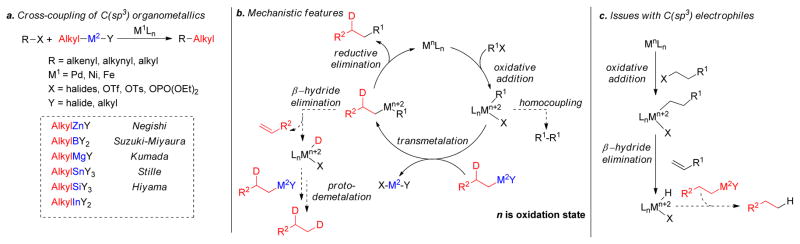
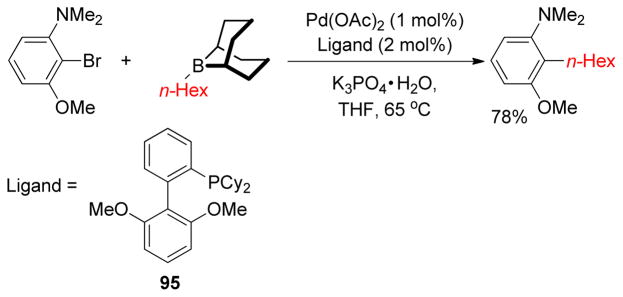
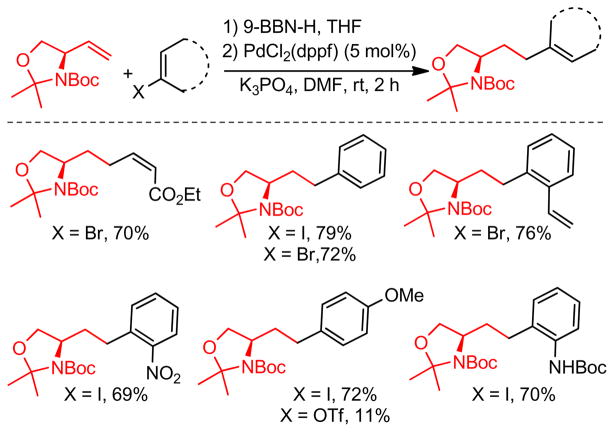
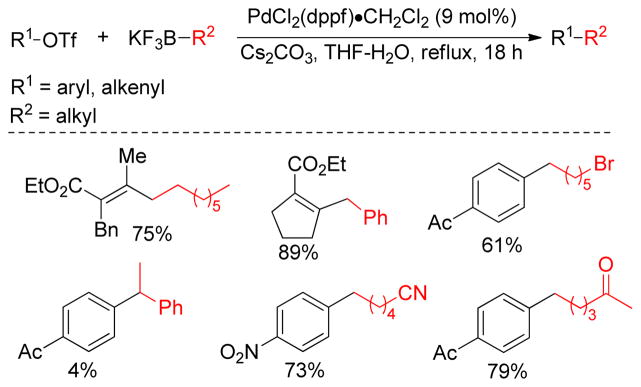
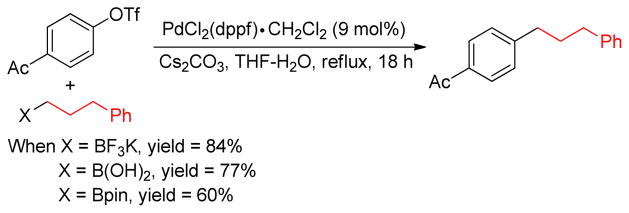
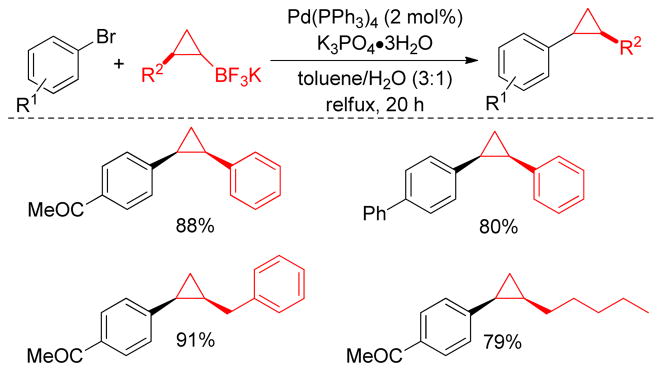
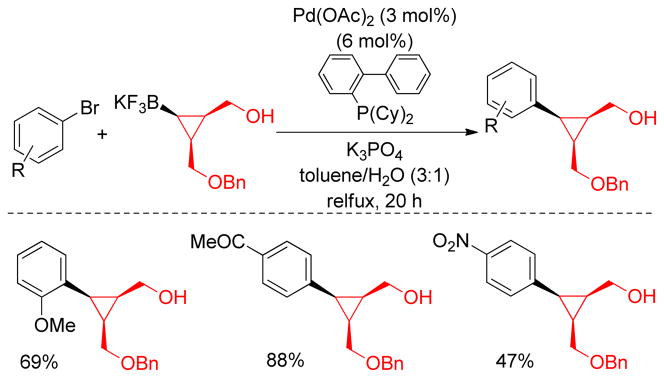
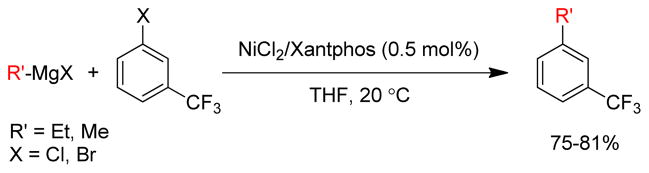
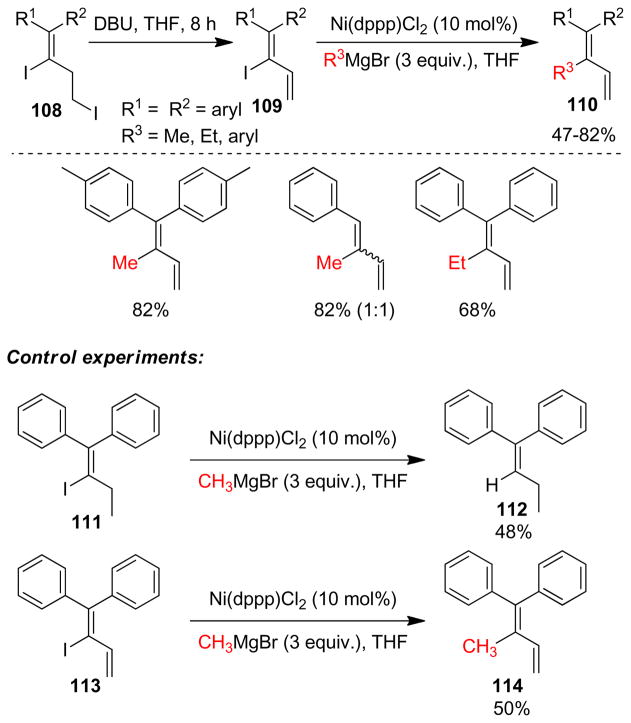
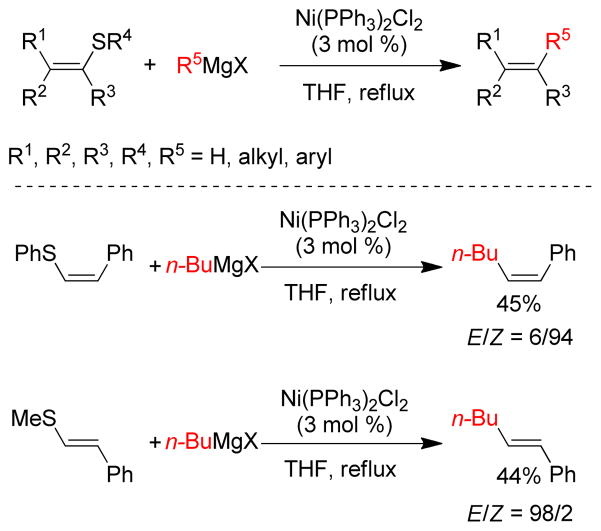
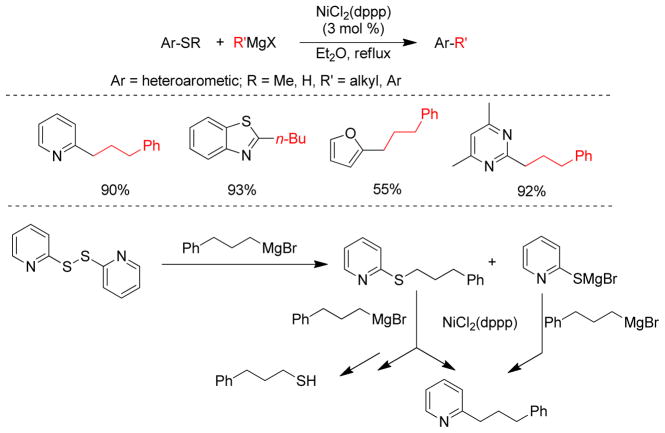
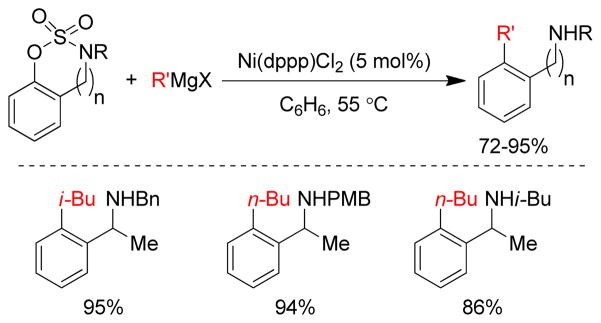
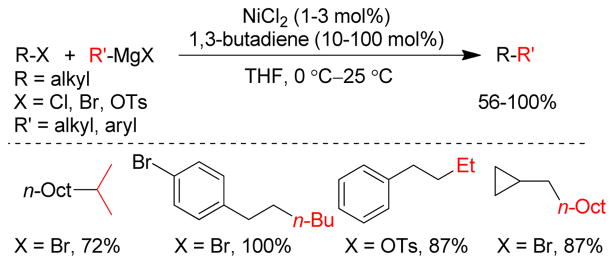
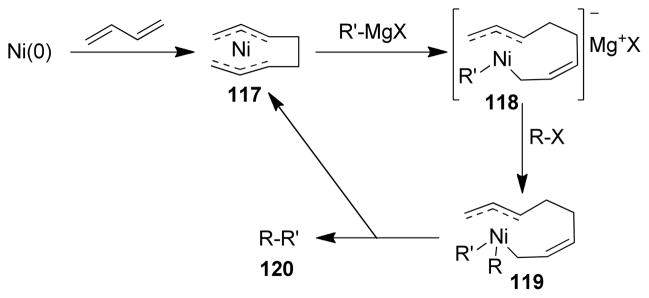
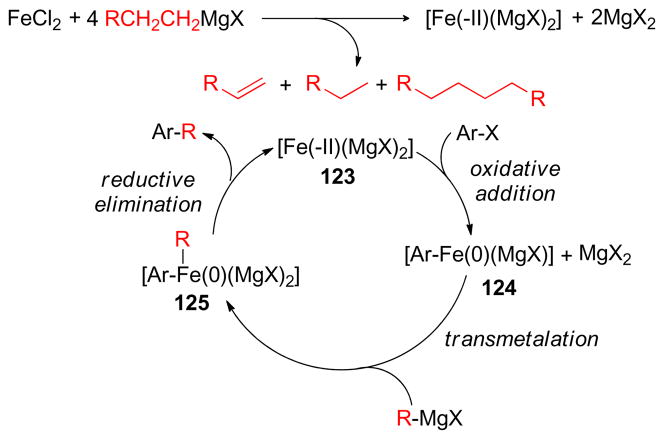
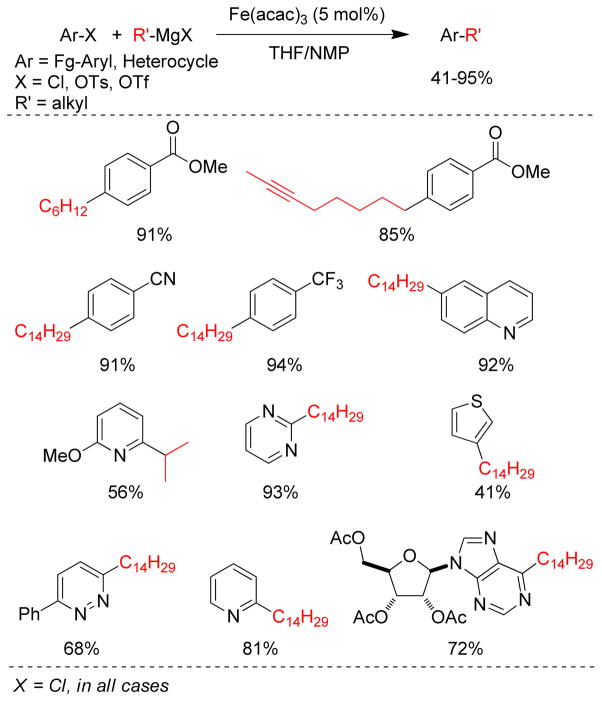
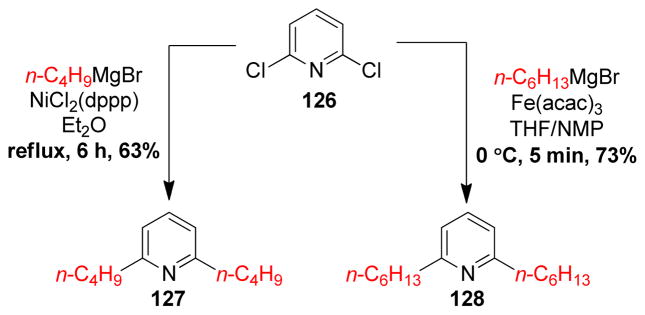
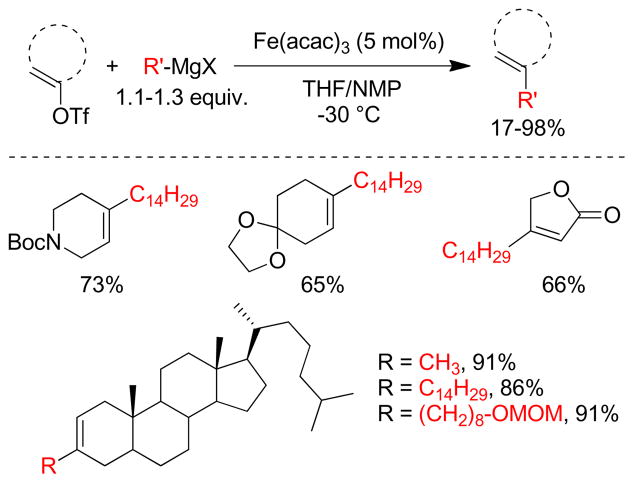
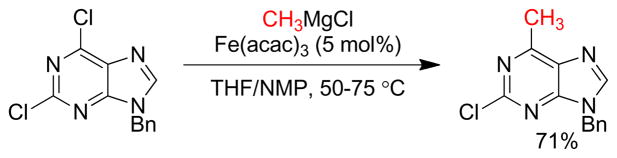
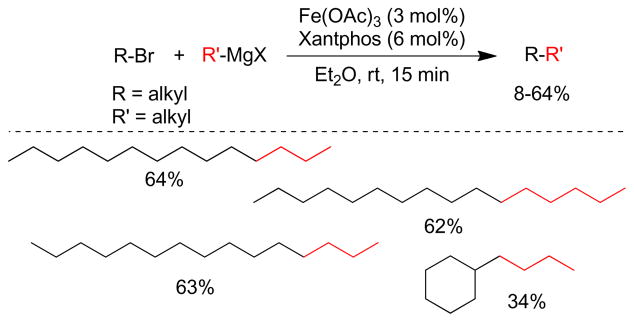
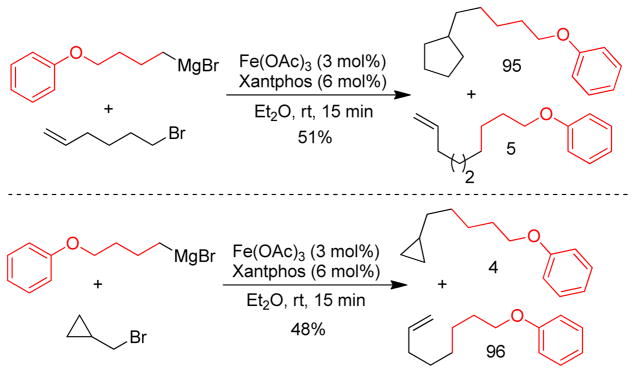
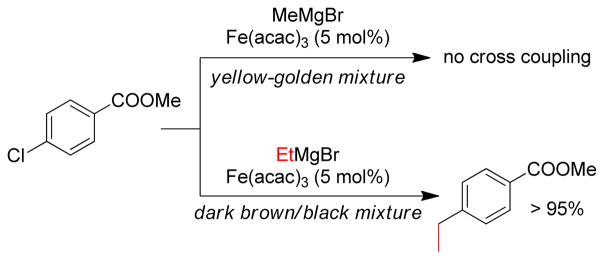
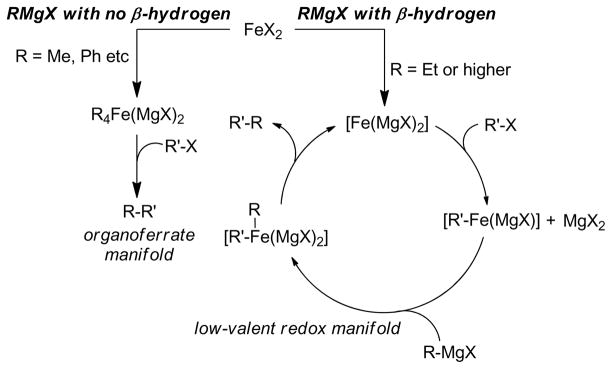
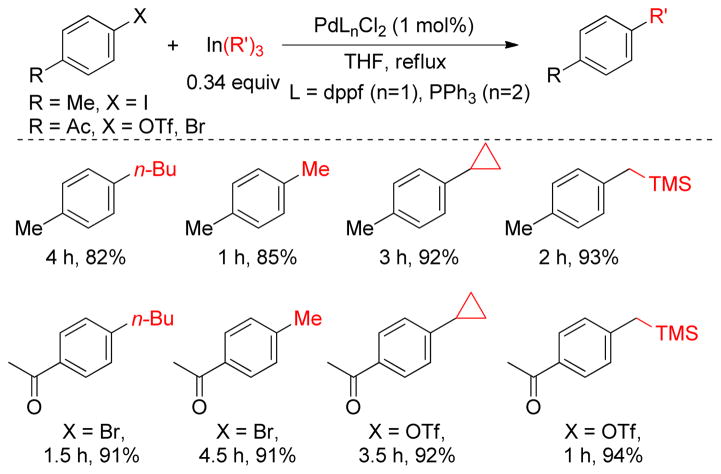
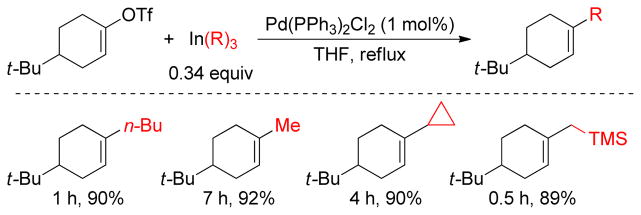
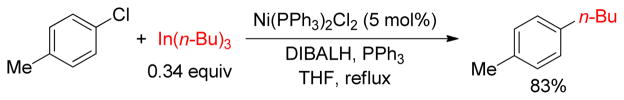
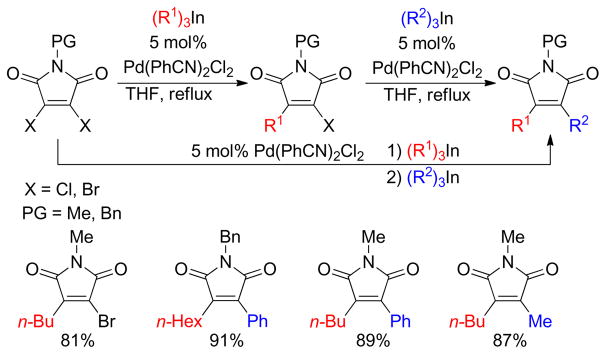
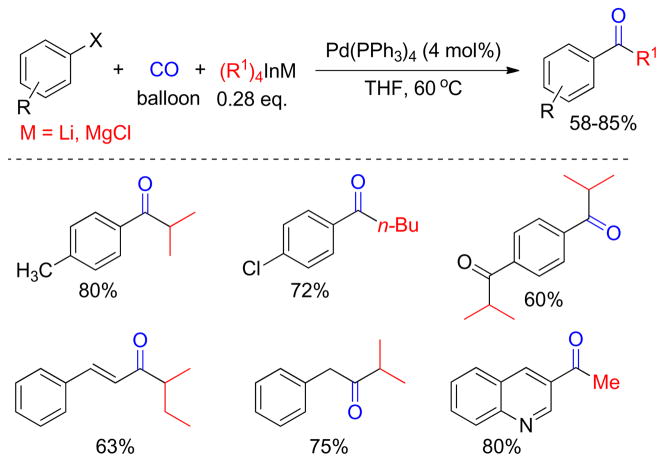
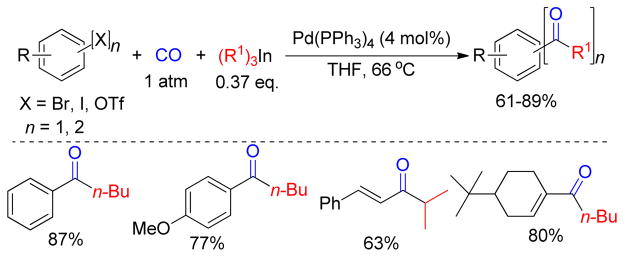
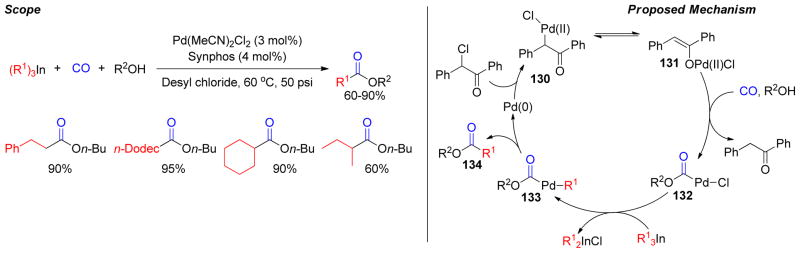
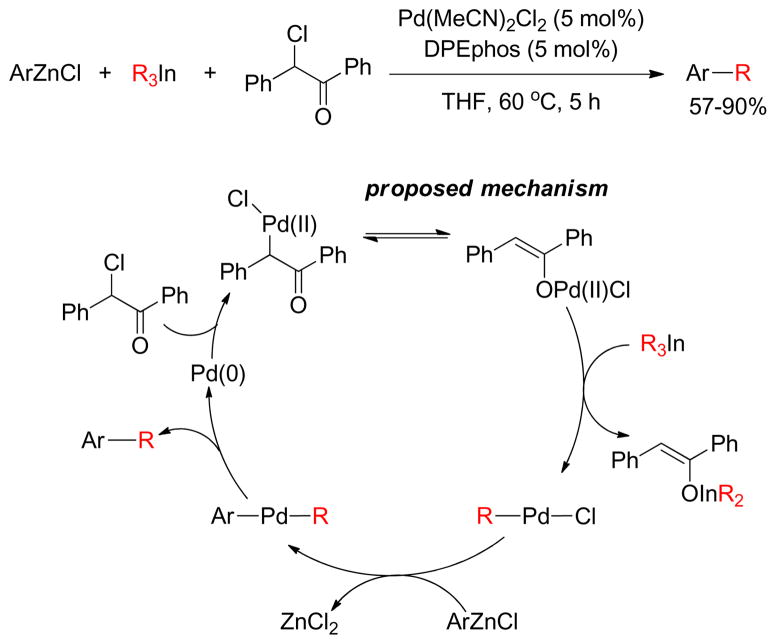
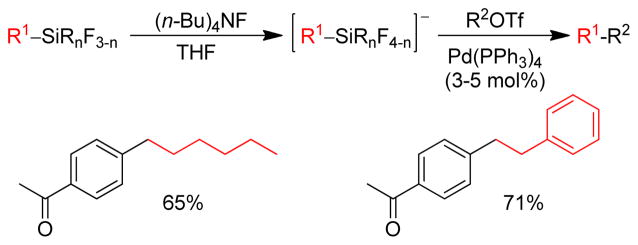
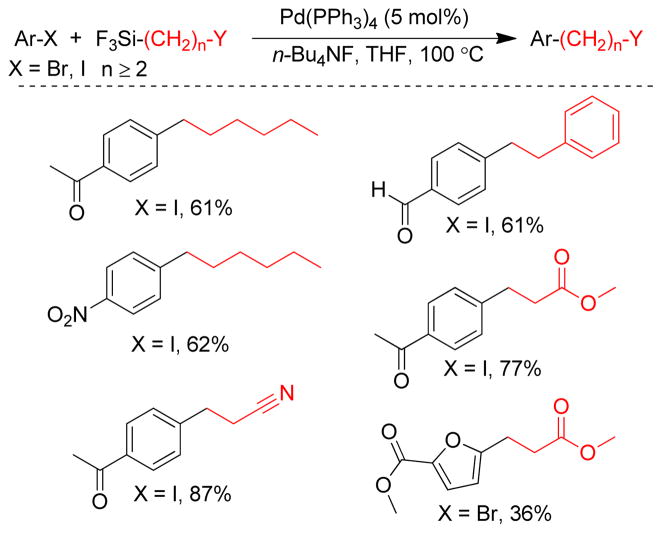
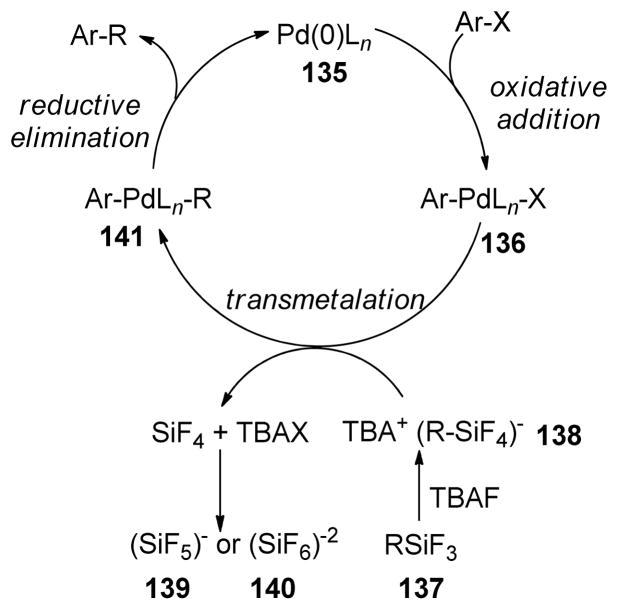
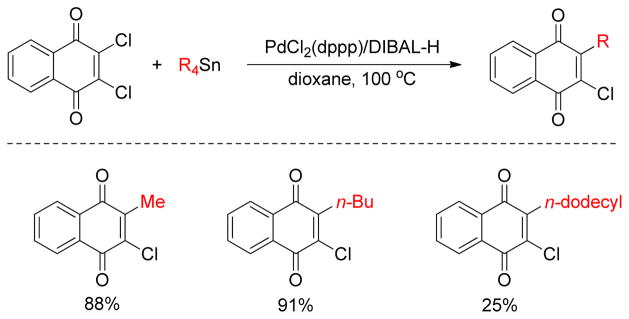
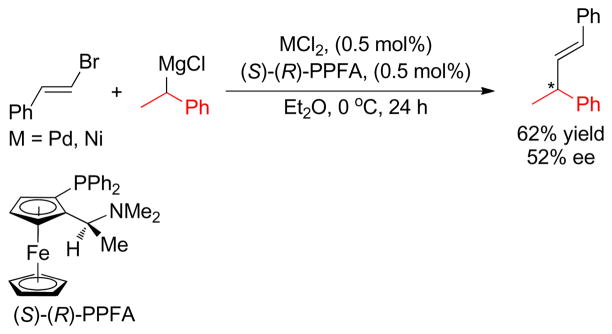
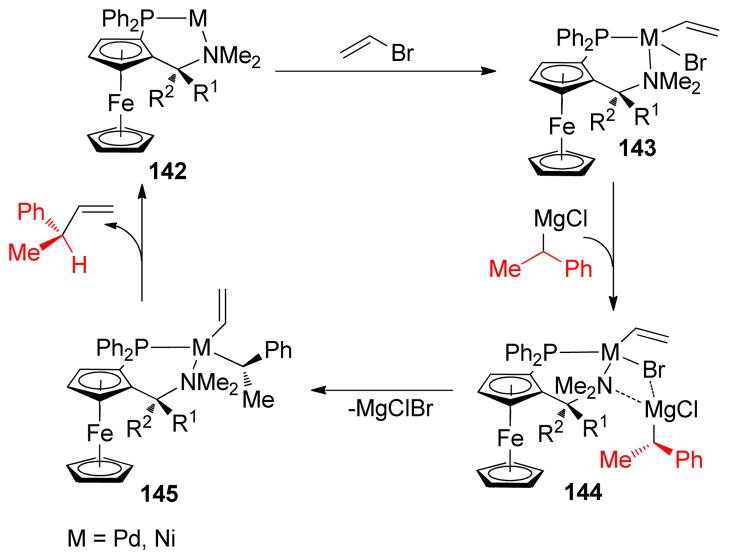
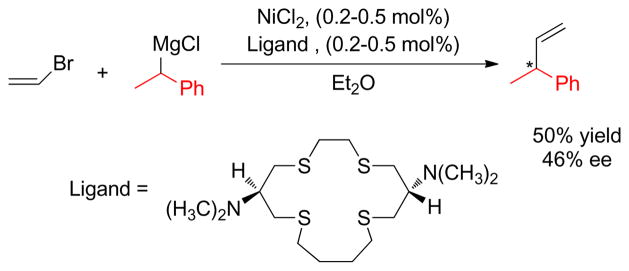
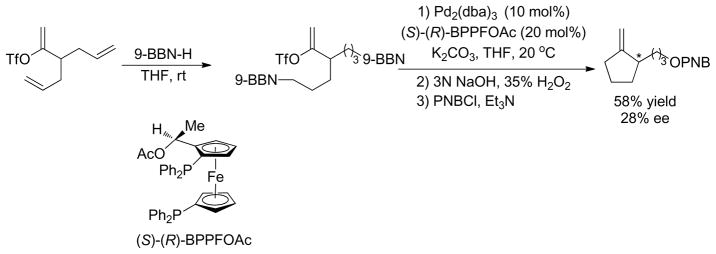
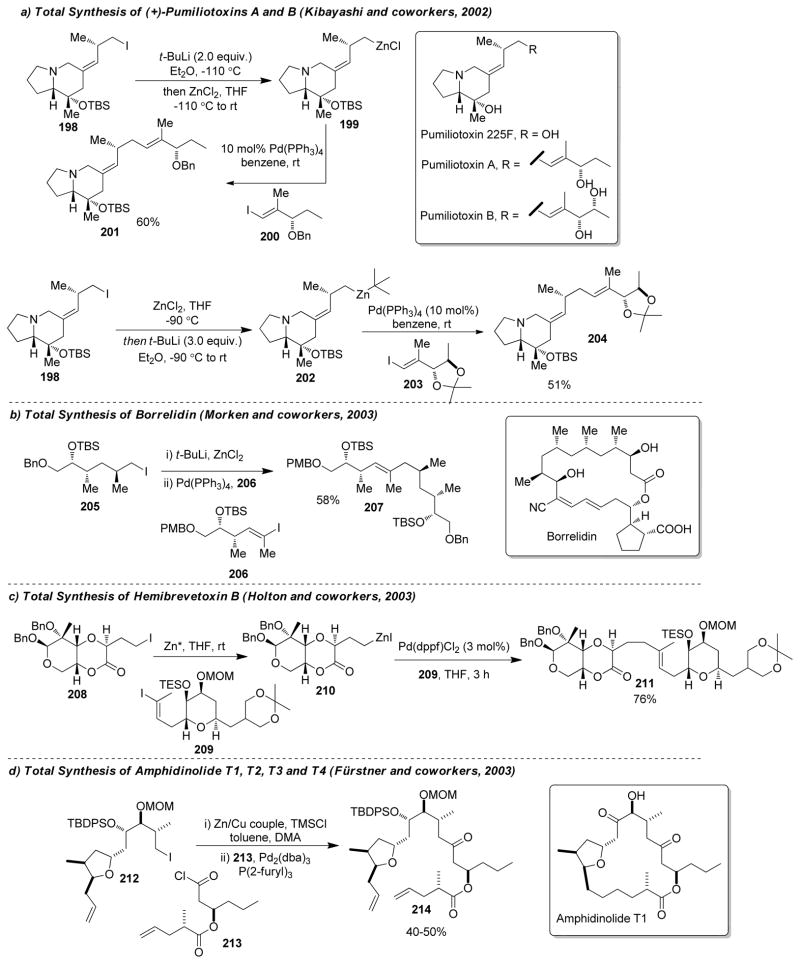
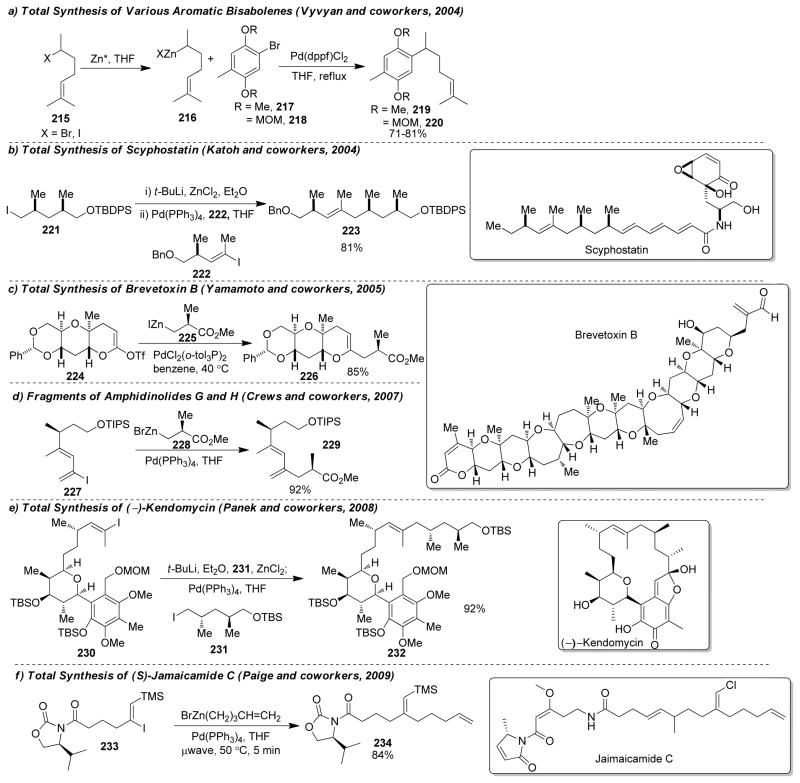
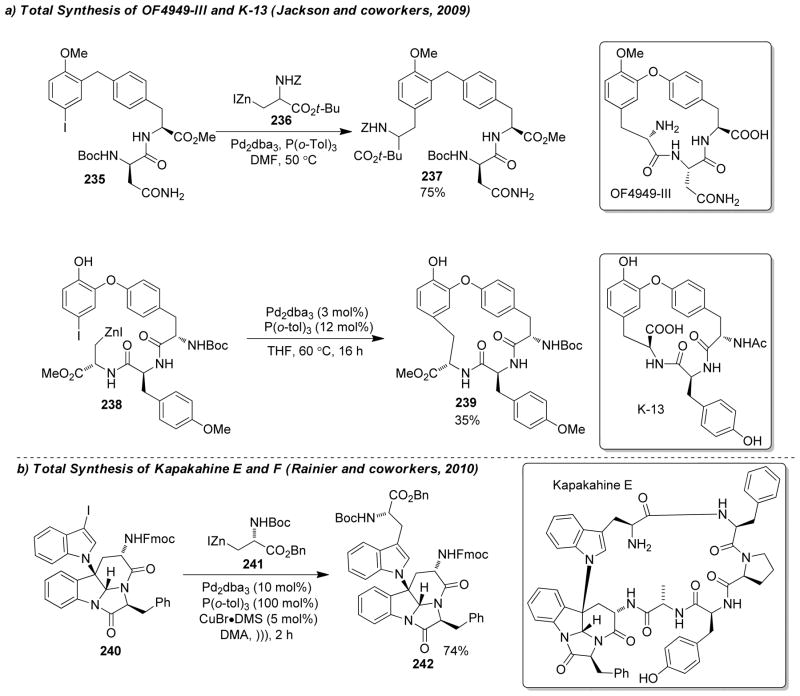
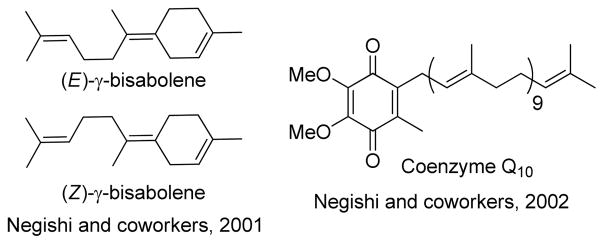
1. Introduction
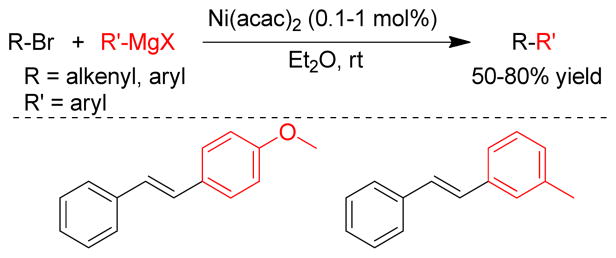
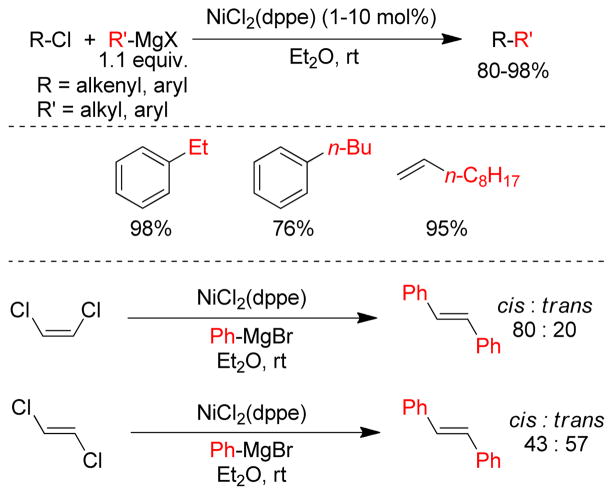
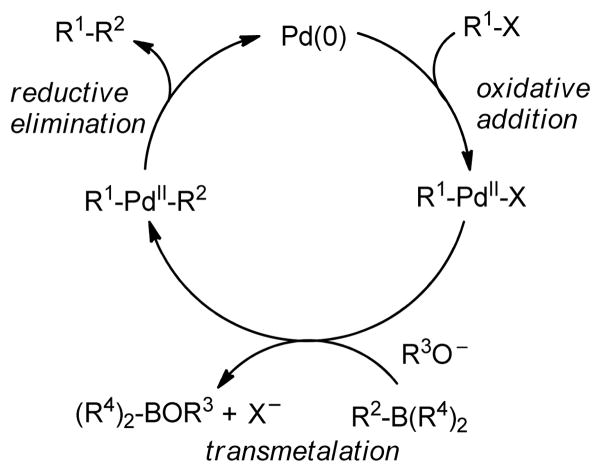
Transition metal-catalyzed cross-coupling reactions of organic electrophiles and organometallic reagents have emerged as a tremendously powerful synthetic tool and the development has reached a level of sophistication that allows for wide range of coupling partners to be combined efficiently.1–3 In the last three decades, this paradigm for carbon-carbon bond construction has allowed chemists to assemble complex molecular frameworks of diversified interests encompassing total synthesis of natural products, medicinal chemistry, and industrial process development as well as chemical biology, materials, and nanotechnology. The emergence of cross-coupling as a popular method in synthesis arises from both the diversity of organometallic reagents utilized in these reactions and the broad range of functional groups which can be incorporated into these reagents.4–11 Since intial submission of this review, the importance of this general class of reactions was recognized by the awarding of the Nobel prize in chemistry to Richard Heck, Ei-ichi Negishi and Akira Suzuki “for palladium-catalyzed cross-couplings in organic synthesis”. This review details transition metal-catalyzed cross-coupling of C(sp3) organometallics with various organic electrophiles (Figure 1a).
Figure 1.
Cross-Coupling of C(sp3)-Organometallics
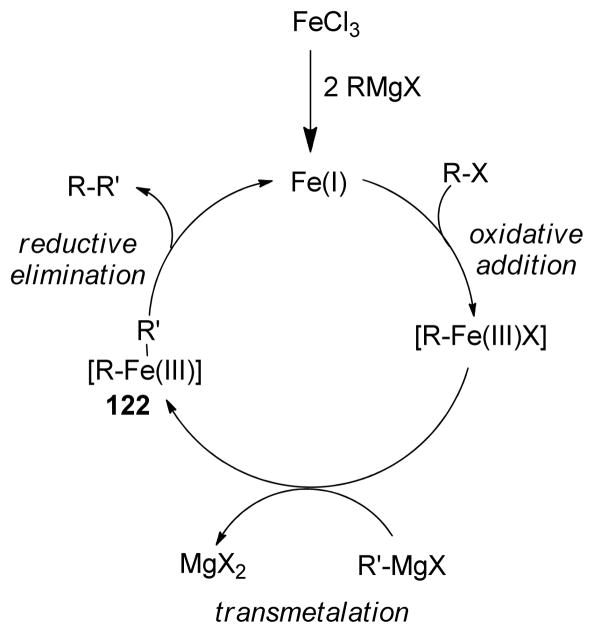
Historically, the use of C(sp3)-organometallics in cross-coupling reactions has suffered from several serious problems, making its development much slower than related C(sp2)-couplings. The issues include but are not limited to: (1) the spontaneous decomposition of alkyl organometallics via β-elimination or by proto-demetalation,12 (2) the necessity to pre-form the organometallic reagents without purification as they are not air stable, making the use of superstoichiometric (3–4 equiv.) amounts necessary to achieve satisfactory conversions, and (3) often slow transmetalation (vide infra), thereby requiring various additives.13 Additionally, evaluation of the general proposed mechanism reveals several features which can lead to undesired side products, especially when considering the emerging area of C(sp3)-C(sp3) cross-couplings between alkyl halides or pseudohalides and alkyl organometallics (Figure 1b,1c).14–18 In general, metal-catalyzed cross-coupling reactions proceed through three critical organometallic processes: (1) oxidative addition of a electrophilic carbonheteroatom bond into the low valent transition metal, (2) transmetalation or displacement of a heteroatom leaving group by the nucleophilic partner and finally, (3) reductive elimination to form a new C-C bond. The use of C(sp3)-electrophiles generally results in slower oxidative addition, which is proposed to be either a nucleophilic substitution (SN2) with Pd-complex19 or radical in nature with Ni20,21 or Fe22,23. Comparatively, oxidative addition of sp or sp2 analogues is faster and generally proceeds in a concerted manner. As illustrated in Figure 1b-c, slow oxidative addition with alkyl electrophiles leads to the formation of homo-coupling products, or β-hydride elimination generating various possible side products. Finally, sluggish reductive elimination also can lead to competing β-hydride elimination.
These issues have made it necessary to identify sometimes complex combination of ligands, metals, and conditions to effectively promote cross-coupling reactions of C(sp3)-organometallics especially with C(sp3)-electrophiles. The C(sp)-C(sp3) coupling between alkynyl electrophile and alkyl organometallic nucleophiles is rarely seen in the literature,24,25 whereas alkynyl organometallics in these cross-coupling reactions are very common. In this review, the synthesis, stability, and transition metal-catalyzed (Pd, Ni, Fe) cross-coupling reactions of sp3-organometallics possessing β-hydrogen(s) using alkylzinc (Negishi-protocol), alkylboron (Suzuki-Miyaura-protocol), alkylmagnesium (Kumadaprotocol), alkyltin (Stille-protocol), alkylsilicon (Hiyama protocol) and alkylindium will be discussed. Besides their detailed development and mechanistic investigations, extension to asymmetric catalysis and applications in total synthesis will be described. It should be noted that organometallic reagents that cannot undergo β-hydride elimination will not be reviewed comprehensively. Co-catalyzed cross-coupling reactions will not be covered in this article as they have been reviewed recently.26 Cu-catalyzed coupling reactions will also not be covered in this review as this could be a subject of a distinct review.
1.1 List of Common Ligands
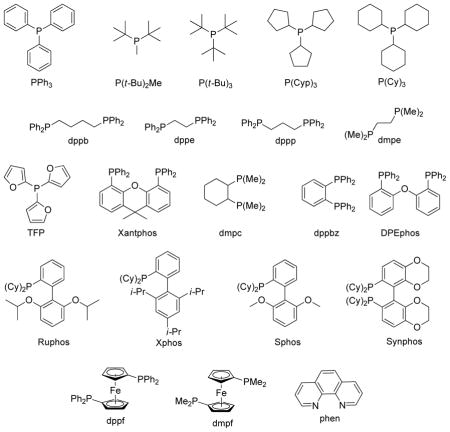
2. Alkylzinc Reagents
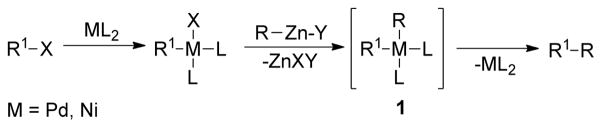
Organozinc reagents of type R2Zn or RZnX are some of the most widely used organometallic reagents in cross-coupling reactions.27 The preparation and systematic studies of their reactivity in addition reactions to general electrophiles such as acid chlorides, aldehydes, ketones, and esters was reported initially in the 1880’s.28 During this time, it was realized that organozinc reagents in general possess low reactivity in addition reactions because of the highly covalent character of the carbon-zinc bond, and zinc has a mild Lewis acidic character to activate electrophiles.29 This low reactivity presents considerable advantages in the preparation of functionalized organozinc reagents to perform chemoselective reactions for the direct introduction of desired functionality into organic scaffolds. Additionally, the empty low-lying p orbitals of zinc make these reagents susceptible to transmetalation reactions with various transition metals. These combined characteristics have allowed organozinc reagents to be a reaction partner for a vast number of cross-coupling reactions, as highlighted by the Pd-catalyzed cross-coupling reactions pioneered by Negishi.30 After smooth transmetalation of a Ni or a Pd salt with organozinc reagents, a reactive intermediate 1 is formed, which readily undergoes reductive elimination to provide cross-coupling products (Scheme 1).31–42 This section will be devoted to the synthesis and reactivity of C(sp3)-alkylzinc reagents in cross-coupling reactions.
Scheme 1.
