidence for their involvement in receptor signaling and transcriptional pathways that link oxidative events to inflammatory responses that underline many neurodegenerative diseases. Recent studies also reveal an important role of cPLA2 in modulating neuronal excitatory functions, sPLA2 in the inflammatory responses, and iPLA2 with childhood neurologic disorders associated with brain iron accumulation. The goal for this review is to better understand the structure and function of these PLA2s and to highlight specific types of PLA2s and their cross-talk mechanisms in these inflammatory responses under physiological and pathological conditions in the CNS.
Keywords: Cytosolic phospholipases A2, Calcium-independent phospholipases A2, Secretory phospholipases A2, NADPH oxidase, NMDA receptor, Inflammatory responses, Cerebral ischemia, Cytokines, Alzheimer’s disease
Introduction
Phospholipases A2 (EC 3.1.1.4) (PLA2s) belong to a superfamily of enzymes responsible for cleaving the sn-2 fatty acids of membrane phospholipids. This reaction results in the release of free fatty acids and lysophospholipids as products. In the healthy cellular system with sufficient supply of ATP and CoASH, the released free fatty acids are readily converted to acyl-CoA and subsequently returned to membrane phospholipids through lysophospholipid acyltransferases. Since arachidonic acid (AA) and docosahexaenoic acid (DHA) are major fatty acids in the sn-2 position of brain phospholipids, the deacylation–reacylation system plays a critical role in controlling the metabolic activity of these polyunsaturated fatty acids.
Although genes encoding multiple forms of PLA2 are expressed in rat brain (Molloy et al. 1998), not all PLA2 function is clarified. Research to understand PLA2s in the central nervous system (CNS) has focused on the group IV cytosolic cPLA2, the group VI Ca2+-independent iPLA2, and the group II secretory sPLA2 (Sun et al. 2004, 2005). With increasing information on the genes and protein expressions of specific PLA2s, enormous effort has been devoted to understand their structure and function, and cellular and subcellular localization. While cPLA2α is ubiquitously expressed in neurons and glial cells, the signaling pathways for regulating its activity may vary depending on cell types. Immunohistochemistry and in situ hybridization studies revealed distinct distribution patterns for cPLA2β, iPLA2, sPLA2-IIA, and sPLA2V among different cell types in rat cerebellum (Shirai and Ito 2004). Studies to unveil the mechanisms of action of different PLA2s and factors regulating their activities in normal physiological and pathophysiological functions have increased precipitously. These studies have demonstrated presence of cross-talks among different groups of PLA2s and together, they are crucial in mediating the oxidative and inflammatory responses in CNS pathologies, including stroke, spinal cord injury, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, and Wallarian degeneration (Adibhatla and Hatcher 2008).
The brain is an immune active organ; microglial cells and astrocytes are active players in mediating cerebral innate immunity (Farina et al. 2007; Olson and Miller 2004). These cells respond to the toll-like receptors, which induce synthesis of cytokines and chemokines. In the peripheral system, sPLA2-IIA has been regarded as a mediator to connect innate and adaptive immunity (Ibeas et al. 2009). In this review, special emphasis is placed on recent studies to understand the role of cPLA2 and sPLA2-IIA in mediating neuroinflammatory responses in neurodegenerative diseases.
Cytosolic Phospholipase A2 (cPLA2)
Group IV cPLA2s is comprised of six known paralogs (cPLA2α, cPLA2β, cPLA2γ, cPLA2δ; cPLA2ε, and cPLA2ζ), each with different molecular weights (Burke and Dennis 2009; Ghosh et al. 2006; Shimizu et al. 2006). Among these paralogs, cPLA2α, a 85 kDa protein comprising of a calcium-binding domain (C2) and a catalytic domain, has been extensively studied (Burke and Dennis 2009; Niknami et al. 2009). The cPLA2β, a 114 kDa protein residing at chromosome 15, has also a calcium binding domain. The cPLA2γ, a 61 kDa protein, does not exhibit a calcium binding domain, but contains a prenyl group. The cPLA2δ has a molecular weight of 92–93 kDa, and has significant homology to the C2 domain and the catalytic domain of cPLA2α. In murine lung tissue, cPLA2ζ shows similar properties as cPLA2α, but has different subcellular localization and targets different types of membrane (Ghosh et al. 2007).
cPLA2α Structure
The crystal structure of cPLA2α revealed a N-terminal calcium-dependent C2 domain for docking the protein to membrane, and a catalytic domain with serine 228 as the active site. A number of serine residues in the catalytic domain are substrates for phosphorylation. Around the active site, there is a “lid region” spanning 415–432 residues. Opening the “lid region” to expose the catalytic site can be mediated by a single phospholipid molecule in the membrane surface (Burke et al. 2008). Although there is a putative pleckstrin homology (PH)-like domain (amino acids 263–354) in cPLA2α, its function in binding membrane anionic phospholipids, such as phosphatidylinositol-4,5-bisphosphate (PIP2), remains unclear. Unlike the C2 domain of protein kinase Cα (PKCα), which is directed to target anionic phospholipids in the inner monolayer of plasma membrane, the C2 domain of cPLA2α appears to target phosphatidylcholine (PC) in Golgi and endoplasmic reticulum (Corbin et al. 2007; Ghosh et al. 2006). There is an intricate interplay between localized pools of target lipids and Ca2+ affinity for activation of cPLA2 under physiological conditions. Ability to produce different cPLA2α mutants has provided important information regarding the role of Ser 505 phosphorylation, the basic residues in the catalytic domain in regulating the interplay among calcium and phosphorylation, and its binding to PIP2 (Casas et al. 2006; Tucker et al. 2009; Le Berre et al. 2006). Mutagenesis of anionic residues located or near the active site lid area suggests that electrostatic repulsion exists between the enzyme and membrane phospholipids (Das and Cho 2002). While binding of PIP2 to the protein is required for conferring full enzyme activity, this binding is not responsible for translocation of the enzyme to the membrane (Stahelin et al. 2007; Tucker et al. 2009).
Besides PIP2, ceramide-1-phosphate has been shown to bind cPLA2α at the C2 domain and function as a positive allosteric activator (Pettus et al. 2004; Subramanian et al. 2005; Stahelin et al. 2007; Shimizu et al. 2009). There is evidence that ceramide-1-phosphate binding increases membrane association of cPLA2, and enhances cPLA2 response to inflammatory agonists such as ATP and A23187, which in turn mediates prostaglandin synthesis (Lamour et al. 2009). These studies further suggest that ceramide-1-phosphate and PIP2 regulate cPLA2α by different mechanisms; the former by increasing the residence time of the enzyme on the membrane, and the latter that increases catalytic efficiency through its ability to penetrate into the membrane (Subramanian et al. 2007).
Regulation of cPLA2α by Phosphorylation and Nitrosylation
The cPLA2α activity is regulated by a number of protein kinases, which confer phosphorylation on different serine residues. For example, Ser 505 is phosphorylated by extracellular signal-regulated kinases (ERK1/2) and p38 mitogen-activated protein kinase (MAPK), Ser 515 by Ca2+/calmodulin-dependent protein kinase II (CaMKII), and Ser 727 by MAPK-interacting kinase (MNK1) or a closely related isoform (Muthalif et al. 2001; Lin et al. 1993; Hefner et al. 2000; Geijsen et al. 2000). In murine astrocytes, G-protein-coupled receptor agonists such as ATP/UTP stimulate cPLA2α phosphorylation through PKC-dependent and independent ERK1/2 pathways (Xu et al. 2002). In norepinephrine (NE)-stimulated vascular smooth muscles cells, phosphorylation of cPLA2α at Ser 515 by CaMKII was required for the phosphorylation of Ser 505 by ERK1/2, and both sites of phosphorylation were shown to be important for AA release (Pavicevic et al. 2008). A number of cellular proteins, e.g., p11, vimentin, PLIP, and NADPH oxidase, are known to bind cPLA2. In the study by Tian et al. (2008), p11, a small molecule and member of the S-100 family, was shown to form hetero-tetramer with annexin A2 and regulate cPLA2α at the Ser 727 site. Phosphorylation of cPLA2α at this site resulted in disruption of the interaction of cPLA2α with these binding proteins, leading to cPLA2α activation (Tian et al. 2008). Okadaic acid is among those that activate cPLAα and stimulate AA release, and this event may occur in the absence of an increase in intracellular calcium (Tucker et al. 2008). Under this condition, heat shock protein 90 (hsp90) together with a p54 kinase was shown to regulate activation of cPLA2 by okadaic acid.
A study using Rhesus monkey cerebellum indicated colocalization of cPLA2α with cyclooxygenase-2 (COX-2) in the plasma membrane of Purkinje cells (Pardue et al. 2003). In macrophage cells, production of prostaglandins is mediated through a cPLA2/COX-2 pathway, which in turn regulates expression of the iNOS gene (Pindado et al. 2007). In human epithelial cells, inducible nitric oxide synthase (iNOS)-derived nitric oxide (NO) was shown to enhance cPLA2α activity through S-nitrosylation (Xu et al. 2008). Site-directed mutagenesis revealed Cys 152 as the critical residue for this post-translational modification. This study further suggests that induction of COX-2 under inflammatory conditions could enhance iNOS-induced cPLA2α S-nitrosylation, as this modification leads to a larger increase in AA release and prostaglandin E2 (PGE2) production (Xu et al. 2008). These results suggest possible formation of a multi-protein complex comprised of cPLA2α, iNOS, and COX-2. Activation of iNOS produces NO that can lead to S-nitrosylation of cPLA2α and COX-2, and together constitute a novel mechanism for exacerbating cellular inflammatory responses.
cPLA2 in Neuronal Excitation
There is evolving evidence for the involvement of cPLA2 in regulating neurite outgrowth and neuronal excitatory functions, both under physiological and pathological conditions. In cultured primary cortical neurons, the stimulation of ionotropic glutamate receptors by N-methyl-d-aspartic acid (NMDA) has been shown to activate cPLA2 and AA release (Shelat et al. 2008). Involvement of cPLA2 in neuronal excitotoxicity may be demonstrated by using neurons from cPLA2 knockout mice, which showed less NMDA-mediated injury as compared to the wild-type controls (Shen et al. 2007). The activation of cPLA2 and AA release is also involved in memory retrieval. Retarding cPLA2 activity by infusing an inhibitor palmitoyl trifluoromethyl ketone (PACOCF3) into the rat hippocampus demonstrated a long-term memory retrieval disturbance (Schaeffer and Gattaz 2007). Interestingly, memory impairment was fully reversed once PLA2 activity was recovered. In line with the NMDA-induced Ca2+ entry and activation of cPLA2, AA released at the synapse has been regarded as a retrograde messenger molecule. Another possible function of AA is its ability to activate PKC, which in turn may phosphorylate various substrates including regulation of cytoskeletal dynamics (Leu and Schmidt 2008).
cPLA2 in Regulation of Inflammatory and Immune Functions
Various stimuli including neurotransmitters, P2Y2 receptor agonists, calcium ionophores, phorbol esters, zymogens, and oxidant compounds have been shown to activate cPLA2. In rat astrocytes, nucleotides such as ATP/UTP could stimulate AA release and enhance prostaglandin production through activation of the P2Y2 receptor (Xu et al. 2003b). Astrocytes also respond to pro-inflammatory cytokines, which cause the transcriptional induction of sPLA2-IIA, COX-2, and iNOS (but not cPLA2) (Xu et al. 2003a). This study further showed that cytokine-primed astrocytes became more sensitive to stimulation by ATP/UTP for AA release and PGE2 synthesis (Xu et al. 2003a). Other factors, such as PLA2-activating protein (PLAA), may also regulate cPLA2 activity, and the production of prostaglandins and tumor necrosis factor-alpha (TNFα) (Zhang et al. 2008). These studies demonstrate multiple factors for regulation of cPLA2, and its link with iNOS and COX-2 in exacerbating inflammatory events under pathological conditions.
