Abstract
Introduction
It has been shown that PDE 5 inhibitors preserve smooth muscle (SM) content and ameliorate the fibrotic degeneration normally seen in the corpora cavernosa after bilateral cavernosal nerve resection (BCNR). However, the downstream mechanisms by which these drugs protect the corpora cavernosa remain poorly understood.
Aim
To provide insight into the mechanism, we aimed to determine the gene expression profile of angiogenesis related pathways within the penile tissue after BCNR with or without continuous sildenafil treatment.
Methods
5-month old Fisher rats were subjected to BCNR or sham operation and treated with or without sildenafil (20 mg/Kg. B.W drinking water) for 3 days or 45 days (n=8 rats per group). Total RNAs isolated from the denuded penile shaft and prostate were subjected to reverse transcription and to angiogenesis real time-PCR arrays (84 genes). Changes in protein expression of selected genes such as epiregulin and CTGF were corroborated by western blot and immunohistochemistry.
Main outcomes measures
Genes modulated by BCNR and sildenafil treatment.
Results
A decreased expression of genes related to SM growth factors such as epiregulin (EREG), platelet derived growth factor (PDGF), extracellular matrix regulators such as metalloproteinases 3 and 9, endothelial growth factors, together with an up-regulation of pro-fibrotic genes such as connective tissue growth factor (CTGF) and TGFβ2 were found at both time points after BCNR. Sildenafil treatment reversed this process by up-regulating endothelial and SM growth factors and down-regulating pro-fibrotic factors. Sildenafil did not affect the expression of EREG, VEGF, PDGF in the ventral prostate of BCNR animals
Conclusions
Sildenafil treatment after BCNR activates genes related to SM preservation and down regulates genes related to fibrosis in the corpora cavernosa. These results provide a mechanistic justification for the use of sildenafil and other PDE5 inhibitors as protective therapy against corporal SM loss and fibrosis after radical prostatectomy.
Introduction
Erectile dysfunction (ED) associated with radical prostatectomy (RP) is a common complication after surgery due to intra-operative injury to the cavernosal nerves(1,2). Although nerve-sparing RP (NSRP) has significantly decreased the rates of post operative ED(2–4) novel approaches for preserving both the normal architecture of the corporal tissue as well as its function continue to undergo investigation because of the still unacceptable high rates of ED with NSRP.
Cavernosal nerve damage ultimately leads to apoptosis of the corporal smooth muscle cells as well as fibrosis of the corporal tissue(5,6). This condition leads to the development of cavernosal venocclusive dysfunction (CVOD)(6) which is sometimes referred to as venous leakage. In this condition, blood entering the corporal tissue during an erectile event fails to remain within the sinusoids mainly because the remaining smooth muscle within the cavernosa is unable to relax sufficiently enough to achieve an intracorporeal pressure high enough to occlude the veins egressing the corpora. This alteration in the corporal histology is initiated immediately after the nerve injury occurs and it is assumed that a certain threshold of smooth muscle impairment/apoptosis has to be reached before a functional impairment i.e. erectile dysfunction due to CVOD occurs (7). Therefore, in this setting, therapies to protect corporal smooth muscle viability either by reducing its apoptosis and/or sustaining its proliferation should be initiated as early as possible after the injury.
It has been previously demonstrated in rats that long term treatment with the phosphodiesterase type 5 (PDE 5) inhibitors such as vardenafil(6), sildenafil(8) and tadalafil(9), preserves corporal smooth muscle and ameliorates fibrotic degeneration normally seen after bilateral cavernosal nerve resection (BCNR). Anecdotally, acute sildenafil treatment is able to down regulate penile hypoxia markers (10). There is still ongoing debate as to the exact role that PDE5 inhibitors play in the post RP setting (11). One school of thought states that these PDE5 inhibitors increase “oxygenation” to the penile tissues (10,12–14) while the other school of thought adheres to the concept that these are simply anti-fibrotic compounds which primarily inhibit the oxidative stress to the cavernosal tissue induced by the neurotomy (6–9,15–18).
Although animal studies support the concept of so called corporal preservation post-prostatectomy, in the clinical setting there is no consensus regarding its benefit, mainly because all the studies performed so far have marked differences in their design that comparisons between studies cannot be made. For example, the study of Schwartz et al(19) reported on 40 patients who had undergone radical prostatectomy and were treated with one of two doses of sildenafil started when the catheter was removed and given every-other-day for 6 months and who had a cavernosal biopsy both prior to and the end of the study period. The results demonstrated not only preservation of corporal histology and absence of fibrosis, but in the higher dose group there was a substantial increased content of corporal smooth muscle cells. Padma Nathan and et al (20) found in a randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil started 4 weeks after the NSRP and administered nightly for 36 weeks, a four fold increase in the return of normal spontaneous erections when compared to the control group although in the control group only 8% had normal erections at the end of the study period. More recently, Montorsi et al (21) in the triple arm REINVENT study where drug treatment was initiated 14 days after the NSRP and given for 9 months, reported that vardenafil was more efficacious in preserving erectile function when given as an on demand rather than as a nightly dose although in the final open label phase of the study, there was no difference in terms of recovery of erections as determined by an on-demand vardenafil trial. In fact, both the daily and on demand treatment for 9 months was no different than the placebo group
From these data, it is not surprising that penile rehabilitation or corporal preservation after RP remains controversial and more research is required in order to establish, at least with PDE5 inhibitors, when to start treatment post RRP, as well as the dose and the appropriate outcome metric to determine efficacy.(22,23)
To date, little is known about the mechanism of action by which long-term PDE5 inhibitors would have a beneficial effect on the corporal tissue. While it is known that elevated levels of cGMP may inhibit smooth muscle apoptosis and/or inhibit collagen deposition, the downstream pathways involved in this process are poorly understood. Angiogenesis, the formation of thin-walled endothelium-lined structures with muscular smooth muscle wall, plays an important role during the adult life span as "repair mechanism" of damaged tissues (24). Emerging evidence has demonstrated that sildenafil can induce angiogenesis after pulmonary hypertension (25), and stroke (26). Moreover, sildenafil has also shown promise in the management of ischemia-reperfusion injury in the heart (27). However, there is no molecular evidence regarding the mechanisms by which sildenafil therapy may confer protection on the corporal smooth muscle after nerve injury or a neuropathy, although Musicki et al has suggested that sildenafil may activative pathways that are involved in the repair of the corporal smooth muscle (28,29)
Expression of angiogenic factors such as vascular endothelial growth factor (VEGF) and its respective receptors are present in human corpus cavernosum, indicating the possibility of mechanisms associated with angiogenesis that could be involved in repairing the penile tissue structure after injuries or diseases.(30,31)
AIMS
The specific aim of this study was to determine a) whether BCNR can cause changes in the gene expression profile involved in fibrosis and smooth muscle biology in the penis and b) which molecular mechanisms that induce preservation of the smooth muscle content and ameliorates fibrosis in rat penile tissue following BCNR are modulated by sildenafil.
Materials and Methods
2.1 Animal surgery and treatments
Five month-old male Fisher 344 rats (Harlan Sprague–Dawley, San Diego, CA) were randomly divided into sham operated, BCNR and BCNR + sildenafil (SIL) groups. Treatment with SIL was initiated immediately after surgery, dissolving the drug in drinking water in a dose of 20 mg/kg/BW.
Animals were sacrificed at 3 days (short term treatment) and at 45 days (long term treatment) after surgery (n=8 each group). The selection of these two time points was based on our previous results where we determined that a) at 3 days post BCNR, histological changes, such as the loss of the corporal smooth muscle cells, are observed while CVOD as determined by cavernosometry cannot be detected, and b) at 45 days post BCNR, marked CVOD as well as marked histological changes are present. (7). Furthermore, when sildenafil is given daily immediately after the BCNR, not only is the corporal histology preserved at 45 days post BCNR, but there is no CVOD (6,8,9).
BCNR was performed as previously described (6–9). In the sham-operated group both cavernosal nerves were identified but not resected. In BCNR, the main cavernosal nerves and ancillary branches were resected by removing a 5-mm segment. All animal experiments were approved by the IACUC committee at our institution.
After the various treatments, rats were then killed by CO2 inhalation. The middle regions of the skin-denuded penile shafts and the ventral prostate of the long term treated animals (n=8 group) were dissected out, frozen or immersed in RNA later (Ambion, Foster City, CA). Penile and ventral prostate total RNAs were isolated by homogenization with Trizol-Reagent (Invitrogen, Carlsbad, CA, USA) purified and stored at −80C until further use.
