Summary
Purpose
Given the high incidence of refractory epilepsy, novel therapeutic approaches and concepts are urgently needed. To date, viral mediated delivery and endogenous expression of antisense sequences as a strategy to prevent seizures has received little attention in epilepsy therapy development efforts. Here we validate adenosine kinase (ADK), the astrocyte-based key negative regulator of the brain’s endogenous anticonvulsant adenosine, as a potential therapeutic target for antisense-mediated seizure suppression.
Methods
We developed adeno-associated virus 8 (AAV8)-based gene therapy vectors to selectively modulate ADK expression in astrocytes. Cell type selectivity was achieved by expressing an Adk-cDNA in sense or antisense orientation under the control of an astrocyte-specific gfaABC1D promoter. Viral vectors where injected into the CA3 of wild-type mice or spontaneously epileptic Adk-tg transgenic mice that overexpress ADK in brain. After virus injection, ADK expression was assessed histologically and biochemically. In addition, intracranial EEG-recordings were performed.
Key Findings
We demonstrate in wild-type mice that viral overexpression of ADK within astrocytes is sufficient to trigger spontaneous recurrent seizures in the absence of any other epileptogenic event, whereas ADK downregulation via AAV8-mediated RNA interference almost completely abolished spontaneous recurrent seizures in Adk-tg mice.
Significance
Our data demonstrate that modulation of astrocytic ADK expression can trigger or prevent seizures, respectively. This is the first study to use an antisense approach to validate ADK as a rational therapeutic target for the treatment of epilepsy and suggests that gene therapies based on the knock down of ADK might be a feasible approach to control seizures in refractory epilepsy.
Keywords: RNAi, gene therapy, adenoassociated virus, AAV8, ADK, seizure
Introduction
Current pharmacotherapy for epilepsy largely relies on the neurocentric concept that an imbalance of neuronal excitation and inhibition is the primary contributor to seizure expression and propagation. Unfortunately, about one third of patients with epilepsies remain refractory to current treatment options that are limited by significant side effects (Vajda, 2007). Additionally, current therapies for epilepsy are largely symptomatic and do not affect the underlying disease processes. Given these deficiencies, novel therapeutic (non-neuronal) targets and new treatment strategies are urgently needed.
Hippocampal sclerosis (i.e. proliferation and hypertrophy of astrocytes) is a pathological hallmark of mesial temporal lobe epilepsy, the most common form of pharmacoresistant epilepsy (Wieser, 2004). Several experimental studies over the past five years suggest an astrocytic basis of epilepsy and that astrocyte dysfunction contributes to epileptogenesis and expression of the epileptic phenotype (Tian et al., 2005; Binder and Steinhauser, 2006; Boison, 2008; Oberheim et al., 2008; Rouach et al., 2008; Vezzani, 2008). In addition, recent studies from our laboratory demonstrated a link between astrogliosis and the upregulation of the adenosine-removing enzyme, adenosine kinase (ADK) (Li et al., 2007; Li et al., 2008b). We demonstrated that increased expression of ADK in astrocytes corresponds with neuronal hyperexcitability in a mouse model of CA3-selective epilepsy. In adult brain, astrocytic ADK, constituting a metabolic reuptake system for adenosine, regulates synaptic levels of the brain’s endogenous anticonvulsant and neuroprotectant adenosine, and an astrocyte-based adenosine-cycle has been proposed (Boison, 2008). Consequently, astrogliotic upregulation of ADK in epilepsy contributes to seizure generation by reducing the tone of the endogenous anticonvulsant adenosine; thus focal adenosine augmentation therapies are effective in seizure suppression (Ren et al., 2007; Boison, 2009a, 2009b). Likewise, transgenic overexpression of ADK or lack of the major inhibitory receptor for adenosine, the adenosine A1 receptor, triggered spontaneous seizures in mice (Li et al., 2007). Therefore, ADK is a logical target for therapies aimed at preventing epileptic seizures.
Given the temporal-spatial coincidence between the upregulation of ADK in astrocytes, and the expression of spontaneous seizures (Li et al., 2007; Li et al., 2008b), targeted knock down of ADK specifically in astrocytes constitutes a rational therapeutic approach. Significant advances in adeno associated virus (AAV)-mediated transgene delivery have been made in recent years. AAV-based delivery of galanin or NPY showed prominent seizure suppression in vivo(Richichi et al., 2004; McCown, 2006b, 2006a; Foti et al., 2007). In addition to these overexpression studies, AAV mediated knockdown of the N-methyl-D-aspartate receptor (NMDAR) using an antisense RNA, modulated seizure thresholds when injected into the inferior collicular cortex of rats (Haberman et al., 2002). AAV has an extremely broad host range, capable of infecting most cell types, including neurons and astrocytes (Kaplitt et al., 1994; Peel et al., 1997; Klein et al., 1998; Alisky and Davidson, 2000; Alisky et al., 2000; McCown, 2005, , 2006a, 2006b). Given the size-restrictions of the AAV-system, astrocyte-selective gene expression can be achieved by using a truncated version of the glial fibrillary acidic protein (GFAP) promoter (designated gfaABC1D), which consists of only 680bp of the original promoter but provides the same level of expression and cell type specificity as the full length promoter (Lee et al., 2008).
Aiming to develop a gene therapy based treatment for epilepsy that specifically targets astrocytic ADK, we designed AAV8 vectors that express Adk-cDNA in either sense or antisense orientation under the control of a gfaABC1D promoter to overexpress or knock down ADK in astrocytes, respectively. Here, we demonstrate seizure generation using the ADK-overexpressing sense vector and seizure suppression using the ADK-knockdown antisense vector. These findings indicate that targeted knock down of ADK constitutes an effective and rational approach for antiepileptic therapy.
Methods
Animals
All animal procedures were performed in an AAALAC-accredited facility in accordance with protocols approved by the Legacy Institutional Animal Care and Use Committee and the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. Mice were group housed in ventilated isolator cages with food and water available ad libitum and a 12 hour on/ 12 hour off light cycle.
Cloning of AAV8 expression constructs
To modulate ADK expression in astrocytes, we cloned a set of 2 different expression vectors using the cDNA for the short (cytoplasmic) isoform of ADK that we previously used to generate Adk-transgenic mice (Fedele et al., 2005). The same cDNA was cloned in either the sense (to overexpress ADK) or antisense (to knockdown ADK) orientation into an AAV8-expression plasmid. The Adk-sequence was placed under control of the astrocyte-specific gfaABC1D promoter (Lee et al., 2008). Each construct also contained a 3′ woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) to induce expression of intronless viral messages and increase the stability and level of gene expression (Martin et al., 2002). The resulting plasmids were designated pGfa-Adk-sense (Adk-SS; overexpression of ADK in astrocytes) and pGfa-Adk-antisense (Adk-AS; knockdown of ADK in astrocytes). pGfa-null (AAV-null; containing an “empty” vector construct) was used as a negative control vector.
