Abstract
cAMP/PKA activation represents a key signaling mechanism for neurohormonal stimulation of diversified physiological processes. Using real-time, FRET-based imaging of PKA activity in neonatal cardiac myocytes, we report that sustained activation of PKA induced by β adrenoceptor (βAR) dictates signaling propagation for substrate phosphorylation and myocyte contraction. Activation of βARs in wild-type myocytes induces strong and sustained PKA activities, which are rapidly attenuated upon washing away agonist or adding antagonist to the cells. The sustained PKA activities promote signaling propagation to the sarcoplasmic reticulum for phosphorylation of phospholamban and increases in myocyte contraction. Addition of antagonist after βAR stimulation significantly attenuates PKA phosphorylation of phospholamban, and rapidly reduces contraction rate increases. Moreover, Stimulation of β1AR subtype induces PKA activities similar to those in wild-type cells. In contrast, stimulation of β2AR subtype induces strong initial activation of PKA similar to those induced by β1AR; however the activities are rapidly decreased to baseline levels. The transient PKA activities are sufficient for phosphorylation of the overexpressed β2ARs under agonist stimulation, but not phospholamban. Further analysis reveals that phosphodiesterase 4 is the major family that shapes PKA activities under βAR stimulation. Inhibition of phosphodiesterase 4 extendsβ2AR-induced PKA activities, promotes PKA phosphorylation of phospholamban, and ultimately enhances myocyte contraction responses. Together, our data has revealed insights into kinetics of PKA activities in signaling propagation under neurohormonal stimulation.
Keywords: Protein kinase A phosphorylation, adrenergic receptor, phospholamban, contraction
Introduction
cAMP/PKA activation represents a key signaling mechanism for neurohormonal stimulation controlling diversified physiological processes from cardiac contraction, energy metabolism, to behaviors. Activation of adrenergic receptors is the major neurohormonal mechanism controlling cAMP/PKA activities for cardiac performance during stresses. β adrenoceptors (βARs) conduct stimulatory effects by elevation intracellular cAMP levels, and subsequent activation of cAMP-dependent PKA. Upon activation, PKA exerts two major functions in cardiac myocytes: enhancement of cardiac contraction through phosphorylation of plasmalemma and sarcomeric ion channels and myofibril contractile proteins 1, and desensitization of ligand-bound βARs through phosphorylation of activated receptors 2. Given multiple PKA targets within distinct subcellular regions, a spatially confined PKA activity is essential to warrant response specificity. Indeed, signaling proteins including receptors, G-proteins, and adenylyl cyclases, are compartmentalized within specific intracellular regions for appropriate functioning of cAMP/PKA signaling pathway 3. Additionally, PKA is anchored via a kinase anchoring protein close to its specific targets 4. This organization confines cAMP to discrete intracellular regions, leading to activation of selected pools of PKA upon stimulation 5.
The machinery that underpins compartmentalized cAMP signaling has been extensively studied and is only now becoming fully appreciated 6. Fundamental to shape cAMP gradients is activity of the phosphodiesterases (PDEs), a superfamily of enzymes that hydrolyze cAMP. PDEs associate with distinct signaling complexes or subcellular structures to provide functional proximity to cAMP. It is well documented that cAMP gradients are regulated by different PDEs in magnitude, kinetics, and localization, which are proposed to transiently induce local PKA activities for a set of specific substrates 6–8. PDE3 and PDE4 families account for more than 90% of activities for cAMP hydrolysis in hearts 9. PDE4 enzymes are enriched in both M and Z lines in the proximity of βARs 10. Inhibition of PDE4 or deletion of PDE4D results in higher cellular cAMP levels and enhances myocyte contraction responses upon βAR stimulation 10.
Although PKA is responsible for the majority of effects from increased cellular cAMP levels under βAR stimulation, little progress has been made to understand how elevated levels of cellular cAMP are translated into activation of PKA anchored in discrete cellular domains; and less is known how PKA conducts signal propagation for subsequent phosphorylation of substrates in cellular responses. In particular, activation of β2ARs leads to significant increases of cellular cAMP levels, which can be further enhanced when function of PDE4 is disrupted by genetic deletion or pharmacological inhibition 11. However, the increased cAMP levels induced by β2AR activation fails to promote significant increases in PKA phosphorylation of phospholamban and PKA-dependent contraction responses in rat cardiac myocytes 12. In animal hearts, deletion of β2AR genes does not affect myocardium contraction under exercise or perfusion with βAR agonist isoproterenol 13; however stimulation of overexpressed human β2ARs significantly enhances cardiac performance 14. These data suggest that activation of PKA is highly restricted, and is dependent on the stimulation, time, and location.
Here we tested a hypothesis that, in cardiac myocytes, βAR-induced PKA activities are restricted within local vicinities, and can differentially phosphorylate activated receptors and/or substrates based on locations. By using real-time, FRET-based imaging of PKA activities in living cells, we report, for the first time, that sustainability of PKA activation induced by βAR subtypes dictates signaling propagation in cardiac myocytes for substrate phosphorylation and contraction responses.
Materials and Methods
Neonatal and adult myocytes were isolated from mice as previously described 15. Myocytes were infected with viruses to express A-kinase activity reporter (AKAR2.2) to probe PKA activities with methods as previously described 16. In addition, myocyte contraction assay were carried out accordingly in both neonatal and adult cells 15. For western blot, myocytes were stimulated with isoproterenol in the presence or absence of pretreatment of PDE4 and Gi inhibitors. The cell lysates were subjected to western blot with antibodies accordingly. Statistical Analysis: One or Two-way ANOVA and student t-test were performed using Prism accordingly.