In a number of cellular systems, activation of cPLA2 is also under regulation of oxidative stress. Menadione, a redox active compound known to produce intracellular reactive oxygen species (ROS), was shown to stimulate MAPK signaling pathways and activation of cPLA2 (Zhu et al. 2009). Furthermore, oxidant compounds such as H2O2 also led to activation of cPLA2, which in turn alters membrane molecular order and cytoskeletal arrangements (Zhu et al. 2005). It is worth noting that under stimulated conditions, AA released from cPLA2 is a good target for lipid peroxidation, as lipid peroxides contribute to the increased oxidative pool in the cells (Nanda et al. 2007). In human synovial cells, IL-1β and TNFα mediated the increase in PLA2 activity and the release of AA, and this inflammatory event was linked to superoxide production through activation of NADPH oxidase (Chenevier-Gobeaux et al. 2007). Studies with neutrophils demonstrated a complex formation between the C2 domain of cPLA2 with the p47 phox and PX domain of NADPH oxidase (Shmelzer et al. 2008). In human tracheal smooth muscle cells, cigarette smoke extract was shown to induce cPLA2 expression and activation involving NADPH oxidase, MAPKs, AP-1, and NF-κB (Cheng et al. 2009). Since oxidative stress is involved in many neurodegenerative diseases, further studies are needed to link the signaling pathways between NADPH oxidase and cPLA2 (Sun et al. 2007).
Due to the elevated cPLA2 expression in inflamed arthritic joints, mechanisms to reduce cPLA2 overexpression have been explored as a potential therapy for this inflammatory disease (Raichel et al. 2008). In animal models where pain and inflammation are indicated, specific cPLA2 inhibitors have been shown to enhance therapeutic effects (Six et al. 2007). Downregulation of cPLA2 by antisense oligonucleotide in rat spinal cords did not change acute nociception, but rather significantly attenuated formalin-induced hyperalgesia (second phase flinching behavior) (Kim et al. 2008). Annexin A1, an inhibitory protein for cPLA2, has been suggested to offer neuroprotective effects to alleviate damage due to spinal cord injury (Liu et al. 2007). In this study, injection of Annexin A1 (ANXA-1) into an acutely injured spinal cord was shown to inhibit inflammatory reactions and tissue damage through reducing cPLA2 (Liu et al. 2007). The roles of different PLA2 isoforms in spinal chord injury have been reviewed recently (Liu et al. 2007; Titsworth et al. 2008).
cPLA2 in Alzheimer’s Disease (AD)
Although an increase in cPLA2 mRNA expression was observed in the hippocampus of AD brains as compared to age-matched controls (Colangelo et al. 2002), there is considerable information suggesting reduced PLA2 activity in blood samples and in the AD brain (Smesny et al. 2008). A study utilizing magnetic resonance imaging (MRI) also shows evidence of reduced phospholipid turnover in the pre-frontal cortex of AD patients (Forlenza et al. 2007b). A fluorometric assay used to measure PLA2 activity in cerebrospinal fluid (CSF) from AD, vascular (VD), and mixed AD-vascular (MD) demented patients indicated that decreased PLA2 activity was not only found in AD, but also in VD and MD subjects (Smesny et al. 2008). During early stages of AD, the cognitive impairment associated with alterations of cholinergic and glutamatergic activities appears to link to a decrease in both cPLA2 and iPLA2 activities (Schaeffer and Gattaz 2008). Since PLA2 has been implicated in neuronal homeostasis and memory formation, efforts to enhance PLA2 activity may be an important therapeutic approach for treatment of AD (Forlenza et al. 2007b; Schaeffer et al. 2009). However, there is apparent contradiction to this notion, as studies also indicate up-regulation of cPLA2 due to the effects of toxic amyloid-beta. This apparent paradox is likely to be due to the differing stages of AD (early vs. late), and the form of PLA2 and cell type involved in mediating the pathological responses.
Increase in oxidative stress is regarded as an important pathological landmark of AD. Several lines of evidence suggest that the oligomeric form of Aβ1-42 (Abeta) is a major source of oxidative stress, which results in neurotoxicity and synaptic impairment. In primary cortical neurons, Abeta can enhance AA release in neurons through activation of cPLA2 (Shelat et al. 2008; Kriem et al. 2005). Inhibition of cPLA2, both by inhibitors and antisense oligonucleotides, can prevent Abeta-induced cell death in neurons (Kriem et al. 2005). Studies with other cell types also demonstrated ability for oligomeric Abeta to activate signaling pathways involving MAPK and cPLA2, which in turn causes mitochondrial dysfunction and cell death (Kriem et al. 2005; Nicotra et al. 2005; Zhu et al. 2006). Squalestatin (as well as simvastatin) was shown to reduce Abeta-induced activation of cPLA2 and PGE2 production and prevented neuronal death (Bate and Williams 2007).
Soluble Abeta oligomers could induce the activation of both neutral and acidic sphingomyelinase (SMase) (Malaplate-Armand et al. 2006). Inhibition of cPLA2 in cortical neurons, both by antisense oligonucleotides and inhibitors, could rescue Abeta-mediated neuronal apoptosis by inhibiting neutral and acidic SMases (Malaplate-Armand et al. 2006). This study further demonstrated the ability for Abeta to stimulate a redox-active mechanism for activation of cPLA2, which in turn induces neuronal apoptosis through a cPLA2-dependent sphingomyelinase–ceramide pathway.
The cPLA2 can also regulate the release of soluble amyloid precursor protein-α (sAPPα), a protein fragment released upon cleavage of APP by alpha secretase. In SH-SY5Y neuroblastoma cells, sAPPα release induced by muscarinic receptor activation was inhibited by a PLA2 inhibitor (Cho et al. 2006). Treatment of SH-SY5Y neuroblastoma cells with interferon-γ (IFNγ) increased cPLA2 expression and neuronal death in response to Abeta (Bate et al. 2006). In this study, pretreatment with IFNγ sensitized neurons to the toxic effects of Abeta or HuPrP82-146 (a neurotoxic peptide found in Prion disease), and increased PGE2 production. This effect is attributed to an increase in the levels of cPLA2. The IFNγ effect could be found in cortical and cerebellar neurons, as well as in SH-SY5Y cells. After a single episode of oxidative stress induced by intracerebroventricular injection of ferrous ammonium citrate to the rat hippocampus, a significant increase in the number of pyramidal neurons that were immunolabeled for cPLA2 and stained for 4-hydroxynonenal (HNE) were observed (Ong et al. 2005a). 24S-Hydroxycholesterol is elevated in Alzheimer’s diseased brain. In a primary human co-culture of neurons and glia, 24S-hydroxycholesterol elevated the expression of a proinflammatory gene family that included βAPP, COX-2, and cPLA2 along with heat shock protein 70, of which this effect was partially inhibited by simvastatin (Alexandrov et al. 2005). Using a lipidomic approach, an increase in AA and its metabolites was observed in a transgenic mouse model of AD with neuronal expression of familial AD mutant hAPP (hAPP mice). The increase in AA correlated with increased activated cPLA2 in the hippocampus of AD human brains and hAPP mice. Interestingly, crossing hAPP mice with mice deficient in cPLA2α was shown to significantly reduce the learning and memory deficits observed in the hAPP mice (Sanchez-Mejia et al. 2008).
Calcium-Independent Phospholipase A2 (iPLA2)
The group VI iPLA2 is probably the most abundant type of PLA2 in cells. Not only are they responsible for regulation of membrane phospholipid homeostasis in cells, they also play important roles in intracellular signal transduction in the CNS. There are multiple isoforms of group VI iPLA2 (VIA-F), of which some have splice variants with different ankyrin repeats, suggesting protein–protein interactions (reviewed by Burke and Dennis 2009). Therefore, although calcium is not required for iPLA2 activity, their activity may be regulated by calcium binding proteins. For example, GVIA iPLA2 has a binding domain for calmodulin, which negatively regulates its activity (Wang et al. 2005; Jenkins et al. 2006). Some iPLA2 also possess lysophospholipase and transacylase activities, as others require binding to ATP for stabilization. GVIA iPLA2 (85 kDa) has three splice variants and eight ankyrin repeats, a linker region, and a catalytic domain. While GVIA-1 seems to be present largely in the cytoplasm, the other splice variant, GVIA-2 iPLA2 is membrane bound (Hsu et al. 2009). In INS-1 cells, there is evidence for presence of GVIA iPLA2 in mitochondria and plays a role in protecting mitochondrial-induced apoptotic pathway by staurosporine (Seleznev et al. 2006).
Expression and Physiological Roles of iPLA2s
In the brain, the basal expression and activity of group VI iPLA2s are higher than either cPLAs or sPLA2s (Molloy et al. 1998; Farooqui et al. 1999). Both in vivo and in vitro evidence supports that DHA, which is highly enriched in brain phospholipids, is released by iPLA2 (Green et al. 2008). Using siRNA and pharmacological inhibitors, study by Strokin’s group demonstrated the ability for ATP, glutamate, and lipopolysaccharide (LPS) to release DHA in astrocytes, and attributed this to the involvement of iPLA2β (VIA-2)(Strokin et al. 2006). In astrocytes, the cAMP/PKA pathway is involved in the stimulus-induced activation of iPLA2 and release of DHA (Strokin et al. 2006). In rat hippocampal slices, iPLA2 and DHA could provide neuroprotection against toxicity due to OGD (Strokin et al. 2006). This beneficial effect of iPLA2 is contrasted to its detrimental role in mediating oxidative and apoptotic pathways in mitochondria (Seleznev et al. 2006). Differences in DHA and AA metabolism in the brain were observed in rats deprived of dietary n – 3 polyunsaturated fatty acids for 15 weeks (Rao et al. 2007). The decrease in DHA and increase in AA turnover was in agreement with the decrease in iPLA2 and increase in cPLA2 and sPLA2.
