Abstract
In Saccharomyces cerevisiae, transport of arginine into the vacuole has previously been shown to be facilitated by a putative H+/arginine antiport. We confirm that transport of arginine into isolated yeast vacuoles requires ATP and we demonstrate a requirement for a functional vacuolar H+-ATPase. We previously reported that deletion of BTN1 (btn1-Δ), an ortholog of the human Batten disease gene CLN3, resulted in a decrease in vacuolar pH during early growth. We report that this altered vacuolar pH in btn1-Δ strains underlies a lack of arginine transport into the vacuole, which results in a depletion of endogenous vacuolar arginine levels. This arginine transport defect in btn1-Δ is complemented by expression of either BTN1 or the human CLN3 gene and strongly suggests a function for transport of, or regulation of the transport of, basic amino acids into the vacuole or lysosome for yeast Btn1p, and human CLN3 protein, respectively. We propose that defective transport at the lysosomal membrane caused by an absence of functional CLN3 is the primary biochemical defect that results in Batten disease.
The neuronal ceroid-lipofuscinoses (NCLs) are the most common group of progressive neurodegenerative diseases in children, with an incidence as high as 1 in 12,500 live births (1, 2). The NCL disorders are inherited in an autosomal recessive manner, with mutations in seven distinct genes resulting in pathologically similar disease with a different age of onset (reviewed in refs. 3 and 4). The NCLs are characterized by the accumulation of autofluorescent hydrophobic material in the lysosomes of neurons, and to a lesser extent, other cell types (5, 6). The juvenile form of NCL (JNCL) results from mutations in the CLN3 gene, which codes for a transmembrane protein (7). Progression of JNCL is characterized by a decline in mental abilities, increased severity of seizures, blindness, loss of motor skills, and premature death. Protein sequencing and immunological studies have revealed that subunit c of mitochondrial ATP synthase is the major component of the lysosomal storage material in JNCL (8, 9); however, the molecular basis behind this storage and the disease remains unknown.
Genes encoding predicted proteins with high sequence similarity to CLN3 have been identified in mouse, dog, rabbit, Caenorhabditis elegans, and the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe (refs. 10–12; Swiss-Prot accession no. Q29611, GenBank accession no. U92812, and GenBank accession no. Z49335). We previously reported that the corresponding S. cerevisiae gene, denoted BTN1, encodes a nonessential protein that is 39% identical and 59% similar to human CLN3 (13). Deletion of BTN1 (btn1-Δ) had no effect on the degradation of mitochondrial ATP synthase subunit c. Subsequent studies revealed that Btn1p was localized to the vacuole (14, 15) and that btn1-Δ resulted in a decreased vacuolar pH at the outset of growth (15). This alteration in vacuolar pH became normalized through growth, most likely through up-regulation of HSP30 and BTN2, the only two genes to have altered expression through growth in btn1-Δ, as compared with normal. Therefore, through coordinate gene expression, pH homeostasis is maintained in btn1-Δ strains. Importantly, the underlying effect of altered pH homeostasis in btn1-Δ is complemented by the human CLN3 gene, suggesting that the primordial function of Btn1p/CLN3 is conserved (16).
We recently reported that Btn2p interacts biochemically and functionally with Rsg1p, a down-regulator of the Can1p arginine and lysine plasma membrane permease (17, 18). Rsg1p was shown to localize to a distinct structure toward the cell periphery, and strains lacking Btn2p (btn2-Δ) had altered localization of Rsg1p. Furthermore, btn2-Δ strains, like rsg1-Δ strains, were sensitive for growth in the presence of the arginine analog canavanine, and both btn2-Δ and rsg1-Δ strains had elevated rates of uptake of [14C]arginine, which resulted in increased intracellular levels of arginine. Moreover, overexpression of BTN2 decreased the rate of arginine uptake. Collectively, these results indicate that altered levels of Btn2p can modulate arginine uptake through localization of the Can1p arginine permease regulatory protein, Rsg1p. Btn2p was originally identified as being up-regulated in btn1-Δ, and a genetic interaction between btn1-Δ and btn2-Δ was confirmed by both the suppression of the canavanine sensitivity and the elevated rate of uptake of arginine displayed by a btn2-Δ rsg1-Δ strain. We therefore speculated that up-regulation of BTN2 in btn1-Δ strains facilitated either a direct or an indirect effect on intracellular arginine levels.
