Abstract
Embryonic stem cell (ESC) differentiation is an excellent model to study chromatin changes at developmentally regulated loci. Differentiating mouse and human ESCs increase genome-wide acetylation (euchromatic) and tri-methylation (heterochromatic) of lysine 9 on histone H3. The Oct4 locus is euchromatic when expressed in undifferentiated ESCs and heterochromatic after differentiation. Brachyury T, a mesoderm-specific transcription factor, is not yet expressed in undifferentiated cells, where its locus has “bivalent” tri-methyl lysine 4 and lysine 27 modifications. During directed differentiation to pre-cardiac mesoderm, the activated brachyury locus has high levels of tri-methyl lysine 4 (euchromatin), switching to heterochromatin after gene silencing. Thus, ESC differentiation is accompanied by genome-wide commitment to euchromatin or heterochromatin. Undifferentiated hESCs bivalently modify the brachyury locus, activate it to euchromatin during mesoderm induction and subsequently repress it to heterochromatin, demonstrating, to our knowledge, the first analysis of chromatin dynamics at a locus essential for mesoderm and endoderm differentiation.
Keywords: Chromatin, Oct4, Brachyury, Histones, Electron Microscopy
Introduction
The development of a mammalian organism requires the establishment of hundreds of different cell types, each with specific gene-expression patterns (Li, 2002). Each distinct cell type retains the same genetic material as the original totipotent undifferentiated cell from which it arose. Thus epigenetic mechanisms, heritable influences on gene expression independent of DNA sequence, are required to explain this process (Li, 2002; Rasmussen, 2003). Chromatin changes such as DNA methylation and histone post-translational modification are epigenetic mechanisms implicated in mammalian development (Li, 2002). Locus-specific modifications on histones, such as acetylation and methylation, are proposed to form a code defining the potential transcriptional state of a cell (Turner, 2002). When such modifications are blocked, proper development cannot proceed. For example, targeted disruption of histone deacetylase-1 in mice results in embryonal lethality at E10.5(Lagger et al., 2002). Likewise, disruption of G9a, an enzyme producing de novo methylation of lysine 9 on histone H3, results in embryo death between E9.5 and E12.5(Tachibana et al., 2002). Knockdown of genes responsible for de novo (Okano et al., 1999) or maintenance(Lei et al., 1996) methylation of DNA also result in embryonic lethality.
While the observed embryonic lethalities demonstrate the importance of forming appropriate epigenetic patterns in development, embryonic stem cells offer a means to address these questions more mechanistically. The differentiation of both human (Thomson et al., 1998) and mouse (Nagy et al., 1993) embryonic stem cells in-vitro provides readily manipulable systems to study chromatin modifications during the establishment of new cell lineages and identities.
In the current study we sought to identify genome-wide and locus-specific modifications in chromatin that accompany embryonic stem cell differentiation. We found that differentiation resulted in a global increase of some of the histone modifications associated with both euchromatin and heterochromatin, consistent with a “locking in” of the differentiated phenotype. The Oct4 locus, which is active only in the undifferentiated cell, was associated with euchromatin modifications in pluripotent cells and with heterochromatin modifications in differentiated cells. The not-yet-expressed mesoderm-specific brachyury locus (Chris Showell, 2004) has an intermediate level of both euchromatin- and heterochromatin-associated modifications in the undifferentiated. When induced to form pre-cardiac mesoderm, we show that activation of the brachyury T locus during the earliest moments in human differentiation is accompanied by an increase in tri-methylation of lysine 4 on histone H3, followed by transcriptional silencing and high association with tri-methylated lysine 9 and 27 on histone H3.
Results
Transmission electron microscopy
Undifferentiated human (H7) and mouse (R1) embryonic stem cells as well as their embryoid body progeny were observed using transmission electron microscopy, noting the complexity of the population, the cytoplasmic-to-nuclear cross-sectional area, and the relative abundance of euchromatin and heterochromatin. The populations of undifferentiated human (figure 1a) and mouse (figure S1a) embryonic stem cells were striking in their cytoplasmic simplicity, the prominence of their nuclei and their relatively uniformity as compared to the more differentiated populations. The undifferentiated nuclei comprised a significant portion of the cross sectional area and were euchromatin-rich with prominent nucleoli (figures 1a and S1a). Undifferentiated mouse and human embryonic stem cells' scant cytoplasm was almost entirely composed of electron-dense rosettes of glycogen and the slightly less densely stained and smaller free ribosomes (figures 1b and S1b). In the human undifferentiated cytoplasm there were occasional lipid droplets (figure 1b) and mitochondria that generally had a simple structure with few cristae (figure 1a). The undifferentiated mouse embryonic stem cell cytoplasm (figure S1b) contained more mitochondria and less lipid but was otherwise similar to that of the human cells.
