Abstract
Rationale
Beyond the amelioration of deprivation-induced impairments, and in contrast to effects on attentional processes, the cognitive-enhancing properties of nicotine on working memory (WM) operations remain unclear.
Objectives
In an effort to elucidate potential enhancing effects, we explored the impact of transdermal nicotine on neural functioning in minimally deprived smokers and, in addition, assessed differences between smokers and non-smokers using a mixed block/event-related fMRI design that attempted to isolate specific central executive operations (attentional switch events) within general WM function (task blocks).
Methods
In task blocks, participants performed a continuous counting paradigm that required the simultaneous maintenance of, and frequent switching of attentional focus between, two running tallies in WM on some trials. Cigarette smokers (n=30) were scanned twice, once each with a nicotine and placebo patch, while non-smokers (n=27) were scanned twice with no patch.
Results
Across both groups, task blocks were associated with bilateral activation, notably in medial and lateral prefrontal cortex (PFC), anterior insula, and parietal regions, whereas individual attentional switch trials were associated with activation in a similar, but predominantly left-lateralized network. Within the smoker group, although nicotine increased heart rate, altered performance and mood, and reduced tobacco cravings, no acute drug (state-like) effect on brain activity was detected for either the task or switch effects. However, relative to non-smokers, smokers showed greater tonic activation in medial superior frontal cortex, right anterior insula, and bilateral anterior PFC throughout task blocks (trait-like effect).
Conclusions
These data suggest smokers require recruitment of additional WM and supervisory control operations during task performance.
Keywords: Nicotine, Central executive, Working memory, Anterior prefrontal cortex, Medial superior frontal cortex, Anterior insula
Introduction
To delineate the cognitive-enhancing properties of nicotine beyond withdrawal reversal, the extent to which nicotine affects cognition has been examined in minimally deprived (<3 h), non-deprived, and non-smokers. Investigation of potential enhancing properties has benefited from a reductionist approach attempting to identify elemental cognitive operations and associated neural substrates influenced by nicotinic mechanisms. Such an approach has identified attentional processes, specifically those related to sustained attention and visuospatial orienting, as the psychological constructs most robustly influenced by nicotine in humans (Hahn et al. 2007; Heishman et al. 2010; Lawrence et al. 2002; Newhouse et al. 2004). In contrast, evidence of nicotine’s ability to augment performance in other domains, particularly working memory (WM), has been less definitive. While nicotine administered to non-smokers has been reported to improve performance and alter functional activity in WM-related brain regions (Kumari et al. 2003), the majority of studies have failed to detect nicotine effects when assessing ex- (Ernst et al. 2001a), never- (Kleykamp et al. 2005), or non-deprived smokers (Myers et al. 2008). This paucity of positive results with human participants does not parallel observations from rodent studies that have more consistently demonstrated nicotine-induced improvements in WM (Levin et al. 2006; Rezvani and Levin 2001).
Interpretation of the human literature pertaining to nicotine-induced WM enhancement is challenging not only because of methodological differences across studies but also because the tasks employed often engage multiple cognitive processes. For example, the WM model of Baddeley and Hitch (1974) has been elaborated to include theoretical constructs such as a central executive, the phonological loop, the visuospatial sketchpad, and an episodic buffer (Baddeley 2000; 2003). Within this model, the central executive is perhaps the most important component, but also the most poorly understood, and is responsible for the allocation of attentional resources as dictated by task demands. Commonly employed WM tasks, such as the n-back paradigm, are generally unable or not specifically designed to interrogate isolated WM constructs. As such, more reductionist methodologies attempting to fractionate WM operations according to Baddeley’s theoretical model may provide additional insight into the nature of nicotine’s effects.
Interrogation of the central executive has proven challenging because separating specific, elemental executive functions from general WM operations is non-trivial. Here, in an attempt to isolate executive functions, we employed a concurrent counting task that required the simultaneous maintenance of, and frequent switching of attentional focus between, two running tallies residing in WM (Garavan 1998). The switching of attention between counts in WM is regarded as one tractable function falling under the rubric of the central executive (Garavan et al. 2000). Variations of this task have been used in parametric block-design fMRI studies in an attempt to isolate neural correlates of the central executive (Kübler et al. 2003, 2005; Sylvester et al. 2003). However, with block designs, it is not possible to disambiguate sustained activity associated with task performance from transient event-related attentional switch signals. Alternatively, mixed block/event-related designs (Chawla et al. 1999) allow for the decomposition of sustained and transient activity (Visscher et al. 2003). As such, we employed a mixed-design paradigm to dissociate brain activity associated with general task-related (e.g., stimulus-attending, count-updating, sub-vocal rehearsal) from central executive operations (e.g., attentional switching).
In addition to the acute (state-like) effects of nicotine, a secondary interest of the current investigation was the impact of smoking history (trait-like effect) on neural correlates of WM. Independent of recency of tobacco use or nicotine administration, multiple studies have reported that WM performance is reduced in smoking, relative to non-smoking participants (Ernst et al. 2001b; Greenstein and Kassel 2009; Jacobsen et al. 2005, 2007). These results suggest that trait-like differences in brain function may exist between smokers and non-smokers, which could reflect long-term consequences of smoking or etiological precursors leading to habitual nicotine use. It has been suggested that transient versus protracted exposure to nicotine differentially affects neural functioning (Mansvelder et al. 2006). Thus, understanding the short- and long-term consequences of nicotine on precisely defined cognitive processes is important for guiding development of more effective smoking prevention and treatment strategies as well as therapeutic interventions for age- and disease-related cognitive impairment.
The current study assessed the impact of nicotine in minimally deprived smokers and potential differences between smokers and non-smokers in a WM paradigm that attempted to dissociate central executive processes from general task functions. We addressed three questions: (1) Can neural correlates of executive control functions be dissociated from general WM operations using a mixed block/event-related design?, (2) Does nicotine augment neural activity associated with executive control and/or general task operations?, and (3) What are the neural consequences associated with an extended smoking history?
Methods
Participants
Data from 30 smoking (16 female) and 27 non-smoking (16 female) participants were analyzed. Smokers were 19–49 years old (32.5 mean±1.6 SEM), nicotine dependent as determined by the Fagerström Test (Heatherton et al. 1991) (5.3±0.4), and reported smoking 22.4±1.2 cigarettes/day (range 14–45) for 15.7±1.6 years (range 3–35). The Wechsler Abbreviated Scale of Intelligence (Wechsler 1999) was used to assess smokers’ IQ (108.7±2.0). Nonsmokers reported no nicotine use within the preceding 12 months, no history of daily smoking, and were matched for age [range 18–50, 28.3±1.7; t(55)=−1.8, p=0.08] and IQ [113.0±2.1; t(55)=1.5, p=0.15]. Participants reported no history of drug dependence (other than nicotine), neurological or psychiatric disorders, or claustrophobia. Recruitment from the general population was conducted through newspaper advertisements, flyers, and referrals. Before beginning the study, participants gave written informed consent to a protocol approved by the Institutional Review Board for the National Institute on Drug Abuse—Intramural Research Program. Participants were remunerated for their participation.
Procedures
Participants completed three visits, an orientation and two scan visits. During the orientation visit, participants gave consent and completed task training on two cognitive tasks [only one, the Central Executive Functioning-Event-Related (CEFER) task is reported herein]. Instructions were initially explained on a bench computer, and one full session of each task was performed in a mock scanner. Participants also received training on computerized questionnaires that were completed during scanning to measure mood states and tobacco craving.