2.2 RT2 Profiler™ PCR array analysis of angiogenesis target genes
Aliquots of penile total RNA from each animal were pooled in their respective experimental groups and then subjected to reverse transcription, and the resulting cDNA was analyzed by the Rat Angiogenesis RT2 Profiler™ Array (SABiosciences Corp., Frederick, MD, USA). Each array contains a panel of 84 primer sets related to angiogenesis and fibrosis plus 5 housekeeping genes and 2 negative controls. Table 1 shows the list of genes studied and its classification based on the function. Real-time PCRs were performed as follows: melting for 10 min at 95 °C, 40 cycles of two-step PCR including melting for 15 s at 95 °C, annealing for 1 min at 60 °C. The raw data were analyzed using the ΔΔCt method following the manufacturer's instructions (SABiosciences Corp., Frederick, MD, USA) (32–34). Only genes that expressed above a ± 2.0 fold change were considered as significant with its respective controls.
Table 1.
List of genes analyzed by RT-PCR array in Gene expression profile of angiogenesis related pathways determined by after 3 and 45 days of Sham, BCNR with or without SIL treatment. A list of 84 genes were analyzed and subdivided according to their function. Taken from SA Bioscience PARN-024 http://www.sabiosciences.com/rt_pcr_product/HTML/PARN-024A.html
| Angiogenic Factors: |
| Growth Factors and Receptors: Angpt1, Bai1, Ctgf, Ereg, Fgf1, Fgf2, Fgf6, Fgf16, Fgfr3, Figf, Flt1, Fzd5, Itgav, Jag1, Kdr, Nrp1, Pgf, Pdgfa, Pdgfb, Tek, Vegfa, Vegfb, Vegfc. |
| Adhesion Molecules: Col18a1, Eng, Itga5, Itgav, Nrp1, Tek. |
| Proteases, Inhibitors and Other Matrix Proteins: Anpep, Col4a3, Fn1, Mmp2, Mmp3, Mmp9, Mmp19, Serpinb5, Serpinf1. |
| Transcription Factors and Others: Angpt2 (Agpt2), Epas1, Mapk14, Tbx4. |
| Other Factors Involved in Angiogenesis: |
| Cytokines and Chemokines: Ccl2, Cxcl1 (GRO), Cxcl2 (GRO2), Cxcl9, Ifna1, Ifnb1, Ifng, Il1b, Il6, Tnf. |
| Other Growth Factors and Receptors: Edg1, Efna5, Egf, Hgf, Igf1, Itgb3, Lep, Mdk, Npr1, Nrp2, , Tgfa, Tgfb1, Tgfb2, Tgfb3, Tgfbr1. |
| Adhesion Molecules: Cdh5, Itgb3, Lama5, Nrp2, Pecam, Thbs4. |
| Proteases, Inhibitors and Other Matrix Proteins: Ecgf1, F2, , Plau, Plg, Timp1, Timp2, Timp3. |
| Transcription Factors and Others: Akt1, Efna1 (Ephrin A1), Efna2, Hif1a, Id1, Id3, Lect1, Ptgs1, Sphk1. |
2.3. Real-time quantitative PCR
Aliquots of 1 µg penile and ventral prostate RNAs from each experimental animal were reverse transcribed using a RNA PCR kit (Applied Biosystems, Foster City, CA, USA). Rat gene PCR primer sets for connective tissue growth factor (CTGF), epiregulin (EREG), Platelet derived growth factor (PDGF), and Vascular endothelial growth factor (VEGF) were obtained from SuperArray Bioscience The Power SYBR® Green PCR Master Mix (Applied Biosystem, Foster City, CA) was used with Step One Plus thermocycler and fluorescent detector lid (Applied Biosystem,) (32–34). The protocol included melting for 15 min at 95 C, 40 cycles of three-step PCR including melting for 15 s at 95°C, annealing for 30 s at 58 °C, elongation for 30 s at 72 °C with an additional detection step of 15 s at 81 °C, followed by a melting curve from 55 to 95 °C at the rate of 0.5°C per 10 s. Samples of 25 ng cDNA were analyzed in quadruplicate in parallel with 4 housekeeping gene controls standard curves (threshold cycle vs. log pg cDNA) were generated by log dilutions of from 0.1 pg to 100 ng standard cDNA (reverse-transcribed mRNA from control animals). Experimental mRNA starting quantities were then calculated from the standard curves and averaged using i-Cycler, iQ software as described previously (33) The ratios of marker experimental gene (e.g. CTGF and EREG mRNA) to RPLP1 mRNA were computed.
2.4 Western blot analysis
Penile tissue homogenates (100 mg tissue) were obtained in T-PER (Pierce, Rockford, IL) and protease inhibitors (3 µM leupeptin, 1 µM pepstatin A, 1mM phenyl methyl sulfonyl fluoride), and centrifuged at 10,000 g for 5 min. Supernatant protein (30–50 µg) were subjected to western blot analyses(6, 35) by 7–10 % Tris-HCl polyacrylamide gel electrophoresis (PAGE) (Bio-Rad, Hercules, CA) in running buffer (Tris/Glycine/SDS). Proteins were transferred overnight at 4°C to nitrocellulose membranes in transfer buffer (Tris/glycine/methanol) and the next day, the non-specific binding was blocked by immersing the membranes into 5% non-fat dried milk, 0.1% (v/v) Tween 20 in PBS for 1hour at room temperature. After several washes with washing buffer (PBS Tween 0.1%), the membranes were incubated with the primary antibodies for 3 hours at room temperature as follows: a) mouse monoclonal α-SMA, as described above (1/1000) (Sigma Aldrich St Louis, MO); b) rabbit polyclonal CTGF 1/5,000 (Ab CAM Cambridge,MA) a polyclonal EREG antibody (Santa Cruz, Santa Cruz, CA); c) glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1/10,000) (Chemicon International, Temecula, CA). The washed membranes were incubated for 1 hour at room temperature with 1/2,000 dilution of secondary antibodies, anti mouse or anti-rabbit respectively, linked to horseradish peroxidase. After several washes, the immunoreactive bands were visualized using the ECL plus western blotting chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). The densitometric analyses of the bands were performed with Image J (NIH, Bethesda, MD). A positive control was run throughout all gels for each antibody to standardize for variations in exposures and staining intensities. Negative controls were performed omitting the primary antibody. Band intensities were determined by densitometry and corrected by the respective intensities for a housekeeping protein, glyceraldehyde phosphate dehydrogenase (GAPDH), upon reprobing.
2.5 immunohistochemistry
Skin-denuded penile shafts were fixed overnight in formalin, washed, and stored in alcohol 70 % at 4 °C until processed for paraffin embedded tissue sections (5 µm). Adjacent tissue sections were used for immunostaining with polyclonal antibody against CTGF (1:5,000) and epiregulin (1:1,000) respectively.
Sections were then incubated with biotinylated anti-Rabbit IgG, respectively, followed by ABC complex (Vector labs, Temecula, CA) and 3,3’diaminobenzidine (Sigma)
2.6 Quantitative image analysis
Quantitative image analysis (QIA) was performed by computerized densitometry using the ImagePro Plus 7.0 program (Media Cybernetics, Silver Spring, MD), coupled to a Leica microscope equipped with an Spot RT digital camera (32–35). For CTGF and EREG staining, only the corpora cavernosa was analyzed in a computerized grid and expressed as % of positive area vs. total area of the corpora cavernosa. In all cases, two fields at 40X, (both sides of the corpora cavernosa) or 4 fields at 200X, were analyzed per tissue section, with at least 4 matched sections per animal and 8 animals per group (6–9,35).
2.7 Statistical analysis
Values were expressed as mean ± SEM. The normality distribution of the data was established using the Wilk-Shapiro test. Multiple comparisons were analyzed by a one way analysis of variance (one way ANOVA) followed by post-hoc comparisons with the Tukey test, according to the GraphPad Prism V 5.0. Differences were considered significant at p < 0.05.
Results
3.1 Effect of BCNR and sildenafil treatment on the expression of α-SMA
In order to verify whether the expression of α-SMA in penile tissue after BCNR plus SIL treatment can be observed under these experimental settings, western blot analysis were carried out in animals that were used for gene expression analyses. Figure 1 shows that by 45 days after BCNR, α-SMA expression was decreased by 57% compared to the sham group while the addition of SIL to the BCNR group was able to preserve the smooth muscle content levels even 45 days after BCNR.
Figure 1. Verification of the effect of bilateral cavernosal nerve resection and sildenafil treatment on the smooth muscle content in the rat corpora cavernosa.