Results
Stimulation of βARs induces sustained increases in PKA activities in cardiac myocytes
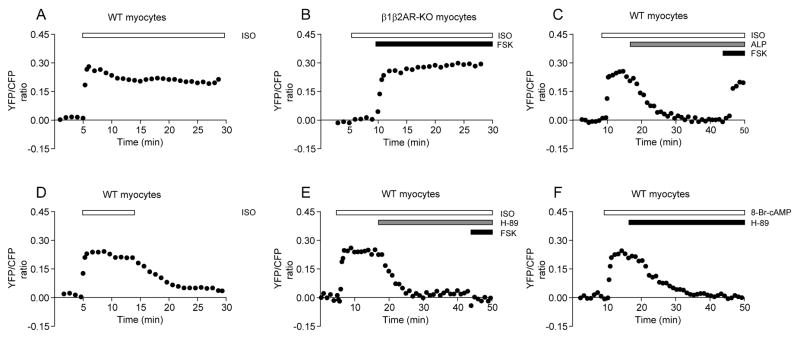
We have recently successfully analyzed PKA-mediated phosphorylation with AKAR2.2 in living cells 16. Recombinant adenovirus delivery of the PKA biosensor in neonatal mouse cardiac myocytes yielded more than 70% positive cells, a much more efficient method than plasmid transfection (Online Figure I). Stimulation of βARs with isoproterenol induced a rapid and sustained increase in FRET ratio in wild-type myocytes (t1/2 = 7.68 ± 2.28 sec and initial increase = 0.27 ± 0.02, n = 22, Figure 1A and Online Figure I), but not in myocytes isolated from mice lacking both β1 and β2AR genes in which the increase was recuperated by direct stimulation of adenylyl cyclases with forskolin (Figure 1B, n = 15). The increase was sustained at high levels (Figure 1A). Similar responses were observed in myocytes with plasmid transfection (t1/2 = 7.80 ± 1.74 sec and initial increase = 0.26 ± 0.03, n = 5, Online Figure I). The agonist-induced increases in FRET ratio were rapidly reversed by addition of βAR-specific antagonist alprenolol (Figure 1C) or by washing away isoproterenol (Figure 1D), indicating agonist-occupancy is necessary to maintain elevated PKA activities. In contrast, addition of alprenolol did not block future increase in FRET ratio induced by forskolin (Figure 1C). The increases in FRET ratio induced by both isoproterenol and forskolin were rapidly attenuated by addition of a PKA specific inhibitor H-89 that also blocked future stimulation by forskolin (Figure 1E). In addition, a cAMP analogue 8-br-cAMP induced rapid increases in FRET ratio, which were also attenuated by addition of H-89 (Figure 1F). Together, our data suggest that the AKAR reporter expressed in mouse neonatal cardiac myocytes is fully responsive to diversified stimuli, and displays dynamic changes in FRET ratio.
Figure 1.
Dynamic PKA activities upon βAR stimulation in neonatal cardiac myocytes. Myocytes expressing the PKA reporter AKAR2.2 are stimulated with different drugs. Time profiles of PKA activities after βAR stimulation with isoproterenol in wild-type (A) or β1β2AR-KO (B) myocytes, or with additional stimulation with forskolin. (C) Addition of βAR antagonist alprenolol rapidly decreases isoproterenol-induced PKA activities, but does not prevent further stimulation by forskolin. (D) Removal of isoproterenol after stimulation rapidly decreases isoproterenol-induced PKA activities. (E) Addition of PKA inhibitor H-89 rapidly decreases isoproterenol-induced PKA activities and prevents further stimulation by forskolin. (F) Addition of H-89 rapidly decreases cAMP analog (8-Br-cAMP) stimulated PKA activities. Traces represent average of FRET ratios from different cells.
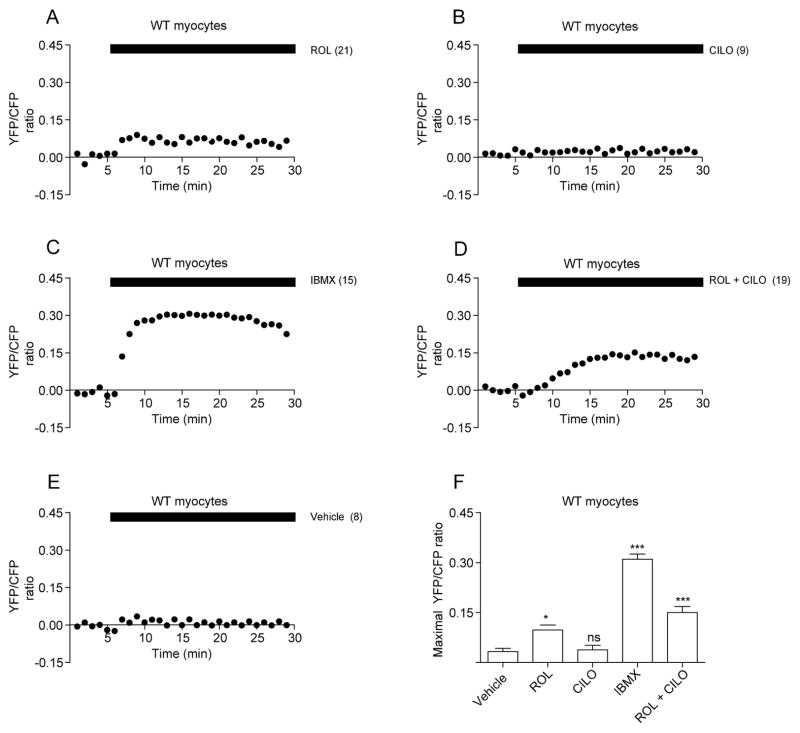
Recent progress has significantly improved our understanding of how cellular cAMP induced by adrenergic stimulation is confined within subcellular domains by PDE activities. We sought to examine the effects of PDE4 and PDE3, two major families expressed in hearts 9, on PKA FRET ratio at resting state or after βAR activation. At resting wild-type myocytes, inhibition of PDE4 with rolipram, but not inhibition of PDE3 with cilostamide or vehicle alone, increased baseline levels of FRET ratio (Figure 2A, 2B, and 2E, respectively); suggesting PDE4 plays a key role in controlling baseline PKA activities. Surprisingly, inhibition of all PDE activity with IBMX promoted much higher increases in FRET ratio than those with rolipram (Figure 2C and 2F), indicating potential involvement of other non-PDE4 families in maintaining baseline PKA activities. Interestingly, rolipram and cilostamide together promoted higher increases in FRET ratio than rolipram alone, though the levels were still significantly lower than those induced by IBMX (Figure 2D and 2F), indicating a synergistic effect between PDE3 and PDE417, and supporting involvement of additional PDE families in maintaining baseline PKA activities in myocytes.
