Abstract
Schizophrenia is a neurodevelopmental disorder characterized by deficits in cognitive processes mediated by the circuitry of the dorsolateral prefrontal cortex (DLPFC). These deficits are associated with a range of alterations in DLPFC circuitry, some of which reflect the pathology of the illness and others of which reflect the neuroplasticity of the brain in response to the underlying disease process. This article reviews disturbances in excitatory and inhibitory components of DLPFC circuitry from the perspective of developmental neuroplasticity and discusses their implications for the identification of novel therapeutic targets.
Keywords: GABA, glutamate, prefrontal coitex, working memory
Abstract
La esquizofrenia es un trasiorno del neurodesarrollo que se caracieriza por déficit en los procesos cognitivos, los que están mediados por los circuitos de la corteza prefrontal dorsolateral (CPFDL), Estos déficit están asociados con una amplia gama de alteraciones en los circuitos de la CPFDL; algunos de ellos reflejan la patología de la enfermedad y otros cómo la neuroplasticidad cerebral responde al proceso patológico subyacente. En este artículo se revisan las alteraciones en los componentes excitatorios e inhibitorios de los circuitos de la CPFDL desde la perspectiva de la neuroplasticidad del desarrollo y se discuten sus implicancias en la identificación de nuevas terapias.
Abstract
La schizophrénie est une maladie d'origine neuro-développementale caractérisée par un déficit des processus cognitifs transmis par les circuits du cortex dorsolateral préfrontal (CPFDL), Ces déficits s'associent à un ensemble d'altérations des circuits du CPFDL, dont certains reflètent la physiopathologie du trouble et dont d'autres sont la conséquence de la neuroplasticité cérébrale en réponse au processus pathologique sous-jacent Cet article propose une revue des troubles des composants excitateurs et inhibiteurs des circuits du CPFDL selon une perspective de neuroplasticité développementale, et traite de leurs implications pour l'identification de nouvelles cibles thérapeutiques.
Neuroplasticity can be broadly considered to be the capacity of the brain to change the molecular and structural features that dictate its functions in response to a disease process (or other factors) that disrupts those functions.1 For a disorder such as schizophrenia, the disease process appears to result from a complex interplay of an unknown number of genetic liabilities and environmental risk factors that unleash pathogenetic mechanisms which produce a pathological entity, a conserved set of molecular and cellular disturbances in specific neural circuits. These pathological changes so alter the normal function of the affected circuits that the resulting pathophysiology gives rise to the emergent properties recognized as the clinical features of the illness.2 One approach to dissecting this disease process involves focusing on a well-defined clinical component of the illness. For example, deficits in cognitive abilities are thought to be the core features of schizophrenia because they occur with high frequency in individuals with schizophrenia, are relatively stable over the course of the illness, are independent of the psychotic symptoms of the disorder, are present in a milder form in individuals at genetic risk who do not become clinically ill,3 and are the best predictor of long-term functional outcome.4
Of the domains of cognition affected in schizophrenia, disturbances in working memory, the ability to transiently maintain and manipulate a limited amount of information in order to guide thought or behavior, are accompanied by altered activation of the dorsolateral prefrontal cortex (DLPFC, Figure 1 A, B). The altered activation of the DLPFC under such conditions might be specific to the disease process of schizophrenia because these disturbances are present in medication-naïve individuals with schizophrenia, but not in subjects with other psychotic disorders or major depression.5,6
Figure 1. A) Photograph of an unstained coronal block, containing the prefrontal cortex, cut immediately anterior to the corpus callosum through the left hemisphere of a postmortem human brain. This block also includes the adjacent anterior cingulate gyrus (ACG) of the limbic lobe. The portion of the dorsolateral prefrontal cortex (DLFPC) delineated by the small rectangle is shown at higher magnification in panel B. B) Nissl-stained section showing the typical appearance of six layers or lamina, numbered from the pial surface of the cortex to the underlying white matter, based on the size and packing density of neurons. C) Schematic representation of neurons across cortical layers. Pyramidal neurons (red) represent about 75% of cortical neurons and typically have triangularly-shaped cell bodies, a single apical dendrite directed towards the pial surface, and an array of basilar dendrites. Depending on their laminar location, the axons of pyramidal neurons preferentially provide excitatory projections to different brain regions. Axons that project to the DLPFC from other brain regions also tend to innervate different subsets of cortical layers. For example, axonal projections (green) from the thalamus terminate in layers deep 3 and 4. The remaining -25% of DLPFC neurons are local circuit or interneurons (blue). These neurons use the inhibitory neurotransmitter GABA, and have axons that arborize locally and innervate other neurons in the same area of the prefrontal cortex. Reproduced from ref 1: Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141-165. Copyright©Nature Publishing Group 2008.