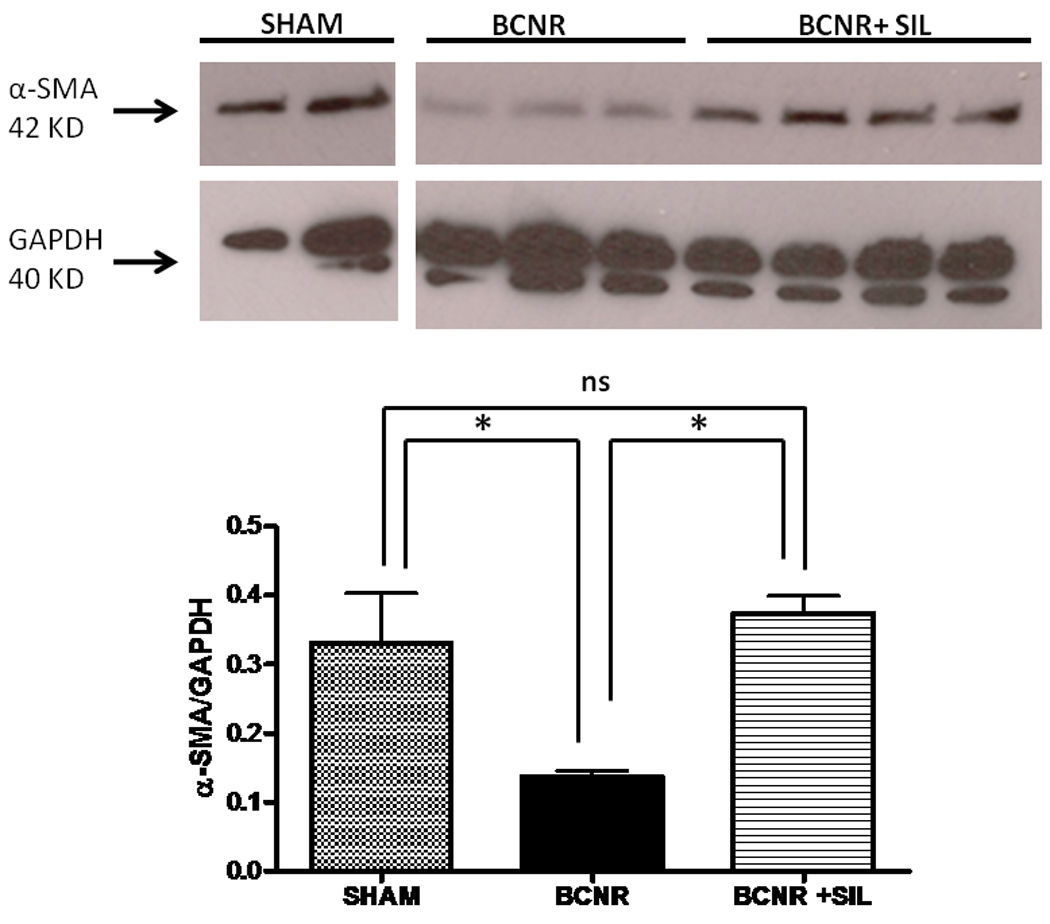
Homogenates from corpora cavernosa tissue, from the same animals employed for gene expression analysis, were subjected to western blot for alpha smooth muscle (α-SMA) expression. SHAM: sham-operated animals; BCNR: animals subjected to cavernosal nerve resection. BCNR+SIL: treated with sildenafil in drinking water and killed at 45 days after surgery. * p<0.05.
3.2 Short term treatment with sildenafil modulates the expression of genes involved in both the preservation of the smooth muscle content and the amelioration of fibrosis
The impact of BCNR and SIL treatment on the gene expression profile of angiogenesis-related markers in penile tissue was studied at 3 days after surgery. The profile of the fold difference between BCNR and sham operated animals showed a down regulation of approximately 13 genes related to smooth muscle and endothelial function, whereas an up regulation of 4 genes involved in development of fibrosis was found after 3 days of BCNR. Table 2 shows the list of genes with its respective fold changes that were down-regulated or up-regulated by BCNR in comparison with the sham operated animals (Left column). We found that 3 days after BCNR, there is an up-regulation of genes related to fibrosis such as connective tissue growth factor (CTGF) and transforming growth factor beta 2 (TGFβ2). In addition, a down regulation of growth factor related genes such as a potent smooth muscle proliferator, epiregulin (EREG); neurotrophic and pro-angiogenic factors such as Ephrin 2 and 5, Platelet derived growth factor a (PDFGa), endothelial cell growth factor 1 (ECGF1), proteases such as metallopeptidases 9 and 3 (MMPS 3 and 9) and the metalloproteinase tissue inhibitors (TIMP) among others were observed.
Table 2. Changes in gene expression profile of angiogenesis related pathways determined by RT-PCR array after 3 days of Sham, BCNR and SIL treatment.
Pooled penile Total RNA from 3 days Sham BCNR and BCNR+SIL were subjected to RT PCR array utilizing the Rat Angiogenesis RT2 Profiler™ PCR Array. List of genes up or down regulated) by BCNR n comparison with Sham and BCNR+SiL in comparison with BCNR. The ratios between the Sham vs. BCNR and BCNR vs. BCNR+SIL were normalized by RPLP1, RPLP3 and LDH housekeeping genes and were calculated for the assays performed in quadruplicates. Genes that were +2.0 fold up regulated are labeled in red, genes down regulated by −2.0 are labeled in blue. Genes regulated by less than ± 2 fold are labeled in black. It is considered significant those genes that shows ±2 fold changes
| Sham vs BCNR | BCNR vs. SIL | ||
|---|---|---|---|
| Symbol | Gene name | Fold change | Fold change |
| Akt1 | Thymoma viral proto-oncogene 1 | −2.65 | 1.73 |
| Angpt1 | Angiopoietin 1 | 2.14 | −2.48 |
| Anpep | Alanyl (membrane) aminopeptidase | −2.65 | 2.31 |
| Ctgf | Connective tissue growth factor | 3.35 | −2.45 |
| Ecgf | Endothelial cell growth factor 1 (platelet-derived) | −2.27 | 1.81 |
| Efna2 | Ephrin A2 | −3.15 | 3.32 |
| Efna5 | Ephrin A5 | −2.01 | 1.33 |
| Egf | Epidermal growth factor | 1.51 | 2.16 |
| Ereg | Epiregulin | −4.77 | 2.01 |
| Fn1 | Fibronectin 1 | −2.04 | −1.38 |
| Itga5 | Integrin alpha 5 | −2.01 | 1.42 |
| Kdr | Kinase insert domain protein receptor | 1.22 | 2.12 |
| Mmp3 | Matrix metallopeptidase 3 | −2.43 | 1.42 |
| Mmp9 | Matrix metallopeptidase 9 | −7.49 | 3.51 |
| Pdgfa | Platelet derived growth factor, alpha | −2.43 | 3.63 |
| Serpinb5 | Serine (or cysteine) peptidase inhibitor, | 2.77 | −2.61 |
| TGFb2 | Transforming growth factor, beta 2 | 6.04 | −2.89 |
| Thbs4 | Thrombospondin 4 | −4.61 | 1.02 |
| Timp2 | Tissue inhibitor of metalloproteinase 2 | −2.35 | 1.14 |
Short term treatment with SIL after BCNR reversed the gene profile to an up-regulation of 8 tissue growth factor genes and down regulation of 3 pro-fibrotic genes compared to BCNR at 3 days after surgery (right column). Short term treatment with SIL was able to down regulate the expression of pro-fibrotic genes such as CTGF and Tgfβ2 in comparison to BCNR. Moreover, genes related to tissue growth factor such as EREG, Epidermal growth factor (Egf), and PDGF were up-regulated by SIL.
The comparison of the gene expression profile between the sham and BCNR+SIL animals did not show any significant differences, indicating that short term treatment with SIL after BCNR normalize the gene expression profile.
3.3 Long term treatment with sildenafil promotes changes in the gene expression profile related to mechanism involved in angiogenesis and amelioration of fibrosis
The changes in the gene expression profile seen at 3 days were also seen at 45 days post BCNR and SIL. Table 3 shows the list of genes, which were up or down regulated by 2 fold when compared to the sham-operated group (left column). A down regulation of 11 genes related to endothelial and smooth muscle growth factors, as well as smooth muscle protein content was found after 45 days BCNR while 7 genes related to inflammatory cytokines and pro-fibrotic markers were up-regulated after BCNR.
Table 3. Changes in Gene expression profile of angiogenesis related pathways determined by RT-PCR array after 45 days BCNR and SIL treatment.