Cross-Couplings with Alkylzinc Reagents
2.1. Synthesis of Alkylzinc Reagents
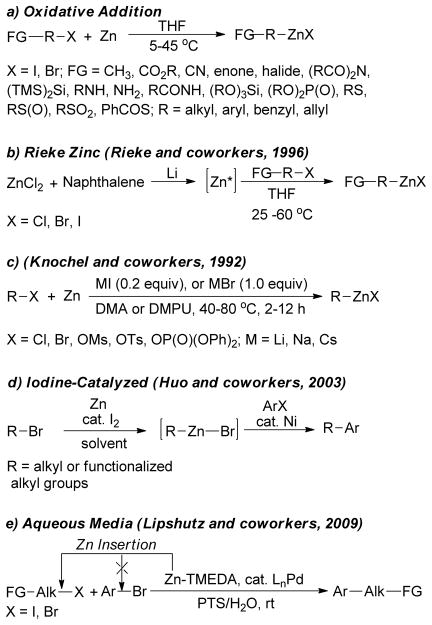
The zinc insertion into alkyl halides represents the most convenient and widely used method for the preparation of alkylzinc reagents.43 In 1849, Frankland discovered that the heating of ethyl iodide with zinc produces highly pyrophoric diethylzinc that could be safely stored under a hydrogen atmosphere.44,45 During preparation of zinc reagents, a faster rate of insertion was observed by activating zinc successively with 1,2-dibromoethane (4–5 mol%)46,47 and/or chlorotrimethylsilane (3 mol%)46,47,48–50 prior to addition of the alkyl halide in THF. The Normant group reported the preparation of alkylzinc using Et2O as solvent.51 Alternative methods of metal activation include washing with aqueous HCl,52 using Zn-Cu53–56 Zn-Cu-Ag couples,57,58 or Zn-Hg alloys,59 ultrasound irradiation,60 metal-solvent co-condensation,61,62 sacrificial Zn-anodes,63 pulsed sonoelectro-chemical reduction of zinc salts,64 and reduction of zinc salts on titanium dioxide.65 A wide range of organic functionalities, such as esters,46,66–86 ketones,46,66 cyanides,46,66–73,78,80–84,86–89 halides,46,66,73,79,82,86N,Nbis( trimethylsilyl)amino groups,90 primary and secondary amino groups,91 amides and phthalimides,91,92 sulfoxides,93 sulfides,93 sulfones,93,94 thioesters,94 boronic esters,76,79,82 enones95,96 and phosphates70,97 are compatible under this mild zinc insertion protocol (Figure 2a). In contrast, carboxylic acid, hydroxyl, nitro and azide groups present in the alkyl moiety potentially prevent zinc insertion. It is feasible to generate Rieke zinc metal in situ by reduction of zinc chloride with lithium naphthalenide in THF (Figure 2b).98–104 Using catalytic amount of alkali metal iodides or stoichiometric amount of alkali metal bromides, zinc insertion into alkyl chlorides, bromides, sulfonates, phosphates etc… was achieved in polar solvents (Figure 2c).105 Huo reported an efficient and general procedure for the preparation of alkylzinc reagents and Ni-catalyzed coupling with aryl halides (Figure 2d).106 An interesting example of the preparation of alkylzinc reagents directly from zinc metal and primary alkyl halides in water at room temperature was reported using diamines as promoter, followed by Pd-catalyzed cross-couplings with aryl bromides (Figure 2e).112,113,107 Transition metal salts also catalyze the zinc insertion process.108–110 Boron-zinc exchange to afford functionalized dialkylzinc reagents is another attractive methodology.111,112
Figure 2.
Standard Methods of Preparation for Alkylzincs Reagents
2.2. Stability of Alkylzinc Reagents
The stability of the preformed organometallics is an important factor for the optimization of subsequent reaction conditions and yield. It is a general observation that most cross-coupling reactions require an excess (3–4 equiv) of alkylzinc reagents to achieve satisfactory yields. At the same time, alkylzinc reagents have usually been prepared in solvents of moderate Lewis basicity such as THF113 or DMA,114 and the benefits of using polar aprotic solvents as a minor component of the solvent mixture such as DMSO/THF, NMP/THF have been highlighted.39 Another crucial parameter for stability is the structure of the alkylzinc reagents. Functionalized alkylzinc reagents containing one or multiple functionalities have differing degrees of decomposition tendency, depending on the nature and position of the functional group. Generally, they undergo decomposition via β-hydride elimination to form olefins, or via protonation to form alkanes.
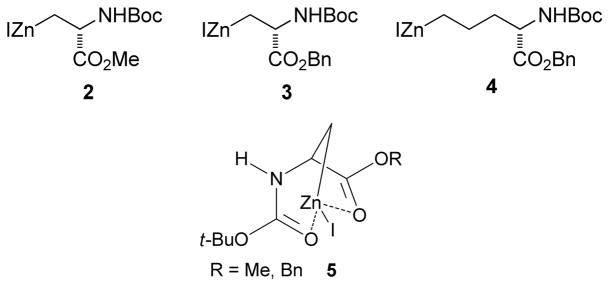
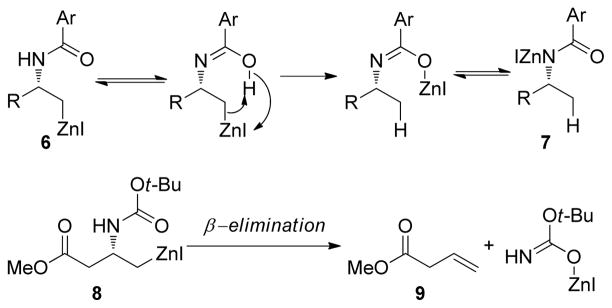
Amino acid-derived alkylzinc reagents are an important class of reaction partners in Negishi coupling reactions as they provide concise routes to the synthesis of numerous biologically active molecules.115 The Jackson group has studied the structure stability relationship as well as the solvent dependent stability of these functionalized alkylzinc reagents.12,116 In this study, a series of functionalized alkylzinc reagents derived from α-amino acids were prepared by gentle heating (35 ºC) in THF and the zinc was activated by 1,2-dibromoethane or chlorotrimethylsilane (Figure 3).
Figure 3.
α-Amino Acid-Derived Alkylzinc Reagents (Jackson and coworkers, 1998)
From NMR studies in THF-d8, it was evident that samples undergo decomposition at different rates upon heating at 50 °C to form alkanes and alkenes through protonation and β-elimination, respectively. The zinc reagents 2 and 3 are the most stable (relatively) whereas 4 is susceptible to β-elimination. This high stability of 2 and 3 is best accounted for invoking an internally chelated structure 5 that voids the necessary conformation for elimination (either a syn or anti arrangement of the C-Zn and C-NHBoc bonds) (Figure 3). In contrast, compound 4 contains zinc remote to the α-center, which diminishes the potential for chelating possibilities.
Interestingly, the stability of these reagents is relatively high in DMF as compared to THF. It has been observed that the intramolecular coordination of the carbamate carbonyl group to zinc is responsible for the faster β-elimination since zinc can act as an internal Lewis acid. The intramolecular coordination of the carbamate carbonyl group to zinc appears to be completely suppressed in DMF to improve their stability. Their reactivity in subsequent cross-coupling reactions is also enhanced in DMF as compared to THF.
It has been found that decomposition of β-benzamido alkylzinc iodide 6 occurs by self-protonation of the carbon-zinc bond to form 7 through first-order kinetics.12 In contrast, the carbamate derivative 8 decomposes by a first-order elimination process to form 9 (Figure 4). The homologous reagent also decomposes at a faster rate compared to 8 by β-elimination. In conclusion, the structure of alkylzinc reagents and the solvent used for their preparation have a pronounced effect on their stability and reactivity.
Figure 4.
Decomposition of Amino Acid Derived Alkylzinc Reagents (Jackson and coworkers, 2008)
2.3. Cross-Coupling Reactions of Alkylzinc Reagents
Typically, transition metal-catalyzed cross-coupling reactions proceed through three consecutive steps as outlined in the introduction: (1) oxidative addition, (2) transmetalation and (3) reductive elimination to afford product (Figure 1). The alkylzinc reagents exhibit an excellent ability to undergo transmetalation due to the presence of an empty low-lying p orbital of zinc. Consequently, Pd(II) and Ni(II)-catalyzed cross-coupling reactions of alkylzinc reagents with aryl or alkenyl,117 acyl,113 or alkyl halides118–120 or pseudohalides121 have proved to be powerful tools in vast areas of synthesis.
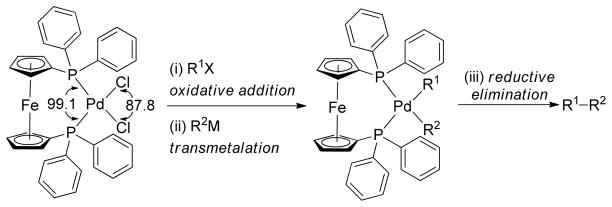
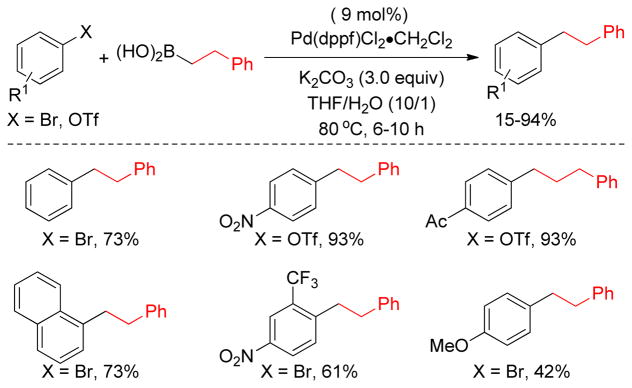
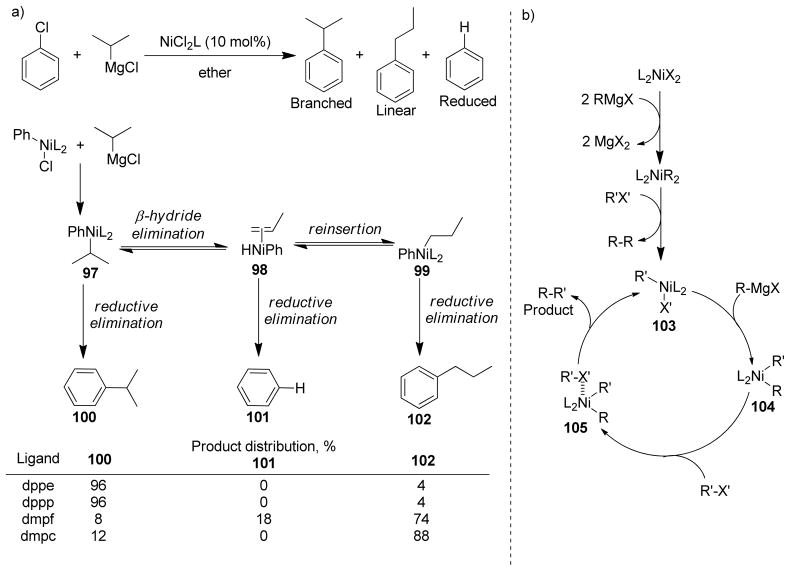
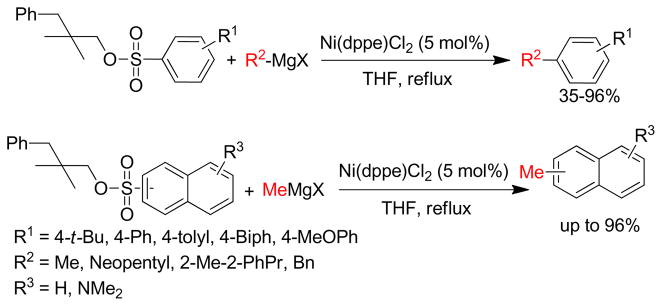
The cross-coupling of secondary and tertiary alkyl reagents has been found to be especially difficult because it is accompanied by the isomerization of the alkyl group and/or reduction of the halide.122 This isomerization and reduction must occur via σ-π interconversions of σ-alkylmetal intermediates through β-hydride elimination to give a hydrido-olefin complex followed by readdition to the olefin.123–125 Therefore, selective cross-couplings are realized by faster reductive elimination than β-hydride elimination. The undesired side reactions could be suppressed to some extent by an appropriate choice of catalyst that could potentially accelerate the reductive elimination pathway, providing a lower energy transition state or elevating the ground state energy.126–130 The isomerization of the coupling partners from a trans arrangement to cis is necessary for reductive elimination to occur.131 Bidentate phosphine ligands with a large bite angle (P-Pd-P) enforce the coupling partners into a cis geometry in a squareplaner Pd(II) complex, thereby increasing the rate of reductive elimination. Thus, PdCl2(dppf), was expected to be an excellent catalyst for cross-coupling as it has a large bite angle (∠P-Pd-P = 99.07°) to facilitate reductive elimination (Scheme 2).132 Indeed, the Hayashi group found that PdCl2(dppf) is a highly active catalyst compare to similar Pd-catalysts with other bidentate phosphine ligands providing excellent product selectivity in the cross-coupling of bromobenzene and primary or secondary butylzinc chloride (Table 1).
Scheme 2.
PdCl2(dppf)-Catalyzed Negishi Coupling (Hayashi and coworkers, 1984)
Table 1.
Cross-Coupling of Organozinc Reagents with Bromobenzene (Hayashi and coworkers, 1984)
 | |||||
|---|---|---|---|---|---|
| Catalyst | Ra | Time (h) | Yield (%)b |
||
| sec-BuPh | n-BuPh | Recovered PhBr | |||
| PdCl2(dppf) | sec-Bu | 20 | 100 | 0 | 0 |
| Pd(PPh3)4 | sec-Bu | 24 | 1 | 2 | 78 |
| PdCl2(PPh3)2 | sec-Bu | 22 | 3 | 3 | 87 |
| PdCl2(dppp) | sec-Bu | 22 | 13 | 3 | 79 |
| NiCl2(PPh3)2 | sec-Bu | 22 | 1 | 4 | 59 |
| NiCl2(dppp) | sec-Bu | 22 | 45 | 7 | 44 |
| PdCl2(dppf) | n-Bu | 22 | 100 | 0 | |
| PdCl2(PPh3)2 | n-Bu | 24 | 34 | 13 | |
| PdCl2(dppp) | n-Bu | 24 | 66 | 0 | |
| PdCl2(dppb) | n-Bu | 21 | 90 | 0 | |
| NiCl2(PPh3)2 | n-Bu | 21 | 42 | 22 | |
| NiCl2(dppp) | n-Bu | 21 | 3 | 78 | |
2.0 equiv zinc reagent was used;
GC yields
2.4. Pd-Catalyzed Cross-Coupling Reactions of Alkylzinc Reagents
In 1977, Negishi reported that organozinc reagents undergo Ni- or Pd-catalyzed cross-coupling reactions, providing a general and mild procedure for the synthesis of biaryls and diarylmethines with high chemo- and regioselectivity as well as high cross- versus homocoupling ratios.30 Although this Negishi protocol has been extensively studied using alkynyl,133 aryl, alkenyl,34,117,134 methyl,135 allyl,136 and benzylzinc30,65,90,137 reagents, the frequent use of more challenging alkylzinc reagents containing β-hydrogen(s) as a cross-coupling partner has only evolved more recently. Our discussion in this section will be limited to the use of alkylzinc reagents as cross-coupling partners in Pd(0) catalysis and are generally presented in chronological order.
2.4.1. Pd-Catalyzed C(sp2)-C(sp3) Negishi Coupling with Alkylzinc Reagents
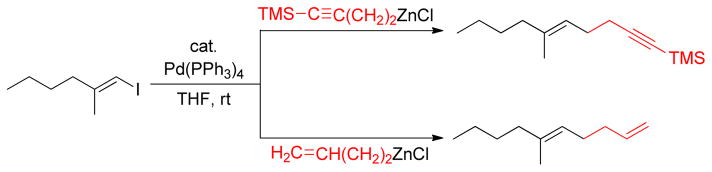
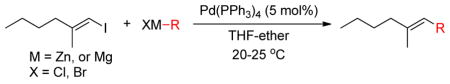
The 1,5-diene moiety can be prepared via allyl-allyl coupling reactions.138–140 Negishi reported a Pd-catalyzed cross-coupling reactions of homoallylic and homopropargylic alkylzinc reagents with alkenyl halides, which provides an effective route for the synthesis of 1,5-dienes and 1,5-enynes (Scheme 3) that constitute the core structure of several terpenoids.141,142 It was also observed that primary and secondary alkylzinc reagents are more selective towards cross-coupling compared to the corresponding alkylmagnesium reagents (Table 2, entries 1 vs. 2 and 3 vs. 4). Homoallylic and homopropargylic alkylzincs are even better coupling partners, providing cross-coupling products in high yields (Table 2, entries 5 & 7).31 The highly regio- and stereoselective product formation through this protocol is noteworthy.143
Scheme 3.
Negishi Coupling with Homoallylic and Homopropargylic Alkylzinc Reagents (Negishi and coworkers, 1980)
Table 2.
Negishi Coupling with Alkylzinc and Alkylmagnesium Reagents (Negishi and coworkers, 1980)
 | ||||
|---|---|---|---|---|
| Entry | Organometallic reagent | Time (h) | Product yield (%) | |
| Cross-coupled | De-iodo | |||
| 1 | n-BuZnCI | 2 | 76 | 2 |
| 2 | n-BuMgBr | 2 | 25 | 51 |
| 3 | sec-BuZnCI | 16 | 68 | 15 |
| 4 | sec-BuMgBr | 16 | 40 | 35 |
| 5 | H2C=CH(CH2)2ZnCI | 16 | 81 | tr |
| 6 | H2C=CH(CH2)2MgBr | 16 | 21 | 37 |
| 7 | TMSC≡C(CH2)2ZnCI | 2 | 91 | tr |
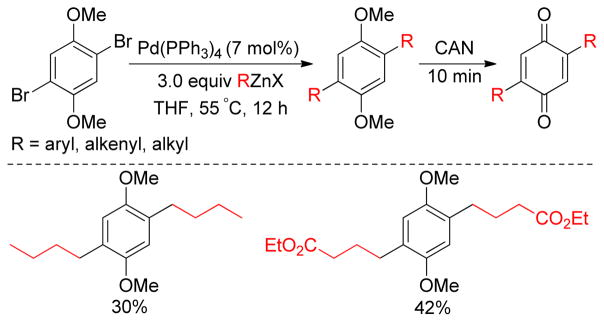
Symmetrical 2,5-disubstituted benzoquinones are synthesized in a convenient route by Pd-catalyzed double Negishi coupling reactions between simple or functionalized alkylzinc and 2,5-dibromo-1,4- dimethoxy benzene, followed by oxidative demethylation with ceric ammonium nitrate (CAN) (Figure 5).144 The precursor 2,5-dibromo-1,4-dimethoxy benzene was easily synthesized by bromination (Br2/AcOH) of 1,4-dimethoxybenzene.
Figure 5.
Synthesis of Symmetrical 2,5-dialkyl Benzoquinones via Negishi Coupling (Bäckvall and coworkers, 1998).
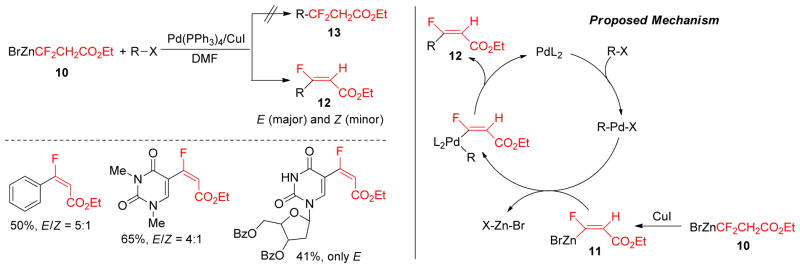
Perfluorinated organometallics are an important class of coupling partners as fluorine can be easily introduced into the organic backbone via cross-coupling reactions.145,146 The Qing group prepared the fluorine containing alkylzinc reagent 10 from the corresponding alkyl bromide by the treatment with zinc dust in anhydrous DMF at ambient temperature.147 It undergoes Pd(0)/Cu(I)-cocatalyzed Negishi cross-coupling with aryl or alkenyl iodides or bromides to produce β-fluoro-α, β-unsaturated ester 12 (Figure 6). Mechanistic studies revealed that the alkylzinc reagents first produce (Z)-1-fluoro-2- (ethoxycarbonyl)-ethenylzinc reagent 11 via Cu(I)-catalyzed stereoselective elimination and this subsequently undergoes the Pd-catalyzed cross-coupling to produce 12 in lieu of direct cross-coupling to produce 13 (Figure 6).
Figure 6.
Negishi Coupling of Ethyl 3-bromo-3,3-difluoropropionate (Qing and coworkers, 2000)
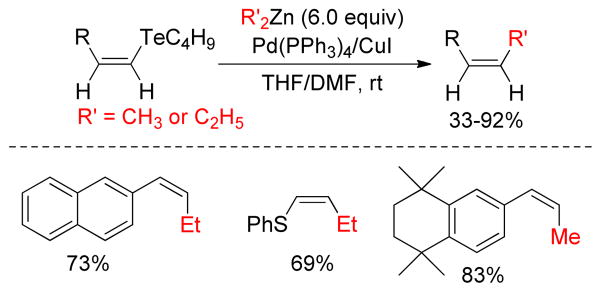
Organotellurium species undergo efficient Te/metal-exchange reactions in a regio- and stereoselective manner.148 This unique property is utilized for the preparation of organozinc reagents by reaction with dimethyl or diethylzinc reagents. Although this Te/Zn-exchange reaction is not quite a general method to obtain vinylzinc reagents, a new carbon-carbon bond is formed when the reaction is carried out employing CuI/Pd(PPh3)4 in DMF (Figure 7).149
Figure 7.
Negishi Coupling of Unsaturated Organotellurium Compounds (Dabdoub, and coworkers, 2000)
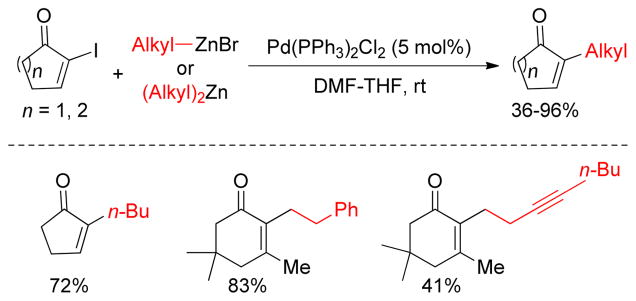
Negishi reported a Pd-catalyzed monosubstitution of cyclic α-iodoenones with organozinc reagents. Several five and six membered α-iodoenones underwent cross-coupling reactions with a number of dialkylzinc or alkylzinc halide reagents in the presence of a catalytic amount of Pd(PPh3)2Cl2 (Figure 8).150
Figure 8.
Negishi Coupling of Cyclic α-Iodoenones (Negishi and coworkers, 2000)
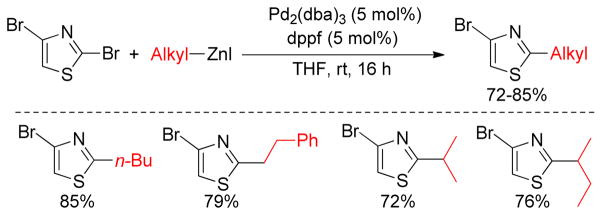
Dibromothiazole compounds undergo Pd-catalyzed Negishi cross-coupling reactions in a regio-selective manner as reported by Bach and coworkers. The bromine atom at C-2 is more susceptible to oxidative addition, allowing for a mild reaction with alkylzincs using Pd2(dba)3/dppf to provide a series of 2-alkyl-substituted thiazole analogues leaving the other C-Br intact for further functionalization (Figure 9).151
Figure 9.
Regioselective Negishi Coupling of 2,4-Dibromothiazoles (Bach and coworkers, 2002)
Pd-catalyzed cross-coupling reactions were applied to the synthesis of azamacrocycles. Suitably functionalized 2-bromopyridines were reacted with alkylzinc reagents in the presence of catalytic Pd(PPh3)2Cl2 in THF to produce 2,6-difunctionalized pyridines which were converted to azamacrocycles after a couple of functional group interconversions followed by intramolecular N-alkylation (Scheme 4).152
Scheme 4.
Synthesis of Azamacrocycles via Negishi Coupling (Skerlj and coworkers, 2002)
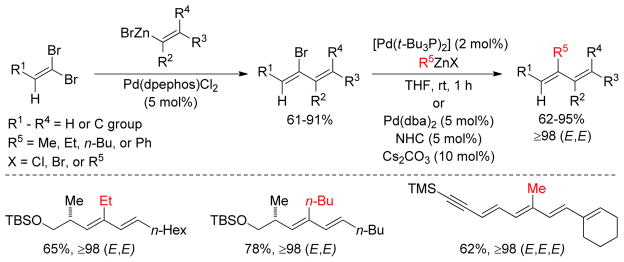
Two Negishi couplings were performed consecutively for the stereoselective synthesis of enynes from the corresponding 1,1-dibromo-1-alkenes or 1,1-dichloro-1-alkenes. A trans-selective alkenylation was accomplished with alkenylzinc chloride or bromide catalyzed by Pd(DPEPhos)Cl2 followed by a Pd(Pt- Bu)2-catalyzed stereospecific methylation or ethylation with dimethylzinc/methylzinc halide or diethylzinc/ethylzinc halide respectively (Figure 10). Alternatively, a [Pd2(dba)3]/NHC ligand combination could be used to alkylate the second halide stereospecifically.153 Following almost the same protocol, (1E)-2-methyl-1,3-ene-ynes were synthesized from the trans-selective Negishi coupling of 1,1-dibromo-1-alkenes with alkynylzinc reagents followed by stereospecific alkylation with alkylzincs.154
Figure 10.
Stereoselective Negishi Couplings (Negishi and coworkers, 2004)
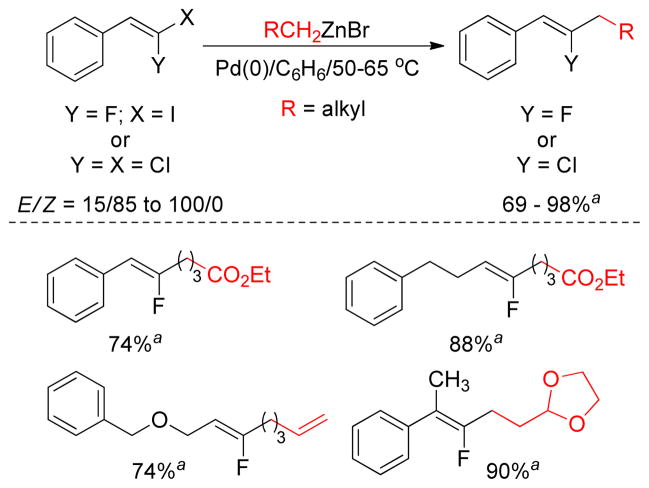
1-Fluoro-1-haloalkenes undergo Pd-catalyzed Negishi cross-couplings with primary alkylzinc bromides to give fluoroalkenes. Excellent trans-stereoselectivity is observed to provide the Z-fluoroalkenes in most cases.155 Interestingly, tertiary alkylzincs also produce the desired fluoroalkenes in high yields (Figure 11). Although PdCl2(dppb) gave the highest yields, the best stereochemical outcome was observed with Pd(PPh3)4 in favor of Z-fluoroalkene.
Figure 11.
Negishi Coupling of Dihaloalkenes (Wnuk and coworkers, 2006)
aisolated yields were based on E-isomer only.
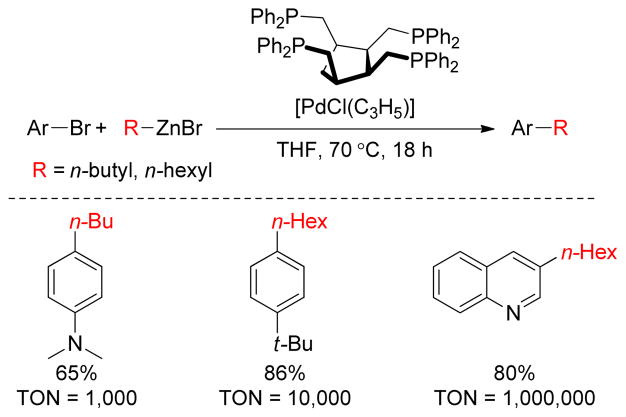
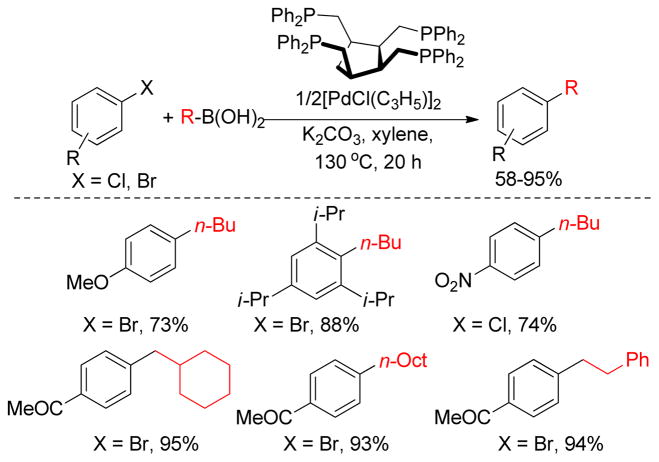
The catalytic system using tetraphosphine cis,cis,cis-1,2,3,4- tetrakis(diphenylphosphinomethyl)cyclopatane (tedicyp) as a ligand and [Pd(C3H5)Cl]2 as the metal precatalyst are active for Negishi cross-coupling reactions between aryl halides and alkylzinc bromides as reported by Doucet and coworkers (Figure 12).156 A wide variety of aryl bromides react with aryl or alkylzinc reagents providing good yields and high turnover numbers (TONs up to 1 × 106).
Figure 12.
High TON in Pd(0)-Catalyzed Negishi Couplings (Doucet and coworkers, 2006)
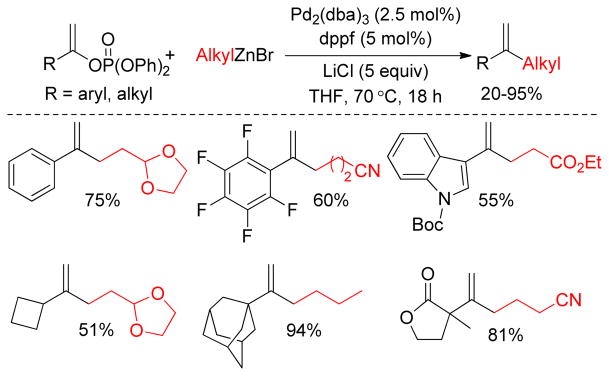
A series of aryl or alkyl vinyl phosphates undergo Pd(0)-catalyzed Negishi cross-coupling reactions with alkylzinc reagents to produce the corresponding 1,1-aryl,alkyl or 1,1-alkyl,alkyl disubstituted alkenes respectively.157 In this coupling, the counter ion (chloride or bromide) of the alkylzinc reagent has a pronounced effect. It was found that alkylzinc bromides, in the presence of LiCl, are active towards the cross-couplings whereas alkylzinc bromides by themselves are less reactive or inactive. This is in good agreement with the observation of Buchwald and coworkers.158 It is assumed that during the preparation of the organozinc chloride from the corresponding organochloride via lithium-zinc exchange at least an equivalent amount of lithium chloride is formed that augments the concentration of a more reactive anionic Pd(0) complex in equilibrium with its neutral species. As a mechanistic probe, addition of an excess (5 equiv) of lithium chloride was found to effectively promote cross-couplings with alkylzinc bromides in combination with Pd2dba3/dppf in THF at 70 °C (Figure 13). Furthermore, it was observed that alkylzinc reagents are less reactive compared to their arylzinc counterparts.
Figure 13.
Negishi Coupling of Alkenyl Phosphates: (Skrydstrup and coworkers, 2007)
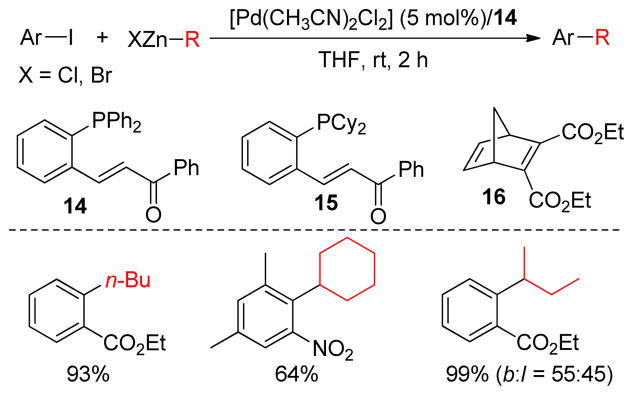
C(sp2)-centers with higher electronegativity or adjacent double bonds have an accelerating effect on the rate of cross-coupling reactions. This is proposed to arise from faster reductive elimination via the transfer of metal d electrons to the π* orbitals of the olefin or aryl group.159,160 As a result, competitive β-hydride elimination is suppressed. Based on this concept, the Lei group designed a chelating ligand containing a phosphine and an electron-deficient olefin for Pd-catalyzed Negishi couplings (Figure 14). Superior effects of the ligands were observed not only in terms of higher yields but also faster reaction rates under mild conditions.161 Thus, a series of aryl iodides underwent cross-couplings with primary, secondary and even tertiary alkylzincs at ambient temperature. A kinetic study with a 1:1 mixture of ligand 14 and PdCl2(CH3CN)2 was performed using the cross-coupling between ethyl-2-iodobenzoate and cyclohexylzinc chloride as the model reaction.162 The kinetic data revealed that the rate constant for reductive elimination of [Ar-Pd-Csp3] was > 0.3 s−1, which was about 4 or 5 orders of magnitude greater than values using [Pd-(dppbz)] or [Pd(PPh3)2] (dppbz = 1,2-bis(diphenylphosphino)-benzene). The rate enhancement was proposed to be a result of the π-acidity of the ligand. As expected the same reaction with the saturated analog of the ligand resulted in not only a slower rate but also the formation of a substantial amount of ethyl benzoate. The formation of ethyl benzoate was a clear indication that the ligand promotes reductive elimination and suppresses competitive β-hydride elimination. During a subsequent mechanistic investigation by Lei and coworkers, a second transmetalation step in the Negishi coupling was revealed and its competition with reductive elimination was disclosed.163 The isomerization of the secondary dialkylzinc such as i-Pr2Zn to a linear one was significantly suppressed using the catalytic combination of [Pd(CH3CN)2Cl2] and ligand 14 (Figure 14). Similarly, ligand 15 was used for the Negishi coupling between aryl bromides and alkylzinc chlorides. Using the same concept, a highly electron-deficient diene ligand 16 was also employed for the Pd-catalyzed cross-couplings of aryl iodides and alkylzinc reagents.164
Figure 14.
Negishi Coupling using π-Acceptor Ligands (Lei and coworkers, 2007)
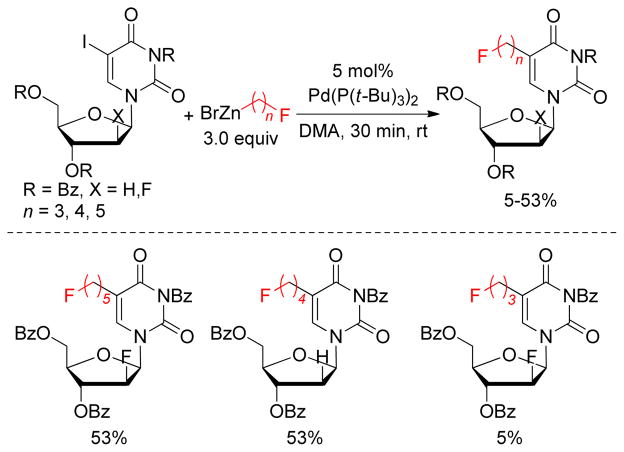
A library of biologically active 5-fluoroalkylated pyrimidine nucleosides was synthesized by a Pd(Pt- Bu3)2-catalyzed cross-coupling reaction between protected 5-iodo-2′S-deoxyuridine nucleosides and fluorinated, unactivated alkylzinc reagents (Figure 15).165 This methodology provides a direct route to synthesize F18-radiolabeled 5-fluoroalkylated pyrimidine nucleosides that could be used as probes for noninvasive in vivo molecular imaging.166–168 Although yields are modest, mild reaction conditions for the cross-coupling reactions of sensitive and densely functionalized molecules are noteworthy.
Figure 15.
Negishi Coupling of 5-iodo-pyrimidine Nucleosides (Kung and coworkers, 2008)
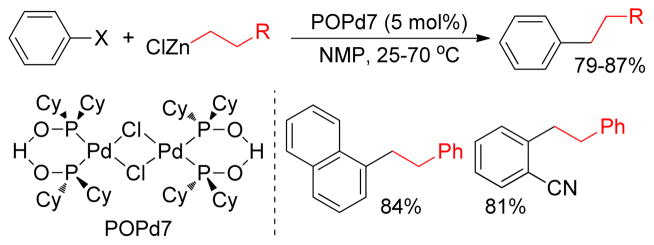
Wolf and coworkers recently reported the Negishi cross-coupling of organozinc reagents and aryl halides catalyzed by Pd-phosphinous (POPd7) acid in NMP (Figure 16). A wide range of functional groups were compatible under the reaction conditions.169
Figure 16.
Pd-phosphinous Acid-Catalyzed Negishi Couplings (Wolf and coworkers, 2008)
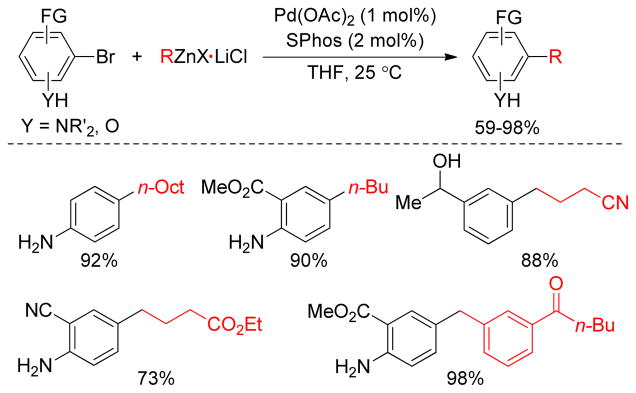
The cross-coupling of unsaturated halides bearing acidic protons with organozinc reagents is an ongoing challenge. The Knochel group reported that Pd(OAc)2/SPhos is an excellent catalyst combination for the Negishi cross-coupling reactions of substrates bearing acidic protons (Figure 17).170,171 Thus a variety of bromo- or iodo-anilines, alcohols, and acidic phenols underwent cross-coupling upon slow addition (90 min) of the organozinc reagents at 25 °C. The relative kinetic basicity of the organozinc reagents is in the order: arylzinc halide > alkylzinc halide > benzylzinc halide as determined by the addition of i-PrOH to the organozinc reagents and subsequent quenching with CuCN/allyl bromide in THF.170
Figure 17.
Negishi Coupling of Aryl Halides Bearing Acidic Protons (Knochel and coworkers, 2008)
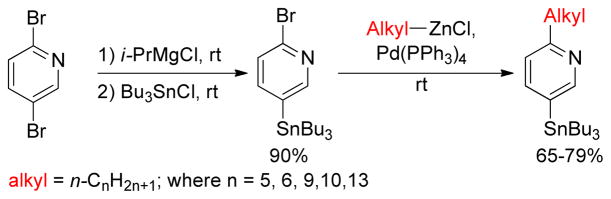
A highly chemoselective Negishi cross-coupling reaction between alkylzinc reagents and 2-bromo- 5(or 6)-tri-n-butylstannylpyridines was reported by Twieg group.172 The Pd(PPh3)4-catalyzed cross-coupling occurred at ambient temperature selectively at the 2-position, leaving the tri-n-butylstannyl group intact for further Stille couplings (Scheme 5).
Scheme 5.
Chemoselective Negishi Coupling of 2-bromo-pyridinylstannanes (Twieg and coworkers, 2008)
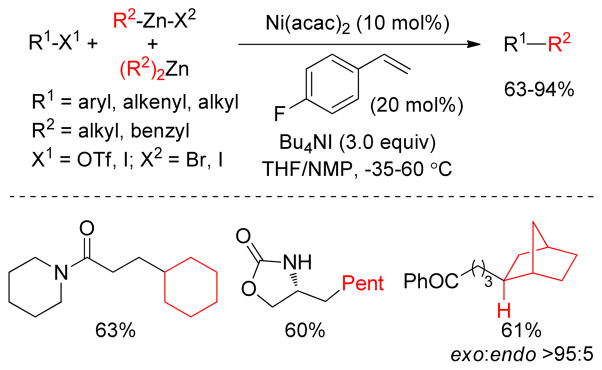
The Knochel group observed a positive effect of tetra-n-butylammonium iodide on Pd(0)- and Ni-catalyzed Negishi cross-couplings. A series of aryl or alkenyl triflates underwent a Pd(dba)2/dppf (7 mol%)-catalyzed cross-coupling with benzyl173 and/or alkylzinc halides using three equivalents of Bu4NI.174 To explore the impact of Bu4NI on the conversion of the starting material, experiments were performed with differing amounts of Bu4NI. It was found that the cross-coupling between benzylzinc bromides and p-Cl-phenyl triflate produced 2.4% of the coupling product with 0.1 equiv, 38% with 1.0 equiv and 90% with 3.0 equiv of Bu4NI. More recently, Marder and Lei reported that Pd-nanoparticles were formed from Pd(OAc)2 and Bu4NBr under these reaction conditions (Figure 18).175 Transition metal nanoparticles can provide a more accessible and active surface area to interact with substrates that increase the catalytic activity.176 Suitable stabilizers such as commercially available tetraalkylammonium halides are frequently used in nano-Pd catalysis to prevent agglomeration and precipitation of the catalyst.177 Thus, a combination of Pd(OAc)2 and Bu4NBr catalyzed the cross-coupling between aryl iodide and alkylzinc efficiently at room temperature as well as at −20 ºC in excellent yield within 1 h (Figure 18). Presumably, Pd(OAc)2 was reduced to Pd(0) by the alkylzinc reagent under the reaction conditions. The Pd(0)-species was then suspended as nanoparticles (PdNPs) and stabilized by Bu4NBr. The reaction between ethyl-2-iodobenzoate and CyZnCl was monitored by in situ IR. It was found that the reaction reached 60% completion after 30 seconds and 100% after 2 min. Several experiments support that Pd(NPs) are formed including the observation that PPh3 acts as a catalyst poison.
Figure 18.
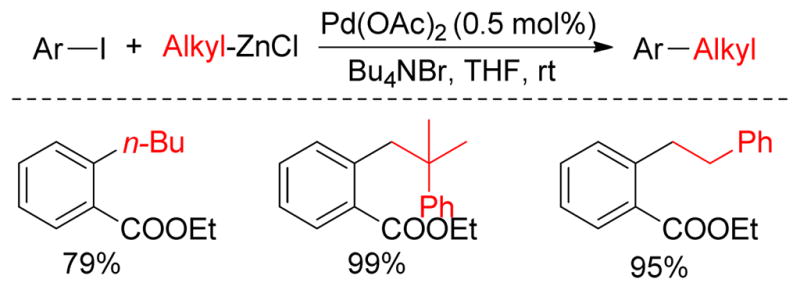
Pd-nanoparticle-catalyzed Negishi couplings (Lei and coworkers, 2008)
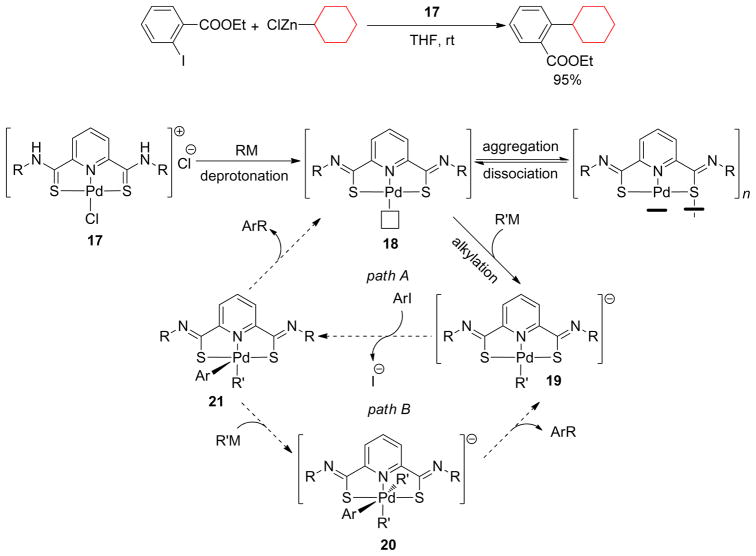
Yang and Lei identified a highly efficient alkylated pincer thioimido-Pd complex intermediate in Negishi coupling by in situ IR, 1H and 13C NMR studies. Contrary to the Pd(OAc)2/Bu4NBr catalytic system that forms Pd(NPs) as a true active species, the thioimido-Pd(II) complex 17 reacted with CyZnCl to form an alkylated, anionic species 19 that catalyzed cross-coupling further with ethyl-2- iodobenzoate (Scheme 6).178 The long induction time (~ 60 min) was due to the formation of 18. Once it was formed the subsequent coupling is complete within 20 min.
Scheme 6.
Pincer thioimido-Pd complex-Catalyzed Negishi Couplings (Lei and coworkers, 2008)
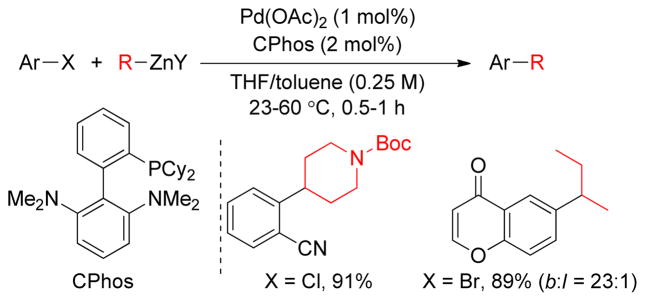
The Buchwald group reported an efficient Negishi coupling between secondary alkylzinc halides and aryl bromides and chlorides catalyzed by Pd(OAc)2 and an electron-rich ligand CPhos (Figure 19). Excellent selectivity for branched versus linear products using CPhos was observed due to the slow relative rates of β-hydride elimination-reinsertion versus reductive elimination.125
Figure 19.
Negishi Coupling of Secondary Alkylzinc Halides (Buchwald and coworkers, 2009)
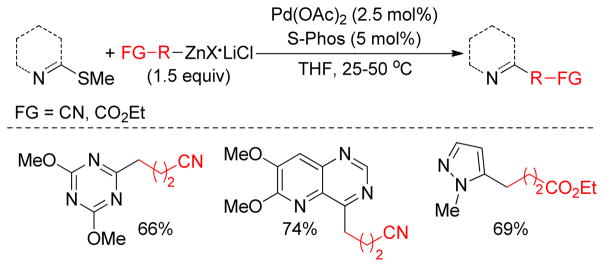
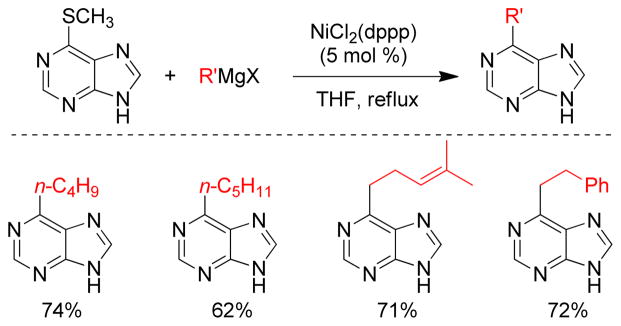
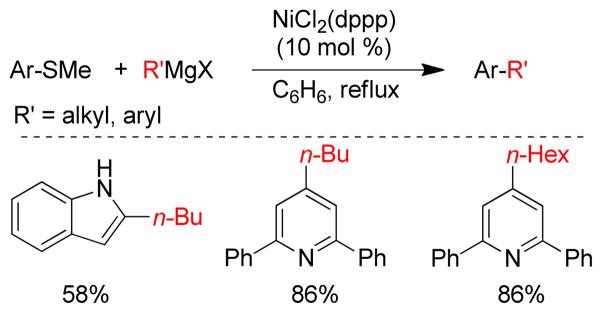
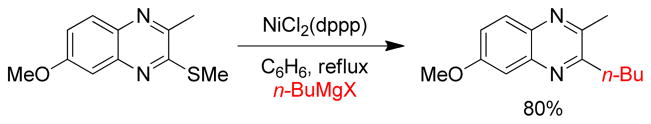
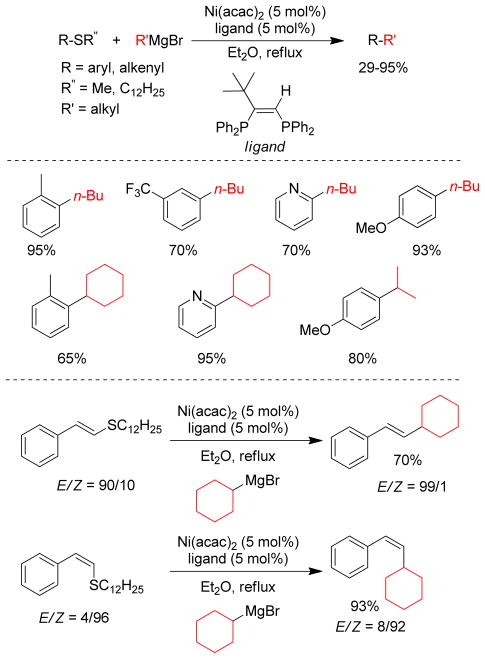
In 1979, the Wenkert group first reported the transmetalation of Grignard reagents containing unsaturated thioethers in transition metal-catalyzed cross-coupling reactions.179 The Fukuyama coupling is another illustration of this concept that converts thioesters to ketones by reacting with organozincs in the presence of catalytic Pd.180 The Knochel group exploited this concept of C-S bond activation to show that various thiomethyl-substituted N-heterocycles, i.e. pyridines, pyrimidines, pyrazines, pyridazines, benzothiazoles etc., undergo Pd-catalyzed cross-coupling reactions with organozinc reagents using Pd(OAc)2/SPhos (Figure 20).181 For alkylzincs, the cross-couplings required heating at 50 °C for completion. More recently, the same group reported that Ni(acac)2/DPEPhos could catalyze this reaction at room temperature. Several 2-substituted oxazoles were synthesized using a Ni-catalyzed cross-coupling of 2-methylthio-oxazole and various organozinc reagents.182 A Pd-catalyzed cross-coupling with thiomethylated alkynes and alkylzinc reagents was also reported by this group.183 Furthermore, Stambuli reported the synthesis of unsymmetrical 2,5-disubstituted oxazoles from the corresponding 2,5-dimethylthio-oxazole by chemoselective Pd- and Ni-catalyzed cross-couplings with organozinc reagents in one pot.184
Figure 20.