Figure 1. Ultrastructure of Undifferentiated and Differentiated Human Embryonic Stem Cells.
(A): A representative undifferentiated human embryonic stem cell with a large euchromatin-rich nucleus and scant cytoplasm. The nucleus contains a prominent nucleolus and strands and clumps of heterochromatin distributed throughout. Rare mitochrondria with simple structure and few cristae are present. (B): Undifferentiated cytoplasm is simple and primarily comprised of glycogen, free ribosomes and lipid droplets. Organelles are rare and generally less mature in structure. (C): A cell after 8 days of differentiation shows a significant increase in cytoplasm area. The nucleus is slightly condensed and contains more heterochromatin that is distributed in a diffuse granular pattern and a dense rim inside the nuclear envelope. (D): A typical differentiation day 8 cell contains far more complex cytoplasm than the undifferentiated cell, with increases in protein synthetic machinery including Golgi apparatus and rough endoplasmic reticulum. (E): A cell after 17 days of differentiation contains a nucleus with finely dispersed heterochromatin, a less prominent nucleolus and granular distribution with a dense rim. The cytoplasm comprises significantly more area than the undifferentiated cell's. (F): A group of cardiomyocytes after 17 days of differentiation contains highly specialized organelles, including contractile apparatus and desmosomes.
Abbreviations: Ds = Desmosome. Gly = Glycogen. MF = myofilaments. Nu = Nucleus. GA = Golgi Apparatus. RER = Rough Endoplasmic Reticulum.
During embroid body differentiation there were dramatic increases in the diversity and complexity of the cells. The nuclei of differentiating human embryonic stem cell (figures 1c and 1e) typically had more granular “salt and pepper” chromatin with a dense rim of heterochromatin at the nuclear periphery that was less prominent in the undifferentiated cells. In the differentiating mouse embryoid bodies (figures S1c and S1e) the nuclei became more varied, with both more heterochromatin and reduced cross-sectional area. The cytoplasm of differentiated human or mouse cells was more complex than that of the undifferentiated cells, showing extensive rough endoplasmic reticulum (RER) and Golgi apparatus (GA) (figure 1d), indicative of brisk synthesis and secretion of protein. The differentiating cells exhibited mitochondria that were more abundant and structurally mature (figure S1d). Later points in differentiation produced highly specialized cell types, such as human cardiomyocytes observable at Day 17 with myofibrils of contractile apparatus (MF) and desmosomes (DS) (figure 1f) or mouse cells with abundant clathrin coated pits (CCP) and rough endoplasmic reticulum (figure S1f).
Histone Tail Modifications During Embryonic Stem Cell Differentiation
A panel of histone tail modifications associated with euchromatin (acetyl lysine 9, mono-, di and tri- methyl lysine 4 on histone H3 and acetylated histone H4) or heterochromatin (mono-, di and tri- methyl lysine 9 and tri-methyl lysine 27 on histone H3) were probed by Western blot of whole cell lysates from a time course of human and mouse embryonic stem cell differentiation. Many of the chromatin modifications probed were present but showed no significant changes over the course of differentiation (data not shown). However, acetylation and tri-methylation of lysine 9 on histone H3 both showed significant genome-wide increases during differentiation as compared to levels found in undifferentiated pluripotent cells.
Acetylation of lysine 9 on histone H3, a modification associated with active euchromatin (Struhl, 1998), dramatically increased genome-wide from nearly undetectable levels as both human (Figure 2a) and mouse (Figure 2b) embryonic stem cells exited the pluripotent state. Lysine 9 acetylation was elevated by Day 4 of differentiation, reaching at plateau at Day 4 in mouse cells and Day 17 in human cells. Tri-methlyation of lysine 9 on histone H3, a functional component of silenced heterochromatin and binding partner for heterochromatin protein 1 (Lachner et al., 2001; Nakayama et al., 2001; Stewart et al., 2005), was almost undetectable in the pluripotent state of both human and mouse embryonic stem cells (figures 2c and 2d). In differentiating human embryonic stem cells lysine 9 tri-methylation rose steadily until Day 17 (figure 2c). The pattern in mouse embryonic stem cell differentiation was a more complex oscillatory pattern, with the overall moving average amount of the tri-methyl lysine 9 increasing (figure 2d), with a statistical significant increase at embryoid body day 8. The period of this oscillation was about 4 days (two feeding cycles.)