Virus-production and delivery
Recombinant AAV8 was packaged in cultures of HEK 293T cells. Approximately 1.5 × 107 293T cells were seeded into 150 cm dishes in complete DMEM supplemented with 10% fetal bovine serum, 1 mM MEM sodium pyruvate, 0.1 mM MEM nonessential amino acids solution, and 0.05% Penicillin-Streptomycin (5,000 units/ml). At 24 h media was changed to culture media containing 5% FBS and cells were transfected with using Polyfect (Qiagen, Valencia, CA): Three separate plasmids were used: 1) Adeno helper plasmid (pFΔ6), 2) AAV helper encoding the Rep 2 and Cap 8 sequences for serotype 8 (pAR8) (Broekman et al., 2006), and 3) one of three AAV transgene plasmids described above containing an expression cassette flanked by the AAV8 inverted terminal repeats. After culturing cells for 48 h at 37° C, 5% CO2, cells were harvested and pelleted by centrifugation. The pellet was resuspended in 10 mM Tris, pH 8.0 and chilled on ice. Cells were lysed by repeated freeze-thaw cycles followed by treatment with 50 U benzonase (Novagen, CA) and 0.5% sodium deoxycholate for 30 min at 37° C. Virus was purified by density gradient centrifugation in iodixinol (Zolotukhin et al., 1999). Two buffer exchanges with artificial CSF were performed. The purified virus was then concentrated in artificial CSF by centrifugation in Amicon Ultra-15 Centrifugal Filter Units. The final preparation was sterile filtered through a milipore syringe filter. The titer of each virus (genomic particles/ml) was determined by quantitative RT-PCR using primers and a probe specific for the WPRE sequence. Virus vector infusion was performed under isoflurane anesthesia with 68.5% N2O, 30% O2, and 1.5% isoflurane. Using stereotactic coordinates (AP = −2.18 mm; ML = −2.6 mm; DV = −2.5 mm with bregma as reference), viral particles were unilaterally injected into the CA3. A 5-μl Hamilton syringe with a 34-gauge stainless steel injector (Plastics One Inc) was used to inject 2 μl of concentrated viral solutions (1012 genomic particles/ml) at a rate of 1 μl/min. The needle was left in place for an additional 2 minutes after infusion to minimize reflux.
EEG Monitoring. EEG electrodes were implanted five to six weeks after virus was injected
Briefly, bipolar stainless steel electrodes (insulated except for 80–100μm vertically exposed at the tip; tip diameter 5-μm; vertical tip separation 200–250μm; Plastics One Inc.) were bilaterally implanted using stereotactic coordinates (AP = −2.18 mm; ML = ±2.6 mm; DV = −2.5 mm with bregma as reference) into the CA3 of wild type mice injected withsaline, Adk-SS and AAV-null virusandAdk-tg mice injected with Adk-AS and AAV-null virus. A cortical screw electrode was placed over the frontal cortex and a ground electrode over the cerebellum. All electrodes were secured to the skull with dental cement. After recovery from surgery all animals were continuously recorded by EEG for 24 to 48 hours (mean of 31 hours). The first 12 hours of recordingswere routinely discarded to exclude potential surgery artifacts. All scored EEG segments contained only minimal noise or movement artifacts (<5%). We have previously documented in long-term EEG recordings over 9 days beginning 12 hours after electrode implantation that EEG-data are robust and are dependent on ADK expression levels, but are not influenced by the timepoint of analysis (Li et al., 2007). Likewise, naive (no injection) wild-type control mice (n = 2) with bilateral intrahippocampal electrodesdisplayed similar baseline activity, irrespective of whether they were recorded 12 h or 1 week post-operative. Electrical brain activity was monitored using a Nervus EEG recording system connected with a Nervus Magnus 32/8 Amplifier, and filtered (high-pass filter 0.3 Hz cut off, low-pass 100 Hz). The digital EEG signal was recorded, stored and visualized using a NicoletOne-System (Viasys Healthcare Inc). An observer unaware of the experimental treatment performed quantification of EEG records.EEG seizure activity was assessed unilateral to the virus-injected site in wild type mice and bilaterally in virus-injected Adk-tg mice. EEG seizure activity was defined as high-amplitude rhythmic discharges that clearly represented a new pattern of tracing lasting for more than 5s (repetitive spikes, spike-and-wave discharges, or slow waves). Epileptic events occurring with an interval less than 5s without the EEG returning to baseline were defined as belonging to the same seizure. Seizures were primarily electrographic in nature, but frequently accompanied by arrest or staring episodes, but were never accompanied by convulsions; due to the lack of convulsions in these animals, seizure quantification was performed exclusively by intrahippocampal EEG-recordings.
Histology
Animals were sacrificed within 24 hours of the last EEG recording session by transcardial perfusion with 0.9% NaCl followed by 4% paraformaldehyde in PBS. Considering that seizures in our models are frequent (> 1 seizure per hour), animals were perfused in close proximity (<60 min) to the last seizure. Brains were removed and post fixed in the same fixative at 4°C for 3 days before being cut into 40 μm coronal sections using a vibratome. At least six sections from each brain representing different levels of the hippocampal formation (AP = from −1.34 mm to −2.70 mm with bregma as reference; Franklin and Paxinos, 1997) were then mounted onto gelatine-coated slides and subjected to immunohistochemical detection of ADK (1:4000; see Gouder et al., 2004 for characterization), GFAP (1:15,000; MAB360; Chemicon International), and [SMI-310] 200 kDA+160kDA Neurofilament (1:200; ab24570; Abcam). Previously published procedures were used for immunohistochemical detection of GFAP and ADK (Studer et al., 2006). The neurofilament immunohistochemistry was developed to green fluorescence using secondary fluorescein (FITC) conjugated goat anti-mouse antibodies (1:100; 115-095-166; Jackson ImmunoResearch) using standard procedures (Studer et al., 2006; Li et al., 2008b). Digital images of ADK immunohistochemistry on 3,3′-diaminobenzidine(DAB) stained slices were acquired using a Zeiss AxioPlan inverted microscope equipped with an AxioCam 1Cc1 camera (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Dual immunohistofluorescence images were acquired using a Leica DMLB fluorescence microscope (Leica Microsystems Inc., Bannockburn, IL) and Bioquant Nova version 5.50.8 software (R&M Biometrics).
ADK-densitometry
For semi-quantitative analysis of ADK expression, brain sections from C57BL/6 mice injected with Adk-SS were analyzed using ImageJ software (NIH). Briefly, digital images of ADK staining developed with DAB were acquired with a Zeiss AxioPlan inverted microscope equipped with an AxioCam 1Cc1 camera. The levels of ADK immunoreactive material were measured in corresponding fields from both the ipsi- and contralateral sides of Adk-SS injected hippocampi by analyzing fields encompassing the CA3 region across 3 sections from each animal. The area of CA3 measured was held constant between the ipsi- and contralateral sides of each section. Levels of ADK were initially measured as arbitrary density units and subsequently expressed relative to the contralateral ADK measurement within each section. Data analysisis expressed as the mean ± SEM of ADK levels relative to the contralateral injected hippocampus.