Negishi Coupling of Thiomethyl-substituted Heterocycles (Knochel and coworkers, 2009)
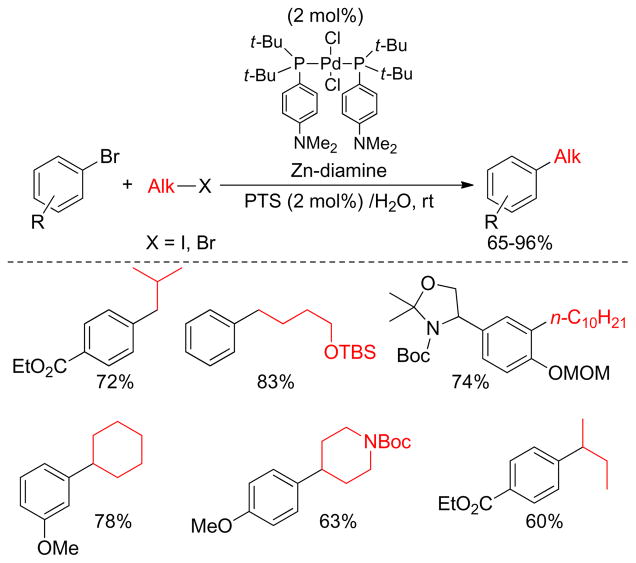
Lipshutz and coworkers reported the in situ preparation of alkylzinc reagents in water catalyzed by tetramethylethylenediamine (TMEDA) and subsequent cross-coupling reactions with arylbromides. A combination of a sterically hindered and electron-rich Pd-catalyst along with a surfactant (PTS) in water formed a highly active aqueous micellar catalyst for the Negishi cross-coupling (Figure 21).107 This methodology offers a low-waste technology for C(sp2)-C(sp3) bond constructions in the absence of stoichiometrically preformed organometallic coupling partners and organic solvents. Similarly, a one pot protocol using a Pd-PEPPSI-catalyst for a cross-coupling between aryl chlorides, bromides and triflates and alkylzinc reagent was reported by the Knochel group.185
Figure 21.
Negishi Couplings in Aqueous Medium (Lipshutz and coworkers, 2009)
2.4.2. Pd-Catalyzed C(sp3)-C(sp3) Negishi Coupling with Alkylzinc Reagents
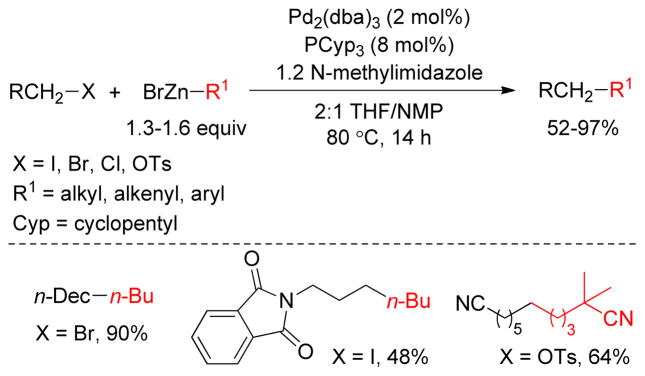
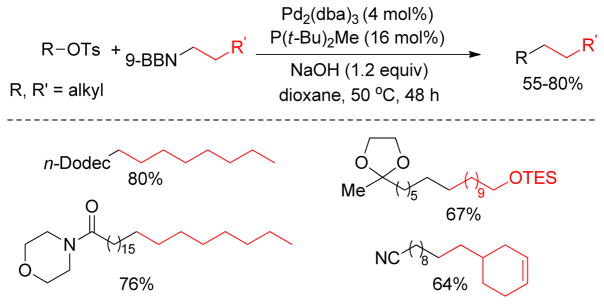
Over the past two decades, significant progress has been achieved in the area of C(sp3)-C(sp3) cross-coupling between alkyl organometallics and alkyl electrophiles. The Fu group has made key contributions by identifying a set of electron-rich hindered ligands capable of promoting oxidative addition and reductive elimination to furnish cross-coupling products. As an example, Pd2(dba)3/PCyp3/NMI was found to be an effective catalyst combination for the cross-coupling of alkylzinc reagents with alkyl halides and tosylates (Figure 22).121 The addition of NMI improved yields to some extent, perhaps through activation of alkylzinc halides toward transmetalation.
Figure 22.
Negishi Coupling with Unactivated Alkyl Halides and Tosylates (Fu and coworkers, 2003)
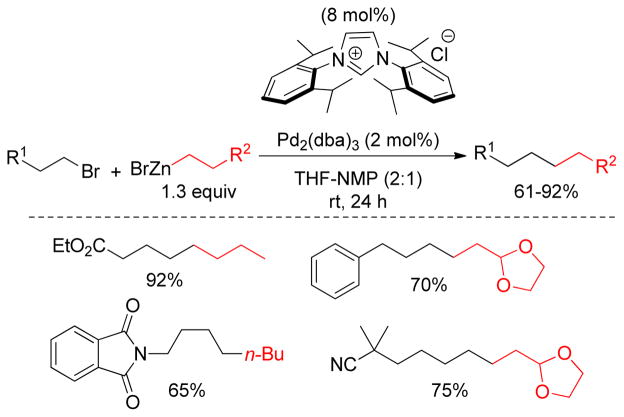
As discussed earlier, one of the main problems in alkyl-alkyl cross-coupling is the reluctance of saturated carbon-halogen bonds to undergo oxidative addition compared to aryl, vinyl, benzyl, or allyl halides and competing β-hydride elimination from the organometallic intermediates. To overcome these obstacles, an electron-rich Pd-center is often employed for effective oxidative addition, wherein N-heterocyclic carbenes (NHCs) are an alternative to electron-rich phosphine ligands due to their σ-donicity and steric properties.186,187 Fu and coworkers reported low yields when a NHC ligand having modest sized substituents was used in a Negishi coupling.121 In contrast, Organ and coworkers reported good to excellent yields using sterically more hindered NHC ligands (Figure 23).188
Figure 23.
Alkyl-Alkyl Negishi Coupling using NHC-ligands (Organ and coworkers, 2005)
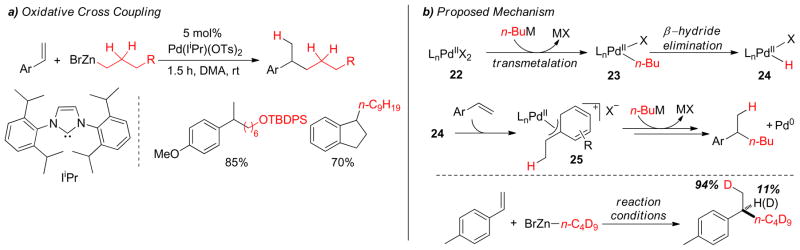
The use of an NHC ligand (Ii-Pr) in an oxidative Pd(II)-catalyzed C(sp3)-C(sp3) cross-coupling was recently reported by the Sigman group (Figure 24).189 In this chemistry, styrenes were utilized as synthons for alkyl halides in an overall hydroalkylation process with alkylzinc reagents to form primary-secondary carbon-carbon bonds under oxidative conditions. The reaction is proposed to proceed by transmetalation to form complex 23 followed by β-hydride elimination to form a Pd-hydrido species 24. Insertion of the Pd-hydride into the conjugated alkene presumably generates a π-benzyl species 25 which undergoes transmetalation to form the product. Benzoquinone was used as a terminal oxidant that regenerates the active Pd(II)-species to complete the catalytic cycle (Figure 24). The cationic nature of the metal center was found to be crucial in this transformation, which is in contrast to the involvement of electron-rich metal centers in C(sp3)-C(sp3) couplings with alkyl halides. To illustrate the origin of hydrogen in the product, a deuterated alkylzinc reagent was subjected to the cross-coupling reaction. Nearly one deuterium incorporation was observed predominantly (94%) at the methyl position, and at the methine position to some extent (11%). This phenomenon indicates that the insertion of alkene occurs with either regiochemistry, but the resultant Pd-alkyl species likely rearranges to the more stabilized π-benzyl intermediate via β-hydride elimination-reinsertion.
Figure 24.
Oxidative C(sp3)-C(sp3) Coupling (Sigman and coworkers, 2009)
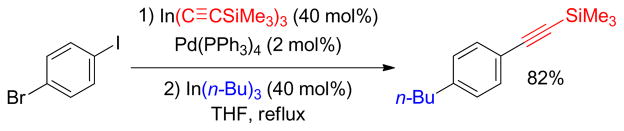
2.4.3. Pd-Catalyzed C(sp)-C(sp3) Negishi Coupling with Alkylzinc Reagents
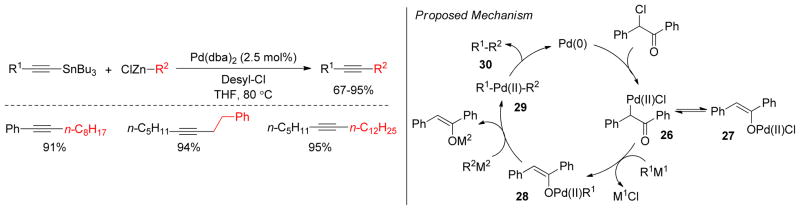
Although C(sp2)-C(sp3) couplings have been studied extensively, there are few examples of C(sp)- C(sp3) couplings using alkynyl electrophiles and alkyl organometallics.190 One such example is the Pd-catalyzed oxidative cross-coupling of terminal alkynyltin reagents with alkylzincs to generate alkylated alkynes through double transmetalation (Figure 25).24 Mechanistically, the oxidative addition of decyl chloride to Pd(0) generates C-bound Pd-enolate chloride 26 that undergoes tautomerization into the O-bound Pd-enolate chloride 27. Sequential transmetalation with zinc and tin reagents produces the C(sp)- Pd-C(sp3) intermediate 29, which undergoes reductive elimination to yield the desired cross-coupling product 30 (Figure 25). Kinetic studies by in situ IR revealed that the rate of cross-coupling is much faster than that of the corresponding homocoupling. In addition, the rate of stilbenyloxyzinc chloride formation is almost equal to the consumption of desyl chloride and the formation of the cross-coupling product (R1–R2).
Figure 25.
Oxidative Cross-Coupling through Double Transmetalation (Lei and coworkers, 2006)
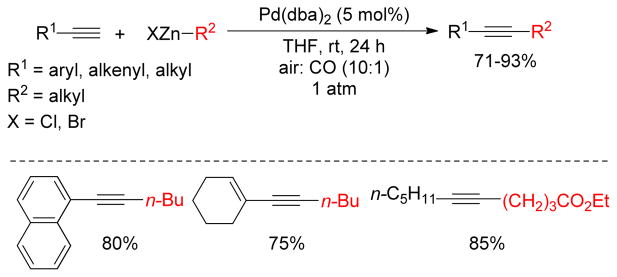
Similarly, C(sp)-C(sp3) coupling of terminal alkynes with alkylzincs using Pd(dba)2 were accomplished under oxidative conditions by the same group (Figure 26).25 A substantial amount of diyne formation was observed using air as the oxidant along with Pd(dba)2. However, cross-coupling products were obtained in good to high yields using a (10:1) mixture of air/CO. Presumably, the well-known π-acidic ligand CO facilitates the reductive elimination in this process. A similar accelerating effect was observed using dibenzylideneacetone (dba) in lieu of CO. A nice practical advantage of using CO as the π-acidic ligand was that no rigorous column chromatography for separation was required.
Figure 26.
Oxidative Cross-Coupling with Terminal Alkynes (Lei and coworkers, 2010)
2.4.4. Formation of Ketones via Pd-Catalyzed Negishi Coupling with Alkylzinc Reagents
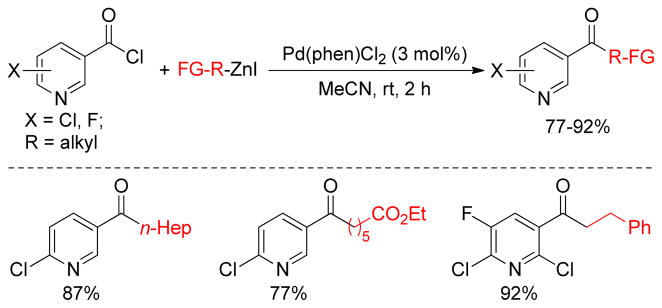
The Pd-catalyzed acylation of organozinc reagents using carboxylic acid chlorides was reported in early 1980’s.113,191 As a recent example, pyridine carboxylic acid chlorides were coupled with alkylzinc reagents catalyzed by Pd(phen)Cl2 to afford pyridyl ketones. Interestingly, only the acid chlorides react while the 2-chloroazine moiety remains intact under the reaction conditions (Figure 27).192
Figure 27.
Formation of Ketones via Negishi Coupling (Ohno and coworkers, 2009)
2.5. Ni-Catalyzed Cross-Coupling Reactions of Alkylzinc Reagents
The use of Ni-complexes in Negishi type cross-coupling reactions was initially reported simultaneously with the Pd variants. As described below, the Ni-catalyzed variants are quite powerful on an array of substrate types.
2.5.1. Ni-Catalyzed C(sp2)-C(sp3) Negishi Coupling with Alkylzinc Reagents
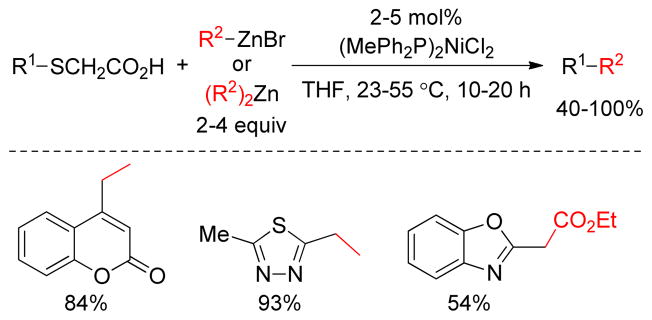
Liebeskind reported a biologically relevant, Ni-catalyzed cross-coupling of a thioglycolate series with organozinc reagents based on the principle of metal-thiolate activation. A series of thioglycolic acids were synthesized conveniently by base-catalyzed thioether formation of thioarenes and 2- bromoethanesulfonic acid and underwent cross-coupling with organozincs in the presence of catalytic (MePPh2)2NiCl2 in THF at 25–50 °C (Figure 28).193 Control experiments suggested the crucial role of Zn2+ presumably as a thiolate ion scavenger to prevent poisoning of the metal catalyst in these cross-coupling reactions.
Figure 28.
Negishi Coupling of Thioglycolic Acids (Liebeskind and coworkers, 1999)
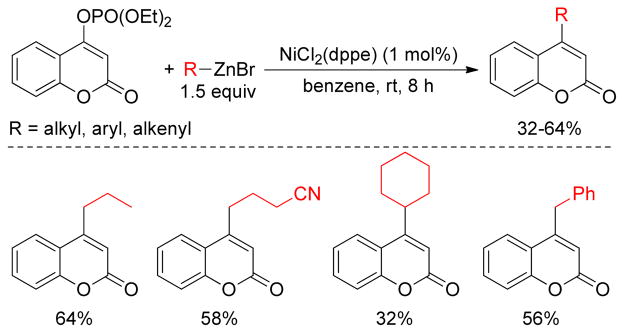
The Yang group reported Pd-catalyzed cross-coupling reactions of arene and vinyl-sulfonates with organozincs to produce 4-substituted coumarins.194 Switching from Pd to Ni resulted in the facile coupling of organozinc reagents with vinyl-phosphates. Thus, a series of 4-substituted coumarins were obtained from the corresponding 4-phosphate-coumarins (Figure 29).
Figure 29.
Negishi Coupling of 4-diethylphosphonooxycoumarins (Yang and coworkers, 2001)
Ni-catalyzed Negishi coupling of amino-heteroaryl chlorides with alkylzinc reagents was reported by Walters (Figure 30).195 The alkylzinc reagents could be commercially available dialkylzincs or alkylzinc halides, or they could be conveniently generated in situ from diethylzinc and primary alkyl bromides in the presence of NiCl2(dppp) as catalyst, which also catalyzes the cross-coupling reaction.
Figure 30.
Negishi Coupling of Amino-heteroaryl Chlorides (Walters, 2005)
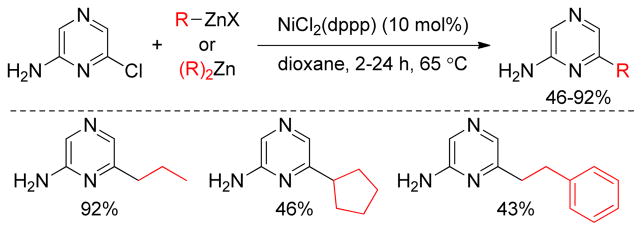
Pd-catalyzed Negishi coupling of substrates bearing basic nitrogen substituents has proved to be unsuccessful in general. The Knochel group has developed a Ni-catalyzed protocol for aminoalkylations of arenes. The lithium salts of amino alkyl Grignard reagents were converted to the corresponding alkylzinc reagents in situ by treatment with ZnBr2. Subsequently, Ni(acac)2/DPEPhos–catalyzed cross-coupling with an aryl or a heteroaryl (pseudo)halides introduced the aminoalkyl moiety into the arene system (Scheme 7).196
Scheme 7.
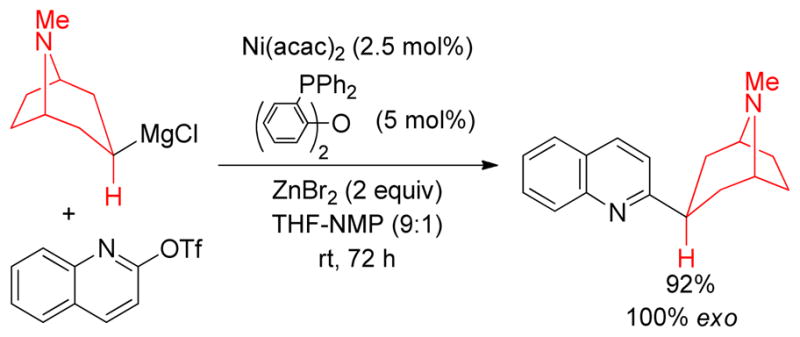
Aminoalkylation of Heteroarenes (Knochel and coworkers, 2007)
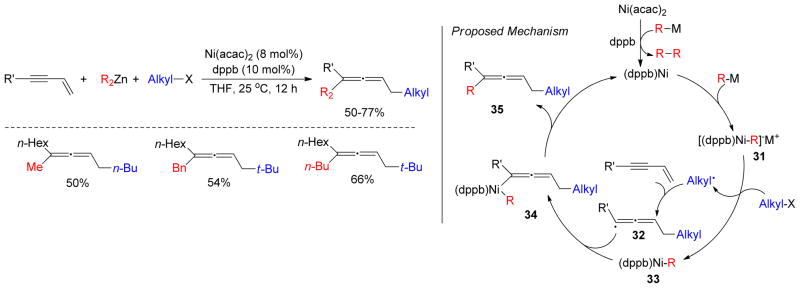
Recently, the Terao group reported a Ni-catalyzed regioselective three-component coupling of alkyl halides, arylacetylenes, or enynes with organomagnesium or organozinc reagents (Figure 31).197 Mechanistically, it was proposed that alkyl radicals are generated in situ from the alkyl halides by single electron transfer from nickelate complex 31. The alkyl radical undergoes addition to the unsaturated C-C system forming 32 (at terminal double bond in the case of enynes) followed by generation of 34 through combination of 32 and Ni-complex 33. Finally, reductive elimination from Ni-complex 34 provides trisubstituted olefins 35 (or allenes).
Figure 31.
Ni-Catalyzed Three-Component Coupling (Terao and coworkers, 2009)
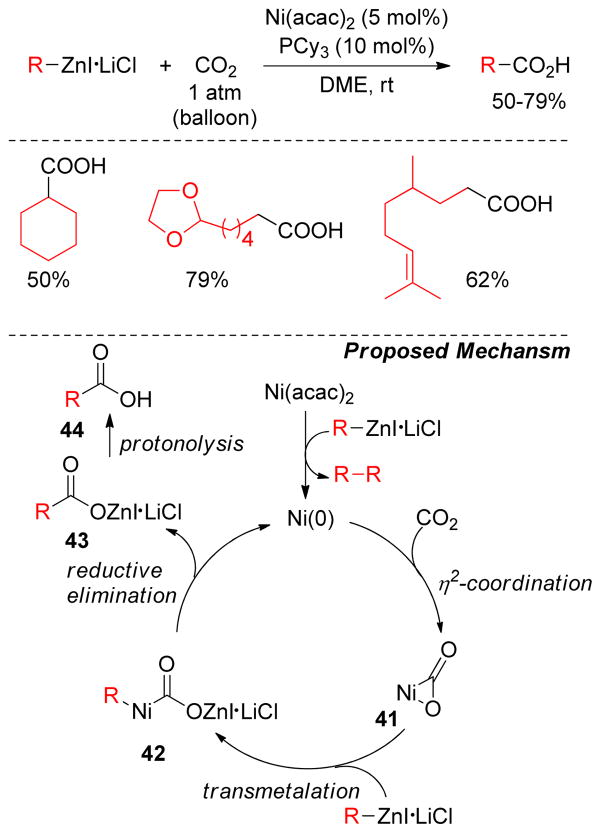
2.5.2. Ni-Catalyzed Carboxylations with Alkylzinc Reagents
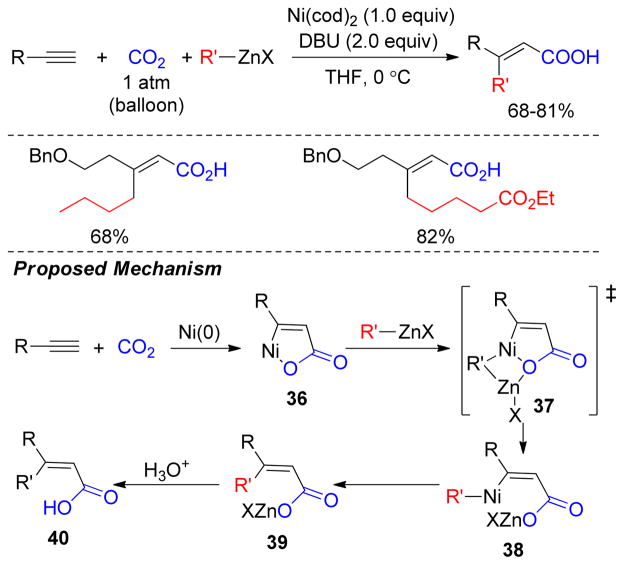
A Ni(0)-mediated three component coupling of alkynes, alkylzinc reagents and CO2 has been reported to yield β,β′-disubstituted, unsaturated carboxylic acids (Figure 32).198 Mechanistically, the reaction of Ni, alkyne, and CO2 forms an oxonickelacycle 36 that undergoes transmetalation with alkylzinc reagents to produce intermediate 38. Reductive elimination of the β,β′-disubstituted, unsaturated carboxylate 39 is followed by hydrolysis to release the product 40 (Figure 32). Various functionalized alkylzinc reagents also underwent this alkylative carboxylation of alkynes whereas diethylzinc produced corresponding β-monosubstituted acids via β-hydride elimination.
Figure 32.
Alkylative Carboxylation of Alkynes (Mori and coworkers, 2001)
Oshima and coworkers reported an efficient Ni-catalyzed carboxylation of alkylzinc reagents for the production of saturated carboxylic acids. Lithium chloride salts of alkylzinc reagents underwent carboxylations smoothly under ambient pressure of CO2, catalyzed by Ni(acac)2 and PCy3 (Figure 33).199 The mechanism is proposed to proceed by initial reduction of Ni(acac)2 with the organozinc reagent. Ni(0) subsequently reacts with CO2 to produce η2-coordinated complex 41, which undergoes transmetalation with the organozinc reagent to generate reactive intermediate 42 upon a rapid reductive elimination, alkylcarboxylate 43 and active Ni(0) species are formed (Figure 33). The transmetalation and reductive elimination are thought to be facilitated by the highly electron-rich and bulky PCy3 ligand. Recently, this protocol has been revisited by the Dong group and they found that [Ni(PCy3)2(N2)] is an efficient catalyst for the carboxylation of alkylzinc halides as well as alkylzinc halide-lithium salts.200
Figure 33.
Carboxylation of Alkylzinc Reagents (Oshima and coworkers, 2008)
2.5.3. Ni-Catalyzed C(sp3)-C(sp3) Negishi Coupling with Alkylzinc Reagents
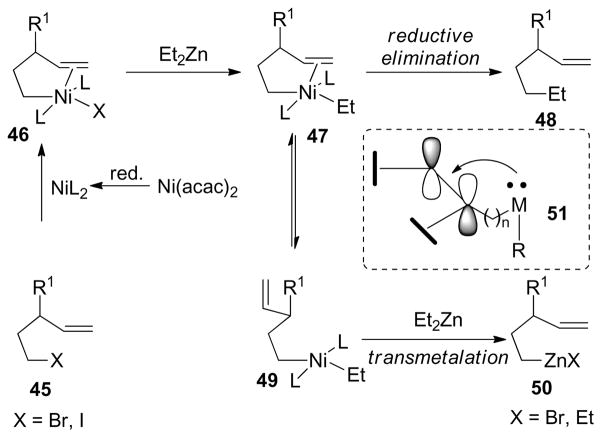
The Ni-catalyzed cross-coupling reaction between an alkyl organometallic reagent and an alkyl halide has been an active area of research during the past two decades. During the study of Ni-catalyzed Negishi couplings, the Knochel group observed that the presence of unsaturation or an electron withdrawing functional group on the alkyl halide accelerates the rate of reaction and produces the desired cross-coupling product in higher yields. Therefore, it was suspected that the remote double bond could act as an additional ligand for the Ni intermediates in the cross-coupling reaction. To gain more insight, the corresponding saturated alkylzinc reagent was submitted to the same cross-coupling conditions where a negligible amount of product formation was observed. Based on these observations, it is proposed that the catalytically active species L2Ni(0) generated by in situ reduction of Ni(acac)2 undergoes an oxidative addition of the alkyl halide to produce a Ni(II) complex 46, in which the double bond is coordinated to the metal center (Scheme 8). After ligand exchange with diethyl zinc, complex 47 is formed where the double bond accepts the d electrons of the Ni (51), resulting in a faster reductive elimination reaction. In the absence of the double bond (or if it is sterically encumbered), a Ni complex of type 49 is formed that undergoes further transmetalation to generate a new alkylzinc reagent 50, which can also undergo cross-coupling to give the undesired product. Alkyl halides bearing double bonds can be utilized in a substrate-controlled cross-coupling with dialkylzinc reagents as demonstrated by the Knochel group (Figure 34).201
Scheme 8.
Hypothesis for Substrate-Controlled Negishi Coupling (Knochel and coworkers, 1999)
Figure 34.
Substrate-Controlled Alkyl-Alkyl Negishi Coupling (Knochel and coworkers, 1996)
Similarly, functional groups such as ketones, cyanides, esters, and amides have positive effects on related cross-couplings. Besides the strategic control over reductive elimination by substrates that contain double bond(s), external additives were also effective in promoting cross-coupling reactions. It was found that additives such as acetophenones, benzophenones, and styrenes with electron withdrawing substituents accelerate the reaction (Figure 35).159 Among all of these additives, p- and m-trifluoromethyl substituted styrenes were the most effective in affording high yields at fast rates (Figure 35).159
Figure 35.
Ni-Catalyzed Alkyl-Alkyl Negishi Couplings (Knochel and coworkers, 1998)
The Knochel group reported a Ni(II)-catalyzed cross-coupling of benzyl zinc reagents with alkyl iodides in combination with p-fluorostyrene and Bu4NI as additives (Figure 36).174 The role of p-fluorostyrene was quite evident based on the preceding discussion; however, the role of Bu4NI is not clear although it is necessary for improved rates and yields. The same protocol was applied to the coupling of functionalized primary and secondary alkylzincs with primary alkyl halides.174 Recently, the same group has discovered that isopropyl iodide accelerates Negishi cross-couplings.202
Figure 36.
Ni-Catalyzed Alkyl-Alkyl Negishi Couplings (Knochel and coworkers, 2002)
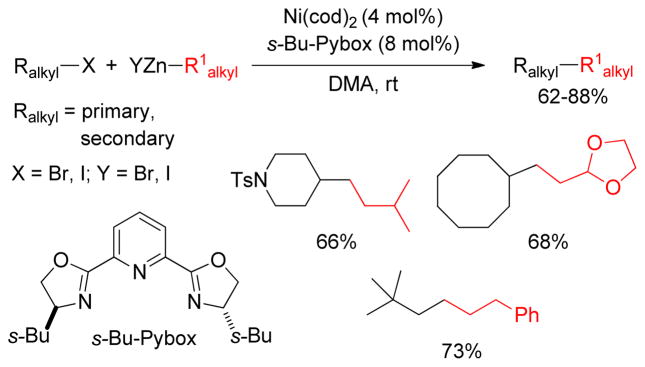
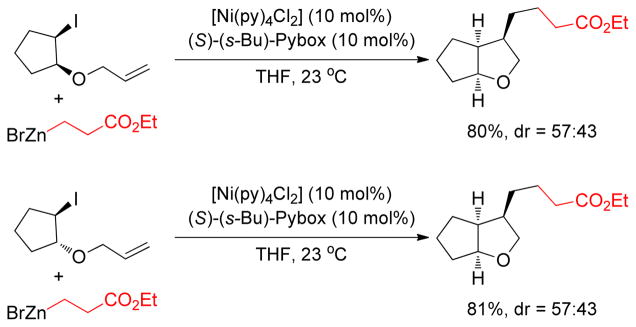
Fu and coworkers reported Ni-catalyzed C(sp3)-C(sp3) cross-coupling reactions of diverse alkylzinc reagents with unactivated secondary alkyl halides under very mild conditions. Ni(cod)2 and s-Bu-Pybox catalyzed the Negishi couplings in DMA at room temperature (Figure 37). Gratifyingly, the same catalytic system was also employed in the Negishi coupling with primary alkyl halides highlighted by the use of neopentyl iodide as a reaction partner.203
Figure 37.
Negishi Coupling of Secondary Alkyl Halides (Fu and coworkers, 2003)
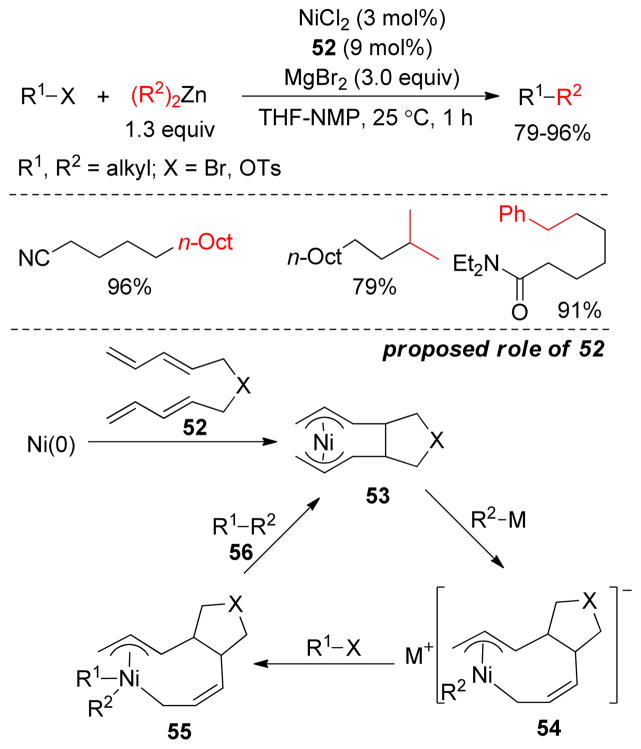
A number of primary alkyl halides and a tosylate underwent cross-coupling with dialkylzinc reagents at room temperature in NMP using catalytic NiCl2 in combination with MgBr2 and tetraene 52 (Figure 38).204 It was found that the electron-withdrawing tetraene additive 52 has a pronounced effect on reaction outcome, compared to conventional additives such as Bu4NBr, Bu4NI, p-fluorostyrene, isoprene, and i-PrI. It was speculated that a bis-π-allyl-Ni structure 53 is formed by oxidative cycloaddition of Ni(0) with the two butadiene moieties of 1,3,8,10-tetraene 52. An organomagnesium or zinc reagent attacks the bis-π-allyl-Ni complex to generate the η,η-octadienediylnickelate complex 54, which then reacts with the alkyl halide to yield complex 55. Cross-coupling product 56 is obtained via the subsequent reductive elimination of complex 55, and complex 53 is regenerated to complete the catalytic cycle (Figure 38).
Figure 38.
1,3,8,10-Tetraenes in Negishi Coupling (Kambe and coworkers, 2004)
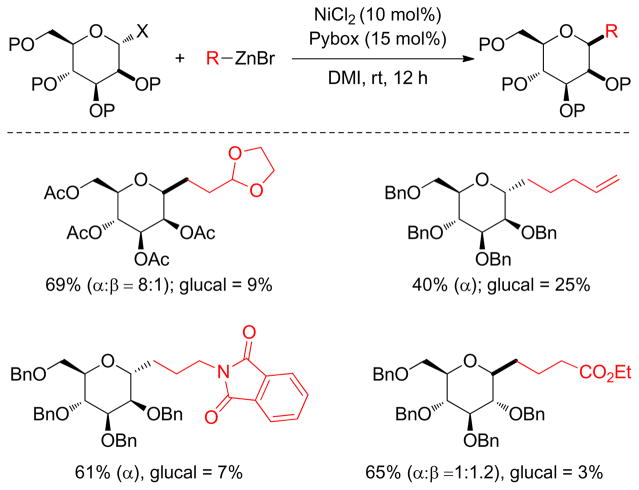
Gagné and coworkers reported another example of a Ni-catalyzed Negishi coupling between functionalized alkylzinc reagents and secondary glycosidic halides.205 It was observed that NiCl2 with an achiral Pybox ligand lead to good product yields, and mannosyl halides were diastereoselective for retentive C1-alkylation. In the case of acetyl-protected glycosides, α-bromides were the reaction partners, whereas benzyl-protected α-bromoglycosides were more reactive and in that case α-chlorides provided good yields and selectivity (Figure 39).
Figure 39.
C1-Alkylation of Glycosides (Gagné and coworkers, 2007)
The Cárdenas group utilized the proposed intermediacy of alkyl radicals in a Ni-catalyzed radical cyclization/cross-coupling process. This lead to the successful construction of substituted cyclic ethers via cascade formation of C(sp3)-C(sp3) bonds by cyclization and cross-coupling reactions of iodoalkanes with alkylzinc halides (Figure 40).206
Figure 40.
Ni-Catalyzed Radical Cyclization (Cárdenas and coworkers, 2007)
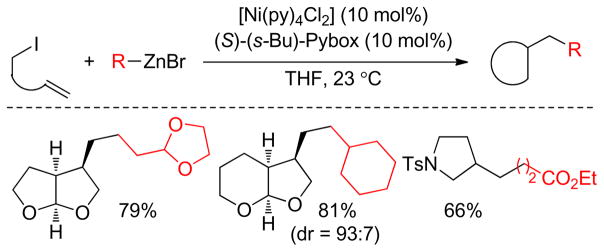
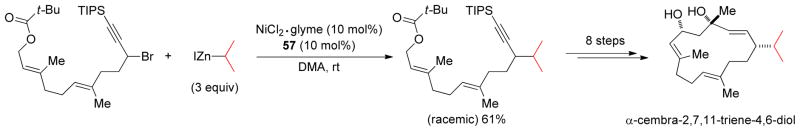
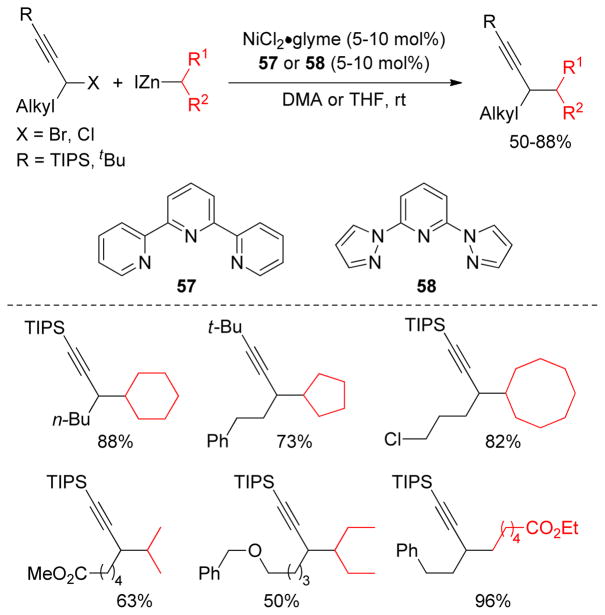
Cross-coupling reactions between secondary electrophiles and secondary alkyl organometallics are very problematic due to the presumed steric interactions associated with the process. The Ready group reported a Cu-catalyzed α-alkylation of α-chloro ketones with secondary alkylzinc reagents at room temperature.207 Fu and coworkers developed a Ni-catalyzed Negishi coupling with secondary alkylzinc reagents and secondary propargylic halides at room temperature using a combination of 10 mol% Ni-complex, and 10 mol% terpyridine 57 in DMA.208 More hindered alkylzinc reagents required the use of 5 mol% of Ni-complex and 5 mol% of 2,6-bis(N-pyrazolyl)pyridine 58 as ligand in THF. Bulky substituents on the alkyne were found to be crucial to avoid diyne formation via homocoupling. This methodology has been extended into the formal total synthesis of α-cembra-2,7,11-triene-4,6-diol (Scheme 9).
Scheme 9.
Secondary-Secondary Negishi Coupling in a Formal Total Synthesis of α-cembra-2,7,11-triene-4,6-diol (Fu and coworkers, 2008)
2.5.4. Mechanistic Insights in Ni-Catalyzed Cross-Couplings
Alkyl halides are known to undergo oxidative addition to Ni-catalysts via a radical pathway.209 Cárdenas and coworkers conducted tandem cyclization-cross-coupling reactions.206 It was observed that the cis and trans-isomers of a secondary alkyl halide containing a tethered olefin underwent intramolecular cyclizations followed by cross-coupling to give the same degree of cis/trans selectivity, indicating loss of stereochemical fidelity (Scheme 10).206 The same type of selectivity is observed under reported radical cyclization conditions,210 implicating the formation of a planar radical intermediate.
Scheme 10.
Stereochemistry in Tandem Cyclization-Cross-Couplings (Cárdenas and coworkers, 2007)
The same phenomenon was also observed under Fu’s conditions where cross-coupling of both exo- and endo-2-bromonorbornane with various organometallic reagents produced the exo product predominantly.211 Therefore, enantioselective cross-coupling reactions, which are discussed later, with racemic secondary alkyl halides, undergo stereoconvergence rather than a kinetic resolution.
Vicic and coworkers carried out an extensive study to probe the mechanism of Ni-catalyzed Negishi couplings.20,212 It was observed that a (terpyridine)nickel(0) complex does not react with alkyl halides by simple oxidative addition (two electron process) followed by transmetalation to afford cross-coupling product.21 The mono methyl Ni-complex is formed by ligand-induced loss of ethane followed by a comproportionation with another molecule of a dimethyl Ni(II) species (Scheme 11).212
Scheme 11.
Identification of Mono Methyl Nickel-Complex (Vicic and coworkers, 2005)
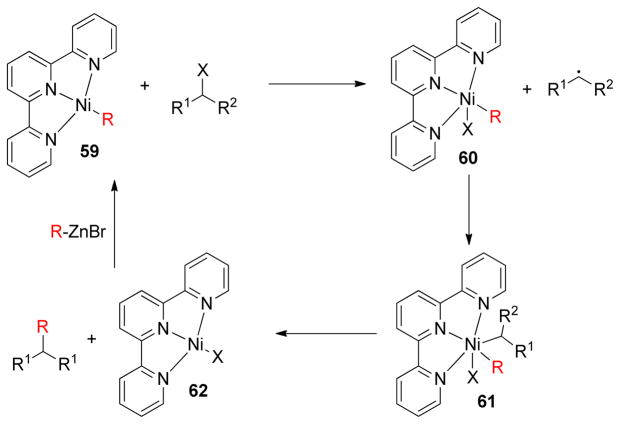
In contrast, the mechanism involving a secondary alkyl iodide and (terpyridyl)Ni(alkyl) complex 59 was proposed to proceed via a single electron transfer from the ligand to the alkyl halide generating a new metal complex 60 and an alkyl radical (Scheme 12). In EPR investigations, it was observed that the radical in the complex remains mainly on the ligand (g = 2.021 ± 0.002), and the alkyl radical remains in close proximity to the metal center. A subsequent oxidative radical addition to the metal center gives a putative Ni(III)-dialkyl complex 61 that yields the cross-coupling product via reductive elimination along with the complex 62.
Scheme 12.
Radical Mechanism in Ni-Catalyzed Alkyl-Alkyl Cross-Couplings (Vicic and coworkers, 2006)
Further DFT calculations performed by Phillips and coworkers also suggest the involvement of a single electron process in Ni-catalyzed alkyl-alkyl Negishi couplings.213 It was computed that the traditional two-electron redox mechanism is energetically unfavorable. Moreover, the halogen atom transfer to the metal center is the rate-determining step. The use of secondary electrophiles led to a faster decomposition of a Ni(III) complex compared to the reductive elimination.
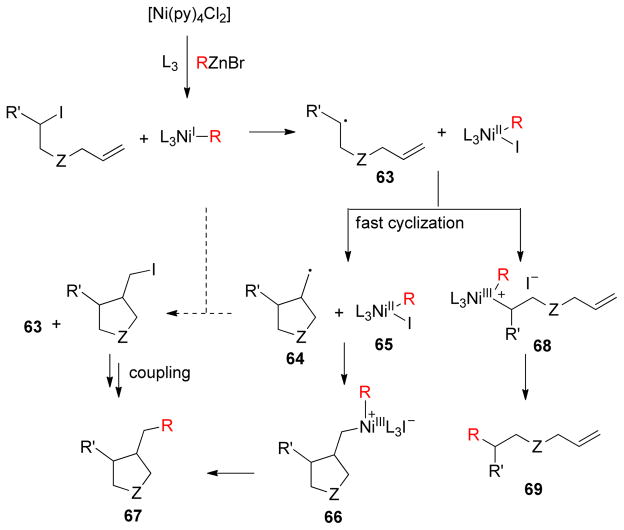
Finally, a plausible mechanism for the cyclization/coupling reaction described by Cárdenas was proposed based on computational and experimental results.206,214 Free radicals of type 63 are readily formed from alkyl iodides in the presence of Ni salts, which in some cases cyclize to yield intermediate 64. If the cyclization is slow, faster coordination to a NiII-complex to give a dialkyl-Ni(III) intermediate 68 may occur. This species would then undergo reductive elimination to give simple coupling product 69. Alternatively, intermediate 64 could react with the starting iodide to give 63 and 66 that could undergo subsequent coupling to give final product 67 (Scheme 13).
Scheme 13.
Proposed Mechanism for Tandem Radical Cyclization Cross-Coupling (Cárdenas and coworkers, 2007)
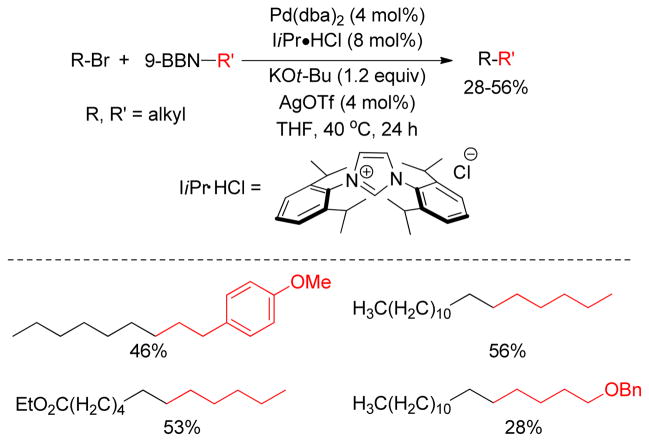
The role of additives in alkyl-alkyl Negishi couplings has also been investigated.199 The Organ group performed a titration study with the various amounts of LiBr doped into the alkyl-alkyl cross-coupling reaction mixture.215 It was observed that the rate of cross-coupling increases with the amount of LiBr added, and a sharp increase was observed at appoximately 1.0 equiv of LiBr added. In addition to the presumed break up of polymeric zinc aggregates, LiBr converts it to the tetracoordinated zincate complexes, which is an active transmetalating agent.
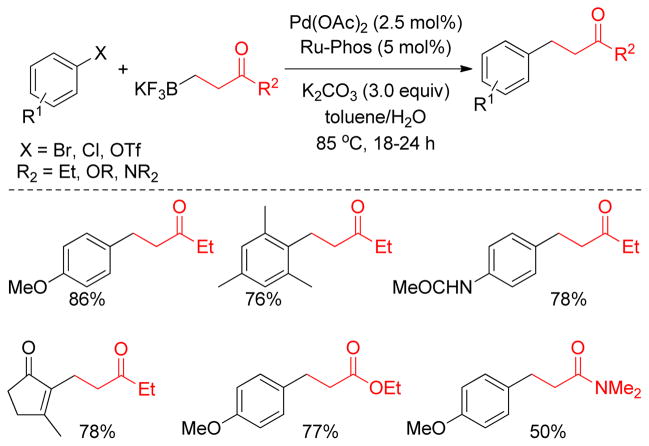
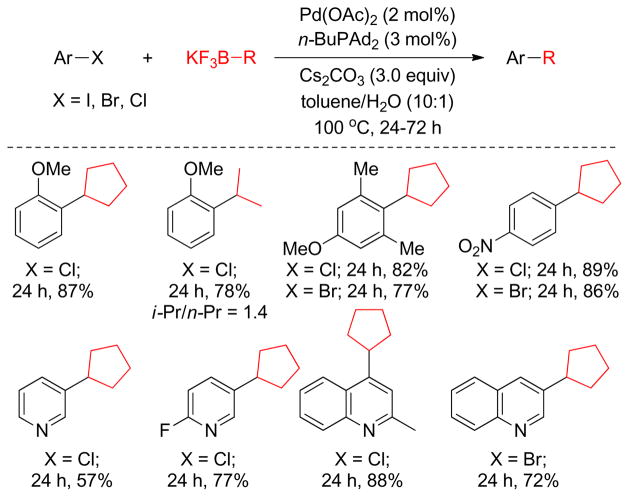
3. Cross-Coupling with Alkylboron Reagents
3.1. Introduction
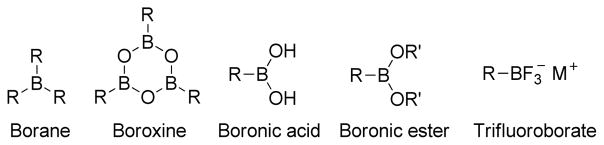
In the context of transition metal-catalyzed C-C bond forming reactions, the Suzuki-Miyaura coupling using organoboron compounds has emerged as a powerful tool due to its operational simplicity, environmentally benign nature, and the thermal stability of the transmetalating agents.117,216,217 The broad functional group tolerance compared to Negishi or Kumada protocols, which utilize organozinc and organomagnesium reagents respectively, is an added advantage of this protocol.117,216 Although organoboron compounds are highly electrophilic, the organic groups on boron are weakly nucleophilic. Additionally, the coordination of a negatively charged base to the boron atom can increase the nucleophilicity of the organic moiety on boron.218 Interestingly, Suzuki and Miyaura simultaneously realized that organoboron compounds, even organoboronic acids and esters, have sufficient reactivity for the transmetalation to various metals such as silver(I),219 magnesium(II),220 zinc(II),221–223 aluminum(II),224,225 tin(IV),226 copper(I)227,228 and mercury(II).229 Since the first report in 1986 of the cross-coupling reaction between alkylboron reagents and aryl and alkenyl halides in the presence of Pd(0) and a base,230 the B-alkyl Suzuki-Miyaura cross-coupling has become one of the most popular cross-coupling protocols in organic synthesis.231,232
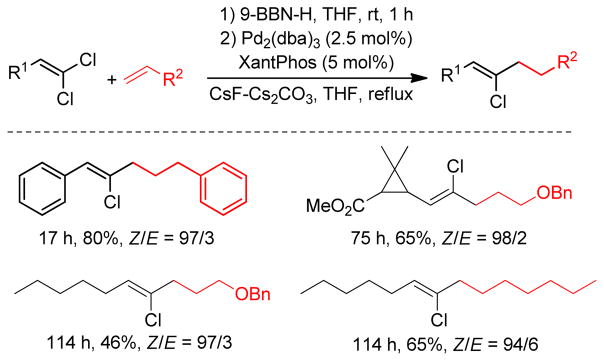
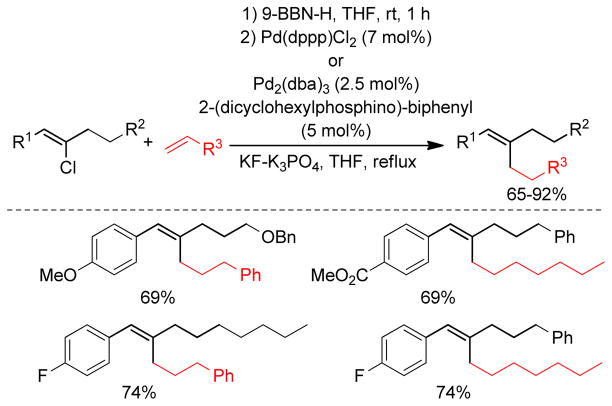
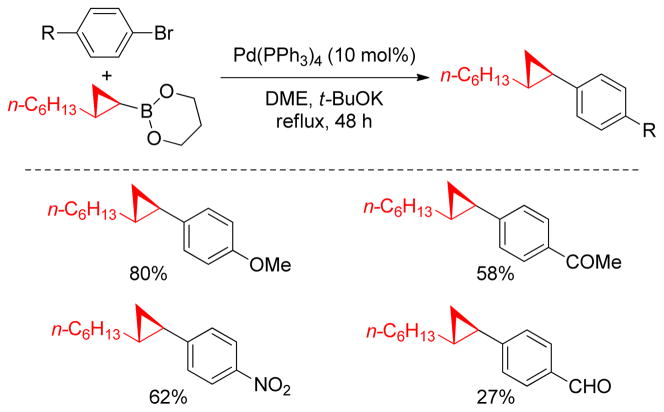
Primary alkyl borane compounds are easily synthesized by hydroboration of terminal alkenes in a highly chemo-, regio- and stereoselective manner.233 They are generally reactive species and undergo cross-couplings under diverse reaction conditions, but secondary alkylborons, especially secondary alkylboronic acids or trifluoroborates, are less reactive with the exception of cyclopropylboron derivatives that possess significant sp2-character.234 In spite of their low reactivity, the use of alkylboronic acids or trifluoroborates has become common practice in cross-coupling reactions due to their excellent compatibility with a wide range of functional groups.
