Abstract
During transcription elongation, RNA polymerase (RNAP) occasionally loses its grip on the growing RNA end and backtracks on the DNA template. Prokaryotic Gre factors rescue the backtracked ternary elongating complex through stimulation of an intrinsic endonuclease activity, which removes the disengaged 3′ RNA segment. By using RNA-protein crosslinking in defined ternary elongating complexes, site-directed mutagenesis, discriminative biochemical assays, and docking of the two protein structures, we show that Gre acts by providing two carboxylate residues for coordination of catalytic Mg2+ ion in the RNAP active center. A similar mechanism is suggested for the functionally analogous eukaryotic SII factor. The results expand the general two-metal model of RNAP catalytic mechanism whereby one of the Mg2+ ions is permanently retained, whereas the other is recruited ad hoc by an auxiliary factor.
Keywords: catalytic magnesium, Gre proteins
Prokaryotic transcription factors GreA and GreB have attracted much attention in the context of their role in the basic mechanism of elongation (1–7). The biochemical activity of Gre proteins is to effect internal cleavage of RNA during transcription elongation. In backtracked ternary elongating complex (TEC), RNA polymerase (RNAP) slides in the reverse direction (8–10), unwinding the DNA/RNA hybrid and the upstream DNA duplex and extruding a single-stranded 3′ RNA segment (Fig. 1A). The latter blocks the substrate binding site of the active center (11) so that TEC stalls, but it can be reactivated through the cutting of the disengaged 3′ RNA segment by nuclease activity intrinsic to the enzyme (12). Gre factors strongly stimulate the cleavage (1); however, their mechanism of action remains unknown.
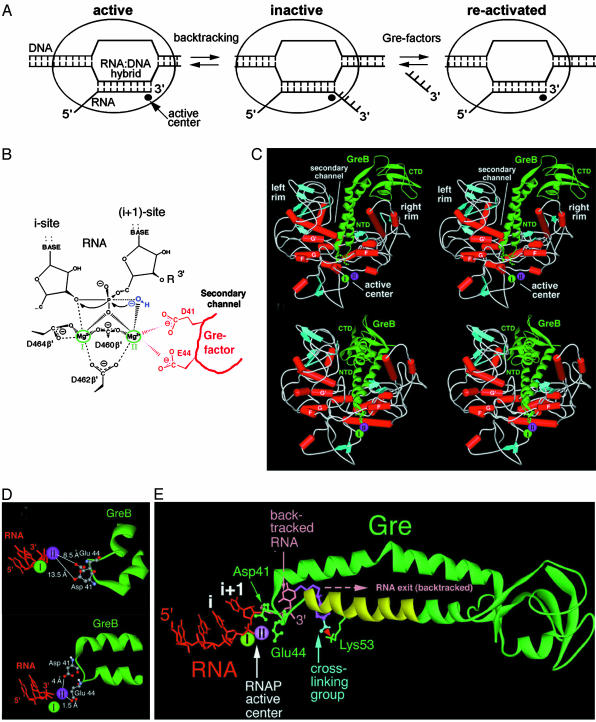
Fig. 1.
Mechanism of RNA cleavage in TEC assisted by Gre factors. (A) Schematic representation of TEC undergoing arrest and rescue. (B) The two-metal model of the active center (13) for the reaction of RNA cleavage assisted by Gre protein. RNA residues flanking the scissile phosphate occupy i and i + 1 sites of the active center. R symbolizes the extruded 3′ single-stranded RNA segment in backtracked TEC. Two Mg2+ ions required for catalysis are circled in green, coordinated by three Asp residues of RNAP β′-subunit. The attacking water molecule is shown in blue. The two acidic residues of Gre factors, which according to the present study recruit Mg-II, are represented in red. The relative orientation and distance between the interacting groups and residues is in accordance with x-ray structure and molecular modeling. (C) Docking of GreB factor into RNAP secondary channel (stereoview for crossed eyes) based on the low-resolution structure (14) of the Gre–RNAP complex (Upper) and the results of the present study (Lower). Green, GreB; white (segments with no particular secondary structure), RNAP (only relevant sections of β′-subunit are shown); red, α-helices; cyan, β-strands; light green, Mg-I; magenta, Mg-II. The residues Asp-41 and Glu-44 of Gre factor are shown in ball-and-stick representation. The helices are letter-marked reflecting the evolutionary conserved motifs of β′-subunit. The right and left rims represent the opposite sides of the rim of the secondary channel. (Upper) The N-terminal domain of Gre (NTD) is tilted ≈45° relative the bottom of the secondary channel, whereas the C-terminal domain (CTD) contacts the right part of the rim (β′ residues 960-1010; T. aquaticus numbering). (Lower) The NTD is nearly parallel to the bottom of the secondary channel, and CTD is submerged into the secondary channel. (D) Relative position of RNA, catalytic Mg2+ ions, and Asp-41, Glu-44 residues of Gre B in the active center of RNAP according to the present model (Lower) and the low-resolution cocrystal (Upper) (14). (E) Structural model of crosslinking of Gre factor to RNA in backtracked TEC. Green, GreB; red, RNA in RNA/DNA hybrid; pink, the disengaged 3′ terminal nucleotide; magenta, the crosslinking spacer; cyan, the crosslinking group; light green, Mg-I; light magenta, Mg-II. The catalytic residues of Gre factor D41 and E44 and the crosslinked Lys-53 are indicated. The red arrow indicates crosslink. The region of Gre where the crosslink in TEC from 3′ RNA terminus was mapped previously (19) is highlighted in yellow.