ADK Western blot analysis
Aqueous extracts from whole contra- and ipsilateral hemispheres of Adk-SS injected C57BL/6 hippocampi were prepared by homogenizing and solubilizing the tissue in radioimmunoprecipitation assay (RIPA) buffer (Thermo Scientific, CA) and by removing unsolubilized material by centrifugation (104×g, 15 min at 4°C). The protein content in the supernatants was determined using a commercial Bradford assay (Sigma, MO). 50 μg of protein extract was separated by electrophoresis on an SDS–10% PAGE gel and blotted onto a polyvinylidene fluoride (PVDF) membrane according to standard procedures. The blots were probed for 1 h at room temperature with a 1:4000 dilution of the polyclonal rabbit anti-ADK antibody in 5% blocking reagent in TBST (10 mM Tris, 150 mM NaCl, 0.05% Tween 20 in H2O). After washing (3×5 min in TBST), blots were then probed with a peroxidase-linked anti-rabbit IgG (1:8000 in TBST). To control for equal loading the blots were reprobed with monoclonal mouse anti-GAPDH antibodies (1:500, TBST) followed by goat anti-mouse HRP antibodies (1:2000, TBST). Bands were visualized with a commercial enhanced bioluminescence detection method (ECL) kit (PerkinElmer Life Sciences, MA).
Statistics
Data were analyzed by a one way ANOVA using the Statview data analysis software (Abacus Concepts, Inc., Berkeley, CA, USA). Analysis of variance was performed for each experiment. Student-Newman-Keul’s and t-test procedures were used posthoc for individual comparisons. Differences were considered significant when p<0.05. Data was expressed as group means ± SEM.
Results
Cell-type specific modulation of ADK expression
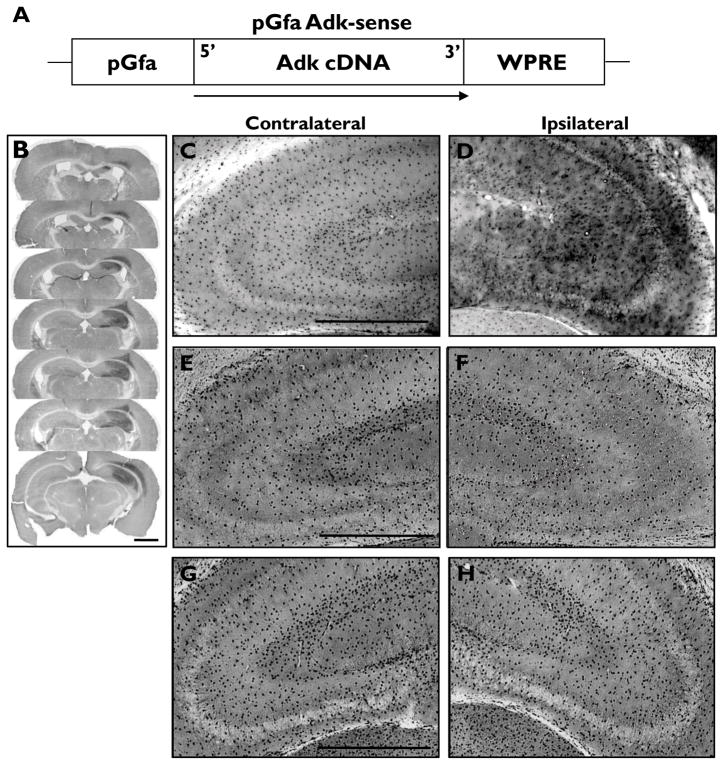
Adenosine kinase is expressed predominantly by astrocytes in the adult brain, and is the key regulator of the endogenous anticonvulsant adenosine (Boison, 2008; Boison et al., 2009). We wanted to test the hypothesis that modulation of ADK expression and activity could influence seizure activity. Towards this goal, we first constructed two novel AAV8 vectors that expressed Adk-cDNA in either sense or antisense orientation. Astrocyte specific expression of the ADK sense and antisense constructs was driven by the gfaABC1D promoter (Fig 1A, 3A). Adult male C57BL/6 wild-type mice were injected with AAV8-pGfa-Adk-sense (Adk-SS) or AAV8-pGfa-Adk-antisense (Adk-AS) unilaterally into the CA3 region of the hippocampus. Control animals were injected with a corresponding AAV not containing an expression cassette for Adk (AAV-null) or saline. Immunohistochemical detection of ADK with DAB enhancement 5–6 weeks after virusinjection was used to confirm that the virus was delivered to the CA3 region within the hippocampus. In regards to the Adk-SS virus, a robust increase in ADK protein was identified ipsilateral to the virus injection site (Fig. 1B, D) compared to levels observed in either the contralateral ADK-SS hippocampus (Fig. 1C) or the AAV-null and saline injected controls(Fig. 1E-H). Analysis of serial coronal brain sections (Fig. 1B) revealed ADK overexpression extends throughout the caudo-rostral extent of the hippocampal formation. Overexpression of ADK was confined to the ipsilateral lateral aspect of the hippocampal formation encompassing the entire CA3 region (Fig. 1B-D). In addition, immunoreactivity was found in cortical structures dorsal to the injected hippocampus (Fig. 1B).
Figure 1.
Overexpression of ADK by unilateral injection of the astrocytic AAV- Adk-SS virus. (A) Schematic illustration of the pGfa-Adk-sense (Adk-SS) vector coding region. The Adk-cDNA is oriented in the sense direction (5′ to 3′) under control of the truncated astrocyte specific gfaABC1D promoter (pGfa). The transcriptional woodchuck hepatitis virus transcriptional regulatory element (WPRE) is placed downstream of the Adk-cDNA to induce expression of intronless viral messages and to increase the stability and level of gene expression. (B-H) Immunohistochemistry using ADK primary antibodies and DAB enhancement in wild-type mice injected with either Adk-SS or AAV-null and naive non-injected controls. (B) Coronal sections of wild-type mouse subjected to unilateral injection of Adk-SS virus into the CA3 region of the hippocampus illustrate the rostral to caudal and median to lateral extent of virus spread. (C, D) Higher magnification images of the contra- and ipsilateral hippocampus of Adk-SS injected wild-type mice with increased astrocytic ADK expression ipsilateral to the injection site (panel D) compared to the non-injected contralateral side (panel C). (E-H) ADK immunoreactivity in contra- (panels E, G) and ipsilateral (panels F, H) hippocampus of AAV-null (panels E, F) and saline (panels G, H) injected wild-type mice with comparable ADK expression in the contra- (panel G) and ipsilateral sides (G, H). Scale bars = 1mm B; 500 μm C-H; 300 μm.
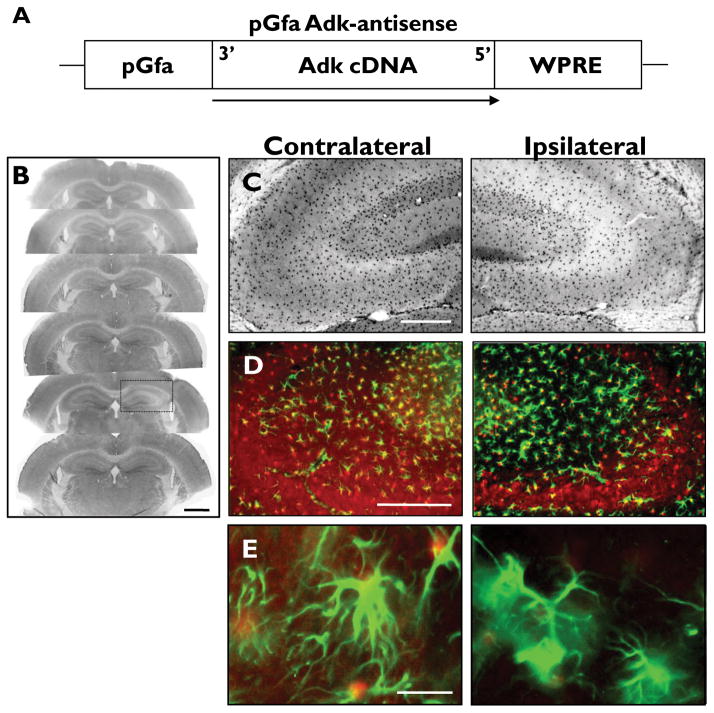
Figure 3.