MRI scanning was performed during visits two and three, which were separated by a minimum of 2 days (11.9±1.9, range 2–63). Participants were instructed not to ingest alcohol or use psychoactive drugs or over-the-counter medications 24 h before visits and not to consume more than a half-cup of a caffeinated beverage within 12 h. Smokers’ last cigarettes were smoked within 1 h before the visit, i.e., between ~2.5–3 h pre-scan. Participants were tested for pregnancy if female, recent drug (Triage®; Biosite, San Diego, CA, USA) and alcohol use (Alco-Sensor IV Breathalizer; Intoximeters, Inc., St. Louis, MO, USA), and for expired carbon monoxide (CO) levels (Vitalograph Breath CO monitor, Lenexa, KS, USA). Participants were excused from the study if testing positive for pregnancy or recent drug/alcohol use. A nicotine (21 mg/24 h; Nicoderm®; GlaxoSmithKline, Research Triangle Park, NC, USA) or placebo patch (obtained from GSK) was applied to the upper back of smokers ~2 h before task performance. Patch order was single-blind and counterbalanced across participants. Non-smokers were scanned twice with no patch. The two MRI sessions, lasting ~1.5–2 h each, began with the CEFER task which was followed by an anatomical scan and a second cognitive task (see supplementary information).
Mood states (Parrott et al. 1996) and tobacco cravings (Heishman et al. 2003) were assessed using self-report measures collected immediately before and after fMRI data collection while participants were positioned in the scanner (see supplementary information). Within 10 min of scan completion, a venous blood sample (5 ml) was drawn from smokers and assayed for nicotine levels (Shakleya and Huestis 2009). Additional physiological measures collected from smoking participants included heart rate (HR) at pre-patch and 30, 60, and 120 min post-patch time points.
Task
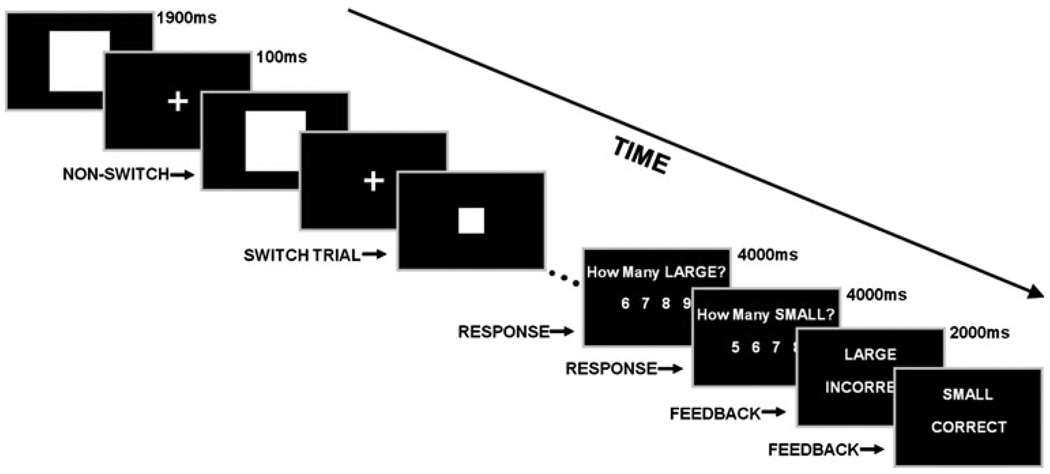
The CEFER task, a concurrent counting task implemented in a mixed fMRI paradigm, was designed to isolate one precisely defined function of the central executive, that is, attentional switching between items in verbal WM. On any given trial of the task, one of two counts was required to be updated depending on the nature of the stimuli presented, while across trials WM demands were held constant as both counts were required to be maintained in WM. The pertinent manipulation in the task was the order in which stimuli were presented, which dictated whether a switch between counts was required.
Participants were presented with serial sequences of large (L) and small (S) squares and instructed to keep running tallies pertaining to the number of each type shown. During the presentation sequence, the current square could either be the same as the previous (NON-SWITCH event) or different (SWITCH event). For example (Fig. 1), an L square could be presented, followed by another L (L-L, NON-SWITCH), and then an S square (L-S, SWITCH). In ten, ~30-s (26–34 s) counting blocks, squares were serially presented (13–17 squares/block) in M-sequences (Buracas and Boynton 2002) designed to produce optimally distributed switch events (i.e., L-S or SL; five to nine switches/block). Squares were displayed for 1,900 ms and separated by a fixation point presented for 100 ms to clearly delineate successive presentations. At the end of each counting block, participants reported how many L and, subsequently, how many S squares had been displayed by selecting one of four potential answers. Answers for both L and S counts remained visible for 4 s each. Feedback for the two separate counts was then presented (2 s each). Counting blocks alternated with 30-s rest blocks. Two seconds before the end of each rest period, a “get ready” notice signaled the start of the next counting block. The task lasted ~12.5 min and both began and ended with a rest period.
Fig. 1.
CEFER task schematic. Participants kept running counts pertaining to the number of large (L) and small (S) squares presented and reported those tallies at the end of the counting blocks. During the presentation sequence, the current square could either be the same as (e.g., L-L; NON-SWITCH) or different than (e.g., L-S; SWITCH) the previous item. In the example given, the running tallies would be updated as follows: trial 1 (1 L, 0 S), trial 2 (2 L, 0 S), trial 3 (2 L, 1 S) …trial n. Feedback was subsequently presented. Counting blocks (~30 s) were interspersed with resting blocks (30 s)
Image acquisition
Data were acquired with a 3-T Siemens Allegra scanner (Erlangen, Germany). Thirty-nine 4-mm-thick slices covering the whole brain were obtained in the sagittal plane using a gradient echo, echo-planar imaging sequence [repetition time (TR)=2,000 ms; echo time (TE)=27 ms; flip angle (FA)=80°; 64×64 matrix; field of view= 220 mm] sensitive to T2*-weighted blood oxygenation level-dependent (BOLD) effects. In addition, whole-brain sagittal T1-weighted structural images (MPRAGE, 1 mm3 voxels; TR=2,500 ms; TE=4.38 ms; FA=8°) were acquired during each session for anatomical reference.
Image processing and analyses
Processing and analyses of fMRI data were performed using the AFNI software package (Cox 1996). Functional data were motion corrected by rigid-body registration of each three-dimensional volume to a base volume and then aligned with the high-resolution anatomical images. The time series were normalized to percent signal change and subsequently analyzed by voxel-wise multiple regression using regressors convolved with a model hemodynamic response function and its first derivative. A general linear model was constructed that included the following regressors: (1) TASK (block), modeling counting blocks to capture sustained activity; (2) SWITCH (event-related), which modeled switch trials to capture transient activity; (3) response and feedback, to account for additional variance in the time series; and (4) six motion nuisance regressors to account for residual head movement. More specifically, the TASK regressor modeled sustained activity for the duration of the “task-on” intervals, encompassing both switch and non-switch trials, versus the “task-off” rest periods. The SWITCH regressor modeled transient activity for trials on which a switch occurred versus all other times, including task-on intervals (non-switch events) as well as rest. For each subject and session, the voxel-wise amplitude of signal change relative to baseline associated with the regressors (β value) was determined. The resulting β maps were re-sampled to a resolution of 3 mm3, normalized into standard space (Talairach and Tourneaux 1988), and spatially blurred using a Gaussian filter with 3 mm full width at half maximum to a final blur of ~7 mm.