Previously, we suggested a unified catalytic mechanism based on nucleotidyl transfer reaction and involving the same active center for the reactions of phosphodiester bond formation and RNA degradation (13). In our model, the triad of conserved aspartate residues (D460, D462, and D464 in β′-subunit of RNAP Escherichia coli) coordinates two Mg2+ ions (Fig. 1B), one of which (Mg-I) is permanently retained in the enzyme, whereas the other (Mg-II), is bound transiently and has to be recruited ad hoc for each act of catalysis. In the polymerization reaction, Mg-II is recruited by the incoming substrate; in the exonuclease reaction it is recruited by a noncomplementary NTP; and in pyrophosphorolysis, by pyrophosphate. According to the model, Gre factors could stimulate RNA cleavage by affecting, directly or indirectly, recruitment of Mg-II in the backtracked TEC. Recently Opalka et al. (14) showed that GreB protein binds to RNAP in such a way that its protruding domain could reach the vicinity of the active center via the “secondary channel” in the RNAP structure, which normally serves as entry route for NTP substrates. However, the resolution of that analysis was insufficient to decipher precise contacts in TEC so as to distinguish between direct or allosteric effect of Gre. In this article we present experimental proof for direct action mechanism, an instance of material intervention by a regulatory factor into the workings of an enzyme's active site.
Materials and Methods
GreB Mutations. E41Q and D44N mutations were generated in pET15 derivative plasmid containing the greB gene with the His-6 tag at the 3′ terminus by using the QuikChange XLSite-Directed Mutagenesis Kit (Stratagene). His-6-GreB was purified as described (15), but mutant proteins were purified as WT GreB. The samples of WT and mutant GreB were loaded onto a HiTrap Chelating HP 5-ml column (Amersham Pharmacia Biosciences), and bound proteins were eluted in binding buffer supplemented with 500 mM imidazole. The eluates then were applied onto a 6-ml butyl-Toyopearl TSK 650M column (Supelco, Park Bellefonte, PA) and processed as described in ref. 15.
Transcriptional Complexes. Defined TECs were obtained by RNAP “walking” on the 5′ biotinylated T7A1 promoter fragment immobilized on neutravidin-coated agarose (Pierce). The 370-bp DNA-promoter fragment with 5′ biotin TEC was generated by PCR amplification from promoter-containing pTZ19 plasmid. The open promoter complex was obtained by mixing of a 1-μl suspension of neutravidin agarose with immobilized T7A1-promoter with 1 pmol of RNAP in 2 μl of transcription buffer (TB; 50 mM Tris·HCl, pH 7.9/100 mM KCl/10 mM MgCl2/1 mM 2-mercaptoethanol). The mixture was incubated for 10 min at 37°C and washed with cold TB (3 × 1 ml). To prepare TEC-13A with labeled penultimate residue, 10 μl of “start” mixture [10 μM ApUpC RNA primer (Oligos, Etc., Wilsonville, OR)/13 μM ATP/13 μM GTP (Amersham Pharmacia)] was added to obtain first TEC-11A stalled for the absence of the next substrate. After 2.5 min of incubation at 37°C, the suspension was washed with cold TB (6 × 1 ml), the volume was adjusted to 20–25 μl, and 2 μl of [α-32P]CTP (3,000 Ci/mmol, 3 μM; NEN Life Science Product) was added to the reaction. After a 5-min incubation at room temperature, the beads were washed with cold TB without MgCl2, followed by addition of ATP and MgCl2 to final concentrations of 25 μM and 1 mM, respectively, and subsequent incubation for 15 min at 0°C. The mixture was washed with TB (6 × 1 ml) and used in cleavage experiments. In the case of the internal labeling of RNA to the solution of open complex, the start mixture (see above) containing 1 μl of [α-32P]ATP (3,000 Ci/mmol, 3 μM; NEN Life Science Product) was added to obtain TEC-11A. Then the obtained TEC was walked by appropriate NTP to the desired register as described above. TEC14C was obtained by the same protocol but with [α-32P]CTP in 14 position. For crosslinking purposes, the reactive CTP analog (0.1 mM) was incorporated in the 3′ RNA terminus (with TEC labeled internally) during 30 min at 21°. The crosslink with reagent I was achieved by UV-irradiation at 254 nm during 3 min, with reagent II as described in ref. 16. Both reagents were synthesized by using protocols described in ref. 17.