Immunohistochemical studies demonstrated high levels of iPLA2 expression in the hippocampus, cerebellum, and brain stem, with lower expression in the thalamus and hypothalamus (Shirai and Ito 2004; Ong et al. 2005b). Enzyme activity is present in the cytosolic and nuclear fraction. Immunolabeling showed iPLA2 on the nuclear envelop of neurons, dendrites, and axon terminals (Ong et al. 2005b). It is worth noting that in the rat hippocampus, there was an age-dependent decrease in mRNA levels of iPLA2, but not cPLA2 (Aid and Bosetti 2007).
Although these enzymes do not require Ca2+ for activity, they can modulate calcium homeostasis by promoting replenishment of intracellular calcium stores (Wilkins and Barbour 2008). Using brain slices from rat cerebellum and Ca2+-sensitive fluorescent dye Fluo-4, BEL, and specific antisense oligodeoxynucleotide, iPLA2 was shown to be involved in the store-operated calcium entry in rat cerebellar astrocytes (Singaravelu et al. 2006). These results suggest that iPLA2 activates the formation of lysophospholipids in astrocytes, which causes openings of the Ca2+ channels in the plasma membrane to refill Ca2+ stores, and thus allows normal Ca2+-signaling. Another important role of iPLA2s in synaptic plasticity is the regulation of hippocampal AMPA receptor (Menard et al. 2005). Hippocampal injection of BEL attenuated short-term and long-term memory in inhibitory avoidance learning (Schaeffer and Gattaz 2005).
iPLA2 in Oxidative and Apoptotic Pathways
There is evidence that oxidant compounds can regulate iPLA2 activity (Song et al. 2006; Xu et al. 2003b). Furthermore, iPLA2 isoforms are involved in oxidant-induced cell death (Song et al. 2006; Peterson et al. 2007). In human astrocyte A172 cells, cytosolic iPLA2β (VIA-2), and microsomal iPLA2γ (VIB) are present and participate in PC remodeling. R-bromoenol lactone (BEL), an iPLA2γ inhibitor, but not S-BEL (an iPLA2β inhibitor) was shown to inhibit the lipid remodeling process. Exposure of A172 cells to H2O2 or tert-butylhydroperoxide (TBHP) induced time- and concentration-dependent increase in cell death. Pretreatment with BEL prior to H2O2 or TBHP significantly increased oxidant-induced cell death, which is due to accelerated loss of ATP, but not formation of ROS (Peterson et al. 2007). Endoplasmic reticulum iPLA2 was shown to be involved in oxidant-induced cell death and lipid peroxidation, which was attributed to iPLA2γ, an isoform present in endoplasmic reticulum of different tissues including the brain (Kinsey et al. 2005). The emerging role of iPLA2 in apoptosis has been reviewed recently (Balsinde et al. 2006).
iPLA2 in Inflammatory Responses
There is increasing evidence that iPLA2 plays a role in inflammatory responses. The study by Kuwata’s group using siRNA in rat fibroblast 3Y1 cells showed the involvement of iPLA2γ, but not cPLA2 in cytokine-induced expression of sPLA2-IIA (Kuwata et al. 2007). iPLA2 is also required for iNOS expression in glial cell activation by LPS (Won et al. 2005). Mechanism for inhibition of iPLA2 could prevent inflammatory mediators in pulmonary microvascular endothelium (Rastogi and McHowat 2009). The link between iPLA2 and the sPLA2 pathway further suggests the ability for iPLA2 to modulate prostaglandin production (Strokin et al. 2007). DHA, the major fatty acid released from iPLA2, is the substrate for synthesis of neuroprotectin 1 (NPD1) and resolvin, molecules with protective effects in cell survival, and the resolution of inflammation (Lukiw and Bazan 2008, 2006; Schwab et al. 2007). These studies are in agreement with the different PLA2 types in regulating the release and metabolism of AA and DHA in brain (Rapoport 2008).
iPLA2 in Neurodegenerative Diseases
In a study using primary neurons, treatment with iPLA2 inhibitors demonstrated the role of this enzyme in neurite outgrowth and neuronal viability (Forlenza et al. 2007a). The availability of iPLA2β knockout mice provided evidence for the importance of this enzyme in neurodegenerative processes. Mice with iPLA2β deficiency developed normally, but showed severe motor dysfunction. Examinations of the nervous system revealed widespread degeneration of axons and/or synapses together with the presence of spheroids and vacuoles (Shinzawa et al. 2008). An age-dependent disruption in axonal membrane homeostasis and accumulation of ubiquitinated proteins was shown at the onset of motor impairment, and was correlated with an increase in ubiquitin-positive spheroids in the brain (Malik et al. 2008). Interestingly, macrophages from iPLA2β knockout mice have reduced sensitivity to apoptosis induced by cholesterol loading, further supporting the role for iPLA2β in mitochondria function and ER stress (Bao et al. 2007).
iPLA2 has been implicated in the pathogenesis of neurodegenerative disorders with iron dyshomeostasis. Mutations of gene encoding iPLA2 are associated with two childhood neurologic disorders: infantile neuroaxonal dystrophy (INAD) and idiopathic neurodegeneration with brain iron accumulation (NBIA) (Morgan et al. 2006; Gregory et al. 2008). Besides iron accumulation, patients defective in iPLA2 also exhibit a variety of phenotypes including cerebellar atrophy, increased Lewy bodies, and neurofibrillary tangles (Kurian et al. 2008). Axonal swelling was also observed in INAD patients found in Chinese populations (Wu et al. 2009). Recent reports linked mutations in iPLA2beta to dystonia–parkinsonism, a neurodegenerative disorder characterized by Parkinsonism, dystonia, and cognitive impairment (Paisan-Ruiz et al. 2009). The iPLA2 is also involved in Wallerian degeneration and axon regeneration. Using FKGK11, a highly selective inhibitor of VIA iPLA2, iPLA2 was shown to be involved in the early stages of myelin breakdown in the sciatic nerve crush, whereas cPLA2 plays a greater role in myelin clearance by macrophages (Lopez-Vales et al. 2008). Although cPLA2 and iPLA2 are similarly important in the onset of experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis), it is iPLA2 that plays a critical role in the progression of the disease (Kalyvas et al. 2009). In addition to cPLA2, a reduced iPLA2 activity was also found in AD brains, particularly in the hippocampus. A recent review concluded that the down-regulation of cPLA2 and iPLA2 activities in the early stages of AD might contribute to memory deficit through attenuation of glutamatergic and cholinergic signaling (Schaeffer and Gattaz 2008). Further studies are necessary to characterize the expression and/or activity of PLA2 isoforms in different stages of AD, and to reveal the mechanisms underlying their roles in the onset and/or progression of AD.
Secretory Phospholipase A2 (sPLA2)
The mammalian sPLA2 enzymes are small molecular weight proteins (~14–19 kDa, except sPLA2-III) with several disulfide bridges (6–8) and possess a strongly conserved active site. There are many isoforms of sPLA2s in mammals including groups IA-B, IIA-F, III, V, IX, X, XIA-B, XII, XIII, and XIV (Burke and Dennis 2009). A variety of biological functions has been proposed for these sPLA2s based on in vitro studies, but few studies have demonstrated their in vivo role, an area needing further investigation.
Some mammalian sPLA2s have a close relation to the sPLA2 in snake and bee venom. Instead of using a classical acyl enzyme intermediate like serine proteases, sPLA2s utilize the catalytic site His and the assistance of an Asp to polarize a bound H2O, which then attacks the carbonyl group. The sPLA2s are calcium-dependent, and the Ca2+ ion is bound in the conserved Ca2+ binding loop to provide stabilization for the transition state (Verheij et al. 1981; Dennis 1994). The structure, kinetics, and catalytic mechanisms of sPLA2s have been reviewed recently (Lambeau and Gelb 2008).
sPLA2 Receptors
Some sPLA2s may have more physiological functions outside of their catalytic activity, as some may behave as ligands for receptors. Both M-type (muscular) and N-type (neuronal) receptors have been identified using the neurotoxic snake venom sPLA2 (OS2) as a ligand (Lambeau and Lazdunski 1999). Although the N-type receptors have not been cloned, a variety of neuronal proteins, including calmodulin and 14-3-3 proteins, can compete with OS2 binding (Kovacic et al. 2007). The M-type sPLA2 receptor is a 180-kDa protein that has been cloned from different species including humans (Lambeau and Lazdunski 1999). This receptor belongs to the superfamily of C-type lectins, which contains one or more carbohydrate recognition domains and is expressed in various tissues (Lambeau and Lazdunski 1999). The M-type sPLA2 receptor can be upregulated by various inflammatory stimuli (Hanasaki and Arita 2002; Beck et al. 2006). Some sPLA2 inhibitors may also block the binding of sPLA2 to the M-type receptors (Boilard et al. 2006).
sPLA2 Groups in the CNS
sPLA2-IB
The sPLA2-IB is found abundantly in the digestive tract where it hydrolyzes phospholipids in the intestinal lumen for aiding dietary lipid absorption (Carey et al. 1983). In the mammalian brain, sPLA2-IB appears to be mainly neuronal, and is more abundant in the cortex and hippocampus (Kolko et al. 2006). The sPLA2-IB expression is induced by kainic acid and has been regarded as a neuronal intercellular signal modulator (Kolko et al. 2005). The sPLA2-IB has also been found to act as an endogenous PLA2 receptor ligand leading to cell proliferation, cell migration, and in releasing lipid mediators (Hanasaki and Arita 2002; Lambeau and Lazdunski 1999).
sPLA2-IIA
sPLA2-IIA was first identified in atherosclerotic lesions, and is a known inflammatory marker protein in atherosclerosis. Due to its involvement in inflammatory processes in the peripheral systems, the IIA isoform is the most studied of the group II sPLA2s (Murakami et al. 1997; Murakami and Kudo 2002). Many group II genes are clustered in chromosome 1 (Suzuki et al. 2000). In most cell types, sPLA2-IIA is not constitutively expressed under resting conditions. RT–PCR and in situ hybridization studies showed a low expression of sPLA2-IIA mRNA in different brain regions, but higher levels in the cerebellum, especially in the Purkinje cells (Molloy et al. 1998; Shirai and Ito 2004). Western blot analysis also demonstrated low levels in the cortex as well as in the spinal chord (Svensson et al. 2005; Adibhatla et al. 2005). Although its cellular localization has not been investigated in detail, this enzyme has been implicated in contributing to several important processes in the brain including regulation of neurotransmitter release, neuritogenesis, and neuronal apoptosis (Farooqui et al. 2006; Mathisen et al. 2007).