In the present study, we clearly demonstrate that btn1-Δ results in a deficiency in the intracellular, and in particular vacuolar, levels of the basic amino acids arginine and lysine, as compared with normal. Furthermore, we show that the previously characterized ability for arginine to be transported into the vacuole is absent from vacuoles derived from btn1-Δ strains. Therefore, transport of arginine into the vacuole is ATP-dependent and requires a functional vacuolar H+-ATPase and the presence of Btn1p. Furthermore, expression of either Btn1p or human CLN3 restores the ability to transport arginine into btn1-Δ vacuoles, suggesting that Btn1p/CLN3 may transport, or mediate transport of, basic amino acids into the vacuole or lysosome. We propose that the underlying lysosomal defect in Batten disease is an alteration in transport of basic amino acids into this organelle.
Materials and Methods
Yeast Strains and Growth. Yeast strains used in this study are listed in Table 1. Yeast strains were grown as indicated in YPD (1% Bacto yeast extract/2% Bacto peptone/2% glucose) medium. Synthetic medium or synthetic medium with galactose instead of glucose, where indicated, lacking or containing uracil, when 1052-derived plasmids are present or absent, respectively, was also used for complementation studies.
Table 1. Yeast strains used in this study.
| Genotype | Strain |
|---|---|
| MATαhis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-11718 |
| MATαbtn1-Δ::KANMXhis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-13048 |
| MATαbtn1-Δ::KANMXhis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 + pAB1052 | Y001 |
| MATα btn1-Δ::KANMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 + DAP142 (pAB1052-BTN1) | Y002 |
| MATα btn1-Δ::KANMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 + DAP139 (pAB1052-CLN3) | Y003 |
| MATα cup5-Δ::KANMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-12074 |
| MATα vmal3-Δ::KANMX his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | B-13046 |
Extraction of Yeast Amino Acids. A standard procedure for extraction of total amino acids was used (19). In summary, cells at OD600 = 1.6 were harvested, twice washed in distilled water, resuspended in AA buffer [which is composed of 2.5 mM potassium phosphate buffer (pH 6.0) containing 0.6 M sorbitol, 10 mM glucose, and 0.2 M CuCl2], and incubated for 10 min at 30°C. This buffer permeabilizes the plasma membrane and causes leakage of cytosolic amino acids (19). The cell suspension was collected by filtration on membrane filters (0.45 μm, Millipore) and washed four times with AA buffer lacking 0.2 M CuCl2. These filtrates were combined and represent the cytosolic fraction (19). Cells retained on the filter were resuspended in water and boiled for 15 min and centrifuged at 5,000 × g for 5 min, and the supernatant was collected as the vacuolar fraction.
Amino Acid Analysis. Yeast cytosolic and vacuolar fraction supernatants were analyzed by the Hewlett Packard Aminoquant system. It consists of an automated precolumn derivatization sampler, a narrow-bore HPLC, a fluorescence detector, and a dedicated Hewlett Packard computer. A report is generated for each sample that includes the following data: picomole yields of amino acids, nanogram yields of amino acids, and mole percent. A Hewlett Packard ODS Hypersil 5 μm (200 × 2.1 mm) column was used for the separation of amino acids after precolumn derivatization as described below. Amino acids were separated by using two buffer systems. Buffer A consisted of 0.01 M acetate/triethylamine buffer (pH 7.2) containing 0.3% tetrahydrofuran, and buffer B contained 0.01 M sodium acetate (pH 7.2), methanol, and acetonitrile in a ratio of 1:2:2 (vol/vol).
Isolation of Yeast Vacuoles. Yeast vacuoles were obtained by using the method of Ohsumi and Anraku (20) with some modifications. Yeast cells were grown to OD600 = 0.6–0.7 in medium, as indicated, then washed once with sterile distilled water and then once with 1 M sorbitol. Cells were converted to spheroplasts by suspending the cell pellet in 100 ml of 1 M sorbitol containing 400 units of Zymolyase 100T (ICN Pharmaceuticals). The culture was gently shaken for 90 min at 30°C. Spheroplasts were collected by centrifugation at 800 × g for 5 min and then washed twice in 1 M sorbitol. All subsequent manipulations were carried out at 4°C. The pellet was suspended in 25 ml of buffer X (10 mM Tris–Mes, pH 6.9/0.1 mM MgCl2/12% Ficoll 400) and homogenized by six or seven strokes in a Dounce homogenizer. The lysate was cleared by centrifugation at 26,600 × g for 35 min. The top layer was collected in a Dounce homogenizer containing 6 ml of buffer X, and clumps were broken up by six or seven strokes. The homogenate was transferred to an ultracentrifuge tube and layered with 6 ml of buffer Y (10 mM Tris–Mes, pH 6.9/0.1 mM MgCl2/8% Ficoll 400). The mixture was centrifuged at 26,600 × g for 30 min. The top layer was collected and dispersed in a tube containing 6 ml of 2× buffer Z (1× buffer Z is 10 mM Tris–Mes, pH 6.9/5 mM MgCl2/25 mM KCl). Vacuoles were converted to vesicles by adding 2 vol of 1× buffer Z, and a pellet was obtained by centrifugation at 26,600 × g for 20 min. Purity of the isolated vacuoles was verified by confocal microscopy and conformed to our previous use of Western analysis with vacuolar markers (21).