Endonuclease Cleavage. For endonuclease cleavage, the sample of stalled [32P]-labeled TEC in 10 μl of TB without MgCl2 was diluted by 10 μl of TB containing different MgCl2 concentrations, and the mixture was incubated at 21°C. GreA- and GreB-stimulated cleavage was performed by using 10 nM factors (unless specified otherwise in Fig. 2). The reaction times were chosen to allow <20% of conversion (unless specified otherwise). The cleavage was stopped by adding 1 vol of the loading buffer (7 M urea/20 mM EDTA/0.25% bromophenol blue/0.25% xylene cyanol), and reaction products were resolved on a 20% PAGE (20:3). The radioactivity of the resolved products was determined by phosphorimaging and quantified with software from Molecular Dynamics.
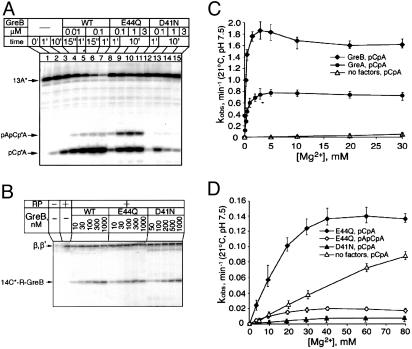
Fig. 2.
Properties of WT and mutant GreB factors. (A) Transcript cleavage activity. [32P]-labeled TEC-13 obtained as described above was incubated in the absence or in the presence of indicated concentrations of GreB in TB. The original 13-nt RNA as well as 2- and 3-nt cleavage product are marked. (B) Crosslinking of Gre factors in TEC-14 containing photoreactive group (reagent I, see Fig. 3B) attached to the 3′ terminal RNA residue. The position of the crosslinked GreB and RNAP β- and β′-subunits are indicated. (C and D) The rate of RNA degradation in TEC13 in the absence or in the presence of Gre at indicated concentrations of Mg2+.
Fe2+ Cleavage. The stalled TEC in TB without MgCl2 was supplemented with 20 μM of Fe(NH4)2(SO4)2 and 10 mM DTT. The reaction mixture was incubated for 10 min at 21°C, stopped by adding of 1 vol of the loading buffer (7 M urea/20 mM EDTA/0.25% bromophenol blue/0.25% xylene cyanol), and analyzed by 20% PAGE (20:3).
Modeling. Modeling was performed by using the program weblab viewerlight 4.0 (Molecular Simulations, Waltham, MA). The atomic coordinates for Thermus aquaticus RNAP and GreA factor from E. coli were from Protein Data Bank (PDB ID codes 1HQM and 1GRJ, correspondingly). The coordinates of the atomic model of Gre/RNAP binary complex were from Seth Darst (The Rockefeller University, New York).
Results
Gre Factors Strongly Increase the Retention of Mg-II at RNAP Active Center. To test the hypothesis about increased binding of Mg2+, we measured the rate of RNA cleavage in stalled TEC containing 13-nt RNA (TEC-13) in the presence or in the absence of GreA and GreB factors at different concentrations of Mg2+. Cleavage of the transcript in backtracked TEC-13 results in the accumulation of the pCpA product (Fig. 2 A, lanes 1–3), which is greatly stimulated by GreB (Fig. 2 A, lanes 4–7) or GreA (data not shown).
In the presence of GreA or GreB, the rate of hydrolysis sharply increased over the submillimolar range of Mg2+ concentrations, reaching a plateau above 3–5 mM (Fig. 2C). The apparent dissociation constant for Mg-II calculated from these data were in the range 0.2–0.3 mM for GreB (Table 1) and ≈0.5 mM for GreA (data not shown). In the absence of Gre, the increase of Mg2+ concentration up to 60 mM gave a linear response in the rate of RNA hydrolysis (Fig. 2 C and D, open triangles) reflecting an Mg2+ binding constant higher than 60 mM. Thus, Gre factors increase TEC's affinity for the weakly bound catalytic Mg2+ >200- to 300-fold.