Figure 2. Genome-wide Increases in Histone H3 Tail Acetylation and Tri-Methylation During Embryonic Stem Cells Differentiation.
Graphs depict mean western blot band density normalized to both pan-histone H4 band intensity and each time-course's maximum observed density ± standard error. * = p < 0.05 by Student's t-test. Typical blot image are provided below each graph. (A): Acetyl lysine 9 on histone H3 is nearly undetectable in undifferentiated human embryonic stem cells and increases steadily from differentiation day 4 to 17. (B): Acetylated lysine 9 on histone H3 increases significantly during the first four days of mouse embryonic stem cell differentiation and remains consistently elevated thereafter. (C): Tri-methylation of lysine 9 on histone H3 is low in undifferentiated human embryonic stem cells and increases significantly during differentiation. (D): Tri-methylation of lysine 9 on histone H3 is low in mouse embryonic stem cells differentiation and shows an oscillatory but generally increasing level during differentiation.
Abbreviations: AK9 = Acetyl Lysine 9 on Histone H3. TMK9 = Tri-Methyl Lysine 9 on Histone H3. H4 = pan H4 normalization control. ESC = Embryonic stem cell.
The genome-wide and population-wide increases in opposing acetylation and trimethylaton of lysine 9 on histone H3 with differentiation, while other markers of euchromatin and heterochromatin showed no significant change during this time, suggests distinct meanings for these lysine 9 on histone H3 modifications in the resolution of developmentally regulated genes to either active euchromatin or silenced heterochromatin respectively.
Locus-specific histone tail modifications during “spontaneous” ESC differentiation
Recent studies have described “bivalent” modification, with both euchromatic trimethylation of lysine 4 and heterochromatic tri-methylation of lysine 27 on histone H3 present, of developmentally regulated loci in undifferentiated mouse and human embryonic stem cells (Azuara et al., 2006; Bernstein et al., 2006; Guenther et al., 2007; Spivakov and Fisher, 2007). We were curious if such modifications were present in undifferentiated cells at the brachyury T locus, because of the key role this transcription factor plays in mesoderm differentiation (Izumi et al., 2007). We therefore performed quantitative chromatin immunoprecipitation (ChIP) and compared changes at the brachyury locus to those at another developmentally regulated locus, Oct 4, a key transcription factor of pluripotent stem cells (Nichols et al., 1998). Antibodies recognizing euchromatin associated acetyl lysine 9 (AcK9) and tri-methyl lysine 4 (TMK4) on histone H3 as well as heterochromatin associated tri-methyl lysine 27 (TMK27) were all used in chromatin immunoprecipitation to determine the patterns of histone modification present.
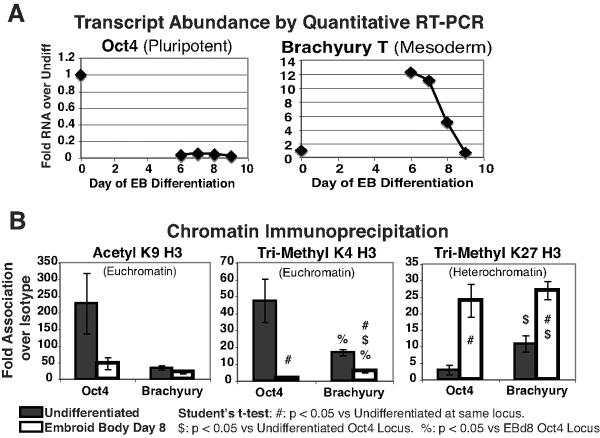
Analysis of mRNA expression by qRT-PCR demonstrated that, as expected, the Oct4 locus was vigorously transcribed only in the undifferentiated cell population, with a rapid decline during differentiation (Figure 3a). In the undifferentiated cells, Oct4 demonstrated features of an active locus. We observed very high levels of association with euchromatin markers such as acetyl lysine 9 (227-fold over IgG Control) and trimethyl lysine 4 (47-fold over IgG control), and low levels of association with the heterochromatin modification of tri-methyl lysine 27 (only 3-fold over IgG control). After eight days of embryoid body differentiation, the Oct4 locus showed changes characteristic of silencing. There was an 8-fold increase in association with the heterochromatin associated tri-methyl lysine 27 (24-fold over IgG control) compared to undifferentiated cells. In addition, there was a 33-fold reduction in tri-methyl lysine 4 and a 4.7-fold reduction in acetylated lysine 9 compared to the undifferentiated cells (Figure 3b). Thus, Oct4 locus was definitively active in the pluripotent state and definitively silenced in the differentiated state.