Figure 2.
PDE3 and PDE4 modulate PKA activities at resting state in neonatal cardiac myocytes. Wild-type myocytes expressing AKAR2.2 are treated with PDE inhibitors. Effects of PDE4 inhibitor rolipram (A), PDE3 inhibitor cilostamide (B), general PDE inhibitor IBMX (C), rolipram and cilostamide (D), or vehicle (E) on AKAR2.2 FRET ratio (PKA activities) at resting state in wild-type cardiac myocytes. The maximal effects of PDE inhibitors on AKAR2.2 FRET ratio are plotted in (F). Traces represent the average of the FRET ratios from different cells. * p<0.05, and *** p<0.001 when compared to vehicle control by one-way ANOVA.
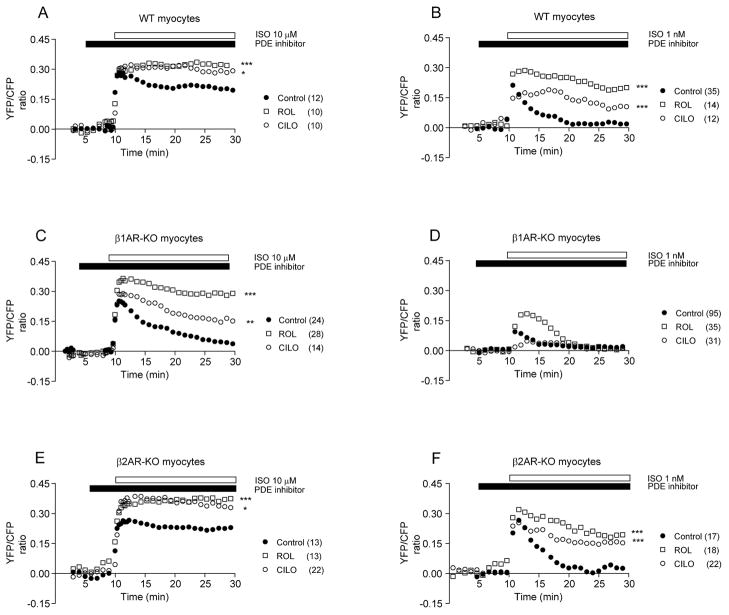
We then examined the effects of rolipram and cilostamide on AKAR FRET increases induced by βAR in wild-type myocytes. At both saturating concentration (10μM) and non-saturating physiological concentration (1nM), isoproterenol induced similar initial increases in FRET ratio (Figure 3A and 3B). However, the FRET increases induced by 1nM of isoproterenol underwent rapid attenuation whereas the ones induced by 10μM of isoproterenol remained high (Figure 3A and Online Figure II). Pretreatment with either rolipram or cilostamide blocked attenuation of FRET ratio induced by 10μM of isoproterenol; however rolipram was more effective in maintaining the FRET ratio increases induced by 1nM of isoproterenol than cilostamide (Figure 3B and Online Figure II). These data confirm that PDE4 are the major phosphodiesterases involved in maintaining sustained PKA activities upon βAR agonist stimulation.
Figure 3.
PDE3 and PDE4 regulate PKA activities induced by βAR subtypes in neonatal cardiac myocytes. Myocytes expressing AKAR2.2 are treated with or without PDE inhibitors before addition of isoproterenol to activate βARs. (A–B) Effects of PDE inhibitors on the increases in FRET ratio induced by either 10μM or 1nM of isoproterenol in wild-type myocytes. (C–D) Effects of PDE inhibitors on the increases in FRET ratio induced by either 10μM or 1nM of isoproterenol in β1AR-KO cardiac myocytes. (E–F) Effects of PDE inhibitors on the increases in FRET ratio induced by either 10μM or 1nM of isoproterenol in β2AR-KO cardiac myocytes. Traces represent the average of the FRET ratios from different cells. * p<0.05, ** p<0.01, and *** p<0.001 when compared to controls by two-way ANOVA.