In humans, sPLA2-IIA has been implicated in the pathogenesis of many inflammatory and infectious diseases (Adibhatla and Hatcher 2008; Titsworth et al. 2008). Depending on the cell types and species, sPLA2-IIA gene expression is typically upregulated by cytokines, including TNFα, IL-1β, IL-6, and bacterial toxins, and is downregulated by growth factors (Andreani et al. 2000). The sPLA2-IIA induction can also be blocked by anti-inflammatory cytokines as well as glucocorticoids (Touqui and Alaoui-El-Azher 2001). Besides C/EBP and NF-κB, which are major transcriptional factors, other factors such as AP-1, CREB, STAT, and PPARγ may also regulate induction of sPLA2-IIA (Couturier et al. 1999). IL-1β-induced sPLA2-IIA mRNA expression in astrocytes is dependent on ERK1/2 and PI-3 kinase (but not p38 MAPK), and ROS from NADPH oxidase (Jensen et al. 2009).
In agreement with evidence that AD pathology is marked by increased inflammatory responses, sPLA2-IIA mRNA expression was significantly increased in the hippocampus in AD brains as compared with age-matched controls (Moses et al. 2006). Furthermore, immunohistochemical studies indicated that sPLA2-IIA overexpression is localized in astrocytes (Moses et al. 2006).
Studies also showed an increase in sPLA2-IIA mRNA and protein expression in different stroke models (Adibhatla and Hatcher 2007; Lin et al. 2004). In a rat focal cerebral ischemia model induced by occlusion of the middle cerebral artery, increased sPLA2-IIA mRNA expression was found in an early phase at 30 min after reperfusion, and also in a later phase at 3 days after reperfusion (Lin et al. 2004). The sPLA2-IIA immunore-activity was colocalized with GFAP-positive cells in the penumbral area at 3 days after reperfusion. Interestingly, although ischemia–reperfusion also increased expression of COX-2 mRNA, expression of cPLA2 mRNA was not altered (Lin et al. 2004). In agreement with the increase in inflammatory response, treatment with an TNFα antibody or IL-1 receptor antagonist was shown to significantly attenuate infarction volume and inhibited the increase in sPLA2-IIA in cerebral ischemia (Adibhatla and Hatcher 2007). These results support the role of sPLA2 in ischemic injury (Adibhatla and Hatcher 2008), and justify the use of PLA2 inhibitors as neuroprotective agents against cerebral ischemia-induced neuronal lesions and apoptosis (Cunningham et al. 2004; Yagami et al. 2002).
sPLA2-IIC, IIE, and IIF
The sPLA2-IIC has been found in rats, mice, and humans; however, sPLA2-IIC in humans appear to be a non-functional pseudogene (Tischfield et al. 1996). RT–PCR studies have detected group IIC expression in all regions of the rat brain as well as in the spinal chord (Chen et al. 1997).
The sPLA2-IIE shows the highest homology, and has the most similar in vitro enzymatic properties with groups IIA and IID (Valentin et al. 1999; Suzuki et al. 2000). In mammalian cells, sPLA2-IIE can be induced in response to proinflammatory stimuli, which further promotes AA release and eicosanoid generation. Compared to group IIA, sPLA2-IIE has lower expression levels in physiological states. Unlike IIA and IID, which are more widespread among tissues, sPLA2-IIE transcripts were limited to the brain, heart, lung, and placenta, and elevated expression was found in alveolar macrophage-like cells in the lungs of endotoxin-treated mice (Suzuki et al. 2000). In the brain, the localization of sPLA2-IIE is mainly neuronal, and the highest level can be found in the hippocampus and the cerebral cortex (Kolko et al. 2006).
sPLA2-III
Group III is unique as it has both N-terminal and C-terminal domains with a central sPLA2 domain homologous to bee venom group III sPLA2. Apparently, the mammalian sPLA2-III is processed into the mature and active form consisting of only the central sPLA2 domain (Valentin et al. 2000; Murakami et al. 2003). The sPLA2-III has been found in both the peripheral and CNS, in the vascular endothelium of various tissues, and in alveolar epithelium and macrophages (Masuda et al. 2008; Mounier et al. 2008; Murakami et al. 2005). Group III sPLA2 appears to possess neuritogenic and neurotropic action, and plays a role in mediating neuronal outgrowth (Masuda et al. 2008). However, injections of sPLA2-III into the cervical dorsolateral funiculus resulted in dose-dependent demyelination, loss of oligodendrocytes and astrocytes, as well as axonopathy (Titsworth et al. 2007).
sPLA2-V and X
The sPLA2-V may also play a role in lipid mediator production and phagocytosis in macrophages (Balboa et al. 1996; Balestrieri et al. 2006; Satake et al. 2004), in atherosclerosis development (Bostrom et al. 2007), and in lung surfactant hydrolysis (Ohtsuki et al. 2006). The sPLA2-V is present at low levels in most regions of the rat brain, but at higher levels in the hippocampus (Molloy et al. 1998; Kolko et al. 2006). In addition, sPLA2-V has been identified in the nuclei of neuronal and glial cells in the rat brain cortex (Nardicchi et al. 2007). In astrocytes, TNFα was shown to enhance the mRNA expression of sPLA2-V and sPLA2-IIA with different time-courses (Thomas et al. 2000).
The sPLA2-X has been demonstrated in the human spleen, thymus, peripheral leukocytes, and brain, and appears to be involved in functions relating to immunity and inflammation (Cupillard et al. 1997). The sPLA2-X is considered to be the most potent hydrolyser of the outer plasma membrane (Murakami et al. 1998; Hanasaki et al. 1999; Bezzine et al. 2000). Despite low levels of expression in peripheral neurons, sPLA2-X also appears to have neuritogenic effects and can promote neuronal outgrowth and survival, possibly through the release of LPC (Nakashima et al. 2004; Masuda et al. 2005, 2008). The sPLA2-X also plays a role in airway inflammation and remodeling induced by allergens (Henderson et al. 2007), and in myocardial ischemia/reperfusion injury (Fujioka et al. 2008). However, more future studies are needed to investigate the role of this sPLA2 in neural inflammation processes.
Cross-Talks Among Different Groups of PLA2
Cross-talk between sPLA2s and either cPLA2s or iPLA2s is an important characteristic of PLA2s. In the CNS, there are examples of independent activation, and positive and negative cooperations among different PLA2 isoforms. For example, both cPLA2 and sPLA2 may be upregulated in neuroinflammation by cytokine induction. Ventricular infusion of LPS increased brain cPLA2 and sPLA2 activities as well as levels of AA, PGE2, and PGD2 (Rosenberger et al. 2004). Exposure of astrocytes in cultures to cytokines resulted in an increased expression of sPLA2 and COX-2, but not COX-1 and cPLA2, along with a time-dependent increase in PGE2 (Xu et al. 2003b). Although astrocytes could respond to ATP or phorbol ester, which stimulated cPLA2 phosphorylation and AA release, a relatively small increase in PGE2 was observed. However, when astrocytes were first treated with cytokines to induce sPLA2 expression, subsequent exposure to ATP or phorbol ester markedly increased the levels of PGE2 (Xu et al.2003b). A number of studies, including one with human astrocytoma cells demonstrated involvement of cPLA2 in inflammatory induction of sPLA2 (Hernandez et al. 1998). However, because of the broad specificity of these inhibitors, studies using small interfering RNA have been helpful to clarify differences between cPLA2 and iPLA2. In fact, studies with rat fibroblasts unveiled the involvement of group VIB iPLA2 and not cPLA2 in the inflammatory regulation of sPLA2 (Kuwata et al. 2007). Similar studies should be carried out with other inflammatory cells including those in the CNS.
Concluding Remarks
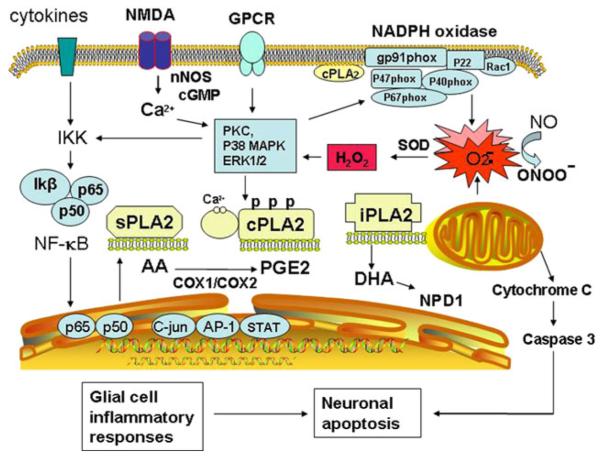
These studies have demonstrated the important role of PLA2, not only in maintaining cell membrane phospholipid homeostasis, but also in regulating oxidative and inflammatory processes in physiological and pathological conditions. It is recognized that multiple types of PLA2 are present in individual cells, and despite similar catalytic function, these enzymes exhibit wildly different properties and target different subcellular membranes and specific fatty acids within the phospholipids. An integration of signaling pathways for cPLA2, iPLA2, and sPLA2 in mediating cellular inflammatory responses is shown in Fig. 1. Furthermore, a summary of recent studies describing the involvement of these PLA2s in physiological and pathological functions in the CNS is indicated in Table 1.
Fig. 1.
Phospholipases A2 in oxidative and inflammatory pathways
Table 1.