pGfa- Adk-antisense virus selectively knocks down ADK expression in astrocytes. (A) Schematic illustration of the pGfa-Adk-antisense (Adk-AS) vector coding region. The Adk-cDNA is oriented in the antisense direction (3′ to 5′) under control of the truncated astrocyte specific gfaABC1D promoter (pGfa). The transcriptional woodchuck hepatitis virus transcriptional regulatory element (WPRE) is placed downstream of the Adk-cDNA to induce expression of intronless viral messages and increase the stability and level of gene expression. (B-E) Coronal sections of wild-type mice subjected to unilateral injection of Adk-AS virus into the CA3 region of the hippocampus. (B, C) ADK immunohistochemistry with ADK primary antibodies and DAB enhancement. (B) Unilateral injection of Adk-AS causes localized knockdown of ADK in CA3 (black box). (C) Higher magnification image shows that Adk-AS decreases astrocytic ADK expression ipsilateral to the injection site (right panels) compared to the non-injected contralateral side (left panels). (D, E) Dual immunofluorescence analysis using ADK (red) and GFAP (green) primary antibodies. (D) The decreased ADK immunoreactive material in the ipsilateral hippocampus (right panel) is confined to the CA3. (E) Higher magnification image shows that ADK is uniformly knocked down within GFAP-positive astrocytes (right panel). Scale bars = 1 mm B, 250 μm B, C; 10 μm D.
Confirmation that ADK overexpression was confined to glia was demonstrated by dual immunohistofluorescence detection of ADK and the astrocyte marker, GFAP (Fig. 2A, B). ADK was overexpressed throughout the entire CA3, areas that are densely associated with GFAP labeled astrocytes (Fig. 2A, right panel). Additionally, the injection of Adk-SS yielded an increase in ADK expression that extended throughout the cytoplasm and into the peripheral cell processes of GFAP labeled astrocytes (Fig. 2B, right panel), consistent with the overexpression of the cytoplasmic variant of ADK. This is in contrast to the endogenous ADK expression profile in the contralateral non-injected site with ADK being largely confined to the nuclear region in non-infected astrocytes (Fig. 2A, B, left panel). ADK expression was not observed in neurons based on the absence of ADK colocalization with neurofilament labeled neuronal processes (Fig. 2C).
Figure 2.
pGfa-Adk-sense virus expression is restricted to astrocytes. (A-C) Coronal sections of wild type mice unilaterally injected with Adk-SS into CA3 and subjected to dual immunofluorescence analysis using primary antibodies against ADK (panels A-C; red) and either GFAP (panels A, B; green) or neurofilament (panel C; green). (A) The increased ADK immunoreactivity in the ipsilateral CA3 (right panel) is confined to regions of GFAP staining. Note the absence of reactive astrogliosis evidenced by comparable GFAP staining in the contra-and ipsilateral hippocampus (GFAP insets). (B) Higher magnification image shows that viral ADK is overexpressed throughout the astrocyte cell body (in line with the expression of the cytoplasmic isoform of ADK) and extends into the peripheral processes (right panel), while endogenous ADK (largely the nuclear isoform) is largely confined to the nucleus and cell body of astrocytes (left panel). (C) Immunohistochemistry with neurofilament shows that endogenous (left panel) and overexpressed ADK (right panel) is excluded from neuronal processes. Scale bars = 250 μm A, inlay; 10 μm B, C.
In contrast to the Adk-SS, following injection with the Adk-AS virus a decreasein ADK expression was identified in the CA3 region of the ipsilateral hippocampal formation (Fig. 3B, C).The decrease in ADK expression was modest; however, there was a significant 4.0 ± 1.2 % change versus the contralateral side (TDF,7 = 75.354, p < 0.0001).This decrease in ADK expression was confirmed to be preferentially knocked down in astrocytes using dual immunohistofluorescence with ADK and GFAP antibodies (Fig. 3D, E). These data demonstrate that under these conditions, ADK expression levels can be modulated in vivo in a cell type specific manner. Furthermore, viral mediated overexpression or knockdown of ADK occur independent of reactive astrogliosis, which is evident from the uniform GFAP protein expression in both the ipsi- and contralateral hippocampus (Fig. 2A insets & 3D,E).
Overexpression of ADK in astrocytes triggers seizures
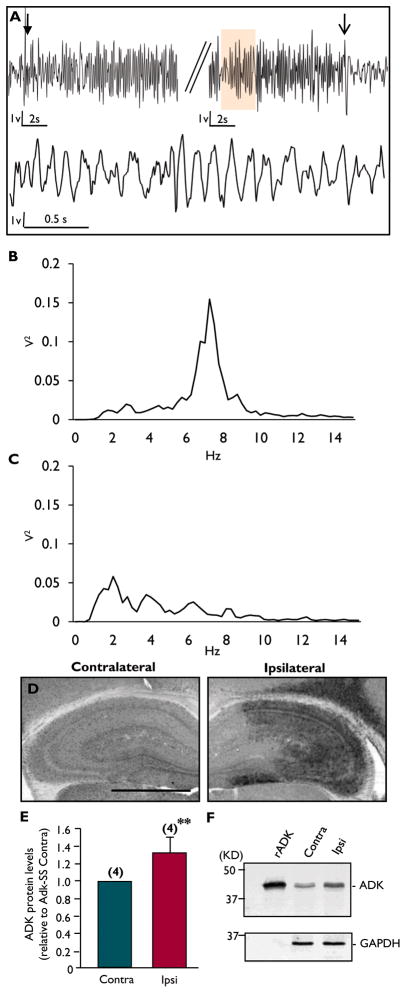
Spatial and temporal coincidence ofastrogliosis, overexpressed ADK, and focal seizures has been described in post status epilepticus models in mice (Gouder et al., 2004; Li et al., 2007; Li et al., 2008b). These studies suggest that overexpression of ADK might be involved in seizure generation; however, the contribution of other epileptogenic events to seizure generation, such as astrogliosisper se, mossy fiber sprouting, granule cell dispersion, or ectopic dentate neurons, could not be excluded. Therefore, our goal was to molecularly dissect ADK expression from other potential epileptogenetic events. To accomplish this, we injected adult male C57BL/6 wild-type mice with the Adk-SS virus to induce the overexpression of ADK in astrocytes in the absence of any other epileptogenetic event. Control animals received injections with AAV-null virus or saline. All injections were performed unilaterally into the CA3 region. Five to six weeks after injection, all animals were subjected to continuous EEG-monitoring using intrahippocampal bipolar electrodes placed bilaterally into the CA3 region. Representative EEG traces from the ipsilateral hippocampus of Adk-SS injected wild-type mice illustrate that increases in ADK protein are associated with spontaneous recurrent electrographic seizures (Fig. 4A). The spontaneous electrographic seizure activity does spread to the contralateral hippocampus (data not shown). The seizures are characterized by a gradual increase in amplitude and frequency that becomes rhythmic at the beginning of the seizure (upper trace, closed arrow); while the end of the seizure has a distinct drop in amplitude and frequency (upper trace, open arrow). The rhythmic nature of the Adk-SS induced seizures is depicted in the lower trace, which is a high resolution component of the seizure depicted in the upper panel (orange color indicates corresponding regions in the traces). Power spectral analysis of the single event depicted in Fig. 4A indicates a predominant frequency of around 7 Hz in the theta band range: 4 – 7 Hz (Fig. 4B), which is in contrast to background EEG activity with a dominance of lower amplitudes and a frequency in the Delta band range: up to 4 Hz) (Fig 4C). Seizures in the Adk-SS injected hippocampus were frequent in terms of the average number of seizures per hour (1.23 +/− 0.21 seizures/ h, n = 6)with an average sz duration of 23 +/− 6.5 s, compared to AAV-null virus (n=8) and saline (n = 5) injected mice that showed no seizure activity.