Table 1. Kinetic parameters of intrinsic and Gre-stimulated endonuclease reaction catalyzed by the WT and mutant E813A, D814A RNAPs.
| Relative rate
|
||||||
|---|---|---|---|---|---|---|
| RNAP | GreB | Vmax, min-1 | At 10 mM MgCl2 | At 60 mM MgCl2 | Kd, nM (GreB) | Kd, mM (Mg2+) |
| WT | WT | 25-35*† | 2,000-3,500 | 330-580 | 170 | 0.2-0.3 |
| WT | E44Q | 0.14-0.16*‡ | 4-6 | 1.3-2 | 100 | 9-12 |
| WT | D41N | 0.3-0.6 × 10-2*‡ | 0.1-0.12 | 0.05-0.07 | 180 | 15-20 |
| WT | — | 1.0-1.3 × 10-2§ | 1 | 1 | — | >60 |
| ED/AA | WT | 3.4-4.6*† | 150-200 | — | 150 | 0.1-0.3 |
At saturating concentration of MgCl2.
Calculated based on the cleavage rate measured in the presence of 10 nM GreB and Kd (GreB).
The rate measured at 3 μM GreB. All measurements have been performed at pH 7.5, 21°C.
At 10 mM MgCl2.
Testing of the Allosteric Mechanism. The effect of increased Mg2+ binding in the presence of Gre could be either allosteric or direct, whereby Gre protein could directly donate residues for Mg2+ coordination. The notion of an indirect allosteric mechanism of Gre action was initially put forward in our previous communication based on the observation that destruction of a particular salt bridge within the RNAP molecule led, in the absence of Gre, to a conformational shift that made D814 residue of the β′-subunit available for additional coordination of Mg-II (13). By extension, it was not inconceivable that on binding, Gre induced the breakage of same salt bridge, thus making D814 available for coordination. To explore this possibility, we determined the Mg2+-dependence of the mutant RNAP carrying the double substitution D814A, E813A, which eliminates the potential coordinating residue(s). The apparent dissociation constant for Mg-II in the Gre-stimulated RNA cleavage reaction is the same for mutant and WT RNAP (Table 1), which argues against the allosteric mechanism based on the loss of the D814 salt bridge.
Docking of GreB into RNAP. From both the model of bacterial TEC (11) and the x-ray crystal structure of eukaryotic TEC (18), Gre can reach the active center only through the secondary channel. That GreB indeed can bind in the secondary channel follows from the recent low-resolution structure of RNAP–GreB binary complex (14).
However, binding of Gre in the vicinity of the nucleotide pore has to be reconciled with the view that RNA exits from the same pore during backtracking; intuitively one could imagine a steric clash. To explore this issue, we attempted to dock the high-resolution crystal structure of Gre protein to that of RNAP in the context of backtracked TEC, aiming to bring the two invariant acidic residues (Asp-41 and Glu-44) on the tip of Gre's protruding domain into a close proximity of Mg-II. The additional requirement was to provide enough room for RNA exit and to allow for the contacts of the backtracked RNA with Gre's “basic patch.”
It turned out that in the published model (Fig. 1C Upper) derived from low-resolution structure of the binary GreB–RNAP complex (14) the two proteins could not be docked so that Gre's tip reached Mg-II without major steric clashes; Asp-41 and Glu-44 could not be brought closer to Mg-II than 13.5 Å and 8.5 Å, respectively (Fig. 1D Upper), which is too far away for coordination. Moreover, when we attempted to introduce conformational changes into the rigid GreB structure so that Gre's tip could be squeezed deeper in the active center, the pore became completely blocked, not allowing for the exit of RNA (data not shown). Thus, the reported structure (14) is incompatible with the direct action mechanism.
However, we found that in another orientation, in which GreB was turned by ≈120° relative to RNAP, both the direct coordination of Mg-II by Asp-41/Glu-44 and the exit of the backtracked RNA were possible (Fig. 1C Lower). This assumed orientation of Gre protein in TEC is hereafter referred to as the working “direct-contact” structural model to distinguish it from the “no-contact” model derived from the low-resolution structure of binary complex (14).
RNA-GreB Contacts in TEC. A model of GreB–TEC complex obtained by docking, although instructive, is by no means conclusive. To see how close GreB comes to the active center in the actual GreB–TEC complex in solution, we resorted to RNA-protein crosslinking. Previously Koulich et al. (19) mapped a crosslink of the 3′ terminus within the segment between residues 47 and 63 of Gre's protruding domain (marked in yellow on Fig. 1E). However, for structural interpretation of a crosslink one needs to know the precise register of RNA, which can move back and forth because of backtracking.
To this end, we used Fe2+-mediated cleavage of RNA in TEC (9, 20), which occurs when the resident Mg-I in the active center is substituted with Fe2+. Hydroxyl radicals generated by coordinated Fe2+ cleave the phosphodiester bond between the i and i + 1 sites, leaving the phosphate attached to the 3′ terminus. The experiments were carried out with complexes TEC-12 and TEC-14, known to differ in their propensity to backtracking.