Involvement of cPLA2, iPLA2, and sPLA2 in inflammatory responses in the CNS
| cPLA2 | ||
|---|---|---|
| 1 | Involved in long term memory retrieval | (Schaeffer and Gattaz 2007) |
| 2 | AA as a retrograde signal in growth and dynamics of retinotectal arbors | (Leu and Schmidt 2008) |
| 3 | AA as an important cofactor for synovial NADPH oxidase | (Chenevier-Gobeaux et al. 2007) |
| 4 | As anti-inflammatory therapy for collagen-induced arthritis | (Raichel et al. 2008) |
| 5 | Involvement in spinal nociceptive processes | (Kim et al. 2008) |
| 6 | Involve in cognitive function—implication for Alzheimer’s disease | (Schaeffer and Gattaz 2008; Sanchez-Mejia et al. 2008) |
| 7 | Involved in Abeta-mediated increase in AA release | (Chalimoniuk et al. 2007; Shelat et al. 2008) |
| 8 | Mediates NMDA-stimulated electrophysiological responses of hippocampal neurons | (Shen et al. 2007) |
| 9 | Contributes to Abeta-induced mitochondrial dysfunction and neuronal apoptosis | (Kriem et al. 2005; Zhu et al. 2006) |
| 10 | Regulates Abeta-induced activation of neutral and acidic sphingomyelinase | (Malaplate-Armand et al. 2006) |
| 11 | Involved in muscarinic receptor-mediated sAPPalpha release | (Cho et al. 2006) |
| iPLA2 | ||
|---|---|---|
| 1 | Release of DHA | (Lukiw et al. 2005; Schwab et al. 2007; Green et al. 2008) |
| 2 | Neuroprotection against toxicity due to oxygen glucose deprivation (OGD) | (Strokin et al. 2006) |
| 3 | Regulates store-operated calcium entry in cerebellar astrocytes | (Singaravelu et al. 2006) |
| 4 | Regulates hippocampal AMPA receptor and learning and memory | (Menard et al. 2005) |
| 5 | Acquisition of short- and long-term memory in hippocampus | (Schaeffer and Gattaz 2005) |
| 6 | Involved in oxidant-induced cell death | (Xu et al. 2003b; Song et al. 2006; Peterson et al. 2007) |
| 7 | Induces expression of sPLA2-IIA in fibroblasts | (Kuwata et al. 2007) |
| 8 | Involved in LPS-induced NF-κB activation and iNOS gene expression | (Won et al. 2005) |
| 9 | Mouse model of iPLA2β deficiency—severe motor dysfunction and axon degeneration | (Shinzawa et al. 2008; Malik et al. 2008) |
| 10 | Genetic defect of iPLA2β—childhood neurologic disorders: infantile neuroaxonal dystrophy (INAD) and idiopathic neurodegeneration with brain iron accumulation (NBIA) |
(Morgan et al. 2006; Gregory et al. 2008; Kurian et al. 2008) |
| 11 | Wallerian degeneration and axon regeneration | (Lopez-Vales et al. 2008) |
| 12 | Experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis | (Kalyvas et al. 2009) |
| sPLA2 | ||
|---|---|---|
| 1 | Role in innate immunity, cross-talk with cPLA2 or iPLA2 | (Kuwata et al. 2007) |
| 2 | sPLA2-1B, induced in brain by kainic acid | (Kolko et al. 2005) |
| 3 | sPLA2-IIA and ROS generation in mitochondria mediates glutamate-induced neuronal death | (Mathisen et al. 2007) |
| 4 | sPLA2-IIA in inflammation—transcriptional regulation by pro-inflammatory cytokines | (Andreani et al. 2000; Touqui and Alaoui-El-Azher 2001; Adibhatla and Hatcher 2007, 2008; Jensen et al. 2009) |
| 5 | Increased sPLA2-IIA mRNA and immunoreactivity in AD brain | (Moses et al. 2006) |
| 6 | Increase in sPLA2-IIA mRNA and protein in cerebral ischemia models | (Lin et al. 2004; Adibhatla and Hatcher 2007) |
| 7 | Involved in spinal cord injury | (Liu et al. 2007; Titsworth et al. 2008) |
| 9 | sPLA2-III—promotes neuronal outgrowth and survival | (Masuda et al. 2008) |
| 10 | sPLA2-III—induces axonopathy | (Titsworth et al. 2007) |
Under physiological conditions, cPLA2 is engaged in rapid responses to a number of exogenous stimuli, including receptor agonists and oxidant compounds. Its activation is associated with the release of AA, an important lipid mediator serving multiple functions. The link between cPLA2 and calcium-dependent signaling pathways renders this enzyme one of the most important in health and diseases in CNS. In particular, information about involvement of cPLA2 in neuronal excitatory processes has wild implications in neurological diseases including AD.
Advances in technology have led to a better understanding of the different types of iPLA2 and their role in mediating cell apoptosis process. These tools also help to distinguish between iPLA2 and cPLA2. While cPLA2 prefers the release of AA, there is strong evidence that iPLA2 is responsible for release of DHA, a precursor for synthesis of docosanoids and NPD1. However, more studies are needed to understand target phospholipids and mechanisms for the release of DHA. It is also recognized that aberrant mutations of iPLA2 genes occur in humans resulting in two identified neurological diseases, infantile neuroaxonal dystrophy (INAD), and idiopathic neurodegeneration with brain iron accumulation (NBIA). More studies to understand how iPLA2 alters membrane phospholipids or membrane domains may help to provide strategy for alleviating these neurological symptoms.
Many sPLA2 subtypes are present in the CNS although their role in innate immunity has not been explored. Increase in inflammation is the underlying cause of many neurological disorders. Similar to the peripheral systems, sPLA2-IIA is considered to be an important inflammatory protein induced in the brain under different pathological conditions. Although sPLA2s are present in neurons and glial cells, little is known about their role in mediating innate immunology of these cells. With increasing molecular tools available for investigating the genetics, molecular structure, and physical properties of these enzymes, more studies will target toward specific inhibitors for PLA2s, and in understanding their role in regulating physiological and pathological functions in the CNS. Expectedly, these future studies will provide important information and novel mechanisms for prevention and treatment of neurodegenerative diseases in the CNS.
Acknowledgment
This study was supported by P01 AG018357 from NIH.
Abbreviations
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- AA
Arachidonic acid
- BEL
Bromoenol lactone
- CaMKII
Ca2+/Calmodulin-dependent protein kinase II
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- COX-2
Cyclooxygenase-2
- DHA
Docosahexaenoic acid
- ERK
Extracellular signal-regulated kinases
- HNE
4-Hydroxynonenal
- hsp90
Heat shock protein 90
- iNOS
Inducible nitric oxide synthase
- IFNγ
Interferon-γ
- IL-1β
Interleukin 1β
- INAD
Infantile neuroaxonal dystrophy
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MNK1
MAPK-interacting kinase
- MRI
Magnetic resonance imaging
- NBIA
Idiopathic neurodegeneration with brain iron accumulation
- NMDA
N-Methyl-d-aspartic acid
- NO
Nitric oxide
- NPD1
Neuroprotectin 1
- PACOCF3
Palmitoyl trifluoromethyl ketone
- PIP2
Phosphatidylinositol-4,5-bisphosphate
- PGE2
prostglandin E2
- PKC
Protein kinase C
- PLA2
Phospholipases A2
- SMase
sphingomyelinase
- ROS
Reactive oxygen species
- TBHP
tert-Butylhydroperoxide
- TNFα
Tumor necrosis factor-alpha
Contributor Information
Grace Y. Sun, Department of Biochemistry, University of Missouri, 117 Schweitzer Hall, Columbia, MO 65211, USA; Department of Pathology and Anatomical Sciences, University of Missouri, Columbia, MO 65211, USA
Phullara B. Shelat, Department of Biochemistry, University of Missouri, 117 Schweitzer Hall, Columbia, MO 65211, USA
Michael B. Jensen, Department of Biochemistry, University of Missouri, 117 Schweitzer Hall, Columbia, MO 65211, USA
Yan He, Department of Biochemistry, University of Missouri, 117 Schweitzer Hall, Columbia, MO 65211, USA.
Albert Y. Sun, Department of Pathology and Anatomical Sciences, University of Missouri, Columbia, MO 65211, USA
Agnes Simonyi, Department of Biochemistry, University of Missouri, 117 Schweitzer Hall, Columbia, MO 65211, USA.
References
- Adibhatla RM, Hatcher JF. Secretory phospholipase A2 IIA is up-regulated by TNF-alpha and IL-1alpha/beta after transient focal cerebral ischemia in rat. Brain Research. 2007;1134:199–205. doi: 10.1016/j.brainres.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Adibhatla RM, Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Reports. 2008;41:560–567. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Tureyen K. CDP-choline liposomes provide significant reduction in infarction over free CDP-choline in stroke. Brain Research. 2005;1058:193–197. doi: 10.1016/j.brainres.2005.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid S, Bosetti F. Gene expression of cyclooxygenase-1 and Ca(2+)-independent phospholipase A(2) is altered in rat hippocampus during normal aging. Brain Research Bulletin. 2007;73:108–113. doi: 10.1016/j.brainresbull.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov P, Cui JG, Zhao Y, Lukiw WJ. 24S-hydroxycholesterol induces inflammatory gene expression in primary human neural cells. Neuroreport. 2005;16:909–913. doi: 10.1097/00001756-200506210-00007. [DOI] [PubMed] [Google Scholar]
- Andreani M, Olivier JL, Berenbaum F, Raymondjean M, Bereziat G. Transcriptional regulation of inflammatory secreted phospholipases A(2) Biochimica et Biophysica Acta. 2000;1488:149–158. doi: 10.1016/s1388-1981(00)00117-7. [DOI] [PubMed] [Google Scholar]
- Balboa MA, Balsinde J, Winstead MV, Tischfield JA, Dennis EA. Novel group V phospholipase A2 involved in arachidonic acid mobilization in murine P388D1 macrophages. The Journal of Biological Chemistry. 1996;271:32381–32384. doi: 10.1074/jbc.271.50.32381. [DOI] [PubMed] [Google Scholar]
- Balestrieri B, Hsu VW, Gilbert H, Leslie CC, Han WK, Bonventre JV, et al. Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. The Journal of Biological Chemistry. 2006;281:6691–6698. doi: 10.1074/jbc.M508314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde J, Perez R, Balboa MA. Calcium-independent phospholipase A2 and apoptosis. Biochimica et Biophysica Acta. 2006;1761:1344–1350. doi: 10.1016/j.bbalip.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bao S, Li Y, Lei X, et al. Attenuated free cholesterol loading-induced apoptosis but preserved phospholipid composition of peritoneal macrophages from mice that do not express group VIA phospholipase A2. The Journal of Biological Chemistry. 2007;282:27100–27114. doi: 10.1074/jbc.M701316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C, Kempster S, Last V, Williams A. Interferongamma increases neuronal death in response to amyloid-beta1-42. Journal of Neuroinflammation. 2006;3:7. doi: 10.1186/1742-2094-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C, Williams A. Squalestatin protects neurons and reduces the activation of cytoplasmic phospholipase A2 by Abeta(1–42) Neuropharmacology. 2007;53:222–231. doi: 10.1016/j.neuropharm.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Beck S, Beck G, Ostendorf T, et al. Upregulation of group IB secreted phospholipase A(2) and its M-type receptor in rat ANTI-THY-1 glomerulonephritis. Kidney International. 2006;70:1251–1260. doi: 10.1038/sj.ki.5001664. [DOI] [PubMed] [Google Scholar]
- Bezzine S, Koduri RS, Valentin E, Murakami M, Kudo I, Ghomashchi F, et al. Exogenously added human group X secreted phospholipase A(2) but not the group IB, IIA, and V enzymes efficiently release arachidonic acid from adherent mammalian cells. The Journal of Biological Chemistry. 2000;275:3179–3191. doi: 10.1074/jbc.275.5.3179. [DOI] [PubMed] [Google Scholar]