Assay of Arginine Transport. Arginine uptake assays were performed in the manner described by Ohsumi and Anraku (20). Accumulation of [14C]arginine by vacuole vesicles at each time point, where indicated, or at time 0 and at 30-sec increments, was assayed in a 100-μl reaction mixture composed of 25 mM Tris–Mes (pH 7.4), 4 mM MgCl2, 25 mM KCl, 0.3 mM ATP unless otherwise indicated, 3.33 μCi of [14C]arginine (348 mCi/mmol; 1 mCi = 37 MBq) unless otherwise indicated, and 30 μg of protein. The reaction occurred at room temperature and was stopped by adding 5 ml of cold 1× buffer Z. Vacuole vesicles were collected on a 0.22-μm-pore nylon membrane (Millipore). The radioactivity retained on the membrane was quantified in a scintillation counter (Beckman). The protein content of vacuolar preparations was measured with Bradford assays (Bio-Rad).
Results
Decreased Intracellular Levels of Arginine and Lysine, Particularly in the Vacuole of btn1-Δ Strains. The fact that BTN2 was up-regulated in btn1-Δ strains, and that Btn2p has a role in modulating intracellular arginine levels, prompted us to measure intracellular levels of all amino acids in btn1-Δ cells. In Table 2 we show levels of detectable amino acids in btn1-Δ cells and isogenic wild-type controls. Furthermore, we fractionated cells in such a way as to delineate the distribution of these amino acids in the cytosol and vacuole. The intracellular amino acid levels are in line with previous reports of levels of amino acids in yeast, and we confirm that the predominantly occurring amino acids in yeast vacuoles are the basic amino acids arginine and lysine (20). Moreover, there is a clear decrease in the levels of arginine and lysine of ≈10-fold in btn1-Δ vacuoles as compared with normal. Interestingly, other than a 2-fold decrease in glutamic acid levels in btn1-Δ vacuoles as compared with normal, there are no significant differences in the levels of other detectable amino acids.
Table 2. Intracellular levels of amino acids in normal BTN1+ (B-11718) and btn1-Δ (B-13048) cells indicate that levels of arginine and lysine are significantly decreased in the vacuoles of btn1-Δ strains.
| Intracellular free amino acid content, nmol per 108 cells
|
||||
|---|---|---|---|---|
|
BTN1+
|
btn1-Δ
|
|||
| Amino acid | Vacuole | Cytosol | Vacuole | Cytosol |
| Ala | 31 ± 5 | 4 ± 1 | 24 ± 3 | 5 ± 1 |
| Asn | — | — | — | — |
| Asp | 4 ± 1 | >1 | 4 ± 1 | — |
| Arg | 117 ± 14 | 5 ± 1 | 18 ± 5 | — |
| Cys | — | — | — | — |
| Gly | 25 ± 5 | 3 ± 1 | 20 ± 4 | 3 ± 1 |
| Gln | 2 ± 1 | — | 3 ± 1 | — |
| Glu | 42 ± 4 | — | 16 ± 4 | — |
| His | — | — | — | — |
| Ile | 5 ± 1 | 1 ± 1 | 3 ± 1 | 1 ± 1 |
| Leu | 4 ± 1 | — | 3 ± 1 | >1 |
| Lys | 103 ± 8 | 12 ± 4 | 11 ± 3 | 2 ± 1 |
| Met | 3 ± 1 | 10 ± 2 | 2 ± 1 | 11 ± 3 |
| Phe | 2 ± 1 | — | 2 ± 1 | — |
| Pro | 8 ± 2 | 2 ± 2 | 6 ± 2 | 3 ± 1 |
| Ser | 15 ± 3 | 7 ± 3 | 15 ± 3 | 7 ± 3 |
| Thr | — | 5 ± 1 | — | 6 ± 2 |
| Trp | — | — | — | — |
| Tyr | 2 ± 1 | — | — | — |
| Val | 12 ± 2 | 2 ± 1 | 10 ± 2 | 2 ± 1 |
Results are presented as mean ± SD of four independently derived samples. Differences in vacuolar arginine and lysine levels in BTN1+ and btn1-Δ are statistically significant with P < 0.05 as determined by standard t test.