Pooled penile Total RNA from 45 days Sham, BCNR and BCNR+SIL were subjected to RT PCR array utilizing the Rat Angiogenesis RT2 Profiler™ PCR Array.. The ratios between the sham vs. BCNR and BCNR vs. BCNR+SIL were normalized by RPLP1, RPLP3 and LDH housekeeping genes and were calculated for the assays performed in quadruplicates Genes that were +2.0 fold up regulated are labeled in red, genes down regulated by −2.0 are labeled in blue. Genes regulated by less than ± 2 fold are labeled in black.
| Sham vs BCNR | BCNR vs. SIL | ||
|---|---|---|---|
| Symbol | Gene name | Fold change | Fold change |
| Ctgf | Connective tissue growth factor | 2.99 | −3.00 |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | 2.01 | −2.01 |
| Efna1 | Ephrin A1 | 1.22 | 2.09 |
| Efna2 | Ephrin A2 | 1.26 | 2.06 |
| Epas1 | Hypoxia fibroblast factor | 2.00 | −2.00 |
| Ereg | Epiregulin | −14.83 | 6.57 |
| Fn1 | Fibronectin 1 | −4.33 | −2.44 |
| Id1 | Inhibitor of DNA binding 1 | −2.00 | 1.25 |
| Ifnb1 | Interferon beta 1, fibroblast | 2.00 | 4.26 |
| Il1b | Interleukin 1 beta | 2.01 | −1.16 |
| Itga5 | Integrin alpha 5 | −2.13 | 2.43 |
| Mapk14 | Mitogen activated protein kinase 14 | 3.00 | −1.10 |
| Mmp9 | Matrix metallopeptidase 9 | −1.50 | 8.21 |
| Npr1 | Natriuretic peptide receptor 1 | −3.40 | 4.08 |
| Nrp1 | Neuropilin 1 | −1.45 | 3.04 |
| Nrp2 | Neuropilin 2 | −2.49 | 5.12 |
| Pdgfa | Platelet derived growth factor, alpha | −2.00 | 2.00 |
| Pdgfb | Platelet derived growth factor, B polypeptide | −3.28 | 2.00 |
| Pecam | Platelet/endothelial cell adhesion molecule | −2.62 | −1.64 |
| Thbs4 | Thrombospondin 4 | −4.18 | −1.27 |
| Timp2 | Tissue inhibitor of metalloproteinase 2 | −2.13 | 1.17 |
| Tnf | Tumor necrosis factor | 2.20 | 2.4 |
| Vegfa | Vascular endothelial growth factor A | −1.20 | 2.76 |
| Vegfb | Vascular endothelial growth factor B | −1.36 | 2.67 |
Forty-five days of treatment with SIL starting immediately after surgery induced an up-regulation of a variety of growth factors when compared to the BCNR group and gellatinases as well as a down regulation of pro-fibrotic factors. An up-regulation of 14 genes mostly related to tissue growth factors and a down regulation of 4 genes related to fibrosis and hypoxia were modulated in the 45 days SIL treatment group. Table 3 right columns shows the list of genes which were ±2 fold up or down regulated by SIL in comparison to BCNR at forty five days after surgery. The genes that were up-regulated by greater than two fold were EREG, MMP9, PDGFa, PDFGb, VEGF a and b among others whereas the genes down regulated by SIL treatment were CTGF, chemokine 2 EPAS and fibronectin.
3.4 Short and long term sildenafil treatment hampers the expression of pro-fibrotic factor CTGF induced by BCNR
In order to corroborate the findings observed by PCR array in pooled samples, real time PCR assays on total penile RNA extracted from each experimental animal were performed on selected genes that were highly regulated by short and long term BCNR and BCNR+ SIL treatment.
Figure 2A shows the expression of CTGF mRNA at 3 days. Its expression was increased at 3 days following BCNR, indicating that the genes involved in development of fibrosis are starting to be up-regulated. Short term treatment with SIL kept the CTGF gene expression at the same level as sham operated animals, indicating that SIL treatment appears to exert some sort of protection against the development of corporal fibrosis.
Figure 2. Short term treatment with sildenafil modulates the expression of connective tissue growth factor (CTGF) in the corpora cavernosa after BCNR.
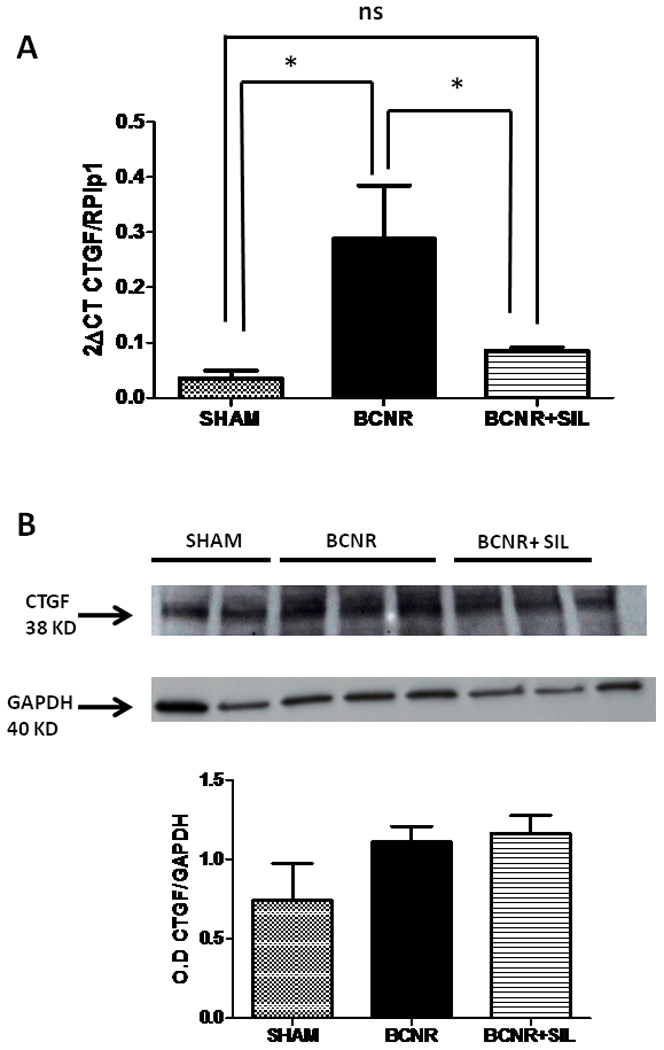
Top: Total RNA was extracted from 3 days Sham, BCNR and BCNR+ SIL were subjected to real time PCR for CTGF (SA Bioscience) normalized by RPLP1 housekeeping genes. Bottom: homogenates from corpora cavernosa were subjected to western blot analysis for CTGF (Ab Cam) normalized by GAPDH with the correspondent densitometric analysis. * p<0.05
In order to verify that the changes observed at the transcriptional levels are also reflected at the translational level, western blots analysis were carried out in the same experimental settings. Figure 2B shows that no changes in the expression of CTGF were found after 3 days of BCNR, and SIL treated animals
The same pattern of expression albeit more pronounced is also seen after 45 days treatment with SIL. Figure 3 A shows an up regulation of CTFG gene expression by 4.4 folds after BCNR compared to the sham while SIL treatment was able to reduce CTGF gene expression comparable to the sham levels. Moreover, CTGF expression determined by western blot was up-regulated by 1.7 fold when compared to the sham group. SIL treatment was able to significantly keep CTGF expression at the level found in the sham operated animals (Figure 3B)
Figure 3. Long term treatment with Sildenafil modulates the expression of CTGF in the corpora cavernosa after BCNR.