3.2. Synthesis of Alkylboron Reagents
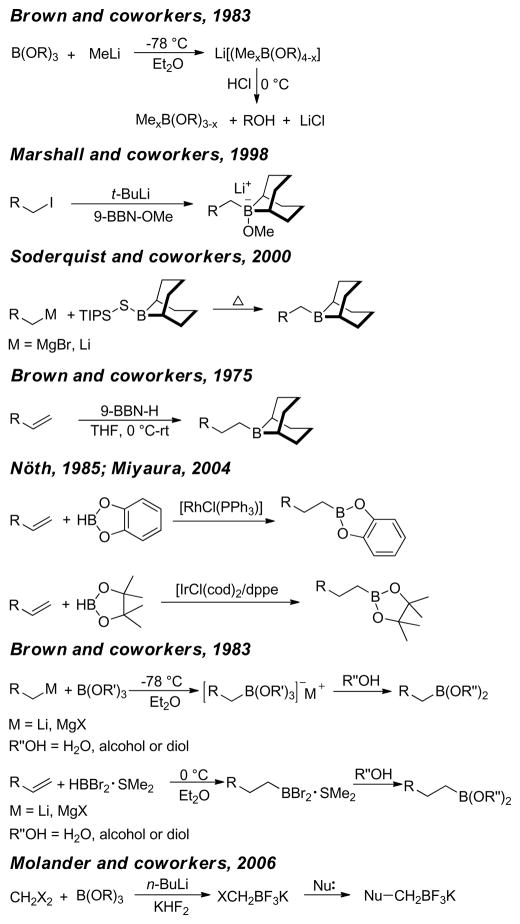
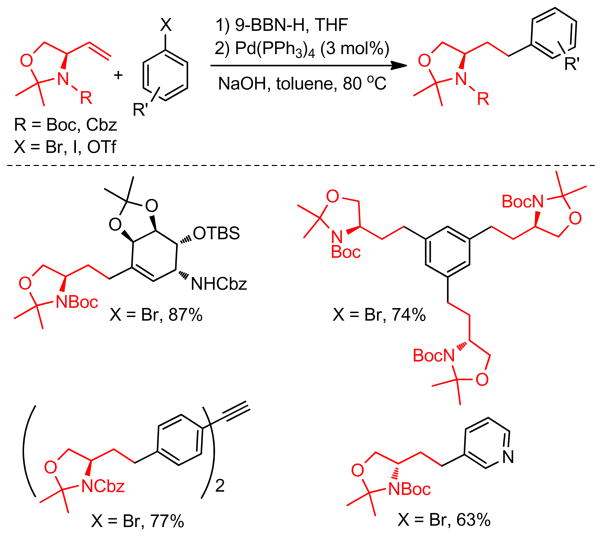
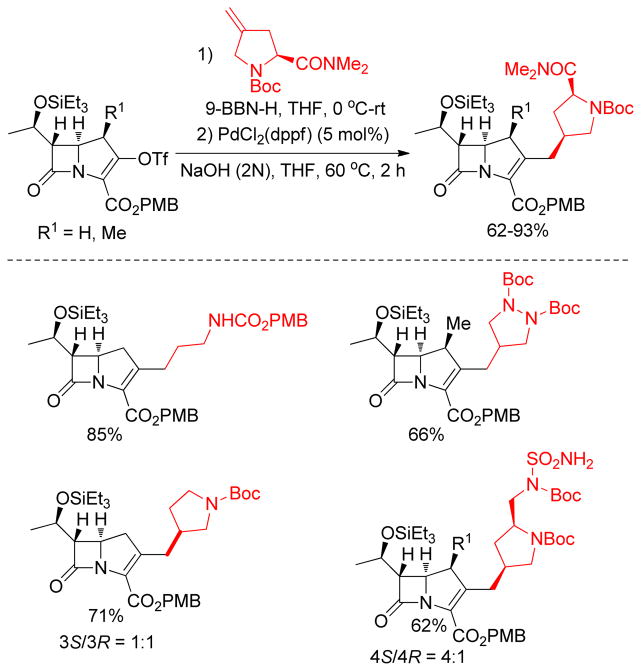
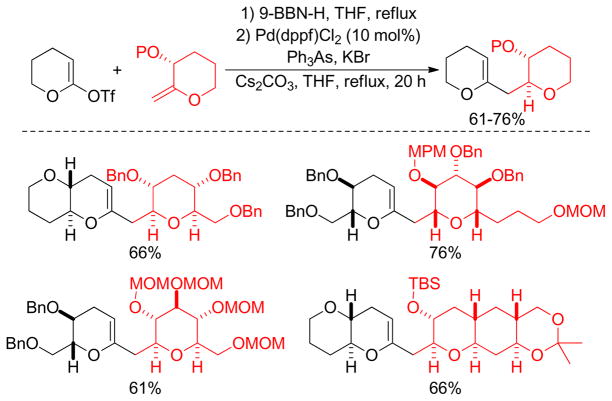
Classically, alkylboron reagents have been synthesized from the corresponding alkyllithium or alkylmagnesium reagents by reaction with suitable boron compounds. The Brown group reported a simple and efficient preparation of boronic esters, in which the corresponding alkyl halides were treated with methyllithium and quenched with trialkoxyborane at low temperature (−78 °C).235 Acidification with anhydrous HCl at 0 °C furnished the corresponding alkylboronic acids. Triisopropylborate was found to be the most effective furnishing diisopropylborates in quantitative yields. Similarly, treatment of alkyl iodides with t-BuLi followed by trapping with 9-methoxy-9-borabicyclo[3.3.1]nonane produces the corresponding alkylboron compounds in situ, which undergo cross-coupling reactions without further purification.236 Soderquist and coworkers used (TIPS)S-9-BBN instead of B-MeO-9-BBN. (Figure 43).237 A more common and popular approach to the synthesis of alkylboron reagents is through hydroboration of the corresponding alkene, which offers operational simplicity and a high degree of chemo-, regio-, and diastereoselectivity (Figure 43). The terminal alkylboron compounds are formed selectively through the anti-Markovnikov addition of the boron from the less hindered side of the alkene. Catecholborane (HBcat)238,239 or pinacolborane (HBpin)240 can also be used to synthesized the corresponding alkyl boronic ester under rhodium- or iridium-catalysis respectively (Figure 43). The Hartwig group also reported the Ti-catalyzed hydroboration of alkenes.241 Mioskowski and coworkers reported the synthesis of polymer-bound alkylboranes that could be purified by filtration, washing with anhydrous THF, and drying under vacuum for subsequent Pd-catalyzed cross-coupling reactions.242 Alkylboronic acids are prepared by the reaction of Grignard reagents or alkyllithium reagents with trihaloboranes and trialkoxyboranes, followed by acidification. Instead of the acidic work up, the reaction can be quenched with different alcohols to form the corresponding esters in high yields (Figure 43).235 The Brown group reported a convenient preparation of alkyl boronic acids and esters by the hydroboration of alkenes with dibromoborane-dimethylsulfide, followed by treatment with water or alcohols (Figure 43).235,243 A recent literature account described the preparation of alkyl trifluoroborates via halomethyltrifluoroborates.244
Figure 43.
Preparation of Common Alkylboron Reagents
3.3. Stability of Alkylboron Reagents
Alkylation reactions can be achieved under Suzuki-Miyaura conditions using alkylboron components as the coupling partner that transfers the alkyl group to the organic framework. One of the leading candidates to serve this purpose is B-alkyl-9-BBN. However, this reagent is not air stable, which makes it relatively difficult to handle, isolate, and purify.245 Typically, cross-coupling reactions with B-alkyl-9-BBN are carried out in situ immediately after hydroboration of the alkene. In addition, carrying out a cross-coupling reaction with alkyl-9-BBN produces significant waste. As an alternative to alkyl-9-BBN, alkylboronic acids, esters, as well as trifluoroborates are extremely useful. They can be easily prepared as described in the previous section and stored (Figure 43). Alkylboronic acids are frequently used in superstoichiometric amounts to achieve satisfactory yields of the cross-coupling products. This is due to the fact that under the cross-coupling conditions, they can decompose either via protodeboration, or β-elimination. The corresponding esters of boronic acids can be employed as coupling partners, but very often they require activation by thallium bases, such as TlOH or Tl2CO3.246 In contrast, potassium organotrifluoroborates are stable under air and moisture indefinitely without any special precautions. Several functionalized organotrifluoroborates can be easily synthesized from various organoboron intermediates by addition of KHF2.244,247
3.4. General Mechanistic Aspects
In 1986, Suzuki and Miyaura reported the cross-coupling between alkylboron reagents and aryl halides.230 Since its disclosure, it has been an attractive solution to challenging synthetic problems and has been used frequently in total synthesis for the construction of complex molecular frameworks.231,232 Like other cross-coupling reactions, this reaction typically proceeds through (1) oxidative addition of aryl, alkenyl or alkyl halides or pseudohalides to a low-valent metal complex; (2) transmetalation with the alkylboron component; and (3) reductive elimination to give the cross-coupling product and metal complex for use in the next catalytic cycle. In most cases, oxidative addition is the turnover limiting step. The electronic character of the alkyl groups as well as the nature of the halide or pseudohalide greatly influences the rate of oxidative addition. Fu and coworkers have shown that the relative rate of oxidative addition of the electrophile is in the order I ≫ Br > OTf ≫ Cl.248
3.4.1. Catalysts for Suzuki-Miyaura Cross-Coupling Reactions
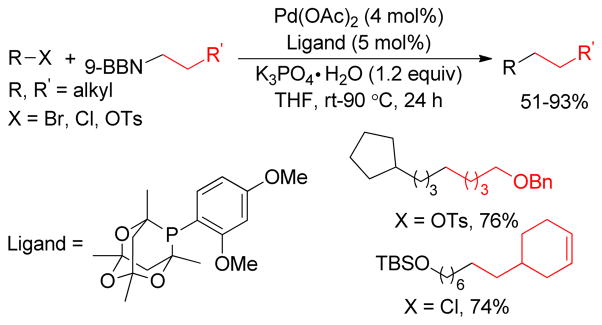
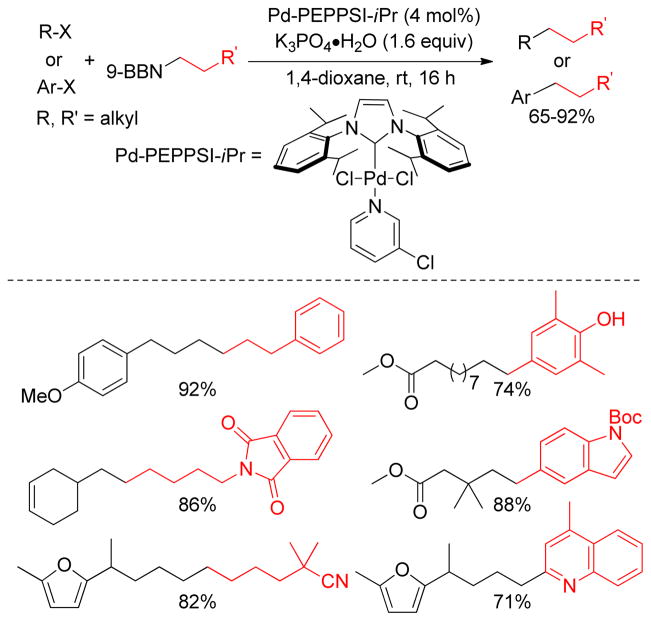
A rigorous literature survey revealed that among all other electrophiles217,249 the alkyl electrophiles16,120 are the most challenging in B-alkyl Suzuki-Miyaura coupling due to the sluggish oxidative addition and deleterious β-hydride elimination. The most frequently used catalysts in B-alkyl Suzuki-Miyaura coupling with aryl, alkenyl, benzyl and allyl halides and triflates are PdCl2(dppf) and Pd(PPh3)4,249 but they have been found to be unsuccessful for alkyl-alkyl couplings. In the last decade, the Fu group has made significant progress in this field using electron-rich and sterically hindered phosphine ligands, as well as replacing Pd- with Ni-catalysts.248 The Buchwald group also developed a series of electron-rich ligands for the Pd-catalyzed Suzuki couplings of various substrates including aryl chlorides and alkylboranes that were previously considered to be an unreactive.250,251
3.4.2. Role of Bases in the Suzuki-Miyaura Cross-Coupling Reaction
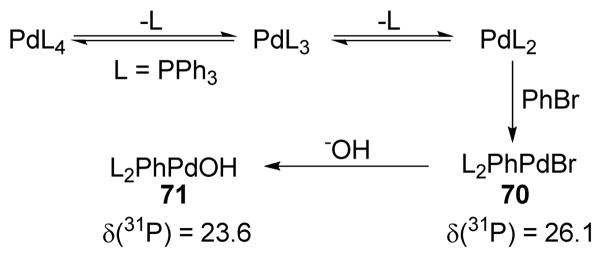
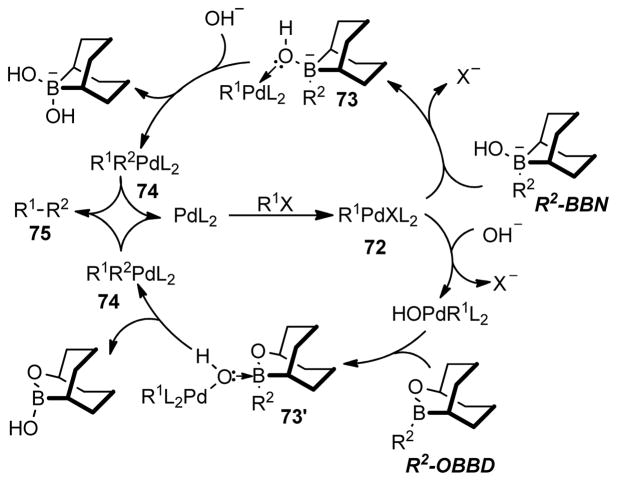
It has been found that the choice of base(s) is crucial in the Suzuki-Miyaura coupling reaction. In their initial reports, the Suzuki group demonstrated the solvent and base dependence of the conversion for different boron derivatives.246,252 Stronger bases such as NaOH, TlOH, and NaOMe perform well in THF/H2O solvent systems, whereas K2CO3 and K3PO4 are more efficient in DMF. Soderquist and coworkers253 have shown that the base is involved in several steps, such as (1) formation of the borate complex, (2) hydrolysis of the RPdIIX intermediate to the more reactive RPdIIOH species, which was evident from 31P NMR spectroscopy (Scheme 15). (The chemical shift in the 31P NMR for Pd(PPh3)4 appears at δ = 18.0 (broad singlet) whereas it shifts to δ = 26.1 after oxidative addition (70)). The adduct is then hydrolyzed by base to form 71 with δ = 23.6) (3) complexation of HOBR2 byproducts that can compete for base with the trialkyl borane, (4) acceleration of the rate of reaction for the OBBD derivatives (Scheme 16), and (5) regeneration of the catalyst.
Scheme 15.
Role of Bases in Suzuki-Miyaura Coupling (Soderquist and coworkers, 1998)
Scheme 16.
Modified Suzuki-Miyaura Catalytic Cycle (Soderquist and coworkers, 1998)
Based on Soderquist’s study, it was proposed that the oxidative addition of phenyl bromide is the rate limiting step in the case of alkyl-9-BBN derivatives. The halide ion is displaced by the base to form a hydroxo μ2-bridged intermediate 73. The coordination of the Pd species with the hydroxyl group of the intermediate 73 accelerates the transmetalation with boron with retention of configuration through a four-centered transition state (Scheme 18). After fast reductive elimination, the cross-coupling product is formed. In contrast, the formation of PdPh(PPh3)2OH is rate-limiting in the case of less Lewis acidic OBBD-derivatives. The reaction proceeds through the formation of the μ2-bridged species 73′ that collapses spontaneously to 74, which on reductive elimination gives the cross-coupling product. Thus, a more detailed catalytic cycle for the B-alkyl Suzuki coupling was proposed, which demonstrates the crucial role of base and boron derivatives (Scheme 16).
Scheme 18.
Mechanism of Transmetalation (Soderquist and coworkers, 1998)
3.4.3. Boron Derivatives in the Suzuki-Miyaura Cross-Coupling Reactions
Generally, unhindered electron-rich organoboranes and electron-deficient vinyl or aryl halides or triflates are the most efficient reaction partners for the B-alkyl Suzuki reaction. Although 9-BBN-H is the most commonly used hydroborating agent, disiamylborane and dicyclohexylborane are also used in this reaction. The rate of transmetalation of a primary alkyl group on boron is much higher than that of a secondary alkyl group. The coupling reactions with secondary alkylborons provide only moderate yields (Table 3).246,252
Table 3.
Suzuki Coupling of Iodobenzene with Alkyl Boranesa
| Borane | Baseb | Solvent | Yield (%) |
|---|---|---|---|
| n-Oct-9-BBN | NaOH | THF/H2O | 99 |
| n-Oct—B(Sia)2 | NaOH | THF/H2O | 82 |
| NaOH | THF/H2O | 93 | |
| (n-Oct)3B | NaOH | THF/H2O | 98 |
| KOH | THF/H2O | 40 | |
| KOH | THF/H2O | 65 | |
| KOH | THF/H2O | 55 | |
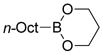 |
NaOH | THF/H2O | 1 |
| TIOH | THF/H2O | 75 | |
| TI2CO3 | THF | 60 | |
| TIOH | benzene/H2O | 93 | |
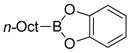 |
KOH | THF/H2O | trace |
| TIOH | THF/H2O | 41 | |
| TI2CO3 | THF | 93 | |
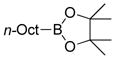 |
TIOH | THF/H2O | 34 |
| TI2CO3 | THF | trace | |
| n-Oct—B(OH)2 | TIOH | THF/H2O | trace |
3 mol% PdCl2(dppf) was used at 50 °C,
3 equiv of NaOH, KOH, TlOH and 1.5 equiv of Tl2CO3 were used.
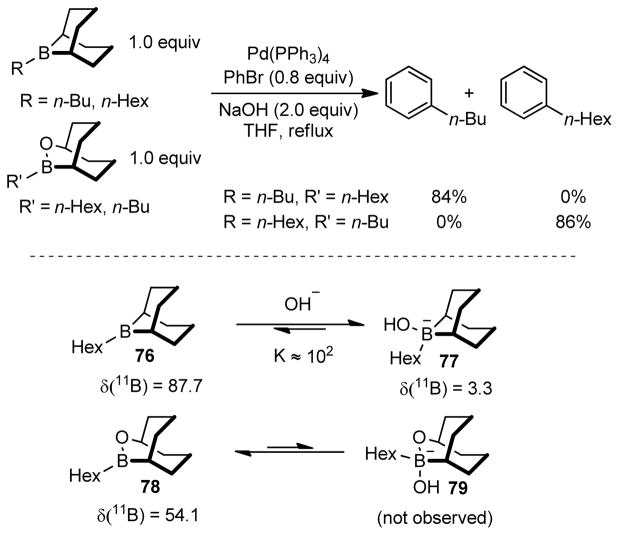
The Soderquist group observed differences in rate when varying the structure of boron reagents. The B-alkyl-9-BBN derivative was found to be significantly more reactive towards bromobenzene than the corresponding B-alkyl-OBBD counterpart.253 Their different Lewis acidities accounted for the observed reactivity, which was examined in the study of borane-borate equilibria. The boranes were titrated with NaOH and the changes in chemical shift in their 11B NMR spectra were recorded (Figure 44). The corresponding chemical shift of 9-BBN-H (76) was shifted from δ = 87.7 to δ = 12.0, δ = 6.0 and δ = 3.3 upon the addition of one, two, and three equivalents of NaOH respectively. No change in chemical shift was observed upon further addition of NaOH. In contrast, there was no observable change in 11B chemical shift of the OBBD counterpart (78) upon treatment with NaOH. These results clearly indicate that the 9-BBN derivative is much more Lewis acidic than OBBD. Therefore, the borate of the corresponding 9-BBN participates in the transmetalation step, whereas OBBD-derivatives undergo transmetalation without further complex formation with base (Scheme 16).
Figure 44.
Rate-Dependence on Boron Derivatives (Soderquist and coworkers, 1998)
Soderquist and coworkers also observed differences in reaction kinetics under Suzuki coupling conditions. For the reaction of PhBr with [(HO)Bu-9-BBN]−, a first-order rate dependence on [PhBr] was observed, indicating that the oxidative addition was the turnover-limiting step. In contrast, a first-order dependence on base and zero-order dependence on [PhBr] and [Bu-OBBD] was observed for Bu-OBBD derivative. Therefore, the hydrolysis of [BrPdPh(PPh3)2] is the proposed turnover-limiting step in this case (Figure 44).
3.4.4. Stereochemistry of Oxidative Addition
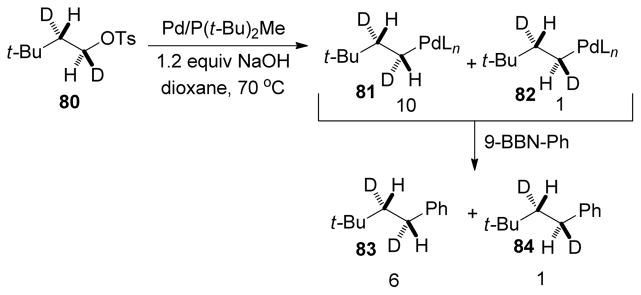
The stereochemistry at the oxidation step in the B-alkyl Suzuki-Miyaura coupling is highly dependent on the metal complexes used for the cross-coupling. Recently, Fu and coworkers demonstrated that the oxidative addition process in Pd-catalyzed Suzuki-Miyaura cross-couplings proceeds predominantly via inversion of configuration.19 In their mechanistic investigation (Scheme 17), the diastereomerically pure tosylate 80 was subjected to the optimized reaction conditions without alkyl-9-BBN. From NMR analysis, it was determined that the oxidative addition was predominantly associated with inversion of configuration [inversion (81)/retention (82) = 10:1], and the primary kinetic isotope effect for the subsequent β-hydride elimination was found to be kH/kD = 3. In the next step, Ph-9-BBN was added to the reaction mixture and the cross-coupling products (83, 84) were obtained with inversion of configuration, indicating retention of configuration at the reductive elimination step.19
Scheme 17.
Stereochemistry of the Pd-Catalyzed Oxidative Addition of Alkyl Tosylates (Fu and coworkers, 2002)
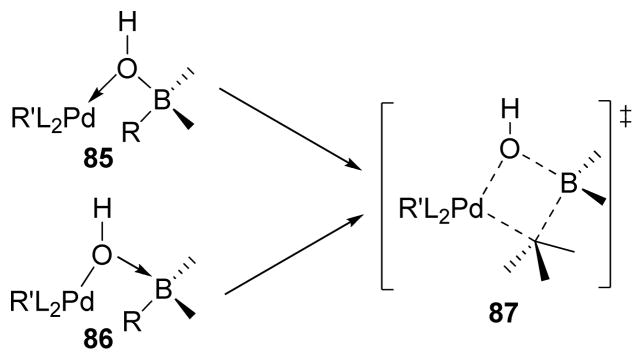
3.4.5. Stereochemistry of Transmetalation
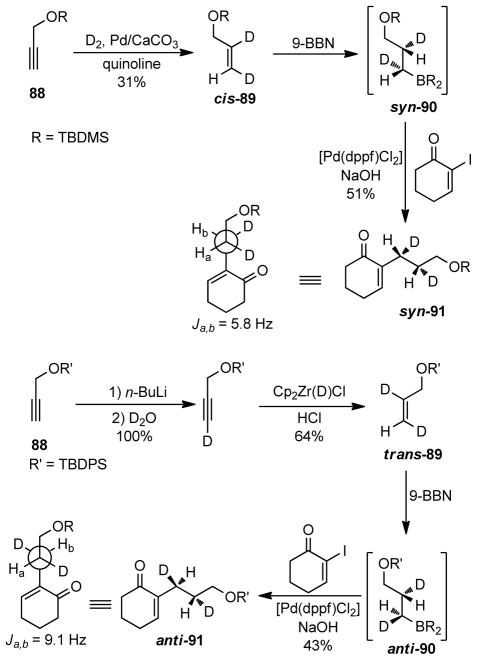
Woerpel254 and Soderquist253 independently studied the stereochemistry of transmetalation in the B-alkyl Suzuki-Miyaura coupling. The Soderquist group proposed that the transmetalation proceeds with retention of configuration through the formation of a four-membered cyclic transition state 87 (Scheme 18).253 From deuterium-labeling experiments, the Woerpel group also showed that transmetalation to Pd proceeds with retention of configuration (Scheme 19).254 In this experiment, diastereomeric dideuterioalkenes cis-89 and trans-89 were synthesized, and underwent hydroboration followed by Pd-catalyzed cross-coupling with α-iodocyclohexenone. The stereochemistry of the corresponding coupling products was assigned by 1H NMR coupling constants (for syn isomer Jab = 5.8 Hz, anti isomer Jab = 9.1 Hz). The syn-coupling product (syn-91) was obtained from the cis-90 isomer, whereas the trans-coupling product (trans-91) was obtained from the corresponding trans-90. As the hydroboration is a syn-addition process, it was concluded that transmetalation in B-alkyl Suzuki-Miyaura cross-coupling proceeds through retention of configuration, which is in agreement with Soderquist’s findings.
Scheme 19.
Stereochemistry in Suzuki-Miyaura Couplings (Woerpel and coworkers, 1998)
3.4.6. Choice of Bases and Boron Substituents in Suzuki-Miyaura Couplings
Various alkylboranes have been used in the B-alkyl Suzuki-Miyaura cross-coupling reactions (See Table 3). 9-BBN-derivatives are the most commonly used. Different bases are used to activate them, and no reaction is observed in the absence of base.246,252 Therefore, selection of a suitable base is crucial to obtain high yields. The Kishi group first observed rate-enhancing effect of thallium salts in the C(sp2)-C(sp3) Suzuki reactions.255 In their early reports, Suzuki and Miyaura also observed a dramatic effect of different bases with different boron compounds. Therefore, thallium salts were found to be important for several Suzuki-Miyaura coupling reactions.256–258
3.5. B-Alkyl Suzuki-Miyaura Cross-Coupling with Alkyl Boranes
3.5.1. B-Alkyl Suzuki-Miyaura Cross-Coupling with Aryl and Alkenyl Halides
Generally, aryl or alkenyl iodides or bromides are the most frequently used coupling partners in Suzuki-Miyaura cross-couplings,249 whereas aryl or alkenyl chlorides have traditionally been less common due to their low reactivity. However, the development of electron-rich and sterically hindered catalysts has overcome the slow oxidative addition of these substrates and made these cross-coupling reactions more effective.16
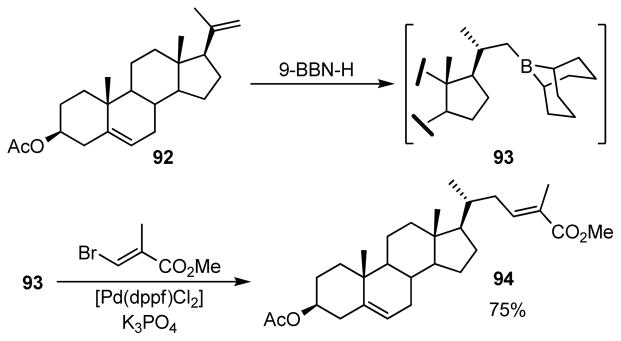
In their seminal report in 1989, Suzuki and Miyaura reported the cross-coupling reaction of B-alkyl-9-BBNs readily generated in situ from the corresponding alkenes and 9-BBN-H with a range of aryl and alkenyl halides, performed in the presence of a catalytic amount of PdCl2(dppf) and base, such as sodium hydroxide, potassium carbonate, and phosphates.252 High stereoselectivity was observed for the alkene products obtained from the reaction of alkenyl halides and B-alkyl boranes. Several common functional groups were found to be compatible under these mild reaction conditions. The stereoselective hydroboration of a C20-C21-methylene steroid 92 with 9-BBN-H produced predominantly C21-boryl steroid 93. This underwent cross-coupling with ethyl (E)-β-bromomethacrylate to give 94 in 75% yield (Scheme 20). Interestingly, the hydroboration occurred chemoselectively at the less hindered C19-C20 double bond in the presence of C5–C6 double bond.
Scheme 20.
Suzuki-Miyaura Coupling with a Steroidal Moiety (Suzuki and coworkers, 1989)
The Buchwald group developed an exceptional Pd catalyst for the Suzuki-Miyaura cross-coupling of challenging aryl halides (Scheme 21).259 An electron-rich biaryl ligand 95 was designed to provide the necessary steric interactions and electron density. Furthermore, X-ray analysis suggests an important interaction of the ipso-carbon of the arene with Pd. Employing this ligand, cross-coupling between sterically hindered aryl halides and aryl boronic acids or alkylboranes was accomplished with low catalyst loadings.259
Scheme 21.
Suzuki-Miyaura Coupling with Electron-rich and Sterically Hindered Aryl Bromides (Buchwald and coworkers, 2004)
The Taylor group demonstrated the synthesis of non-natural amino acids via B-alkyl Suzuki-Miyaura cross-coupling (Figure 45).260 The precursor olefin for the hydroboration was synthesized by Wittig olefination of Garner’s aldehyde. The alkylboranes formed in situ underwent cross-coupling with alkenyl or aryl halides in the presence of K3PO4 and a catalytic amount of PdCl2(dppf). The coupling products could easily be converted to the corresponding amino esters via Jones oxidation followed by methylation with diazomethane.
Figure 45.
Suzuki-Miyaura Coupling on Amino Acid Synthons (Taylor and coworkers, 1999)
Johnson and coworkers subsequently reported the synthesis of nonproteinogenic α-amino acids and amino alcohols following a similar protocol (Figure 46).261 In this case, it was observed that a catalytic amount of Pd(PPh3)4 was more efficient than PdCl2(dppf). Aryl triflates were found to be excellent coupling partners under these reaction conditions, whereas in the Taylor’s report, they were inefficient and only a small amount (11%) of the coupling product was isolated. All coupling products were converted to their corresponding amino acids using various oxidation protocols. Following the same procedure, Overman and Kamatani reported the synthesis of phenethyl and homoallylic amines from protected vinyl amines and aryl or alkenyl halides, respectively.262
Figure 46.
Suzuki-Miyaura Coupling on Amino Acid Synthons (Johnson and coworkers, 2000)
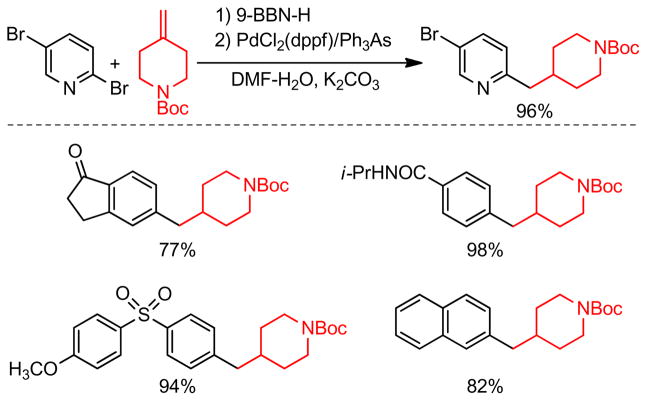
A B-alkyl Suzuki-Miyaura coupling procedure for the synthesis of biologically active 4-benzyl piperidines and related substances was reported by Vice and coworkers (Figure 47).263 The hydroboration of N-Boc-4-methylene piperidine, followed by reaction with aryl bromides, iodides, and triflates in the presence of a catalytic amount of PdCl2(dppf)/AsPh3 produced a series of biologically relevant coupling products. Interestingly, 2,5-dibromopyridine underwent cross-coupling regioselectively at the 2-position, producing the corresponding coupling product in high yield.
Figure 47.
Cross-Coupling with N-Boc-4-methylene Piperidines (Vice and coworkers, 2001)
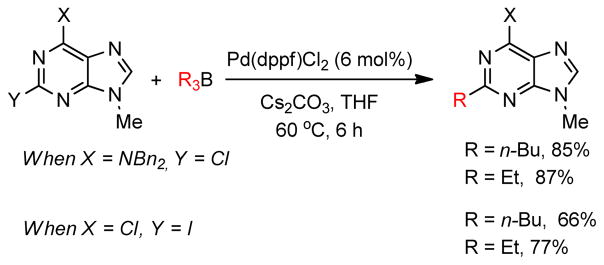
A Pd-catalyzed direct alkylation of 2-halopurines with Et3B or Bu3B was developed by Piersanti and coworkers (Scheme 22).264 The alkylation of these particular substrates was challenging but highly desirable, as it offers a concise route for the synthesis of ST1535, a potent adenosine A2A receptor antagonist. Fortunately, applying a B-alkyl Suzuki-Miyaura cross-coupling in the presence of Cs2CO3 and catalytic Pd (dppf)Cl2 in THF afforded 2-alkylated purines in high yields. A comparative study with other organometallics revealed that, with the exception of tetra-n-butyltin, no other organometallics, including n-butylboronic acids, were successful in producing the desired product.
Scheme 22.
Alkylation of 2-halopurines (Piersanti and coworkers, 2010)
Tan and coworkers developed a flexible and efficient method to convert glycals to C1-substituted glycals using a B-alkyl Suzuki-Miyaura cross-coupling approach (Figure 48).265 A wide range of C1-iodo substituted glycals were alkylated directly with a variety of in situ generated alkylboranes in the presence of NaOH (aq) and a catalytic amount of Pd(dppf)Cl2. It was observed that upon preincubation of the alkylborane with base prior to the addition of a mixture of C1-iodoglycal and Pd(dppf)Cl2, the Pd-mediated reduction of the alkene iodide product was substantially suppressed.
Figure 48.
Suzuki Coupling on C1-iodo-glycals (Tan and coworkers, 2004)
Interestingly, when 1,1-dichloro-1-alkenes were cross-coupled with B-alkyl-9-BBN, the chloride trans to the alkene chain reacted preferentially (Figure 49).266 A detailed ligand study was performed, which showed that the larger bite angle of the ligand resulted in an improved ratio of the monoalkylation product. Thus, moving from dppb (∠P-Pd-P = 98°) to DPEPhos (∠P-Pd-P = 102°), the yield as well as selectivity were dramatically improved. Finally, using XantPhos (∠P-Pd-P = 111°) in combination with Pd2(dba)3 and K3PO4-KF as base couple afforded excellent yields of the monoalkylated products. The stereoselectivity was also very good in most cases (Z/E = 90/10-98/2). Interestingly, when the monoalkylation product was subjected to further alkylation with Pd(dppp)Cl2 or 2-(dicyclohexylphosphino)biphenyl, the unsymmetrical dialkylation product was obtained in good yields (Figure 50).266
Figure 49.
Suzuki Coupling for Selective Monoalkylations (Roulland and coworkers, 2007)
Figure 50.
Suzuki Coupling for the Synthesis of Trisubstituted Olefins (Roulland and coworkers, 2007)
3.5.2. Cross-Coupling with Aryl and 1-Alkenyl Triflates
Aryl or 1-alkenyl triflates are also an efficient class of coupling partners in B-alkyl Suzuki-Miyaura cross-coupling reactions. Suzuki and coworkers first reported that 1-alkenyl or aryl triflates underwent cross-coupling reactions with various alkylboranes in the presence of K3PO4 and a catalytic amount of Pd(PPh3)4 or PdCl2(dppf) in dioxane in high yields.252 Alkylboranes containing several functional groups, derived in situ from the reaction of the corresponding alkene with 9-BBN-H were also compatible under the reaction conditions.
Narukawa and coworkers reported an efficient synthesis of 2-alkyl carbapenems via Pd-catalyzed alkylation of carbapenem-2-yl triflate with B-alkyl-9-BBN (Figure 51).267 The alkylboranes were prepared in situ by reaction of 9-BBN-H with an alkene. Since the hydroboration occurs stereoselectively from the less hindered side of the olefin, the alkylborane obtained from the corresponding chiral olefin gave a single isomer that was incorporated into the carbapenem moiety without racemization through the subsequent cross-coupling reaction.
Figure 51.
Suzuki Coupling in Carbapenem Analog Synthesis (Narukawa and coworkers, 1997)
The Pd-catalyzed B-alkyl Suzuki coupling has been successfully employed for the construction of the trans-fused polyether framework that constitutes the core of several natural products.268 Sasaki and coworkers reported that cyclic vinyl triflates and a highly functionalized B-alkyl-9-BBN underwent cross-coupling in the presence of Cs2CO3, KBr, and a catalytic amount of Pd(dppf)Cl2/AsPh3 (Figure 52).269
Figure 52.
Suzuki Coupling in Polyether Synthesis (Sasaki and coworkers, 1998)
The Stevens group applied the B-alkyl Suzuki-Miyaura cross-coupling reaction in the synthesis of functionalized 8-methylquinoacridines, which were methylated with methyl iodide to generate telomerase-inhibitors, 8,13-dimethylquinoacridinium iodides (Scheme 23). Alkylboranes were prepared in situ by reaction of 9-BBN-H with allyl acetate or N-allyltrifluoroacetamide, which was subsequently treated with chloro- and triflate-substituted 8-methylquinoacridines. The cross-coupling occurred chemoselectively with the triflate functionality leaving the chloride intact when Cs2CO3 and catalytic amount of Pd(OAc)2 and PPh3were used.270
Scheme 23.
Suzuki Coupling in Quinoacridine-derivative Synthesis (Stevens and coworkers, 2003)
An interesting example of Suzuki-Miyaura cross-coupling between arenediazonium o-benzenesulfonamides and alkylboranes was reported by the Dughera group.271 In this study, arenediazonium o-benzenesulfonamides were reacted with triethyl- or tri-n-butylborane in the presence of Pd(OAc)2, Pd(PPh3)2Cl2, or Pd(dppf)Cl2 to afford ethyl or n-butyl arenes in high yields (Figure 53). No base was required for this transformation. It was observed that although high yields of the cross- coupling products were obtained using tetramethyltin reagents, ethyl or n-butyl analogues produced mainly protonated arenes via β-hydride elimination.
Figure 53.
Suzuki Coupling with Arenediazonium o-benzenesulfonamides (Dughera and coworkers, 2008)
3.5.3. Intramolecular Suzuki-Miyaura Cross-Coupling and Macrocyclization
An interesting extension of the Suzuki-Miyaura cross-coupling process is the formation of five- or six-membered rings via intramolecular cross-coupling.252 The Suzuki group first reported the intramolecular reaction of aryl or alkenyl halides with B-alkyl-9-BBN derivatives to form five- and six-membered rings. However, the authors were unable to construct larger rings through this protocol due to oligomerization of the coupling partners at high substrate concentration. Compounds containing a vinyl halide and a terminal olefin linked through a two- or three-carbon tether can also be cyclized to form five- and six-membered exocyclic alkenes (Table 4 and 5).272
Table 4.
Cyclization of Haloalkenes via Intramolecular Suzuki Coupling (Suzuki and coworkers, 1989)
| Entry | Haloalkene | Product | Yield (%) |
|---|---|---|---|
| 1 |  |
 |
86 |
| 2 |  |
 |
66 |
| 3 |  |
 |
68 |
| 4 |  |
 |
70 |
| 5 |  |
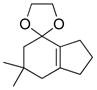 |
84 |
Hydroboration was carried out with 9-BBN-H in THF at 0 °C-rt. for 4 h then cross-coupled intramolecularly in the presence of Pd(dppf)CI2 (1.5 mol%) and NaOH (3.0 equiv).
Table 5.
Synthesis of Exocyclic Alkenes (Suzuki and coworkers, 1992)a
| Entry | Haloalkene | Product | Yield (%) |
|---|---|---|---|
| 1 | 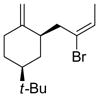 |
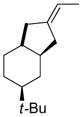 |
68 |
| 2 | 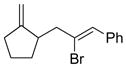 |
 |
83 |
| 3 |  |
 |
51 |
| 4 |  |
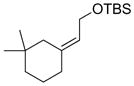 |
60 |
Hydroboration was carried out with 9-BBN-H in THF at 0 ºC-rt. for 4 h then cross-coupled intramolecularly in the presence of Pd(dppf)Cl2 (1.5 mol%) and NaOH (3.0 equiv).
Soderquist and coworkers extended this idea when they performed tandem cross-coupling reactions with symmetrical bis-boranes and geminal dibromides (Figure 54).273 The intermolecular followed by intramolecular cross-couplings produced six-membered ring systems with exocyclic double bonds in moderate to good yields. The construction of five-membered rings was unsuccessful using this protocol.
Figure 54.
Formation of Six-Membered Rings with Exocyclic Double Bonds (Soderquist and coworkers, 1995)
Applying a high dilution technique (0.003 M), macrocyclization was achieved via intramolecular B-alkyl Suzuki-Miyaura cross-coupling with substrates containing aryl or alkenyl iodides and terminal double bonds. The double bond was converted to the corresponding terminal B-alkyl component in situ for subsequent cross-coupling in the presence of Cs2CO3 and a catalytic amount of Pd(dppf)Cl2/AsPh3 in THF (Table 6).258 The Halcomb group accomplished the total synthesis of (+)-phomactin via late stage B-Alkyl Suzuki macrocyclization.274
Table 6.
Macrocyclization via Suzuki-Miyaura Coupling (Danishefsky and coworkers, 2000)a
| Entry | Haloalkene | Product | Yield (%) |
|---|---|---|---|
| 1 | 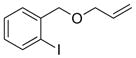 |
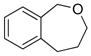 |
22 |
| 2 | 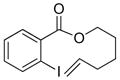 |
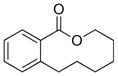 |
23 |
| 3 | 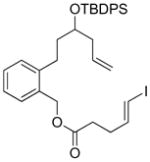 |
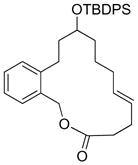 |
41 |
| 4 | 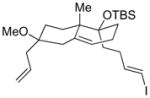 |
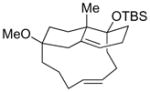 |
40, 60b |
| 5 | 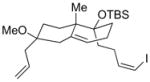 |
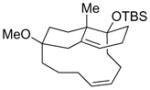 |
46 |
Hydroboration was carried out with 9-BBN-H in THF at 23 °C-rt for 1.5 h then cross-coupled (slow addition for 5 h) intramolecularly in the presence of Pd(dppf)Cl2 (20 mol%), AsPh3 (20 mol%), Cs2CO3 (3.0 equiv) and H2O (40 equiv) in THF:DMF (10:1) (0.003 M).
H2O (5 equiv) and preincubation with TlOEt (3 equiv).
3.5.4. Carbonylative Suzuki-Miyaura Cross-Coupling Reactions
In 1991, Suzuki and coworkers reported a carbonylative cross-coupling for the synthesis of α,β-unsaturated ketones from alkenyl halides and B-alkylboranes using a catalytic amount of Pd.275 In the same year, they reported the synthesis of unsymmetrical ketones via carbonylative cross-coupling between alkyl iodides and alkylboranes under an atmosphere of carbon monoxide (Figure 55). It was found that the reaction was accelerated by visible light, which could be due to the involvement of a radical process in the oxidative addition step.276 Tan and coworkers reported a carbonylative Suzuki-Miyura cross-coupling for the synthesis of C1-substituted glycals from the corresponding iodo-glycals (Scheme 25).265
Figure 55.
Carbonylative Suzuki-Miyaura Couplings
Scheme 25.
Carbonylative Suzuki Coupling of 2-iodo-glycal (Tan and coworkers, 2004)
3.5.5. Suzuki-Miyaura Alkyl-Alkyl Cross-Coupling Reactions
As in many other cross-coupling reactions, alkyl electrophiles are the most problematic substrates, and difficulties are especially exasperated in C(sp3)-C(sp3) couplings. In 1992, the Suzuki group first reported the C(sp3)-C(sp3) couplings using B-alkyl-9-BBN and alkyl halides (Figure 56). In a previous report, rate acceleration was observed for the carbonylative cross-coupling reaction in the presence of light,276 which suggested that the oxidative addition to the alkyl halide proceeds through a radical process. Although there was no induction of light in the cross-coupling of 9-octyl-9-BBN with 6-iodo-1-hexene, the formation of nonylcyclopentane as a byproduct along with the cross-coupling product suggests a radical pathway.277 To explore the effect of organometallic reagents, a series of n-butyl organometallic reagents were subjected to cross-coupling with 1-iododecane under the optimized reaction conditions, and 9-butyl-9-BBN was found to be the best coupling partner, providing 50% of the desired product.
Figure 56.
Radical Oxidative Addition (Suzuki and coworkers, 1992)
Fu and coworkers have made substantial progress in the field of alkyl-alkyl Suzuki-Miyaura cross-coupling. Exploiting PCy3 as the ligand, the oxidative addition of the Pd complex to alkyl halides was accelerated and cross-couplings with B-alkyl-9-BBN were accomplished at room temperature. Gratifyingly, it was discovered that Pd(OAc)2/PCy3 (1:2) in the presence of K3PO4•H2O provided cross-coupling products in high yields (Figure 57).278 Besides the mild reaction conditions, triumph over β-hydride elimination, which is a major challenge of using alkyl electrophiles bearing β-hydrogens, was a major achievement of this protocol. Under a slightly modified catalytic combination of Pd2(dba)3 and PCy3 in the presence of CsOH•H2O, alkyl-alkyl Suzuki-Miyaura cross-couplings with more challenging alkyl chlorides were also accomplished at elevated temperature (90 °C) in dioxane (Figure 58).279
Figure 57.
Alkyl-Alkyl Suzuki-Miyaura Couplings with Alkyl Bromides (Fu and coworkers, 2001)
Figure 58.
Alkyl-Alkyl Suzuki-Miyaura Couplings with Alkyl Chlorides (Fu and coworkers, 2002)
Although alkyl bromides and chlorides underwent cross-coupling successfully using PCy3 as the ligand, alkyl tosylates were unreactive using the same reaction conditions. After optimization of the reaction conditions for these substrates, it was observed that a slightly different trialkylphosphine ligand, Pt-Bu2Me, was able to provide cross-coupling products in high yields (Figure 59). During this study, it was observed that the reaction was exceptionally sensitive towards the cone angle of the ligands employed. Finally, a bench-stable trialkyl phosphonium salt, [HPt-Bu2Me]BF4, and Pd(OAc)2 in the presence of NaOH were discovered to be effective for the cross-coupling between alkyl tosylates and B-alkyl-9-BBN.19
Figure 59.