Task and switch effect analyses
Across all participants, separate second-level voxel-wise one-sample t tests (df=56) were performed to identify TASK- and SWITCH-related neural correlates. The β weights from the smokers’ nicotine and placebo scans, as well as from the non-smokers’ session 1 and 2 scans, were first averaged together. A voxel-wise threshold of p<0.00001 was applied to the t test activation maps which, combined with a minimum cluster size of 13 voxels (351 µl), maintained the overall probability of a false positive at αcorrected<0.001 (AFNI program, AlphaSim). Such a stringent threshold was used because a large effect size, a relatively large sample size, and the two-session averaged β weights led to high statistical power.
Nicotine and session effect analyses
To examine the effects of nicotine, paired-sample t tests were performed comparing smokers’ (df=29) nicotine and placebo scans. Separate tests examined the impact of nicotine on the TASK and SWITCH effects. Two similar analyses were conducted for the non-smokers (df=26) comparing session 1 and 2 to identify practice effects. For all tests, a voxel-wise threshold of p<0.002 combined with a minimum cluster size of 31 voxels (837 µl) maintained the probability of a false positive at αcorrected<0.01.
Smoker versus non-smoker effect analysis
The impact of smoking history was assessed by comparing the session-averaged TASK and SWITCH effects between smokers and non-smokers. Two separate group-level linear-mixed effects models were constructed to analyze group differences (AFNI program, 3dLME) in this unbalanced design using the R statistical package (R-Development-Core-Team 2009). Linear mixed-effects models included factors for SESSION and GROUP and covariates for age, gender, and reaction time. These analyses output voxel-wise F values and the GROUP effect of interest (smoker two-session average vs. non-smoker two-session average) was equivalent to performing a one-way ANOVA with two levels (df=1,53). A voxel-wise threshold of p<0.002 combined with a minimum cluster size of 31 voxels maintained the probability of a false positive at αcorrected<0.01. Activity from areas showing group differences was extracted as functional regions of interest (ROIs).
Behavioral analyses
Reaction times (RT) and accuracy rates associated with count reporting were averaged over L and S responses and compared across sessions using paired-sample t tests. These “gross” behavioral measures were collected at the end of the counting blocks and do not index trial-by-trial dynamics associated with attentional switching, but rather helped ensure that participants remained on task. Other than the correct/incorrect feedback following count reporting, there were no other manipulations to keep participants engaged in the task. However, overall accuracy rates (89±1%) were well above chance levels (25%) and all participants achieved accuracies greater than 65%, suggesting compliance with instructions and adequate performance. Given a priori expectations that nicotine/practice effects would improve performance, these data were tested for session differences using one-tailed tests.
Results
Physiological measures
CO levels confirmed that smokers smoked their last cigarette within 1 h before each visit (nicotine=25.5±1.8 ppm; placebo=25.0±1.9). As expected, the nicotine patch elevated HR (see supplementary material) and resulted in greater post-scan plasma concentrations [nicotine=33.7±2.1 ng/ml; placebo=4.6±1.0; t(29)=12.6, p<0.001].
Self-report measures
Visual analog ratings collected from smoking participants before and after (TIME) the blinded nicotine and placebo scans (SESSION) indicated that drug administration was associated with alterations in mood states. Smokers reported feeling less tense [F(1,29)=5.8, p=0.02], irritated [F(1,29)=4.6, p=0.04], and dissatisfied [F(1,29)=6.2, p=0.02] during the nicotine scan as indicated by TIME×SESSION interactions. Bonferroni-corrected post hoc tests indicated no nicotine versus placebo differences at pre-scan (ts<2.3, ps>0.05), suggesting that participants were not in a withdrawn state immediately before the CEFER task. However, at post-scan, under nicotine, smokers reported lower levels of tension [t(29)=−3.6, p=0.002], irritation [t(29)=−3.3, p=0.004], and dissatisfaction [t(29)=−3.4, p=0.004], consistent with the emergence of affect-related withdrawal symptoms during the placebo scan after task completion. Non-smokers reported no change in levels of tension, irritation, or dissatisfaction. Both groups indicated feeling more tired, drowsy, distracted, and hungry at the end of the scan (TIME main effect, Fs>6.2, ps<0.02).
Assessment of smokers’ tobacco craving scores indicated nicotine administration was associated with changes in craving. Smokers reported greater levels of craving both during the placebo versus nicotine session [SESSION effect, F(1,29)=5.7, p=0.02] and also after, in comparison to before, scanning [TIME effect, F(1,29)=10.8, p=0.003]. During the nicotine session, higher nicotine plasma concentrations were associated with lower levels of self-reported craving at both pre- [r(29)=−0.4, p=0.03] and post-scan time points [r(29)=−0.4, p=0.03].
Behavioral measures
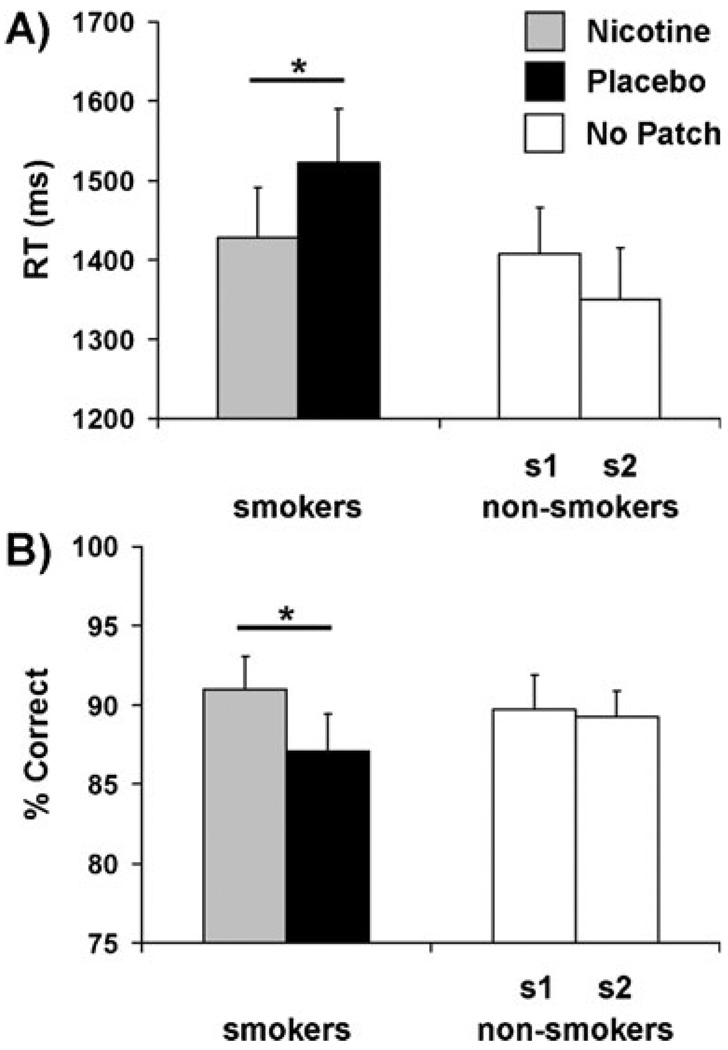
Within the smoker group, RTs associated with count reporting (Fig. 2a) were significantly faster during the nicotine (1,428±63 ms), in comparison to the placebo session (1,523±67); t(29)=1.8, p=0.04, one-tailed. However, there was no association between nicotine plasma levels and RT during the active-patch session; r(29)=−0.17, p=0.4. Non-smokers’ RTs did not differ between sessions 1 (1,408±58 ms) and 2 (1,350±65 ms), suggesting the absence of a practice effect; t(26)=0.9, p=0.2, one-tailed. Similarly, parsing the smokers into two cohorts based on patch order indicated the lack of a practice effect in that group (see supplementary material). No group difference was detected when comparing session-averaged smoker (1,475±58 ms) and non-smoker RTs (1,379± 53 ms); t(55)=1.2, p=0.2, two-tailed.