β2AR induces significant, but transient PKA activities in cardiac myocytes
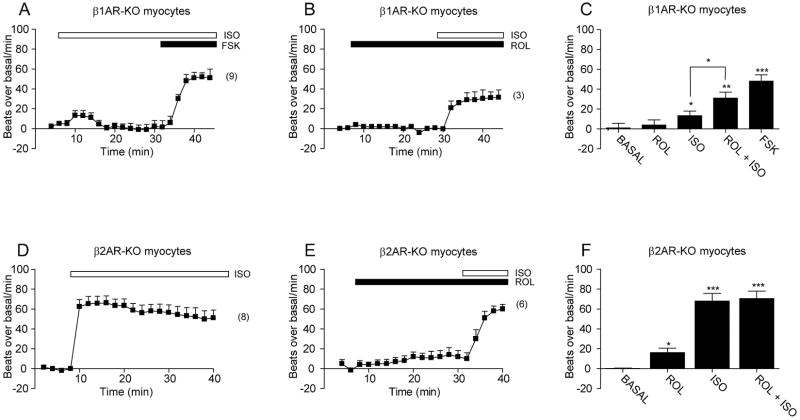
In cardiac myocytes, cAMP accumulation induced by β2ARs seems to be localized and restricted, and its access to PKA has produced seemingly conflicting observations on PKA-dependent substrate phosphorylation and contraction responses 7, 10–12. Therefore, it is of great interest to detect PKA activities induced by β2ARs in living myocytes. We took advantage of myocytes isolated from mice lacking either β1AR or β2AR gene, which have been very useful models to analyze βAR subtype-specific signaling without complication of simultaneous activation of both subtypes 11. Stimulation of β2ARs in β1AR-KO myocytes with isoproterenol (10μM) induced strong initial increases in FRET ratio with both magnitude (0.25 ± 0.02) and t1/2 (7.38 ± 2.0 sec, n = 24) similar to those in wild-type cells. However, the increased FRET ratio underwent a rapid decrease to baseline levels (t1/2 = 320 ± 12 sec, Figure 3C and Online Figure II). Stimulation of β1ARs in β2AR-KO myocytes induced sustained increases in FRET ratio (t1/2, 8.76 ± 1.3 sec and initial response 0.26 ± 0.03, n = 13) similar to those in wild-type cells (Figure 3E and Online Figure II). However, isoproterenol (10μM) induced comparable increases in cAMP accumulation in β1AR-KO andβ2AR-KO myocytes (Online Figure III). Therefore, it is unlikely that sustained PKA activities under β1AR activation are due to saturated cAMP accumulation. In contrast, isoproterenol (1nM) induced transient increases in FRET ratio in both β1AR-KO (t1/2 = 6.78 ± 1.77 sec and initial response 0.08 ± 0.03, n = 96, Figure 3D and Online Figure II) and β2AR-KO myocytes (t1/2 = 13.26 ± 2.91 sec and initial response 0.25 ± 0.01, n = 17) (Figure 3F and Online Figure II). To determine whether the difference between AKAR FRET responses induced by βAR subtypes is due to the lower expression levels of β2AR in cardiac myocytes, we overexpressed mouse β2AR in β1AR-KO myocytes (Online Figure IV). The extra receptors failed to promote sustained FRET responses induced by either 1nM or 10μM of isoproterenol, though they enhanced magnitude of FRET responses induced by 1nM of isoproterenol (Online Figure IV). Therefore, activation of β2AR induces transient PKA activities; and the lower receptor expression levels may in part contribute to lower PKA activities when stimulated with 1nM of isoproterenol in myocytes.
We further investigated whether PDE activities play a role in shaping PKA activity induced by βAR subtypes. At resting state, rolipram induced small increases in baseline AKAR FRET ratio in β2AR-KO, but not β1AR-KO myocytes, whereas cilostamide did not affect in either cell type (Online Figure V). In β1AR-KO myocytes, rolipram significantly enhanced magnitude and duration of increases in FRET ratio induced by both 10μM (Figure 3C and Online Figure II) and 1nM (Figure 3D and Online Figure II) of isoprotereonol. Cilostamide moderately enhanced only duration of increases in FRET ratio induced by 10μM of isoproterenol (Figure 3C and Online Figure II). In β2AR-KO myocytes, both rolipram and cilostamide significantly enhanced magnitude and duration of FRET responses induced by 10μM of isoproterenol (Figure 3E and Online Figure II). However, rolipram was more effective in maintaining the FRET ratio increases induced by 1 nM of isoproterenol than cilostamide (Figure 3F and Online Figure II). Together, these data indicate that PDE4 are the major phosphodiesterases involved in shaping both magnitude and duration of PKA activities upon stimulation of βAR subtypes in cardiac myocytes.
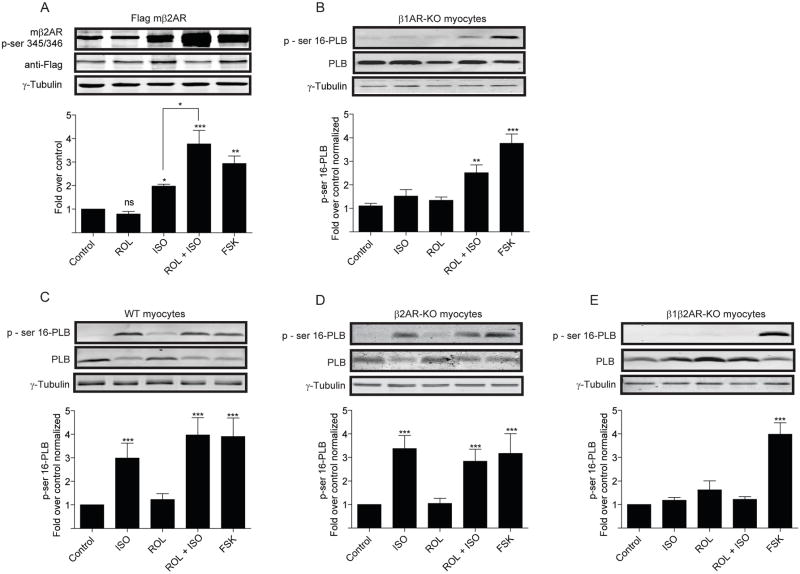
Sustained PKA activities induced by βAR subtypes promotes PKA phosphorylation of phospholamban on sarcoplasmic reticulum and contraction responses
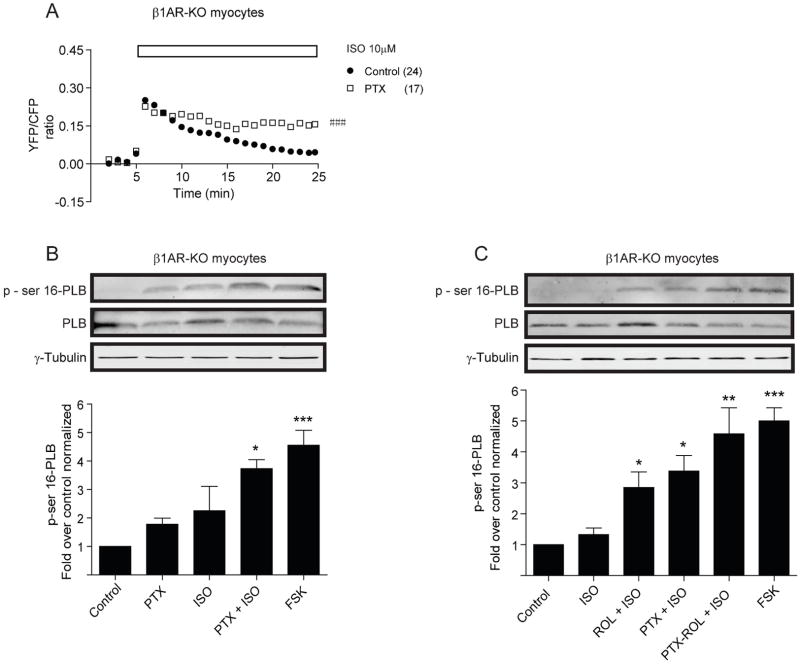