This review examines alterations in components of excitatory and inhibitory neurotransmission in DLPFC circuitry that might contribute to the impairments in working memory in schizophrenia. Each mediator is considered from the perspective of which alterations reflect the disease process and which might be neuroplastic responses of the affected circuits. Although additional studies are required, existing data suggests that many of the alterations described below are probably also present in other cortical regions that are dysfunctional in schizophrenia.7
Neuroplasticity of excitatory cortical connections in schizophrenia
Excitatory connections in the DLPFC are altered in schizophrenia
The disease process of schizophrenia appears to involve deficient glutamate -mediated excitatory neurotransmission through the N-methyl-D-aspartic acid (NMDA) receptor.8,9 NMDA receptor antagonists such as phencyclidine (PCP) or ketamine increase both positive and negative symptoms in patients with schizophrenia, and the administration of subanesthetic doses of ketamine to healthy individuals produces thought disorder and other features similar to those seen in schizophrenia.10
In addition, systemic administration of NMDA receptor antagonists disrupts working memory in rats,11 and application of an NMDA receptor antagonist to the DLPFC impairs working memory performance in monkeys.12 However, although postmortem studies have reported alterations in measures of glutamate receptor binding, transcription, and subunit protein expression in several brain regions in subjects with schizophrenia,13 such findings for mRNA and protein levels of NMDA receptor subunits in the DLPFC have been limited in magnitude and not always replicated, suggesting that other components of NMDA receptor signaling might be affected in the illness.14
Anatomical studies do support the presence of input-specific alterations of excitatory connections in the DLPFC in schizophrenia. In the DLPFC, pyramidal neurons (Figure 1 C) are the principal source of glutamate neurotransmission, as well as the targets of the majority of glutamate-containing axon terminals. Although the number of these neurons does not appear to be altered in schizophrenia,15,16 neuronal density in the DLPFC has been reported to be increased in schizophrenia.17 Increased cell packing density has been interpreted as evidence of a reduction in the amount of cortical neuropil, the axon terminals, dendritic spines, and glial processes that occupy the space between neurons.18 Consistent with this interpretation, synaptophysin protein, a marker of axon terminals, has been reported to be decreased in the DLPFC of subjects with schizophrenia.19,21 Furthermore, gene expression profiling studies have found reduced tissue levels of gene transcripts that encode proteins involved in the presynaptic regulation of neurotransmission.22
Dendritic spines are the principal targets of excitatory synapses to pyramidal neurons. Although most dendritic spines present are stable in number during adulthood,23 they are subject to a number of neuroplastic changes, such as a loss of their presynaptic excitatory input. In schizophrenia, dendritic spine density in pyramidal neurons has been reported to be lower in the DLPFC24,25; understanding the nature of these neuroplastic responses requires knowledge of the specific circuits that are affected and the developmental mechanisms that might underlie these changes.
Reduced excitatory connections in schizophrenia are specific to a subset of pyramidal neurons
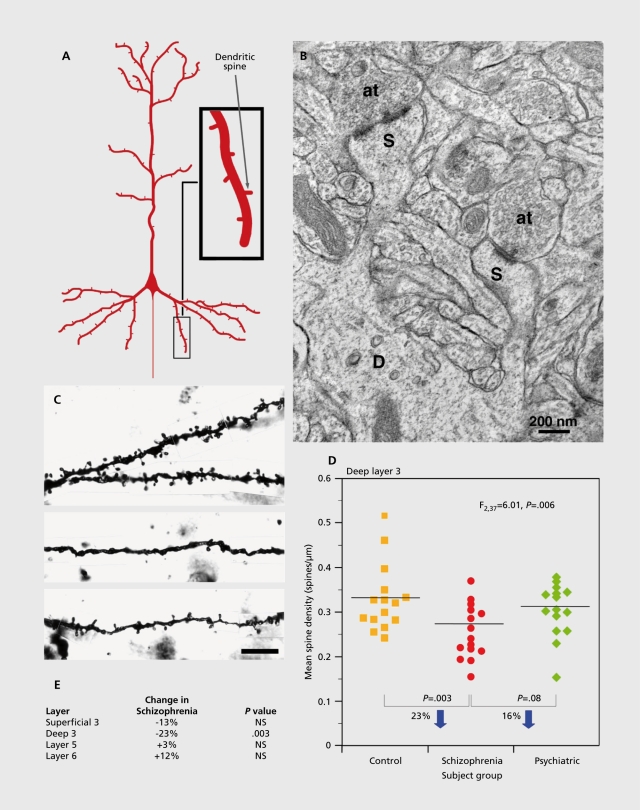
Pyramidal neurons can be divided into subgroups based on the brain region targeted by their principal axonal projection and the sources of their excitatory inputs; both of these characteristics are associated with the location of pyramidal cell bodies in different layers of the cortex (Figure 1 C). For example, many pyramidal cells in layers 2 to 3 send axonal projections to other cortical regions, pyramidal neurons in layer 5 tend to project to the striatum and other subcortical structures, and pyramidal neurons in layer 6 furnish projections primarily to the thalamus.26 Studies of basilar dendritic spine density on Golgi-impregnaled pyramidal neurons in each cortical layer of the DLPFC in the same cohort of subjects found a significant effect of diagnosis on spine density only for pyramidal neurons in deep layer 3 (Figure 2).25,27
Figure 2. Pyramidal neuron dendritic spines in the human DLPFC. A) Schematic diagram illustrating the dendritic tree and dendritic spines on a prototypic pyramidal neuron. B) Electron micrograph showing a dendrite (D) with two spines (S). Each spine receives an asymmetric (presumably excitatory) synapse from an axon terminal (at). C) Golgi-impregnated basilar dendrites and spines on deep layer 3 pyramidal neurons from a normal comparison (top) and two subjects with schizophrenia (bottom). Note the reduced density of spines in the subjects with schizophrenia in these extreme examples. D) Scatter plot demonstrating the lower density of spines on the basilar dendrites of deep layer 3 pyramidal neurons in the DLPFC of subjects with schizophrenia relative to both normal and psychiatrically-ill comparison subjects. E) Laminar-specificity of the spine density differences in the same subjects. Reproduced from ref 1: Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141-165. Copyright© Nature Publishing Group 2008.