Fig. 2.
Nicotine altered gross behavioral measures. a During the nicotine session (gray), smokers’ RTs (ms) were faster relative to the placebo session (black), *p<0.05, one-tailed. Within the non-smoker group (no patches administered, open bars), there was no significant RT difference between session 1 (s1) and 2 (s2). b During the nicotine session, smokers’ count reports were more accurate (% Correct) than under placebo, *p<0.05, one-tailed. No difference in accuracy rates were detected between non-smokers’ session 1 and 2
In parallel with the RT measure, smokers’ accuracy (Fig. 2b) was greater during the nicotine (91.0±2.1%), relative to the placebo session (87.1±2.3%); t(29)=2.0, p=0.03, one-tailed. However, accuracy was not related to plasma nicotine levels during the active-patch session; r (29)=0.29, p=0.1. Non-smokers’ accuracy rates did not differ between sessions 1 (89.2±2.2%) and 2 (89.3±1.6%);t(26)=0.1, p=0.5, one-tailed. Dividing smokers into two groupsagain indicated the lack of a practice effect (see supplementary material). No group difference between session-averaged accuracy was detected [smokers=89.0±2.0%; non-smokers=89.2±1.6%; t(55)=−0.7, p=0.9, two-tailed].
Imaging results
Task and switch effects
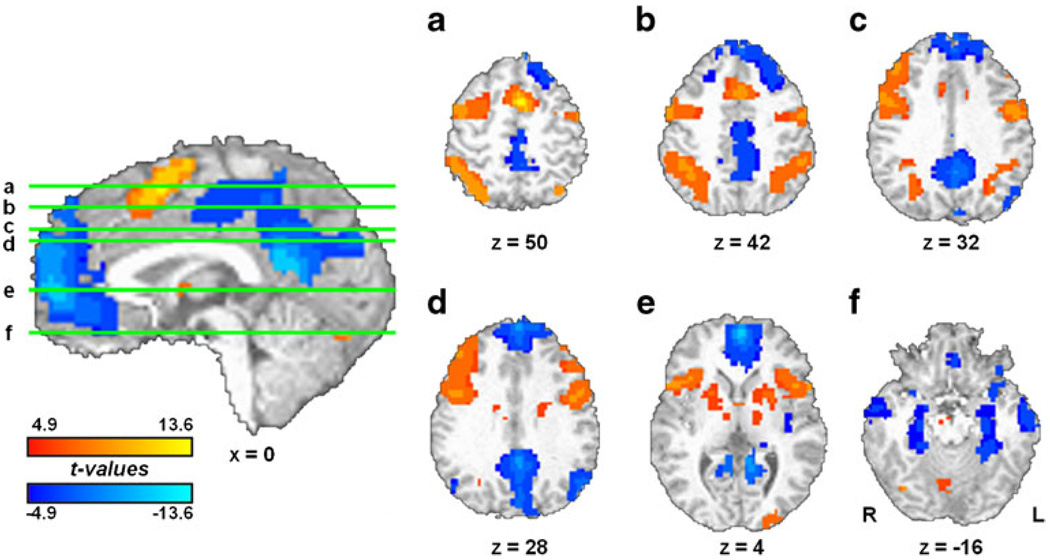
Task performance was associated with increased activity in a bilateral network encompassing areas related to WM (Fig. 3). Task-induced activations were observed notably in medial superior frontal cortex extending into supplemental motor area (msFC/SMA), right and left lateral PFC, and bilateral anterior insula/frontal operculum (aI/fO), putamen, parietal, and cerebellar regions. Substantial task-induced deactivations were also observed in areas consistent with the default-mode network, including rostral–medial PFC, posterior cingulate cortex (PCC), and bilateral parietal, temporal, and parahippocampal regions.
Fig. 3.
Tonic neural activity. TASK blocks were associated with bilateral activation (red scale) notably in msFC/SMA, lateral PFC, aI/fO, and parietal regions. Substantial deactivations (blue scale) were observed in rostral–medial PFC, PCC, and temporal regions. See supplementary information for complete list
Attention switching was associated with increased activity in a predominately left-lateralized frontoparietal network (Fig. 4). Switch-induced activity was noted in msFC/SMA, left lateral PFC, left superior parietal, and right superior frontal regions (Table 1). These switch-related regions, showing activation beyond that accounted for by the TASK regressor, presumably subserved the central executive function of attentional switching within verbal WM. In addition, deactivations were also noted in rostral–medial PFC and PCC.
Fig. 4.
Phasic neural activity. SWITCH trials were associated with activation (red) in a predominantly left-lateralized network consisting of msFC/SMA, left lateral PFC, left superior parietal, and right superior frontal regions. Switch-induced deactivations (blue) were noted in rostral–medial PFC and PCC. Numbering corresponds to that shown in Table 1
Table 1.
Regions showing significant SWITCH effects (phasic activity)
| Brain region | Side | Activation peak (x, y, z, in millimeters) | Brodmann area(s) | Volume (µl) | |
|---|---|---|---|---|---|
| Activations | |||||
| 1 | msFC/SMA | L | −4, 12, 48 | 6/32/8 | 5,670 |
| 2 | Lateral PFC | L | −54, 8, 32 | 9 | 6,372 |
| 3 | Superior parietal | L | −40, −58, 48 | 7/40 | 3,915 |
| 4 | Middle frontal gyrus | R | 30, −4, 60 | 6 | 1,026 |
| 5 | Inferior parietal | R | 38, −48, 48 | 40 | 459 |
| Deactivations | |||||
| 6 | Rostral–medial PFC | B | 0, 56, 2 | 10/32 | 4,266 |
| 7 | Posterior cingulate | B | −4, −42, 20 | 30/31/23 | 2,268 |
| 8 | Inferior parietal lobule | R | 56, −60, 32 | 39 | 351 |
Numbering corresponds to that shown in Fig. 4
B bilateral, L left, R right
Nicotine and session effects
Nicotine-induced changes in brain activation were not detected when comparing the TASK or SWITCH effects between nicotine and placebo conditions, even at the liberal voxel-wise threshold of p<0.01. An ad hoc analysis failed to identify any TASK- or SWITCH-related brain regions correlated with nicotine plasma levels. In the non-smoker group, no differences between sessions 1 and 2 were detected with respect to the TASK or SWITCH effects.