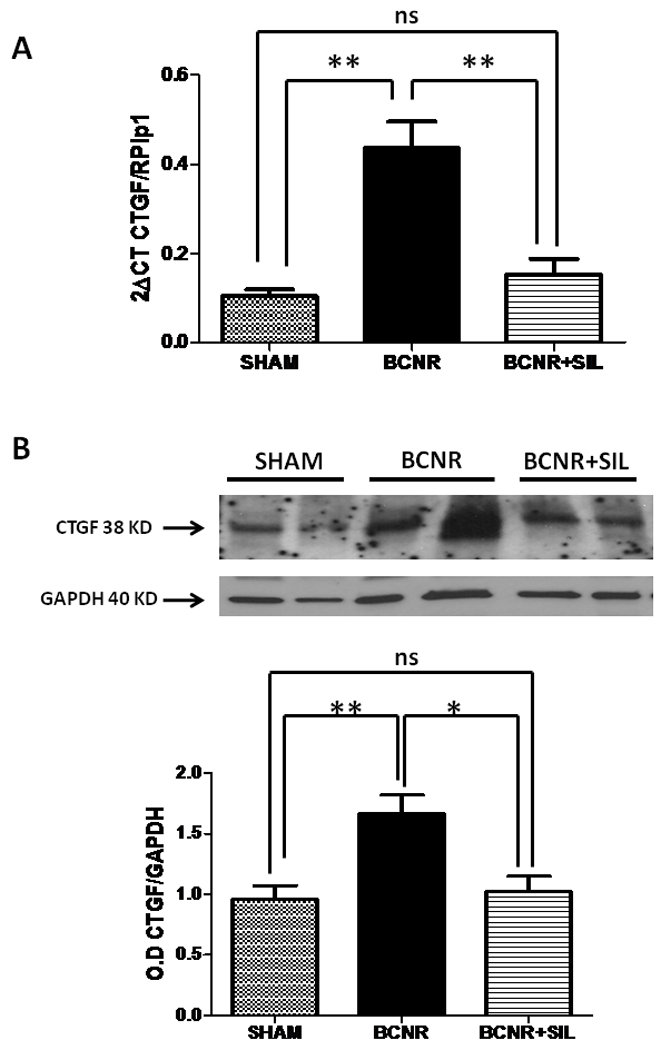
Top: Total RNA was extracted from 45 days Sham, BCNR and BCNR+ SILDE were subjected to real time PCR for CTGF normalized by RPLP1 housekeeping genes. Bottom: homogenates from corpora cavernosa as in figure 1 were subjected to western blot analysis for CTGF (Ab Cam) normalized by GAPDH with the correspondent densitometric analysis. * p<0.05; ** p<0.01
In order to determine that the changes in CTGF expression were restricted to the corpora cavernosa, we performed immunohistochemistry in another set of experimental animals in which we previously demonstrated the presence of CVOD 45 days after performing BCNR and its subsequent amelioration by daily SIL treatment. Figure 4 top shows that in sham operated animals, CTGF was virtually not expressed, although some staining was found in few fibroblasts of the tunica albuginea. BCNR induced a more generalized CTGF expression mainly in the smooth muscle cells of the corpora cavernosa while its expression was reduced by SIL treatment. Figure 4 bottom shows the results determined by image analysis.
Figure 4. Long term treatment with sildenafil decreases the immunohistochemical expression of CTGF in the corpora cavernosa after BCNR.
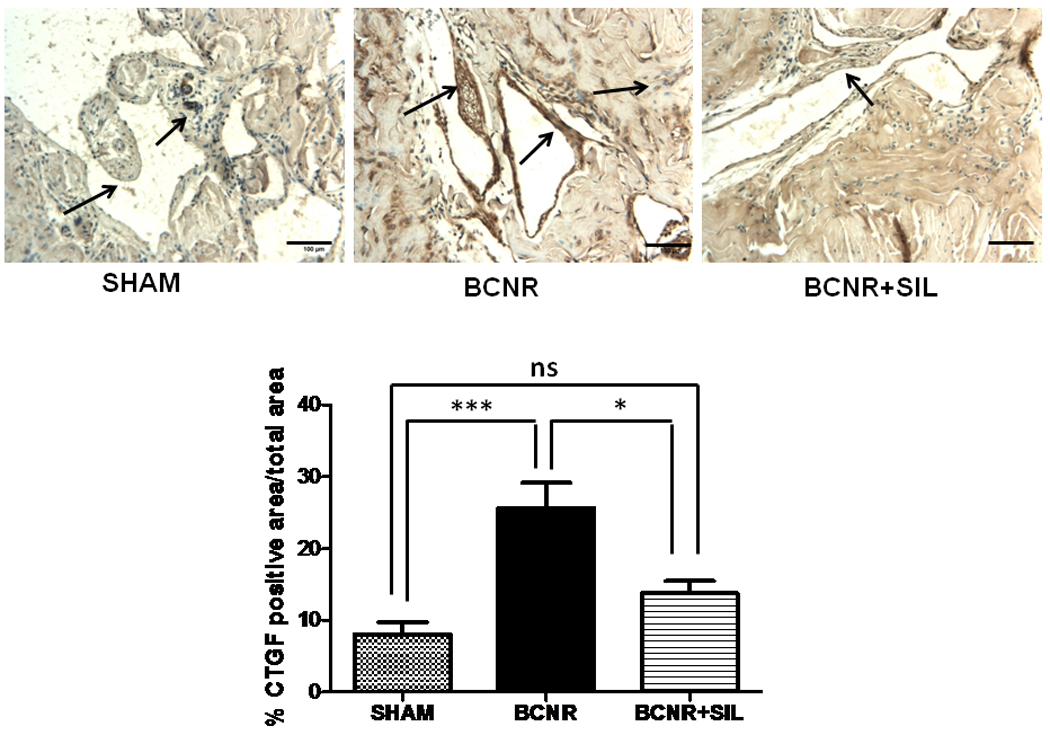
Paraffin embedded penile sections from 45 days Sham, BCNR and BCNR+SIL were immunostained for CTGF and subsequent detection by DAB. Representative pictures (Magnification 200X, Bar=100 µm) bottom: Image Analysis determined by % positive CTGF area over total area corpora cavernosa * p<0.05
3.6 Sildenafil promotes smooth muscle preservation by up regulating epiregulin (EREG) expression
It has been established that EREG is an important regulator of smooth muscle biology and proliferation. By PCR array analysis, we found that EREG expression is highly modulated by short and long term treatment with SIL after BCNR.
In order to confirm our findings, a real time PCR analysis was carried out at 3 days after BCNR and subsequent SIL treatment. Figure 5 A shows that 3 days after BCNR, EREG mRNA expression was decreased by 70% compared to sham operated animals. This effect was reversed by SIL since it induced a 3.5 fold increase in EREG expression compared to the BCNR only group. These findings were corroborated at the protein level as determined by western blot, with a reduction in the expression of EREG by BCNR. Short term treatment with SIL was able to preserve the EREG expression at the same level as the sham operated animals. (Figure 5B)
Figure 5. Short term treatment with sildenafil regulates the expression of epiregulin (EREG) in the corpora cavernosa after BCNR.
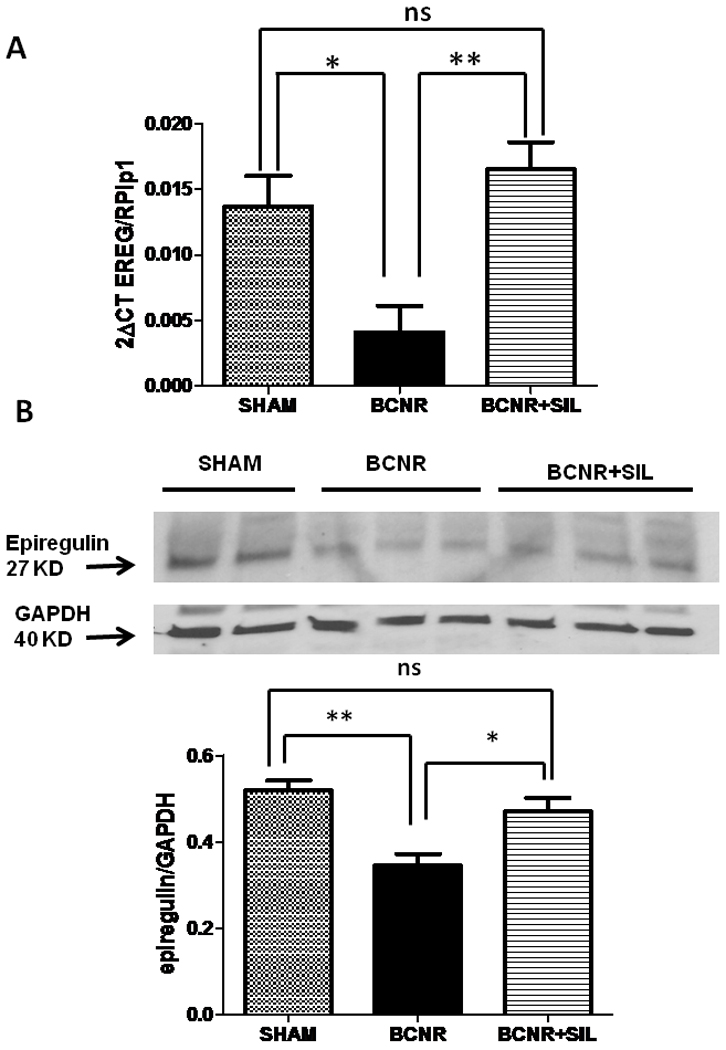
Top: Total RNA was extracted from 3 days Sham, BCNR and BCNR+ SIL were subjected to real time PCR for CTGF (SA Bioscience) normalized by RPLP1 housekeeping genes. Bottom: homogenates from corpora cavernosa were subjected to western blot analysis for CTGF (Ab Cam) normalized by GAPDH with the correspondent densitometric analysis. * p<0.05
A more pronounced effect was seen 45 days after surgery, EREG mRNA expression was down regulated by BCNR compared to the sham and SIL was able to up-regulate the EREG mRNA expression by 4.6 fold compared to BCNR only. (Fig 6A) A similar pattern was observed at 45 days after BCNR and SIL treatment. As determined by western blot analysis, BCNR decreased the expression of EREG by 30% compared to the sham group while SIL was able restore these levels up to the sham group.