The functional integrity of the pyramidal neurons with lower dendritic spine densities may be reflected in changes in their somal volume. For example, shifts in somal size may indicate disturbances in neuronal connectivity, given that somal size has been shown to be correlated with measures of a neuron's dendritic tree28 and axonal arbor.29 Indeed, the mean cross-sectional somal area of the Golgi-impregnated, deep layer 3 pyramidal neurons was 9% smaller in the subjects with schizophrenia relative to normal control subjects.25 Consistent with this observation, the mean somal volume of Nisslstained pyramidal neurons in DLPFC deep layer 3 was also 9% smaller in a different cohort of subjects with schizophrenia.30 Similarly, in another study, the mean somal size of all layer 3 neurons in DLPFC area 9 was smaller in subjects with schizophrenia, and was accompanied by a decrease in the density of the largest neurons in deep layer 3, without a change in somal volume in layer 5.31 Furthermore, in both primary and association auditory cortices, somal volumes of deep layer 3, but not of layer 5, pyramidal neurons were smaller in schizophrenia.32,33 Together, these findings suggest that in schizophrenia: i) basilar dendritic spine density is lower and somal volume is smaller in deep layer 3 pyramidal neurons; ii) these alterations are specific to or at least most prominent in deep layer 3; iii) this pattern of alterations is not restricted to the DLPFC; and iv) these differences reflect the underlying disease process and not confounding factors.
The contribution of developmental plasticity to dendritic spine alterations in schizophrenia
Dendritic spine density on DLPFC layer 3 pyramidal neurons undergoes a substantial decline during adolescence in primates.34 Consistent with the findings that dendritic spines are the main site of excitatory synaptic input onto pyramidal cells and that all mature dendritic spines contain an excitatory synapse,35 the number of excitatory synapses declines in a similar age-related fashion in both monkey and human DLPFC.36,37 In humans, this synaptic pruning is thought to underlie the decrease in cortical gray matter thickness that occurs during adolescence.38,39 Interestingly, the late developmental refinements in excitatory connectivity are more marked in layer 3 than in the deeper cortical layers,36 suggesting that they may be associated with the apparent laminas-specific alterations in spine density in schizophrenia. The observation of alterations in the expression of certain synaptic proteins in schizophrenia suggested the possibility that the exuberant synapses present before adolescence somehow compensated for a dysfunction in excitatory transmission in individuals with schizophrenia.40 Alternatively, such alterations in synaptic protein expression might disturb the mechanisms of adolescence-related synapse elimination leading, for instance, to excessive synapse pruning and decreased spine number in the illness.41,42
The potential contribution of excitatory synapse pruning during adolescence to disease-related changes in DLPFC function depends, in part, on the functional properties of the synapses that are pruned. During early brain development, pruned synapses are functionally immature. Immature glutamate synapses are relatively weak and their maturation involves an activity-dependent increase in strength. Such activity-dependent strengthening might underlie synapse stabilization, and thus mark for elimination the immature synapses that are not strengthened.43,44 However, recent findings in the developing monkey DLPFC indicate that the excitatory-inputs to layer 3 pyramidal neurons mature functionally during the age range when they are present in high density and before synaptic pruning begins.45 Thus, these data suggest that the substantial remodeling of excitatory connectivity of the primate DLPFC during adolescence primarily involves the elimination of mature synapses, and that some other factor, such as the neuronal source of input, somehow tags mature synapses for pruning.46 Thus, the presence of functionally mature synapses prior to adolescence supports the hypothesis that the excess in excitatory synapse number prior to adolescence might be able to compensate for a molecular based dysfunction of these synapses in individuals with schizophrenia, and thereby forestall the appearance of the clinical features of the illness until synapse number falls below some critical threshold.40
Neuroplasticity of inhibitory cortical connections in schizophrenia
Prefrontal inhibitory neurotransmission is altered in schizophrenia
Studies from multiple laboratories have consistently found lower levels of the mRNA for the 67 kilodalton isoform of glutamic acid decarboxylase (GAD67), the principal synthesizing enzyme for y-aminobutyric acid (GABA), in the DLPFC of subjects with schizophrenia.16,22-47,52 At the cellular level, the expression of GAD67 mRNA was not detectable in -25% to 35% of GABA neurons in layers 15 of the DLPFC, but the remaining GABA neurons exhibited normal levels of GAD67 mRNA.16-47 Similarly, expression of the mRNA for the GABA membrane transporter (GAT1), a protein responsible for reuptake of released GABA into nerve terminals, was decreased in a similar minority of GABA neurons.53 These findings suggest that both the synthesis and reuptake of GABA are lower in a subset of DLPFC neurons in schizophrenia.