As can be seen from Fig. 3A, Fe2+-mediated cleavage in TEC-12 resulted in the removal solely of the 3′ terminal residue (lane 1), reflecting predominant positioning of the 3′ terminus in the i + 1 site. In other words, TEC-12 does not backtrack. By contrast, in TEC-14, three degradation products were observed (Fig. 3A, lane 8), reflecting an equilibrium whereby the 3′ terminus fluctuated between the i + 1 site and two backtracked positions, by 1 and 2 nt (Fig. 3A Upper). The same result was obtained with TEC-12 carrying crosslinking residues at 3′ RNA's termini (Fig. 3A, lanes 3–6) as well as in TEC-14 (data not shown). Hence TEC-12 and TEC-14 provide a system to assess RNA–GreB interaction in relation to backtracking or lack thereof.
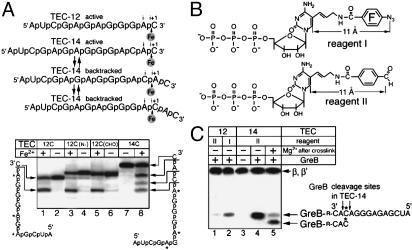
Fig. 3.
Crosslinking of GreB from the RNA 3′ terminus in TEC. (A Upper) Schematic representation of Fe2+-mediated cleavage reaction. Position of RNA in the active center (i and i + 1 sites) and the site of Fe2+ ion action are shown. The disengaged 3′ terminus is tilted to reflect the loss of base pairing through backtracking. (Lower) Electrophoretic analysis of Fe2+-mediated RNA degradation products in TEC-12 bearing crosslinking or natural cytidine residues at the 3′ terminus and natural TEC-14. The sequence of the RNA species is presented on the sides. The arrows connect cleavage sites with corresponding degradation products. (B) The structure of photoreactive nonspecific (reagent I) and Lys-specific (reagent II) substrate analogs used for crosslinking. (C) Electrophoretic analysis of GreB and RNAP crosslinking products in TEC-12 and TEC-14. The position of RNAP β- and β′-subunits as well as that for GreB with the attached RNA is marked. The vertical arrows show GreB-stimulated cleavage sites in crosslinked RNA, and the asterisk depicts the radioactive RNA residue.
Thus, we performed RNA-protein crosslinking in TEC-12 and TEC-14 by using two probes incorporated into the 3′ terminus: the nonspecific reagent I and Lys-specific reagent II (Fig. 3B). As can be seen from the radioautograph of the crosslinking products (Fig. 3C) both the β- and β′-subunits of RNAP and GreB protein crosslinked to RNA, providing internal reference to observed crosslinking to GreB.
In TEC-12, reagent II (Fig. 3C, lane 1) was much less efficient in crosslinking to GreB than reagent I (lane 2), reflecting the absence of Lys residues in the crosslink range of the probe (≈11 Å) in the nonbacktracked complex. Yet, the relatively strong crosslinking of the nonspecific reagent I indicates that the distance between the C5 carbon of the RNA base in the i + 1 site and the surface of GreB should be ≤11 Å. This experimental value agrees with the 7-Å distance between the C5 carbon and the carboxylate of Asp-41 in Gre B (Fig. 1 CLower and D Lower) in our assumed direct-contact model and is somewhat less than the distance of 12.6 Å in the no-contact model based on the low-resolution structure of binary complex (14) (Fig. 1 C Upper and D Upper).
In backtrack-prone TEC-14, Lys-specific reagent II gave strong crosslink to GreB (Fig. 3C, lane 4). The two Lys residues in the target 47–63 segment (19) (marked in yellow in Fig. 1E) are Lys-52 or Lys-53. To interpret this result in terms of the direct-contact model, one should allow both for deep penetration of GreB into the active center and RNA exit into the secondary channel, between the factor and the wall of the secondary channel as shown in Fig. 1E. Notably, in the context of this model, Lys-52 is unreachable, so only the crosslink to Lys-53 is shown. It should be emphasized that the crosslink to Lys-53 is compatible with both the direct-contact and the no-contact structures, so by itself it does not constitute proof of direct contact. The important point is that in the backtracked complex, the Lys-53 crosslink does not contradict the model whereby RNA can exit and GreB directly contact Mg-II at the same time.
That the Lys-specific crosslink in TEC-14 was indeed attributable to 1- to 2-nt backtracking was further verified by inducing RNA cleavage after crosslinking. To induce cleavage, we added Mg2+ to the precrosslinked complex. This induction had two effects (Fig. 3C, lane 5): (i) the appearance of a new crosslinked species caused by shortening of the tethered RNA tag and (ii) a significant loss of overall radioactivity (≈70%) in the crosslinked material, indicating that the major cleavage occurred between C12 and A13 (in this experiment, 32P-label was in C12). Thus, ≈70% of Lys-specific crosslinking in TEC-14 occurred from the complex backtracked by 1 nt as is shown in Fig. 1E.