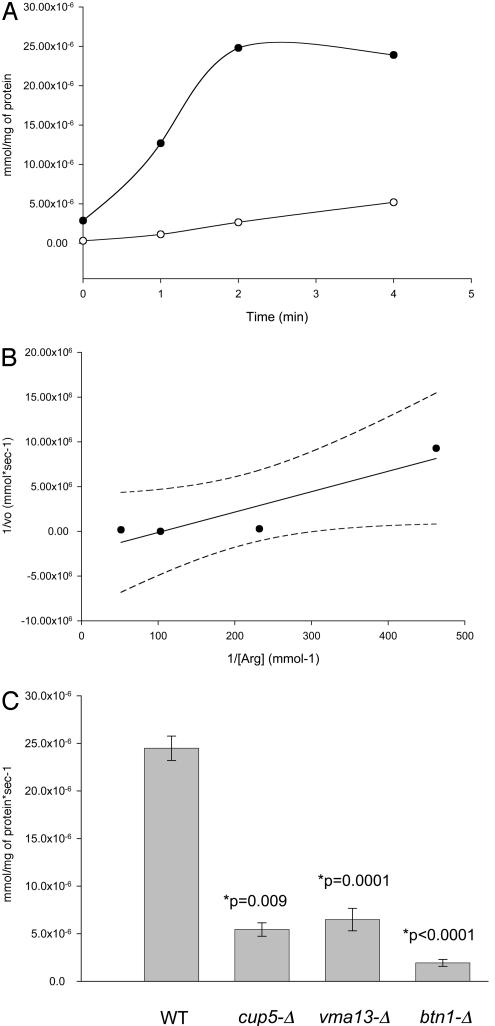
Transport of Arginine into the Yeast Vacuole. A simple explanation for depletion of arginine and lysine levels in the vacuoles of btn1-Δ cells would be a lack of transport across the vacuolar membrane of these amino acids. Transport of arginine into the vacuole has been reported by other groups to be imparted by an H+/arginine antiport in an ATP-dependent manner (20, 22). Our initial step was to confirm this transport process in our wild-type yeast strains before establishing whether the depletion in arginine in the vacuole of btn1-Δ strains results from a transport defect at the vacuole. In Fig. 1A we show a typical uptake profile for arginine for vacuoles isolated from a wild-type yeast strain, and we show that this transport depends upon the presence of ATP. By varying the concentration of arginine for the transport assay we have confirmed similar kinetics for uptake previously reported for arginine transport into the vacuole (Fig. 1B). In addition, we have demonstrated that isolated vacuoles from yeast cells lacking a functional vacuolar H+-ATPase, because of deletion of either CUP5 (VMA3), cup5-Δ, or VMA13, vma13-Δ, which encode V0 and V1 subunits of the vacuolar H+-ATPase respectively, have a decrease in the transport of arginine (Fig. 1C). Furthermore, we have demonstrated that an absence of Btn1p, btn1-Δ, results in a decrease in arginine transport into the vacuole. It is worth noting that transport of arginine into btn1-Δ vacuoles is a little less than into those lacking a functional vacuolar H+-ATPase. We have previously reported that btn1-Δ vacuoles do not have a significantly altered activity of vacuolar H+-ATPase (15, 21), suggesting that this lack of arginine transport may be independent of vacuolar H+-ATPase activity. It should also be noted that to validate reproducibility of arginine uptake into isolated vacuoles, this and subsequent uptakes are presented as rate of arginine uptake. Rate of arginine uptake was determined from independent vacuolar isolates as indicated.
Fig. 1.