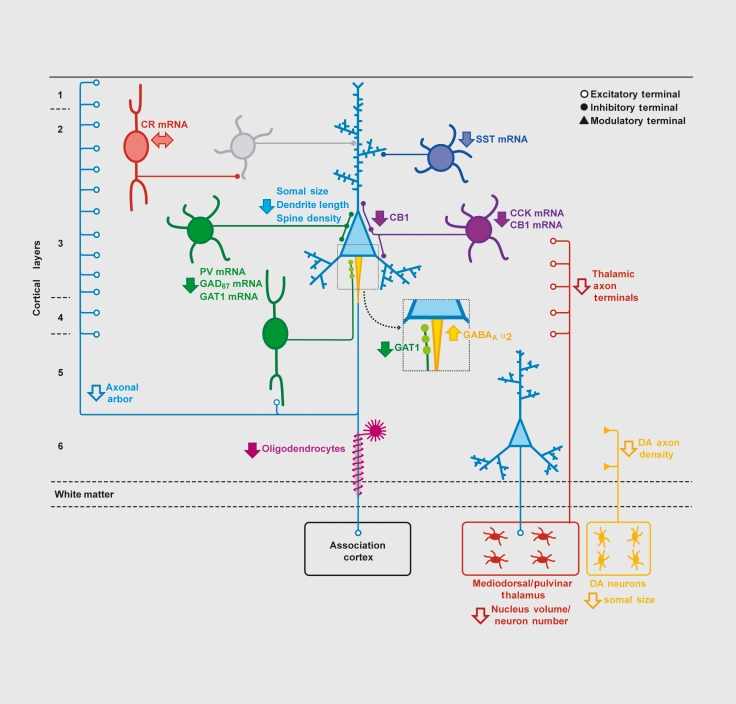
Subclasses of cortical GABA neurons can be distinguished on the basis of a number of molecular, electro-physiological, and anatomical properties. For example, the affected GABA neurons in schizophrenia include the subclass that contain the calcium-binding protein, parvalbumin (PV), which comprise -25% of GABA neurons in the primate DLPFC. PV-containing neurons include fast-spiking chandelier and basket neurons that principally target the axon initial segments and cell body/proximal dendrites, respectively, of pyramidal neurons54,55 (Figure 3). In individuals with schizophrenia the expression level of PV mRNA is reduced, although the number of PV neurons appears to be unchanged56; in addition, approximately half of PV mRNA-containing neurons lack detectable levels of GAD67 mRNA.57 In contrast, the -50% of GABA neurons that express the calcium binding protein calretinin appear to be unaffected.57
Figure 3. Schematic summary of putative alterations in DLPFC circuitry in schizophrenia. Pyramidal neurons (light blue) in deep layer 3 have smaller somal size, shorter basilar dendrites, lower dendritic spine density, and a reduced axonal arbor in schizophrenia. Altered GABA neurotransmission by PV-containing neurons (green) is indicated by expression deficits in several gene products as well as by lower levels of GAT1 protein in the terminals of chandelier neurons and upregulated GABAA receptor α2 subunits at their synaptic targets, the axon initial segments of pyramidal neurons (enlarged square). Expression of the neuropeptide somatostatin (SST) is decreased in GABA neurons (dark blue) that target the distal dendrites of pyramidal neurons. Decreased cholecystokinin (CCK) and cannabinoid receptor 1 (CB1) mRNA levels, and lower CB1 protein in axon terminals, suggest altered regulation of GABA neurotransmission in a subset of basket neurons (purple) that target the cell body and proximal dendrites of pyramidal neurons. Gene expression does not seem to be altered in calretinin (CR)-containing GABA neurons (red) that primarily target other GABA neurons (gray). Putative alterations in thalamic and dopamine (DA) cell bodies and their projections to the DLPFC are also shown. Some studies indicate that the number and/or gene expression in oligodendrocytes is also altered. Not all of the circuitry alterations shown here have been sufficiently replicated or demonstrated to be specific to the disease process of schizophrenia to be considered established “facts;” solid arrows indicate abnormalities supported by convergent and/or replicated observations. Reproduced from ref 2: Lev/is DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009; 119:706-716. Copyright © American Society for Clinical Investigation 2009.
In the DLPFC of subjects with schizophrenia, G ATI immunore activity is selectively reduced in the characteristic axon terminals (cartridges) of PV-containing chandelier neurons.58 In the postsynaptic targets of these axon cartridges, the axon initial segments of pyramidal neurons, immunoreactivity for the GABAA receptor α2 subunit (which is present in most GABAA receptors in this location59) is markedly increased in schizophrenia.60 Several lines of evidence suggest that the reductions in presynaptic GABA markers (GAT1 and PV) and increased postsynaptic GABAA receptors are compensatory responses to a deficit in GABA release from chandelier neurons. For example, PV is a slow calcium buffer that does not affect the amplitude, but accelerates the decay, of Ca2+ transients in GABA nerve terminals.61,62 Thus, PV decreases the residual Ca2+ levels that normally accumulate in nerve terminals and facilitate GABA release during repetitive firing.61 Studies in PV-deficient mice have demonstrated that a decrease in PV increases residual Ca2+ and favors synaptic facilitation.61,63 Furthermore, the enhanced facilitation of GABA release from fast-spiking neurons with reductions in PV is associated with increased power of gamma oscillations63 (which is, as explained below, deficient in schizophrenia). Similarly, the blockade of GABA reuptake via GAT1 prolongs the duration of inhibitory postsynaptic currents (IPSCs) when synapses located close to each other are activated synchronously64; the resulting prolongation of IPSCs increases the probability of IPSC summation, enhances the total efficacy of IPSC trains, and thereby augments GABA signaling. The upregulation of the postsynaptic GABAA receptors that contain α2, subunits would be expected to increase the efficacy of the GABA that is released from chandelier neurons. Thus, the combined reduction of PV and GAT1 proteins in chandelier cell axon cartridges, and the upregulation of postsynaptic GABAA receptors, appear to represent neuroplastic responses that might act synergistically to increase the efficacy of GABA neurotransmission at pyramidal neuron axon initial segments during the types of repetitive neuronal activity associated with working memory.