Smoker versus non-smoker effect
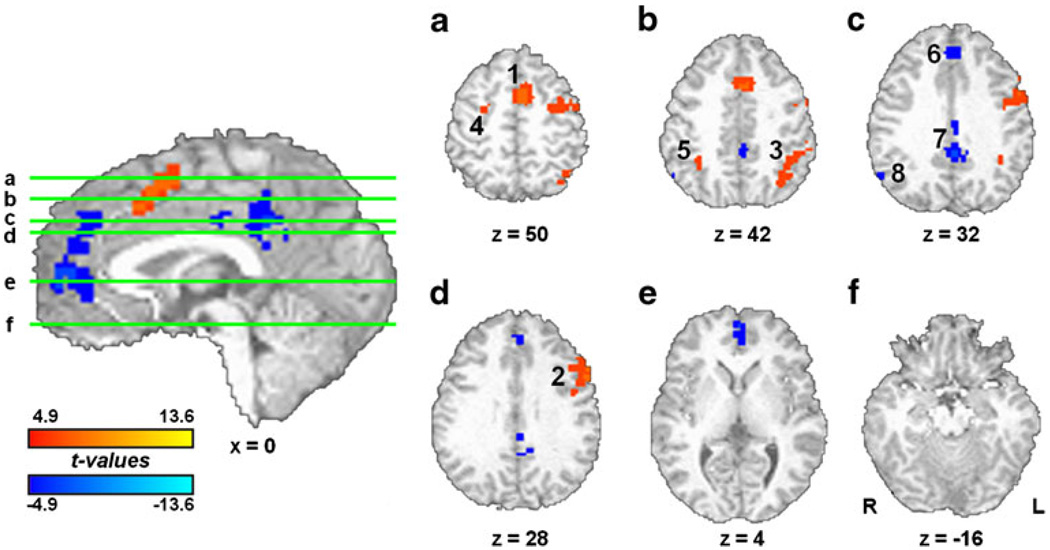
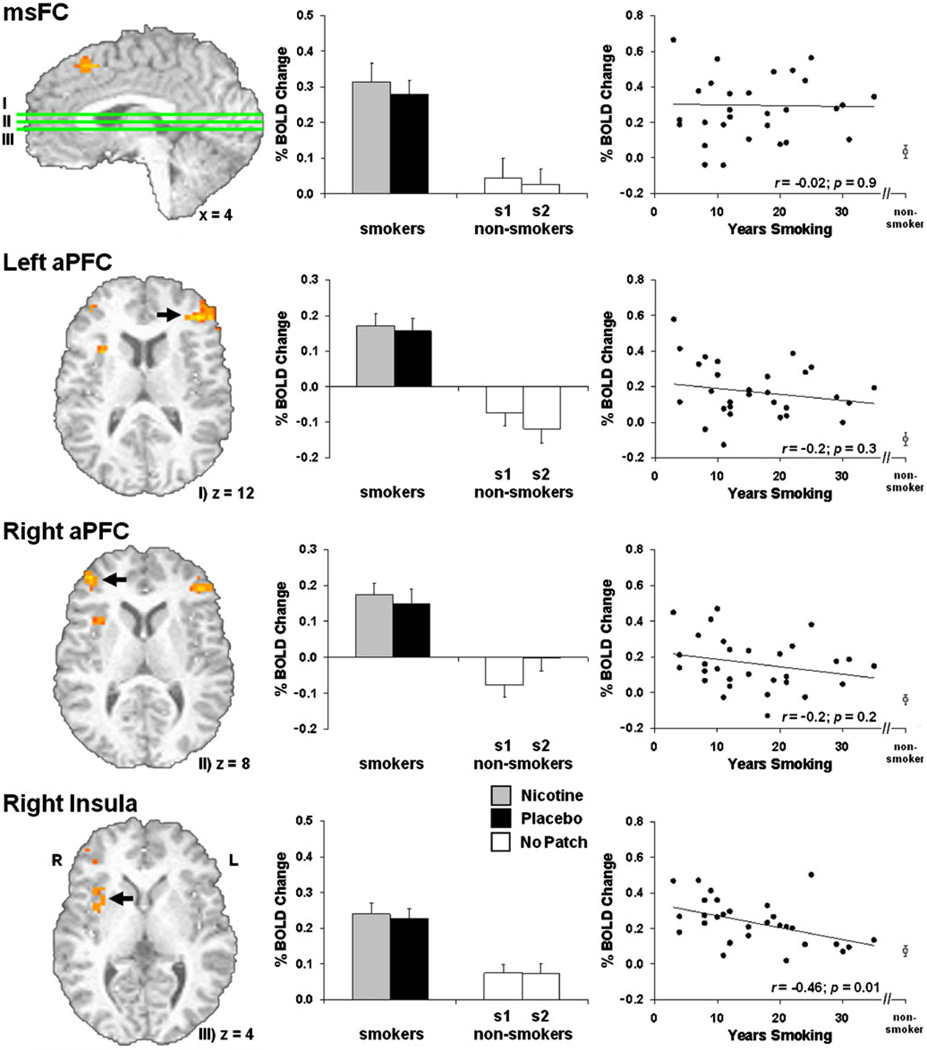
Comparison of the TASK effect between smokers and non-smokers (controlling for age, gender, and RT) suggested differences in brain activation related to WM control operations. Smokers showed greater activation in msFC, left and right anterior PFC, and right aI/fO (Fig. 5, Table 2). Activity from these ROIs was extracted and examined for nicotine and practice effects (Fig. 5, middle). Similar to the whole-brain analyses above, no drug or practice effects were detected. Correlation analyses between ROI activity and number of years smoking (Fig. 5, right) indicated that right aI/fO activation was negatively related to duration of smoking [r(29)=−0.46, p=0.01], i.e., the longer participants reported smoking, the closer their insula activation levels were to those of non-smokers. Negative correlations between lifetime usage (packs per day × number of years smoking) and task-related activity were also detected for right aI/fO [r(29)=−0.4, p=0.03] and right anterior PFC [r(29)=−0.38; p=0.04]. Activity from none of the ROIs was correlated with Fagerström scores (rs<0.25, ps>0.2). No SWITCH effect differences were observed between smokers and non-smokers.
Fig. 5.
Task-related smoker versus non-smoker differences. During TASK blocks, smokers showed greater activation in msFC, left and right aPFC, and right aI/fO (left; see also Table 2). Activity from these functional regions of interest was not modulated by nicotine or practice effects (middle). Correlations between ROI activity and number of years smoking (right) indicated that the longer participants reported smoking, the closer their right insula activation levels came to those of non-smokers (right, bottom)
Table 2.
Regions showing increased activation in the smoker group during TASK blocks (tonic activity). See also Fig. 5
| Brain region | Side | Activation peak (x, y, z, in millimeters) | Brodmann area(s) | Volume (µl) | |
|---|---|---|---|---|---|
| 1 | msFC | R | 6, 24, 48 | 8/6 | 891 |
| 2 | Anterior PFC | L | −42, 38, 12 | 10/46 | 1,998 |
| 3 | Anterior PFC | R | 42, 48, 8 | 10/46 | 837 |
| 4 | Insula | R | 32, 12, 12 | 13 | 1,188 |
Discussion
This study examined the impact of acute nicotine administration and chronic smoking on WM-related operations using a mixed block/event-related fMRI paradigm that allowed for the dissociation of tonic (TASK) and phasic (SWITCH) BOLD signal changes. Consistent with the known pharmacological effects of nicotine, active-patch administration resulted in increased heart rate, elevated self-reported mood, decreased levels of tobacco craving, and improvements in gross measures of behavioral performance1. However, no nicotine (state-like) effects within the smoker group were detected when comparing activity associated with either the TASK or SWITCH regressors. In contrast, smokers versus non-smokers (trait-like effect) showed greater sustained activation throughout TASK performance in msFC, right aI/fO, and bilateral anterior PFC (aPFC); activity which was not modulated by acute nicotine administration.