Taken together, these results, although they do not constitute rigorous proof of direct contact, demonstrate that such a model is feasible in the functional i + 1 and backtracked GreB–TEC complexes in solution.
Substitution of the Conservative Carboxylate Residues at the Tip of GreB. The two GreB residues that, according to the direct-contact model, coordinate Mg-II are Asp-41 and Glu-44. To test their actual role, we engineered their substitution mutations with Asn and Gln, respectively. Both mutant proteins displayed significantly reduced capacity to stimulate RNA cleavage reaction (Fig. 2 A, lanes 8–11 and 12–15, respectively; note the different incubation times). This effect could not be attributed to reduced binding of the Gre protein as was demonstrated by a crosslinking experiment performed in TEC-14 with reagent I (Fig. 2B) at the 3′ terminus. Quantification of crosslinking at increasing concentrations of Gre factors (Fig. 2B) indicated that both WT and mutant GreB proteins bind to TEC with approximately the same affinity (see Kd values in Table 1), which was comparable to that determined previously for WT GreB (15). The loss of the stimulatory activity of GreB resulting from the two mutations was three to four orders of magnitude at the standard Mg concentration of 10 mM, and was two to four orders of magnitude at the saturating concentration of 60 mM (see relative reaction rates in Table 1). This decrease was in the same range that had been reported for mutations of carboxylate residues holding Mg2+ in the nuclease center of DNA polymerases (21). From the residual activity of E44Q and D41N mutants, we calculated the dissociation constant for Mg-II to be 9–12 mM and 15–20 mM, respectively, in contrast with the value of 0.2–0.3 mM value in the WT (Fig. 2D and Table 1).
Discussion
The principal conclusion from this work is that Gre proteins directly participate in the RNA cleavage reaction by donating acidic residues to coordinate catalytic Mg-II in RNAP active center. The following considerations argue in favor of this interpretation.
We found that GreB causes >3,000-fold increase in RNA cleavage rate (see Vmax values in Table 1). The bulk of this effect could be attributed to the increased retention (at least 200-fold, compare Kd with and without GreB in Table 1) of the weakly bound Mg-II. In principle, this effect could be either allosteric or direct. We ruled out the allosteric mechanism at least with regard to the particular model that we proposed previously, which attributed the Gre effect to the breakage of a salt bridge in RNAP molecule. The bridge involving the β-subunit residue D814 was eliminated by the double substitution D814A, E813A with no effect on the retention of Mg-II, which argues against the allosteric model.
On the other hand, the effect of the E44Q and D41N substitutions in the Gre protein on the retention of Mg-II strongly supports the direct mechanism of Gre action. The magnitude of the mutation's effect is comparable to that seen for the model complexes of the Mg2+ with low-molecular weight ligands. Thus, the dissociation constant of the metal ion with tridentate citrate anion (Cit3–) is 0.1 mM, whereas the same constant for bidentate oxalate (Ox2–) or tartrate (Tart2–) are ≈30 and 100 times higher, respectively (22). Substitutions E44Q and D41N decreased the retention of Mg-II in the GreB–TEC complex 30–60 times and 50–100 times, respectively (Table 1), strongly supporting the view that these residues directly participate in coordination of Mg-II.
It should be noted, however, that the observed decrease in retention of Mg2+ alone would not be sufficient to fully account for the much greater reduction of the GreB-stimulating effect caused by the mutations. One can imagine that the mutations could have an additional effect on the orientation of Mg-II in the active center and/or on the orientation of the attacking water molecule. For example, in DNA polymerase I, the same glutamate residue holds Mg2+ and coordinates the water through a hydrogen bond facilitating hydrolysis (23).
The crosslinking results presented in this article establish principal topological relationship between components of the GreB–TEC complex by demonstrating (i) close contact between GreB and the 3′ terminus of RNA in the nonbacktracked complex (TEC-12) and (ii) the ability of RNA to reach a more distant point on the “side” of GreB in the backtracked complex (TEC-14). In other words, whereas GreB binds so that it reaches the active center with its tip, it still leaves room for RNA 3′ terminus passage through the nucleotide pore and into the secondary channel.