However, the persistence of cognitive impairments in individuals with schizophrenia suggests that these neuroplastic changes in GABA neurotransmission from chandelier neurons are insufficient as compensatory responses. Alternatively, it is possible that compensation at chandelier cell synapses is not effective because additional interneuron subclasses are also functionally deficient in schizophrenia.65 Consistent with this interpretation, other findings indicate that alterations in PVcontaining GABA neurons cannot account for all of the observed findings in postmortem studies of schizophrenia. For example, the levels of GAD67 and GAT1 mRNAs are reduced to comparable degrees in layers 2-5 ,47,53 even though the density of PV neurons is much greater in layers 3 and 4 than in layers 2 and 5.66 In addition, PV mRNA expression was reduced in layers 3 and 4, but not in layers 2 and 5, in subjects with schizophrenia.57 Indeed, other studies have found lower tissue concentrations of the mRNAs for the neuropeptides somatostatin (SST) and cholecystokinin (CCK) in the DLPFC of subjects with schizophrenia (Figure 3). 51
In the cortex, SST is expressed by GABA neurons located in layers 2 and 5 that do not express PV or CR.67 CCK is also heavily expressed in GABA neurons that do not contain either PV or SST located principally in layers 2-3 of the primate prefrontal cortex.68
Interestingly, the axon terminals of CCK-containing large basket neurons, which target selectively pyramidal neuron cell bodies, contain type I cannabinoid receptors (CB1R),69 and the mRNA and protein levels of CB1R are also lower in schizophrenia.70 Because activation of the CB1R suppresses GABA release from the terminals of CCK neurons, the downregulation of this receptor may represent a compensatory response to reduce the ability of endogenous cannabinoids to decrease GABA release from CCK/CBlR-containing axon terminals.70
Altered GABA neurotransmission in PV-containing neurons impairs prefrontal network synchrony in schizophrenia
Reduced GABA signaling from PV-containing GABA neurons to the perisomatic region of pyramidal neurons in the DLPFC might contribute to the pathophysiology of working memory dysfunction via the following mechanisms. First, the activity of DLPFC GABA neurons is essential for normal working memory function in monkeys.71,72 Second, PV-positive GABA neurons and pyramidal neurons share common sources (eg, thalamic afferents) of excitatory input.55 The resulting feed-forward, disynaptic inhibition creates a time window during which the number of excitatory inputs required to evoke pyramidal neuron firing must occur.73 Third, both chandelier and basket neurons target multiple pyramidal neurons,74 enabling them to use this timing mechanism to synchronize the activity of local populations of pyramidal neurons.75 Fourth, networks of PV-positive GABA neurons, formed by both chemical and electrical synapses, give rise to oscillatory activity in the gamma band range, the synchronized firing of a neuronal population at 30 to 80 Hz.76,77 Interestingly, gamma band oscillations in the human DLPFC increase in proportion to working memory load,78 and in subjects with schizophrenia, prefrontal gamma band oscillations are reduced bilaterally during a working memory task.79 Thus, a deficit in the synchronization of pyramidal cell firing, resulting from impaired regulation of pyramidal cell networks by PV-positive GABA neurons, may contribute to reduced levels of induced gamma band oscillations, and consequently to impairments in cognitive tasks that involve working memory in subjects with schizophrenia.65 Interestingly, CCK/CB1R- and PV-containing cells provide convergent sources of perisomatic inhibition to pyramidal neurons that play specific roles in shaping network activity, including complementary roles in regulating gamma band oscillations.80 Thus, alterations in CCK-containing basket cells could also contribute to impaired gamma oscillations in schizophrenia.