Alkyl-Alkyl Suzuki Couplings with Alkyl Tosylates (Fu and coworkers, 2002)
The Capretta group synthesized a series of phosphaadamantane ligands via P-arylation of 1,3,5,7-tetramethyl-2,4,8-trioxa-6-phosphaadamantane and employed them in Pd-catalyzed cross-coupling reactions. A highly active catalyst combination was found for the alkyl-alkyl Suzuki-Miyaura cross-coupling using alkyl halides and tosylates as electrophiles. Thus, catalytic Pd(OAc)2 in combination with 1,3,5,7-tetramethyl-6-(2,4-dimethoxyphenyl)-2,4,8-trioxa-6-phosphaadamantane in the presence of K3PO4•H2O facilitated the C(sp3)-C(sp3) coupling in high yields (Figure 60).280
Figure 60.
Alkyl-alkyl Suzuki-Miyaura Couplings with Alkyl Halides (Capretta and coworkers, 2004)
Caddick and coworkers first reported the use of N-heterocyclic carbenes in a Pd-catalyzed alkyl-alkyl Suzuki-Miyaura coupling. Using this protocol, alkyl bromides were treated with B-alkyl-9-BBNs to provide cross-coupling products in modest yields (Figure 61).281 Later, a modified NHC-based catalyst, Pd-PEPPSI-IiPr, was used to obtain high yields from the corresponding alkyl bromides and B-alkyl-9-BBN (Figure 62).282,283
Figure 61.
Alkyl-Alkyl Suzuki Coupling with Alkyl Iodides (Caddick and coworkers, 2004)
Figure 62.
Alkyl-alkyl Suzuki couplings with alkyl halides (Organ and coworkers, 2008)
The use of secondary alkyl halides as the electrophile in cross-coupling reactions is extremely rare due to their unreactive nature. Fu and coworkers were able to accomplish this challenging transformation, utilizing a Ni-catalyzed Suzuki-Miyaura cross-coupling. It was found that a catalytic amount of NiCl2•glyme, in combination with commercially available trans-N,N′-dimethyl-1,2-cyclohexanediamine in the presence of KOt-Bu and i-BuOH at room temperature is the best catalytic system for this reaction (Figure 63).284 Thus, a series of B-alkyl-9-BBN was cross-coupled with a wide variety of secondary alkyl bromides under the optimized reaction conditions affording high yields. From a 11B NMR study, it was confirmed that KOt-Bu and i-BuOH activate the alkylborane for transmetalation with Ni via the formation of a tetravalent ate complex, which is in good agreement with Soderquist’s earlier observation.253 Recently, the same group reported that after modification of the Ni-source, ligand, and the solvent that unreactive secondary alkyl chlorides also underwent Suzuki coupling at room temperature providing high yields.285
Figure 63.
Alkyl-Alkyl Suzuki-Miyaura Couplings with Secondary Alkyl Bromides (Fu and coworkers, 2007)
In summary, alkyl-alkyl Suzuki-Miyaura cross-couplings have been successfully accomplished using several metal complexes and electron-rich as well as sterically hindered ligands. The electron-rich metal center generally overcomes slow oxidative addition to alkyl halides and steric crowding of the ligands provides the necessary interactions to stabilize the metal center and suppress β-hydride elimination. In addition to primary alkyl halides, secondary alkyl halides were also successfully coupled with alkylboranes. Ultimately, the use of bench stable ligands for such challenging cross-coupling reactions has made the protocols accessible to the entire community.
3.5.6. B-Alkyl Suzuki-Miyaura Cross-Coupling for the Synthesis of Ketones and Amides
The direct ketone or amide formation from organometallic reagents with acid chlorides, activated esters or potentially formamide derivatives is an attractive method owing to its simplicity and reliability. However, organometallic reagents used for this approach have been limited mainly to magnesium, lithium, or alkylzinc reagents (Fukuyama coupling).180 The use of less reactive alkylboron reagents for these transformations is highly desirable due to their high functional group tolerance.
The Liebeskind group reported the synthesis of ketones from activated thioesters via C-S bond scission and C-C bond formation. Coupling of thiol esters and B-alkyl-9-BBN derivatives was achieved in the presence of stoichiometric Cu(I)-thiophene-2-carboxylate (CuTc) and catalytic Pd(PPh3)4 (Figure 64). Unlike in the case of boronic acids, B-alkyl-9-BBN required only activation by base for this transformation, and no oxygen source was found to be necessary. A series of alkyl-alkyl and aryl-alkyl ketones were prepared in high yields from the corresponding thioesters.286
Figure 64.
Formation of Ketones from Thioesters (Liebeskind and coworkers, 2004)
The Takemoto group reported a one pot amidation of alkenes via Pd-catalyzed coupling of carbamoyl chlorides with alkylboranes derived from the in situ reaction of alkenes and 9-BBN-H in a highly stereoselective manner (Figure 65). A series of functionalized alkylboranes was prepared and reacted to afford good to excellent yields.287
Figure 65.
Formation of Amides from Carbamoyl Chlorides (Takemoto and coworkers, 2007)
3.6. Suzuki-Miyaura Cross-Coupling Reactions with Alkylboronic Acids
Alkyl or arylboronic acids exist in equilibrium with their trimeric cyclic anhydrides, boroxines.288 The equilibrium has some influence on the coupling process, but it is difficult to determine the concentrations of boronic acid and boroxine during the catalytic reactions. Furthermore, boronic acids can degrade via hydrolysis or protonolysis. Consequently, most of the Suzuki-Miyaura coupling protocols employ superstoichiometric amounts of boronic acids to ensure complete conversion of the electrophilic coupling partner. Advantages of using organoboronic acids in cross-coupling reactions are the ease of purification via recrystallization prior to reaction, compatibility with air and moisture, and generally long shelf-life.
3.6.1. Suzuki-Miyaura Coupling with Primary Alkylboronic Acids
In 1989, Suzuki and coworkers reported the cross-coupling between alkyl boronic esters with 1-alkenyl or aryl halides in the presence of thallium hydroxide or thallium carbonate and catalytic PdCl2(dppf) or Pd(PPh3)4 (Figure 66).246 A serious limitation of this protocol is the use of toxic thallium bases that was found to be crucial, as in their absence extremely low conversion was observed. In search for alternative bases, Falck and coworkers found that high yields of the cross-coupling products with alkylboronic acids and aryl bromides were achieved using PdCl2(dppf) as a catalyst and K2CO3 as a base. The reaction unfortunately required a superstoichiometric (2.5 equiv) amount of Ag2O as an additive.13 In the same year, Bellina and coworkers also reported the regioselective cross-coupling of 3,4-dibromo-furanones with alkylboronic acids in the presence of 5 mol% PdCl2(MeCN)2, 20 mol% AsPh3 and 3.0 equiv of Ag2O to afford the corresponding 4-alkylation product.289
Figure 66.
Suzuki-Miyaura Coupling of Alkylboronic Acids (Falck and coworkers, 2001)
In 2002, the Molander group developed reaction conditions for the Suzuki-Miyaura cross-coupling with phenethylboronic acids and aryl triflates or halides, which do not require Ag2O. A catalytic amount of Pd(dppf)Cl2•CH2Cl2 in combination with 3.0 equiv K2CO3 in THF/H2O furnished the cross-coupling products in high yields (Figure 67).290 Electron-deficient aryl triflates led to high yields while electron-rich triflates were less effective.
Figure 67.
Suzuki Coupling of Phenethylboronic Acids (Molander and coworkers, 2002)
To overcome the problem of slow oxidative addition to electron-rich aryl triflates and chlorides, the Hartwig group developed an air stable, sterically-hinderedferrocenyl dialkylphosphine ligand for Pd-catalyzed cross-coupling reactions.291 With this catalyst, a series of alkyl boronic acids were coupled successfully with various aryl chlorides and bromides. Gratifyingly, the cross-coupling of electron-rich, 2-chloroanisole with n-butylboronic acid catalyzed by a 1:1 mixture of Pd(dba)2 and the ferrocenyl ligand in the presence of K3PO4 also afforded high yields at 100 °C in toluene (Figure 68). A secondary boronic acid was coupled with p-t-butylbromobenzene to afford the unisomerized coupling product in moderate yield, although the secondary alkylboronic acids are prone to isomerize to the linear form via β-hydride elimination and reinsertion. A similar ferrocene-based ligand and reaction protocol was reported by the Chan and Kwong group.292
Figure 68.
Suzuki Coupling with Aryl Halides (Hartwig and coworkers, 2002)
Phosphite ligands have also been used in B-alkyl Suzuki-Miyaura cross-coupling reactions. The bulky ligands undergo facile orthopalladation in situ, which forms an active species for the cross-coupling reactions. Therefore, a catalytic amount (0.5 mol%) of Pd(OAc)2 and P(OC6H3-2,4-t-Bu2)3 in the presence of K3PO4 constituted effective conditions to carry out the cross-coupling between electron-rich 4-bromo anisole and n-butylboronic acid (Figure 69).293 High turnover numbers were observed when electron-deficient aryl bromides, such as 4-bromoacetophenone (TON = 10 000), was used. An indolyl phosphine ligand coordinated to Pd2(dba)3 catalyzed the same reaction to afford excellent yields.294
Figure 69.
Suzuki Coupling with Phosphite Ligands (Bedford and coworkers, 2003)
Cross-coupling reactions in aqueous media are often desirable in the field of organic and organometallic chemistry, as they provide a manifold to develop more environmentally benign protocols for the incorporation of desired functionality. To perform a reaction in an aqueous medium, water soluble ligands, as well as suitable additives, are necessary for optimal performance.295 Nájera and coworkers developed a water soluble di-(2-pyridyl)methylamine-based Pd-dichloride complex for the Suzuki-Miyaura cross-coupling and related reactions in aqueous medium (Figure 70).296 Utilizing this catalyst, trimethylboroxine and n-butylboronic acid were coupled with bromo- and chloroarenes in refluxing water with K2CO3 and Bu4NBr as additives. The reaction was accomplished in a shorter reaction time under microwave irradiation, providing comparable yields.
Figure 70.
Suzuki Coupling in Aqueous Medium (Nájera and coworkers, 2004)
Doucet and coworkers reported the use of [PdCl(C3H5)]2/cis,cis,cis-1,2,3,4-tetrakis(diphenylphosphinomethyl)cyclopentane (tedicyp) in Suzuki-Miyaura cross-coupling reactions of alkylboronic acids with aryl bromides or chlorides (Figure 71).297,298 The low catalyst loading and high turnover number (TON up to 10,000) of this protocol are noteworthy. A wide range of functional groups were well tolerated under the reaction conditions.
Figure 71.
Suzuki Coupling with Tedicyp Ligand (Doucet and coworkers, 2004)
The Buchwald group developed an electron-rich biaryl ligand for general Suzuki-Miyaura cross-coupling reactions.250,251 Ma et al. used the same ligand, along with catalytic PdCl2(PhCN)2, for the cross-coupling of n-butylboronic acids with chlorobenzylidenelactone in refluxing toluene (Scheme 26).299
Scheme 26.
Suzuki Coupling with Chlorobenzylidenelactone (Ma and coworkers, 2006)
Delaude and coworkers tested the cross-coupling between n-butylboronic acid and unprotected 3-bromo-2,4,6-trimethylaniline under Fu’s conditions, using the sterically hindered and electron-rich P(t-Bu)3 ligand along with Pd2(dba)3 (Scheme 27).300 The corresponding cross-coupled product was isolated in 63% yield after refluxing in dioxane for 24 h. Although the yield was far from quantitative, the result was encouraging, considering the steric hindrance imposed by the two methyl groups flanking the bromine as well as the unprotected aniline moiety.
Scheme 27.
Suzuki Coupling of Hindered Aryl Bromides (Delaude and coworkers, 2007)
The Ranu group also reported a simple and efficient ligand-free Suzuki coupling in aqueous medium. The sodium tetrachloropalladate (Na2PdCl4) precatalyst is reduced to Pd(0) by organoboronic acids in aqueous medium to form Pd nanoparticles (PdNPs), which were stabilized by sodium dodecyl sulfate (Figure 72). Using K3PO4 as a base, the cross-coupling reaction between aryl bromides and iodides and alkylboronic acids was accomplished at 100 °C within 25 min.301
Figure 72.
Pd Nanoparticle-Catalyzed Suzuki Couplings (Ranu and coworkers, 2009)
The Fu group has reported a C(sp3)-C(sp3) Suzuki-Miyaura cross-coupling of alkyl halides with alkylboronic acids at room temperature. It was observed that a catalytic amount of Pd(OAc)2/P(t-Bu)2Me in the presence of KOt-Bu afforded alkyl-alkyl cross-coupling products in high yields (Figure 73).302 Interestingly, the oxidative addition of alkyl halides was accomplished under very mild conditions, furnishing intermediates that were stable enough to undergo transmetalation instead of decomposition via β-hydride elimination. One such oxidative addition product 96 was isolated and characterized by X-ray crystallography. When the oxidative addition product 96 was treated with a boronic acid, the cross-coupling product was formed with comparable yield.
Figure 73.
Alkyl-Alkyl Suzuki Couplings with Alkylboronic Acids (Fu and coworkers, 2002)
3.6.2. Suzuki-Miyura Coupling with Secondary Alkylboronic Acids
While primary alkylboronic acids have been explored in Suzuki-Miyaura cross-coupling reactions, secondary alkylboronic acids have been sparsely studied. This is due to the fact that transmetalation of these hindered substrates is extremely slow failing to yield cross-coupling products. In contrast, cyclopropyl boronic acids have been used successfully, as they possess a substantial amount of s-character on the exocyclic C-B bond, and their geometry prevents β-hydride elimination.
Deng and Wang reported Suzuki couplings of trans-2-butylcyclopropylboronic acid with aryl bromides. The reactions gave stereodefined trans-2-cyclopropylarenes in high yields with both electron-rich and electron-deficient aryl bromides.303 Cyclopropaneboronic acid itself has also been coupled with aryl bromides, irrespective of their electronics, in the presence of catalytic Pd(OAc)2 and the electron-rich PCy3 (Figure 74).304
Figure 74.
Suzuki Coupling with Substituted Cyclopropylboronic Acids.
Fu and coworkers reported a Suzuki-Miyaura cross-coupling between cyclopentylboronic acid and p-chlorotoluene (Scheme 28). This challenging transformation was made possible by employing P(t-Bu)3 with catalytic Pd2(dba)3.248
Scheme 28.
Suzuki Coupling with Cyclopentylboronic Acid (Fu and coworkers, 2000)
3.7. Suzuki-Miyaura Cross-Coupling with Alkylboronic Esters
In general, alkylboronic esters are even less reactive than alkylboronic acids and often fail to produce cross-coupling products. Higher catalyst loadings and more forcing reaction conditions are often required for these cross-couplings to proceed. However, alkylboronic esters are important coupling partners in Suzuki coupling reactions, as they can offer neutral reaction conditions (pKa of boronic acid ~ 9), and only the monomeric form is the active species. In addition, high yields of boronic esters are isolated by the simple addition of an alcohol or diol to the boronic acids.
After the first report in 1989 by the Suzuki group, several examples of Suzuki coupling with alkylboronic esters have been reported (Figure 75).246 In 1996, Marsden and Hildebrand reported the stereoselective coupling of a cyclopropylboronate with several p-substituted electron-deficient or electron-rich aryl bromides in the presence of catalytic Pd(PPh3)4 (Figure 76).305 In 1997, Charette and coworkers reported the cross-coupling between cyclopropylboronate and iodocyclopropanes to generate contiguous cyclopropanes (Figure 77).306 These reactions proceed smoothly in the presence of Pd(OAc)2 with PPh3 in DME with t-BuOK as base. In 2001, de Meijere and coworkers reported the same reaction between 2-cyclopropylborolane and aryl halides under identical reaction conditions. Although a wide range of aryl iodides were tolerated and furnished cross-coupling products, electron-deficient p-bromonitrobenzene or methyl-2-iodobenzoate gave no coupling products.307
Figure 75.
Suzuki Coupling with Alkylboronic Esters (Suzuki and coworkers, 1989)
Figure 76.
Suzuki Coupling with Cyclopropylboronic Esters (Marsden and coworkers, 1996)
Figure 77.
Suzuki Coupling with Cyclopropylboronic Esters (Charette and coworkers, 1997)
In line with the previous observations, alkylboronic acids are poorer nucleophiles than alkenyl- and arylboronic acids, and alkylboronic esters are even less reactive in Suzuki-Miyaura cross-couplings. This is demonstrated in the Suzuki-couplings of vinyl triflates derived from N-alkoxycarbonyl lactams and alkylboron derivatives (Table 7).308 An accelerating effect of Ag(I) salt was also observed in these transformations.
Table 7.
Suzuki Coupling with Alkyl Boron Derivativesa (Occhiato and coworkers, 2001)
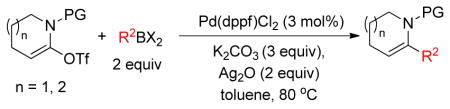 | |||
|---|---|---|---|
| Substrate | Boronic acid or ester | Product | Yield (%) |
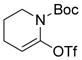 |
 |
88 | |
 |
 |
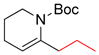 |
0 |
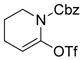 |
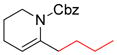 |
93 | |
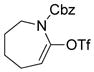 |
 |
22 | |
Andrus and coworkers demonstrated that N,N-bis-(2,6-diisopropylphenyl)dihydroimidazolium chloride with Pd(OAc)2 was an effective catalytic system for the cross-coupling between aryldiazonium tetrafluoroborates and alkyl catecholborane at room temperature in the absence of base.309 The aryldiazoniumtetrafluoroborates were generated in situ by the treatment of aryl amines with t-butyl nitrite followed by BF3•OEt2 at 0 °C in THF (Figure 78). Interestingly, despite having bromine in the alkyl catechol borane moiety, cross-coupling with aryldiazonium salts occurs selectively, leaving the bromine intact. This suggests that oxidative addition of allyl bromide is slow under these reaction conditions, whereas aryldiazonium salts readily furnish cross-coupling products in high yields.
Figure 78.
Suzuki Coupling with Catecholboranes (Andrus and coworkers, 2001)
In the same year, Falck and coworkers demonstrated that when a mixed alkylboronate containing primary and secondary alkyl chains was subjected to Suzuki-Miyaura cross-coupling conditions with aryl bromides or alkenyl triflates, the n-alkylaryl product was isolated in excellent yield (Scheme 29).310 This suggests that the secondary alkyl group undergoes β-hydride elimination and reinsertion to give the n-alkyl isomer under the reaction conditions, followed by a normal cross-coupling reaction. Aryl triflates were also tested with this mixed alkylboronate and similar results were consistently observed.
Scheme 29.
Suzuki Coupling with Mixed Alkylboronic Esters (Falck and coworkers, 2001)
In summary, alkylboronic esters are used as coupling partners less frequently due to their lower reactivity. In many cases, they afford modest yields and sometimes fail to give products, whereas their alkylboronic acid counterparts give better yields of the cross-coupling products under the same reaction conditions.
3.8. Suzuki-Miyaura Cross-Coupling with Alkyltrifluoroborates
Organotrifluoroborates are an interesting class of coupling partners in Suzuki-Miyaura cross-coupling reactions.311 The Molander group has made substantial contributions to their preparation and applications in cross-couplings. Potassium organotrifluoroborates are easily synthesized by addition of KHF2 to various organoboron intermediates, and several functionalized trifluoroborates can be synthesized easily from readily available starting materials. Nearly all organotrifluoborates synthesized to date can be stored indefinitely without special precautions.
In 2001, Molander and coworkers reported the Pd-catalyzed B-alkyl Suzuki-Miyaura cross-coupling between potassium alkyltrifluoroborates and aryl or alkenyltriflates.312 Besides the simple potassium alkyltrifluoroborates, a number of functionalized variants were also synthesized and readily underwent the cross-coupling reaction with aryl or alkenyltriflates. It was observed that electron-deficient aryl and alkenyl triflates underwent cross-couplings efficiently in the presence of catalytic amounts of Pd(dppf)Cl2·CH2Cl2 and Cs2CO3 as a base (Figure 79). Electron-rich triflates were not suitable coupling partners. The aqueous conditions for this transformation were found to be necessary presumably due to the formation of alkylboronic acid in situ, which undergoes the coupling process.313 To probe this hypothesis, an 11B NMR study was performed. When the potassium-3-phenylpropylfluoroborate was exposed to water for 4 days at room temperature, the 11B NMR revealed a new peak at δ 33.5 ppm in addition to the original resonance at δ 5.6 ppm. This observation indicated that the alkylboronic acid (whose 11B NMR signal typically appears at δ 31.5 ppm) or a fluorinated active species such as RBF3−, RBF2 (OH)−, and/or RBF(OH)2− could be generated in situ, and be the active species in fluoride-mediated coupling of boronic acids. Interestingly, it was observed that the corresponding alkylboronic acid and ester also underwent cross-coupling under identical reaction conditions, affording similar yields. This observation has great synthetic potential as it implies that the cross-coupling reactions in general could be performed with potassium alkyltrifluoroborates instead of the corresponding alkyl boronic acid or esters.
Figure 79.
Suzuki Coupling with Alkyl Potassium Trifluoroborates (Molander and coworkers, 2001)
In 2003, the same group demonstrated the synthesis of alkyltrifluoroborates and their use in the Pd-catalyzed Suzuki-Miyaura coupling with aryl halides and triflates. Along with potassium methyltrifluoroborate and potassium trimethylsilyltrifluoroborate, potassium-3-substituted-propyltrifluoroborates were also reacted with electron-deficient aryl halides and triflates to afford moderate to good yields.314 Similarly to the previous results, electron-rich electrophiles proved to be more difficult substrates. In general, the products were formed efficiently in refluxing THF or THF-H2O. Further heating led to the formation of protiodehalogenation and protiodeboration products, and ultimately resulted in an inseparable mixture of several products and poor yields of the desired cross-coupling product. During the coupling of aryl triflates with various alkylboron reagents it was observed that potassium alkyltriflouroborates provided better yield (Scheme 30).312
Scheme 30.
Comparative Study with Boron Derivatives (Molander and coworkers, 2001)
The Deng group reported the reaction of substituted potassium cyclopropyltrifluoroborates with aryl bromides. A series of electron-deficient as well as electron-rich aryl bromides underwent Pd-catalyzed cross-coupling with retention of configuration to provide the corresponding disubstituted cyclopropanes in high yields (Figure 80).315 Subsequently, the Charette group also reported a stereospecific cross-coupling between disubstituted cyclopropyltrifluoroborates and aryl bromides to furnish trisubstituted cyclopropanes in high yields (Figure 81).316
Figure 80.
Suzuki Coupling with Potassium Trifluoroborates (Deng and coworkers, 2004)
Figure 81.
Suzuki Coupling with Substituted Cyclopropyl Potassiumtrifluoroborates (Charette and coworkers, 2005)
The Molander group reported the synthesis of ketone-, ester-, and amide- containing potassium trifluoroboratohomoenolates and their cross-coupling reactions with aryl halides and triflates. These boron salts are particularly challenging in cross-coupling processes due to the formation of metallacyclopropanoxides and facile β-hydride elimination that leads to Heck-type products. Gratifyingly, it was observed that aryl bromides, chlorides, triflates and alkenyl bromides afforded high yields irrespective of their electronic nature (Figure 82). A series of functionalized potassium alkyltrifluoroborates underwent coupling with relatively unreactive aryl chlorides to furnish excellent yields.317
Figure 82.
Suzuki Coupling with Potassium Trifluoroboratohomoenolates (Molander and coworkers, 2008)
In 2008, Molander and coworkers demonstrated the rare cross-coupling of secondary alkyltrifluoroborates with aryl chlorides (Figure 83).318 The reaction conditions for this challenging transformation were optimized using parallel microscale experimentation. Aryl chlorides, irrespective of electron-donating or electron-withdrawing substituents, underwent cross-coupling efficiently under the optimized reaction conditions. In the case of branched acyclic alkyltrifluoroborates, e.g. with potassium i-propyltrifluoroborate, a mixture of branched and linear (1.4:1 for 2-chloroanisole, 8.2:1 for 4-chloroanisole) products were isolated. During the course of this study, enhanced nucleophilicity of the alkyltrifluoroborates compared to their alkylboronic acid and boronic ester counterparts was experienced and the reactivity order for the aryl or alkenyl component was found to be Br > OTf ≫ Cl.
Figure 83.
Suzuki Coupling with Secondary Potassiumtrifluoroborates (Molander and coworkers, 2008)
3.9. Conclusion
It is quite evident that considerable progress has been achieved in B-alkyl Suzuki-Miyaura cross-coupling reactions. Various boron sources have been used successfully as coupling partners. Several catalyst systems with high activity and chemo- and regioselectivity are now available for this widely used process. The success in alkyl-alkyl Suzuki-Miyaura cross-coupling is also noteworthy. However, a number of challenges still need to be addressed. Although there are a few examples of Suzuki-Miyaura cross-coupling with relatively inert and inexpensive aryl chlorides, general catalytic systems for these substrates are in high demand. Additionally, an efficient catalytic system for the general class of secondary alkylboron reagents has yet to be developed. A more detailed mechanistic understanding is essential for less efficient alkylboronic acids or esters to make them effective towards cross-coupling. At the same time, cost-effective catalysts are in high demand for successful application in industrial processes.
4. Cross-Couplings with Alkylmagnesium Reagents
4.1. Introduction
Organomagnesium reagents were first discovered by Grignard in 1900319,320 and since then, they have occupied a central role in synthetic organic chemistry.1–3,18,321–324 Despite their extensive use, the main focus of these reagents has been on nucleophilic addition and substitution reactions. The pioneering studies of Kharasch and Fuchs opened new synthetic avenues for Grignard reagents.325 In 1972, Kumada and coworkers and Corriu and Masse independently demonstrated the synthetic utility of Grignard reagents in Ni-catalyzed cross-coupling reactions. However, the modest functional group tolerance of Grignard reagents compared to that of corresponding boron and zinc reagents has limited their use in synthesis. In this section of the review, the synthesis, stability, and cross-coupling reactions of alkylMg reagents will be discussed. This will exclude cross-coupling reactions of reagents unable to undergo β-hydride elimination such as ArMgX, MeMgX, PhCH2MgX, TMSCH2MgX and alkynylMgX.
4.2. Synthesis and Stability
The most common method to synthesize organomagnesium reagents is via Mg-halogen exchange. Though there have been significant developments in the preparation of functionalized arylMgX and alkenylMgX, the preparation of functionalized alkylMgX has not received much attention. Since the preparation of functionalized arylMgX, alkenylMgX and heteroarylMgX is discussed in detail by Knochel and coworkers in their review,326 this will be excluded from discussion here.
Unfunctionalized alkylMgX can be synthesized via Mg-halogen exchange reactions with the corresponding alkyl halides. Similar methods can be applied to the synthesis of the polyfunctionalized alkylMgX but few examples of such processes have been reported.327–332
4.3. Ni-Catalyzed Cross-Coupling Reactions with Alkylmagnesium Reagents
4.3.1. Kumada Cross-Coupling Reactions via Carbon-Halogen Bond Activation
In 1972, Kumada and coworkers333–335 as well as Corriu and Masse336 independently reported the Ni-catalyzed coupling reaction with organomagnesium reagents. This transformation has come to be known as the Kumada-Corriu cross-coupling. 1–3,15,17,18,335–337 Corriu and Masse used vinyl bromides, chlorides and aryl bromides with arylMgBr and Ni(acac)2 as a catalyst (Figure 84).336
Figure 84.
Seminal Report (Corriu and Masse, 1972)
In the same year, Kumada and coworkers reported the cross-coupling of aryl and vinyl chlorides with alkyl- and arylMgBr using Ni(dppe)Cl2 in high yields (Figure 85).123,335 It was also observed that only bidentate ligands gave reasonable yields of the cross-coupled products. The reaction was not found to be highly stereospecific.
Figure 85.
Seminal report (Kumada and coworkers, 1972)
Use of sec-alkylMgX resulted in mixture of products as shown in Figure 86.123 It was postulated that more electron-rich ligands favored the formation of the linear over the branched product. The proposed mechanism for the undesired (linear) product formation is depicted in Figure 86a. Oxidative addition of PhCl followed by transmetalation with i-PrMgCl gives intermediate 97. Intermediate 97 can either undergo reductive elimination to give the desired branched product 100, or it can β-hydride eliminate to give 98, which then rearranges to intermediate 99, which upon reductive elimination gives undesired product 102.
Figure 86.
a) Mechanistic Studies, b) Proposed Mechanism
In 1976, Kumada and coworkers reported a full account of this work.337 The proposed mechanism is described in figure 86b, starting with the reaction of L2NiX2 with 2 equiv of RMgX to give a diorganonickel species, which undergoes reductive elimination to produce Ni(0). The resulting Ni(0) undergoes oxidative addition to give Ni-complex 103. Intermediate 103 transmetalates with another equiv of RMgX to give intermediate 104, which then coordinates with an alkyl halide to give penta-coordinated Ni-species 105. Intermediate 105 undergoes reductive elimination to give the cross-coupled product as well as regenerate the active catalyst 103. The scope of this transformation is extremely broad with one major limitation, namely that substitution of organic halides and on the Grignard reagents are restricted to those that cannot react with Grignard reagents. The most striking feature of this chemistry is that even alkyl Grignard reagents containing β-hydrogens are compatible under the reaction conditions to give cross-coupled product and do not undergo β-hydride elimination (Figure 87).337 In the same year, Kumada and coworkers extended this methodology to include β-bromovinylethers as substrates to give the corresponding aldehyde after coupling and hydrolysis (Figure 88).338
Figure 87.
Cross-Coupling of Aryl and Alkenyl Halides (Kumada and coworkers, 1976)
Figure 88.
Cross-Coupling of β-bromovinylethers (Kumada and coworkers, 1976)
Mono and dichlorinated benzenes were used by Kumada to give corresponding mono and dialkylated products respectively. Tamborski and coworkers extended this work to trichlorinated benzenes to give trialkyl benzenes (Figure 89).339 They observed a significant decrease in the rate of the reaction when monoalkylated dichlorobenzene was used as a substrate, which indicates that the substitutions do not occur in a stepwise fashion (Figure 89). Furthermore, this limits sequential functionalization to obtain unsymmetrical tri-alkyl benzenes. Bochmann reported the Ni-catalyzed alkylation of di-, tri- and polychlorobiphenyls.340
Figure 89.
Trialkylation of Trichlorobenzenes (Tamborski and coworkers, 1983)
Though these reactions are useful, the selective alkylation of one halogen atom, for dihalobenzenes that possess two identical halo groups, was not achieved. In 1983, Tam and Reddy developed a Ni catalyst that gave high selectivity for the monoalkylation of dichlorobenzene with 1 equiv of R-MgX (Scheme 31).341 The use of the tridentate ligand “triphos” [triphos = bis(2-(dipheny1phosphino)ethyl)phenylphosphine] was necessary to achieve high selectivity. Manabe and Wang addressed the issue of selectivity by using phenol as the directing group to achieve a highly selective cross-coupling reaction, where the halogen next to the phenol is activated and reacts to give the corresponding alkylated product (Scheme 32).342 Interestingly, the o-fluoro group was more reactive than a p-bromo group (106), which indicates that the directing effects of hydroxyl group take priority over the inherent reactivity of the halo groups. The proposed transition state is shown in scheme 37, where the coordination of Mg to the phenol is postulated to facilitate oxidative addition of Ni to the adjacent C-X bond.342
Scheme 31.
Selective Alkylation of Dichlorobenzene (Tam and coworkers, 1983)
Scheme 32.
Phenol-Directed Alkylation of Halobenzenes (Manabe and coworkers, 2009)
Scheme 37.
Initial Report (Kumada and coworkers, 1981)
Liao and coworkers reported a unique tridentate amido diphosphino ligand for Ni-catalyzed cross-coupling. Using this pincer-type complex, they were able to couple EtMgCl and n-BuMgCl with aryl halides in moderate yields (Scheme 33).343 Saint-Jalmes and Roques reported a unique use of the Kumada coupling reaction to synthesize 3-alkyl-trifluoromethylbenzenes, where NiCl2/Xantphos is used as the catalyst and the reaction can be scaled up to 2L (Scheme 34).344
Scheme 33.
Tridentate Amido Diphosphino Ligand for Ni-Catalyzed Cross-Coupling (Liao and coworkers, 2006)
Scheme 34.
Synthesis of Alkylated Trifluoromethylbenzenes (Saint-Jalmes and Roques, 2006)
Claesson and coworkers initially reported the Ni-catalyzed synthesis of multi-substituted 1,3-butadienes from the corresponding phosphates.345 Shi and Shao reported the use of Ni(dppp)Cl2 as an effective catalyst for the synthesis of multi-substituted 1,3-butadienes.346 In this case, diiodo-substrates of type 108 were first treated with DBU to give the corresponding dienes 109, which were subsequently treated with the Ni catalyst and R-MgX to give the desired cross-coupled products in good yields (Figure 90). To probe the role of the diene, the following control experiments were conducted: the use of a substrate lacking the diene (111) resulted in only dehalohydrogenation to give product (112), whereas a substrate with a diene (113) gave the desired product (114). These experiments suggest that the diene is necessary to promote the Kumada coupling reaction under these conditions.346,347
Figure 90.
Synthesis of Multi-substituted 1,3-Butadienes (Shi and Shao, 2005)
4.3.2. Kumada Cross-Coupling Reactions via Carbon-Sulfur Bond Activation
Cross-coupling reactions with organosulfur compounds348 are promising because of their ease of synthesis and availability; however, such reactions are not well developed. The strong carbon-sulfur and metal-sulfur bonds result in slow oxidative addition and transmetalation compared to organic halides. Hence, strong σ-donating ligands as well as higher temperatures are required for a successful reaction.348
In 1979, Takei and coworkers first reported the cross-coupling between aryl and alkenyl sulfides and arylMgBr (Figure 91).349 The use of 3 mol% Ni(PPh3)2Cl2 was found to be effective for the coupling of various organosulfides with Grignard reagents. Under these conditions, no reaction with alkyl sulfides as substrates was observed. Interestingly, excellent retention of stereochemistry was observed when isomerically pure alkenes were used.
Figure 91.
Alkylation of Organosulfides (Takei and coworkers, 1979)
Later, the scope was expanded to include allyl sulfides and heterocyclic sulfides.350 Ni(dppp)Cl2 (3 mol%) was the catalyst of choice for the coupling of various alkylMgBr with heterocyclic sulfides. The use of bidentate dppp was essential for the successful cross-coupling with alkylMgX, presumably because of its ability to prevent β-hydride elimination of the alkyl Grignard as demonstrated by Kumada.123 Thiols and disulfides were also successfully coupled to give products in good yields (Figure 92). Interestingly, for the reaction of 2,2′-dipyridyl disulfide with PhCH2CH2MgBr, formation of PhCH2CH2SH was observed. Based on this observation, a mechanism for the reaction with disulfides was proposed (Figure 92).350
Figure 92.
Scope and Proposed Mechanism (Takei and coworkers, 1979)
In 1985, Takei and coworkers reported the synthesis of 6-substituted purine bases via cross-coupling between 6-(methylthio)-purine and Grignard reagents (Figure 93).351 In the same year, Wenkert and coworkers reported the preparation of 4-alkylpyridines from the corresponding thiopyridines and 2-alkylindoles from the corresponding thioindoles (Figure 94).352
Figure 93.
Synthesis of Substituted Purines (Takei and coworkers, 1985)
Figure 94.
Cross-Coupling of Thioaryls with Grignard reagents (Wenkert and coworkers, 1985)
In 2005, Ila and coworkers reported the synthesis of an unsymmetrical quinoxaline.353 To demonstrate the synthetic utility of their method they showcased the cross-coupling of 2-thioquinoxaline with n-BuMgBr to give 2-butylquinoxaline in good yield (Scheme 35).353 In 2008, Oshima and coworkers reported Ni-catalyzed (5 mol%) cross-coupling of sulfides with alkyl Grignards.354 The use of bidentate (Z)-3,3-dimethyl-2-bis(diphenylphosphino)but-1-ene ligand was optimal for coupling (Figure 95). The most striking feature of this protocol is the use of sec-alkyl Grignards as coupling partners. Moreover, the reaction of an alkenylsulfide and an alkylMgBr occurs with retention of configuration under the reaction conditions.
Scheme 35.
Synthesis of Unsymmetrically Substituted Quinoxaline (Ila and coworkers, 2005)
Figure 95.
Cross-Coupling of Sulfides with Alkyl Grignards (Oshima and coworkers, 2008)
In 2005, Park and coworkers demonstrated that neopentyl arenesulfonates could be used in Ni-catalyzed cross-coupling reactions (Scheme 36).355 Interestingly, arenesulfonates are unreactive in the Pd-catalyzed variant, allowing for stepwise functionalization of the bromoarenesulfonates. Under the optimized conditions, MeMgBr, t-BuCH2MgBr and BnMgBr undergo the cross-coupling reaction in good yields. It should be noted that only alkylMgBr reagents with no β-hydrogens are used in this report. This methodology was later extended to the use of a Ni-NHC (IiPr) system.356 NHC ligands are better σ-donors than phosphines, which facilitates oxidative addition of the relatively unreactive C-S bond.
Scheme 36.
Use of Neopentyl Arenesulfonates in Cross-Coupling (Park and coworkers, 2005)
4.3.3. Kumada Cross-Coupling Reactions via Carbon-Oxygen Bond Activation
The conversion of a C-O bond to the corresponding C-C bond holds high synthetic value because of their ease of synthesis and high stability. In 1981, Kumada and coworkers reported a Ni-catalyzed cross-coupling reaction between aryl phosphonates with various arylmagnesium reagents.357 Only n-BuMgBr was used as an alkyl coupling partner in this seminal publication (Scheme 37).
In 1999, Fiaschisi and coworkers reported the Ni-catalyzed cross-coupling of vinyl triflates with alkylMgBr.358 They observed a significant impact of the ligand’s bite angle on the reaction outcome, which was more pronounced in the case of coupling with sec-alkylMgBr (Figure 96). The use of sec-alkylMgBr is complicated by the formation of n-alkyl product 116, which was suppressed by use of ligands with smaller bite angles (dppe, dppp) as compared to ligands with larger bite angles (dppf, dppb) (Figure 97). Moreover, under the optimized conditions, trisubstituted vinyl triflates also react to give the corresponding products in good yield.
Figure 96.
Cross-Coupling of Vinyl Triflates (Fiaschisi and coworkers, 1999)
Figure 97.
Effect of Ligand Bite Angle on Product Distribution
Bäckvall and coworkers have reported the cross-coupling of dienyl phosphates with Grignard reagents in the presence of 1 mol% Ni(dppe)Cl2 or Ni(dppp)Cl2 (Figure 98).359 Under the optimized conditions, various alkylMgBr, including sec-alkylMgBr, react to give satisfactory yields of the corresponding products. In addition to cyclic dienyl phosphates, one acyclic substrate was found to give the product in 77% yield. Aryltriflates are also viable coupling partners for a Ni-catalyzed cross-coupling as demonstrated by Busacca and coworkers.360
Figure 98.
Synthesis of Substituted Dienes (Bäckvall and coworkers, 1999)
In 2005, DuBois and coworkers first reported the Ni(dppp)Cl2-catalyzed cross-coupling of cyclic sulfamates with organomagnesium reagents (Figure 99).361
Figure 99.
DuBois and coworkers, 2005
4.3.4. C(sp3)-C(sp3) Cross-Coupling with Alkylmagnesium Reagents
C(sp3)-C(sp3)cross-coupling reactions are challenging for the reasons described in the preceding sections of this review. Thus, it was not until 2002, almost 30 years after the initial report of the Kumada-Corriu coupling, that the first successful Ni-catalyzed C(sp3)-C(sp3) coupling reaction was reported by Kambe and coworkers (Figure 100).118,347,362
Figure 100.
C(sp3)-C(sp3) Cross-Coupling Reaction (Kambe and coworkers, 2002)
In 2002, Kambe and coworkers reported the successful cross-coupling of alkyl bromides, chlorides and tosylates with alkylMgBr using a Ni-catalyst. Interestingly, it was found that the use of 1,3-butadiene rather than phosphine ligands led to a competent catalyst. Under the optimized conditions (NiCl2 (1–3 mol %) and butadiene (10–100 mol %)), the products were obtained in good to excellent yields. The butadiene ligand was essential for the observed reaction, as in the absence of butadiene reduction and/or elimination of the substrates was mainly observed (Scheme 38).347
Scheme 38.
Proposed Mechanism (Kambe and coworkers, 2002)
The proposed mechanism for this transformation is shown in scheme 43. NiCl2 is reduced to Ni(0) by reacting with 2 equiv of n-BuMgCl. The resulting Ni(0) complex reacts with 2 equiv of 1,3-butadiene to afford bis-π-allyl Ni complex 117. Intermediate 117 is proposed to react with Grignard reagents to form η1,η3-octadiene-diylnickelate complex 118, which then undergoes oxidative addition of alkyl halides and tosylates to yield dialkylnickel complex 119. Complex 119 reductively eliminates to give desired product 120. In 2010, Fang and coworkers reported DFT calculations on the model substrate for Ni(allyl)2-catalyzed cross-coupling.363 Their study suggested a weak but persistent interaction between Mg and the allyl moiety throughout the catalytic cycle. Moreover, oxidative addition is proposed to be the turnover limiting step and competing β-hydride elimination is much higher in energy compared to reductive elimination.363 Subsequently, Kambe and coworkers reported the use of bis-(η3-allyl)nickel complex as a catalyst for the coupling of alkyl bromides and alkyl tosylates with alkyl Grignard reagents.364
Scheme 43.
Proposed Mechanism (Fürstner and coworkers, 2002)
Kambe and coworkers extended this methodology to alkyl fluorides, where the use of Ni/butadiene as a catalyst provided excellent yields of the cross-coupled products as shown in scheme 39.365 Surprisingly, the reactivity of the alkyl halides increased in the order Cl < F < Br. This trend cannot be explained by the bond energies of the alkyl halides and the magnesium salts formed.
Scheme 39.
C-F Bond Functionalization (Kambe and coworkers, 2003)
Despite substantial advancements in Kumada cross-coupling reactions, functional group tolerance has always been an issue. This can be attributed to the high nucleophilicity/basicity of Grignard reagents. Recently, Kambe and coworkers reported a C(sp3)-C(sp3) cross-coupling reaction using NiX2/butadiene system, where improved functional group tolerance was observed (Figure 101).366
Figure 101.
Scope of Reaction (Kambe and coworkers, 2009)
In 2008, Hu reported a Ni-catalyzed Kumada-Corriu coupling with a unique pincer amido-bis-(amine) ligand.367–371 They observed an unusual reaction of CH2Cl2 and CHCl3 with n-BuMgBr to give the dialkylated or trialkylated products respectively (Scheme 40).372
Scheme 40.
Ni-catalyzed Kumada-Corriu Coupling with a Unique Pincer Amido-bis-(amine) Ligand (Hu and coworkers, 2008)
The reaction involves cleavage of up to three C-Cl bonds to form three new C-C bonds at the same carbon center. The reaction is proposed to follow a radical pathway based on the following observations: 1) the reactions of CH2Cl2 and CHCl3were faster than octyl-Cl, 2) TEMPO inhibits the reaction of 121 with CH2Cl2, and the formation of TEMPO-CH3 was observed; 3) the reaction of 121 with bromomethylcyclopropane gave 1-pentene as the product. The scope of this transformation was later expanded to include other alkyl Grignard reagents (Table 8).368,369 This system was found to be general for the cross-coupling between various alkyl halides and alkylMgCl, and exhibits good functional group tolerance. Interestingly, Grignard reagents containing functional groups also undergo smooth reactions in the presence of 3–9 mol% catalyst in DMA at −35 °C.
Table 8.
Substrate Scope.
 | ||
|---|---|---|
| R-X | R′-MgCI | Yield |
| n-Bu | 85% | |
| n-Bu | 77% | |
| n-Pent | 60% | |
| n-Oct | 79% | |
| n-Pent | 91% | |
 |
n-Bu | 91% |
 |
n-Bu | 93% |
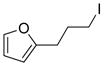 |
n-Bu | 99% |
 |
 |
60% |
4.4. Fe-Catalyzed Kumada Cross-Coupling Reactions
Cross-coupling reactions are typically catalyzed by Pd and Ni salts. But the higher cost of these metals, as well as environmental issues, demand cheaper and more environmentally friendly metals for this transformation. Iron being less expensive than Pd and Ni, as well as its lower toxicity makes it an ideal choice for cross-coupling reactions.373–378
4.4.1. C(sp2)-C(sp3) Cross-Coupling with Alkylmagnesium Reagents
In 1971, Kochi and coworkers378–384 first reported Fe-catalyzed cross-coupling reactions.379,380,383,384 In this seminal report, they described the coupling of vinyl bromides with alkyl Grignard reagents catalyzed by FeCl3 (Figure 102). Utilizing this protocol, Kochi obtained good yields, but the requirement of a large excess of vinyl halide to effect the reaction limited its synthetic utility. Furthermore, they observed decomposition of the catalyst on standing (catalyst aging), which led to diminished yields of products.
Figure 102.
Seminal Report (Kochi, 1971)
Several years later, Kochi published a subsequent report aimed at improving this reaction by employing tris(dibenzoylmethido)iron(III) as the catalyst.381 Indeed, this improved the reaction stoichiometry and catalyst stability to some degree; however, excess vinyl halide was still required. The proposed reaction mechanism is shown in scheme 41.381,382 The catalytically active Fe(I) species is formed in situ by reduction of Fe(III) with 2 equiv of RMgX. The resultant Fe(I) species undergoes oxidative addition of R-X followed by transmetalation with RMgX to give intermediate 122, which upon reductive elimination yields product and regenerates the catalyst.
Scheme 41.
Proposed Mechanism (Kochi, 1971)
The following experiments provide support for the formation of a Fe(I) complex.381 First, a careful study of the amount of methane and ethane gases generated in the reaction with methylmagnesium bromide suggested a stoichiometric relationship Fe(III) + nCH3MgBr = Fe(III-n) + XCH4 + YC2H6, where n = X + 2Y. The value of n according to the above equation provides the change in oxidation state of iron. The value of n was found to be close to 2, which supports reduction of Fe(III) to Fe(I). Secondly, the ESR spectrum of Fe(dbm)3 after treatment with excess EtMgBr gave <g> = 2.08. This value matches the well-characterized HFe(I)(dppe)2 complex,385 providing additional evidence for the presence of Fe(I).
Vinyl sulfones and allyl phosphates can also serve as viable coupling partners with Grignard reagents as demonstrated by Julia386 and Yamamoto,387,388 respectively. Despite this early report of a Fe-catalyzed cross-coupling reaction with alkylMgX, almost 30 years passed from the original report before the development of synthetically useful protocols. Cahiez and Avedissia reported that the reaction of a vinyl bromide with octylMgBr in the presence of 3 mol% Fe(acac)3 in THF afforded the desired alkene in only 40% yield (Scheme 42).389 However, the addition of N-methyl-2-pyrrolidinone (NMP) as a co-solvent enhanced the yield to 87%. An even more drastic increase in yield was observed for the reaction between a vinyl chloride and BuMgX (Scheme 42).389
Scheme 42.
Effect of NMP on Reaction Outcome (Cahiez and coworkers, 1998)
Suprisingly, the nature of the iron salt had little effect on the reaction outcome. The only requirement found was that the catalyst should be soluble in THF, as illustrated by the ability of various iron catalysts (Fe(acac)3, Fe(dpm)3, Fe(dbm)3, and FeCl3), to catalyzed the reaction with isolated yields in excess of 85%. In contrast, Fe2(SO4)3, gave a poor yield of 31%, as it is sparingly soluble in THF (Figure 103). Interestingly, the nature of the halide also had little effect on product yield. The scope of the reaction is broad with tolerance for a vast array of functional groups including esters and ketones. Vinyl phosphonates are also viable substrates under these conditions. Furthermore, this protocol is highly stereospecific, (Z)-and-(E)-1-chlorooct-1-enes afforded the corresponding Z- and E-olefins with excellent stereoisomeric purity.
Figure 103.
Scope (Cahiez and Coworkers, 1998)
In 2002, Fürstner and coworkers reported the reaction of aryl chlorides, tosylates and triflates with alkylMgBr, which represents substrate classes that were unreactive prior to this report.378,390,391 In this report, they proposed a modified mechanism for the Fe-catalyzed coupling reaction in the presence of Grignard reagents. FeCl2 reacts with 4 equiv of RMgX to give a new species of the formal composition [Fe(−II)(MgX)2], an “inorganic Grignard reagent”, which is readily soluble in ethereal solvents.392 In complex 123, the iron is proposed to have a formal charge of −2.391 The nucleophilic iron “super-ate” complex is proposed to oxidatively add to aryl chlorides to give species 124. Intermediate 124 undergoes transmetalation with another equivalent of RMgX to form species 125, which upon reductive elimination gives product and regenerates the active catalyst (Scheme 43).