We hypothesize that transient PKA activities induced by β2ARs are sufficient to phosphorylate the agonist-occupied receptors, but have limited access to phospholamban at distance. Isoproterenol (10μM) induced significant increases of PKA phosphorylation of serine 345 and 346 on β2ARs overexpressed in β1AR-KO myocytes, which peaked at 3 minutes (Figure 4A and Online Figure VI). Rolipram significantly enhanced the maximal increases in PKA phosphorylation induced by isoproterenol (Figure 4A). In contrast, isoproterenol had minimal increase in the levels of PKA phosphorylation of phospholamban in β1AR-KO myocytes (Figure 4B and Online Figure 6B), which was significantly promoted by rolipram (Figure 4B and Online Figure VI). Moreover, isoproterenol also induced significant increases in PKA phosphorylation of AKAR2.2 expressed in β1AR-KO myocytes (Online Figure VI). In addition, isoproterenol induced significant increases in PKA phosphorylation of phospholamban in both wild-type and β2AR-KO myocytes, which were not further enhanced by rolipram (Figure 4C and 4D). In β1β2AR-KO myocytes, isoproterenol failed to induce increase in levels of PKA phosphorylation of phospholamban (Figure 4E). Together, these data indicate that PKA activities induced by β2AR activation are primarily restricted at plasma membrane domains which are accessible to the activated β2ARs, as well as AKAR2.2 within the proximity of signaling domains. Inhibition of PDE4 promotes sustained PKA activities for phosphorylation of phospholamban on sarcoplasmic reticulum.
Figure 4.
Activation of βAR induces phosphorylation of local and distant PKA substrates in neonatal myocytes. (A) Phosphorylation of ser345/346 of β2AR expressed in β1AR-KO myocytes after 3 minutes of isoproterenol stimulation in the presence or absence of rolipram or after forskolin stimulation. (B–E) Phosphorylation of ser16 of phospholamban in β1AR-KO, wild-type, β2AR-KO, β1β2AR-KO myocytes after 10 min of isoproterenol stimulation in the presence or absence of rolipram treatment or after 10 min of forskolin stimulation. * p<0.05, ** p<0.01, and *** p<0.001 when compared to controls or as indicated by student t-test.
We further examined the role of sustained PKA activities in βAR-induced myocyte contraction responses. We found that overexpressing AKAR2.2 did not affect the β2AR-induced contraction rate responses in myocytes (Online Figure VII), indicating a minimal perturbation on the βAR signaling, and supporting the utility of AKAR2.2 FRET responses as indicators for physiological PKA activities in myocytes. In β1AR-KO myocytes, stimulation of β2AR induced small increases in contraction rate which followed by a rapid decrease to baseline levels (Figure 5A). An additional of forskolin promoted further increases in contraction rate (Figure 5A). Rolipram did not change baseline rates, but significantly enhanced maximal increases induced by isoproterenol (Figure 5B and 5C). In contrast, in β2AR-KO myocytes, stimulation of β1AR induced sustained increases in contraction rate (Figure 5D). Rolipram promoted small increases at baseline, but did not further enhance maximal increases induced by isoproterenol (Figure 5E and 5F).
Figure 5.
PDE4 affects βAR subtype-induced neonatal myocyte contraction responses. Myocyte contraction rates are measured upon isoproterenol stimulation (10 μM) in the absence or presence of rolipram. (A–B) Activation of β2AR induces small and transient increases in contraction rate in β1AR-KO myocytes. Rolipram promotes higher and sustained increases in contraction rate induced by isoproterenol. (C) The increases in baseline and maximal contraction rates in panel A and B are compared among different groups. (D–E) Activation of β1AR induces significant increases in contraction rate in β2AR-KO myocytes. Rolipram induces small increases in baseline contraction rate, but does not promote higher increases in contraction rate induced by isoproterenol. (F) The increases in baseline and maximal contraction rates in panel D and E are compared among different groups. * p<0.05, ** p<0.01, and *** p<0.001 when compared to controls or as indicated by student t-test.