The contribution of developmental plasticity to GABA neuron alterations in schizophrenia
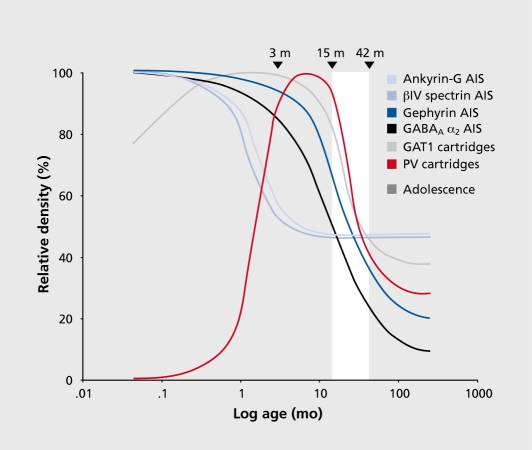
In the monkey prefrontal cortex DLPFC, the density of symmetric, presumably GABA, synapses rises rapidly during the third trimester of gestation and perinatal period until stable, adult levels are achieved at 3 months postnatal.36 In contrast, pre- and postsynaptic markers of the functional properties of chandelier axon inputs to the axon initial segment (AIS) of pyramidal neurons exhibit a very protracted maturation. Presynaptically, immunoreactivities for the calcium-binding protein PV and GAT1 in chandelier axon cartridges are not detectable or low at birth, rise (albeit with different developmental time courses) to peak levels early in postnatal development that are sustained until -15 months of age, and then rapidly decline during adolescence until stable adult levels are achieved.34,81,82 Since chandelier cartridges are readily visualized with Golgi staining across postnatal development,83 these changes in PV and GAT1 immunoreactivity are likely to reflect shifts in the concentration of these proteins rather than changes in the presence of, or in the density of, axon terminals within chandelier axon cartridges.82 Post-synaptically, GABAA receptors containing α2 subunits predominate in pyramidal neuron AIS especially in cortical layers 2-4.84 The density of pyramidal neuron AIS immunoreactive for oc2 subunits is high at birth, then significantly declines during adolescence before achieving stable adult levels:82 These findings indicate that both pre- and postsynaptic markers of GABA neurotransmission undergo significant changes during postnatal development, suggesting that the capacity to synchronize pyramidal neuron output in the prefrontal cortex (PFC) might be in substantial flux until adulthood.
The significance of these changes depends, at least in part, on how GABA synapses are stabilized at the AIS while the functional properties of GABA neurotransmission at this location are changing during postnatal development. For example, pyramidal neuron AIS contain specific proteins that regulate synapse structure and receptor clustering: i) Ankyrin-G, an adaptor molecule that links various membrane proteins to the cytoskeleton, is localized to AIS,85 and interactions between ankyrin-G and the cell adhesion molecule, neurofascin, are required for the formation and stabilization of GABA synapses at AIS86; ii) piV spectrin, which is localized to the AIS of pyramidal neurons through its direct interaction with ankryin-G,87 is a critical component in the organization and stabilization of membrane proteins at the AIS88; iii) The scaffolding protein gephyrin facilitates the preferential accumulation of gephyrin-GABAA receptor clusters, especially those containing α2 subunits.89 In monkey DLPFC, ankyrin-G and βiV spectrin-labeled AIS decline in density during the first 6 months postnatal, but then remain stable, whereas the density of gephyrin-labeled AIS is stable through early postnatal development and then then markedly declines during adolescence.90 Thus, molecular determinants of the structural features that define GABA inputs to pyramidal neuron AIS in monkey PFC undergo distinct developmental trajectories, with different types of changes occurring during the perinatal period and adolescence (Figure 4).
Figure 4. Schematic summary of the trajectories of pyramidal neuron axon initial segments and chandelier neuron axon cartridges labeled with different markers across postnatal development in area 46 of monkey PFC. Lines for each marker represent the percent maximal value achieved plotted against age in months after birth on a log scale. Arrowheads demarcate the indicated ages in months, and the white area indicates the approximate age range corresponding to adolescence in this species. GAT1 indicates GABA membrane transporter 1 ; PV indicates parvalbumin. Reproduced from ref 90: Cruz DA, Lovallo EM, Stockton S, Rasband M, Lewis DA. Postnatal development of synaptic structure proteins in pyramidal neuron axon initial segments in monkey prefrontal cortex. J Comp Neurol. 2009;514:353-367“Copyright©Wiley-Liss 2009.
This complex and protracted postnatal maturation of the inputs from PV-containing GABA neurons in the primate DLPFC provides a number of opportunities for even subtle disturbances to have their effects amplified as they alter the trajectories of the developmental events that follow. For example, the marked developmental changes in the axon terminals of PV-containing chandelier neurons, and their postsynaptic receptors, during the perinatal period and adolescence, raises the possibility that the alterations in schizophrenia of these markers reflect a disturbance in these patterns of development.
These temporal correlations may explain how a range of environmental factors (eg, labor-delivery complications, urban place of rearing, and marijuana use during adolescence) are all associated with increased risk for the appearance of schizophrenia later in life. Although speculative, the current literature raises the possibility that the GABA-related disturbances in schizophrenia represent an arrest of normal developmental trajectories. For example, recent studies indicate that mRNA expression for the al subunit of the GABAA receptor in the DLPFC increases across postnatal development, whereas the expression of the α2 subunit declines.91 Thus, the elevated α2 60 subunit expression60 and the decreased α1 mRNA levels92 in schizophrenia might reflect a developmental dysregulation of GABAA receptor a subunit expression, where the changes in subunit expression with age fail to undergo their full course. Under this scenario, the alterations of DLPFC circuitry in schizophrenia may render it unable to support higher levels of working memory load, rendering the impaired performance in schizophrenia analogous to the immature levels of working memory function seen in children.93,94
Neuroplastic responses as targets for treatment
The findings reviewed above indicate that working memory and related cognitive impairments in schizophrenia are likely the result of a complex set of alterations in prefrontal excitatory and inhibitory circuitry. Some of these alterations appear to be deleterious causes or consequences of disturbances in the functional architecture of the DLPFC and interconnected brain regions, whereas others may be best explained as compensatory responses. In each case, they reflect the morphological and molecular neuroplasticity of DLPFC circuitry in a disease state. Understanding whether the disease-related change in a given molecule is a consequence or compensation in the disease process has important implications both for the nature of activity of the drugs designed against that target and for the potential therapeutic value of the target. For example, is the neuroplastic capacity of cortical circuitry sufficiently limited that pharmacological augmentation of a compensatory response is feasible? The results of a recent proofof -concept clinical trial suggest that this may be the case. For example, the idea that GABAA receptors α2 subunits are upregulated in pyramidal neurons due to a deficit in GABA input from chandelier neurons led to the use of a novel, positive allosteric modulator of this receptor subtype that improved both working memory function and prefrontal gamma band oscillations in a small randomized controlled trial of subjects with schizophrenia.95 Given the marked developmental changes that occur in each of these systems during adolescence, this type of pharmacological intervention may have particular value as a treatment strategy for high-risk adolescents in the prodromal phase of the illness. However, the effectiveness and safety of such interventions requires a fuller understanding of the maturation of these neural circuits, of the functional consequences of these circuitry changes and of the vulnerability of these developmental processes to pharmacological agents.