Figure 4.
pGfa Adk-sense virus induces spontaneous seizures in wild-type mice that are correlated to ADK overexpression. (A) Intrahippocampal EEG traces from the ipsilateral CA3 region of a wild-type mouse injected with Adk-SS virus. The upper panel is a representative sample of a seizure lasting > 20 sec showing the beginning (closed arrow) and distinct end of the seizure (open arrow). The lower trace represents a higher resolution component of the seizure in the upper panel (orange color). (B) Power spectral analysis of the complete seizure depicted in panel A showing peak activity between 7–8 Hz. (C) Power spectral analysis of baseline EEG activity. (D) Immunohistochemistry for ADK with DAB enhancement on a coronal section from a wild-type mouse subjected to unilateral injection of Adk-SS into the CA3 region of the hippocampus. Note that Adk-SS overexpresses astrocytic ADK ipsilateral to the injection site (right panel) compared to the non-injected contralateral side (left panel). (E) Densitometry analysis of ADK immunoreactive cells from the contra- and ipsilateral CA3 regions of Adk-SS injected mice. Data presented as mean± SEM. **p<0.01 (F) Western blot analysis using ADK primary antibodies on protein extracts from the contra- and ipsilateral hippocampal formation of a wild-type mouse unilaterally injected with Adk-SS. Recombinant ADK (rADK) was used as a positive control. GAPDH was used as loading control. Scale bar = 1 mm.
To confirm that the increased incidence of seizure activity in the Adk-SS group coincided with ADK overexpression, we subsequently assessed ADK expression levels by immunohistofluorescence in Adk-SS injected wild-type mice (Fig. 4D). Semi-quantitative analysis of ADK identified a significant increase in ADK expression (145% of normal) in the ipsilateral CA3 hippocampal region of Adk-SS injected wild type mice compared to the non-injected contralateral side of Adk-SS mice (TDF,2 = 11.6, ** p = 0.0073, Fig. 4E).The magnitude of viral mediated ADK overexpression corresponds with our previous findings that ADK enzymatic activity is increased by 177% and CA3 ADK immunoreactivity by 125% following either intra-hippocampal or amygdaloidkainic acid injection, respectively (Gouder et al., 2004; Li et al., 2008b).These results were confirmed by Western blot analysis of ipsi- and contralateral hippocampal protein extracts from Adk-SS injected mice (Fig. 4F, n = 2).
Our data indicate that overexpression of ADK in astrocytes in the absence of astrogliosis or any other epileptogenic event is sufficient to trigger electrographic seizures. Thus, overexpression of ADK appears to be a cause for, rather than a consequence of, chronic recurrent seizure activity. These findings demonstrate that ADK is a rational antiepileptic target for therapeutic intervention.
Antisense-mediated knock down of ADK expression within astrocytes, inhibits spontaneous seizures in Adk-tg mice
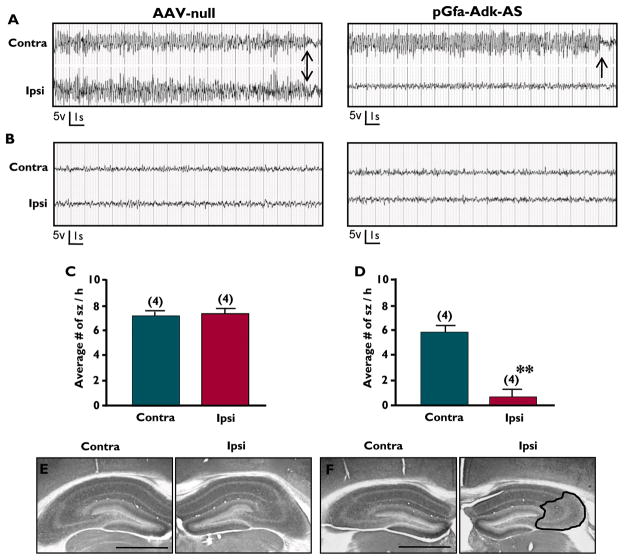
To further test the hypothesis that knock down of ADK expression can indeed ameliorate seizures, we used a transgenic model of spontaneous recurrent seizures. Adk-tg mice (Li et al., 2007) show a global, brain-wide overexpression of ADK (141% of normal). Furthermore, these mice are hypersensitive to ischemic or seizure-induced cell death (Pignataro et al., 2007; Li et al., 2008b) and exhibit spontaneous recurrent bilateral electrographic hippocampal seizures at a baseline rate of 4.8 ± 1.5 seizures per hour with each seizure lasting on average 26.7 ± 13.2 seconds (Li et al., 2008b). Importantly, seizures in this model are frequent and occur synchronized in both hippocampi simultaneously. Thus, Adk-tg mice constitute an ideal model to test whether Adk-AS vectors can ameliorate seizures that are linked to the overexpression of ADK. Adult male Adk-tg mice received unilateral CA3 injections of the astrocyte-specificAdk-AS virus or the empty control AAV-null virus. Five to six weeks after virus injection, all animals were subjected to bilateral EEG recordings using bipolar electrodes implanted into both the ipsi- and contralateral CA3. Representative EEG traces from the AAV-null virus injected Adk-tg mice display the characteristic recurrent bilateral electrographic seizures (Fig. 5A, left panel), compared to baseline levels observed in the interictal periods (Fig. 5B, left panel). Conversely, transgenic mice injected with Adk-AS have a substantial unilateral decrease in seizure activity ipsilateral to the virus injection site with 0.6 ± 0.6 seizures / h (note: only one animal had seizures, the remaining animals were seizure-free), compared to 5.8 ± 0.5 seizures / h on the contralateral (non-injected)side (TDF,6 = 6.5, ** p = 0.006, Fig. 5A, D).Although the analysis of baseline EEG activity prior to virus injection was precluded by technical reasons, maintenance of regular seizures in the contralateral hippocampus of Adk-AS injected mice was comparable to baseline seizure rates in untreated Adk-tg mice, thus the maintenance of seizure activity in the contralateral hippocampus of the Adk-AS injected mice can be considered as surrogate baseline. The interictal period was comparable between the AAV-null and Adk-AS injected mice in both hippocampal hemispheres (Fig. 5B). Immunohistochemistry for ADK was performed to qualitatively assess the knockdown efficiency of the Adk-AS virus. The dramatic decrease in seizure activity was accompanied by a decrease in ADK expression in the Adk-AS injected transgenic mice, which was not evident in the AAV-null injected mice (Fig. 5E, F). Specifically, there was a 3.1% decrease in ADK expression levels in the ipsilateral hippocampus of the Adk-AS injected side(Fig. 5F) compared to the contralateral non-injected hippocampus. Considering that the Adk-tg mouse lacks the nuclear isoform of ADK but has ubiquitous overexpression of the cytoplasmic isoform of ADK (in neurons and in astrocytes) our data indicate that a decrease of cytoplasmic ADK in astrocytes alone is sufficient to suppress seizures in Adk-tg mice. Cumulatively, our data demonstrate that an antisense-mediated knockdown of ADK constitutes a rational approach for seizure suppression.