Isolated vacuoles from yeast display ATP-dependent and vacuolar H+-ATPase-dependent transport of arginine into the vacuole. (A) Uptake of [14C]arginine into isolated vacuoles from wild-type yeast (B-11718) in the presence (•) and absence (○) of ATP. Results shown are typical of those obtained from six independently derived uptake experiments. (B) Lineweaver–Burk plot with 95% confidence limits, showing first-order kinetic uptake, Km = 0.02 mM, of [14C]arginine into isolated vacuoles from wild-type yeast, B-11718. (C) Rate of uptake of [14C]arginine into isolated vacuoles from wild-type (WT) yeast, B-11718; yeast lacking the CUP5-encoded V0 subunit of the vacuolar H+-ATPase (cup5-Δ), B-12074; yeast lacking the VMA13-encoded V1 subunit of the vacuolar H+-ATPase (vma13-Δ), B-13046; and yeast lacking Btn1p (btn1-Δ), B-13048. Results were obtained from four independently derived preparations of vacuoles from each strain. All isolated vacuoles shown were prepared from strains grown on YPD.
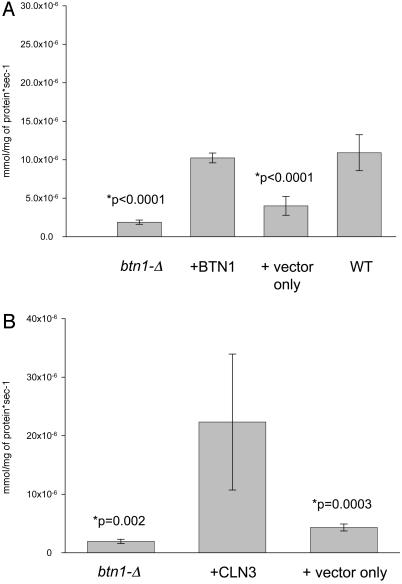
Lack of Arginine Transport into the Vacuole of btn1-Δ and Complementation by Human CLN3 of the Arginine Transport Defect in btn1-Δ. Having established that btn1-Δ vacuoles had a decreased ability to transport arginine into the vacuole, we wanted to determine whether this depended upon BTN1, and whether human CLN3 could restore transport activity. To do this we switched to a more defined growth medium, namely synthetic medium. In Fig. 2, it is clear that btn1-Δ vacuoles still have a reduced capacity to transport arginine into the vacuole. Importantly, in Fig. 2 A, we show that reintroduction of BTN1 restores or complements vacuolar arginine transport into isolated vacuoles of btn1-Δ to the level observed in control wild-type vacuoles grown under identical conditions. Furthermore, by inducing overexpression of the human CLN3 gene product under control of the GAL1 promoter, vacuolar arginine transport activity is again returned or complemented in btn1-Δ vacuoles (Fig. 2B).
Fig. 2.
Isolated vacuoles from btn1-Δ cells display a lack of transport into the vacuole that is complemented by yeast BTN1 and overexpression of human CLN3. (A) Rate of uptake of [14C]arginine into isolated vacuoles from btn1-Δ, B-13048; btn1-Δ with plasmid-borne BTN1, B-13048 plus DAP142 (pAB1052-BTN1), Y002; btn1-Δ with plasmid vector only, B-13048 plus pAB1052, Y001; and wild-type, B-11718. Results were obtained from four independently derived preparations of vacuoles from each strain. Strains were grown on synthetic complete medium minus uracil, except for B-13048 and B-11718, which had uracil added to the medium to facilitate growth. (B) Rate of uptake of [14C]arginine into isolated vacuoles from btn1-Δ, B-13048; btn1-Δ with plasmid-borne human CLN3, B-13048 plus DAP (pAB1052-CLN3), Y003; and btn1-Δ with plasmid vector only, B-13048 plus pAB1052, Y001. Results were obtained from four independently derived preparations of vacuoles from each strain. Strains were grown on synthetic complete medium lacking uracil, followed by washing and resuspension into synthetic complete galactose medium lacking uracil for 4 hr for induction purposes. Note that, for B-13048, uracil was added to the medium to facilitate growth.
Discussion
This study further demonstrates the utility of the yeast model for Batten disease. The fact that human CLN3 complements defective vacuolar arginine transport in btn1-Δ strains strongly suggests that human CLN3 has the same function as Btn1p in yeast. Although active transport of arginine across the yeast vacuolar membrane has previously been reported (19), a systematic comparison of all yeast transport proteins failed to suggest that Btn1p was a transporter (23). However, the data presented here make it tempting to speculate that Btn1p and perhaps human CLN3 facilitate vacuolar and lysosomal transport of arginine, respectively.