Task and switch effects
Following and expanding on the work of Garavan and co-workers (Garavan et al. 2000; Kübler et al. 2003, 2005), the CEFER task was designed to isolate one precisely defined central executive function, attentional switching. The displacement and reallocation of attentional resources from the currently available count (i.e., the one updated on the previous trial) to the one outside of focus (i.e., the one simply maintained on the previous trial) confers a sizeable “cognitive cost” in comparison to when no such switch is required (Garavan 1998). While multiple bottom-up (e.g., repetition effects) and top-down processes (e.g., central executive operations) contribute to this switch cost (Gehring et al. 2003), it has been viewed as an indication that people do not have immediate and simultaneous access to all items in WM. Thus, on switch trials, an internal focus of attention is considered to be shifted from one count to the other. The task is thought to provide a probe of executive functions isolated from general WM operations since storage and rehearsal demands are held constant across trials and the pertinent manipulation relates to attentional switches (Garavan et al. 2000).
Neural correlates of executive control functions appeared to be dissociable from general task and/or WM operations. Task performance was associated with sustained bilateral activation, notably in msFC/SMA, aI/fO, lateral PFC, and parietal cortices. These activations are presumably related to global cognitive processes required by the task (e.g., maintaining task rules, attending to stimuli, updating count values, and count rehearsal) and not specifically to the central executive function of attentional switching. A similar frontoparietal co-activation has been repeatedly associated with WM performance and top-down attention (Cabeza and Nyberg 2000; Corbetta and Shulman 2002; Kübler et al. 2003; Naghavi and Nyberg 2005; Owen et al. 2005). In addition to these activations, substantial deactivations were observed in rostral–medial PFC, PCC, and bilateral parietal, temporal, and parahippocampal regions. These deactivations encompass regions belonging to the “default system” (Buckner et al. 2008) and may reflect the suppression of spontaneous self-generated mental activity (Gusnard et al. 2001) during counting blocks. Such sustained activations and deactivations have been commonly observed in mixed fMRI paradigms (Dosenbach et al. 2006) and recapitulate results from a functional connectivity study demonstrating two anti-correlated networks in the resting brain (Fox et al. 2005). The current activations strongly overlap with the “task-positive” network, and the deactivations with the “task-negative” network, described by Fox and colleagues (2005).
Switch trials, in contrast, were associated with increased phasic activity in a predominantly left-lateralized network consisting of msFC/SMA, left lateral PFC, and left superior parietal areas. These brain regions, showing transient switch-related signal changes, reflect the putative neural correlates of attention shifting, one tractable function of the central executive. Previous studies employing separate block and/or event-related designs have also identified a frontoparietal set of cortical regions related to attentional shifts in WM (Garavan et al. 2000; Kübler et al. 2003, 2005; Li et al. 2004; Sylvester et al. 2003), but were not able to decompose tonic and phasic aspects of neural activity.
While traditional views of executive functioning have focused on the frontal lobes, the emerging perspective is that such operations are associated with a distributed frontoparietal network (Collette and Van der Linden 2002). Based on results from neuroimaging studies parametrically manipulating switching frequency within blocks, Garavan and co-workers have argued that attentional switching recruits the entire WM network (Garavan et al. 2000; Kübler et al. 2003, 2005). In fact, when using a block design to assess attentional switching within and between verbal and visuo-spatial WM, extensive bilateral activations were observed with minimal differences across modalities (Kübler et al. 2003). The frontoparietal activity reported here is generally consistent with previous studies, further suggesting that attentional switching is instantiated by a distributed neuroanatomy as opposed to a specific and unique locus. However, the current results deviate from previous ones in that switch-related activity was predominately left-lateralized as opposed to bilateral. Tasks involving verbal WM have been associated with left hemispheric activations, whereas visuospatial WM has typically been related to the right hemisphere (Baddeley 2003; Smith et al. 1996). As such, it is suggested that the current mixed design may have advantages over previous parametric block designs with respect to isolating neural correlates of executive functioning from general task operations.
Nicotine’s effect on neural correlates of WM
Nicotine did not appear to augment neural activity associated with executive control (SWITCH effect) or general WM operations (TASK effect). The lack of a nicotine effect, beyond the amelioration of withdrawal, is consistent with most (Barr et al. 2008; Ernst et al. 2001a, b; Kleykamp et al. 2005; Myers et al. 2008), but not all (Kumari et al. 2003), studies assessing the WM-enhancing properties of nicotine in minimally and non-deprived smokers, and non-smokers.
More protracted periods of smoking deprivation (>12 h) than assessed herein impair WM performance with smoking, nicotine, or varenicline ameliorating such deficits (Ernst et al. 2001a; Jacobsen et al. 2005; Jacobsen et al. 2007; Mendrek et al. 2006; Patterson et al. 2009; Xu et al. 2005). On the other hand, nicotine administration following abstinence has been reported to produce null effects (Myers et al. 2008) and to even impair deprived-smokers’ WM abilities (Greenstein and Kassel 2009; Jacobsen et al. 2004). Shedding light on these behavioral inconsistencies, a growing literature on the functional neuroanatomy underlying abstinence effects indicates smoking deprivation decreases efficiency in brain regions associated with WM, including ACC, lateral prefrontal, and parietal cortices (Ernst et al. 2001a; Jacobsen et al. 2007; Xu et al. 2005). Furthermore, smoking (Xu et al. 2006), nicotine (Ernst et al. 2001a), and varenicline (Loughead et al. 2010) appear to attenuate these deprivation-induced regional alterations. Imaging investigations stratified by specific genetic polymorphisms have also led to the emerging appreciation that inconsistencies in behavioral results may not only arise from methodological differences (e.g., task specifics, nicotine dose/route of administration) but also from genetic variations among participants modulating the impact of abstinence (Loughead et al. 2009) and nicotine (Jacobsen et al. 2006) on WM-related brain regions. Thus, the emerging view is that abstinence likely results in impaired WM performance and altered regional brain activity which can be reversed by nicotine or nicotinic analogues, an effect that may contribute to the perpetuation of smoking behaviors (Patterson et al. 2010).
Less clear are the cognitive enhancing properties of nicotine beyond withdrawal reversal in the WM domain. For instance, nicotine-induced improvements in n-back performance have not been detected when assessing ex-(Ernst et al. 2001a) or never-smokers (Ernst et al. 2001b; Kleykamp et al. 2005). Additionally, task dissociations in both non-deprived smokers (Myers et al. 2008) and non-smokers (Barr et al. 2008) have demonstrated null effects on WM performance in the face of nicotine-induced behavioral improvements in sustained attention tasks. In contrast, Kumari and co-workers (2003) showed that subcutaneous nicotine to never-smokers improved n-back performance and increased activity in ACC and frontoparietal cortices. However, Lawrence and colleagues (2002) suggested that nicotine-induced behavioral alterations, albeit in another task domain (sustained attention), resulted from increased activation in posterior cortical and subcortical regions presumably related to visual attention rather than modulation of frontal regions subserving WM operations. In fact, it has been proposed that positive effects of nicotine on WM, such as those reported by Kumari et al. (2003), may be mediated by attentional enhancement rather than true mediation of WM operations (Newhouse et al. 2004; Warburton and Rusted 1993). Nicotine-induced WM enhancement may only be observed in situations involving high attentional demands or in participants already performing sub-optimally because of nicotine abstinence or disease states (Newhouse et al. 2004).