Figure 3. mRNA expression and histone tail modifications at the pluripotent Oct4 and mesodermal brachyury T loci.
(A): Transcript abundance in undifferentiated and differentiated human ESCs. qRT-PCR was performed to determine abundance of Oct4 or brachyury T transcripts in undifferentiated human ESCs or embryoid bodies differentiated for 6–9 days. Transcript abundance is presented as fold over that observed in undifferentiated cells. Oct4 transcript is abundant in the undifferentiated cells and largely absent after six or more days of differentiation. Brachyury T mRNA is not significantly expressed in the undifferentiated cells, peaks in abundance at approximately day 6 and declines to very low levels by day 9.
(B): Chromatin immunoprecipitation of undifferentiated human embryonic stem cells or day 8 embryoid bodies. Antibodies recognizing acetylated lysine 9 (euchromatin), trimethylated lysine 4 (euchromatin) or tri-methylated lysine 27 (heterochromatin) on histone H3 were used for precipitation. The resulting precipitate was amplified by quantitative PCR with primers for highly conserved regulatory regions of the Oct4 or brachyury T loci. Euchromatin marker acetyl lysine 9 on histone H3 is strongly associated with the highly expressed Oct4 locus in undifferentiated cells and minimally associated after differentiation. The brachyury locus (TSS+1300, near the translation start site) was minimally associated with acetylated lysine 9 in the undifferentiated or differentiated cells. Euchromatic tri-methyl lysine 4 on histone H3 has strong association with the highly transcribed undifferentiated Oct4 locus and minimal association after differentiation. In contrast, the brachyury locus showed intermediate levels of euchromatic tri-methyl lysine 4 in the undifferentiated state, followed by minimal association after differentiation. Heterochromatin marker tri-methyl lysine 27 on histone H3 is minimally associated with active Oct4 locus and strongly associated after silencing in differentiated cells. In contrast, the brachyury locus had intermediate levels of trimethyl lysine 27 heterochromatin modification in the undifferentiated state, followed by strong association after silencing during differentiation.
# = p < 0.05 as compared to the undifferentiated association at this same locus. $ = p < 0.05 as compared to the association with the undifferentiated Oct4 locus. % = p < 0.05 as compared to the assocation with the embryoid body day 8 differentiated Oct4 locus.
Abbreviations: K4 = Lysine 4. K9 = Lysine 9. K27 = Lysine 27. H3 = histone H3. Undiff = undifferentiated cell. EB = embroid body. qRT-PCR = quantitative reverse transcriptase polymerase chain reaction. PCR = polymerase chain reaction.
In contrast to Oct4, brachyury T transcript was not significantly expressed in the undifferentiated cells, showed increased expression at six days of embryoid body differentiation, and declined significantly by day 8 when samples were collected for chromatin immunoprecipitation (Figure 3a). We observed that in the undifferentiated population brachyury T had intermediate levels of both heterochromatin-associated trimethylation of lysine 27 and euchromatin-associated tri-methylation of lysine 4 on histone H3, a “bivalent” pattern similar to that described at other developmentally regulated loci in human and mouse embryonic stem cells. The methyl modifications were roughly halfway between those observed for Oct4 in the active vs. silenced states, suggesting the locus is poised for either rapid activation or silencing. Low levels of euchromatin-associated acetyl lysine 9 were observed at the brachyury T locus in the undifferentiated cell. After 8 days of differentiation the brachyury T locus was similar to the silenced Oct4 locus (Figure 3b), with high levels of the heterochromatin modification tri-methyl lysine 27 and relatively low levels of the euchromatin markers acetyl lysine 4 and tri-methyl lysine 4.