Figure 5. pGfa Adk-antisense virus suppresses spontaneous seizures in Adk-tgmice.
(A, B)Representative bilateral EEG traces during a seizure (panel A) and an interictal period (panel B) from the CA3 of Adk-tg mice injected with either the AAV-null control virus (left panels) or the Adk-AS virus (right panels). (A) Adk-AS suppresses spontaneous seizure activity in the ipsilateral injected hippocampus, but not in the contralateral non injected side. Arrows denote distinct seizure end. (B) The interictal period is similar between the AAV-null and Adk-SS mice.(C) Quantification of seizure frequency in AAV-null mice shows no significant difference between the injected ipsilateral (7.4 ± 0.4 sz / h) and non-injected contralateral (7.3 ± 0.3 sz / h) CA3. (D) The average number of seizures per hour was significantly reduced in the Adk-AS injected CA3 (ipsilateral, 0.6 ± 0.6sz / h) compared to the uninjected CA3 (contralateral, 5.8 ± 0.5 sz / h). Note that 3 of 4 mice treated with Adk-AS were seizure-free in the ipsilateral CA3. Data presented as mean± SEM. **p<0.01. (E, F) Immunohistochemistry for ADK with DAB enhancement on a coronal section from an Adk-tg mouse subjected to unilateral injection with either AAV-null (panel E) or Adk-AS (panel F) virus into the CA3 subfield. Note that Adk-AS decreases astrocyte ADK expression ipsilateral to the injection site (F, right panel, black outline) compared to the non-injected contralateral side (F, left panel); while there is no change in ADK expression following AAV-null injection (E). Scale bars = 1 mm.
Discussion
To test the hypothesis that levels of ADK in astrocytes govern the excitability of the hippocampal formationwe developed a novel viral expression system that facilitates the overexpression and knock down of ADK specifically in astrocytes. Using the Adk-SS virus, we established that ADK overexpression within astrocytes is sufficient to cause spontaneous recurrent electrographic seizures independent of any additional epileptogenic factors. More importantly, using Adk-AS, in spontaneously epileptic Adk-tg mice, we found that antisense-mediated knock down of ADK in astrocytes effectively suppressed spontaneous recurrent seizures. Thus, using a vector system that specifically targets astrocytes, we not only identify astrocytes as a target for viral gene delivery approaches, but we also demonstrate that AAV-based modulation of astrocyte function is sufficient to modify hippocampal excitability.
Within the concept of the tripartite synapse, astrocytes are key regulators of purinergic signaling and of synaptic activity (Araque et al., 1999; Pascual et al., 2005; Fellin et al., 2006a; Fellin et al., 2006b; Halassa et al., 2007). The existence of two types of equilibrative nucleoside transporters within the astrocyte membrane allow the rapid exchange of synaptic and astrocytic pools of the endogenous purine adenosine, which has well established anticonvulsant properties (Boison et al., 2009). Given the lack of an energy-driven transporter-mediated reuptake system for adenosine, the astrocyte-based enzyme ADK fulfills the role of a metabolic reuptake system for adenosine by phosphorylation of adenosine into 5′-adenosine-monophosphate (AMP) and thereby removing it from the synaptic pool of adenosine (Boison, 2007b, , 2008, , 2010b). Astrogliosis, accompanied by dysregulation of the adenosine system is a well-established hallmark of the epileptic brain (Gouder et al., 2004; Fedele et al., 2005; Li et al., 2007; Boison, 2008; Li et al., 2008a; Li et al., 2008b; Boison et al., 2009; Etherington et al., 2009; Boison, 2010b). In particular, astrogliosis has been linked to the overexpression of ADK and the incidence of spontaneous and recurrent electrographic seizures (Li et al., 2007; Boison, 2008; Li et al., 2008a; Li et al., 2008b).
Previous studies suggested that the increase in astrocytic ADK might be sufficient to cause spontaneous recurrent seizures and epilepsy. In a mouse model of CA3-selective epileptogenesis, where kainic acid is administered by intraamygdaloid injection, a spatial and temporal coincidence of astrogliosis and overexpressed ADK within the ipsilateral CA3 was linked to spontaneous and recurrent electrographic seizures (Li et al., 2007; Li et al., 2008b). The experiments conducted here with the astrocytic-specificAdk-SS and Adk-AS viruses establish that ADK serves as a key regulator of neuronal excitability and seizures. First, using the Adk-SSvector we specifically overexpressed the short cytoplasmic isoform of ADK (Cui et al., 2009) in astrocytes. As a result ADK expression expands into the cellular processes of astrocytes (Fig. 2C) and abundance of the short isoform of the enzyme is increased in the Western Blot (Fig. 4E). In contrast to Adk-SS induced overexpression of ADK as described here, overexpression of ADK in chronic epilepsy affects both isoforms (nuclear and cytoplasmic) of ADK (Li et al, 2008). Thus, our present results suggest, that overexpression of the short isoform is sufficient to trigger seizures. Second, our data suggest that seizure parameters are dictated by the amount of ADK expressed within astrocytes. In the Adk-tg mice we previously established that ADK protein levels are increased by 141% cumulatively in both astrocytes and neurons (Li et al., 2007). Conversely, wild type mice injected with Adk-SS have a 145% increase of ADK in astrocytes alone because the virus is under control of the gfaABC1D promoter, and because adult wild-type mice do not express ADK in most neurons (Studer et al. 2006). The robust ADK-SS mediated increase in astrocytic ADK expression was shown here to besufficient to induce seizures at a rate of 1.2± 0.2 seizures / h lasting on average 23.0±6.4seccompared to4.8±1.5 seizures / h lasting 26.7 ± 13.2 secin Adk-tg mice. Third, by administering the Adk-AS virus to Adk-tg mice we demonstrate that the knockdown of ADK in astrocytes per se is sufficient to suppress spontaneous seizures despite residual expression of ADK in neurons. Specifically, in 3 out of the 4 Adk-tg mice injected with Adk-AS any seizure activity was completely abrogated. The single Adk-AS injected Adk-tg mouse that did have remaining seizures also experienced a reduction in seizure duration compared to the AAV-null injected Adk-tg controls (data not shown). Thus we provide proof for the first time that (i) via a novel vector system ADK expression can be modulated in a specified cell-type (i.e. astrocytes) and(ii) an increase in spontaneous electrographic seizure activity is solely linked to ADK expression in astrocytes.