Indirect support for the notion that nicotine does not affect the central executive component of WM assessed herein comes from a series of reports by Rusted, Marchant, and co-workers identifying a pharmacological dissociation between attentional switching and another psychological construct, prospective memory (i.e., remembering to perform delayed intentions). Specifically, these researchers suggest that nicotine does not modulate attentional switching but improves measures of prospective memory, whereas the psychostimulant modafinil appears to improve attentional switching but produce no effect on prospective memory (Marchant et al. 2008, 2009; Rusted et al. 2005, 2009). The current results and extant literature support the interpretation that nicotine provides minimal or no cognitive benefit, other than withdrawal reversal, on central executive or general WM-related operations.
A useful heuristic framework to understand nicotine’s cognitive enhancing properties comes from Posner and colleagues (Posner and Petersen 1990) who suggested that aspects of attention can be fractionated into three rather independent domains (Fan et al. 2002) and linked to spatially and neurochemically dissociable brain networks (Fan et al. 2005). These aspects of attention are related to (1) alerting operations, associated with thalamic, frontal, and parietal regions and mediated by the norepinephrine system (Fan et al. 2002); (2) orienting functions involving frontal and parietal areas impacted by acetylcholine transmission (Posner and Rothbart 2007); and (3) executive control processes associated with medial and lateral PFC and influenced by dopaminergic functioning (Fan et al. 2005). Previously, nicotine administered to minimally deprived cigarette smokers under the current procedures2 has been shown to modulate alerting and orienting aspects of cognition as assessed by a rapid visual information processing task (Lawrence et al. 2002) and a visuospatial orientation task (Hahn et al. 2007), respectively. In the current study, no nicotine-induced changes in brain activation were detected when probing executive control operations. Based on these three reports, we suggest that the beneficial effects of nicotine may be specific to alerting and orienting aspects of attention and do not generalize to control operations. To address this conjecture, it is of interest to assess the impact of nicotine on these aspects of cognition within a single imaging task (Fan et al. 2002) similar to investigations with mecamylamine (Thienel et al. 2009a) and scopolamine (Thienel et al. 2009b).
Smoker versus non-smoker effect
The neural consequences of an extended smoking history appeared to be increased sustained activation in msFC, right aI/fO, and bilateral aPFC throughout task performance. These increased activations were identified using age as a covariate, suggesting that, although the smokers as a whole were slightly older than the non-smokers (p=0.08), age-related increased recruitment of prefrontal regions (Cabeza et al. 2004) was unlikely to fully account for group differences.
Another possible interpretation of this activity enhancement is that smoking participants were less efficient at mobilizing cognitive resources required for task completion necessitating the recruitment of additional WM and supervisory control areas (i.e., compensatory activation). Consistent with this position, reduced gray matter volume and/or density in regions associated with WM operations have been detected in smokers (Brody et al. 2004). Recent work by Dosenbach and colleagues employing meta-analyses of mixed fMRI designs (Dosenbach et al. 2006), resting state functional connectivity assessments (Dosenbach et al. 2007), and network connectivity approaches (Dosenbach et al. 2008) has identified a network consisting of msFC, aI/fO, and aPFC that displays sustained activation over the duration of task blocks. This network is thought to be related to the deployment and maintenance of capacity-limited cognitive resources involved with supervisory control operations that instantiate the rules of association between stimuli and responses (Sakai 2008). Smokers’ increased sustained activity in this cingulo-opercular–aPFC network may thus reflect the need for increased cognitive resources necessary to guide goal-directed behavior during task performance.
A third, more speculative interpretation of the increased activations observed in the smoker group is that such activity may be a manifestation of an additional ongoing cognitive process not present in the non-smokers. Specifically, others have suggested that ruminative drug-related thoughts activate a WM-like cortical network (Bonson et al. 2002; Kübler et al. 2005) and that such a tonic cognitive process may negatively impact WM capacity and hence performance (Hester and Garavan 2009). We hypothesize that the greater sustained activation in bilateral aPFC, near the junction of BA 10/46, is consistent with such ruminative thoughts. Similar aPFC activations have been observed during task switching (Braver et al. 2003; Kübler et al. 2003), in tasks requiring the integration of sub-task processing outcomes with information stored in WM (De Pisapia and Braver 2008; De Pisapia et al. 2007), and during prospective memory operations (Reynolds et al. 2009; Simons et al. 2006). Koechlin and colleagues (1999) speculated that aPFC may be important for planning and reasoning, particularly when exogenous or endogenous stimuli (e.g., intrusive thoughts) interrupt task performance. More recently, De Pisapia and Braver (2008) proposed that a region of left aPFC, almost identical in anatomical location to that observed here, may be related to the maintenance of task-relevant information and protection of that information from sub-task interference. The increased aPFC activity observed here may thus reflect increased WM control processes associated with the maintenance of task-relevant information and its protection from internally generated intrusive thoughts related to tobacco and/or prospective memory operations associated with future smoking intentions. Smokers may have been “cued” to experience such intrusive thoughts as they were queried about tobacco cravings while lying in the scanner immediately before task onset. Although we observed no behavioral differences between groups, compromised WM capacity due to ruminative drug-related thoughts could be one potential explanation of decreased WM performance in smokers noted in previous studies (Ernst et al. 2001b; Greenstein and Kassel 2009; Jacobsen et al. 2005, 2007).
It is also noteworthy that similar regions to those showing greater sustained activation in the smoker group have previously been related to craving and urges to smoke. For instance, positive correlations between self-reported craving and activity in dorsal medial PFC and aPFC have been reported (McClernon et al. 2005, 2009). With respect to the insula, damage to this region has been associated with sudden and sustained smoking cessation and an absence of urges to smoke after quitting (Naqvi et al. 2007). Imaging studies have demonstrated that insula activity is correlated with self-reported smoking urges (Brody et al. 2002) and higher right insula activity during a simple decision-making task is associated with an increased propensity for drug relapse (Paulus et al. 2005). The view that insula is involved with the subjective awareness of bodily sensations is consistent with these observations (Craig 2009). The negative correlation between right insula activation and number of years smoking indicates that the longer participants reported smoking, the closer their activation levels were to those of non-smokers. This observation suggests that those who become regular smokers may be predisposed to habitual tobacco use and that over the course of time, approach normative levels, consistent with a self-medication hypothesis (Evans and Drobes 2009). That is, smokers may begin to smoke to down-regulate elevated insula activity.
Taking a reductionist approach, we examined the extent to which a contemporary model of WM functioning could provide a framework for understanding the short- and long-term consequences of nicotine use. Employing a mixed block/event-related fMRI paradigm and a concurrent counting span task, neural correlates of executive control functioning were able to be dissociated from general WM operations. This fMRI design may be better able to isolate central executive functions than previously employed methodologies. Nicotine did not appear to augment neural correlates of executive control or general task operations. A working hypothesis to guide future research, synthesized from the results of the current and methodologically compatible studies, is that the cognitive-enhancing properties of nicotine may be specific to alerting and orienting domains of attention and may not generalize to executive control operations. Finally, increased frontal activation in brain regions previously associated with the deployment of cognitive resources and WM control was identified as neural consequence of an extended smoking history. We speculate that smokers may experience higher WM demands during task performance as a result of intrusive thoughts related to smoking.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Abuse—Intramural Research Program. We thank all NIDA-IRP staff members who assisted in data collection, the NIH Fellow’s Editorial Board for suggestions on this manuscript, and Hugh Garavan.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-010-2013-6) contains supplementary material, which is available to authorized users.