Although we show that a consequence of lacking arginine transport in btn1-Δ cells is arginine depletion in the vacuole, levels of amino acids, and in particular altered vacuolar levels of arginine and lysine, have previously been described in yeast strains with defective vacuolar function (24, 25). However, these other studies only measured amino acid levels, not transport into the vacuole. Therefore, we cannot exclude the possibility that a disturbance in vacuolar function in btn1-Δ strains is a part of a general cellular response to functional stress of this organelle that results in a decrease in the sequestration of basic amino acids. For example, an alternative method of altering vacuolar acidification has been reported, such that transport of 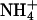 as a protonophore can facilitate equilibration of pH across plasma and vacuolar membranes (26). Therefore an alternative interpretation of decreased vacuolar content, and transport of basic amino acids into the vacuole, may be that this is an elaborate mechanism that equilibrates the observed abnormally acidic pH in the vacuoles of btn1-Δ strains (15). There is precedent to suggest in btn1-Δ strains that a shift to balance intracellular levels of arginine may be occurring. BTN2, which encodes a coiled-coil protein, is up-regulated in btn1-Δ strains, and Btn2p has been shown to be necessary for the localization of Rsg1p, a downregulator of arginine uptake at the plasma membrane through the Can1p permease (16). Furthermore, increased expression of BTN2 has been shown to directly decrease arginine uptake into cells (16). Thus, as btn1-Δ strains have increased expression of BTN2, one could predict that btn1-Δ elicits a decrease in intracellular levels of arginine at both the vacuole and the plasma membrane. First, up-regulation of Btn2p may modulate uptake of arginine through interaction with Rsg1p, and second, there is a decrease in the vacuolar levels of arginine, which presumably is transported in from the cytosol. In addition, the decreased vacuolar levels of arginine may result directly from a decrease in uptake of arginine mediated by the increased expression of BTN2. This supposition predicts that increased levels of Btn2p should cause a decrease in the degree of arginine uptake into btn1-Δ cells from the outside environment. However, this prediction is not borne out, as btn1-Δ and normal strains show similar rates of uptake for [14C]arginine at the plasma membrane (16). Up-regulation of Btn2p may therefore serve to modulate the intracellular level of arginine at a level necessary for normal cell survival. Such a hypothesis suggests that btn1-Δ strains have a requirement that they keep arginine levels diminished, or that btn1-Δ strains have a requirement for arginine uptake that is energetically too expensive for the cell to comply to. The fact that the elevated uptake of arginine shown by a btn2-Δ rsg1-Δ strain is suppressed by btn1-Δ strongly suggests that the absence of Btn1p leads a cell to keep intracellular levels of arginine and lysine down, for reasons yet to be ascertained. A key to understanding this may be determining the reason for vacuolar sequestration of basic amino acids. Further exploration of genetic interactions between btn1-Δ and amino acid utilization, and whether Btn2p is involved in the regulation of utilization of certain amino acids, may be informative. For example, the Schizosaccharomyces pombe homolog to Rsg1p, Rhb1p, has been independently shown to have a role in regulating response to nitrogen limitation (27).
as a protonophore can facilitate equilibration of pH across plasma and vacuolar membranes (26). Therefore an alternative interpretation of decreased vacuolar content, and transport of basic amino acids into the vacuole, may be that this is an elaborate mechanism that equilibrates the observed abnormally acidic pH in the vacuoles of btn1-Δ strains (15). There is precedent to suggest in btn1-Δ strains that a shift to balance intracellular levels of arginine may be occurring. BTN2, which encodes a coiled-coil protein, is up-regulated in btn1-Δ strains, and Btn2p has been shown to be necessary for the localization of Rsg1p, a downregulator of arginine uptake at the plasma membrane through the Can1p permease (16). Furthermore, increased expression of BTN2 has been shown to directly decrease arginine uptake into cells (16). Thus, as btn1-Δ strains have increased expression of BTN2, one could predict that btn1-Δ elicits a decrease in intracellular levels of arginine at both the vacuole and the plasma membrane. First, up-regulation of Btn2p may modulate uptake of arginine through interaction with Rsg1p, and second, there is a decrease in the vacuolar levels of arginine, which presumably is transported in from the cytosol. In addition, the decreased vacuolar levels of arginine may result directly from a decrease in uptake of arginine mediated by the increased expression of BTN2. This supposition predicts that increased levels of Btn2p should cause a decrease in the degree of arginine uptake into btn1-Δ cells from the outside environment. However, this prediction is not borne out, as btn1-Δ and normal strains show similar rates of uptake for [14C]arginine at the plasma membrane (16). Up-regulation of Btn2p may therefore serve to modulate the intracellular level of arginine at a level necessary for normal cell survival. Such a hypothesis suggests that btn1-Δ strains have a requirement that they keep arginine levels diminished, or that btn1-Δ strains have a requirement for arginine uptake that is energetically too expensive for the cell to comply to. The fact that the elevated uptake of arginine shown by a btn2-Δ rsg1-Δ strain is suppressed by btn1-Δ strongly suggests that the absence of Btn1p leads a cell to keep intracellular levels of arginine and lysine down, for reasons yet to be ascertained. A key to understanding this may be determining the reason for vacuolar sequestration of basic amino acids. Further exploration of genetic interactions between btn1-Δ and amino acid utilization, and whether Btn2p is involved in the regulation of utilization of certain amino acids, may be informative. For example, the Schizosaccharomyces pombe homolog to Rsg1p, Rhb1p, has been independently shown to have a role in regulating response to nitrogen limitation (27).