To support their mechanistic proposal, Fürstner and coworkers conducted a key control experiment. When nonpassivated iron metal Fe(0)* powder was used, which was prepared by reduction of FeCl3 with potassium, no reaction with substrate was observed (Scheme 44). Whereas, when excess Grignard reagent was added to the reaction mixture containing Fe(0)*, the desired product was formed. These experiments rule out an active catalytic species with Fe in the zero oxidation state and support their proposed mechanism.391
Scheme 44.
Control Experiments (Fürstner and coworkers, 2002)
The scope of this transformation was found to be broad, and commercially available, air and moisture stable Fe(acac)3 could be used as the pre-catalyst (Figure 104). Of most interest, the reaction of 126 in the presence of a Ni-catalyst proceeds in 6 h at reflux in ether, whereas with a Fe-catalyst it was completed in 5 min with a better yield (Scheme 45).391 Vinyl triflates were also found to undergo the cross-coupling under the reported conditions (Figure 105).393 Furthermore, monoalkylation of a dichloroarene was also achieved using Fe(acac)3 as a catalyst in THF/NMP as solvent.393 Hocek and coworkers utilized Fe-catalyzed cross-coupling for preparation of 6-methyl purine bases and nucleosides (Scheme 46).394 Tam and coworkers reported the synthesis of 2-substituted bicyclic alkenes utilizing an Fe-catalyzed cross-coupling of alkenyl triflates with alkyl Grignard reagents.395
Figure 104.
Scope of Iron-Catalyzed Cross-Coupling (Fürstner and coworkers, 2002)
Scheme 45.
Superiority of Fe-Catalyzed Reaction over Ni-Catalyzed Reaction (Fürstner and coworkers, 2002)
Figure 105.
Cross-Coupling Reaction with Vinyl Triflates (Fürstner and coworkers, 2004)
Scheme 46.
Synthesis of 6-Methylpurine Bases (Hocek and coworkers, 2003)
Alami and coworkers utilized an Fe-catalyzed cross-coupling for the synthesis of substituted quinolines, which show anti-retroviral activity against HIV-1 and HTLV-1 transformed cells (Scheme 47).396 The optimized reaction conditions were also effective for the reaction of substituted chloroenynes with alkyl Grignards. Using similar conditions, Olsson and coworkers reported the cross-coupling of imidoyl chlorides with Grignard reagents (Scheme 48).397 Vogel and Volla reported the cross-coupling of alkenyl sulfonyl chlorides with Grignard reagents using Fe(acac)3 as catalyst.348,398,399
Scheme 47.
Cross-Coupling of Chloroenynes with Grignard Reagents (Alami and coworkers, 2004)
Scheme 48.
Cross-Coupling of Imidoyl Chlorides with Grignard Reagents (Olsson, 2006)
Skrydstrup reported the cross-coupling of heteroaromatic sulfonates and phosphates with alkyl Grignards using FeCl3 using a THF/NMP mixture as a solvent (Scheme 49).400
Scheme 49.
Cross-Coupling of Heteroaromatic Sulfonates with Alkyl Grignards (Skrydstrup and coworkers, 2009)
Recently, Shi and coworkers reported the cross-coupling of alkenyl carboxylates with Grignard reagents in the presence of FeCl2 (1 mol%) and H2IMes•HCl (2 mol%) as a ligand (Figure 106).401 Interesingly, the counterion of the Grignard reagent was highly important, as n-hexylMgCl reacts cleanly, but n-hexylMgBr gave no product, which can be overcome by addition of excess LiCl.401
Figure 106.
Cross-Coupling of Alkenyl Carboxylates with Grignard Reagents (Shi and coworkers, 2009)
4.4.2. C(sp3)-C(sp3) Cross-Coupling with Alkylmagnesium Reagents
Iron-catalyzed C(sp3)-C(sp3)coupling reactions of unactivated alkyl electrophiles face various issues, including homocoupling, disproportionation, and β-elimination. These issues hinder the development of a successful iron-catalyzed C(sp3)-C(sp3)cross-coupling. In 2007, Chai and coworkers reported the first C(sp3)-C(sp3) coupling of unactivated alkyl halides with alkyl Grignard reagents.402 In their optimization study, they found that use of 3 mol% of Fe(OAc)2 and 6 mol% of Xantphos in Et2O gave the best results. Using these conditions, various unfunctionalized alkyl bromides coupled with alkyl Grignards in moderate yields of 46–64% (Figure 107). The reaction is proposed to proceed via a radical pathway based on the results shown in Scheme 50.402 Reaction of alkylMgBr with hexylbromide and cyclopropylbromide gave radical-induced cyclization and ring opened products, respectively. Both of these experiments suggest the formation of an alkyl radical from the corresponding alkyl halide.
Figure 107.
Iron-catalyzed C(sp3)-C(sp3) Coupling Reaction (Chai and coworkers, 2007)
Scheme 50.
Control Experiments (Chai and coworkers, 2007)
4.4.3. Mechanistic Considerations
Despite these impressive advances, the mechanistic understanding of iron-catalyzed cross-coupling reactions is not well developed. Various proposals with catalytic cycles shuttling between Fe(-2)/Fe(0),22,374,390,391,393,403 Fe(0)/Fe(+2)382,404 or Fe(+1)/Fe(+3)381,382,405,406 have been proposed and discussed briefly above. Moreover, the catalyst is generated in situ and is prone to rapid degradation on isolation attempts. Kochi proposed a catalytic cycle where the active catalytic species is formally a Fe(I) species.389,391–393 Recenty, Norrby and coworkers supported this claim based on their findings.23 From computational studies the authors found unfavorable thermodynamics for reductive elimination of Fe(−II) to Fe(0), while the energy barrier for reductive elimination in the case of Fe(III) to Fe(I) was only 10 kJ/mol. The energy barrier for reductive elimination of Fe(II) to Fe(0) was also found to be prohibitively high. Based on these findings as well as systematic study of change in oxidation state of iron and Hammett plots suggest Fe(I) as the active catalyst in Fe-catalyzed cross-coupling reaction with alkylMgX.23
In contrast, Fürstner proposed a catalytic cycle,391 in which the active catalytic species is formally in the −2 oxidation state. Recently, Fürstner reported a full account of their mechanistic study and proposed two different active species for different Grignard reagents (with and without β-hydrogens) based on the following observations.22
Fürstner and coworkers observed that the treatment of methyl 4-chlorobenzoate with EtMgBr (or higher alkyl Grignard reagents) in the presence of Fe(acac)3 or FeCln (n = 2, 3) as the precatalyst affords the desired product in excellent yield at 0 °C, whereas MeMgBr fails to react even under more forcing conditions (Scheme 51). Interestingly, the appearance of the reaction mixtures is also noticeably different: the reaction containing MeMgBr leads to a yellow-colored solution that slowly darkens, whereas the reaction containing EtMgBr instantaneously generates a brown/black and turbid mixture, which eventually transforms to black-violet as the reaction proceeds. This observation indicates the presence of two different active catalytic species for Grignard reagents with β-hydrogens as compared to reagents without β-hydrogens.22
Scheme 51.
Different Reactivity for MeMgBr and EtMgBr
To gain insight into the nature of the catalytic species, Fürstner and coworkers isolated the Fe complex. On treatment of ethereal solutions of FeCl3 with excess MeLi at low temperatures a red Fe-complex was isolated, which was exceptionally sensitive. X-ray analysis provided the structural composition as [(Me4Fe)(MeLi)][Li(OEt2)]2 (129). This complex was found to exhibit similar reactivity as observed with the reaction of MeMgBr in presence of FeX3, suggesting that 129 might be the reactive iron species which acts as the nucleophilic partner in methylation reactions. It was postulated that other analogs such as aryl Grignards, vinyl Grignards, etc. (not containing β-hydrogen) might also lead to similar reactive species.22
Bogdanovi and coworkers have proposed that Grignard reagents containing a β-hydrogen react with FeX2 to give dark-brown bimetallic clusters of the formal composition [Fe(-II)(MgX)2]n.392 Various attempts to further characterize the iron species failed. Based on a model study conducted by Fürstner and coworkers, [Fe(-II)(MgX)2]n is proposed to be the active catalytic species for Grignard reagents containing β-hydrides. The proposed mechanism for the Fe-catalyzed cross-coupling with various alkylMgX is shown in scheme 53.
Scheme 53.
Proposed Mechanism for Iron-Catalyzed Cross-Coupling
4.4.4. Kumada Cross-Coupling for Ketone Formation
Direct addition of Grignard reagents to acid chlorides is limited by the subsequent addition to the product ketones to give the tert-alcohol products. Addition of metal salts was found to be effective to improve selectivity for the ketone product over the tertiary alcohol.191 In 2000, Taurino and coworkers reported a polymer-supported Fe-catalyst, Fe(AAEMA)3, which was effective for the coupling reaction between hexanoyl chloride and butylmagnesium chloride (Figure 109).407 This catalyst showed similar activity to the homogeneous Fe(acac)3 catalyst. Furthermore, low leaching of iron was observed and the recovered Fe(AAEMA)3 gave similar results on subsequent uses.
Figure 109.
Polymer Supported Iron Catalyst for Ketone Formation (Taurino and coworkers)
In 2004, Fürstner reported a Fe(acac)3 system, which showed excellent functional group tolerance. Representative examples are shown in figure 110.393
Figure 110.
Cross-Coupling of Acid Chlorides with Grignard Reagents (Fürstner and coworkers, 2004)
4.5. Pd-Catalyzed Kumada Cross-Coupling Reactions with Alkylmagnesium Reagents
In 1975, Murahashi and coworkers408–410 were the first to demonstrate the use of Pd in cross-coupling reactions of vinyl halides with CH3MgBr.408 Despite significant advances in Ni- and Fe-catalyzed Kumada cross-couplings, Pd-catalyzed alkyl Kumada cross-couplings are limited. This could be attributed to the high reactivity of Grignard reagents and the higher propensity of Pd-alkyl species to undergo β-hydride elimination compared to the corresponding Ni-alkyl species.
4.5.1.C(sp2)-C(sp3) Cross-Coupling with Alkylmagnesium Reagents
In 1984, Hayashi and coworkers demonstrated a significant effect of ligand bite angle on the reaction outcome for primary and secondary alkyl Grignards and zinc reagents (see section 2.3).132 The use of dppf as ligand gave optimal results for both primary and secondary alkyl Grignards. Since, this initial report dppf has become a very common ligand in both Pd-catalyzed Kumada and Negishi coupling reactions.
In 1991, Katayama and coworkers used Pd(dppf)Cl2 as a catalyst to selectively monoalkylate, dichlorobenzenes (Figure 111).411 They observed that the use of additional dppf ligand gave better results for reactions with alkyl Grignard reagents. It was postulated that the additional ligand blocks the open coordination site on Pd.
Figure 111.
Selective Monoalkylation of Dichlorobenzene (Katayama and coworkers, 1991)
In 2002, Miller reported the Pd-catalyzed cross-coupling of Grignard reagents with enol phosphonates, which were formed in situ from the corresponding ketones using hindered Grignard reagents and used as substrates for sequential cross-coupling reactions with separate Grignard reagents (Figure 112).412 The reaction of the ketone with MesitylMgBr and ClP(O)(OPh)2 gave the corresponding enol phosphonate, which was then treated with n-BuMgBr in the presence of Pd(dppf)Cl2 to give the product in 79% yield.
Figure 112.
Cross-coupling Reaction with Enol Phosphonates (Miller, 2002)
In 2005, Hartwig and coworkers reported a Pd-catalyzed cross-coupling between aryl and vinyl tosylates with Grignard reagents.413 The use of an electron-rich phosphine ligand allows for oxidative addition of aryl and vinyl tosylates (Figure 113). Under the optimized conditions, alkenyl, n-alkyl, branched alkyl, cycloalkyl and benzyl Grignard reagents all couple with electron-rich anisyl tosylates in good to excellent yields. Moreover, vinyl tosylates also react with sec-alkyl Grignards to give products in good yields.413
Figure 113.
Cross-Coupling Reaction of Vinyl and Aryl Tosylates (Hartwig and coworkers, 2005)
Recently, Eycken and coworkers utilized a Pd-catalyzed desulfitative Kumada cross-coupling reaction to generate libraries of 2-(1H)-pyrazinones (Figure 114).414 In this report, few examples using alkyl Grignards are demonstrated. Interestingly, the use of Ni- and Fe-catalysts in the desulfitative Kumada cross-coupling reaction resulted in the formation of homocoupling products, whereas Pd(dba)2 (5 mol%), TFP (tri-2-furylphosphine) (10 mol%) in THF/NMP mixtures gave the desired products in good yields.
Figure 114.
Synthesis of 2-(1H)-Pyrazinones (Eycken and coworkers, 2009)
4.5.2. C(sp3)-C(sp3) Cross-Coupling with Alkylmagnesium Reagents
In 2003, Kambe and coworkers were the first to report a Pd-catalyzed C(sp3)-C(sp3) coupling with alkylMgX.415 The use of 1,3-butadiene as a ligand was found to be essential for this reaction to proceed, similar to the Ni-catalyzed transformation.347,362,364 Under the optimized conditions (1–3 mol% Pd(acac)2, 30–100 mol% butadiene, THF), alkyl bromides and tosylates react with alkylMgX to give products in excellent yields (Figure 115). To showcase the superiority of Pd over Ni as the catalyst, parallel reactions of a chloroalkyltosylate with EtMgBr were conducted (Scheme 54). The Pd-catalyst reacts exclusively with the −OTs leaving the −Cl intact, whereas Ni reacts with partial selectivity.
Figure 115.
Pd-Catalyzed C(sp3)-C(sp3) Coupling (Kambe and coworkers, 2003)
Scheme 54.
Selectivity of Pd- over Ni-Catalyst
4.6. Conclusion
From its discovery in 1972, the Kumada-Corriu cross-coupling has come a long way. Key contributions by scientists in the Ni- and Fe-catalyzed Kumada cross-coupling have made it more synthetically useful. Additionally, recent developments in C(sp3)-C(sp3) cross-coupling as well as the successful use of functionalized reagents are promising and showcase the potential to make this methodology even more general.
5. Cross-Coupling with Alkylindium Reagents
5.1. Introduction
Transition metal-catalyzed cross-coupling reactions of alkyl organometallics and organoelectrophiles are a field of growing interest due to their potential applications in the synthesis of target-oriented molecular frameworks.3,416 Although traditional organometallic reagents are frequently used in cross-couplings, they sometimes suffer from serious limitations, particularly in the case of alkyl transfer reactions.417 In recent years, the synthetic scope of these types of reactions has been continuously expanded by the use of new organic electrophiles, catalysts, and organometallics. Besides the chemo- and stereoselectivity issues they also generate a stoichiometric amount of metal waste that can display serious toxicity. Organoindium reagents were introduced as cross-coupling partners that offer a protocol for C–C bond formation. Importantly, unlike other organometallics, triorganoindiums generate substoichiometric (1/3) metal waste in the alkylation processes. In addition, indium organometallic reagents are substantially less nucleophilic reagents, compared to alkylzinc and alkylmagnesium reagents but possess sufficient transmetalation properties for cross-coupling reactions.418
5.2. Preparation of Trialkylindium Reagents
Indiumorganometallics are readily accessible by different approaches. (1) Transmetalation of dimethylmercury with indium metal in the presence of mercuric chloride leads to the formation of the trimethylindium reagent, but this method is not practically useful due to the involvement of highly toxic materials (Figure 116).419 (2) The metathesis reaction of lithium, magnesium, sodium, aluminum, or zinc organometallics with indium halides is a frequently used protocol for the preparation of triorganoindium reagents. Anhydrous indium(III) chloride is added to a solution of alkyllithium or alkylmagnesium reagents in THF at −78 °C, the reaction mixture is warmed to room temperature and the subsequent cross-coupling reaction is performed without further purification.420 421 (3) Oxidative addition of indium metal to an organic halide is one of the most common procedures for the preparation of organoindium reagents.422 The Hill group isolated an indium metal complex which was formed from the oxidative addition of methyl iodide to indium(I)-species. From an electron paramagnetic resonance (EPR) study, it became evident that the indium insertion to the alkyl halide bond is a radical process.423
Figure 116.
Preparation of Indium Reagents
5.3. Cross-Coupling Reactions of Alkylindium Reagents
Due to low bond strengths in triorganoindiums and the large difference between the heats of formation of trialkylindiums and indium halides, indium has the ability to transfer all three groups in cross-coupling reactions.424 Nevertheless, the highly Lewis acidic character of the indium reagents may lead to an alternative mechanism that involves the formation of Pd–In complex prior to transmetalation.425
Initially, the Nomura group reported a catalyst-free addition of trialkylindium to chloroalkenes. Subsequently, several groups have made significant progress on the use of triorganoindium reagents in metal-catalyzed cross-coupling reactions.424,426 The Sarandeses group reported a Pd-catalyzed cross-coupling of triorganoindiums with aryl or alkenyl iodide and triflate (Figure 117).421 It was revealed that in addition to aryl, alkenyl, and alkynylindium organometallics, trialkylindiums also undergo cross-coupling reactions with a variety of organic electrophiles, such as aryl halides and triflates, vinyl triflates, benzyl bromides, and acid chlorides in the presence of Pd or Ni catalysts. A systematic study revealed that tri-n-butyl, trimethyl, and triallylindium underwent cross-coupling with p-iodotoluene in the presence of 1 mol% Pd(PPh3)2Cl2 in refluxing THF, affording high yields. In addition, the trialkylindiums are able to deliver all three alkyl groups for effective cross-coupling with electrophiles. Therefore, the ratio of trialkylindium reagents to electrophiles could be reduced to 1:3, still obtaining almost quantitative yields. Aryl bromides and triflates as well as alkenyl triflates were also successful coupling partners under the same reaction conditions (Figure 118). In contrast, relatively inert aryl chlorides did not afford any cross-coupled products using Pd catalysts, even with Buchwald or Fu ligands.248,250,427 However, a combination of 5 mol% Ni(PPh3)2Cl2 and DIBAL-H/PPh3 generates Ni(0) in situ, which catalyzes the cross-coupling between p-chlorotoluene and triphenyl or tri-n-butylindium (Scheme 56). Similar to Pd catalysis, all three alkyl groups were transferred to the electrophiles under Ni catalysis. A comparative reactivity study was performed with an aryl iodide, bromide and triflate and the reactivity order was observed as I > Br ≅ OTf. The same order of reactivity is observed in the case of Pd-catalyzed cross-coupling with other organometallics reagents. Therefore, the oxidative addition is proposed to be the turnover-limiting step similar to many other cross-coupling processes.
Figure 117.
Cross-Coupling with Aryl Halides (Sarandeses and coworkers, 2001)
Figure 118.
Cross-Coupling with Alkenyl Triflates (Sarandeses and coworkers, 2001)
Scheme 56.
Ni-Catalyzed Cross-Coupling with Aryl Chlorides (Sarandeses and coworkers, 2001)
In 2002, utilizing the chemoselective nature of organoindium reagents, a sequential one-pot cross-coupling with oligohaloarenes was accomplished. For example, p-iodobromobenzene was reacted first with TMS-protected triacetyleneindium in the presence of catalytic Pd(PPh3)4 followed by a second cross-coupling with tributylindium to afford disubstituted benzene in high yields (Scheme 57).428
Scheme 57.
Sequential Cross-Coupling (Sarandeses and coworkers, 2002)
Considering the low reactivity of indium organometallics, the Oshima group hypothesized that cross-couplings could be performed in aqueous media (Scheme 58).429 As a proof of concept, 1.0 equiv of triphenylindium was reacted with 3.0 equiv of 3-iodoanisole in aqueous THF (THF:H2O = 6:1). After work-up and purification, 2.1 equiv of the cross-coupled product was isolated along with 0.9 equiv of unreacted 3-iodoanisole. This observation led to the conclusion that at least two of the three phenyl groups could participate in the cross-coupling reaction under these aqueous reaction conditions. It was further confirmed by NMR experiments that triphenylindium converted to diphenylindium hydroxide upon addition of water. The cross-coupling of diethylindium chloride with p-nitro-iodobenzene in the presence of Pd2(dba)3•CHCl3 afforded excellent yield under the optimized reaction conditions.
Scheme 58.
Cross-Coupling in Aqueous Media (Oshima and coworkers, 2001)
The Sarandeses group reported a regio- and stereoselective Pd-catalyzed cross-coupling reaction of indium organometallics with 1,1-dihaloalkenes and stereo-defined 1-haloalkenes (Figure 119, 120).430 Triorganoindium reagents were stereospecifically coupled with stereodefined alkenyl iodides in good yields and short reaction times using Pd catalysts. Additionally, the Pd-catalyzed cross-coupling reaction of R3In (0.9 equiv) with 1,1-dibromo-1-alkenes gave coupling products in high yields (Figure 120). Interestingly, when the reaction was performed with 0.4 equiv of aryl-, vinyl- or alkynylindium derivatives, trans-monosubstituted products were obtained selectively in moderate to good yields. These selective cross-couplings were performed with [Pd2dba3]/P(2-furyl)3 (1:1, 2 mol%) at room temperature. The resulting (Z)-monobromoalkenes were further functionalized by cross-coupling reactions with various triorganoindium reagents at room temperature in the presence of [Pd(t-Bu3P)2] as a catalyst, to provide trisubstituted olefins in good yields.
Figure 119.
Stereoselective Cross-Coupling with Iodoalkenes (Sarandeses and coworkers, 2008)
Figure 120.
Cross-Coupling with 1,1-Dibromoalkenes (Sarandeses and coworkers, 2008)
In 2009, the same group reported the synthesis of unsymmetrical 3,4-disubstituted maleimides by a sequential one-pot Pd-catalyzed cross-coupling reaction of indium organometallics with 3,4-dihalomaleimides (Figure 121). Disubstituted products were obtained in high yields and selectivity.431
Figure 121.
Cross-Coupling with Maleimides (Sarandeses and coworkers, 2009)
5.4. Carbonylative Cross-Coupling With Trialkylindium Reagents
In 2003, the Lee group reported a Pd-catalyzed cross-coupling of aryl halides, triflates, benzyl bromides and acid chlorides with tetraorganoindate complexes.432 The tetraorganoindates were prepared in situ by the reaction of indium trichloride and alkyl, aryl or alkenyl lithium or magnesium bromides. It was observed that 0.28 equiv of indate complexes were able to furnish almost quantitative yields of the cross-coupled products. Primary as well as secondary alkyl groups were transferred successfully, whereas tertiary alkyls did not form any cross-coupling products. When the coupling reaction was performed under CO atmosphere the corresponding carbonylative cross-coupling product was obtained in good yield (Figure 122).433
Figure 122.
Carbonylative Cross-Couplings (Lee and coworkers, 2004)
The same group also reported a Pd-catalyzed synthesis of unsymmetrical ketones using trialkyl- and triarylindiums via carbonylative cross-coupling with a variety of organic electrophiles.434 Only 0.37 equivalents of alkylindium were required to afford high yields of ketones using one atmosphere of CO (Figure 123). Interestingly, when p-bromoiodobenzene was reacted with 0.37 equiv of trimethylindium under a CO atmosphere in the presence of 4 mol% Pd(PPh3)4, p-bromoacetophenone was formed selectively. The subsequent addition of 0.37 equiv of triphenylindium generated 4-phenylacetophenone in 75% overall yield. In the following year, modified conditions for ketone formation were reported using tetraorganoindate complexes.434 This protocol allows for the use of secondary alkyl groups to afford unsymmetrical ketones in high yields.
Figure 123.
Carbonylative Cross-Couplings (Lee and coworkers, 2004)
5.5. Oxidative Cross-Couplings with Trialkylindium Reagents
The Lei group reported a Pd-catalyzed oxidative carbonylation protocol for a broad range of primary and secondary alkyl and aryl indium reagents under mild conditions.435 The alkyl indium reagents exhibited compatibility to a wide range of functional groups, whereas the alkyl Grignard precursor precludes their involvement in such reactions in many cases because of the high reactivity and basicity. Unlike conventional cross-coupling protocols, desyl chloride was used as an oxidant to avoid slow oxidative addition and β-hydride elimination. Mechanistic investigations under stoichiometric and catalytic conditions provided evidence for the oxidative addition of desyl chloride to Pd(0) followed by rapid tautomerization to give Pd species 131 (Figure 124). Subsequent displacement of the enolate group in 131 by ROH, followed by CO insertion gave alkoxycarbonyl Pd complex 132. Transmetalation with alkylindium and reductive elimination furnished the desired alkyl esters 134.
Figure 124.
Oxidative Carbonylative Cross-Couplings (Lei and coworkers, 2008)
Exploiting the oxidative addition of desyl chloride to Pd(0) complex, the C(sp2)-C(sp3) cross-coupling of arylzinc reagents with trialkylindium reagents was accomplished in the following year by the same group. Analysis of the preliminary kinetic data revealed that alkylindium reagents rapidly undergo transmetalation after oxidation with desyl chloride. A second transmetalation with an arylzinc reagent and reductive elimination yields the cross-coupling product (Scheme 59).436
Scheme 59.
Oxidative Cross-Couplings (Lei and coworkers, 2009)
5.6. Formation of Ketones from Acid Chlorides
Sarandeses reported ketone formation via the cross-coupling of triorganoindium and acid chlorides. Besides triaryl and alkynylindium reagents, trimethylindium also afforded a near quantitative yield with benzoyl chloride (Scheme 60).421
Scheme 60.
Cross-Coupling with Acid Chlorides (Sarandeses and coworkers, 2001)
The Liebeskind group reported the synthesis of ketones via a Pd-catalyzed cross-coupling of thioesters and alkylindium reagents (Figure 125).437 This protocol offers an advantage over other existing methods, in that due to the thiophilicity of indium, no added Cu was required and no base activation was necessary. It was observed that with 0.40 equiv of a trialkylindium reagent less than 5% yield was observed, whereas upon increasing the amount of the indium reagent to 1.2 equiv, very high yields (95%) were achieved. Unfortunately, this process requires 3.6 equiv of the alkyl group from the indium reagent. This problem was addressed by using a non-transferring tert-butyl “dummy ligand” on indium. Employing 0.6 equiv of this mixed trialkylindium bearing two transferring alkyl groups, good yields of the ketones were achieved.
Figure 125.
Cross-Coupling with Thioesters (Liebeskind and coworkers, 2005)
5.7. Conclusion
Triorganoindium reagents have been successfully used in cross-coupling reactions. Due to their low reactivity, alkylindium reagents provide a high degree of chemo-, regio and stereoselectivity and good functional group compatibility. Above all, they are attractive alternatives as they are environmentally benign and generate substoichiometric (0.33 equiv) metal waste. In addition, due to their water compatibility, aqueous conditions can be used to perform cross-coupling reactions.
6. Cross-Coupling Reactions with Alkylsilicon Reagents
Hiyama first reported the use of an organosilicon in a cross-coupling reaction in 1988.438 Since then, substantial advancements have been made in the cross-coupling of alkynylsilicons and arylsilicons, while the use of alkylsilicons is still limited.439–442 The strong C-Si bond requires higher temperatures along with activation of the organosilicon reagent for successful transmetalation, which restricts their use. However, alkylsilicons have several advantages over other alkylmetals: 1) low toxicity, 2) high stability to air and moisture and 3) easy handling and preparation. In this part of the review, the seminal report on the use of alkylsilicons in cross-coupling as well as significant advancements will be discussed in chronological order.
In 1988, Hiyama and coworkers reported the first example of alkylation of aryl halides with alkylsilicon.438 They showcased the use of tris(diethylamino)sulfonium difluorotrimethylsilicate (TASF) as a source of a methyl group in cross-coupling with various aryl halides. The [Pd(allyl)Cl]2 was an effective catalyst for this transformation. The Pd-catalyzed methylation reaction using TASF has excellent functional group tolerance (Figure 126).
Figure 126.
Methylation Reaction of Aryl Halides (Hiyama and coworkers, 1988)
Several years later, Hiyama and coworkers reported the use of vinyl and aryl triflates in the cross-coupling reaction with various organosilicons.443,444 TBAF as the source of fluoride was essential for the successful reaction. Fluoride is proposed to react with the organosilicon reagent to generate pentavalent silicon, which undergoes transmetalation with the palladium complex. The Hiyama group expanded the scope of this transformation to various functionalized aryl halides and organosilicons (Figure 127, 128).445–447 They observed that Pd(PPh3)4 in THF at 100 °C was the best catalyst for the desired transformation. Moreover, excess TBAF was required for activation of the alkylsilicon.
Figure 127.
Cross-Coupling of Aryl Triflate with Alkylsilicon (Hiyama and coworkers, 1990)
Figure 128.
Cross-Coupling of Aryl halides with Alkylsilicon (Hiyama and coworkers, 1997)
The proposed mechanism for this transformation is depicted in scheme 61.446 The oxidative addition of the aryl halide results in the formation of Pd-aryl complex 136, which undergoes transmetalation with activated alkylsilicon 138 to give Pd complex 141 and SiF4. Upon reductive elimination, the product is released and the active catalyst 135 is regenerated. The SiF4 is proposed to react with an equivalent of TBAF to either form SiF5− (139) or SiF6−2(140) species. The existence of these species was confirmed by 19F-NMR.448,449
Scheme 61.
Proposed Mechanism (Hiyama and coworkers, 1997)
Recently, Bach and Schweizer reported a highly regioselective monoalkylation of dibromo heteroarenes.450 Bidentate phosphine ligands gave mainly the undesired hydrodebromination product, whereas monodentate phosphines gave the desired product. P(2-furyl)3 was the optimal ligand, giving the best selectivity for the desired product. Extended reaction times and elevated temperatures were required due to slow transmetalation of alkylsilicon. Under the optimized conditions, an array of dibromo-substituted heterocycles was successfully employed.450
In 2010, Hiyama and coworkers reported the use of 2-(2-hydroxyprop-2-yl)phenyl-substituted alkylsilanes in cross-coupling reactions (Figure 129).451 These reagents have advantages over the widely used polyfluorinated alkylsilicons such as stability under acidic as well as basic conditions. The authors observed that the use of Cu(hfacac)2 as a cocatalyst gave improved results, which suggests that an alkyl Cu species formed in situ from alkylsilicon might be the active transmetalating agent. Under the optimized conditions, various functionalized alkylsilicon reagents cross-coupled to give products in good yields. Furthermore, challenging sec-alkylsilicons also yielded the desired products.
Figure 129.
2-(2-Hydroxyprop-2-yl)phenyl-Substituted Alkylsilanes in Cross-Coupling Reaction.
7. Cross-Coupling Reactions with Alkyltin Reagents
Stille cross-couplings with organotin reagents are some of the most frequently used methods to form C–C bonds.3,416 In practice though, aryl -or alkenyltin reagents are commonly used as coupling partners, while alkyl transfer is mostly limited to methyl group transfer using SnMe4.452 Although there are a few examples of benzyl or methoxymethyl group transfer from tin reagents, alkyl groups containing β-hydrogens such as n-butyl are extremely rare453 and very often they provide low yields or fail to give any cross-coupling products.417,452,454 This is in contrast to the SnMe4 reagent, which produces the corresponding coupling products in high yields under the same reaction conditions. Alkyltin reagents can be prepared conveniently via the oxidative addition of alkyl halides, or Grignard additions to ClSnMe3.455
In 1983, the Tam group reported the Pd-catalyzed Stille coupling of 2,3-dichloro-1,4-naphthoquinones to afford 2-alkylated products (Figure 130).456 During alkylation it was observed that tetraalkyltin reagents containing alkyl groups such as methyl or n-butyl, were reactive enough to provide high yields, however, the reaction was slow with larger alkyl groups. For example, tetra-dodecyltin provided only 25% of the cross-coupling product after refluxing in dioxane for six days. The same order of reactivity was also observed in the case of alkylation of arenediazonium o-benzenedisulfonamides.271 Using Pd-catalysis, tetramethyltin afforded substituted toluenes in high yields, whereas tetra-n-butyltin afforded only low or moderate yields under identical reaction conditions.
Figure 130.
2-Alkylation of 2,3-Dichloro-1,4-naphthoquinone (Tam and coworkers, 1983)
Ohta and coworkers demonstrated alkylations of pyrazines by a Pd-catalyzed Stille coupling. Pyrazine or pyrazine N-oxides were alkylated using tetra-n-butyltin, tetra-n-pentyltin and tetra-n-octyltin reagents, providing good to high yields (Figure 131).457
Figure 131.
2-Alkylation of Pyrazines (Ohta and coworkers, 1989)
Fouquet and coworkers illustrated an attractive advancement in Stille couplings.458 Considering the low reactivity, inherent toxicity and difficulties in separation of tetraalkyltin reagents, they prepared activated tin reagents in situ by simple oxidative addition of alkyl halides to low valent tin, which underwent a subsequent Pd2(dba)3-catalyzed cross-coupling reaction (Figure 132). Gratifyingly, only TBAF was used as an additive, no additional ligand was required on Pd, and relatively less toxic inorganic tin byproducts were separated from the organic materials by simple filtration. A number of n-alkyl iodides and bromides underwent cross-couplings with aryl iodides and bromides providing high yields. Unfortunately, secondary alkyl bromides did not furnish any products. Perfluorinated alkyls were also tested and afforded the corresponding cross-coupling products under even milder reaction conditions. Chiappe and coworkers also reported ligandless Stille couplings in ionic liquids.459 A single example of Stille cross-coupling between an alkyltin chloride and an aryl chloride appeared in Fu’s general protocol for Stille couplings.460
Figure 132.
Stille Coupling of Activated Alkyltin Reagents (Fouquet and coworkers, 2005)
In conclusion, although alkylative Stille couplings with SnMe4 are well demonstrated, higher alkyltin reagents are limited in use due to their low reactivity and alkyl transfer capability. In addition, due to the toxicity of tin, Stille couplings for alkylations have been substantially substituted by Negishi and Suzuki protocols.
8. Asymmetric Cross-Coupling Reactions with Alkyl Organometallics
8.1. Introduction
The synthesis of both natural and unnatural organic compounds in optically active form is a central challenge in chemistry. Enantioselective cross-coupling reactions are powerful tools for the assembly of diverse building blocks in a stereocontrolled manner via C-C bond formation.461 Strategically, it can be 1) reagent-controlled, where the organometallic reagent itself is stereo- or diastereoselective; or 2) catalyst-controlled. The latter is the most powerful method for chiral induction, as it requires only a catalytic amount of precious chiral ligands on the metal centers and is highly tunable.462 Cross-coupling with reactants containing remote stereocenters on their backbone will not be discussed in this section.
8.2. Organometallic Reagent-Controlled Enantioselective Cross-Coupling Reactions
In reagent-controlled enantioselective cross-coupling, an alkyl organometallic reagent with a well-defined stereocenter is prepared and undergoes a subsequent cross-coupling with a prochiral substrate to provide an optically active product. The downside to this strategy is that depending on the reaction mechanism and reaction conditions, the stereochemical fidelity can be poor or inversion may occur. In 1990, the Hiyama group observed the influence of temperature and solvent on the stereochemistry of the cross-coupling reaction of chiral alkylsilanes with aryl triflates.463 The cross-coupling between (S)-phenyl-1-(trifluorosilyl)ethane (34% ee) with 4-acetylphenyl triflate took place smoothly in the presence of 5 mol% Pd(PPh3)4 and 2.0 equiv of TBAF in THF to afford the cross-coupled product with retention of configuration at 50 °C (32% ee), and an inversion of configuration at 75 °C (Figure 133). Furthermore, the inversion of configuration was observed predominantly in a polar solvent system (HMPA-THF) even at 50 °C, whereas retention was still maintained in comparatively less polar DMF and DMSO at 50 °C. Presumably, the Pd-catalyzed cross-coupling of an organosilane with a triflate involves a pentacoordinate silicate intermediate which is generated by nucleophilic attack of a fluoride ion to an organosilicon compound. The retention of configuration in THF at low temperature is ascribed to a fluoride ion-assisted cyclic four-centered transition state, whereas at higher temperatures or in HMPA-THF, the fluoride bridge is cleaved and a back-side attack of the Pd-complex occurs, leading to inversion (Figure 133).
Figure 133.
Hiyama Coupling of Chiral Alkyl Silanes (Hiyama and coworkers, 1990)
Crudden and coworkers reported the Pd-catalyzed cross-coupling of chiral secondary alkylboronic esters with aryl iodides.464 The chiral boronic esters were synthesized by Rh-catalyzed hydroboration of alkenes wiht excellent enantiomeric ratios. A subsequent cross-coupling with aryl iodides in the presence of 0.08 equiv Pd2(dba)3 and 1.0 equiv Ag2O afforded coupling products in high yields and with retention of configuration (Figure 134). The Deng group465 and the Gevorgyan group466 also reported a Pd-catalyzed cross-coupling of chiral cyclopropylboronic acids with aryl iodides and aryl bromides, producing the corresponding cross-coupling products in high yields and with retention of configuration. Interestingly, the primary alkylboron remains intact under the reaction conditions.
Figure 134.
Suzuki Coupling with Retention of Configuration (Crudden and coworkers, 2009)
While the enantioenriched 1-arylethylboronic esters underwent cross-coupling with retention of configuration, an inversion of configuration was observed in the case of Pd-catalyzed cross-coupling between α-(acetylamino)benzylboronic esters and aryl bromides (Figure 135).467 Presumably, due to the strong intramolecular coordination of the carbonyl group to boron, the Pd-complex cannot attack from the same side of boron via p-orbital overlap (TS2). Alternatively, a backside attack on the carbon center of α-(acetylamino)benzylboronic esters (TS1) leads to the inversion of configuration in the final product (Scheme 62). β-hydride elimination is not an issue in these substrates.
Figure 135.
Suzuki Coupling with Inversion of Configuration (Suginome and coworkers, 2010)
Scheme 62.
Proposed Mechanism for Inversion of Configuration
Campos and coworkers reported a reagent-controlled enantioselective α-arylation of N-Boc-pyrrolidines (Figure 136). The enantioenriched zinc reagent was prepared in situ via deprotonation of N-Boc pyrrolidine by s-BuLi and (−)-sparteine followed by treatment with zinc chloride at −78 °C. The reagent was then cross-coupled at room temperature with aryl halides in the presence of 5 mol% Pd(OAc)2 and 6 mol% t-Bu3P-HBF4 in MTBE, affording high yield and enantioselectivities of the α-arylated products.468 The high degree of stereoselectivity of the cross-coupled product suggests that the secondary zinc reagents are configurationally stable under the reaction conditions and undergo facile transmetalation with the Pd-species. Since numerous biologically active molecules contain N-protected pyrrolidines in their backbone, this methodology can offer a concise and practical route for their synthesis. An illustrative example is the synthesis of a glucokinase activator (Scheme 63).469 Recently, Knochel and coworkers reported a Pd-catalyzed diastereoselective Negishi coupling with substituted cycloalkylzinc reagents.470
Figure 136.
Negishi Coupling with Chiral Alkylzinc Reagent (Campos and coworkers, 2006)
Scheme 63.
Synthesis of Gluocokinase Activator (Campos and coworkers, 2008)
8.3. Catalyst-Controlled Enantioselective Cross-Coupling Reactions
Catalyst-controlled enantioselective cross-coupling is the most powerful technique to afford optically active cross-coupled products, as it requires only substoichiometric chiral ligands.471 In 1982, Kumada and coworkers reported the asymmetric cross-coupling of secondary alkyl Grignard reagents with organic halides catalyzed by chiral ferrocenyl-phosphine complexes of Ni and Pd.472 It was observed that the ferrocene planar chirality was a dominant factor over the chiral carbon center attached to one of the cyclopentyl rings, and a dimethylamino group vicinal to the chiral carbon center was required to achieve high degrees of enantioselectivity (Scheme 64). Mechanistically, it is speculated that the ferrocenyl metal complex undergoes oxidative addition to the alkenyl halide to generate a tetracoordinated complex 143 (Scheme 65). When the Grignard reagent approaches, the dimethylamino group dissociates from metal center and coordinates with the magnesium atom of the Grignard reagent to form the diastereomeric intermediate 144. This coordination occurs selectively with one of the enantiomers of the racemic Grignard reagent, and facile transmetalation occurs to form the diorganonickel- or Pd-intermediate 145. The stereoselectivity of the reaction is determined at the transmetalation step, and coordination with the pendant dimethylamino group is influential.
Scheme 64.
Asymmetric Kumada Coupling with Chiral Ferrocenylphosphine Ligand (Kumada and coworkers, 1982)
Scheme 65.
Coordination-Assisted Chiral Induction Model (Kumada and coworkers, 1982)
In 1986, the Kellogg group designed, developed, and synthesized a series of chiral, macrocyclic sulfide- and sulfide/alkylamine-containing ligands for Ni-catalyzed cross-coupling reactions with Grignard reagents (Scheme 66).473 After ligand evaluation, it was observed that an L-cysteine-derived tetradentate ligand provided moderate yield (50%) with 46% enantiomeric excess of the cross-coupled product between vinyl bromide and 1-phenyl-1-chloroethane (Scheme 66). The square planar geometry provided by this ligand at the Ni(0) and diorganonickel intermediates was found to be crucial for effective asymmetric induction, as the open chain analogue of the ligand afforded only 8% ee of the same product.
Scheme 66.
Asymmetric Kumada Coupling with Chiral Sulfide Ligand (Kellogg and coworkers, 1986)
Shibasaki and coworkers developed an intramolecular asymmetric Suzuki-Miyaura cross-coupling reaction to construct cyclopentane derivatives (Scheme 67).474 Prochiral 9-BBN derivatives of vinyl triflates were prepared by hydroboration of the corresponding alkenes and subsequently cross-coupled in the presence of Pd2(dba)3·CHCl 3, (S)-(R)-BPPFOAc and K2CO3 in THF at 40 °C. The product was isolated after the subsequent oxidative work-up and benzoylation in overall 58% yield with 28% ee.
Scheme 67.
Asymmetric Synthesis of Cyclopentane Derivatives (Shibasaki and coworkers, 1998)
In 2001, Lemaire reported an asymmetric Kumada-Corriu coupling of 1-phenylethylmagnesium chloride with vinyl bromide in the presence of NiCl2 and quinphos ligand (Scheme 68).475 A satisfactory enantiomeric excess was obtained (85%) at 0 °C along with a moderate yield (50%) of the cross-coupling product.
Scheme 68.
Asymmetric Kumada Coupling with Quinphos Ligand (Lemaire and coworkers, 2001)
In 2004, Aoyama also reported the Pd-catalyzed asymmetric cross-coupling of 1-phenylethylmagnesium and (E)-β-bromostyrene using an N-Ar axially chiral ligand (Figure 137). Interestingly, this protocol offered the chemoselective cross-coupling of vinyl bromides in the presence of aryl halides.476
Figure 137.
Asymmetric Kumada Coupling (Aoyama and coworkers, 2004)
From the above examples, it is quite evident that asymmetric induction in cross-coupling is very difficult and, in most cases, moderate to good enantiomeric excess of the cross-coupled products was observed. Recently, Fu and coworkers developed a series of Ni-catalyzed enantioselective cross-coupling reactions between alkyl organometallics and different types of secondary electrophiles using chiral nitrogen-based ligands. In a mechanistic study, Vicic and coworkers illustrated that Ni-catalyzed cross-couplings proceed in a stereoconvergent manner instead of through a kinetic resolution. Therefore, the oxidative addition of alkyl halides most likely occurs through a radical pathway and a planar radical intermediate has been proposed.20,21 The stereochemistry of the cross-coupling product can then be determined by the configuration of the ligand used. In 2005, the Fu group reported the enantioselective Negishi coupling of benzylic halides in the presence 10 mol% NiBr2•diglyme and 13 mol% (S)-(i-Pr)-Pybox in DMA at 0 °C (Figure 138).477 The high yields and excellent enantioselectivity of the cross-coupled products are noteworthy. In the same year, they also reported an enantioselective Negishi coupling of secondary α-bromo amides in the presence of 10 mol% NiCl2•glyme and 13 mol% (R)-(i-Pr)-Pybox in DMI/THF at 0 °C.478
Figure 138.
Asymmetric Negishi coupling with Benzylic Halides (Fu and coworkers, 2005)
The Fu group also reported a Ni-catalyzed enantioselective cross-coupling of secondary allylic chlorides and primary alkylzinc bromides. Consiglio and coworkers have previously reported Ni-catalyzed, asymmetric Kumada-Corriu couplings with cyclic allyl phenyl ethers, but the yields and enatioselectivities were poor.479 Furthermore, the substrate scope was limited to cyclic allyl phenyl ethers to avoid regioselectivity issues. Using Fu’s conditions high yields and excellent regio- and enantioselectivities were obtained using (S)-BnCH2-Pybox as a chiral ligand (Figure 139).480 As a showcase, this methodology has been applied to the formal synthesis of fluvirucinine A1, where excellent regio-, diastereo-, and enantioselectivity have been achieved (Scheme 69).
Figure 139.
Asymmetric Negishi Coupling with Allylic Chlorides (Fu and coworkers, 2008)
Scheme 69.
Formal Total Synthesis of Fluvirucinine A1 (Fu and coworkers, 2008)
In 2008, the Fu group demonstrated the stereoconvergent, enantioselective alkyl-alkyl Suzuki cross-coupling of unactivated homobenzylic halides catalyzed by Ni-complexes. It was hypothesized that the chiral Ni-complex should differentiate between the alkyl groups of benzylCH2 and alkyl groups attached to secondary bromides. Therefore, proper placement of the aryl groups was found to be important for chiral induction, as deviation from the homobenzylic position produced low enantioselectivity. During this study it was noted that when an ether is present in the electrophile, low enantioselectivity was observed (Figure 140).481 It was therefore hypothesized that, the Ni-complex might coordinate with the oxygen of the ether moiety. Using this concept, the protocol was extended into the asymmetric alkyl-alkyl cross-coupling of acylated halohydrins under slightly modified reaction conditions, where a simple phenyl-substituted diamine ligand was used in place of the electron-deficient (m-CF3-C6H4) substitution on the diamine ligand.482
Figure 140.
Asymmetric Suzuki Coupling with Homobenzylic Bromides (Fu and coworkers, 2008)
8.4. Conclusion
In conclusion, enantioselective cross-coupling reactions with different electrophiles and alkyl organometallics have been demonstrated and more attention is being devoted to this field. Recent advancements using Fu’s conditions are noteworthy. However, sparse mechanistic information has been reported on these reactions which may be limiting the extension to broader reaction classes.
9. Applications of Alkyl Organometallic Reagents in Total Synthesis
In recent years, alkyl organometallics have been used extensively in the total synthesis of complex natural products. High functional group tolerance, mild coupling conditions, and the ability to utilized novel disconnections have made cross-coupling a mainstream fragment coupling technology. The application of cross-coupling reactions in total synthesis has been extensively reviewed by Danishefsky231 and Nicolaou232 in 2001 and 2005, respectively. In this final section of the review, representative examples of alkyl boron, alkyl zinc, and alkyl magnesium compounds used in total synthesis will be covered in chronological order starting from 2001 to 2010.
9.1. Total Synthesis with Alkylboron Reagents
In 2002, Mandal reported the total synthesis of (−)-ebelactone A using a Suzuki-Miyaura cross-coupling (Figure 141a).483 The diastereoselective hydroboration of fragment 146 with Still’s hydroboration protocol gave compound 147.484 Late stage cross-coupling was achieved between compound 147 and 148 using catalytic Pd(dppf)Cl2 in 70% isolated yield as a single diastereomer. In 2002, Marshall and coworkers utilized a B-alkyl Suzuki-Miyaura cross-coupling as a key step in their total synthesis of (−)-callystatin A (Figure 141b).485 The union of subunits 150 and 151 was achieved in 73% yield using 5 mol% Pd(dppf)Cl2, AsPh3, Cs2CO3, DMF/H2O.
Figure 141.
Total Synthesis using Alkylboron Reagents (2002–2004).
In 2005, Sasaki and coworkers reported the total synthesis of marine polyether gymnocin-A486 using Suzuki-Miyaura cross-coupling methodology developed in their lab.269 They have reported the synthesis of various natural products containing cyclic ether moieties using their methodology.487,488 Since all of them follow the same general concept, we will cover only their synthesis of brevenal (Figure 141c).487 Hydroboration of compound 153 followed by reaction with compound 152 in the presence of Cs2CO3 and Pd(dppf)Cl2 gave the desired product in good yield. Successful use of the Suzuki-Miyaura reaction in the late stage of a total synthesis of a molecule of this complexity clearly showcases the high chemoselectivity and mildness of these procedures.
Another elegant example of the late stage use of a Suzuki-Miyaura cross-coupling was demonstrated by Lee and coworkers (Figure 141d). During the total synthesis of kendomycin, cross-coupling between compound 155 and compound 156, using [Pd(dppf)Cl2] as the catalyst in a ether/THF/DMF solvent mixture, gave product 157 in excellent yield.489 Sequential functional group manipulations transformed intermediate 157 into the natural product. In 2004, Cossy and coworkers reported the total synthesis of (+)-(3′S, 2′R)-zaopatanol using the Suzuki-Miyaura reaction (Figure 141e).490 In this case, use of Pd(PPh3)2Cl2 with K3PO4 in dioxane at 85 °C gave the desired product in 74% yield.