Molecular modeling (docking) showed that such topology is incompatible with the no-contact model of TEC–GreB complex deduced from the reported low-resolution structure of the binary RNAP–GreB complex (14). However, the notion of direct action of Gre would entail no major steric conflicts once GreB rotates by 120° relative to RNAP as envisaged by our direct-contact model. Thus, the structure seen in the binary crystal could represent an intermediate state of GreB–RNAP complex on the way to the final fit involving rotation of GreB. One can speculate that GreB moves into the catalytic location when triggered by backtracked RNA and otherwise resides in the catalytically incompetent location where it does not interfere with nucleotide addition. Such transition could be a simple shift of rigid GreB in the secondary channel or involve some structural deformation of the protein molecule(s).
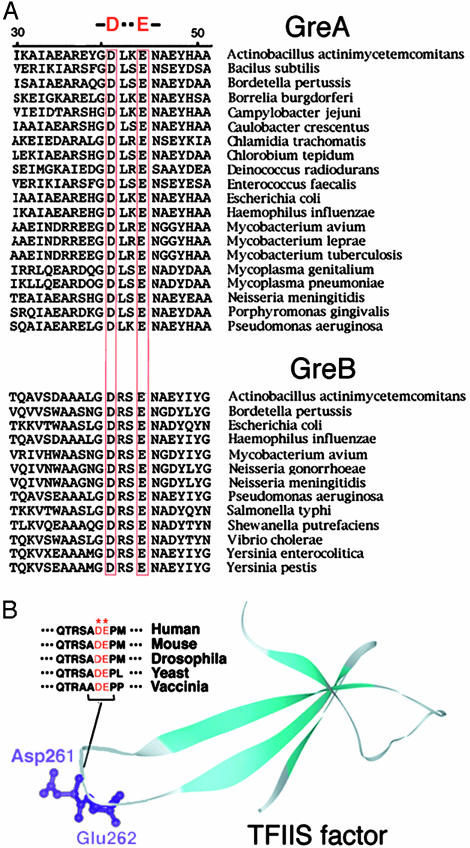
From sequence comparisons, Asp-41 and Glu-44 are evolutionarily invariant in both GreA and GreB protein families in prokaryotes (Fig. 4A). The eukaryotic analog of Gre factors is the elongation factor TFIIS (a.k.a., SII), which also acts through transcript cleavage (24, 25). There is no sequence homology between TFIIS and Gre. However, inspection of the three-dimensional structure of TFIIS (26) reveals an extended protrusion (Fig. 4B), which can be docked to the secondary channel of yeast Pol II the way it was done for Gre proteins. This protrusion also contains evolutionarily invariant aspartate and glutamate residues at the tip. It has been shown that substitution of these residues abolished TFIIS stimulation activity (27). We suggest that the mechanism of TFIIS action is the same as for Gre factors, despite their different evolutionary origins. While this manuscript was in revision, a paper by Cramer and coworkers (28) demonstrated that in cocrystal structure of yeast Pol II and TFIIS the conservative acidic residues in the tip of the factor reside in close proximity to the active center. This result together with the data of our article strongly suggest the direct mechanism for both factors. It is worth noting that in the model presented in that study, the tip of TFIIS and exiting RNA adopt a similar arrangement in the pore of secondary channel as in our model.
Fig. 4.
Evolutional conservation of the catalytic residues in transcript-cleavage factors. (A) Sequence alignment of Gre factors. D41 and E44 residues are marked (19). (B) Structure of TFIIS factor. Cyan, β-strands; gray, loops. Functionally important residues (Asp-261 and Glu-262) are shown in ball-and-stick representation and highlighted. The atomic coordinates were taken from Protein Data Bank (PDB ID code 1TFI). Sequence alignment for the residues constituting the tip of the functionally important loop of the factor is presented.
The demonstration of Gre's role in recruiting Mg2+ for RNA cleavage extends and reinforces the two-metal model of the active center that envisages different auxiliary factors bringing in Mg-II for different types of catalytic activity (13). For the reactions of polymerization, pyrophosphorolysis, and 3′ terminal hydrolysis of RNA, these cofactors are small molecules that coordinate Mg-II by their phosphates. The remarkable distinction of GreB is that it represents an instance when a protein factor directly donates residues for building up of a functional active site of another enzyme.
Acknowledgments
We are grateful to S. Darst for providing the coordinates of the Gre/RNAP atomic model before publication and for helpful discussion. We also thank K. Severinov (Rutgers University, Piscataway, NJ) for providing GreB coding plasmids. This work was supported by National Institutes of Health Grants GM49242 and GM30717 (to A.G.) and Russian Academy of Sciences Grant RFFI 0204-48525 (to V.N.).
Abbreviations: RNAP, RNA polymerase; TEC, ternary elongating complex; TB, transcription buffer.