Expression of developmentally regulated genes during cardiac directed differentiation
With our observation of “bivalent” modification of the brachyury T locus in undifferentiated human ESCs, we wished to understand how this pattern is resolved during the transcriptional activation and subsequent silencing of this locus. Embryoid body differentiation cannot produce a pure enough population of brachyury T-expressing cells to permit a meaningful lysate-based chromatin immunoprecipitation during the activation phase. Our group has recently adapted a protocol that permits directed differentiation of human ESCs towards cardiomyocytes, employing the sequential addition of Activin A and BMP4 in defined media to a high density monolayer of undifferentiated H7 human embryonic stem cells (Laflamme et al., 2007). We hypothesized that this differentiation protocol would result in an initial direction of the population towards brachyury T-positive mesendoderm, and successful differentiation would come as the expense of Sox1-positive definitive ectoderm. We tested this hypothesis by analyzing messenger RNA samples by quantitative reverse transcription PCR (qRT-PCR) with primers amplifying Oct4 (pluripotent), brachyury T (mesendoderm), or Sox1 (ectoderm) transcripts. Following addition of activin A and BMP4, Oct4 transcripts rapidly decrease from levels observed in undifferentiated colonies or that observed in the high-density monolayer (day 0; figure 4). Brachyury T expression showed a sharp spike at day 2 with a rapid decline thereafter. As hypothesized, Sox1 was not significantly expressed at any time point during cardiac directed differentiation. In contrast, during a directed differentiation protocol towards retinal ectoderm (Lamba et al., 2006) we observed a significant spike in Sox1 expression, and low levels of brachyury T expression (data not shown). These data demonstrate that activin A/BMP4 treatment results in a population of highly enriched mesodermal cells expressing brachyury T.
Figure 4. RNA Abundance of Key Developmentally Regulated Loci During Cardiac Directed Differentiation of H7 Human Embryonic Stem Cells.
Human embryonic stem cells were plated into a high-density monolayer, switched to RPMI supplemented with B27 and sequentially exposed to Activin A and BMP4. RNA was collected from the cells in colony propagation (undifferentiated), on the day of induction (day 0), day 2, day 5, day 7, day 10 and day 14. SYBR Green quantitative reverse transcription PCR was performed with primers amplifying Oct4, Sox1 (ectoderm marker) or Brachyury T (mesoderm marker). Values are reported as fold over undifferentiated, with the left vertical-axis for Sox1 and Brachyury and the right vertical-axis for Oct4. Oct4 (open circles and a dashed line) rapidly declined with induction of differentiation, becoming nearly undetectable by day 5. Sox1 shows no significant expression at any time point observed. Brachyury T shows a spike of expression around two days after induction, followed by a rapid decline to nearly undetectable levels by seven days after induction.
Locus-specific histone tail modifications in cardiac mesoderm directed differentiation
To observe the chromatin changes required to activate expression at the brachyury T locus, we performed chromatin immunoprecipitation on the undifferentiated cells in a high density monolayer (day 0), mesendoderm-committed cells (day 2) and post-brachyury T differentiated cells (day 7) using antibodies recognizing euchromatic acetylated lysine 9 and tri-methylated lysine 4 on histone H3 as well as heterochromatic tri-methyl lysine 9 and 27 on histone H3. The precipitated DNA was quantitatively amplified using primers annealing to three locations in the brachyury T locus: about 800 base pairs upstream of the transcription start site, over the transcription start site and about 1300 base pairs downstream of the transcription start site (figure 5). To confirm the performance of the lysine 9 antibody, precipitates were also amplified with primers annealing to the heterochromatic ALU repeat. As expected, strong association at the ALU loci was observed with histone H3 Lysine 9 tri-methylation but not Lysine 27 trimethylation (supplemental figure S2).
Figure 5. Chromatin Changes During Activation of the Brachyury T Locus in Directed Differentiation.
Graphs depict fold increase in association over isotype control for the given modification. Error bars represent the standard error of the mean (n=3 for all data points). Gray bars are for undifferentiated cells in a high-density monolayer on the day of induction, White bars for brachyury T expressing cells two days after induction and black bars are for later differentiated cells no longer expressing brachyury T.