Since activated β2AR can couple to Gi protein in cardiac myocytes, we also examined the effect of Gi inhibitor pertussis toxin (PTX) on β2AR-induced AKAR FRET responses in β1AR-KO myocytes. Pretreatment with PTX did not change the initial increases in AKAR FRET ratio induced by isoproterenol, but significantly attenuated the FRET decrease (Figure 6A). Moreover, pretreatment with either PTX or rolipram was sufficient to enhance the levels of β2AR-dependent PKA phosphorylation of phospholamban (Figure 6B and 6C). Pretreatment with both drugs together promoted slightly higher levels of PKA phosphorylation of phospholamban than those with either drug alone, but the difference is not significant (Figure 6C).
Figure 6.
Inhibition of Gi protein affects PKA activities induced by β2AR in neonatal β1AR-KO cardiac myocytes. (A) Pretreatment with Gi inhibitor pertussis toxin (PTX) prolongs the increases in AKAR2.2 FRET ratio upon stimulation with 10μM of isoproterenol. (B) Pretreatment with Gi inhibitor PTX enhances the levels of PKA phosphorylation of phospholamban at rest state and after with 10μM of isoproterenol. (C) Pretreatment with either Gi inhibitor PTX or PDE4 inhibitor rolipram is sufficient to enhance the levels of PKA phosphorylation of phospholamban induced by 10μM of isoproterenol. ### p<0.001, when compared to controls by two-way ANOVA. * p<0.05, ** p<0.01, and *** p<0.001 when compared to basal controls student t-test.
Sustained PKA activities are necessary for maintaining increases in myocyte contraction responses
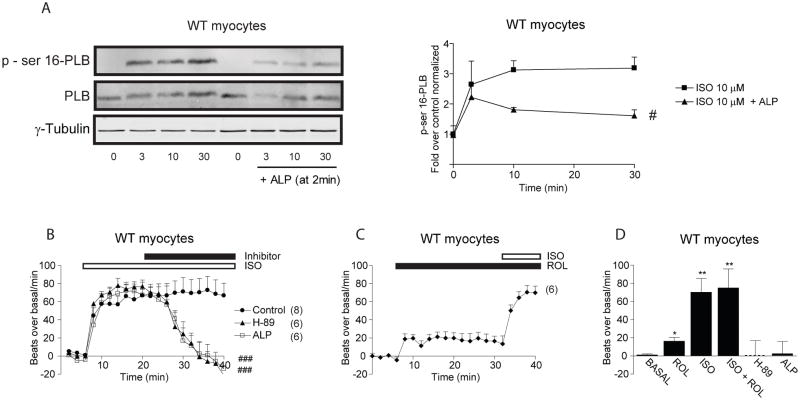
The effects of rolipram on PKA activities and PKA phosphorylation of phospholamban induced by β2ARs raised an intriguing question whether sustained PKA activities are necessary for propagation of signal to sarcoplasmic reticulum for substrate phosphorylation. Since addition of alprenolol can rapidly attenuate increases in FRET ratio induced by isoproterenol in wild-type myocytes, we examined effect of alprenolol on isoproterenol-induced PKA phosphorylation of phospholamban by addition of antagonist after stimulation. Stimulation of βARs induced time-dependent increases in PKA phosphorylation of phospholamban in wild-type myocytes that peaked at 10 minutes (Figure 7A). However, addition of alprenolol after isoproterenol stimulation significantly attenuated the increases in PKA phosphorylation of phospholamban (Figure 7A). Meanwhile, isoproterenol induced sustained increases in myocyte contraction rate; addition of alprenolol rapidly attenuated the increases to baseline (Figure 7B and 7D). Similarly, addition of PKA inhibitor H-89 after isoproterenol stimulation also rapidly attenuated the increases in myocyte contraction rate to baseline (Figure 7B and 7D). In contrary, rolipram induced small increases in contraction rate at baseline, but did not further enhance the maximal increases induced by isoproterenol (Figure 7C and 7D).
Figure 7.
Sustained PKA activities are necessary for increasing PKA phosphorylation of phospholamban and neonatal cardiac myocyte contraction. (A) Addition of alprenolol after 2 min of isoproterenol stimulation significantly attenuates the phosphorylation of ser16 of phospholamban in wild-type myocytes. (B) Isoproterenol induces sustained contraction rate increases in wild-type cardiac myocytes, which are rapidly decreased by addition of alprenolol or H-89. (C) Inhibition of PDE4 with rolipram induces small increases in contraction rate at baseline, but does not further enhance isoproterenol-induced increases in contraction rate. (D) The increases in baseline and maximal contraction rates in panel B and C are compared among different groups. # p<0.05 and ### p<0.001 when comparing the time-course curves to controls by two-way ANOVA. * p<0.05 and ** p<0.01 when compared to the basal levels by student t-test.
βAR subtypes induces distinct PKA activities in adult cardiac myocytes
To examine whether the observed PKA activities induced by βAR subtypes are preserved during different developmental stages, adult cardiac myocytes were isolated for FRET studies. Stimulation with 10μM of isoproterenol induced sustained increases in FRET ratio (Figure 8A and 8C) and significant increases in shortening in adult wild-type and β2AR-KO myoyctes (Online Figure VIII). Rolipram did not further enhance FRET responses in both cell types (Figure 8A and 8C). In contrast, stimulation with 10μM of isoproterenol induced small and transient increases in FRET ratio (Figure 8E), but minimal increase in myocyte shortening in β1AR-KO myocytes (Online Figure VIII); the transient FRET responses were enhanced and prolonged by rolipram (Figure 8E). In comparison, stimulation with 1nM of isoproterenol induced transient increases in FRET ratio in wild-type and β2AR-KO (Figure 8B and 8D) but not in β1AR-KO myocytes (Figure 8F), and failed to enhance myocyte shortening in all three cell types (Online Figure VIII). The transient increases in FRET ratio were prolonged by rolipram (Figure 8B and 8D). Together, these data indicated the PKA activities induced by βAR subtypes in adult myocytes are similar to those in neonatal myocytes, with smaller PKA activities under β2AR activation in adult myocytes.