Figure 6. Long term treatment with sildenafil regulates the expression of EREG in the corpora cavernosa after BCNR.
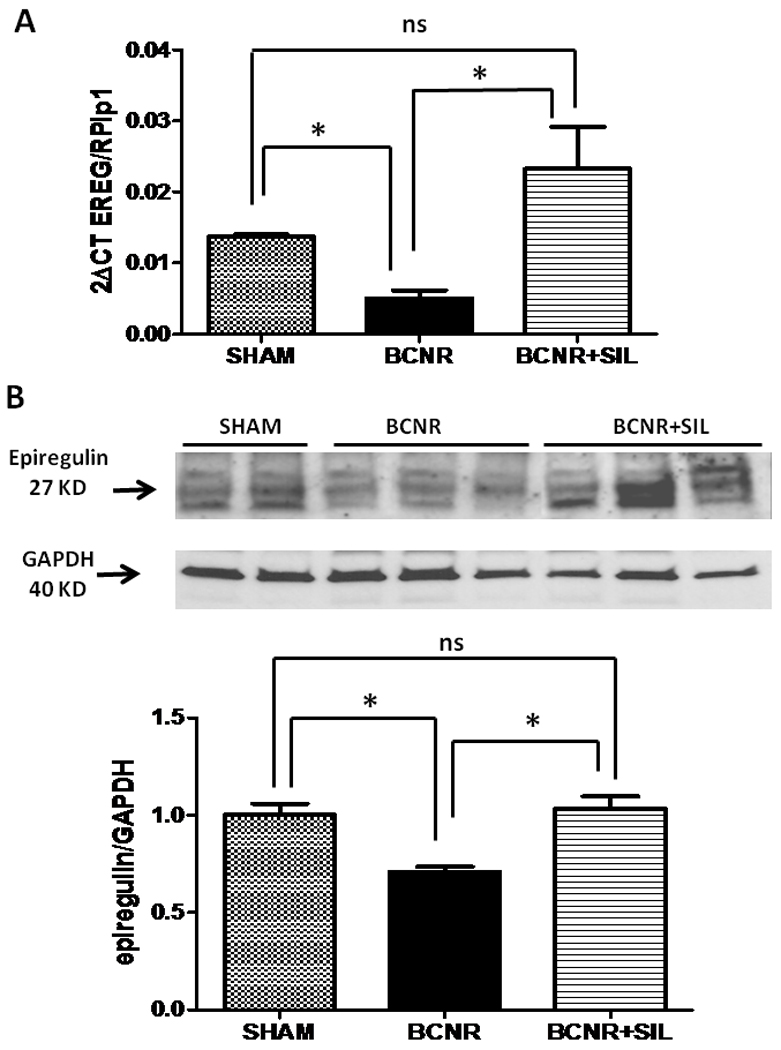
Top: Total RNA was extracted from 45 days Sham, BCNR and BCNR+ SIL were subjected to real time PCR for CTGF normalized by RPLP1 housekeeping genes. Bottom: homogenates from corpora cavernosa as in figure 1 were subjected to western blot analysis for EREG (Santa Cruz) normalized by GAPDH with the correspondent densitometric analysis. * p<0.05; ** p<0.01
In order to determine that the changes in the EREG expression by BCNR and SIL treatment were restricted to the corpora cavernosa, we studied the EREG expression also by immunohistochemistry. EREG was localized in the corporal smooth muscle cells in the sham operated animals. BCNR alone induced a profound decrease in the expression of EREG which was up regulated by long term treatment with SIL. (Fig. 7).
Figure 7. Long term treatment with sildenafil up-regulates the expression of EREG in the corpora cavernosa after BCNR determined by Immnunohistochemistry.
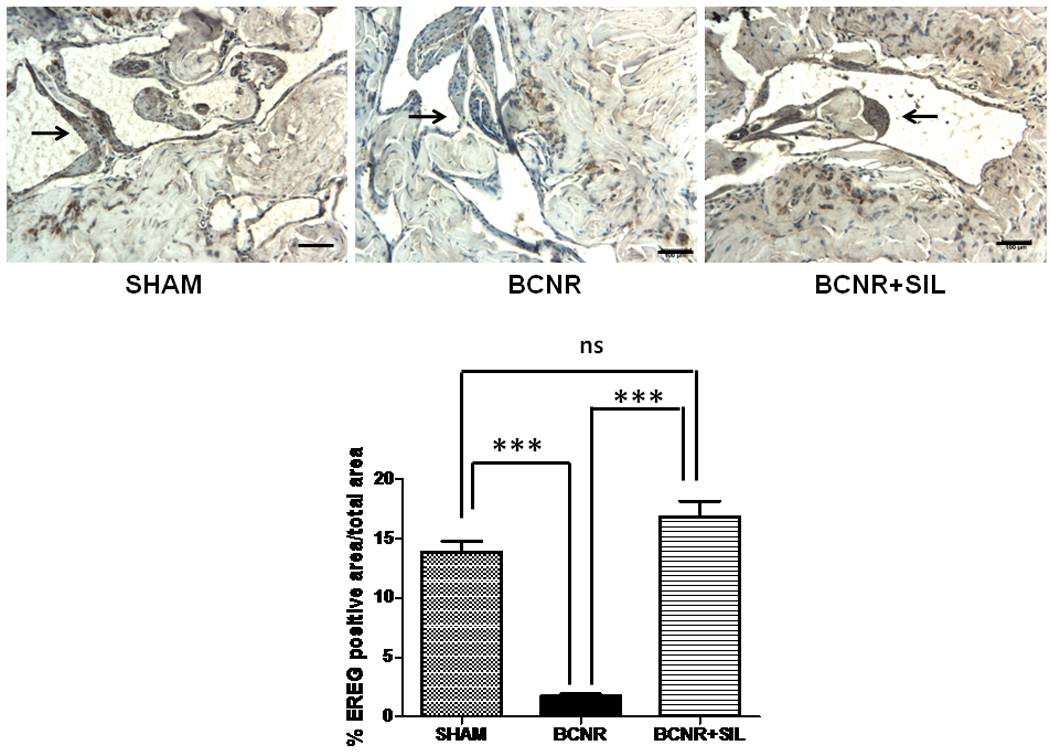
Paraffin embedded penile sections from 45 days Sham, BCNR and BCNR+SIL were immunostained for CTGF and subsequent detection by DAB. Representative pictures (Magnification 200X, Bar=100 µm).Bottom: Image analysis determined by % EREG positive area/total area. *** p<0.001
3.7 Sildenafil treatment did not affect the gene expression profile in the ventral prostate of BCNR operated animals
In order to rule out whether SIL treatment has angiogenic properties that could promote the abnormal growth of a tumor in close related organs, an experiment was carried out to determine the expression of angiogenic factors in the ventral prostate of sham, BCNR and BCNR+SIL animals. The gene expression profile for EREG, PDGFa, and VEGFa was studied in animals subjected to 45 days BCNR and SIL treatment. No changes in the expression of EREG was found at this time point (2Δ CT EREG/RPLP13A Sham 0.000553983± 0.0000891155; BCNR: 0.000669183 ± 0.0000422529 BCNR+SIL: 0.000572527± 0.000092736 In addition, no changes in the expression of PDGFa (2Δ CT PDGF/RPLP13A Sham 0.0298± 0.000475; BCNR: 0.00239 ± 0.000486; BCNR+SIL: 0.00348± 0.000295 and VEGFa (2Δ CT VEGFa/RPLP13 Sham 0.0098± 0.0016; BCNR: 0.0101 ± 0.0018; BCNR+SIL: 0.0103± 0.00035) were found in the ventral prostate of the animals, indicating that at least in normal ventral prostatic tissue SIL has no effect in up-regulating angiogenic factors.