In 2006, Smith and coworkers reported the total synthesis of the indole diterpenoid (+)-nodulisporic Acid F (Figure 142a).491 The alkyl side chain was introduced by Suzuki-Miyaura cross-coupling between compound 162 and tether 163. The reaction of 161 with 9-BBN dimer in toluene gave the corresponding alkyl borane 162, which reacts smoothly with vinyl bromide 163 to furnish 164 in 69% yield. Dai and coworkers synthesized amphidinolide Y, a 17-membered cytotoxic macrolide (Figure 142b).492 The treatment of compound 165 with 9-BBN gives intermediate 166, which was then subjected to Suzuki-Miyaura cross-coupling to give the desired product in 70% yield. Use of the Aphos ligand493,494 gave better results compared to dppf and allowed for the use of lower catalyst loading. In 2008, Roulland reported the synthesis of (+)-oocydine A, a cytotoxic and phytopathogenic compound, by means of the B-alkyl Suzuki-Miyaura reaction (Figure 142c).495 Utilizing a methodology developed in their laboratory,266 they were able to accomplish the coupling of two highly functionalized fragments, 167 and 9-BBN derivative of 168. It should be noted that, using 1,1-dichloroalkene 167, the chloride trans to the alkyl chain reacts preferentially, giving the E-isomer of 169 in excellent yield and selectivity. Kigoshi and coworkers also achived the synthesis of haterumalide HA ((+)-oocydine A), a potent cytotoxic marine macrolide, using Suzuki-Miyaura cross-coupling (Figure 142d).496 The use of 9-BBN dimer gave better results than 9-BBN under optimized reaction conditions.
Figure 142.
Total Synthesis using Alkylboron Reagents (2006–2009).
In 2009, Williams and coworkers reported a unique use of the B-alkyl Suzuki-Miyaura cross-coupling in the total synthesis of 4-hydroxydictyolactone (Figure 142e).497 They utilized a cross-coupling protocol to construct a macrocycle from highly functionalized late stage intermediate 173. During their optimization, they observed a significant influence of the protecting group on the reaction outcome. Interestingly, the use of Pd(PPh3)4 instead of the widely used Pd(dppf)Cl2 gave better results for the Suzuki macrocyclization event.
Mycestericin A, a potent immunosuppresant, was synthesized by Chiba and coworkers (Figure 143a).498 The Negishi cross-coupling between lactone 176 and the alkylzinc derivative of 175 gave the product 177 in low yield. This was proposed to be due to the presence of base-sensitive functionalities (lactone and acetyl groups) in compound 176. In contrast, the Suzuki-Miyaura cross-coupling led to a 77% yield of the desired product 179. Jatrophane was efficiently synthesized using a Suzuki-Miyaura cross-coupling (Figure 143b).499 Hiersemann and coworkers used 7 mol% Pd(dppf)Cl2, 20 mol% Ph3As and Cs2CO3 in THF:DMF:H2O at 80 °C to effect the highly efficient coupling of vinyl iodide 181 with the alkyl borane derived from 180.
Figure 143.
Total Synthesis Using Alkylboron Reagents (2009)
Barrero and coworkers reported the first total synthesis of potent anti-inflammatory (+)-myrrhanol-A in 2009 (Figure 143c).500 They used a late stage B-alkyl Suzuki-Miyaura cross-coupling to assemble the advanced fragments 183 and 184 to give product 185 in 90% yield as a single regioisomer. Subsequent deprotection of ethers afforded the natural product. Another elegant use of a late stage B-alkyl Suzuki-Miyaura cross-coupling was demonstrated by Fürstner and coworkers in their total synthesis of spirastrellolide F methyl ester.501,502 Two highly functionalized fragments 186 and 187 were coupled under mild conditions to give key intermediate 188 in a respectable 75% yield.
An alternative version of the B-alkyl Suzuki coupling was used by Bonazzi and coworkers in a synthesis of the polyketide anguinomycin (Scheme 70).503 Lithiation of primary iodide 189, followed by an addition of 9-methoxy-BBN gave the intermediate borate 190, which was coupled with iodoalkene 191 in high yield (Scheme 70).
Scheme 70.
Total Synthesis of Anguinomycin (Bonazzi and Coworkers, 2010)
Other molecules which have been synthesized using the B-alkyl Suzuki-Miyaura cross-coupling are depicted in Figure 144.274,504–509
Figure 144.
Application of B-alkyl Suzuki-Miyaura Cross-Coupling
9.2. Total Synthesis with Alkylzinc Reagents
In 2002, Panek and coworkers reported the total synthesis of oleandolide, the aglycon of the macrolide antibiotic oleandomycin (Scheme 71).510 The failure of the Suzuki-Miyaura cross-coupling to efficiently provide key intermediate 196 prompted the authors to explore a Negishi cross-coupling. The rationale behind this change was that transmetalation from Zn to Pd is faster than the transmetalation from B to Pd, and a cleaner and faster reaction might be achieved.134 Indeed, reaction of alkylzinc 197 with vinyl triflate 195 gave the cross-coupled product 196 in 82% isolated yield.
Scheme 71.
Total Synthesis of Oleandolide (Panek and coworkers, 2002)
In 2002, Kibayashi reported an elegant use of the Negishi coupling reaction for the synthesis of (+)-Pumiliotoxins A and B511 from common precursor 198 (Figure 145a). This was the first example of cross-coupling between a highly functionalized homoallylic organozinc such as 199 and a vinyl iodide like 200. The common intermediate 198 was treated with ZnCl2 in presence of t-BuLi to give the corresponding alkylzinc intermediate 199. The reaction of intermediate 199 with 200 in the presence of Pd(PPh3)4 gave compound 201 in 60% yield. When the same methodology was applied to the synthesis of 204, it resulted in the formation of the desired coupling product in low yield (28%) together with a complex mixture. The low yield was presumably due to the Lewis acid sensitive acetal group, which could react in the presence of IZnCl generated from the cross-coupling. To overcome this problem, dialkylzinc 202 was cross-coupled with 203 to give 51% yield of the desired product 204. Sequential functional group manipulations transformed intermediates 201 and 204 into the corresponding natural products.511
Figure 145.
Total Synthesis using Alkylzinc Reagents (2002–2003).
In 2003, Morken and coworkers reported the total synthesis of borrelidin, a potent angiogenesis inhibitor with an IC50 of 0.8 nM (Figure 145b).512 Alkyl iodide 205 was converted to the corresponding alkylzinc in situ and subjected to the coupling conditions to give 207 in 58% yield. A similar coupling strategy (coupling between alkyl iodide and vinyl iodide) was used by Altmann and coworkers for the total synthesis of trans-epothilone A.513 For the total synthesis of hemibrevetoxin B, the coupling of two fragments was accomplished utilizing a Negishi cross-coupling by Holton and coworkers (Figure 145c).514 The authors observed a significant effect of the method of zinc activation on the reaction outcome. When iodine-zinc exchange was performed through Pd catalysis or Mn/Cu515 mixed metal catalysis, the yield of 211 was low and variable, whereas when the zinc reagent 210 was prepared following a Rieke zinc protocol,98 a high yield of 211 was obtained.
In 2003, Fürstner utilized an acyl Negishi cross-coupling between the highly functionalized alkylzinc derivative of 212 and enantiopure acyl chloride 213, in the total syntheses of amphidinolides T1, T2, T3 and T4 (Figure 145d).516 The alkyl iodide 212 was treated with Zn/Cu couple, which was activated with TMSCl immediately prior to use, to give the alkylzinc. The alkylzinc generated in situ was treated with acyl chloride 213 in the presence of Pd2(dba)3 as catalyst and P(2-furyl)3 as ligand to give the desired product 214 in modest yield. The reaction was sensitive to the conditions used, e.g. only toluene as solvent with DMA as the additive was successful; also, the use of coupling partners other than alkylzincs did not result in the formation of the desired product.
In 2004, Vyvyan and coworkers reported the total synthesis of various aromatic bisabolene natural products via Pd-catalyzed Negishi cross-coupling (Figure 146a).517 The reaction of sec-alkylzinc 216 with aryl bromides 217 and 218, respectively gave the corresponding products in good yields. Scyphostatin, a powerful and specific inhibitor of neutral sphingomyelinase, has been synthesized by various groups using the Negishi coupling (Figure 146b).518–522 One representative example is shown in Figure 146b, where the coupling of vinyl iodide 222 with the alkylzinc generated in situ from 221 provided the desired product 223 in 81% yield.
Figure 146.
Total Synthesis using Alkylzinc Reagents (2004–2009).
During the total synthesis of brevetoxin B, Yamamoto and coworkers523 used a Negishi cross-coupling to install the side chain 225 on fragment 224 (Figure 146c). In 2007, Crews and coworkers utilized a Negishi cross-coupling to the synthesize the C3-C18 fragment of amphidinolides G and H, where the treatment of compound 227 with alkylzinc 228 in the presence of Pd(PPh3)4 gave the coupled product 229 in 92% yield (Figure 146d).524 The same alkylzinc (228) was used by Pilli and coworkers in the total synthesis of (−)-delactomycin.525
The total synthesis of (−)-kendomycin, a potential antiosteoporotic agent, was achieved by Panek and coworkers utilizing a Negishi cross-coupling (Figure 146e).526 The reaction of 230 with the alkylzinc derived from 231 in the presence of Pd(PPh3)4 gave product 232 in 92% yield. In Paige and coworker’s approach towards the synthesis of (S)-jamaicamide C, a Negishi reaction was used to introduce the alkyl chain (Figure 146f).527 The reaction of vinyl iodide 233 with alkylzinc gave the product 234 in good yield.
In 2009, Jackson and coworkers used a Negishi cross-coupling as a key step in the synthesis of OF4949-III and K-13 (Figure 147a).528 OF4949-III was synthesized by reaction of compound 235 with alkylzinc 236 to give 237 in 75% yield. This provided the full carbon skeleton of OF4949-III and only a few functional group manipulations were needed to furnish the final product. Impressively, K-13 was synthesized via an intramolecular Negishi cross-coupling of highly functionalized compound 238, though in this case a lower yield was observed.
Figure 147.
Total Synthesis using Alkylzinc Reagents (2009–2010).
Recently, Rainier and coworkers synthesized kapakahines E and F, potent cytotoxic compounds, utilizing a key Negishi cross-coupling (Figure 147b).529 The reaction between 3-iodoindole 240 and well known alkylzinc 241 gave the desired product 242 in 74% yield, effectively completing the synthesis of the kapakahine dimeric tryptophan core. Other molecules which have been synthesized using the Negishi cross-coupling are depicted in Figure 148.
Figure 148.
9.3. Total Synthesis with Alkylmagnesium Reagents
In 2002, Danishefsky and coworkers demonstrated the use of a Kumada cross-coupling for synthesis of 17- and 18-membered ring homologues of epothilone B (Figure 149a).532 They successfully coupled 243 and 244, using Pd(dppf)Cl2 as catalyst, to give 245 in good yield. In 2003, Fürstner and coworkers demonstrated the first example of Fe-catalyzed cross-coupling between a vinyl triflate 246 and alkyl-MgBr (247) during their synthesis of latrunculin B (Figure 149b).533 The reaction affords the desired product in excellent yield. Furthermore, the reaction is highly stereoselective and proceeds very rapidly under notably mild conditions.
Figure 149.
Total Synthesis using AlkylMg Reagents (2003–2010).
To further demonstrate the utility of Fe-catalyzed cross-coupling, Fürstner and coworkers reported the total synthesis of muscopyridine (Figure 149c).534 Here, compound 251 was functionalized to give 254 in one pot with two different alkyl Grignards. First, 251 was treated with 250 to give intermediate 253 after reaction with more reactive C-OTf bond. Upon completion, 6-heptenylmagnesium bromide was added to the same reaction mixture to give 254 in 80% overall yield. Fürstner also utilized an iron-catalyzed cross-coupling for the total synthesis of the macrocyclic spermidine alkaloid isooncinotine.535
In 2004, Fürstner and coworkers demonstrated another example of Fe-catalyzed cross-coupling for the synthesis of FTY720 (Figure 149d).536 Here, the coupling of aryl triflate 255 and octyl-MgBr was catalyzed by commercially available Fe(acac)3 to give the desired product 256 in 84% yield. This reaction showcases the chemoselectivity of Fe-catalyzed reactions toward C-OTf bond in the presence of an ester functionality. In 2005, Mulzer and coworkers demonstrated the use of Pd-catalyzed Kumada cross-coupling in the total synthesis of 15-deoxy-16-(m-tolyl)-17,18,19,20-tetranorisocarbacyclin.537
Cahiez and coworkers reported an efficient route for the synthesis of terminal conjugated dienes by coupling of dienol phosphates with Grignard reagents. The methodology was used for the synthesis of Diparopsis castanea pheromone. The iron-catalyzed cross-coupling between 257 and alkylMgX (258) gave the desired product in 79% yield with excellent stereoselectivity.538 The sex pheromone of female moss, Bombyx mori, was synthesized stereospecifically by Ni-catalyzed cross-coupling of vinyl bromide with PrMgBr.539
10.0. Summary and Outlook
As demonstrated in this review, the use of alkylorganometallic reagents in metal-catalyzed cross-coupling has seen a rapid acceleration in the development of catalytic procedures using a wide range of coupling partners, metal catalysts, and diverse organometallics. This is highlighted by the general use of these processes to access key building blocks for synthesis and applications to complex fragment couplings in target synthesis. An especially exciting development is in the area of asymmetric catalysis where methods have been developed to set isolated alkyl chiral centers. Furthermore, recent progress in the use of iron catalysts is noteworthy.
While progress has been considerable over the last decade, in our opinion, there are numerous issues that need to be resolved, including further development of C(sp3)-C(sp3) cross-couplings to cover a broader scope (especially secondary electrophiles), enhanced scope for enantioselective variants, and understanding of the underlying processes. There clearly is a dearth of mechanistic understanding particularly in Ni- and Fe-catalyzed processes. We believe the most important synthetic developments will most likely arise from future mechanistic studies and detailed understanding.
Figure 41.
Secondary-Secondary Negishi Couplings (Fu and coworkers, 2008)
Figure 42.
Various Organoboron Reagents
Figure 108.
Proposed Mechanisms
Scheme 14.
Suzuki-Miyaura Catalytic Cycle
Scheme 24.
Intramolecular Suzuki-Miyaura Couplings
Scheme 52.
Proposed Mechanism for Reaction of Grignard Reagents Containing β-hydrogen with FeX2
Scheme 55.
Catalytic Cycle for Triorganoindium Reagents
Acknowledgments
We thank the National Institutes of Health for support (NIGMS RO1GM3540). We thank Susanne Podhajsky and Laura Steffens for help with editing.
Biographies

Sigman Biography: Matt Sigman received a B.S. in chemistry from Sonoma State University in 1992 before obtaining his Ph.D. at Washington State University with Professor Bruce Eaton in 1996. He then moved to Harvard University to complete an NIH postdoctoral stint with Professor Eric Jacobsen. In 1999, he joined the faculty of the University of Utah where his research group has focused on the development of new synthetic methodology.

Jana Biography: Ranjan Jana is a native of India. He received his B.Sc. in Chemistry from Midnapur College (West Midnapur, India) in 2000 and M.Sc. in 2002 from Vidyasagar University. After attaining his Ph.D. in Organic Chemistry from the Indian Association for the Cultivation of Science (Kolkata, India) under the direction of Professor B. C. Ranu, Dr. Jana moved to Israel for postdoctoral studies at Bar-Ilan University under the supervision of Professor S. Braverman. He joined the Tunge at the University of Kansas and Center for Environmentally Beneficial Catalysis in 2008. Currently, he is working as a postdoctoral fellow in the Sigman group at the University of Utah and working on the development of small molecules for breast cancer therapy as well as asymmetric catalysis.

Pathak Biography: Tejas Pathak was born in Baroda, India. In 2003, he received his B.Sc. degree from Maharaja Sayajirao University of Baroda, where he received four gold medals for an excellent academic record. In 2005, he received his M.Sc. degree from the Indian Institute of Technology Madras (IIT-M) under the mentorship of Professor G. Sundarajan. Then, in 2006, he joined the Department of Chemistry at the University of Utah, where he is working in Professor Matthew Sigman’s research laboratory. His current research project involves development of Pd-catalyzed asymmetric alkene difunctionalization reactions.
References
- 1.de Meijere A, Diederich F, editors. In Metal-Catalyzed Cross-Coupling Reactions, Second Completely Revised and Enlarged Edition. Vol. 2. Wiley-VCH Verlag GmbH & Co; KGaA: 2004. [Google Scholar]
- 2.de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions, Second, Completely Revised and Enlarged Edition. Vol. 1. Wiley-VC CH Verlag GmbH & Co; KGaA: 2004. [Google Scholar]
- 3.Diederich F, Stang PJ, editors. Metal-catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinhein, Germany: 1998. [Google Scholar]
- 4.Knochel P, Leuser H, Gong LZ, Perrone S, Kneisel FF. Chem Organozinc Compd. 2006:287. [Google Scholar]
- 5.Knochel P, Leuser H, Gong L-Z, Perrone S, Kneisel FF. Polyfunctional zinc organometallics for organic synthesis. 2005;1 [Google Scholar]
- 6.Fouquet E, Herve A. Polyfunctional tin organometallics for organic synthesis. 2005;1 [Google Scholar]
- 7.Shimizu M, Hiyama T. Polyfunctional silicon organometallics for organic synthesis. 2005;1 [Google Scholar]
- 8.Knochel P, Krasovskiy A, Sapountzis I. Polyfunctional magnesium organometallics for organic synthesis. 2005;1 [Google Scholar]
- 9.Knochel P, Ila H, Korn TJ, Baron O. Functionalized organoborane derivatives in organic synthesis. 2005;1 [Google Scholar]
- 10.Knochel P, Kopp F. Handbook of functionalized organometallics. 2005;1 [Google Scholar]
- 11.Stephenson GR. Polyfunctional electrophilic multihapto-organometallics for organic synthesis. 2005;2 [Google Scholar]
- 12.Rilatt I, Jackson RFW. J Org Chem. 2008;73:8694. doi: 10.1021/jo801754k. [DOI] [PubMed] [Google Scholar]
- 13.Zou G, Reddy YK, Falck JR. Tetrahedron Lett. 2001;42:7213. [Google Scholar]
- 14.Frisch AC, Beller M. Angew Chem, Int Ed. 2005;44:674. doi: 10.1002/anie.200461432. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji J. Palladium in Organic Synthesis. Top Organomet Chem. 2005;14 [Google Scholar]
- 16.Netherton MR, Fu GC. Adv Synth Catal. 2004;346:1525. [Google Scholar]
- 17.Cárdenas DJ. Angew Chem, Int Ed. 1999;38:3018. doi: 10.1002/(sici)1521-3773(19991018)38:20<3018::aid-anie3018>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Cárdenas DJ. Angew Chem, Int Ed. 2003;42:384. doi: 10.1002/anie.200390123. [DOI] [PubMed] [Google Scholar]
- 19.Netherton MR, Fu GC. Angew Chem, Int Ed. 2002;41:3910. doi: 10.1002/1521-3773(20021018)41:20<3910::AID-ANIE3910>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Anderson TJ, Jones GD, Vicic DA. J Am Chem Soc. 2004;126:8100. doi: 10.1021/ja0483903. [DOI] [PubMed] [Google Scholar]
- 21.Jones GD, McFarland C, Anderson TJ, Vicic DA. Chem Commun. 2005:4211. doi: 10.1039/b504996b. [DOI] [PubMed] [Google Scholar]
- 22.Fürstner A, Martin R, Krause H, Seidel G, Goddard R, Lehmann CW. J Am Chem Soc. 2008;130:8773. doi: 10.1021/ja801466t. [DOI] [PubMed] [Google Scholar]
- 23.Kleimark J, Hedström A, Larsson P-F, Johansson C, Norrby P-O. ChemCatChem. 2009;1:152. [Google Scholar]
- 24.Zhao Y, Wang H, Hou X, Hu Y, Lei A, Zhang H, Zhu L. J Am Chem Soc. 2006;128:15048. doi: 10.1021/ja0647351. [DOI] [PubMed] [Google Scholar]
- 25.Chen M, Zheng X, Li W, He J, Lei A. J Am Chem Soc. 2010;132:4101. doi: 10.1021/ja100630p. [DOI] [PubMed] [Google Scholar]
- 26.Cahiez Gr, Moyeux A. Chem Rev. 2010;110:1435. doi: 10.1021/cr9000786. [DOI] [PubMed] [Google Scholar]
- 27.Knochel P, Singer RD. Chem Rev. 1993;93:2117. [Google Scholar]
- 28.Wagner G, Saytzeff A. Justus Liebigs Annalen der Chemie. 1875;175:351. [Google Scholar]
- 29.Negishi E-i. Organometallics in Organic Syntheses, Vol. 1: General Discussions and Organometallics of Main Group Metals in Organic Synthesis. 1980 [Google Scholar]
- 30.Negishi E-i, King AO, Okukado N. J Org Chem. 1977;42:1821. [Google Scholar]
- 31.Negishi E-i, Valente LF, Kobayashi M. J Am Chem Soc. 1980;102:3298. [Google Scholar]
- 32.Kobayashi M, Negishi E-i. J Org Chem. 1980;45:5223. [Google Scholar]
- 33.Negishi E-i. Acc Chem Res. 1982;15:340. [Google Scholar]
- 34.Negishi E-i, Bagheri V, Chatterjee S, Luo FT, Miller JA, Stoll AT. Tetrahedron Lett. 1983;24:5181. [Google Scholar]
- 35.Grey RA. J Org Chem. 1984;49:2288. [Google Scholar]
- 36.Nakamura E, Kuwajima I. Tetrahedron Lett. 1986;27:83. [Google Scholar]
- 37.Sato T, Naruse K, Enokiya M, Fujisawa T. Chem Lett. 1981:1135. [Google Scholar]
- 38.Tamaru Y, Ochiai H, Yoshida Z. Tetrahedron Lett. 1984;25:3861. [Google Scholar]
- 39.Tamaru Y, Ochiai H, Nakamura T, Tsubaki K, Yoshida Z. Tetrahedron Lett. 1985;26:5559. [Google Scholar]
- 40.Tamaru Y, Ochiai H, Sanda F, Yoshida Z. Tetrahedron Lett. 1985;26:5529. [Google Scholar]
- 41.Tamaru Y, Ochiai H, Nakamura T, Yoshida Z. Tetrahedron Lett. 1986;27:955. [Google Scholar]
- 42.Tamaru Y, Ochiai H, Nakamura T, Yoshida Z. Angew Chem Int Ed. 1987;26:1157. [Google Scholar]
- 43.Rieke RD, Hanson MV. Organozinc Reagents. 1999:23. [Google Scholar]
- 44.Frankland E. Justus Liebigs Ann Chem. 1849;71:171. [Google Scholar]
- 45.Elschenbroich C, Salzer A. In Organometallics: A Concise Introduction. 2. 1992. Rev. Ed. [Google Scholar]
- 46.Knochel P, Yeh MCP, Berk SC, Talbert J. J Org Chem. 1988;53:2390. [Google Scholar]
- 47.Erdik E. Tetrahedron. 1987;43:2203. [Google Scholar]
- 48.Gawronski JK. Tetrahedron Lett. 1984;25:2605. [Google Scholar]
- 49.Picotin G, Miginiac P. Tetrahedron Lett. 1987;28:4551. [Google Scholar]
- 50.Picotin G, Miginiac P. J Org Chem. 1987;52:4796. [Google Scholar]
- 51.Meyer C, Marek I, Courtemanche G, Normant J-F. Tetrahedron. 1994;50:11665. [Google Scholar]
- 52.Cornforth JW, Cornforth RH, Popják C, Gore IY. Biochem J. 1958;69:146. doi: 10.1042/bj0690146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gladstone JH. J Chem Soc, Trans. 1891;59:290. [Google Scholar]
- 54.Job A, Reich R. Bull Soc Chim Fr. 1923;33:1414. [Google Scholar]
- 55.Krug RC, Tang PJC. J Am Chem Soc. 1954;76:2262. [Google Scholar]
- 56.Renshaw RR, Greenlaw CE. J Am Chem Soc. 1920;42:1472. [Google Scholar]
- 57.Boland W, Schroer N, Sieler C, Feigel M. Helv Chim Acta. 1987;70:1025. [Google Scholar]
- 58.Avignon-Tropis M, Pougny JR. Tetrahedron Lett. 1989;30:4951. [Google Scholar]
- 59.Elphimoff-Felkin I, Sarda P. Org Synth. 1977;56:101. [Google Scholar]
- 60.Han BH, Boudjouk P. J Org Chem. 1982;47:5030. [Google Scholar]
- 61.Murdock TO, Klabunde KJ. J Org Chem. 1976;41:1076. [Google Scholar]
- 62.Klabunde KJ, Murdock TO. J Org Chem. 1979;44:3901. [Google Scholar]
- 63.Sibille S, Ratovelomanana V, Nedelec JY, Perichon J. Synlett. 1993:425. [Google Scholar]
- 64.Durant A, Delplancke J-L, Winand R, Reisse J. Tetrahedron Lett. 1995;36:4257. [Google Scholar]
- 65.Stadtmueller H, Greve B, Lennick K, Chair A, Knochel P. Synthesis. 1995:69. [Google Scholar]
- 66.Majid TN, Knochel P. Tetrahedron Lett. 1990;31:4413. [Google Scholar]
- 67.Berk SC, Knochel P, Yeh MCP. J Org Chem. 1988;53:5789. [Google Scholar]
- 68.Berk SC, Yeh MCP, Jeong N, Knochel P. Organometallics. 1990;9:3053. [Google Scholar]
- 69.Yeh MCP, Knochel P, Butler WM, Berk SC. Tetrahedron Lett. 1988;29:6693. [Google Scholar]
- 70.Chen HG, Gage JL, Barrett SD, Knochel P. Tetrahedron Lett. 1990;31:1829. [Google Scholar]
- 71.Yeh MCP, Knochel P. Tetrahedron Lett. 1989;30:4799. [Google Scholar]
- 72.Retherford C, Yeh MCP, Schipor I, Chen HG, Knochel P. J Org Chem. 1989;54:5200. [Google Scholar]
- 73.Tucker CE, Rao SA, Knochel P. J Org Chem. 1990;55:5446. [Google Scholar]
- 74.Knochel P, Chou TS, Chen HG, Yeh MCP, Rozema MJ. J Org Chem. 1989;54:5202. [Google Scholar]
- 75.Knochel P, Chou TS, Jubert C, Rajagopal D. J Org Chem. 1993;58:588. [Google Scholar]
- 76.Knochel P. J Am Chem Soc. 1990;112:7431. [Google Scholar]
- 77.Waas JR, Sidduri A, Knochel P. Tetrahedron Lett. 1992;33:3717. [Google Scholar]
- 78.Retherford C, Knochel P. Tetrahedron Lett. 1991;32:441. [Google Scholar]
- 79.Rozema MJ, Sidduri A, Knochel P. J Org Chem. 1992;57:1956. [Google Scholar]
- 80.Knochel P, Rozema MJ, Tucker CE, Retherford C, Furlong M, Rao SA. Pure Appl Chem. 1992;64:361. [Google Scholar]
- 81.Rao SA, Knochel P. J Org Chem. 1991;56:4591. [Google Scholar]
- 82.Rozema MJ, Rajagopal D, Tucker CE, Knochel P. J Organomet Chem. 1992;438:11. [Google Scholar]
- 83.Yeh MCP, Sun ML, Lin SK. Tetrahedron Lett. 1991;32:113. [Google Scholar]
- 84.Yeh MCP, Tau SI. J Chem Soc, Chem Commun. 1992:13. [Google Scholar]
- 85.Yeh MCP, Sheu BA, Fu HW, Tau SI, Chuang LW. J Am Chem Soc. 1993;115:5941. [Google Scholar]
- 86.Jubert C, Knochel P. J Org Chem. 1992;57:5431. [Google Scholar]
- 87.Yeh MCP, Tsou CJ, Chuang CN, Lin HC. J Chem Soc, Chem Commun. 1992:890. [Google Scholar]
- 88.Yeh MCP, Knochel P. Tetrahedron Lett. 1988;29:2395. [Google Scholar]
- 89.Majid TN, Yeh MCP, Knochel P. Tetrahedron Lett. 1989;30:5069. [Google Scholar]
- 90.Chen HG, Hoechstetter C, Knochel P. Tetrahedron Lett. 1989;30:4795. [Google Scholar]
- 91.Knoess HP, Furlong MT, Rozema MJ, Knochel P. J Org Chem. 1991;56:5974. [Google Scholar]
- 92.Yeh MCP, Chen HG, Knochel P. Org Synth. 1992;70:195. [Google Scholar]
- 93.Rao SA, Tucker CE, Knochel P. Tetrahedron Lett. 1990;31:7575. [Google Scholar]
- 94.Rao SA, Chou TS, Schipor I, Knochel P. Tetrahedron. 1992;48:2025. [Google Scholar]
- 95.Rao CJ, Knochel P. J Org Chem. 1991;56:4593. [Google Scholar]
- 96.Knochel P, Rao CJ. Tetrahedron. 1993;49:29. [Google Scholar]
- 97.Retherford C, Chou TS, Schelkun RM, Knochel P. Tetrahedron Lett. 1990;31:1833. [Google Scholar]
- 98.Zhu L, Wehmeyer RM, Rieke RD. J Org Chem. 1991;56:1445. [Google Scholar]
- 99.Zhu L, Rieke RD. Tetrahedron Lett. 1991;32:2865. [Google Scholar]
- 100.Rieke RD, Uhm SJ, Hudnall PM. J Chem Soc, Chem Commun. 1973:269. [Google Scholar]
- 101.Rieke RD, Li PT-J, Burns TP, Uhm ST. J Org Chem. 1981;46:4323. [Google Scholar]
- 102.Rieke RD, Uhm SJ. Synthesis. 1975:452. [Google Scholar]
- 103.Rieke RD. Science. 1989;246:1260. doi: 10.1126/science.246.4935.1260. [DOI] [PubMed] [Google Scholar]
- 104.Rieke RD, Hanson MV, Brown JD, Niu QJ. J Org Chem. 1996;61:2726. doi: 10.1021/jo952104b. [DOI] [PubMed] [Google Scholar]
- 105.Jubert C, Knochel P. J Org Chem. 1992;57:5425. [Google Scholar]
- 106.Huo S. Org Lett. 2003;5:423. doi: 10.1021/ol0272693. [DOI] [PubMed] [Google Scholar]
- 107.Krasovskiy A, Duplais C, Lipshutz BH. J Am Chem Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stadtmüller H, Lentz R, Tucker CE, Stüdemann T, Dörner W, Knochel P. J Am Chem Soc. 1993;115:7027. [Google Scholar]
- 109.Bluemke TD, Piller FM, Knochel P. Chem Commun. 2010;46:4082. doi: 10.1039/c001845g. [DOI] [PubMed] [Google Scholar]
- 110.Vettel S, Vaupel A, Knochel P. J Org Chem. 1996;61:7473. doi: 10.1021/jo960808v. [DOI] [PubMed] [Google Scholar]
- 111.Langer F, Schwink L, Devasagayaraj A, Chavant P-Y, Knochel P. J Org Chem. 1996;61:8229. doi: 10.1021/jo961129n. [DOI] [PubMed] [Google Scholar]
- 112.Jackson RFW, Oates LJ, Block MH. Chem Commun. 2000:1401. [Google Scholar]
- 113.Knochel P, Singer RD. Chem Rev. 1993;93:2117. [Google Scholar]
- 114.Evans DA, Bach T. Angew Chem Int Ed. 1993;32:1326. [Google Scholar]
- 115.Shimokawa K, Iwase Y, Miwa R, Yamada K, Uemura D. J Med Chem. 2008;51:5912. doi: 10.1021/jm800741n. [DOI] [PubMed] [Google Scholar]
- 116.Jackson RFW, Moore RJ, Dexter CS, Elliott J, Mowbray CE. J Org Chem. 1998;63:7875. [Google Scholar]
- 117.Negishi E-i. Handbook of Organopalladium Chemistry for Organic Synthesis. 2002;1 [Google Scholar]
- 118.Terao J, Kambe N. Bull Chem Soc Jpn. 2006;79:663. [Google Scholar]
- 119.Terao J, Kambe N. Acc Chem Res. 2008;41:1545. doi: 10.1021/ar800138a. [DOI] [PubMed] [Google Scholar]
- 120.Luh T-Y, Leung M-k, Wong K-T. Chem Rev. 2000;100:3187. doi: 10.1021/cr990272o. [DOI] [PubMed] [Google Scholar]
- 121.Zhou J, Fu GC. J Am Chem Soc. 2003;125:12527. doi: 10.1021/ja0363258. [DOI] [PubMed] [Google Scholar]
- 122.Rudolph A, Lautens M. Angew Chem, Int Ed. 2009;48:2656. doi: 10.1002/anie.200803611. [DOI] [PubMed] [Google Scholar]
- 123.Tamao K, Kiso Y, Sumitani K, Kumada M. J Am Chem Soc. 1972;94:9268. [Google Scholar]
- 124.Kiso Y, Tamao K, Kumada M. J Organometal Chem. 1973;50:C12. [Google Scholar]
- 125.Han C, Buchwald SL. J Am Chem Soc. 2009;131:7532. doi: 10.1021/ja902046m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goldberg KI, Yan J, Breitung EM. J Am Chem Soc. 1995;117:6889. [Google Scholar]
- 127.Roy AH, Hartwig JF. J Am Chem Soc. 2001;123:1232. doi: 10.1021/ja0034592. [DOI] [PubMed] [Google Scholar]
- 128.Roy AH, Hartwig JF. J Am Chem Soc. 2003;125:13944. doi: 10.1021/ja037959h. [DOI] [PubMed] [Google Scholar]
- 129.Frech CM, Milstein D. J Am Chem Soc. 2006;128:12434. doi: 10.1021/ja064945d. [DOI] [PubMed] [Google Scholar]
- 130.Johnson JB, Bercot EA, Rowley JM, Coates GW, Rovis T. J Am Chem Soc. 2007;129:2718. doi: 10.1021/ja067845g. [DOI] [PubMed] [Google Scholar]
- 131.Gillie A, Stille JK. J Am Chem Soc. 1980;102:4933. [Google Scholar]
- 132.Hayashi T, Konishi M, Kobori Y, Kumada M, Higuchi T, Hirotsu K. J Am Chem Soc. 1984;106:158. [Google Scholar]
- 133.Negishi E-i, Anastasia L. Chem Rev. 2003;103:1979. doi: 10.1021/cr020377i. [DOI] [PubMed] [Google Scholar]
- 134.Negishi E-i, Takahashi T, Baba S, Van Horn DE, Okukado N. J Am Chem Soc. 1987;109:2393. [Google Scholar]
- 135.Herbert JM. Tetrahedron Lett. 2004;45:817. [Google Scholar]
- 136.Baba Y, Toshimitsu A, Matsubara S. Synlett. 2008:2061. [Google Scholar]
- 137.Rottländer M, Knochel P. Tetrahedron Lett. 1997;38:1749. [Google Scholar]
- 138.Biellmann JF, Ducep JB. Tetrahedron Lett. 1969;10:3707. [Google Scholar]
- 139.Grieco PA, Masaki Y. J Org Chem. 1974;39:2135. [Google Scholar]
- 140.Zhang P, Brozek LA, Morken JP. J Am Chem Soc. 2010;132:10686. doi: 10.1021/ja105161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Devon TK, Scott AI. Handbook of Naturally Occurring Compounds, Vol. 1: Acetogenins, Shikimates, and Carbohydrates. 1975 [Google Scholar]
- 142.Devon TK, Scott AI. Handbood of Naturally Occurring Compounds, Vol. 2: Terpenes. 1972 [Google Scholar]
- 143.Negishi E-i, Liou S-Y, Xu C, Huo S. Org Lett. 2002;4:261. doi: 10.1021/ol010263d. [DOI] [PubMed] [Google Scholar]
- 144.Palmgren A, Thorarensen A, Bäckvall J-E. J Org Chem. 1998;63:3764. [Google Scholar]
- 145.Burton DJ, Lu L. Top Curr Chem. 1997;193:45. [Google Scholar]
- 146.Davis CR, Burton DJ. Organozinc Reagents. 1999:57. [Google Scholar]
- 147.Peng S, Qing F-L, Li Y-Q, Hu C-M. J Org Chem. 2000;65:694. [Google Scholar]
- 148.Terao J, Kambe N, Sonoda N. Tetrahedron Lett. 1996;37:4741. [Google Scholar]
- 149.Dabdoub MJ, Dabdoub VB, Marino JP. Tetrahedron Lett. 2000;41:433. [Google Scholar]
- 150.Negishi E-i, Tan Z, Liou S-Y, Liao B. Tetrahedron. 2000;56:10197. [Google Scholar]
- 151.Bach T, Heuser S. J Org Chem. 2002;67:5789. doi: 10.1021/jo025661o. [DOI] [PubMed] [Google Scholar]
- 152.Skerlj RT, Zhou Y, Wilson T, Bridger GJ. J Org Chem. 2002;67:1407. doi: 10.1021/jo0109523. [DOI] [PubMed] [Google Scholar]
- 153.Zeng X, Qian M, Hu Q, Negishi E-i. Angew Chem, Int Ed. 2004;43:2259. doi: 10.1002/anie.200353022. [DOI] [PubMed] [Google Scholar]
- 154.Shi J-c, Zeng X, Negishi E-i. Org Lett. 2003;5:1825. doi: 10.1021/ol030017x. [DOI] [PubMed] [Google Scholar]
- 155.Andrei D, Wnuk SF. J Org Chem. 2006;71:405. doi: 10.1021/jo051980e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kondolff I, Doucet H, Santelli M. Organometallics. 2006;25:5219. [Google Scholar]
- 157.Hansen AL, Ebran J-P, Gøgsig TM, Skrydstrup T. J Org Chem. 2007;72:6464. doi: 10.1021/jo070912k. [DOI] [PubMed] [Google Scholar]
- 158.Milne JE, Buchwald SL. J Am Chem Soc. 2004;126:13028. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]
- 159.Giovannini R, Stüdemann T, Dussin G, Knochel P. Angew Chem, Int Ed. 1998;37:2387. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2387::AID-ANIE2387>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 160.Giovannini R, Stüdemann T, Devasagayaraj A, Dussin G, Knochel P. J Org Chem. 1999;64:3544. doi: 10.1021/jo982317b. [DOI] [PubMed] [Google Scholar]
- 161.Luo X, Zhang H, Duan H, Liu Q, Zhu L, Zhang T, Lei A. Org Lett. 2007;9:4571. doi: 10.1021/ol701995t. [DOI] [PubMed] [Google Scholar]
- 162.Zhang H, Luo X, Wongkhan K, Duan H, Li Q, Zhu L, Wang J, Batsanov AS, Howard JAK, Marder TB, Lei A. Chem Eur J. 2009;15:3823. doi: 10.1002/chem.200802209. [DOI] [PubMed] [Google Scholar]
- 163.Liu Q, Lan Y, Liu J, Li G, Wu Y-D, Lei A. J Am Chem Soc. 2009;131:10201. doi: 10.1021/ja903277d. [DOI] [PubMed] [Google Scholar]
- 164.Liu Q, Duan H, Luo X, Tang Y, Li G, Huang R, Lei A. Adv Synth Catal. 2008;350:1349. [Google Scholar]
- 165.Chacko A-M, Qu W, Kung HF. J Org Chem. 2008;73:4874. doi: 10.1021/jo800444y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blasberg R. Eur J Cancer. 2002;38:2137. doi: 10.1016/s0959-8049(02)00390-8. [DOI] [PubMed] [Google Scholar]
- 167.Peñuelas I, Boán Jose F, Marti-Climent Josep M, Sangro B, Mazzolini G, Prieto J, Richter Jose A. Mol Imaging Biol. 2004;6:225. doi: 10.1016/j.mibio.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 168.Serganova I, Blasberg R. Nucl Med Biol. 2005;32:763. doi: 10.1016/j.nucmedbio.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 169.Xu H, Ekoue-Kovi K, Wolf C. J Org Chem. 2008;73:7638. doi: 10.1021/jo801445y. [DOI] [PubMed] [Google Scholar]
- 170.Manolikakes G, Muñoz Hernandez C, Schade MA, Metzger A, Knochel P. J Org Chem. 2008;73:8422. doi: 10.1021/jo8015852. [DOI] [PubMed] [Google Scholar]
- 171.Manolikakes G, Schade MA, Munoz Hernandez C, Mayr H, Knochel P. Org Lett. 2008;10:2765. doi: 10.1021/ol8009013. [DOI] [PubMed] [Google Scholar]
- 172.Getmanenko YA, Twieg RJ. J Org Chem. 2008;73:830. doi: 10.1021/jo701812t. [DOI] [PubMed] [Google Scholar]
- 173.Piber M, Jensen AE, Rottländer M, Knochel P. Org Lett. 1999;1:1323. [Google Scholar]
- 174.Jensen AE, Knochel P. J Org Chem. 2002;67:79. doi: 10.1021/jo0105787. [DOI] [PubMed] [Google Scholar]
- 175.Liu J, Deng Y, Wang H, Zhang H, Yu G, Wu B, Zhang H, Li Q, Marder TB, Yang Z, Lei A. Org Lett. 2008;10:2661. doi: 10.1021/ol8007342. [DOI] [PubMed] [Google Scholar]
- 176.Moreno-Manas M, Pleixats R. Acc Chem Res. 2003;36:638. doi: 10.1021/ar020267y. [DOI] [PubMed] [Google Scholar]
- 177.Reetz MT, Westermann E. Angew Chem, Int Ed. 2000;39:165. doi: 10.1002/(sici)1521-3773(20000103)39:1<165::aid-anie165>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 178.Liu J, Wang H, Zhang H, Wu X, Zhang H, Deng Y, Yang Z, Lei A. Chem Eur J. 2009;15:4437. doi: 10.1002/chem.200802238. [DOI] [PubMed] [Google Scholar]
- 179.Wenkert E, Ferreira TW, Michelotti EL. J Chem Soc, Chem Commun. 1979:637. [Google Scholar]
- 180.Tokuyama H, Yokoshima S, Yamashita T, Fukuyama T. Tetrahedron Lett. 1998;39:3189. [Google Scholar]
- 181.Metzger A, Melzig L, Despotopoulou C, Knochel P. Org Lett. 2009;11:4228. doi: 10.1021/ol9017003. [DOI] [PubMed] [Google Scholar]
- 182.Melzig L, Metzger A, Knochel P. J Org Chem. 2010;75:2131. doi: 10.1021/jo1001615. [DOI] [PubMed] [Google Scholar]
- 183.Melzig L, Stemper J, Knochel P. Synthesis. 2010:2085. [Google Scholar]
- 184.Lee K, Counceller CM, Stambuli JP. Org Lett. 2009;11:1457. doi: 10.1021/ol900260g. [DOI] [PubMed] [Google Scholar]
- 185.Sase S, Jaric M, Metzger A, Malakhov V, Knochel P. J Org Chem. 2008;73:7380. doi: 10.1021/jo801063c. [DOI] [PubMed] [Google Scholar]
- 186.Diez-González S, Marion N, Nolan SP. Chem Rev. 2009;109:3612. doi: 10.1021/cr900074m. [DOI] [PubMed] [Google Scholar]
- 187.Kantchev EAB, O’Brien CJ, Organ MG. Angew Chem, Int Ed. 2007;46:2768. doi: 10.1002/anie.200601663. [DOI] [PubMed] [Google Scholar]
- 188.Hadei N, Kantchev EAB, O’Brien CJ, Organ MG. Org Lett. 2005;7:3805. doi: 10.1021/ol0514909. [DOI] [PubMed] [Google Scholar]
- 189.Urkalan KB, Sigman MS. J Am Chem Soc. 2009;131:18042. doi: 10.1021/ja908545b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jin L, Zhao Y, Wang H, Lei A. Synthesis. 2008:649. [Google Scholar]
- 191.Dieter RK. Tetrahedron. 1999;55:4177. [Google Scholar]
- 192.Iwai T, Nakai T, Mihara M, Ito T, Mizuno T, Ohno T. Synlett. 2009:1091. [Google Scholar]
- 193.Srogl J, Liu W, Marshall D, Liebeskind LS. J Am Chem Soc. 1999;121:9449. [Google Scholar]
- 194.Wu J, Yang Z. J Org Chem. 2001;66:7875. doi: 10.1021/jo010452+. [DOI] [PubMed] [Google Scholar]
- 195.Walters IAS. Tetrahedron Lett. 2005;47:341. [Google Scholar]
- 196.Melzig L, Gavryushin A, Knochel P. Org Lett. 2007;9:5529. doi: 10.1021/ol702499h. [DOI] [PubMed] [Google Scholar]