Acknowledgments
Cited work conducted by the author was supported by NIH grants MH045156, MH051234 and MH043784.
Selected abbreviations and acronyms
- CCK
cholecystokinin
- DLPFC
dorsolateral prefrontal cortex
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GAT
GABA membrane transporter
- NMDA
N-methyl-D-aspartic acid
- PV
parvalbumin
Disclosure/conflict of interest: David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer, and in 2007-2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, and Neurogen.
REFERENCES
- 1.Lewis DA., Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology Reviews. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 2.Lewis DA., Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia?. Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald AW., III, Carter CS., et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 6.Barch DM., Sheline Yl., Csernansky JG., Snyder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T., Bazmi HH., Mimics K., Wu Q., Sampson AR., Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olney JW., Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 10.Krystal JH., Karper LP., Seibyl JP., et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 11.Verma A., Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudkin KN., Kruchinin VK., Chueva IV. Neurophysiological correlates of delayed visual differentiation tasks in monkeys: the effects of the site of intracortical blockade of NMDA receptors. Neurosci Behav Physiol. 2001;31:207–218. doi: 10.1023/a:1005224610354. [DOI] [PubMed] [Google Scholar]
- 13.Konradi C., Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97:153–179. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristiansen LV., Huerta I., Beneyto M., Meador-Woodruff JH. NMDA receptors and schizophrenia. Cuir Opin Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Thune JJ., Uylings HBM., Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res. 2001;35:15–21. doi: 10.1016/s0022-3956(00)00043-1. [DOI] [PubMed] [Google Scholar]
- 16.Akbarian S., Kim JJ., Potkin SG., et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 17.Selemon LD., Rajkowska G., Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 18.Selemon LD., Goldman-Rakic PS. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 19.Glantz LA., Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia: regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- 20.Karson CN., Mrak RE., Schluterman KO., Stumer WQ., Sheng JG., Griffin WST. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophenia: a possible neurochemical basis for “hypofrontality”. Mol Psychiatry. 1999;4:39–45. doi: 10.1038/sj.mp.4000459. [DOI] [PubMed] [Google Scholar]
- 21.Perrone-Bizzozero NI., Sower AC., Bird ED., Benowitz LI., Ivins KJ., Neve RL. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci USA. 1996;93:14182–14187. doi: 10.1073/pnas.93.24.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mimics K., Middleton FA., Marquez A., Lewis DA., Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 23.Zuo Y., Lin A., Chang P., Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Garey LJ., Ong WY., Patel TS., et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glantz LA., Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Jones EG. Laminar distribution of cortical efferent cells. In: Peters A, Jones EG, eds. Cerebral Cortex. Vol 1. New York, NY: Plenum Press; 1984:521–553. [Google Scholar]
- 27.Kolluri N., Sun Z., Sampson AR., Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 28.Hayes TL., Lewis DA. Magnopyramidal neurons in the anterior motor speech region: Dendritic features and interhemispheric comparisons. Arch Neurol. 1996;53:1277–1283. doi: 10.1001/archneur.1996.00550120089021. [DOI] [PubMed] [Google Scholar]
- 29.Lund JS., Lund RD., Hendrickson AE., Bunt AH., Fuchs AF. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1975;164:287–304. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- 30.Pierri JN., Volk CLE., Auh S., Sampson A., Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 31.Rajkowska G., Selemon LD., Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: A postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 32.Sweet RA., Pierri JN., Auh S., Sampson AR., Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharm. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- 33.Sweet RA., Bergen SE., Sun Z., Sampson AR., Pierri JN., Lewis DA. Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Anderson SA., Classey JD., Condé F., Lund JS., Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 35.Arellano Jl., Espinosa A., Fairen A., Yuste R., DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Bourgeois J-P., Goldman-Rakic PS., Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 37.Huttenlocher PR., Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Giedd JN., Blumenthal J., Jeffries NO., et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 39.Gogtay N., Giedd JN., Lusk L., et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirnics K., Middleton FA., Lewis DA., Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 41.Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence?. J Psychiatry Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 42.Keshavan MS., Anderson S., Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatry Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 43.Katz LC., Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 44.Le Be JV., Markram H. Spontaneous and evoked synaptic rewiring in the neonatal neocortex. Proc. Natl Acad Sci USA. 2006;103:13214–13219. doi: 10.1073/pnas.0604691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez-Burgos G., Kroener S., Zaitsev AV., et al. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- 46.Woo T-U., Pucak ML., Kye CH., Matus CV., Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 47.Volk DW., Austin MC., Pierri JN., Sampson AR., Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 48.Guidotti A., Auta J., Davis JM., et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 49.Vawter MP., Crook JM., Hyde TM., et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto T., Bergen SE., Nguyen QL., et al. Relationship of brainderived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashimoto T., Arion D., Unger T., et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straub RE., Lipska BK., Egan MF., et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 53.Volk DW., Austin MC., Pierri JN., Sampson AR., Lewis DA. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 54.Williams SM., Goldman-Rakic PS., Leranth C. The synaptology of parvalbumin-immunoreactive neurons in primate prefrontal cortex. J Comp Neurol. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- 55.Melchitzky DS., Sesack SR., Lewis DA. Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: Laminar, regional and target specificity of Type I and Type II synapses. J Comp Neurol. 1999;408:11–22. [PubMed] [Google Scholar]