(A) Tri-methylation of Lysine 4 on histone H3. At all three sites in the locus there is a spike in euchromatic modification at 2 days, returning to low levels at day 7.
(B) Acetylation of Lysine 9 on histone H3. No clear trend in observable for this euchromatic modification.
(C) Tri-methylation of Lysine 9 on histone H3. There is a marked increase in this heterochromatic marker after 7 days, when brachyury T is no longer expressed.
(D) Tri-methylation of Lysine 27 on histone H3. This heterochromatin modification increases markedly at 7 days post induction, after brachyury T is no longer expressed.
All three locations in the brachyury locus showed a marked increase in euchromatic tri-methylation of lysine 4 on histone H3 at day 2 (figure 5a), which decreased significantly in the day 7 cells. Lysine 9 acetylation, another euchromatin marker, did not show a clear trend (figure 5b). Both of the heterochromatin-associated modifications, tri-methyl lysine 9 and lysine 27, showed large increases in abundance only in the post-brachyury T day 7 population of cells(figure 5c and 5d). At the brachyury T locus, this initial increase in a euchromatin modification, followed by increases in the heterochromatin modifications is consistent with the locus becoming open during transcription and tightly closed as the locus is transcriptionally silenced.
Discussion
The principle findings of this study are 1) Differentiation of human embryonic stem cells is accompanied by significant ultrastructual changes in both chromatin and cytoplasmic components; 2) Differentiation is associated with genome-wide increases in a subset of euchromatic and heterochromatic modifications, consistent with these histone tail modifications “locking down” large numbers of loci in the genome; 3) The human Oct4 locus, essential for maintenance of pluripotency, is strongly euchromatic in the undifferentiated state and heterochromatic after differentiation; 4) In the undifferentiated state the human brachyury T locus, essential for mesoderm formation, contains “bivalent” euchromatic and heterochromatic methylation modifications, which might poise it for rapid activation or silencing during differentiation; 5) The activated human brachyury locus is strongly associated with an euchromatin modification; 6) The silenced human brachury locus later in differentiation is strongly associated with heterochromatin modifications.
Previous to our work, studies of mouse embryonic stem cells revealed genome-wide increases in heterochromatin-associated tri-methyl lysine 9 on histone H3 with differentiation, as well as apparent blockade of differentiation by administration of histone deacetylase inhibitors (Lee et al., 2004). Our studies showed in both mouse and human embryonic stem cells that differentiation was accompanied by genome-wide increases of tri-methylation of lysine 9 on histone H3. Interestingly we observed a similar increase in euchromatin-associated acetylation at this same residue, suggesting different loci were associated with different modifications. Conversely, other modifications associated with euchromatin, such as tri-methyl lysine 4 or acetylated histone H4, or heterochromatin, such as tri-methyl lysine 27 did not show any significant genome-wide changes. The relatively reduced level of lysine 9 modification suggests that undifferentiated cells have not committed this residue to either activation or silencing at most developmentally regulated loci. It is only in association with differentiation, and the establishment of many distinct patterns of gene expression, that these definitive markers rise, suggesting a role for this modification in locking out inappropriate loci.
Investigators studying undifferentiated mouse ESCs by ChIP (Azuara et al., 2006) (Bernstein et al., 2006) reported a “bivalent” pattern of both activating tri-methyl lysine 4 on Histone H3 and silencing tri-methyl lysine 27 on histone H3 chromatin modifications at loci of to-be-expressed transcription factors such as Pax3 and Hox genes. Based on these results, we hypothesized that undifferentiated human ESCs would show bivalent modifications at the developmentally regulated brachyury T locus. Indeed, we show that bivalent modifications occur in human embryonic stem cells at the brachyury T locus, with both euchromatic tri-methylation of lysine 4 and heterochromatic tri-methylation of lysine 27 on histone H3 present in the undifferentiated cells. (Notably, we also observed the lack of acetylated lysine 9 on histone H3 at this locus in the undifferentiated cell, indicating specificity for the methylation modifications). Understanding how the brachyury T locus is activated and silenced during ESC differentiation will be important to understanding specification of the primary germ lineages.
The multitude of cell types generated in embryoid bodies initially limited our ability to determine modifications present at the activated brachyury T locus in early mes-endodermal cells. With a cardiac-mesoderm directed differentiation protocol we were able to observe, for the first time, the pattern of histone tail modification present at an activated human brachyury T locus and demonstrated a clear increase in tri-methylated lysine 4 on histone H3 accompanied transcriptional activation of the locus.