- 197.Terao J, Bando F, Kambe N. Chem Commun. 2009:7336. doi: 10.1039/b918548h. [DOI] [PubMed] [Google Scholar]
- 198.Takimoto M, Shimizu K, Mori M. Org Lett. 2001;3:3345. doi: 10.1021/ol016585z. [DOI] [PubMed] [Google Scholar]
- 199.Ochiai H, Jang M, Hirano K, Yorimitsu H, Oshima K. Org Lett. 2008;10:2681. doi: 10.1021/ol800764u. [DOI] [PubMed] [Google Scholar]
- 200.Yeung CS, Dong VM. J Am Chem Soc. 2008;130:7826. doi: 10.1021/ja803435w. [DOI] [PubMed] [Google Scholar]
- 201.Devasagayaraj A, Stüdemann T, Knochel P. Angew Chem, Int Ed. 1996;34:2723. [Google Scholar]
- 202.Kienle M, Knochel P. Org Lett. 2010;12:2702. doi: 10.1021/ol1007026. [DOI] [PubMed] [Google Scholar]
- 203.Zhou J, Fu GC. J Am Chem Soc. 2003;125:14726. doi: 10.1021/ja0389366. [DOI] [PubMed] [Google Scholar]
- 204.Terao J, Todo H, Watanabe H, Ikumi A, Kambe N. Angew Chem, Int Ed. 2004;43:6180. doi: 10.1002/anie.200460246. [DOI] [PubMed] [Google Scholar]
- 205.Gong H, Sinisi R, Gagné MR. J Am Chem Soc. 2007;129:1908. doi: 10.1021/ja068950t. [DOI] [PubMed] [Google Scholar]
- 206.Phapale VB, Buñuel E, García-Iglesias M, Cárdenas DJ. Angew Chem, Int Ed. 2007;46:8790. doi: 10.1002/anie.200702528. [DOI] [PubMed] [Google Scholar]
- 207.Malosh CF, Ready JM. J Am Chem Soc. 2004;126:10240. doi: 10.1021/ja0467768. [DOI] [PubMed] [Google Scholar]
- 208.Smith SW, Fu GC. Angew Chem, Int Ed. 2008;47:9334. doi: 10.1002/anie.200802784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Echavarren AM. Angew Chem, Int Ed. 2005;44:3962. doi: 10.1002/anie.200500918. [DOI] [PubMed] [Google Scholar]
- 210.Pandey G, Rao KSSP, Palit DK, Mittal JP. J Org Chem. 1998;63:3968. [Google Scholar]
- 211.Zhou J, Fu GC. J Am Chem Soc. 2004;126:1340. doi: 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]
- 212.Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. J Am Chem Soc. 2006;128:13175. doi: 10.1021/ja063334i. [DOI] [PubMed] [Google Scholar]
- 213.Lin X, Phillips DL. J Org Chem. 2008;73:3680. doi: 10.1021/jo702497p. [DOI] [PubMed] [Google Scholar]
- 214.Phapale VB, Guisan-Ceinos M, Buñuel E, Cárdenas DJ. Chem Eur J. 2009;15:12681. doi: 10.1002/chem.200901913. [DOI] [PubMed] [Google Scholar]
- 215.Achonduh GT, Hadei N, Valente C, Avola S, O’Brien CJ, Organ MG. Chem Commun. 2010;46:4109. doi: 10.1039/c002759f. [DOI] [PubMed] [Google Scholar]
- 216.Miyaura N, editor. Top Curr Chem. 2002. In Cross-Coupling Reactions. A Practical Guide; p. 219. [Google Scholar]
- 217.Suzuki A. J Organomet Chem. 1999;576:147. [Google Scholar]
- 218.Onak T. Organoborane chemistry. Academic Press; 1975. [Google Scholar]
- 219.Brown HC, Snyder CH. J Am Chem Soc. 1961;83:1002. [Google Scholar]
- 220.Kondo K, Murahashi S. Tetrahedron Lett. 1979;20:1237. [Google Scholar]
- 221.Srebnik M. Tetrahedron Lett. 1991;32:2449. [Google Scholar]
- 222.Langer F, Waas J, Knochel P. Tetrahedron Lett. 1993;34:5261. [Google Scholar]
- 223.Oppolzer W, Radinov RN. J Am Chem Soc. 1993;115:1593. [Google Scholar]
- 224.Koesterand R, Benedikt G. Angew Chem. 1962;74:589. [Google Scholar]
- 225.Giacomelli G, Menicagli R, Caporusso AM, Lardicci L. J Org Chem. 1978;43:1790. [Google Scholar]
- 226.George TA, Lappert MF. Chem Commun. 1966:463. [Google Scholar]
- 227.Yamamoto Y, Yatagai H, Moritani I. J Am Chem Soc. 1975;97:5606. [Google Scholar]
- 228.Brown HC, Molander GA. J Org Chem. 1981;46:645. [Google Scholar]
- 229.Larock RC. J Organometal Chem. 1973;61:27. [Google Scholar]
- 230.Miyaura N, Ishiyama T, Ishikawa M, Suzuki A. Tetrahedron Lett. 1986;27:6369. [Google Scholar]
- 231.Chemler SR, Trauner D, Danishefsky SJ. Angew Chem, Int Ed. 2001;40:4544. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 232.Nicolaou KC, Bulger PG, Sarlah D. Angew Chem, Int Ed. 2005;44:4442. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 233.Brown HC. Organic Syntheses via Boranes. 1975 [Google Scholar]
- 234.Rappoport Z. The Chemistry of the Cyclopropyl Group. 1995;2 [Google Scholar]
- 235.Brown HC, Cole TE. Organometallics. 1983;2:1316. [Google Scholar]
- 236.Marshall JA, Johns BA. J Org Chem. 1998;63:7885. doi: 10.1021/jo971900+. [DOI] [PubMed] [Google Scholar]
- 237.Soderquist JA, De Pomar JCJ. Tetrahedron Lett. 2000;41:3537. [Google Scholar]
- 238.Maennig D, Nöth H. Angew Chem Int Ed. 1985;24:878. [Google Scholar]
- 239.Evans DA, Fu GC, Hoveyda AH. J Am Chem Soc. 1992;114:6671. [Google Scholar]
- 240.Yamamoto Y, Fujikawa R, Umemoto T, Miyaura N. Tetrahedron. 2004;60:10695. [Google Scholar]
- 241.He X, Hartwig JF. J Am Chem Soc. 1996;118:1696. [Google Scholar]
- 242.Vanier C, Wagner A, Mioskowski C. Tetrahedron Lett. 1999;40:4335. [Google Scholar]
- 243.Brown HC, Bhat NG, Somayaji V. Organometallics. 1983;2:1311. [Google Scholar]
- 244.Molander GA, Ham J. Org Lett. 2006;8:2031. doi: 10.1021/ol060375a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kotha S, Lahiri K, Kashinath D. Tetrahedron. 2002;58:9633. [Google Scholar]
- 246.Sato M, Miyaura N, Suzuki A. Chem Lett. 1989:1405. [Google Scholar]
- 247.Molander GA, Ham J. Org Lett. 2006;8:2767. doi: 10.1021/ol060826r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Littke AF, Dai C, Fu GC. J Am Chem Soc. 2000;122:4020. [Google Scholar]
- 249.Miyaura N, Suzuki A. Chem Rev. 1995;95:2457. [Google Scholar]
- 250.Old DW, Wolfe JP, Buchwald SL. J Am Chem Soc. 1998;120:9722. [Google Scholar]
- 251.Wolfe JP, Singer RA, Yang BH, Buchwald SL. J Am Chem Soc. 1999;121:9550. [Google Scholar]
- 252.Miyaura N, Ishiyama T, Sasaki H, Ishikawa M, Sato M, Suzuki A. J Am Chem Soc. 1989;111:314. [Google Scholar]
- 253.Matos K, Soderquist JA. J Org Chem. 1998;63:461. doi: 10.1021/jo971681s. [DOI] [PubMed] [Google Scholar]
- 254.Ridgway BH, Woerpel KA. J Org Chem. 1998;63:458. doi: 10.1021/jo970803d. [DOI] [PubMed] [Google Scholar]
- 255.Uenishi Ji, Beau JM, Armstrong RW, Kishi Y. J Am Chem Soc. 1987;109:4756. [Google Scholar]
- 256.Humphrey JM, Aggen JB, Chamberlin AR. J Am Chem Soc. 1996;118:11759. [Google Scholar]
- 257.Frank SA, Chen H, Kunz RK, Schnaderbeck MJ, Roush WR. Org Lett. 2000;2:2691. doi: 10.1021/ol0062446. [DOI] [PubMed] [Google Scholar]
- 258.Chemler SR, Danishefsky SJ. Org Lett. 2000;2:2695. doi: 10.1021/ol0062547. [DOI] [PubMed] [Google Scholar]
- 259.Walker SD, Barder TE, Martinelli JR, Buchwald SL. Angew Chem, Int Ed. 2004;43:1871. doi: 10.1002/anie.200353615. [DOI] [PubMed] [Google Scholar]
- 260.Campbell AD, Raynham TM, Taylor RJK. Tetrahedron Lett. 1999;40:5263. [Google Scholar]
- 261.Sabat M, Johnson CR. Org Lett. 2000;2:1089. doi: 10.1021/ol005645i. [DOI] [PubMed] [Google Scholar]
- 262.Kamatani A, Overman LE. J Org Chem. 1999;64:8743. [Google Scholar]
- 263.Vice S, Bara T, Bauer A, Evans CA, Ford J, Josien H, McCombie S, Miller M, Nazareno D, Palani A, Tagat J. J Org Chem. 2001;66:2487. doi: 10.1021/jo0007682. [DOI] [PubMed] [Google Scholar]
- 264.Bartoccini F, Cabri W, Celona D, Minetti P, Piersanti G, Tarzia G. J Org Chem. 2010;75:5398. doi: 10.1021/jo101027h. [DOI] [PubMed] [Google Scholar]
- 265.Potuzak JS, Tan DS. Tetrahedron Lett. 2004;45:1797. [Google Scholar]
- 266.Liron F, Fosse C, Pernolet A, Roulland E. J Org Chem. 2007;72:2220. doi: 10.1021/jo061908w. [DOI] [PubMed] [Google Scholar]
- 267.Narukawa Y, Nishi K, Onoue H. Tetrahedron. 1997;53:539. [Google Scholar]
- 268.Zheng W, DeMattei JA, Wu J-P, Duan JJW, Cook LR, Oinuma H, Kishi Y. J Am Chem Soc. 1996;118:7946. [Google Scholar]
- 269.Sasaki M, Fuwa H, Inoue M, Tachibana K. Tetrahedron Lett. 1998;39:9027. [Google Scholar]
- 270.Heald RA, Stevens MFG. Org Biomol Chem. 2003;1:3377. doi: 10.1039/b305177n. [DOI] [PubMed] [Google Scholar]
- 271.Barbero M, Cadamuro S, Dughera S. Synthesis. 2008:474. [Google Scholar]
- 272.Miyaura N, Ishikawa M, Suzuki A. Tetrahedron Lett. 1992;33:2571. [Google Scholar]
- 273.Soderquist JA, León G, Colberg JC, Martínez I. Tetrahedron Lett. 1995;36:3119. [Google Scholar]
- 274.Mohr PJ, Halcomb RL. J Am Chem Soc. 2003;125:1712. doi: 10.1021/ja0296531. [DOI] [PubMed] [Google Scholar]
- 275.Ishiyama T, Miyaura N, Suzuki A. Bull Chem Soc Jpn. 1991;64:1999. [Google Scholar]
- 276.Ishiyama T, Miyaura N, Suzuki A. Tetrahedron Lett. 1991;32:623. [Google Scholar]
- 277.Ishiyama T, Abe S, Miyaura N, Suzuki A. Chem Lett. 1992:691. [Google Scholar]
- 278.Netherton MR, Dai C, Neuschuetz K, Fu GC. J Am Chem Soc. 2001;123:10099. doi: 10.1021/ja011306o. [DOI] [PubMed] [Google Scholar]
- 279.Kirchhoff JH, Dai C, Fu GC. Angew Chem, Int Ed. 2002;41:1945. [PubMed] [Google Scholar]
- 280.Brenstrum T, Gerristma DA, Adjabeng GM, Frampton CS, Britten J, Robertson AJ, McNulty J, Capretta A. J Org Chem. 2004;69:7635. doi: 10.1021/jo048875+. [DOI] [PubMed] [Google Scholar]
- 281.Arentsen K, Caddick S, Cloke FGN, Herring AP, Hitchcock PB. Tetrahedron Lett. 2004;45:3511. [Google Scholar]
- 282.Valente C, Baglione S, Candito D, O’Brien CJ, Organ MG. Chem Commun. 2008:735. doi: 10.1039/b715081d. [DOI] [PubMed] [Google Scholar]
- 283.Achonduh GT, Hadei N, Valente C, Avola S, O’Brien CJ, Organ MG. Chem Commun. 2010;46:4109. doi: 10.1039/c002759f. [DOI] [PubMed] [Google Scholar]
- 284.Saito B, Fu GC. J Am Chem Soc. 2007;129:9602. doi: 10.1021/ja074008l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lu Z, Fu GC. Angew Chem, Int Ed. 2010;49:6676. doi: 10.1002/anie.201003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yu Y, Liebeskind LS. J Org Chem. 2004;69:3554. doi: 10.1021/jo049964p. [DOI] [PubMed] [Google Scholar]
- 287.Yasui Y, Tsuchida S, Miyabe H, Takemoto Y. J Org Chem. 2007;72:5898. doi: 10.1021/jo070724u. [DOI] [PubMed] [Google Scholar]
- 288.Miyaura N. Top Curr Chem. 2002;219:11. [Google Scholar]
- 289.Bellina F, Anselmi C, Rossi R. Tetrahedron Lett. 2001;42:3851. [Google Scholar]
- 290.Molander GA, Yun C-S. Tetrahedron. 2002;58:1465. [Google Scholar]
- 291.Kataoka N, Shelby Q, Stambuli JP, Hartwig JF. J Org Chem. 2002;67:5553. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 292.Kwong FY, Chan KS, Yeung CH, Chan ASC. Chem Commun. 2004:2336. doi: 10.1039/b407661c. [DOI] [PubMed] [Google Scholar]
- 293.Bedford RB, Hazelwood SL, Limmert ME, Albisson DA, Draper SM, Scully PN, Coles SJ, Hursthouse MB. Chem Eur J. 2003;9:3216. doi: 10.1002/chem.200304997. [DOI] [PubMed] [Google Scholar]
- 294.So CM, Lau CP, Kwong FY. Org Lett. 2007;9:2795. doi: 10.1021/ol070898y. [DOI] [PubMed] [Google Scholar]
- 295.Inés B, Moreno I, SanMartin R, Domínguez E. J Org Chem. 2008;73:8448. doi: 10.1021/jo8016633. [DOI] [PubMed] [Google Scholar]
- 296.Nájera C, Gil-Moltó J, Karlström S. Adv Synth Catal. 2004;346:1798. [Google Scholar]
- 297.Kondolff I, Doucet H, Santelli M. Tetrahedron. 2004;60:3813. [Google Scholar]
- 298.Doucet H. Eur J Org Chem. 2008:2013. [Google Scholar]
- 299.Ma S, Jiang X, Cheng X, Hou H. Adv Synth Catal. 2006;348:2114. [Google Scholar]
- 300.Maj AM, Delaude L, Demonceau A, Noels AF. Tetrahedron. 2007;63:2657. [Google Scholar]
- 301.Saha D, Chattopadhyay K, Ranu BC. Tetrahedron Lett. 2009;50:1003. [Google Scholar]
- 302.Kirchhoff JH, Netherton MR, Hills ID, Fu GC. J Am Chem Soc. 2002;124:13662. doi: 10.1021/ja0283899. [DOI] [PubMed] [Google Scholar]
- 303.Wang X-Z, Deng M-Z. J Chem Soc, Perkin Trans. 1996;1:2663. [Google Scholar]
- 304.Wallace DJ, Chen C-y. Tetrahedron Lett. 2002;43:6987. [Google Scholar]
- 305.Hildebrand JP, Marsden SP. Synlett. 1996:893. [Google Scholar]
- 306.Charette AB, De Freitas-Gil RP. Tetrahedron Lett. 1997;38:2809. [Google Scholar]
- 307.Löhr S, de Meijere A. Synlett. 2001:489. [Google Scholar]
- 308.Occhiato EG, Trabocchi A, Guarna A. J Org Chem. 2001;66:2459. doi: 10.1021/jo001807c. [DOI] [PubMed] [Google Scholar]
- 309.Andrus MB, Song C. Org Lett. 2001;3:3761. doi: 10.1021/ol016724c. [DOI] [PubMed] [Google Scholar]
- 310.Zou G, Falck JR. Tetrahedron Lett. 2001;42:5817. [Google Scholar]
- 311.Molander GA, Ellis N. Acc Chem Res. 2007;40:275. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]
- 312.Molander GA, Ito T. Org Lett. 2001;3:393. doi: 10.1021/ol006896u. [DOI] [PubMed] [Google Scholar]
- 313.Molander GA, Canturk B. Angew Chem Int Ed. 2009;48:9240. doi: 10.1002/anie.200904306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Molander GA, Yun C-S, Ribagorda M, Biolatto B. J Org Chem. 2003;68:5534. doi: 10.1021/jo0343331. [DOI] [PubMed] [Google Scholar]
- 315.Fang G-H, Yan Z-J, Deng M-Z. Org Lett. 2004;6:357. doi: 10.1021/ol036184e. [DOI] [PubMed] [Google Scholar]
- 316.Charette AB, Mathieu S, Fournier J-F. Synlett. 2005:1779. [Google Scholar]
- 317.Molander GA, Petrillo DE. Org Lett. 2008;10:1795. doi: 10.1021/ol800357c. [DOI] [PubMed] [Google Scholar]
- 318.Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257. doi: 10.1021/ja8031423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Grignard V. C r d l’Acad des Sciences. 1900;130:1322. [Google Scholar]
- 320.Barbier P. C r d l’Acad des sciences. 1899;128:110. [Google Scholar]
- 321.Shinokubo H, Oshima K. Eur J Org Chem. 2004:2081. [Google Scholar]
- 322.Seyferth D. Organometallics. 2009;28:1598. [Google Scholar]
- 323.Knochel P. Grignard Reagents New Developments Edited by Herman G Richey, Jr. 2000;39 [Google Scholar]
- 324.Takahashi T, Kanna K-i. Mod Organonickel Chem. 2005:41. [Google Scholar]
- 325.Kharasch MS, Fuchs CF. J Am Chem Soc. 1943;65:504. [Google Scholar]
- 326.Knochel P, Dohle W, Gommermann N, Kneisel FF, Kopp F, Korn T, Sapountzis I, Vu VA. Angew Chem, Int Ed. 2003;42:4302. doi: 10.1002/anie.200300579. [DOI] [PubMed] [Google Scholar]
- 327.Pierce OR, Meiners AF, McBee ET. J Am Chem Soc. 1953;75:2516. [Google Scholar]
- 328.McBee ET, Roberts CW, Meiners AF. J Am Chem Soc. 1957;79:335. [Google Scholar]
- 329.Lavaire S, Plantier-Royon R, Portella C. Tetrahedron Asymmetry. 1998;9:213. [Google Scholar]
- 330.Inoue A, Shinokubo H, Oshima K. Org Lett. 2000;2:651. doi: 10.1021/ol991403a. [DOI] [PubMed] [Google Scholar]
- 331.Vu VA, Marek I, Polborn K, Knochel P. Angew Chem, Int Ed. 2002;41:351. doi: 10.1002/1521-3773(20020118)41:2<351::aid-anie351>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 332.Terao J, Watabe H, Kambe N. J Am Chem Soc. 2005;127:3656. doi: 10.1021/ja042565r. [DOI] [PubMed] [Google Scholar]
- 333.Tamao K, Sumitani K, Kumada M. J Amer Chem Soc. 1972;94:4374. [Google Scholar]
- 334.Tamao K. J Organomet Chem. 2002;653:23. [Google Scholar]
- 335.Tamao K, Sumitani K, Kumada M. J Am Chem Soc. 1972;94:4374. [Google Scholar]
- 336.Corriu RJP, Masse JP. J Chem Soc, Chem Commun. 1972:144. [Google Scholar]
- 337.Tamao K, Sumitani K, Kiso Y, Zembayashi M, Fujioka A, Kodama S, Nakajima I, Minato A, Kumada M. Bull Chem Soc Jpn. 1976;49:1958. [Google Scholar]
- 338.Tamao K, Zembayashi M, Kumada M. Chem Lett. 1976:1237. [Google Scholar]
- 339.Eapen KC, Dua SS, Tamborski C. J Org Chem. 1984;49:478. [Google Scholar]
- 340.Bochmann M, Creaser CS, Wallace L. J Mol Catal. 1990;60:343. [Google Scholar]
- 341.Reddy GS, Tam W. Organometallics. 1984;3:630. [Google Scholar]
- 342.Wang J-R, Manabe K. Org Lett. 2009;11:741. doi: 10.1021/ol802824b. [DOI] [PubMed] [Google Scholar]
- 343.Liang L-C, Chien P-S, Lin J-M, Huang M-H, Huang Y-L, Liao J-H. Organometallics. 2006;25:1399. [Google Scholar]
- 344.Roques N, Saint-Jalmes L. Tetrahedron Lett. 2006;47:3375. [Google Scholar]
- 345.Sahlberg C, Quader A, Claesson A. Tetrahedron Lett. 1983;24:5137. [Google Scholar]
- 346.Shao L-X, Shi M. Org Biomol Chem. 2005;3:1828. doi: 10.1039/b504071j. [DOI] [PubMed] [Google Scholar]
- 347.Terao J, Watanabe H, Ikumi A, Kuniyasu H, Kambe N. J Am Chem Soc. 2002;124:4222. doi: 10.1021/ja025828v. [DOI] [PubMed] [Google Scholar]
- 348.Dubbaka SR, Vogel P. Angew Chem, Int Ed. 2005;44:7674. doi: 10.1002/anie.200463007. [DOI] [PubMed] [Google Scholar]
- 349.Okamura H, Miura M, Takei H. Tetrahedron Lett. 1979:43. [Google Scholar]
- 350.Takei H, Miura M, Sugimura H, Okamura H. Chem Lett. 1979:1447. [Google Scholar]
- 351.Sugimura H, Takei H. Bull Chem Soc Jpn. 1985;58:664. [Google Scholar]
- 352.Wenkert E, Hanna JM, Jr, Leftin MH, Michelotti EL, Potts KT, Usifer D. J Org Chem. 1985;50:1125. [Google Scholar]
- 353.Venkatesh C, Singh B, Mahata PK, Ila H, Junjappa H. Org Lett. 2005;7:2169. doi: 10.1021/ol0505095. [DOI] [PubMed] [Google Scholar]
- 354.Kanemura S, Kondoh A, Yorimitsu H, Oshima K. Synthesis. 2008:2659. [Google Scholar]
- 355.Cho C-H, Sun M, Seo Y-S, Kim C-B, Park K. J Org Chem. 2005;70:1482. doi: 10.1021/jo048300c. [DOI] [PubMed] [Google Scholar]
- 356.Kim C-B, Jo H, Ahn B-K, Kim C-K, Park K-Y. J Org Chem. 2009;74:9566. doi: 10.1021/jo902151h. [DOI] [PubMed] [Google Scholar]
- 357.Hayashi T, Katsuro Y, Okamoto Y, Kumada M. Tetrahedron Lett. 1981;22:4449. [Google Scholar]
- 358.Busacca CA, Eriksson MC, Fiaschisi R. Tetrahedron Lett. 1999;40:3101. [Google Scholar]
- 359.Karlstroem ASE, Itami K, Bäckvall J-E. J Org Chem. 1999;64:1745. doi: 10.1021/jo982060h. [DOI] [PubMed] [Google Scholar]
- 360.Busacca CA, Eriksson MC, Dong Y, Prokopowicz AS, Salvagno AM, Tschantz MA. J Org Chem. 1999;64:4564. [Google Scholar]
- 361.Wehn PM, Du Bois J. Org Lett. 2005;7:4685. doi: 10.1021/ol051896l. [DOI] [PubMed] [Google Scholar]
- 362.Terao J, Kambe N. Acc Chem Res. 2008;41:1545. doi: 10.1021/ar800138a. [DOI] [PubMed] [Google Scholar]
- 363.Chass GA, Kantchev EAB, Fang D-C. Chem Commun. 2010;46:2727. doi: 10.1039/b922326f. [DOI] [PubMed] [Google Scholar]
- 364.Terao J, Naitoh Y, Kuniyasu H, Kambe N. Chem Commun. 2007:825. doi: 10.1039/b612586g. [DOI] [PubMed] [Google Scholar]
- 365.Terao J, Ikumi A, Kuniyasu H, Kambe N. J Am Chem Soc. 2003;125:5646. doi: 10.1021/ja034201p. [DOI] [PubMed] [Google Scholar]
- 366.Singh SP, Terao J, Kambe N. Tetrahedron Lett. 2009;50:5644. [Google Scholar]
- 367.Vechorkin O, Barmaz D, Proust V, Hu X. J Am Chem Soc. 2009;131:12078. doi: 10.1021/ja906040t. [DOI] [PubMed] [Google Scholar]
- 368.Vechorkin O, Csok Z, Scopelliti R, Hu X. Chem Eur J. 2009;15:3889. doi: 10.1002/chem.200802059. [DOI] [PubMed] [Google Scholar]
- 369.Vechorkin O, Hu X. Angew Chem, Int Ed. 2009;48:2937. doi: 10.1002/anie.200806138. [DOI] [PubMed] [Google Scholar]
- 370.Vechorkin O, Proust V, Hu X. J Am Chem Soc. 2009;131:9756. doi: 10.1021/ja9027378. [DOI] [PubMed] [Google Scholar]
- 371.Vechorkin O, Proust V, Hu X. Angew Chem, Int Ed. 2010;49:3061. doi: 10.1002/anie.200907040. [DOI] [PubMed] [Google Scholar]
- 372.Csok Z, Vechorkin O, Harkins SB, Scopelliti R, Hu X. J Am Chem Soc. 2008;130:8156. doi: 10.1021/ja8025938. [DOI] [PubMed] [Google Scholar]
- 373.Bolm C, Legros J, Le Paih J, Zani L. Chem Rev. 2004;104:6217. doi: 10.1021/cr040664h. [DOI] [PubMed] [Google Scholar]
- 374.Fürstner A, Martin R. Chem Lett. 2005;34:624. [Google Scholar]
- 375.Ito S, Nakamura M. Organomet News. 2007:83. [Google Scholar]
- 376.Mori K. Yuki Gosei Kagaku Kyokaishi. 2010;68:75. [Google Scholar]
- 377.Nakamura M. Kagaku to Kogyo. 2009;62:994. [Google Scholar]
- 378.Sherry BD, Fürstner A. Acc Chem Res. 2008;41:1500. doi: 10.1021/ar800039x. [DOI] [PubMed] [Google Scholar]
- 379.Kochi JK. Acc Chem Res. 1974;7:351. [Google Scholar]
- 380.Kochi JK. J Organomet Chem. 2002;653:11. [Google Scholar]
- 381.Neumann SM, Kochi JK. J Org Chem. 1975;40:599. [Google Scholar]
- 382.Smith RS, Kochi JK. J Org Chem. 1976;41:502. [Google Scholar]
- 383.Tamura M, Kochi JK. Synthesis. 1971:303. [Google Scholar]
- 384.Tamura M, Kochi JK. J Am Chem Soc. 1971;93:1487. [Google Scholar]
- 385.Gargano M, Giannoccaro P, Rossi M, Vasapollo G, Sacco A. J Chem Soc, Dalton Trans. 1975:9. [Google Scholar]
- 386.Fabre JL, Julia M, Verpeaux JN. Tetrahedron Lett. 1982;23:2469. [Google Scholar]
- 387.Yanagisawa A, Nomura N, Yamamoto H. Synlett. 1991:513. [Google Scholar]
- 388.Yanagisawa A, Nomura N, Yamamoto H. Tetrahedron. 1994;50:6017. [Google Scholar]
- 389.Cahiez G, Avedissian H. Synthesis. 1998:1199. [Google Scholar]
- 390.Fürstner A, Leitner A. Angew Chem, Int Ed. 2002;41:609. [Google Scholar]
- 391.Fürstner A, Leitner A, Mendez M, Krause H. J Am Chem Soc. 2002;124:13856. doi: 10.1021/ja027190t. [DOI] [PubMed] [Google Scholar]
- 392.Bogdanovi B, Schwickardi M. Angew Chem, Int Ed. 2000;39:4610. [PubMed] [Google Scholar]
- 393.Scheiper B, Bonnekessel M, Krause H, Fürstner A. J Org Chem. 2004;69:3943. doi: 10.1021/jo0498866. [DOI] [PubMed] [Google Scholar]
- 394.Hocek M, Dvoráková H. J Org Chem. 2003;68:5773. doi: 10.1021/jo034351i. [DOI] [PubMed] [Google Scholar]
- 395.Le Marquand P, Tsui GC, Whitney JCC, Tam W. J Org Chem. 2008;73:7829. doi: 10.1021/jo801384e. [DOI] [PubMed] [Google Scholar]
- 396.Seck M, Franck X, Hocquemiller R, Figadère B, Peyrat J-F, Provot O, Brion J-D, Alami M. Tetrahedron Lett. 2004;45:1881. [Google Scholar]
- 397.Ottesen LK, Ek F, Olsson R. Org Lett. 2006;8:1771. doi: 10.1021/ol0600234. [DOI] [PubMed] [Google Scholar]
- 398.Rao Volla CM, Vogel P. Angew Chem, Int Ed. 2008;47:1305. doi: 10.1002/anie.200704858. [DOI] [PubMed] [Google Scholar]
- 399.Volla CMR, Markovi D, Dubbaka SR, Vogel P. Eur J Org Chem. 2009:6281. [Google Scholar]
- 400.Gøgsig TM, Lindhardt AT, Skrydstrup T. Org Lett. 2009;11:4886. doi: 10.1021/ol901975u. [DOI] [PubMed] [Google Scholar]
- 401.Li B-J, Xu L, Wu Z-H, Guan B-T, Sun C-L, Wang B-Q, Shi Z-J. J Am Chem Soc. 2009;131:14656. doi: 10.1021/ja907281f. [DOI] [PubMed] [Google Scholar]
- 402.Dongol KG, Koh H, Sau M, Chai CLL. Adv Synth Catal. 2007;349:1015. [Google Scholar]
- 403.Fürstner A, Krause H, Lehmann CW. Angew Chem, Int Ed. 2006;45:440. doi: 10.1002/anie.200502859. [DOI] [PubMed] [Google Scholar]
- 404.Cahiez G, Habiak V, Duplais C, Moyeux A. Angew Chem, Int Ed. 2007;46:4364. doi: 10.1002/anie.200700742. [DOI] [PubMed] [Google Scholar]
- 405.Allen RB, Lawler RG, Ward HR. J Am Chem Soc. 1973;95:1692. [Google Scholar]
- 406.Lawler RG, Livant P. J Am Chem Soc. 1976;98:3710. [Google Scholar]
- 407.Dell’Anna MM, Mastrorilli P, Nobile CF, Marchese G, Taurino MR. J Mol Catal A Chem. 2000;161:239. [Google Scholar]
- 408.Yamamura M, Moritani I, Murahashi S-I. J Organomet Chem. 1975;91:C39. [Google Scholar]
- 409.Murahashi S, Yamamura M, Yanagisawa K, Mita N, Kondo K. J Org Chem. 1979;44:2408. [Google Scholar]
- 410.Murahashi S-I. J Organomet Chem. 2002;653:27. [Google Scholar]
- 411.Katayama T, Umeno M. Chem Lett. 1991:2073. [Google Scholar]
- 412.Miller JA. Tetrahedron Lett. 2002;43:7111. [Google Scholar]
- 413.Limmert ME, Roy AH, Hartwig JF. J Org Chem. 2005;70:9364. doi: 10.1021/jo051394l. [DOI] [PubMed] [Google Scholar]
- 414.Mehta VP, Modha SG, Van der Eycken E. J Org Chem. 2009;74:6870. doi: 10.1021/jo901327y. [DOI] [PubMed] [Google Scholar]
- 415.Terao J, Naitoh Y, Kuniyasu H, Kambe N. Chem Lett. 2003;32:890. [Google Scholar]
- 416.Franció G, Leitner W, editors. In Organic synthesis with transition metal complexes using compressed carbon dioxide as reaction medium. Vol. 2 2004. [Google Scholar]
- 417.Farina V, Krishnamurthy V, Scott WJ. Org React. 1997;50:1. [Google Scholar]
- 418.Paquette LA. In Opportunities offered by indium-promoted carbon-carbon bond-forming reactions in water. 1998 [Google Scholar]
- 419.Dennis LM, Work RW, Rochow EG, Chamot EM. J Am Chem Soc. 1934;56:1047. [Google Scholar]
- 420.Clark HC, Pickard AL. J Organometal Chem. 1967;8:427. [Google Scholar]
- 421.Pérez I, Sestelo JP, Sarandeses LA. J Am Chem Soc. 2001;123:4155. doi: 10.1021/ja004195m. [DOI] [PubMed] [Google Scholar]
- 422.Chao L-C, Rieke RD. J Org Chem. 1975;40:2253. [Google Scholar]
- 423.Hill MS, Hitchcock PB, Pongtavornpinyo R. Inorg Chem. 2007;46:3783. doi: 10.1021/ic070183r. [DOI] [PubMed] [Google Scholar]
- 424.Nomura R, Miyazaki S, Matsuda H. J Am Chem Soc. 1992;114:2738. [Google Scholar]
- 425.Fischer RA, Weiß J. Angew Chem, Int Ed. 1999;38:2830. doi: 10.1002/(sici)1521-3773(19991004)38:19<2830::aid-anie2830>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 426.Pena MA, Sestelo JP, Sarandeses LA. Synthesis. 2005:485. [Google Scholar]
- 427.Bhayana B, Fors BP, Buchwald SL. Org Lett. 2009;11:3954. doi: 10.1021/ol9015892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Pena MA, Pérez I, Sestelo JP, Sarandeses LA. Chem Commun. 2002:2246. doi: 10.1039/b206346h. [DOI] [PubMed] [Google Scholar]
- 429.Takami K, Yorimitsu H, Shinokubo H, Matsubara S, Oshima K. Org Lett. 2001;3:1997. doi: 10.1021/ol015975i. [DOI] [PubMed] [Google Scholar]
- 430.Riveiros R, Saya L, Sestelo JP, Sarandeses LA. Eur J Org Chem. 2008:1959. [Google Scholar]
- 431.Bouissane L, Sestelo JP, Sarandeses LA. Org Lett. 2009;11:1285. doi: 10.1021/ol900063p. [DOI] [PubMed] [Google Scholar]
- 432.Lee PH, Lee SW, Seomoon D. Org Lett. 2003;5:4963. doi: 10.1021/ol035883o. [DOI] [PubMed] [Google Scholar]
- 433.Lee SW, Lee K, Seomoon D, Kim S, Kim H, Kim H, Shim E, Lee M, Lee S, Kim M, Lee PH. J Org Chem. 2004;69:4852. doi: 10.1021/jo0495790. [DOI] [PubMed] [Google Scholar]
- 434.Lee PH, Lee SW, Lee K. Org Lett. 2003;5:1103. doi: 10.1021/ol034167j. [DOI] [PubMed] [Google Scholar]
- 435.Zhao Y, Jin L, Li P, Lei A. J Am Chem Soc. 2008;130:9429. doi: 10.1021/ja801116s. [DOI] [PubMed] [Google Scholar]
- 436.Jin L, Zhao Y, Zhu L, Zhang H, Lei A. Adv Synth Catal. 2009;351:630. [Google Scholar]
- 437.Fausett BW, Liebeskind LS. J Org Chem. 2005;70:4851. doi: 10.1021/jo050110u. [DOI] [PubMed] [Google Scholar]
- 438.Hatanaka Y, Hiyama T. Tetrahedron Lett. 1988;29:97. [Google Scholar]
- 439.Hiyama T, Hatanaka Y. Pure Appl Chem. 1994;66:1471. [Google Scholar]
- 440.Denmark SE, Ober MH. Aldrichimica Acta. 2003;36:75. [Google Scholar]
- 441.Spivey AC, Gripton CJG, Hannah JP. Curr Org Synth. 2004;1:211. [Google Scholar]
- 442.Denmark SE, Regens CS. Acc Chem Res. 2008;41:1486. doi: 10.1021/ar800037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Matsuhashi H, Kuroboshi M, Hatanaka Y, Hiyama T. Tetrahedron Lett. 1994;35:6507. [Google Scholar]
- 444.Hatanaka Y, Hiyama T. J Org Chem. 1989;54:268. [Google Scholar]
- 445.Hiyama T, Hatanaka Y. Pure Appl Chem. 1994;66:1471. [Google Scholar]
- 446.Matsuhashi H, Asai S, Hirabayashi K, Hatanaka Y, Mori A, Hiyama T. Bull Chem Soc Jpn. 1997;70:437. [Google Scholar]
- 447.Hatanaka Y, Hiyama T. Tetrahedron Lett. 1990;31:2719. [Google Scholar]
- 448.Klanberg F, Muetterties EL. Inorg Chem. 1968;7:155. [Google Scholar]
- 449.Marat RK, Janzen AF. Can J Chem. 1977;55:3845. [Google Scholar]
- 450.Schweizer SA, Bach T. Synlett. 2010:81. [Google Scholar]
- 451.Nakao Y, Takeda M, Matsumoto T, Hiyama T. Angew Chem, Int Ed. 2010;49:4447. doi: 10.1002/anie.201000816. [DOI] [PubMed] [Google Scholar]
- 452.Milstein D, Stille JK. J Am Chem Soc. 1979;101:4992. [Google Scholar]
- 453.Zhou W-J, Wang K-H, Wang J-X. J Org Chem. 2009;74:5599. doi: 10.1021/jo9005206. [DOI] [PubMed] [Google Scholar]
- 454.Saa JM, Martorell G, Garcia-Raso A. J Org Chem. 1992;57:678. [Google Scholar]
- 455.Stille JK. Pure Appl Chem. 1985;57:1771. [Google Scholar]
- 456.Peet WG, Tam W. J Chem Soc, Chem Commun. 1983:853. [Google Scholar]
- 457.Watanabe T, Hayashi K, Sakurada J, Ohki M, Takamatsu N, Hirohata H, Takeuchi K, Yuasa K, Ohta A. Heterocycles. 1989;29:123. [Google Scholar]
- 458.Herve A, Rodriguez AL, Fouquet E. J Org Chem. 2005;70:1953. doi: 10.1021/jo047907q. [DOI] [PubMed] [Google Scholar]
- 459.Chiappe C, Imperato G, Napolitano E, Pieraccini D. Green Chem. 2004;6:33. [Google Scholar]
- 460.Littke AF, Fu GC. Angew Chem, Int Ed. 1999;38:2411. doi: 10.1002/(sici)1521-3773(19990816)38:16<2411::aid-anie2411>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 461.Hayashi T. J Organomet Chem. 2002;653:41. [Google Scholar]
- 462.Taylor MS, Jacobsen EN. Proc Natl Acad Sci U S A. 2004;101:5368. doi: 10.1073/pnas.0307893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Hatanaka Y, Hiyama T. J Am Chem Soc. 1990;112:7793. [Google Scholar]
- 464.Imao D, Glasspoole BW, Laberge VS, Crudden CM. J Am Chem Soc. 2009;131:5024. doi: 10.1021/ja8094075. [DOI] [PubMed] [Google Scholar]
- 465.Zhou S-M, Deng M-Z, Xia L-J, Tang M-H. Angew Chem Int Ed. 1998;37:2845. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2845::AID-ANIE2845>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 466.Rubina M, Rubin M, Gevorgyan V. J Am Chem Soc. 2003;125:7198. doi: 10.1021/ja034210y. [DOI] [PubMed] [Google Scholar]
- 467.Ohmura T, Awano T, Suginome M. J Am Chem Soc. 2010;132:13191. doi: 10.1021/ja106632j. [DOI] [PubMed] [Google Scholar]
- 468.Campos KR, Klapars A, Waldman JH, Dormer PG, Chen C. J Am Chem Soc. 2006;128:3538. doi: 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]
- 469.Klapars A, Campos KR, Waldman JH, Zewge D, Dormer PG, Chen C-y. J Org Chem. 2008;73:4986. doi: 10.1021/jo8006804. [DOI] [PubMed] [Google Scholar]
- 470.Thaler T, Haag B, Gavryushin A, Schober K, Hartmann E, Gschwind RM, Zipse H, Mayer P, Knochel P. Nat Chem. 2010;2:125. doi: 10.1038/nchem.505. [DOI] [PubMed] [Google Scholar]
- 471.Hayashi T. Asymmetric cross-coupling reactions. 2008 [Google Scholar]
- 472.Hayashi T, Konishi M, Fukushima M, Mise T, Kagotani M, Tajika M, Kumada M. J Am Chem Soc. 1982;104:180. [Google Scholar]
- 473.Vriesema BK, Lemaire M, Buter J, Kellogg RM. J Org Chem. 1986;51:5169. [Google Scholar]
- 474.Cho SY, Shibasaki M. Tetrahedron Asymmetry. 1998;9:3751. [Google Scholar]
- 475.Pellet-Rostaing S, Saluzzo C, Ter Halle R, Breuzard J, Vial L, Le Guyader F, Lemaire M. Tetrahedron Asymmetry. 2001;12:1983. [Google Scholar]
- 476.Horibe H, Fukuda Y, Kondo K, Okuno H, Murakami Y, Aoyama T. Tetrahedron. 2004;60:10701. [Google Scholar]
- 477.Arp FO, Fu GC. J Am Chem Soc. 2005;127:10482. doi: 10.1021/ja053751f. [DOI] [PubMed] [Google Scholar]
- 478.Fischer C, Fu GC. J Am Chem Soc. 2005;127:4594. doi: 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]
- 479.Indolese AF, Consiglio G. Organometallics. 1994;13:2230. [Google Scholar]
- 480.Son S, Fu GC. J Am Chem Soc. 2008;130:2756. doi: 10.1021/ja800103z. [DOI] [PubMed] [Google Scholar]
- 481.Saito B, Fu GC. J Am Chem Soc. 2008;130:6694. doi: 10.1021/ja8013677. [DOI] [PubMed] [Google Scholar]
- 482.Owston NA, Fu GC. J Am Chem Soc. 2010;132:11908. doi: 10.1021/ja105924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Mandal AK. Org Lett. 2002;4:2043. doi: 10.1021/ol020058d. [DOI] [PubMed] [Google Scholar]
- 484.Still WC, Barrish JC. J Am Chem Soc. 1983;105:2487. [Google Scholar]
- 485.Marshall JA, Bourbeau MP. J Org Chem. 2002;67:2751. doi: 10.1021/jo016025d. [DOI] [PubMed] [Google Scholar]
- 486.Tsukano C, Ebine M, Sasaki M. J Am Chem Soc. 2005;127:4326. doi: 10.1021/ja042686r. [DOI] [PubMed] [Google Scholar]
- 487.Fuwa H, Ebine M, Bourdelais AJ, Baden DG, Sasaki M. J Am Chem Soc. 2006;128:16989. doi: 10.1021/ja066772y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Sasaki M. Bull Chem Soc Jpn. 2007;80:856. [Google Scholar]
- 489.Yuan Y, Men H, Lee C. J Am Chem Soc. 2004;126:14720. doi: 10.1021/ja0447154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Taillier C, Bellosta V, Cossy J. Org Lett. 2004;6:2149. doi: 10.1021/ol049433n. [DOI] [PubMed] [Google Scholar]
- 491.Smith AB, III, Davulcu AH, Kürti L. Org Lett. 2006;8:1665. doi: 10.1021/ol060290+. [DOI] [PubMed] [Google Scholar]
- 492.Jin J, Chen Y, Li Y, Wu J, Dai W-M. Org Lett. 2007;9:2585. doi: 10.1021/ol0710360. [DOI] [PubMed] [Google Scholar]
- 493.Dai W-M, Li Y, Zhang Y, Lai KW, Wu J. Tetrahedron Lett. 2004;45:1999. [Google Scholar]
- 494.Dai W-M, Zhang Y. Tetrahedron Lett. 2005;46:1377. [Google Scholar]
- 495.Roulland E. Angew Chem, Int Ed. 2008;47:3762. doi: 10.1002/anie.200800585. [DOI] [PubMed] [Google Scholar]
- 496.Hayakawa I, Ueda M, Yamaura M, Ikeda Y, Suzuki Y, Yoshizato K, Kigoshi H. Org Lett. 2008;10:1859. doi: 10.1021/ol800554f. [DOI] [PubMed] [Google Scholar]
- 497.Williams DR, Walsh MJ, Miller NA. J Am Chem Soc. 2009;131:9038. doi: 10.1021/ja902677t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Yamanaka H, Sato K, Sato H, Iida M, Oishi T, Chida N. Tetrahedron. 2009;65:9188. [Google Scholar]
- 499.Schnabel C, Hiersemann M. Org Lett. 2009;11:2555. doi: 10.1021/ol900819u. [DOI] [PubMed] [Google Scholar]
- 500.Domingo V, Silva L, Diéguez HR, Arteaga JF, Quílez del Moral JF, Barrero AF. J Org Chem. 2009;74:6151. doi: 10.1021/jo901011m. [DOI] [PubMed] [Google Scholar]
- 501.O’Neil GW, Ceccon J, Benson S, Collin M-P, Fasching B, Fürstner A. Angew Chem, Int Ed. 2009;48:9940. doi: 10.1002/anie.200906121. [DOI] [PubMed] [Google Scholar]
- 502.Benson S, Collin M-P, O’Neil GW, Ceccon J, Fasching B, Fenster MDB, Godbout C, Radkowski K, Goddard R, Fürstner A. Angew Chem, Int Ed. 2009;48:9946. doi: 10.1002/anie.200906122. [DOI] [PubMed] [Google Scholar]
- 503.Bonazzi S, Eidam O, Güttinger S, Wach J-Y, Zemp I, Kutay U, Gademann K. J Am Chem Soc. 2010;132:1432. doi: 10.1021/ja9097093. [DOI] [PubMed] [Google Scholar]
- 504.Bodwell GJ, Li J. Angew Chem, Int Ed. 2002;41:3261. doi: 10.1002/1521-3773(20020902)41:17<3261::AID-ANIE3261>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 505.Herb C, Maier ME. J Org Chem. 2003;68:8129. doi: 10.1021/jo035054g. [DOI] [PubMed] [Google Scholar]
- 506.Loiseleur O, Koch G, Cercus J, Schürch F. Org Process Res Dev. 2005;9:259. [Google Scholar]
- 507.Yajima A, Yamaguchi A, Nukada T, Yabuta G. Biosci, Biotechnol, Biochem. 2007;71:2822. doi: 10.1271/bbb.70468. [DOI] [PubMed] [Google Scholar]
- 508.Sánchez LG, Castillo EN, Maldonado H, Chávez D, Somanathan R, Aguirre G. Synth Commun. 2008;38:54. [Google Scholar]
- 509.Archambaud S, Legrand F, Aphecetche-Julienne K, Collet S, Guingant A, Evain M. Eur J Org Chem. 2010:1364. [Google Scholar]
- 510.Hu T, Takenaka N, Panek JS. J Am Chem Soc. 2002;124:12806. doi: 10.1021/ja020853m. [DOI] [PubMed] [Google Scholar]
- 511.Aoyagi S, Hirashima S, Saito K, Kibayashi C. J Org Chem. 2002;67:5517. doi: 10.1021/jo0200466. [DOI] [PubMed] [Google Scholar]
- 512.Duffey MO, LeTiran A, Morken JP. J Am Chem Soc. 2003;125:1458. doi: 10.1021/ja028941u. [DOI] [PubMed] [Google Scholar]
- 513.Altmann K-H, Bold G, Caravatti G, Denni D, Flörsheimer A, Schmidt A, Rihs G, Wartmann M. Helv Chim Acta. 2002;85:4086. [Google Scholar]
- 514.Zakarian A, Batch A, Holton RA. J Am Chem Soc. 2003;125:7822. doi: 10.1021/ja029225v. [DOI] [PubMed] [Google Scholar]
- 515.Klement I, Knochel P, Chau K, Cahiez G. Tetrahedron Lett. 1994;35:1177. [Google Scholar]
- 516.Aïssa C, Riveiros R, Ragot J, Fürstner A. J Am Chem Soc. 2003;125:15512. doi: 10.1021/ja038216z. [DOI] [PubMed] [Google Scholar]
- 517.Vyvyan JR, Loitz C, Looper RE, Mattingly CS, Peterson EA, Staben ST. J Org Chem. 2004;69:2461. doi: 10.1021/jo035778s. [DOI] [PubMed] [Google Scholar]
- 518.Inoue M, Yokota W, Murugesh MG, Izuhara T, Katoh T. Angew Chem, Int Ed. 2004;43:4207. doi: 10.1002/anie.200454192. [DOI] [PubMed] [Google Scholar]
- 519.Tan Z, Negishi E-i. Angew Chem, Int Ed. 2004;43:2911. doi: 10.1002/anie.200353429. [DOI] [PubMed] [Google Scholar]
- 520.Pitsinos E, Athinaios N, Xu Z, Wang G, Negishi E-i. Chem Commun. 2010;46:2200. doi: 10.1039/b920261g. [DOI] [PubMed] [Google Scholar]
- 521.Fujioka H, Sawama Y, Kotoku N, Ohnaka T, Okitsu T, Murata N, Kubo O, Li R, Kita Y. Chem Eur J. 2007;13:10225. doi: 10.1002/chem.200700871. [DOI] [PubMed] [Google Scholar]
- 522.Watanabe K, Oguchi T, Takizawa T, Furuuchi M, Abe H, Katoh T. Heterocycles. 2007;73:263. [Google Scholar]
- 523.Kadota I, Takamura H, Nishii H, Yamamoto Y. J Am Chem Soc. 2005;127:9246. doi: 10.1021/ja051171c. [DOI] [PubMed] [Google Scholar]
- 524.Petri AF, Schneekloth JS, Jr, Mandal AK, Crews CM. Org Lett. 2007;9:3001. doi: 10.1021/ol071024e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Corrêa IR, Jr, Pilli RA. Angew Chem, Int Ed. 2003;42:3017. doi: 10.1002/anie.200351347. [DOI] [PubMed] [Google Scholar]
- 526.Lowe JT, Panek JS. Org Lett. 2008;10:3813. doi: 10.1021/ol801499s. [DOI] [PubMed] [Google Scholar]
- 527.Graf KM, Tabor MG, Brown ML, Paige M. Org Lett. 2009;11:5382. doi: 10.1021/ol9021222. [DOI] [PubMed] [Google Scholar]
- 528.Nolasco L, Perez Gonzalez M, Caggiano L, Jackson RFW. J Org Chem. 2009;74:8280. doi: 10.1021/jo9018792. [DOI] [PubMed] [Google Scholar]
- 529.Espejo VR, Rainier JD. Org Lett. 2010;12:2154. doi: 10.1021/ol100672z. [DOI] [PubMed] [Google Scholar]
- 530.Anastasia L, Dumond YR, Negishi E-i. Eur J Org Chem. 2001:3039. [Google Scholar]
- 531.Lipshutz BH, Bulow G, Fernandez-Lazaro F, Kim S-K, Lowe R, Mollard P, Stevens KL. J Am Chem Soc. 1999;121:11664. [Google Scholar]
- 532.Rivkin A, Njardarson JT, Biswas K, Chou T-C, Danishefsky SJ. J Org Chem. 2002;67:7737. doi: 10.1021/jo0204294. [DOI] [PubMed] [Google Scholar]
- 533.Fürstner A, De Souza D, Parra-Rapado L, Jensen JT. Angew Chem, Int Ed. 2003;42:5358. doi: 10.1002/anie.200352413. [DOI] [PubMed] [Google Scholar]
- 534.Fürstner A, Leitner A. Angew Chem, Int Ed. 2003;42:308. doi: 10.1002/anie.200390103. [DOI] [PubMed] [Google Scholar]
- 535.Scheiper B, Glorius F, Leitner A, Fürstner A. Proc Natl Acad Sci U S A. 2004;101:11960. doi: 10.1073/pnas.0401322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Seidel G, Laurich D, Fürstner A. J Org Chem. 2004;69:3950. doi: 10.1021/jo049885d. [DOI] [PubMed] [Google Scholar]
- 537.Sheddan NA, Mulzer J. Org Lett. 2005;7:5115. doi: 10.1021/ol0515762. [DOI] [PubMed] [Google Scholar]
- 538.Cahiez G, Habiak V, Gager O. Org Lett. 2008;10:2389. doi: 10.1021/ol800816f. [DOI] [PubMed] [Google Scholar]
- 539.Uenishi Ji, Kawahama R, Izaki Y, Yonemitsu O. Tetrahedron. 2000;56:3493. [Google Scholar]