Although a mere depletion of arginine is unlikely to result in the neurologic consequences seen in Batten disease, it could no doubt provide an indicator as to what may be happening to cells lacking a functional CLN3. Interestingly, arginine levels in plasma of a cln3-knockout mouse model of Batten disease as compared with normal have been shown to be decreased at 3 months of age, although as the animals age, levels do plateau to normal (28). Similarly, 3-month-old cln3-knockout mice have decreased levels of arginine in the brain as compared with normal (not shown). Further studies that examine subcellular distribution of arginine in cells derived from the cln3-knockout mouse may provide a further correlation to altered amino acid levels in the lysosome. Such studies will not only provide a greater understanding of the biochemical nature of the CLN3 defect in Batten disease but also clarify potential differences in the function of the yeast vacuole and mammalian lysosome. For example, yeast vacuoles have long been known to sequester a variety of metabolites such as amino acids, and although active transport of amino acids into lysosomes has been demonstrated (29), an understanding of this process has yet to be achieved. A similar role for the lysosome has not been identified.
In summary, Batten disease is characterized by accumulation of lipopigments in the lysosome (5, 6), and we previously speculated that proteins usually targeted to the lysosome for degradation could either accumulate or aggregate because of the disturbance in vacuolar/lysosomal pH. It may be that both altered pH and perhaps altered levels of metabolites such as arginine alter the lysosomal environment so as to inhibit lysosomal function, leading to accumulation of storage material. Human CLN3 localizes to the late endosome/lysosome and in neuronal cells also to synaptic vesicles (30–32). Therefore in neurons an absence of CLN3 and the altered arginine levels we observe may alter lysosomal function and precipitate a disturbance in the trafficking of proteins implicated in neurotransmission, or disturb the metabolic balance responsible for synthesizing amino acid neurotransmitters, contributing to causation of Batten disease.
Acknowledgments
We thank Brian Vanwuykhuyse and Gurrinder Bedi (University of Rochester, Microchemical Protein/Peptide Core Facility) for amino acid analyses. We also thank Tim Curran for technical assistance during the course of this study. This work was supported by National Institutes of Health Grant R01 NS36610 and by the Luke and Rachel Batten Foundation.