The remarkable biological ability of the embryonic stem cell to become any cell type in the body implies a unique epigenetic state. Embryonic stem cells offer a system to uncover key epigenetic changes during early human development. Current biochemical and molecular studies are limited by the complexity of cell types generated when embryoid bodies are induced to differentiate in the presence of serum. The ability to direct the differentiation of ESCs down restricted developmental pathways, e.g. to mesoderm-derived cardiomyocytes or ectoderm-derived neurons, will permit more detailed and more global analyses of epigenetic regulation during differentiation.
Experimental Procedures
Human Embryonic Stem Cell Culture
Undifferentiated H7 (WiCell) human embryonic stem cells were maintained on Matrigel-coated plates in mouse embryo fibroblast conditioned medium as previously described (Xu et al., 2001; McDevitt et al., 2005). Cells were used between passages 32 and 91. To differentiate as embryoid bodies, cells were digested with Collagenase IV (Invitrogen) and mechanically scraped off the plate into clumps and cultured in suspension for four days in the presence of fetal bovine serum (FBS; Biomedia) as previously described (Laflamme et al., 2005). After four days, the embryoid bodies were plated on gelatin-coated plates and maintained in adherent culture until collection as embryoid body outgrowths.
For directed differentiation, cells were replated into a twenty-four well plate at an initial density of 200,000 cells per well, grown to a confluent monolayer in conditioned medium and sequentially exposed to 50 ng/mL Activin A on day 0 and 10ng/mL of BMP4 on day one (both from R&D) in RPMI supplemented with B27 (Invitrogen) as previously described (Laflamme et al., 2007). Cells were subsequently fed every other day with RPMI supplemented with B27 until collection.
Mouse Embryonic Stem Cell Culture
Undifferentiated R1 mouse embryonic stem cells were maintained on gelatin-coated plates in DMEM supplemented with 15% FBS, L-glutamine, non-essential amino acids, monothiolglycerol and leukemia inhibitory factor (LIF) as recently described (Nussbaum et al., 2007). For embryoid body differentiation cells were digested to single cells with 0.05% trypsin in versene and transferred to differentiation media as above but without LIF, cultured overnight in 400-cell, 20 microliter hanging drops on the lid of a 150 mm tissue culture dish with 2 to 4 mL of PBS in the plate. Drops were collected and cultured in suspension for 6 more days in low-attachment plates. On Day 7 of differentiation, embryoid bodies were gravity settled for 5 minutes and plated on gelatin-coated 6-well plates. Embryoid body outgrowths were harvested at various times after differentiation as indicated in the Results.
Western Blot Analysis
Whole cell protein was collected by lysing cells with sample buffer (50mM Tris-HCl pH 7.8, 1% SDS, 10% glycerol) supplemented with Complete™ Protease Inhibitors Cocktail Tablets (Roche). Samples were homogenized with 10 strokes of a 22 gauge needle and quantified with a BCA (bicinchoninic acid) assay (Pierce). Western blots were prepared using 10 micrograms of total cellular protein lysate derived from a time course of embryonic stem cell differentiation and were probed with antibodies recognizing a panel of histone modifications: Acetylation of histone H4; acetylation of histone H3 on lysine 9; and mono-, di- and tri-methylation of lysine 4; and mono-, di- and tri-methylation of lysine 9 of histone H3. Blots were also probed with an antibody recognizing pan-histone H4 for normalization to histone protein abundance (all histone antibodies are from Upstate Biotech. Please see supplementary table S1 for dilutions, catalog and lot numbers).
Protein abundance was quantified by densitometry. To normalize the densitometry data we initially attempted to use a monoclonal pan-histone H3 antibody directed against the N-terminal tail. Our preliminary studies indicated there might be cleavage of the histone H3 tail early in mouse and human embryonic stem cell differentiation. Therefore, we instead normalized densitometry data with a pan-histone H4 antibody, taking advantage of the obligate dimerization of histone H3 and histone H4 in-vivo, and used a more gentle extraction protocol to protect the apparently delicate histone H3 tails.