- Boilard E, Rouault M, Surrel F, Le Calvez C, Bezzine S, Singer A, et al. Secreted phospholipase A2 inhibitors are also potent blockers of binding to the M-type receptor. Biochemistry. 2006;45:13203–13218. doi: 10.1021/bi061376d. [DOI] [PubMed] [Google Scholar]
- Bostrom MA, Boyanovsky BB, Jordan CT, Wadsworth MP, Taatjes DJ, de Beer FC, et al. Group v secretory phospholipase A2 promotes atherosclerosis: Evidence from genetically altered mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:600–606. doi: 10.1161/01.ATV.0000257133.60884.44. [DOI] [PubMed] [Google Scholar]
- Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovascular Drugs and Therapy. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JE, Hsu YH, Deems RA, Li S, Woods VL, Jr., Dennis EA. A phospholipid substrate molecule residing in the membrane surface mediates opening of the lid region in group IVA cytosolic phospholipase A2. Journal of Biological Chemistry. 2008;283:31227–31236. doi: 10.1074/jbc.M804492200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annual Review of Physiology. 1983;45:651–677. doi: 10.1146/annurev.ph.45.030183.003251. [DOI] [PubMed] [Google Scholar]
- Casas J, Gijon MA, Vigo AG, Crespo MS, Balsinde J, Balboa MA. Phosphatidylinositol 4,5-bisphophate anchors cytosolic group IVA phospholipase A2 to perinuclear membranes and decreases its calcium requirement for translocation in live cells. Molecular Biology of the Cell. 2006;17:155–162. doi: 10.1091/mbc.E05-06-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalimoniuk M, Stolecka A, Cakala M, et al. Amyloid beta enhances cytosolic phospholipase A2 level and arachidonic acid release via nitric oxide in APP-transfected PC12 cells. Acta Biochimica Polonica. 2007;54:611–623. [PubMed] [Google Scholar]
- Chen J, Shao C, Lazar V, Srivastava CH, Lee WH, Tischfield JA. Localization of group IIc low molecular weight phospholipase A2 mRNA to meiotic cells in the mouse. Journal of Cellular Biochemistry. 1997;64:369–375. doi: 10.1002/(sici)1097-4644(19970301)64:3<369::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Chenevier-Gobeaux C, Simonneau C, Therond P, Bonnefont-Rousselot D, Poiraudeau S, Ekindjian OG, et al. Implication of cytosolic phospholipase A2 (cPLA2) in the regulation of human synoviocyte NADPH oxidase (Nox2) activity. Life Science. 2007;81:1050–1058. doi: 10.1016/j.lfs.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Cheng SE, Luo SF, Jou MJ, Lin CC, Kou YR, Lee IT, et al. Cigarette smoke extract induces cytosolic phospholipase A2 expression via NADPH oxidase, MAPKs, AP-1, and NF-kappaB in human tracheal smooth muscle cells. Free Radical Biology and Medicine. 2009;46:948–960. doi: 10.1016/j.freeradbiomed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Cho HW, Kim JH, Choi S, Kim HJ. Phospholipase A2 is involved in muscarinic receptor-mediated sAPPalpha release independently of cyclooxygenase or lipoxygenase activity in SH-SY5Y cells. Neuroscience Letters. 2006;397:214–218. doi: 10.1016/j.neulet.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: Transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and proinflammatory signaling. Journal of Neuroscience Research. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- Corbin JA, Evans JH, Landgraf KE, Falke JJ. Mechanism of specific membrane targeting by C2 domains: Localized pools of target lipids enhance Ca2+ affinity. Biochemistry. 2007;46:4322–4336. doi: 10.1021/bi062140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier C, Brouillet A, Couriaud C, Koumanov K, Bereziat G, Andreani M. Interleukin 1beta induces type II-secreted phospholipase A(2) gene in vascular smooth muscle cells by a nuclear factor kappaB and peroxisome proliferator-activated receptor-mediated process. The Journal of Biological Chemistry. 1999;274:23085–23093. doi: 10.1074/jbc.274.33.23085. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Souayah N, Jameson B, Mitchell J, Yao L. Systemic treatment of cerebral cortex lesions in rats with a new secreted phospholipase A2 inhibitor. Journal of Neurotrauma. 2004;21:1683–1691. doi: 10.1089/neu.2004.21.1683. [DOI] [PubMed] [Google Scholar]
- Cupillard L, Koumanov K, Mattei MG, Lazdunski M, Lambeau G. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. The Journal of Biological Chemistry. 1997;272:15745–15752. doi: 10.1074/jbc.272.25.15745. [DOI] [PubMed] [Google Scholar]
- Das S, Cho W. Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2. The Journal of Biological Chemistry. 2002;277:23838–23846. doi: 10.1074/jbc.M202322200. [DOI] [PubMed] [Google Scholar]
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. The Journal of Biological Chemistry. 1994;269:13057–13060. [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in Immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: Their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacological Reviews. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Yang HC, Hirashima Y, Horrocks LA. Determination of plasmalogen-selective phospholipase A2 activity by radiochemical and fluorometric assay procedures. Methods in Molecular Biology. 1999;109:39–47. doi: 10.1385/1-59259-581-2:39. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Mendes CT, Marie SK, Gattaz WF. Inhibition of phospholipase A2 reduces neurite out-growth and neuronal viability. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2007a;76:47–55. doi: 10.1016/j.plefa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Schaeffer EL, Gattaz WF. The role of phospholipase A2 in neuronal homeostasis and memory formation: Implications for the pathogenesis of Alzheimer’s disease. Journal of Neural Transmission. 2007b;114:231–238. doi: 10.1007/s00702-006-0597-0. [DOI] [PubMed] [Google Scholar]
- Fujioka D, Saito Y, Kobayashi T, et al. Reduction in myocardial ischemia/reperfusion injury in group X secretory phospholipase A2-deficient mice. Circulation. 2008;117:2977–2985. doi: 10.1161/CIRCULATIONAHA.107.743997. [DOI] [PubMed] [Google Scholar]
- Geijsen N, Dijkers PF, Lammers JJ, Koenderman L, Coffer PJ. Cytokine-mediated cPLA(2) phosphorylation is regulated by multiple MAPK family members. FEBS Letters. 2000;471:83–88. doi: 10.1016/s0014-5793(00)01373-9. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Loper R, Ghomashchi F, Tucker DE, Bonventre JV, Gelb MH, et al. Function, activity, and membrane targeting of cytosolic phospholipase A(2)zeta in mouse lung fibroblasts. The Journal of Biological Chemistry. 2007;282:11676–11686. doi: 10.1074/jbc.M608458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Progress in Lipid Research. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Green JT, Orr SK, Bazinet RP. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. Journal of Lipid Research. 2008;49:939–944. doi: 10.1194/jlr.R700017-JLR200. [DOI] [PubMed] [Google Scholar]
- Gregory A, Westaway SK, Holm IE, et al. Neurodegeneration associated with genetic defects in phospholipase A(2) Neurology. 2008;71:1402–1409. doi: 10.1212/01.wnl.0000327094.67726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanasaki K, Arita H. Phospholipase A2 receptor: A regulator of biological functions of secretory phospholipase A2. Prostaglandins and Other Lipid Mediators. 2002;68-69:71–82. doi: 10.1016/s0090-6980(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Hanasaki K, Ono T, Saiga A, et al. Purified group X secretory phospholipase A(2) induced prominent release of arachidonic acid from human myeloid leukemia cells. The Journal of Biological Chemistry. 1999;274:34203–34211. doi: 10.1074/jbc.274.48.34203. [DOI] [PubMed] [Google Scholar]
- Hefner Y, Borsch-Haubold AG, Murakami M, et al. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. The Journal of Biological Chemistry. 2000;275:37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Jr., Chi EY, Bollinger JG, et al. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. The Journal of Experimental Medicine. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Burillo SL, Crespo MS, Nieto ML. Secretory phospholipase A2 activates the cascade of mitogen-activated protein kinases and cytosolic phospholipase A2 in the human astrocytoma cell line 1321N1. The Journal of Biological Chemistry. 1998;273:606–612. doi: 10.1074/jbc.273.1.606. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Burke JE, Li S, Woods VL, Jr., Dennis EA. Localizing the membrane binding region of group VIA Ca2+-independent phospholipase A2 using peptide amide hydrogen/deuterium exchange mass spectrometry. The Journal of Biological Chemistry. 2009;284:23652–23661. doi: 10.1074/jbc.M109.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibeas E, Fuentes L, Martin R, Hernandez M, Nieto ML. Secreted phospholipase A2 type IIA as a mediator connecting innate and adaptive immunity: New role in atherosclerosis. Cardiovascular Research. 2009;81:54–63. doi: 10.1093/cvr/cvn234. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Yan W, Mancuso DJ, Gross RW. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2beta. Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phos-pholipase A2 activity. The Journal of Biological Chemistry. 2006;281:15615–15624. doi: 10.1074/jbc.M511623200. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Sheng W, Simonyi A, Johnson GS, Sun AY, Sun GY. Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes. Neurochemistry International. 2009;55:362–368. doi: 10.1016/j.neuint.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyvas A, Baskakis C, Magrioti V, et al. Differing roles for members of the phospholipase A2 superfamily in experimental autoimmune encephalomyelitis. Brain. 2009;132:1221–1235. doi: 10.1093/brain/awp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Fitzsimmons B, Hefferan MP, et al. Inhibition of spinal cytosolic phospholipase A(2) expression by an antisense oligonucleotide attenuates tissue injury-induced hyperalgesia. Neuroscience. 2008;154:1077–1087. doi: 10.1016/j.neuroscience.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey GR, Cummings BS, Beckett CS, Saavedra G, Zhang W, McHowat J, et al. Identification and distribution of endoplasmic reticulum iPLA2. Biochemical and Biophysical Research Communications. 2005;327:287–293. doi: 10.1016/j.bbrc.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kolko M, Christoffersen NR, Barreiro SG, Miller ML, Pizza AJ, Bazan NG. Characterization and location of secretory phospholipase A2 groups IIE, V, and X in the rat brain. Journal of Neuroscience Research. 2006;83:874–882. doi: 10.1002/jnr.20773. [DOI] [PubMed] [Google Scholar]
- Kolko M, Christoffersen NR, Varoqui H, Bazan NG. Expression and induction of secretory phospholipase A2 group IB in brain. Cellular and Molecular Neurobiology. 2005;25:1107–1122. doi: 10.1007/s10571-005-8221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic L, Sribar J, Krizaj I. A new photoprobe for studying biological activities of secreted phospholipases A2. Bioorganic Chemistry. 2007;35:295–305. doi: 10.1016/j.bioorg.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Kriem B, Sponne I, Fifre A, et al. Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-beta peptide. The FASEB Journal. 2005;19:85–87. doi: 10.1096/fj.04-1807fje. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Morgan NV, MacPherson L, et al. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN) Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- Kuwata H, Fujimoto C, Yoda E, Shimbara S, Nakatani Y, Hara S, et al. A novel role of group VIB calcium-independent phospholipase A2 (iPLA2gamma) in the inducible expression of group IIA secretory PLA2 in rat fibroblastic cells. The Journal of Biological Chemistry. 2007;282:20124–20132. doi: 10.1074/jbc.M611883200. [DOI] [PubMed] [Google Scholar]
- Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annual Review of Biochemistry. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends in Pharmacological Sciences. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- Lamour NF, Subramanian P, Wijesinghe DS, Stahelin RV, Bonventre JV, Chalfant CE. Ceramide-1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. The Journal of Biological Chemistry. 2009;284:26897–26907. doi: 10.1074/jbc.M109.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre L, Takano T, Papillon J, Lemay S, Cybulsky AV. Role of phosphatidylinositol 4,5-bisphosphate in the activation of cytosolic phospholipase A2-alpha. Prostaglandins and Other Lipid Mediators. 2006;81:113–125. doi: 10.1016/j.prostaglandins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Leu BH, Schmidt JT. Arachidonic acid as a retrograde signal controlling growth and dynamics of retinotectal arbors. Developmental Neurobiology. 2008;68:18–30. doi: 10.1002/dneu.20561. [DOI] [PubMed] [Google Scholar]
- Lin TN, Wang Q, Simonyi A, et al. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. Journal of Neurochemistry. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Han S, Pei J, Xu LY, Lu PH, et al. Annexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injury. Journal of Neuropathology and Experimental Neurology. 2007;66:932–943. doi: 10.1097/nen.0b013e3181567d59. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R, Navarro X, Shimizu T, Baskakis C, Kokotos G, Constantinou-Kokotou V, et al. Intracellular phospholipase A(2) group IVA and group VIA play important roles in Wallerian degeneration and axon regeneration after peripheral nerve injury. Brain. 2008;131:2620–2631. doi: 10.1093/brain/awn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Bazan NG. Survival signalling in Alzheimer’s disease. Biochemical Society Transactions. 2006;34:1277–1282. doi: 10.1042/BST0341277. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. Journal of Nutrition. 2008;138:2510–2514. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. Journal of Clinical Investigation. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaplate-Armand C, Florent-Bechard S, Youssef I, et al. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiology of Disease. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, et al. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. American Journal of Pathology. 2008;172:406–416. doi: 10.2353/ajpath.2008.070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Murakami M, Takanezawa Y, Aoki J, Arai H, Ishikawa Y, et al. Neuronal expression and neuritogenic action of group X secreted phospholipase A2. The Journal of Biological Chemistry. 2005;280:23203–23214. doi: 10.1074/jbc.M500985200. [DOI] [PubMed] [Google Scholar]
- Masuda S, Yamamoto K, Hirabayashi T, Ishikawa Y, Ishii T, Kudo I, et al. Human group III secreted phospholipase A2 promotes neuronal outgrowth and survival. The Biochemical Journal. 2008;409:429–438. doi: 10.1042/BJ20070844. [DOI] [PubMed] [Google Scholar]
- Mathisen GH, Thorkildsen IH, Paulsen RE. Secretory PLA2-IIA and ROS generation in peripheral mitochondria are critical for neuronal death. Brain Research. 2007;1153:43–51. doi: 10.1016/j.brainres.2007.03.067. [DOI] [PubMed] [Google Scholar]
- Menard C, Valastro B, Martel MA, Chartier E, Marineau A, Baudry M, et al. AMPA receptor phosphorylation is selectively regulated by constitutive phospholipase A(2) and 5-lipoxygenase activities. Hippocampus. 2005;15:370–380. doi: 10.1002/hipo.20061. [DOI] [PubMed] [Google Scholar]
- Molloy GY, Rattray M, Williams RJ. Genes encoding multiple forms of phospholipase A2 are expressed in rat brain. Neuroscience Letters. 1998;258:139–142. doi: 10.1016/s0304-3940(98)00838-6. [DOI] [PubMed] [Google Scholar]
- Morgan NV, Westaway SK, Morton JE, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurode-generative disorders with high brain iron. Nature Genetics. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, et al. Secretory PLA2-IIA: A new inflammatory factor for Alzheimer’s disease. Journal of Neuroinflammation [electronic resource] 2006;3:28. doi: 10.1186/1742-2094-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier CM, Wendum D, Greenspan E, Flejou JF, Rosenberg DW, Lambeau G. Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. British Journal of Cancer. 2008;98:587–595. doi: 10.1038/sj.bjc.6604184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Phospholipase A2. Journal of Biochemistry. 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- Murakami M, Masuda S, Shimbara S, Ishikawa Y, Ishii T, Kudo I. Cellular distribution, post-translational modification, and tumorigenic potential of human group III secreted phospholipase A(2) The Journal of Biological Chemistry. 2005;280:24987–24998. doi: 10.1074/jbc.M502088200. [DOI] [PubMed] [Google Scholar]
- Murakami M, Masuda S, Shimbara S, et al. Cellular arachidonate-releasing function of novel classes of secretory phospholipase A2s (groups III and XII) The Journal of Biological Chemistry. 2003;278:10657–10667. doi: 10.1074/jbc.M211325200. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Critical Reviews in Immunology. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, et al. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. The Journal of Biological Chemistry. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- Muthalif MM, Hefner Y, Canaan S, Harper J, Zhou H, Parmentier JH, et al. Functional interaction of calcium-/calmodulin-dependent protein kinase II and cytosolic phospholipase A(2) The Journal of Biological Chemistry. 2001;276:39653–39660. doi: 10.1074/jbc.M103136200. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Kitamoto K, Arioka M. The catalytic activity, but not receptor binding, of sPLA2s plays a critical role for neurite outgrowth induction in PC12 cells. Brain Research. 2004;1015:207–211. doi: 10.1016/j.brainres.2004.04.069. [DOI] [PubMed] [Google Scholar]
- Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants–a new role as anti-inflammatory molecule. Current Topics in Medicinal Chemistry. 2007;7:765–777. doi: 10.2174/156802607780487623. [DOI] [PubMed] [Google Scholar]
- Nardicchi V, Macchioni L, Ferrini M, Goracci G. The presence of a secretory phospholipase A2 in the nuclei of neuronal and glial cells of rat brain cortex. Biochimica et Biophysica Acta. 2007;1771:1345–1352. doi: 10.1016/j.bbalip.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Nicotra A, Lupo G, Giurdanella G, Anfuso CD, Ragusa N, Tirolo C, et al. MAPKs mediate the activation of cytosolic phospholipase A2 by amyloid beta(25–35) peptide in bovine retina pericytes. Biochimica et Biophysica Acta. 2005;1733:172–186. doi: 10.1016/j.bbalip.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Niknami M, Patel M, Witting PK, Dong Q. Molecules in focus: Cytosolic phospholipase A2-alpha. International Journal of Biochemistry and Cell Biology. 2009;41:994–997. doi: 10.1016/j.biocel.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Ohtsuki M, Taketomi Y, Arata S, et al. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. The Journal of Biological Chemistry. 2006;281:36420–36433. doi: 10.1074/jbc.M607975200. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. Journal of Immunology. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Ong WY, Ling SF, Yeo JF, Chiueh CC, Farooqui AA. Injury and recovery of pyramidal neurons in the rat hippocampus after a single episode of oxidative stress induced by intracerebroventricular injection of ferrous ammonium citrate. Reproduction, Nutrition, Development. 2005a;45:647–662. doi: 10.1051/rnd:2005051. [DOI] [PubMed] [Google Scholar]
- Ong WY, Yeo JF, Ling SF, Farooqui AA. Distribution of calcium-independent phospholipase A2 (iPLA 2) in monkey brain. Journal of Neurocytology. 2005b;34:447–458. doi: 10.1007/s11068-006-8730-4. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Bhatia KP, Li A, et al. Characterization of PLA2G6 as a locus for dystonia–parkinsonism. Annals of Neurology. 2009;65:19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue S, Rapoport SI, Bosetti F. Co-localization of cytosolic phospholipase A2 and cyclooxygenase-2 in Rhesus monkey cerebellum. Brain Research. Molecular Brain Research. 2003;116:106–114. doi: 10.1016/s0169-328x(03)00262-6. [DOI] [PubMed] [Google Scholar]
- Pavicevic Z, Leslie CC, Malik KU. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. Journal of Lipid Research. 2008;49:724–737. doi: 10.1194/jlr.M700419-JLR200. [DOI] [PubMed] [Google Scholar]
- Peterson B, Knotts T, Cummings BS. Involvement of Ca2+-independent phospholipase A2 isoforms in oxidant-induced neural cell death. Neurotoxicology. 2007;28:150–160. doi: 10.1016/j.neuro.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Bielawska A, Subramanian P, et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. The Journal of Biological Chemistry. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- Pindado J, Balsinde J, Balboa MA. TLR3-dependent induction of nitric oxide synthase in RAW 264.7 macrophage-like cells via a cytosolic phospholipase A2/cyclooxygenase-2 pathway. Journal of Immunology. 2007;179:4821–4828. doi: 10.4049/jimmunol.179.7.4821. [DOI] [PubMed] [Google Scholar]
- Raichel L, Berger S, Hadad N, Kachko L, Karter M, Szaingurten-Solodkin I, et al. Reduction of cPLA2alpha overexpression: An efficient anti-inflammatory therapy for collagen-induced arthritis. European Journal of Immunology. 2008;38:2905–2915. doi: 10.1002/eji.200838545. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Jr., Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi P, McHowat J. Inhibition of calcium-independent phospholipase A2 prevents inflammatory mediator production in pulmonary microvascular endothelium. Respiratory Physiology & Neurobiology. 2009;165:167–174. doi: 10.1016/j.resp.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, et al. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. Journal of Neurochemistry. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nature Neuroscience. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, et al. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. The Journal of Biological Chemistry. 2004;279:16488–16494. doi: 10.1074/jbc.M313748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EL, Forlenza OV, Gattaz WF. Phospholipase A(2) activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease. Psychopharmacology (Berlin) 2009;202:37–51. doi: 10.1007/s00213-008-1351-0. [DOI] [PubMed] [Google Scholar]
- Schaeffer EL, Gattaz WF. Inhibition of calcium-independent phospholipase A2 activity in rat hippocampus impairs acquisition of short- and long-term memory. Psychopharmacology (Berlin) 2005;181:392–400. doi: 10.1007/s00213-005-2256-9. [DOI] [PubMed] [Google Scholar]
- Schaeffer EL, Gattaz WF. Requirement of hippocampal phospholipase A2 activity for long-term memory retrieval in rats. Journal of Neural Transmission. 2007;114:379–385. doi: 10.1007/s00702-006-0585-4. [DOI] [PubMed] [Google Scholar]