Several lines of evidence indicate that overexpression of ADK is cause for, rather than consequence of, seizures: (i)The present study demonstrates that (viral) overexpression of ADK as such is sufficient to trigger seizures.(ii) As demonstrated previously, status epilepticusis associated with an acute transient decrease in ADK immunoreactivity between 2 and 24 hours post intrahippocampal KA injection(Gouder et al., 2004). (iii) Chronic overexpression of ADK is a delayed consequence of acute brain injury and coincides with astrogliosis and spontaneous electrographic seizures (Li et al., 2007). (iv) Induced (kindled) seizures in the rat are not associated with increased expression of ADK (unpublished findings). Together, these findings demonstrate that overexpression of ADK can trigger seizures, but that overexpression of ADK is most likely not a compensatory response to seizures.In future studies the Adk-SS vector described here could be employed as a powerful tool to unravel the temporal co-incidence of ADK overexpression and seizure generation and to elucidate the necessary exposure period and to quantify threshold levels of ADK to induce spontaneous seizures.
Together, ourstudies identify ADK as a prime therapeutic target for the treatment of epilepsy. The avenue of pharmacological manipulation of either the adenosine system or ADK has been explored; however, there are distinct limitations associated with adenosinergic drugs. In principle, adenosine A1 receptor (A1R) agonists are very effective in the inhibition of neuronal activity and in the suppression of seizures (Fredholm, 2003; Jacobson and Gao, 2006). However, despite activity in a variety of models, A1R agonists, when given systemically are not potential antiepileptic agents because of profound peripheral, mainly cardiovascular, side effects (Monopoli et al., 1994). Since endogenous adenosine levels increase during times of stress (e.g. lack of oxygen, seizures), agents (e.g. the ADK inhibitor ABT-702) that amplify this site- and event-specific surge of adenosine could provide antiseizure activity similar to that of adenosine receptor agonists (Kowaluk and Jarvis, 2000; McGaraughty et al., 2005). Thus, pharmacological inhibition of ADK is an efficient tool for the inhibition of epileptic seizures (Kowaluk and Jarvis, 2000; Gouder et al., 2004) and chronic pain (McGaraughty and Jarvis, 2006); these successes were associated with an improved therapeutic window compared to A1R agonists (Jarvis et al., 2002). However, the systemic application of ADK inhibitors is associated with risks of liver toxicity (Boison et al., 2002) and brain hemorrhage (Erion et al., 2000; McGaraughty and Jarvis, 2006). Unfortunately, the adverse side effects associated with systemic use of adenosine augmenting agents do not render them as a realistic option for epilepsy treatment. Therefore, focal adenosine augmentation strategies are needed to restrict the potent anticonvulsant potential of adenosine to an epileptogenic brain region. Cell- and polymer-based focal adenosine augmentation therapies have already been explored and were found to provide effective seizure control (Boison, 2009a; Boison and Stewart, 2009). While polymer-, and in particular silk-based adenosine delivery (Wilz et al., 2008; Szybala et al., 2009), are ideally suited to initiate clinical safety- and feasibility tests for focal adenosine-augmentation, these systems need to be further improved to provide long-term delivery options for adenosine. In contrast, a viral ADK antisense approach as proposed here would allow for permanent reversal of ADK-based adenosine dysregulation restricted to a brain region in which ADK levels are pathologically high (i.e. within an epileptogenic focus). This can best be achieved by using viral mediated delivery of antisense sequences, a powerful therapeutic opportunity that has recently been reviewed for its suitability for the treatment of epilepsy (Boison, 2010a). Here we demonstrate a proof of principle for the feasibility of an antisense approach to knockdown ADK. In particular, we show that our novel Adk-AS virus effectively knocks down ADK within astrocytes. Importantly, this strategy almost completely suppressed spontaneous recurrent seizures in Adk-tg mice within the hippocampal CA3 area ipsilateral to the virus-injection site. Seizure suppression in Adk-tg mice via an antisense mechanism targeting ADK is important for two reasons: (i) Validation of ADK as a therapeutic target for seizure control, and (ii) the demonstration that seizures can be suppressed by implementing an antisense based strategy in vivo. However, prior to establishing the ADK-AS virus as a therapeutic option for epilepsy the efficacy of the virus in established post status epilepticus models of epilepsy, which are characterized by an astrogliotic scar and upregulated endogenous ADK, are necessary.
Considering that one third of all epilepsies are resistant to current treatments, the necessity of developing novel therapeutic options is of high priority (Vajda, 2007; Loscher et al., 2008). Gene therapy approaches are considered to hold promise for those epilepsies, in which pharmacotherapy is ineffective and in which rational therapeutic targets are available (Riban et al., 2009). To date research in this arena has predominantly focused on gene therapy approaches that aim to overexpress select anticonvulsant molecules such as NPY, galanin, or a specific subunit of the GABAAR (McCown, 2004, , 2005; Raol et al., 2006; Boison, 2007a; Noe et al., 2007; Vezzani, 2007; Loscher et al., 2008; McCown, 2009; Noe et al., 2009; Riban et al., 2009; Gray et al., 2010). In contrast to those studies, the results reported here constitute a novel and one of only very few antisense approaches shown to modulate hippocampal excitability. In contrast to most viral vector mediated approaches that overexpress a gene of interest, we demonstrate here the proof-of-principle that an antisense strategy can be used to reduce hippocampal excitability in vivo and that ADK is a suitable target. Furthermore our studies selectively target ADK expression in astrocytes opposed to neurons, which are the focus of a majority of antisense directed studies (Haberman et al., 2002). Thus, we not only identify ADK as a viable candidate for epilepsy treatment, but also identify antisense-based therapy as a potential technique that can be employed for seizure suppression.
Acknowledgments
This project was supported by grantsR01NS061844and R01NS058780 from the National Institutes of Health (NIH).
Footnotes
Disclosure of Conflicts of Interest - None of the authors has any conflicts of interest.