- 56.Woo T-U., Miller JL., Lewis DA. Schizophrenia and the pan/album incontaining class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto T., Volk DW., Eggan SM., et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo T-U., Whitehead RE., Melchitzky DS., Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nusser Z., Sieghart W., Benke D., Fritschy J-M., Somogyi P. Differential synaptic localization of two major y-aminobutyric acid type A receptor a subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Volk DW., Pierri JN., Fritschy J-M., Auh S., Sampson AR., Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 61.Collin T., Chat M., Lucas MG., et al. Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci. 2005;25:96–107. doi: 10.1523/JNEUROSCI.3748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller M., Felmy F., Schwaller B., Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci. 2007;27:2261–2271. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vreugdenhil M., Jefferys JG., Celio MR., Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- 64.Overstreet LS., Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis DA., Hashimoto T., Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 66.Condé F., Lund JS., Jacobowitz DM., Baimbridge KG., Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k, or parvalbumin in monkey prefrontal cortex: Distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 67.Morris HM., Hashimoto T., Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Coitex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oeth KM., Lewis DA. Cholecystokinin innervation of monkey prefrontal cortex: An immunohistochemical study. J Comp Neurol. 1990;301:123–137. doi: 10.1002/cne.903010112. [DOI] [PubMed] [Google Scholar]
- 69.Eggan SM., Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- 70.Eggan SM., Hashimoto T., Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rao SG., Williams GV., Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sawaguchi T., Matsumura M., Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- 73.Pouille F., Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 74.Peters A., Proskauer CC., Ribak CE. Chandelier neurons in rat visual cortex. J Comp Neurol. 1982;206:397–416. doi: 10.1002/cne.902060408. [DOI] [PubMed] [Google Scholar]
- 75.Klausberger T., Magill PJ., Marton LF., et al. Brain-state- and cell-type specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 76.Whittington MA., Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 77.Cardin JA., Carlen M., Meletis K., et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Howard MW., Rizzuto DS., Caplan JB., et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 79.Cho RY., Konecky RO., Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hajos N., Katona I., Naiem SS., et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 81.Condé F., Lund JS., Lewis DA. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbuminimmunoreactive neurons. Dev Brain Res. 1996;96:261–276. doi: 10.1016/0165-3806(96)00126-5. [DOI] [PubMed] [Google Scholar]
- 82.Cruz DA., Eggan SM., Lewis DA. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell inputs to pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- 83.Lund JS., Lewis DA. Local circuit neurons of developing and mature macaque prefrontal cortex: Golgi and immunocytochemical characteristics. J Comp Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- 84.Loup F., Weinmann O., Yonekawa Y., Aguzzi A., Wieser H-G., Fritschy JM. A highly sensitive immunofluorescence procedure for analyzing the subcellular distribution of GABAA receptor subunits in the human brain. J Histochern Cytochem. 1998;46:1129–1139. doi: 10.1177/002215549804601005. [DOI] [PubMed] [Google Scholar]
- 85.Kordeli E., Lambert S., Bennett V. Ankyrin G. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chern. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 86.Ango F., Di Cristo G., Higashiyama H., Bennett V., Wu P., Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Yang Y., Ogawa Y., Hedstrom KL., Rasband MN. beta IV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176:509–519. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y., Lacas-Gervais S., Merest DK., Solimena M., Rasband MN. BetalV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J Neurosci. 2004;24:7230–7240. doi: 10.1523/JNEUROSCI.2125-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fritschy JM., Harvey RJ., Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Cruz DA., Lovallo EM., Stockton S., Rasband M., Lewis DA. Postnatal development of synaptic structure proteins in pyramidal neuron axon initial segments in monkey prefrontal cortex. J Comp Neurol. 2009;514:353–367. doi: 10.1002/cne.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hashimoto T., Nguyen QL., Rotaru D., et al. Protracted developmental trajectories of GABAA receptor alpha 1 and alpha 2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hashimoto T., Arion D., Unger T., et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crone EA., Wendelken C., Donohue S., van Leijenhorst L., Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luna B., Garver KE., Urban TA., Lazar NA., Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Devel. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 95.Lewis DA., Cho RY., Carter CS., et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]