- Schaeffer EL, Gattaz WF. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: Participation of the phospholipase A2 enzyme. Psychopharmacology (Berlin) 2008;198:1–27. doi: 10.1007/s00213-008-1092-0. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation–resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleznev K, Zhao C, Zhang XH, Song K, Ma ZA. Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine. The Journal of Biological Chemistry. 2006;281:22275–22288. doi: 10.1074/jbc.M604330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, et al. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. Journal of Neurochemistry. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Shen Y, Kishimoto K, Linden DJ, Sapirstein A. Cytosolic phospholipase A(2) alpha mediates electrophysiologic responses of hippocampal pyramidal neurons to neurotoxic NMDA treatment. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6078–6083. doi: 10.1073/pnas.0605427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Ohto T, Kita Y. Cytosolic phospholipase A2: Biochemical properties and physiological roles. IUBMB Life. 2006;58:328–333. doi: 10.1080/15216540600702289. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tada E, Makiyama T, Yasufuku K, Moriyama Y, Fujino H, et al. Effects of ceramide, ceramidase inhibition and expression of ceramide kinase on cytosolic phospholipase A2alpha; additional role of ceramide-1-phosphate in phosphorylation and Ca2+ signaling. Cellular Signalling. 2009;21:440–447. doi: 10.1016/j.cellsig.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, et al. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: A model of human neurodegenerative disease. Journal of Neuroscience. 2008;28:2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai Y, Ito M. Specific differential expression of phospholipase A2 subtypes in rat cerebellum. Journal of Neurocytology. 2004;33:297–307. doi: 10.1023/B:NEUR.0000044191.83858.f7. [DOI] [PubMed] [Google Scholar]
- Shmelzer Z, Karter M, Eisenstein M, et al. Cytosolic phospholipase A2alpha is targeted to the p47phox-PX domain of the assembled NADPH oxidase via a novel binding site in its C2 domain. The Journal of Biological Chemistry. 2008;283:31898–31908. doi: 10.1074/jbc.M804674200. [DOI] [PubMed] [Google Scholar]
- Singaravelu K, Lohr C, Deitmer JW. Regulation of store-operated calcium entry by calcium-independent phospholipase A2 in rat cerebellar astrocytes. Journal of Neuroscience. 2006;26:9579–9592. doi: 10.1523/JNEUROSCI.2604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six DA, Barbayianni E, Loukas V, et al. Structure-activity relationship of 2-oxoamide inhibition of group IVA cytosolic phospholipase A2 and group V secreted phospholipase A2. Journal of Medicinal Chemistry. 2007;50:4222–4235. doi: 10.1021/jm0613673. [DOI] [PubMed] [Google Scholar]
- Smesny S, Stein S, Willhardt I, Lasch J, Sauer H. Decreased phospholipase A2 activity in cerebrospinal fluid of patients with dementia. Journal of Neural Transmission. 2008;115:1173–1179. doi: 10.1007/s00702-008-0081-0. [DOI] [PubMed] [Google Scholar]
- Song H, Bao S, Ramanadham S, Turk J. Effects of biological oxidants on the catalytic activity and structure of group VIA phospholipase A2. Biochemistry. 2006;45:6392–6406. doi: 10.1021/bi060502a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. The Journal of Biological Chemistry. 2007;282:20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- Strokin M, Chechneva O, Reymann KG, Reiser G. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose deprivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phospholipids by calcium independent phospholipase A2. Neuroscience. 2006;140:547–553. doi: 10.1016/j.neuroscience.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Strokin M, Sergeeva M, Reiser G. Prostaglandin synthesis in rat brain astrocytes is under the control of the n – 3 docosahexaenoic acid, released by group VIB calcium-independent phospholipase A2. Journal of Neurochemistry. 2007;102:1771–1782. doi: 10.1111/j.1471-4159.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. The Journal of Biological Chemistry. 2005;280:17601–17607. doi: 10.1074/jbc.M414173200. [DOI] [PubMed] [Google Scholar]
- Subramanian P, Vora M, Gentile LB, Stahelin RV, Chalfant CE. Anionic lipids activate group IVA cytosolic phospholipase A2 via distinct and separate mechanisms. Journal of Lipid Research. 2007;48:2701–2708. doi: 10.1194/jlr.M700356-JLR200. [DOI] [PubMed] [Google Scholar]
- Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. Journal of Neurochemistry. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: Implications for neurodegenerative diseases. Journal of Lipid Research. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, et al. Phospholipase A2 in astrocytes: Responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Molecular Neurobiology. 2005;31:27–41. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Ishizaki J, Yokota Y, Higashino K, Ono T, Ikeda M, et al. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A(2)s. The Journal of Biological Chemistry. 2000;275:5785–5793. doi: 10.1074/jbc.275.8.5785. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Lucas KK, Hua XY, Powell HC, Dennis EA, Yaksh TL. Spinal phospholipase A2 in inflammatory hyperalgesia: Role of the small, secretory phospholipase A2. Neuroscience. 2005;133:543–553. doi: 10.1016/j.neuroscience.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Thomas G, Bertrand F, Saunier B. The differential regulation of group II(A) and group V low molecular weight phospholipases A(2) in cultured rat astrocytes. The Journal of Biological Chemistry. 2000;275:10876–10886. doi: 10.1074/jbc.275.15.10876. [DOI] [PubMed] [Google Scholar]
- Tian W, Wijewickrama GT, Kim JH, Das S, Tun MP, Gokhale N, et al. Mechanism of regulation of group IVA phospholipase A2 activity by Ser727 phosphorylation. The Journal of Biological Chemistry. 2008;283:3960–3971. doi: 10.1074/jbc.M707345200. [DOI] [PubMed] [Google Scholar]
- Tischfield JA, Xia YR, Shih DM, et al. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics. 1996;32:328–333. doi: 10.1006/geno.1996.0126. [DOI] [PubMed] [Google Scholar]
- Titsworth WL, Liu NK, Xu XM. Role of secretory phospholipase a(2) in CNS inflammation: Implications in traumatic spinal cord injury. CNS & Neurological Disorders Drug Targets. 2008;7:254–269. doi: 10.2174/187152708784936671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titsworth WL, Onifer SM, Liu NK, Xu XM. Focal phospholipases A2 group III injections induce cervical white matter injury and functional deficits with delayed recovery concomitant with Schwann cell remyelination. Experimental Neurology. 2007;207:150–162. doi: 10.1016/j.expneurol.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Touqui L, Alaoui-El-Azher M. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Current Molecular Medicine. 2001;1:739–754. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- Tucker DE, Ghosh M, Ghomashchi F, et al. Role of phosphorylation and basic residues in the catalytic domain of cytosolic phospholipase A2alpha in regulating interfacial kinetics and binding and cellular function. The Journal of Biological Chemistry. 2009;284:9596–9611. doi: 10.1074/jbc.M807299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DE, Gijon MA, Spencer DM, Qiu ZH, Gelb MH, Leslie CC. Regulation of cytosolic phospholipase A2alpha by hsp90 and a p54 kinase in okadaic acid-stimulated macrophages. Journal of Leukocyte Biology. 2008;84:798–806. doi: 10.1189/jlb.0308197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G. On the diversity of secreted phospholipases A(2). Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. The Journal of Biological Chemistry. 1999;274:31195–31202. doi: 10.1074/jbc.274.44.31195. [DOI] [PubMed] [Google Scholar]
- Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G. Novel human secreted phospholipase A(2) with homology to the group III bee venom enzyme. The Journal of Biological Chemistry. 2000;275:7492–7496. doi: 10.1074/jbc.275.11.7492. [DOI] [PubMed] [Google Scholar]
- Verheij HM, Slotboom AJ, de Haas GH. Structure and function of phospholipase A2. Reviews of Physiology Biochemistry and Pharmacology. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ramanadham S, Ma ZA, Bao S, Mancuso DJ, Gross RW, et al. Group VIA phospholipase A2 forms a signaling complex with the calcium/calmodulin-dependent protein kinase IIbeta expressed in pancreatic islet beta-cells. The Journal of Biological Chemistry. 2005;280:6840–6849. doi: 10.1074/jbc.M405287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins WP, 3rd, Barbour SE. Group VI phospholipases A2: Homeostatic phospholipases with significant potential as targets for novel therapeutics. Current Drug Targets. 2008;9:683–697. doi: 10.2174/138945008785132385. [DOI] [PubMed] [Google Scholar]
- Won JS, Im YB, Khan M, Singh AK, Singh I. Involvement of phospholipase A2 and lipoxygenase in lipopolysaccharide-induced inducible nitric oxide synthase expression in glial cells. Glia. 2005;51:13–21. doi: 10.1002/glia.20178. [DOI] [PubMed] [Google Scholar]
- Wu Y, Jiang Y, Gao Z, et al. Clinical study and PLA2G6 mutation screening analysis in Chinese patients with infantile neuroaxonal dystrophy. European Journal of Neurology. 2009;16:240–245. doi: 10.1111/j.1468-1331.2008.02397.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, et al. Prostaglandin E2 production in astrocytes: Regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2003a;69:437–448. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Xu L, Han C, Lim K, Wu T. Activation of cytosolic phospholipase A2alpha through nitric oxide-induced S-nitrosylation. Involvement of inducible nitric-oxide synthase and cyclooxygenase-2. The Journal of Biological Chemistry. 2008;283:3077–3087. doi: 10.1074/jbc.M705709200. [DOI] [PubMed] [Google Scholar]
- Xu J, Weng YI, Simonyi A, Krugh BW, Liao Z, Weisman GA, et al. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachadonic acid release in primary murine astrocytes. Journal of Neurochemistry. 2002;83:259–270. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Yu S, Sun AY, Sun GY. Oxidant-mediated AA release from astrocytes involves cPLA(2) and iPLA(2) Free Radical Biology and Medicine. 2003b;34:1531–1543. doi: 10.1016/s0891-5849(03)00152-7. [DOI] [PubMed] [Google Scholar]
- Yagami T, Ueda K, Asakura K, et al. Human group IIA secretory phospholipase A2 induces neuronal cell death via apoptosis. Molecular Pharmacology. 2002;61:114–126. doi: 10.1124/mol.61.1.114. [DOI] [PubMed] [Google Scholar]
- Zhang F, Sha J, Wood TG, et al. Alteration in the activation state of new inflammation-associated targets by phospholipase A2-activating protein (PLAA) Cellular Signalling. 2008;20:844–861. doi: 10.1016/j.cellsig.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Hu C, Sheng W, Tan KS, Haidekker MA, Sun AY, et al. NADPH oxidase-mediated reactive oxygen species alter astrocyte membrane molecular order via phospholipase A2. Biochemistry Journal. 2009;421:201–210. doi: 10.1042/BJ20090356. [DOI] [PubMed] [Google Scholar]
- Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JC. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. Journal of Neuroscience. 2006;26:11111–11119. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. Journal of Cell Science. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]