References
- 1.Stebbins, C., Borukhov, S., Orlova, M., Polyakov, A., Goldfarb, A. & Darst, S. (1995) Nature 373, 636–640. [DOI] [PubMed] [Google Scholar]
- 2.Borukhov, S., Sagitov, V. & Goldfarb, A. (1993) Cell 72, 459–466. [DOI] [PubMed] [Google Scholar]
- 3.Nudler, E., Goldfarb, A. & Kashlev, M. (1994) Science 265, 793–796. [DOI] [PubMed] [Google Scholar]
- 4.Severinov, K. & Goldfarb, A. (1994) J. Biol. Chem. 269, 31701–31705. [PubMed] [Google Scholar]
- 5.Hsu, L., Vo, N. & Chamberlin, M. (1995) Proc. Natl. Acad. Sci. USA 92, 11588–11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toulme, F., Mosrin-Huaman, C., Sparkowski, J., Das, A., Leng, M. & Rahmouni, A. (2000) EMBO J. 19, 6853–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erie, D., Hajiseyedjavadi, O., Young, M. & von Hippel, P. (1993) Science 262, 867–873. [DOI] [PubMed] [Google Scholar]
- 8.Komissarova, N. & Kashlev, M. (1997) J. Biol. Chem. 272, 15329–15338. [DOI] [PubMed] [Google Scholar]
- 9.Nudler, E., Mustaev, A., Lukhtanov, E. & Goldfarb, A. (1997) Cell 89, 33–41. [DOI] [PubMed] [Google Scholar]
- 10.Reeder, T. & Hawley, D. (1996) Cell 87, 767–777. [DOI] [PubMed] [Google Scholar]
- 11.Korzheva, N., Mustaev, A., Kozlov, M., Malhotra, A., Nikiforov, V., Goldfarb, A. & Darst, S. A. (2000) Science 289, 619–625. [DOI] [PubMed] [Google Scholar]
- 12.Orlova, M., Newlands, J., Das, A., Goldfarb, A. & Borukhov, S. (1995) Proc. Natl. Acad. Sci. USA 92, 4596–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosunov, V., Sosunova, E., Mustaev, A., Bass, I., Nikiforov, V. & Goldfarb, A. (2003) EMBO J. 22, 2234–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opalka, N., Chlenov, M., Chacon, P., Rice, J. W., Wriggers, W. & Darst, S. A. (2003) Cell 114, 335–345. [DOI] [PubMed] [Google Scholar]
- 15.Loizos, N. & Darst, S. (1999) J. Biol. Chem. 274, 23378–23386. [DOI] [PubMed] [Google Scholar]
- 16.Grachev, M. A., Lukhtanov, E. A., Mustaev, A. A., Zaychikov, E. F., Abdukayumov, M. N., Rabinov, I. V., Richter, V. I., Skoblov, Y. S. & Chistyakov, P. G. (1989) Eur. J. Biochem. 180, 577–585. [DOI] [PubMed] [Google Scholar]
- 17.Langer, P., Waldrop, A. & Ward, D. (1981) Proc. Natl. Acad. Sci. USA 78, 6633–6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnatt, A. L., Cramer, P., Fu, J., Bushnell, D. A. & Kornberg, R. D. (2001) Science 292, 1876–1882. [DOI] [PubMed] [Google Scholar]
- 19.Koulich, D., Orlova, M., Malhotra, A., Sali, A., Darst, S. & Borukhov, S. (1997) J. Biol. Chem. 272, 7201–7210. [DOI] [PubMed] [Google Scholar]
- 20.Epshtein, V., Mustaev, A., Markovtsov, V., Bereshchenko, O., Nikiforov, V. & Goldfarb, A. (2002) Mol. Cell 10, 623–634. [DOI] [PubMed] [Google Scholar]
- 21.Derbyshire, V., Pinsonneault, J. & Joyce, C. (1995) Methods Enzymol. 262, 363–385 [DOI] [PubMed] [Google Scholar]
- 22.Lur'e, Yu. (1989) Handbook of Analytical Chemistry (Khimia, Moscow).
- 23.Beese, L. & Steitz, T. (1991) EMBO J. 10, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izban, M. & Luse, D. (1992) Genes Dev. 6, 1342–1356. [DOI] [PubMed] [Google Scholar]
- 25.Reines, D., Ghanouni, P., Li, Q. & Mote, J., Jr. (1992) J. Biol. Chem. 267, 15516–15522. [PMC free article] [PubMed] [Google Scholar]
- 26.Qian, X., Gozani, S., Yoon, H., Jeon, C., Agarwal, K. & Weiss, M. (1993) Biochemistry 32, 9944–9959. [DOI] [PubMed] [Google Scholar]
- 27.Jeon, C., Yoon, H. & Agarwal, K. (1994) Proc. Natl. Acad. Sci. USA 91, 9106–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kettenberger, H., Armache, K. & Cramer, P. (2003) Cell 114, 347–357. [DOI] [PubMed] [Google Scholar]