Figure 8.
PDE4 modulates PKA activities induced by βAR in adult cardiac myocytes. Adult myocytes expressing AKAR2.2 are treated with or without PDE inhibitors before addition of isoproterenol to activate βARs. (A–B) Effects of PDE4 inhibitor rolipram on the increases in FRET ratio induced by either 10μM or 1nM of isoproterenol in adult wild-type myocytes. (C–D) Effects of rolipram on the increases in FRET ratio induced by either 10μM or 1nM of isoproterenol in β2AR-KO myocytes. (E–F) Effects of rolipram on the increases in FRET ratio induced by either 10μM or 1nM of isoproterenol in β1AR-KO myocytes. Traces represent the average of the FRET ratios from different cells. * p<0.05 and *** p<0.001 when compared to controls by two-way ANOVA.
Discussion
Sustained PKA activities induced by βAR activation in cardiac myocytes
The central finding in this study is that activation of βARs in both wild-type and β2AR-KO cardiac myocytes induces sustained PKA activities, drawing strong contrast to reported transient cAMP activities that peak at 1–2 minutes and rapidly decrease within 3–5 minutes in these cells 11, 18. More importantly, the sustained PKA activities are necessary for signal propagation from cell surface to sarcoplasmic reticulum for phosphorylation of substrates such as phospholamban and for myocyte contraction responses. This observation resolves a long-time puzzle that cAMP accumulation induced by βAR activation does not show a linear correlation with receptor-induced myocyte contraction responses 15. It also posts significant implication on a broad range of physiological processes under neurohormonal stimulation. It is generally assumed that magnitudes of signaling are a major determining factor on intensities of physiological responses. However, sustained PKA activities certainly offer advantages for signaling propagation in many physiological processes than supercharged PKA activities. First, it prevents hyperphosphorylation in substrates and related dysfunction. Second, it promotes a gradual build up of signal cross a whole cell during stresses. Third, sustained PKA activities are dependent on continuous stimulation, which is correlated with sustained catecholamine release from nerve in vivo. Withdrawing stimuli leads to a rapid decrease of PKA activities to baseline levels. Together, sustained but not supercharged PKA activities ensure a steady propagation of signal from plasma membrane to deep inside of a cell.
It is not clear how a transient cAMP activity can be converted into a sustained PKA activity. One possible scenario is that an initial surge of cAMP promotes binding to PKA. The high binding affinity to PKA reduces degradation rate of bound cAMP and contributes to slow attenuation of the PKA activity. Meanwhile, agonist isoproterenol-induced PKA activities can be rapidly reversed by addition of antagonist alprenolol or removal of isoproterenol, indicating that occupancy of βARs by agonist is necessary to maintain sustained PKA activities. A continuous production of cAMP must play a role in maintaining sustained PKA activities in cardiac myocytes, which may feed the equilibrium between binding and unbinding of cAMP to PKA. In supporting this notion, cAMP accumulation displays a biphasic response with an initial transient peak followed by a sustained small increase over baseline levels in both cardiac myocytes and HEK293 fibroblasts during extended βAR stimulation 11, 18. Given the comparable cAMP accumulation induced by both β1AR and β2AR, it is unlikely that the β1AR-induced sustained PKA activities are due to saturation of the FRET reporters with super-high accumulation of cAMP. Indeed, inhibition of PDE4 can further enhance the maximal FRET responses. Meanwhile, it is likely that PKA-associated phosphatase activities upon βAR activation 15 play an essential role in shaping FRET ratio responses in our experiments, which remains to be addressed in future studies.
Transient PKA activities induced by β2ARs lead to differential phosphorylation of substrates on plasma membrane and sarcoplasmic reticulum
Another interesting finding in this study is that stimulation of β2ARs induces transient PKA activities that are sufficient to phosphorylate activated receptors on plasma membrane, but not phospholamban on sarcoplasmic reticulum. It has been well-documented that stimulation of β2AR does not make significant contribution on myocardium contraction in animal hearts and isolated myocytes 11, 12. This notion is further supported by biochemical and electrophysiological evidence that β2AR activation has minimal effect on phosphorylation of substrates such as phospholamban 12, and has restricted effect on PKA-dependent activation of L-type calcium channels within vicinities of activated receptors 19. On the other hand, studies show that stimulation of β2ARs induces significant cellular cAMP in both fibroblasts and cardiac myocytes 18, 20, but with limited diffusion in adult cardiac myocytes 7. Here we address this long-standing question by revealing real-time PKA activities induced by β2ARs in living myocytes. Our data show that cAMP induced by β2ARs does have access to PKA, which yields initial maximal activities equivalent to those induced by β1ARs under stimulation with saturated concentration of agonist. However, these increased PKA activities are transient and highly restricted, and only accessible to substrates within local vicinities of activated receptors.
PDE enzymes control baseline PKA activities and shape βAR-stimulated PKA activities
Maintenance of tonic cAMP/PKA activities acts as an essential regulatory mechanism on cellular function at resting state, in particular in excitable neurons and myocytes. However, little evidence is available, in part due to relative insensitivity of measurements on cAMP and PKA activities. Using the PKA reporter, we find that PDE4 is one of major factors that control baseline levels of PKA activities. Inhibition of PDE4 with rolipram increases baseline PKA activities in β2AR-KO myocytes, suggesting spontaneous β1AR activation at resting state. In agreement, inhibition of PDE4 elevates PKA activities to increase phosphorylation of β1ARs expressed in HEK293 fibroblasts 9. Interestingly, inhibition of all PDE enzymes with IBMX induces much higher PKA activities at resting state than those with rolipram. This is, in part, due to synergistic effects between PDE4 and PDE3 in cardiac myocytes 17. However, PKA activities induced by inhibition of PDE3 and PDE4 together are significantly lower than those induced by IBMX, which underscores involvement of other IBMX-sensitive PDE families, such as PDE2 and PDE721, in complicated regulation of tonic cAMP/PKA activities in cardiac myocytes.