References
- 1.Goebel, H. H. (1995) J. Child. Neurol. 10, 424–437. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, P., Dasgupta, A., Siakotas, A. & Dawson, G. (1992) Am. J. Med. Genet. 42, 549–554. [DOI] [PubMed] [Google Scholar]
- 3.Mole, S. E. (1998) Neurobiol. Dis. 5, 287–303. [DOI] [PubMed] [Google Scholar]
- 4.Weimer, J. M., Kriscenski-Perry, E., Elshatory, E. & Pearce, D. A. (2002) Neuromol. Med. 1, 111–124. [DOI] [PubMed] [Google Scholar]
- 5.Haltia, M., Rapola, L., Santavuori, P. & Keranen, A. (1973) J. Neurol. Sci. 18, 269–285. [DOI] [PubMed] [Google Scholar]
- 6.Koenig, H., McDonald, T. & Nellhaus, G. (1964) J. Neuropathol. Exp. Neurol. 23, 191–193. [Google Scholar]
- 7.International Batten Disease Consortium (1995) Cell 82, 949–957. [DOI] [PubMed] [Google Scholar]
- 8.Hall, N. A., Lake, B. D., Dewji, N. N. & Patrick, N. D. (1991) Biochem. J. 275, 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer, D. N., Fearnley, I. M., Walker, J. E., Hall, N. A., Lake, B. D., Wolfe, L. S., Haltia, M., Martinus, R. D. & Jolly, R. D. (1992) Am. J. Med. Genet. 42, 561–567. [DOI] [PubMed] [Google Scholar]
- 10.Lee, R. L., Johnson, K. R. & Lerner, T. J. (1996) Genomics 35, 617–619. [DOI] [PubMed] [Google Scholar]
- 11.Wilson, R., Ainscough, R., Anderson, K., Baynes, C., Berks, M., Bonfield, J., Burton, J., Connell, M., Copsey, T., Cooper, J., et al. (1994) Nature 368, 32–38. [DOI] [PubMed] [Google Scholar]
- 12.Pearce, D. A. (2001) Adv. Genet. 45, 205–216. [DOI] [PubMed] [Google Scholar]
- 13.Pearce, D. A. & Sherman, F. (1997) Yeast 13, 691–697. [DOI] [PubMed] [Google Scholar]
- 14.Croopnick, J. B., Choi, H. C. & Mueller, D. M. (1998) Biochem. Biophys. Res. Commun. 250, 335–341. [DOI] [PubMed] [Google Scholar]
- 15.Pearce, D. A., Ferea, T., Nosel, S. A., Das, B. & Sherman, F. (1999) Nat. Genet. 22, 55–58. [DOI] [PubMed] [Google Scholar]
- 16.Pearce, D. A. & Sherman, F. (1998) Proc. Natl. Acad. Sci. USA 95, 6915–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay, S. & Pearce, D. A. (2002) Eukaryot. Cell 1, 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urano, J., Tabancay, A. P., Yang, W. & Tamanoi, F. (2000) J. Biol. Chem. 275, 11198–11206. [DOI] [PubMed] [Google Scholar]
- 19.Ohsumi, Y., Kitamoto, K. & Anraku, Y. (1988) J. Bacteriol. 170, 2676–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohsumi, Y. & Anraku, Y. (1981) J. Biol. Chem. 256, 2079–2082. [PubMed] [Google Scholar]
- 21.Chattopadhyay, S., Muzzafar, N. E., Sherman, F. & Pearce, D. A. (2000) J. Bacteriol. 182, 6418–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okorokov, L. A., Kulakovskaya, T. V., Lichko, L. P. & Polorotova, E. V. (1985) FEBS Lett. 192, 303–306. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen, I. T., Sliwinski, M. K., Nelissen, B., Goffeau, A. & Saier, M. H., Jr. (1998) FEBS Lett. 430, 116–125. [DOI] [PubMed] [Google Scholar]
- 24.Kitamoto, K., Yoshizawa, K., Ohsumi, Y. & Anraku, Y. (1988) J. Bacteriol. 170, 2687–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gent, D. P. & Slaughter, J. C. (1998) J. Appl. Microbiol. 84, 752–758. [DOI] [PubMed] [Google Scholar]
- 26.Plant, P. J., Manolson, M. F., Grinstein, S. & Demaurex, N. (1999) J. Biol. Chem. 274, 37270–37279. [DOI] [PubMed] [Google Scholar]
- 27.Mach, K. E., Furge, K. A. & Albright, C. F. (2000) Genetics 155, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattopadhyay, S, Curran, T. M., McCall, K., Mooney, R. A. & Pearce, D. A. (2003) Clin. Chim. Acta 332, 145–148. [DOI] [PubMed] [Google Scholar]
- 29.Pisoni, R. L., Thoene, J. G., Lemons, R. M. & Christensen, H. N. (1987) J. Biol. Chem. 262, 15011–15018. [PubMed] [Google Scholar]
- 30.Jarvela, I., Sainio, M., Rantamaki, T., Olkkonen, V. M., Carpen, O., Peltonen, L. & Jalanko, A. (1998) Hum. Mol. Genet. 7, 85–90. [DOI] [PubMed] [Google Scholar]
- 31.Jarvela, I., Lehtovirta, M., Tikkanen, R., Kyttala, A. & Jalanko, A. (1999) Hum. Mol. Genet. 8, 1091–1098. [DOI] [PubMed] [Google Scholar]
- 32.Haskell, R. E., Carr, C. J., Pearce, D. A., Bennett, M. J. & Davidson, B. L. (2000) Hum. Mol. Genet. 9, 735–744. [DOI] [PubMed] [Google Scholar]