While nicotine-induced alterations in behavioral performance were observed, these gross measures collected at the end of the counting blocks are unlikely to be useful indices of the trial-to-trial dynamics associated with attentional switching, but rather may reflect nonspecific effects on motoric responding or psychomotor speed.
That is, last cigarette smoked ~3 h and transdermal patch applied ~2–2.5 h pre-scan. See also supplementary information regarding additional overlap with the Hahn et al. (2007) study.
Conflict of interest The authors declare no conflicts of interest.
Contributor Information
Matthew T. Sutherland, Neuroimaging Research Branch, National Institute on Drug Abuse-Intramural Research Program, NIH/DHHS, 251 Bayview Blvd, Suite 200, Baltimore, MD 21224, USA, sutherlm@nida.nih.gov
Thomas J. Ross, Neuroimaging Research Branch, National Institute on Drug Abuse-Intramural Research Program, NIH/DHHS, 251 Bayview Blvd, Suite 200, Baltimore, MD 21224, USA
Diaá M. Shakleya, Chemistry and Drug Metabolism Section, National Institute on Drug Abuse-Intramural Research Program, NIH/DHHS, 251 Bayview Blvd, Suite 200, Baltimore 21224 MD, USA
Marilyn A. Huestis, Chemistry and Drug Metabolism Section, National Institute on Drug Abuse-Intramural Research Program, NIH/DHHS, 251 Bayview Blvd, Suite 200, Baltimore 21224 MD, USA
Elliot A. Stein, Neuroimaging Research Branch, National Institute on Drug Abuse-Intramural Research Program, NIH/DHHS, 251 Bayview Blvd, Suite 200, Baltimore, MD 21224, USA
References
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Hitch G. Working memory. In: Bower GA, editor. The psychology of learning and motivation. New York: Academic; 1974. pp. 47–89. [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buracas GT, Boynton GM. Efficient design of event-related fMRI experiments using M-sequences. Neuroimage. 2002;16:801–813. doi: 10.1006/nimg.2002.1116. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev. 2002;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Braver TS. Preparation for integration: the role of anterior prefrontal cortex in working memory. NeuroReport. 2008;19:15–19. doi: 10.1097/WNR.0b013e3282f31530. [DOI] [PubMed] [Google Scholar]
- De Pisapia N, Slomski JA, Braver TS. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cereb Cortex. 2007;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HSC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001a;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London ED. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci U S A. 2001b;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H. Serial attention within working memory. Mem Cogn. 1998;26:263–276. doi: 10.3758/bf03201138. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Li SJ, Stein EA. A parametric manipulation of central executive functioning. Cereb Cortex. 2000;10:585–592. doi: 10.1093/cercor/10.6.585. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Bryck RL, Jonides J, Albin RL, Badre D. The mind’s eye, looking inward? In search of executive control in internal attention shifting. Psychophysiology. 2003;40:572–585. doi: 10.1111/1469-8986.00059. [DOI] [PubMed] [Google Scholar]
- Greenstein JE, Kassel JD. The effects of smoking and smoking abstinence on verbal and visuospatial working memory capacity. Exp Clin Psychopharmacol. 2009;17:78–90. doi: 10.1037/a0015699. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Moolchan ET. Tobacco craving questionnaire: reliability and validity of a new multifactorial instrument. Nicotine Tob Res. 2003;5:645–654. doi: 10.1080/1462220031000158681. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009;93:270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Mencl WE, Gelernter J. C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacology. 2006;188:530–540. doi: 10.1007/s00213-006-0469-1. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology. 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Blank MD, Eissenberg T. The effects of nicotine on attention and working memory in never-smokers. Psychol Addict Behav. 2005;19:433–438. doi: 10.1037/0893-164X.19.4.433. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Kübler A, Murphy K, Kaufman J, Stein EA, Garavan H. Coordination within and between verbal and visuospatial working memory: network modulation and anterior frontal recruitment. Neuroimage. 2003;20:1298–1308. doi: 10.1016/S1053-8119(03)00400-2. [DOI] [PubMed] [Google Scholar]
- Kübler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SCR, Simmons A, Sharma T. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Li ZH, Sun XW, Wang ZX, Zhang XC, Zhang DR, He S, Hu XP. Behavioral and functional MRI study of attention shift in human verbal working memory. Neuroimage. 2004;21:181–191. doi: 10.1016/j.neuroimage.2003.08.043. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha 4 beta 2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology. 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Trawley S, Rusted JM. Prospective memory or prospective attention: physiological and pharmacological support for an attentional model. Int J Neuropsychopharmacol. 2008;11:401–411. doi: 10.1017/S146114570700819X. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Kamel F, Echlin K, Grice J, Lewis M, Rusted JM. Modafinil improves rapid shifts of attention. Psychopharmacology. 2009;202:487–495. doi: 10.1007/s00213-008-1395-1. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Hutettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: comparative effects upon cognitive task performance and mood state over 24 h. Hum Psychopharmacol. 1996;11:391–400. [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during descision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- R-Development-Core-Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. ISBN: 3-900051-07-0; http://www.R-project.org. [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cereb Cortex. 2009;19:1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Trawley S, Heath J, Kettle G, Walker H. Nicotine improves memory for delayed intentions. Psychopharmacology. 2005;182:355–365. doi: 10.1007/s00213-005-0109-1. [DOI] [PubMed] [Google Scholar]
- Rusted J, Sawyer R, Jones C, Trawley S, Marchant N. Positive effects of nicotine on cognition: the deployment of attention for prospective memory. Psychopharmacology. 2009;202:93–102. doi: 10.1007/s00213-008-1320-7. [DOI] [PubMed] [Google Scholar]
- Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Shakleya DM, Huestis MA. Simultaneous and sensitive measurement of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human plasma by liquid chromatography–tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877:3537–3542. doi: 10.1016/j.jchromb.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Scholvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44:1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Sylvester CYC, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tourneaux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Thienel R, Kellermann T, Schall U, Voss B, Reske M, Halfter S, Sheldrick AJ, Radenbach K, Habel U, Shah NJ, Kircher T. Muscarinic antagonist effects on executive control of attention. Int J Neuropsychopharmacol. 2009a;12:1307–1317. doi: 10.1017/S146114570999068X. [DOI] [PubMed] [Google Scholar]
- Thienel R, Voss B, Kellermann T, Reske M, Halfter S, Sheldrick AJ, Radenbach K, Habel U, Shah NJ, Schall U, Kircher T. Nicotinic antagonist effects on functional attention networks. Int J Neuropsychopharmacol. 2009b;12:1295–1305. doi: 10.1017/S1461145709990551. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1964–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Warburton DM, Rusted JM. Cholinergic control of cognitive resources. Neuropsychobiology. 1993;28:43–46. doi: 10.1159/000118998. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. 3rd edn. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- Xu JS, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JS, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, Jarvik M, Rodriguez P, Ernst M, London ED. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-Back Task: a preliminary study. Psychiatry Res. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.