Statement on Ethical Publication - We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Alisky JM, Davidson BL. Gene therapy for amyotrophic lateral sclerosis and other motor neuron diseases. Hum Gene Ther. 2000;11:2315–2329. doi: 10.1089/104303400750038435. [DOI] [PubMed] [Google Scholar]
- Alisky JM, Hughes SM, Sauter SL, Jolly D, Dubensky TW, Jr, Staber PD, Chiorini JA, Davidson BL. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport. 2000;11:2669–2673. doi: 10.1097/00001756-200008210-00013. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Boison D. Cell and gene therapies for refractory epilepsy. Current Neuropharmacology. 2007a;5:115–125. doi: 10.2174/157015907780866938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine, astrogliosis and seizures: a new perspective of epileptogenesis. Neuron Glia Biology. 2007b;2:S9–S9. [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009a;85:131–141. doi: 10.1016/j.eplepsyres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics. 2009b;6:278–283. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Inhibitory RNA in epilepsy: Research tool and therapeutic perspectives. Epilepsia. 2010a;51:1659–1668. doi: 10.1111/j.1528-1167.2010.02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine dysfunction and adenosine kinase in epileptogenesis. The Open Neuroscience Journal. 2010b;4:93–101. doi: 10.2174/1874082001004020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Stewart K-A. Therapeutic epilepsy research: from pharmacological rationale to focal adenosine augmentation. Biochem Pharmacol. 2009;78:1428–1437. doi: 10.1016/j.bcp.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB. Adenosine signalling and function in glial cells. Cell Death Differ. 2009 September 18; doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Scheurer L, Zumsteg V, Rulicke T, Litynski P, Fowler B, Brandner S, Mohler H. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99:6985–6990. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Cui XA, Singh B, Park J, Gupta RS. Subcellular localization of adenosine kinase in mammalian cells: The long isoform of AdK is localized in the nucleus. Biochem Biophys Res Commun. 2009;388(1):46–50. doi: 10.1016/j.bbrc.2009.07.106. [DOI] [PubMed] [Google Scholar]
- Erion MD, Wiesner JB, Rosengren S, Ugarkar BG, Boyer SH, Tsuchiya M. Therapeutic potential of adenosine kinase inhibitors as analgesic agents. Drug Dev Res. 2000;50:S14–06. [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Haydon PG. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology (Bethesda) 2006a;21:208–215. doi: 10.1152/physiol.00161.2005. [DOI] [PubMed] [Google Scholar]
- Fellin T, Sul JY, D’Ascenzo M, Takano H, Pascual O, Haydon PG. Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Novartis Found Symp. 2006b;276:208–217. doi: 10.1002/9780470032244.ch16. discussion 217–221, 233–207, 275–281. [DOI] [PubMed] [Google Scholar]
- Foti S, Haberman RP, Samulski RJ, McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of NPY or NPY13-36 suppresses seizure activity in vivo. Gene Ther. 2007;14:1534–1536. doi: 10.1038/sj.gt.3303013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine receptors as targets for drug development. Drug News Perspect. 2003;16:283–289. doi: 10.1358/dnp.2003.16.5.829316. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press, Inc; 1997. [Google Scholar]
- Gouder N, Scheurer L, Fritschy J-M, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Blake BL, Criswell HE, Nicolson SC, Samulski RJ, McCown TJ. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol Ther. 2010;18:570–578. doi: 10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman R, Criswell H, Snowdy S, Ming Z, Breese G, Samulski R, McCown T. Therapeutic liabilities of in vivo viral vector tropism: adeno-associated virus vectors, NMDAR1 antisense, and focal seizure sensitivity. Mol Ther. 2002;6:495–500. doi: 10.1006/mthe.2002.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Mikusa J, Chu KL, Wismer CT, Honore P, Kowaluk EA, McGaraughty S. Comparison of the ability of adenosine kinase inhibitors and adenosine receptor agonists to attenuate thermal hyperalgesia and reduce motor performance in rats. Pharmacol Biochem Behav. 2002;73:573–581. doi: 10.1016/s0091-3057(02)00840-7. [DOI] [PubMed] [Google Scholar]
- Kaplitt M, Leone P, Freese A, Xiao X, Pfaff D, O’;alley K, During M. Adeno-associated virus (AAV) vectors yield safe delivery and long-term expression of potentially therapeutic genes in the adult mammalian brain. Soc Neurosci Abstr. 1994;20:1465. [Google Scholar]
- Klein RL, Meyer EM, Peel AL, Zolotukhin S, Meyers C, Muzyczka N, King MA. Neuron-specific transduction in the rat septohippocampal or nigrostriatal pathway by recombinant adeno-associated virus vectors. Exp Neurol. 1998;150:183–194. doi: 10.1006/exnr.1997.6736. [DOI] [PubMed] [Google Scholar]
- Kowaluk EA, Jarvis MF. Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs. 2000;9:551–564. doi: 10.1517/13543784.9.3.551. [DOI] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron Glia Biology. 2008a;4:91–99. doi: 10.1017/S1740925X09990135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008b;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Gernert M, Heinemann U. Cell and gene therapies in epilepsy - promising avenues or blind alleys? Trends in Neurosciences. 2008;31:62–73. doi: 10.1016/j.tins.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267–275. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- McCown TJ. The clinical potential of antiepileptic gene therapy. Expert Opinion on Biological Therapy. 2004;4:1771–1776. doi: 10.1517/14712598.4.11.1771. [DOI] [PubMed] [Google Scholar]
- McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2005;5:333–338. doi: 10.2174/1566523054064995. [DOI] [PubMed] [Google Scholar]
- McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Molecular Therapy. 2006a;14:63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- McCown TJ. In vivo seizure suppression by adeno-associated virus (AAV)-mediated expression and constitutive secretion of galanin or NPY. Neuropeptides. 2006b;40:145–145. [Google Scholar]
- McCown TJ. Adeno-associated virus vector-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity. Neurotherapeutics. 2009;6:307–311. doi: 10.1016/j.nurt.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Jarvis MF. Purinergic control of neuropathic pain. Drug Development Research. 2006;67:376–388. [Google Scholar]
- McGaraughty S, Cowart M, Jarvis MF, Berman RF. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem. 2005;5:43–58. doi: 10.2174/1568026053386845. [DOI] [PubMed] [Google Scholar]
- Monopoli A, Conti A, Dionisotti S, Casati C, Camaioni E, Cristalli G, Ongini E. Pharmacology of the highly selective A1 adenosine receptor agonist 2-chloro-N6-cyclopentyladenosine. Arzneimittelforschung. 1994;44:1305–1312. [PubMed] [Google Scholar]
- Noe F, Nissinen J, Pitkanen A, Gobbi M, Sperk G, During M, Vezzani A. Gene therapy in epilepsy: The focus on NPY. Peptides. 2007;28:377–383. doi: 10.1016/j.peptides.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Noe F, Frasca A, Balducci C, Carli M, Sperk G, Ferraguti F, Pitkanen A, Bland R, Fitzsimons H, During M, Vezzani A. Neuropeptide Y overexpression using recombinant adeno-associated viral vectors. Neurotherapeutics. 2009;6:300–306. doi: 10.1016/j.nurt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Peel AL, Zolotukhin S, Schrimsher GW, Muzyczka N, Reier PJ. Efficient transduction of green fluorescent protein in spinal cord neurons using adeno-associated virus vectors containing cell type-specific promoters. Gene Ther. 1997;4:16–24. doi: 10.1038/sj.gt.3300358. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riban V, Fitzsimons HL, During MJ. Gene therapy in epilepsy. Epilepsia. 2009;50:24–32. doi: 10.1111/j.1528-1167.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richichi C, Lin EJ, Stefanin D, Colella D, Ravizza T, Grignaschi G, Veglianese P, Sperk G, During MJ, Vezzani A. Anticonvulsant and antiepileptogenic effects mediated by adeno-associated virus vector neuropeptide Y expression in the rat hippocampus. J Neurosci. 2004;24:3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy J-M, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Szybala C, Pritchard EM, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp Neurol. 2009;219:126–135. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu QW, Peng WG, Lin J, Oberheim N, Lou NH, Wang XH, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nature Medicine. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda FJE. Pharmacotherapy of epilepsy: New armamentarium, new issues. Journal of Clinical Neuroscience. 2007;14:813–823. doi: 10.1016/j.jocn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Vezzani A. The promise of gene therapy for the treatment of epilepsy. Expert Rev Neurother. 2007;7:1685–1692. doi: 10.1586/14737175.7.12.1685. [DOI] [PubMed] [Google Scholar]
- Vezzani A. Epileptogenic role of astrocyte dysfunction. Epilepsy Curr. 2008;8:46–47. doi: 10.1111/j.1535-7511.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]