Upon stimulation, β2AR-induced PKA activities display a strong initial increase followed by a rapid decrease to baseline levels. Inhibition of PDE4 significantly reduces attenuation of the increased PKA activities, and reshapes overall responses similar to those induced by β1ARs. Inhibition of PDE4 also completely blocks slow attenuation of β1AR-induced PKA activities. These observations are consistent with preferential association of PDE4D isoforms with β1 and β2 ARs at resting and stimulating conditions 9, 22, 23. PDE4 enzymes have basal activities under resting conditions, which can be enhanced by PKA phosphorylation for cAMP degradation 4, 24, 25. PDE4D8 directly bind to β1ARs, and dissociate from the receptors upon agonist stimulation, indicating constitutive basal PDE4 activities associated with β1ARs at resting state. This is in contrast to the agonist-induced and arrestin-dependent recruitment of PDE4D3 andPDE4D5 to β2ARs, a potential mechanism for activated PDE4 on cAMP degradation which can be further modulated by the coupling of activated β2ARs to Gi protein. The mechanisms on governing βAR subtype-induced PKA activities by PDE4 isoforms remains to be further addressed.
Together, by using real-time, FRET-based imaging of PKA activity in living myocytes, we report, for the first time, that stimulation of βAR subtypes induces distinct temporal profiles of activation of PKA. Activation of βARs induces strong and sustained PKA activities in wild-type myocytes. The sustained PKA activities are dependent on agonist-occupancy of receptors, and are necessary for signaling propagation to sarcoplasmic reticulum for phosphorylation of phospholamban and for myocyte contraction responses. In contrast, activation of β2AR induces a strong activation of PKA that undergoes a rapid decrease after reaching peak levels. The transient PKA activities are sufficient for phosphorylation of the activated receptors, but not phospholamban for myocyte contraction responses. Together, our data has revealed insights into kinetics of PKA activities under neurohormonal stimulation with implication in a broad range of physiological processes.
Supplementary Material
Acknowledgments
The authors would like to thank members of Xiang laboratory for critical reading and comments.
Sources of Funding. This work is supported by NIH HL082846 to YX.
Abbreviations
- AR
adrenoceptor
- PDE
phosphodiesterase
- ISO
isoproterenol
- ROL
rolipram
- CILO
cilostamide
- FSK
forskolin
- ALP
alprenolol
- PLB
phospholamban
Footnotes
Disclosures: None.
See supplementary materials online for more detail.
References
- 1.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190:9–19. doi: 10.1111/j.1365-201X.2007.01693.x. [DOI] [PubMed] [Google Scholar]
- 3.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem Soc Trans. 2005;33:1131–1134. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- 4.McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med. 2006;12:317–323. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 6.Zaccolo M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol. 2006;85:693–697. doi: 10.1016/j.ejcb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- 8.Saucerman JJ, Zhang J, Martin JC, Peng LX, Stenbit AE, Tsien RY, McCulloch AD. Systems analysis of PKA-mediated phosphorylation gradients in live cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103:12923–12928. doi: 10.1073/pnas.0600137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. Embo J. 2008;27:384–393. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 11.Xiang Y, Naro F, Zoudilova M, Jin SL, Conti M, Kobilka B. Phosphodiesterase 4D is required for {beta}2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc Natl Acad Sci U S A. 2005;102:909–14. doi: 10.1073/pnas.0405263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao RP, Hohl C, Altschuld R, Jones L, Livingston B, Ziman B, Tantini B, Lakatta EG. Beta 2-adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to changes in Ca2+ dynamics, contractility, or phospholamban phosphorylation. J Biol Chem. 1994;269:19151–19156. [PubMed] [Google Scholar]
- 13.Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem. 1999;274:16701–16708. doi: 10.1074/jbc.274.24.16701. [DOI] [PubMed] [Google Scholar]
- 14.Milano C, Allen L, Rockman H, Dolber P, McMinn T, Chien K, Johnson T, Bond R, Lefkowitz R. Enhanced myocardial function in transgenic mice overexpressing the s2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 15.De Arcangelis V, Soto D, Xiang Y. Phosphodieasterase 4 and phosphatase 2A differentially regulate cAMP/PKA signaling for cardiac myocyte contraction under stimulation of {beta}1 adrenergic receptor. Mol Pharmacol. 2008;74:1453–62. doi: 10.1124/mol.108.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 17.Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechene P, Mazet JL, Conti M, Fischmeister R, Vandecasteele G. Spatiotemporal dynamics of beta-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases. Circ Res. 2008;102:1091–1100. doi: 10.1161/CIRCRESAHA.107.167817. [DOI] [PubMed] [Google Scholar]
- 18.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 19.Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG. G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels. Biophys J. 2000;79:2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou YY, Cheng H, Bogdanov KY, Hohl C, Altschuld R, Lakatta EG, Xiao RP. Localized cAMP-dependent signaling mediates beta 2-adrenergic modulation of cardiac excitation-contraction coupling. Am J Physiol. 1997;273:H1611–1618. doi: 10.1152/ajpheart.1997.273.3.H1611. [DOI] [PubMed] [Google Scholar]
- 21.Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J. 2005;388:803–811. doi: 10.1042/BJ20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 23.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci U S A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- 25.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. Embo J. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.