Discussion
To the best of our knowledge, this is the first report to examine the gene expression profile after BCNR and its subsequent treatment with a PDE5 inhibitor. Our initial objective was to look for changes in inflammation, ECM remodeling, and angiogenesis related pathways after BCNR and to understand the mechanism by which SIL preserves the smooth muscle and ameliorates fibrosis after BCNR. Our findings demonstrate that both short and long term BCNR promotes down regulation of the gene expression profile of specific tissue growth factors, up-regulation of inflammatory cytokines and activation of pro-fibrotic factors such as CTGF and TGFβ 2. These effects are all counteracted by daily and continuous SIL treatment. It suggests that PDE5 inhibitors ameliorate CVOD after BCNR through the possible activation of downstream genes related to smooth muscle proliferation such as Ereg and Pdgf as well as inhibition of pro fibrotic factors such as CTGF and TGFβ 2. CTGF is known as a pro-fibrotic factor that is activated by TGFβ and ET-1. CTGF activates several signal transduction pathways via surface receptors that modulate the functional activities of fibroblasts, endothelial and smooth muscle cells (36). In vivo, over-expression of CTGF causes extracellular matrix accumulation and promotes tissue fibrosis in skin, lung, kidney, and the vasculature, especially small arteries(36,37). In our experimental setting, we found that up–regulation of CTGF gene expression by BCNR occurred as early as 3 days, although the protein expression is manifested at least by 45 days. This is in agreement with our previous finding that changes in the smooth muscle/ collagen ratio become evident 7 days after BCNR (7), although it is possible that the transcription of the involved genes in this process starts much earlier. Interestingly, CTGF expression is mainly regulated at the transcriptional level which indicates that BCNR will first induce changes in the RNA level which later on will be translated at the protein level (37).
Short and long term treatment with SIL induced a down regulation of CTGF mRNA levels but only the long term treatment reduces the CTGF protein expression as determined by IMM and western blots, thus promoting an anti-fibrotic effect. The down regulation of CTGF was also accompanied by an increase in the gelatinases MMP-9 and MMP-3, indicating that SIL has a role in modulating both extracellular matrix degradation and its production. It has been shown that MMPs are responsible for the cleavage of CTGF (38), and it is possible that SIL may exert an anti-fibrotic action by inducing MMP-9, which in turn will cleave CTGF and decrease collagen deposition. CTGF is also down regulated by NO produced by iNOS (39) indicating that the anti-fibrotic effect of iNOS(40,41) could be mediated through the down regulation of CTGF expression. It is possible that SIL can exert an anti-fibrotic effect by activating MMPs and also by up-regulating iNOS, which in turn would cleave CTGF thereby inhibiting collagen deposition.
CTGF is also modulated by androgens since it has been demonstrated in animals that androgen insufficiency up-regulates CTGF thereby promoting collagen deposition and fibrosis. Vignozzi et al recently reported that cavernosal neurotomy is associated with the development of hypogonadotropic hypogonadism which in animals has been shown to contribute to penile fibrosis (12). It is possible there may be a link between CTGF and testosterone but since we did not measure androgen levels in our experimental setting at this time we cannot state with assurance whether there is such a link. However, in our BCNR animals, visually we did not observe any changes in the size of the androgen dependent ischicavernosus and bulbospongiosus muscles after BCNR with or without SIL treatment. Since it has recently been reported that cavernosal nerve resection seems to be associated with low testosterone levels in this animal model of cavernosal nerve resection, one limitation of our study is that we did not determine the role of any of the known androgen related genes in our assay. Further studies will be required to explore this association.
In the present study, we found that short and long term treatment with SIL after BCNR induces activation of angiogenic factors in the penis such as ephrins, EREG, VEGF and PDGF. We also demonstrated that the expression of EREG is down regulated by BCNR and highly up-regulated by SIL treatment. However, we showed that SIL appears to be effective in restoring the level of angiogenic factors only in the penis and not elsewhere, since in the ventral prostate, the angiogenic gene expression profile was not affected by SIL treatment.
EREG, a member of the epidermal growth factor family, is considered a potent mitogen of smooth muscle cells, renal cells and liver cells (42–45). EREG is a downstream product of the coordinated activation of ERK and p38MAPK in vascular smooth muscle cells (VSMC) and act as an autocrine/paracrine factor for the progression of VSMC dedifferentiation (46,47). SIL treatment increases both extracellular signal regulated kinase 1/2 (ERK1/2) and p38 phosphorylation in a time-dependent manner in Huvec cells (48, 49). Inhibition of MEK b and p38 blocked SIL-induced proliferation and migration, respectively, suggesting that these MAPK members are downstream of PDE5 and mediate the angiogenic effects of SIL(49). It also has been shown that SIL exerts protection of cardiac cells in ischemia and reperfusion through the activation of ERK ½ pathway (50). Our results suggest that after nerve damage, SIL induces up-regulation of EREG in the corpora cavernosa through the activation of ERK ½ and MAPK pathways which in turn stimulate dedifferentiation and proliferation of the smooth muscle cells although further studies need to be performed in order to prove this mechanism.
Even though we consider that the inhibition of fibrotic factors and the activation of angiogenic genes are the key elements involved in the beneficial effect of PDE 5 inhibitors after BCNR, our array results suggest that PDE 5 inhibtiors may also be involved in promoting a neurotrophic and antihypoxic effect since we observed changes in the gene expression of neurotrophic factors such as neuropilin 1 and VEGF and a down regulation of genes involved in hypoxia such as angiopoietin 1 and hypoxia fibroblast factor (EPAS1).
Conclusion
In conclusion, cavernosal nerve damage appears within the corporal tissue to down regulate genes related to SM preservation and activates genes related to fibrosis while both short and long term continuous sildenafil treatment revert this impaired gene expression profile. Taken together, the data from this present study suggest that PDE5 inhibitors could be used as an alternative treatment in disease states where amelioration of fibrosis and neo-vessel growth are desired as seen for example in myopathic states following a neuropathy or neural injury. Further studies addressing these anti-fibrotic and anti-apoptotic effects appear warranted.
Acknowledgement
This work was supported by National Institute of Neurological Disorders and Stroke and the National Institute of General Medicine NINDS/NIGMS SC1NS064611 (MGF), National Center for Research Resources (NCRR) 1U54RR026138-01 (JA). Research Centers in Minority Institutions (RTRN) U54RR022762. National Center on Minority Health & Disparities (NCMHD) UCLA/Drew Project EXPORT 2P20MD000182 (JA).
Footnotes
Conflict of interest: None
References
- 1.Garcia FJ, Brock G. Current state of penile rehabilitation after radical prostatectomy. Curr Opin Urol. 2010;20(3):234–240. doi: 10.1097/MOU.0b013e3283383b02. [DOI] [PubMed] [Google Scholar]
- 2.Tal R, Valenzuela R, Aviv N, Parker M, Waters WB, Flanigan RC, Mulhall JP. Persistent erectile dysfunction following radical prostatectomy: the association between nerve-sparing status and the prevalence and chronology of venous leak. J Sex Med. 2009;6:2813–2819. doi: 10.1111/j.1743-6109.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 3.Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol. 1984;56:694–697. doi: 10.1111/j.1464-410x.1984.tb06149.x. [DOI] [PubMed] [Google Scholar]
- 4.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–427. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 5.Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang DY, Hyun JS, Champion HC, Abdel-Mageed AB, Hellstrom WJ. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Androl. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–435. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, Gonzalez-Cadavid NF. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6:415–428. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovanecz I, Rambhatla A, Ferrini M, Vernet D, Sanchez S, Rajfer J, Gonzalez-Cadavid N. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J. Impot Res. 2008;20:202–212. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 9.Kovanecz I, Rambhatla A, Ferrini MG, Vernet D, Sanchez S, Rajfer J, Gonzalez-Cadavid N. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008;101:203–210. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 10.Vignozzi L, Morelli A, Filippi S, Vannelli GB, Mungai S, Marini M, Boddi V, Forti G, Maggi M. Effect of sildenafil administration on penile hypoxia induced by cavernous neurotomy in the rat. Int J Impot Res. 2008 Jan–Feb;20(1):60–67. doi: 10.1038/sj.ijir.3901596. [DOI] [PubMed] [Google Scholar]
- 11.El-Sakka AI, Yassin AA. Amelioration of penile fibrosis: myth or reality. J Androl. 2010;31(4):324–335. doi: 10.2164/jandrol.109.008730. [DOI] [PubMed] [Google Scholar]
- 12.Vignozzi L, Filippi S, Morelli A, Marini M, Chavalmane A, Fibbi B, Silvestrini E, Mancina R, Carini M, Vannelli GB, Forti G, Maggi M. Cavernous neurotomy in the rat is associated with the onset of an overt condition of hypogonadism. J Sex Med. 2009;6:1270–1283. doi: 10.1111/j.1743-6109.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 13.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–347. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Vignozzi L, Filippi S, Morelli A, Ambrosini S, Luconi M, Vannelli GB, Donati S, Crescioli C, Zhang XH, Mirone V, Forti G, Maggi M. Effect of chronic tadalafil administration on penile hypoxia induced by cavernous neurotomy in the rat. J Sex Med. 2006;3:419–431. doi: 10.1111/j.1743-6109.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 15.Magheli A, Burnett AL. Medscape. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–427. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Cadavid NF, Rajfer J. Experimental models of Peyronie's disease. Implications for new therapies. J Sex Med. 2009;6:303–313. doi: 10.1111/j.1743-6109.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 17.Mostafa T. Oral phosphodiesterase type 5 inhibitors: nonerectogenic beneficial uses. J Sex Med. 2008;5:2502–2518. doi: 10.1111/j.1743-6109.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 18.Burnett AL. Molecular pharmacotherapeutic targeting of PDE5 for preservation of penile health. J Androl. 2008;29:3–14. doi: 10.2164/jandrol.107.003483. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical prostatectomy. J Urol. 2004;171:771–774. doi: 10.1097/01.ju.0000106970.97082.61. [DOI] [PubMed] [Google Scholar]
- 20.Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20(5):479–486. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 21.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, Stief C. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 22.Briganti A, Gallina A, Salonia A, Zanni G, Cestari A, Guazzoni G, Rigatti P, Montorsi F. The case for postoperative PDE-5 inhibitor drug treatment after radical prostatectomy. J Endourol. 2008;22:2025–2027. doi: 10.1089/end.2008.9752. discussion 2035. [DOI] [PubMed] [Google Scholar]
- 23.Deho F, Gallina A, Salonia A, Briganti A, Suardi N, Zanni G, Guazzoni G, Rigatti P, Montorsi F. Prophylaxis of erectile function after radical prostatectomy with phosphodiesterase type 5 inhibitors. Curr Pharm Des. 2009;15(30):3496–3501. doi: 10.2174/138161209789206999. [DOI] [PubMed] [Google Scholar]
- 24.Karamysheva AF. Mechanism of angiogenesis. Biochemistry. 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 25.de Visser YP, Walther FJ, Laghmani el H, Boersma H, van der Laarse A, Wagenaar GT. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res. 2009;29:10–30. doi: 10.1186/1465-9921-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bednar MM. The role of sildenafil in the treatment of stroke. Curr Opin Investig Drugs. 2008 Jul;9(7):754–759. [PubMed] [Google Scholar]
- 27.Bremer YA, Salloum F, Ockaili R, Chou E, Moskowitz WB, Kukreja RC. Sildenafil citrate (viagra) induces cardioprotective effects after ischemia/reperfusion injury in infant rabbits. Pediatr Res. 2005;57:22–27. doi: 10.1203/01.PDR.0000147736.27672.15. [DOI] [PubMed] [Google Scholar]
- 28.Musicki B, Champion HC, Becker RE, Kramer MF, Liu T, Sezen SF, Burnett AL. In vivo analysis of chronic phosphodiesterase-5 inhibition with sildenafil in penile erectile tissues: no tachyphylaxis effect. J Urol. 2005;174:1493–1496. doi: 10.1097/01.ju.0000173006.47623.2c. [DOI] [PubMed] [Google Scholar]
- 29.Musicki B, Champion HC, Becker RE, Liu T, Kramer MF, Burnett AL. Erection capability is potentiated by long-term sildenafil treatment: role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005;68:226–232. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 30.Lin G, Shindel AW, Fandel TM, Bella AJ, Lin CS, Lue TF. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 2010 Jan;105(1):114–120. doi: 10.1111/j.1464-410X.2009.08647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Sun X, Dai Y, Zheng F, Wang D, Huang Y, Bian J, Deng C. Chronic Administration of Sildenafil Modified the Impaired VEGF System and Improved the Erectile Function in Rats with Diabetic Erectile Dysfunction. J Sex Med. 2010;16 doi: 10.1111/j.1743-6109.2010.01844.x. In press. [DOI] [PubMed] [Google Scholar]
- 32.Artaza JN, Sirad F, Ferrini MG, Norris KC. 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J Steroid Biochem Mol Biol. 2010;119:73–83. doi: 10.1016/j.jsbmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200:207–221. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artaza JN, Singh R, Ferrini MG, Braga M, Tsao J, Gonzalez-Cadavid NF. Myostatin promotes a fibrotic phenotypic switch in multipotent C3H 10T1/2 cells without affecting their differentiation into myofibroblasts. J Endocrinol. 2008;196:235–249. doi: 10.1677/JOE-07-0408. [DOI] [PubMed] [Google Scholar]
- 35.Ferrini MG, Kovanecz I, Sanchez S, Vernet D, Davila HH, Rajfer J, Gonzalez-Cadavid NF. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod. 2007;76:915–923. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]
- 36.Abraham D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc. Rheumatology (Oxford) 2008;47 Suppl 5:8–9. doi: 10.1093/rheumatology/ken278. [DOI] [PubMed] [Google Scholar]
- 37.Cicha I, Goppelt-Struebe M. Connective tissue growth factor: Context-dependent functions and mechanism of regulation. Biofactors. 2009;35:200–208. doi: 10.1002/biof.30. [DOI] [PubMed] [Google Scholar]
- 38.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 39.Keil A, Blom IE, Goldschmeding R, Rupprecht HD. Nitric oxide down-regulates connective tissue growth factor in rat mesangial cells. Kidney Int. 2002;62:401–411. doi: 10.1046/j.1523-1755.2002.00462.x. [DOI] [PubMed] [Google Scholar]
- 40.Ferrini MG, Vernet D, Magee TR, Shahed A, Qian A, Rajfer J, Gonzalez-Cadavid NF. Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide. 2002;6:283–294. doi: 10.1006/niox.2001.0421. [DOI] [PubMed] [Google Scholar]
- 41.Ferrini MG, Rivera S, Moon J, Vernet D, Rajfer J, Gonzalez-Cadavid NF. The Genetic Inactivation of Inducible Nitric Oxide Synthase (iNOS) Intensifies Fibrosis and Oxidative Stress in the Penile Corpora Cavernosa in Type 1 Diabetes. J Sex Med. 2010 doi: 10.1111/j.1743-6109.2010.01884.x. in press. [DOI] [PubMed] [Google Scholar]
- 42.Taylor DS, Cheng X, Pawlowski JE, Wallace AR, Ferrer P, Molloy CJ. Epiregulin is a potent vascular smooth muscle cell-derived mitogen induced by angiotensin II, endothelin-1, and thrombin. Proc Natl Acad Sci U S A. 1999;96:1633–1638. doi: 10.1073/pnas.96.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuang S, Yan Y, Daubert RA, Schnellmann RG. Epiregulin promotes proliferation and migration of renal proximal tubular cells. Am J Physiol Renal Physiol. 2007;293:F219–F226. doi: 10.1152/ajprenal.00082.2007. [DOI] [PubMed] [Google Scholar]
- 44.Komurasaki T, Toyoda H, Uchida D, Nemoto N. Mechanism of growth promoting activity of epiregulin in primary cultures of rat hepatocytes. Growth Factors. 2002;20:61–69. doi: 10.1080/08977190290024192. [DOI] [PubMed] [Google Scholar]
- 45.Berasain C, García-Trevijano ER, Castillo J, Erroba E, Santamaría M, Lee DC, Prieto J, Avila MA. Novel role for amphiregulin in protection from liver injury. J Biol Chem. 2005;280:19012–19120. doi: 10.1074/jbc.M413344200. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi M, Hayashi K, Yoshida K, Ohkawa Y, Komurasaki T, Kitabatake A, Ogawa A, Nishida W, Yano M, Monden M, Sobue K. Epiregulin as a major autocrine/paracrine factor released from ERK- and p38MAPK-activated vascular smooth muscle cells. Circulation. 2003;108:2524–2529. doi: 10.1161/01.CIR.0000096482.02567.8C. [DOI] [PubMed] [Google Scholar]
- 47.Draper BK, Komurasaki T, Davidson MK, Nanney LB. Epiregulin is more potent than EGF or TGFalpha in promoting in vitro wound closure due to enhanced ERK/MAPK activation. J Cell Biochem. 2003;89(6):1126–1137. doi: 10.1002/jcb.10584. [DOI] [PubMed] [Google Scholar]
- 48.Yamanaka Y, Hayashi K, Komurasaki T, Morimoto S, Ogihara T, Sobue K. EGF family ligand-dependent phenotypic modulation of smooth muscle cells through EGF receptor. Biochem. Biophys. Res. Commun. 2001;281:373–377. doi: 10.1006/bbrc.2001.4385. [DOI] [PubMed] [Google Scholar]
- 40.Pyriochou A, Zhou Z, Koika V, Petrou C, Cordopatis P, Sessa WC, Papapetropoulos A. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J. Cell. Physiol. 2007;211:197–204. doi: 10.1002/jcp.20929. [DOI] [PubMed] [Google Scholar]
- 41.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J. Biol. Chem. 2008;283(43):29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]