Blots were read using an infra-red florescence scanner (Licor Oddessy system) with a goat-anti-rabbit Alexa-680 secondary antibody (Molecular Probes Lot#34788A at 1:10K). Antibodies were applied in a blocking solution of 0.1% casein in 0.2× PBS, supplemented with 0.1% tween-20 in the primary antibody and 0.1% tween-20 and 0.01% SDS with the secondary antibody. In order to account for changing proportions of nuclear protein to whole-cell protein with differentiation, densitometry values from modification-specific antibodies were normalized to the signal from a pan-histone H4 antibody for each sample followed by a second normalization to the highest intensity of the normalized specific modification observed in a given differentiation. A Student's t-test was used to determine statistical significance of changes as compared to the undifferentiated state.
Chromatin Immunoprecipitation
Samples were prepared and analyzed as recently described by Nelson et al. (Nelson et al., 2006). In brief, cells were cross-linked for 15 minutes at room temperature with 1.42% final concentration of formaldehyde. The cross-linking was quenched by the addition to 125 mM glycine, and cells were scraped off the plates. Cells were centrifuged (2000 × g for 5 minutes) and washed twice with cold PBS and stored at −80°C. Thawed pellets were lysed with IP buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5, 5 mM EDTA, 0.5% v/v NP-40, 1.0% v/v Triton X-100 in deionized water) supplemented with Complete™ Protease Inhibitors Cocktail Tablets (Roche). The nuclear pellet was collected after centrifugation (12,000 × g for 10 minutes), resuspended in IP buffer and sonicated for 6 rounds of 10 one-second pulses with half-second pauses at a power level of 5 (Misonix 3000 sonicator with a micro tip Cat#S3000). Sheared chromatin was distributed such that approximately 2×106 cells-worth was combined with an antibody recognizing acetyl lysine 9; tri-methylation of lysine 4, tri-methyl lysine 9; or tri-methly lysine 27 on histone H3 (Upstate Biotech. Supplementary table S2 for dilutions, catalog and lot numbers) or with an IgG control (Jackson ImmunoResearch Cat#011-000-002, 4 microliters for 44.4 micrograms.) Protein A sepharose beads (Amersham Cat#17-5280-01) were used to collect precipitated nucleosomes. After extensive washes with IP buffer, 100 microliters of 10% w/v Chelex® 100 Molecular Biology Grade Resin (Bio-Rad Cat#142-1253) beads were added to each sample, and the samples were boiled for 10 minutes after a brief vortex. Proteinase K (Invitrogen Cat# 25530-049, 1 microliter of 20 microgram per milliliter) was added and samples were digested 30 minutes at 55°C at 1200 RPM in a shaking heat block, and then boiled for an additional 10 minutes. Genomic DNA in the resultant samples was probed by powerSYBR Green (ABI) quantitative PCR. The signal for each primer pair in an immunoprecipitate was expressed as a fold-increase over the IgG control sample by calculating 2^(Ct(IgG) – Ct(X)). The primer sequences were: Oct4 (F: GACAGACAAACATCATCCCT. R: CTGGTCTAGTGCTTGATTCT) annealed at 56 °C. Brachyury T (TSS +1300) (F: CCAATGAGATGATCGTGACC. R: TTAACCAGAGCGGGAACA) annealed at 60 °C and signal collected at 87 °C; Brachyury T (TSS) (F: GAAAGCAATGACACAGCAGA R: AGGGAAATGGACGGAAATAAG) annealed at 60°C; Brachyury T (TSS − 800) (F: AATATCCATGCATCCCAGACA R: CTCTCCCTTCTCCAACTTACA) annealed at 60°C. All were amplified for 40 cycles.
qRT-PCR for Transcript Abundance
RNA was collected using the Qiagen RNeasy Mini kit (Cat# 74104). Samples were digested with RNAse-free DNAse. Isolated RNA was assessed for quality by agarose gel electrophoresis and quantified by spectrophotometry. One microgram of RNA per reaction was reverse transcribed by SuperScript II Reverse Transcriptase. Resultant cDNA was amplified with powerSybr qPCR master mix (ABI) using primers amplifying Oct4 (F: GGGTTCTATTTGGGAAGGTAT, R: TTCATTGTTGTCAGCTTCCT), Brachyury T (F: CAAATCCTCATCCTCAGTTTG, R: GTCAGAATAGGTTGGAGAATTG.) or Sox1 (F:GGAATGGGAGGACAGGATTT R: AACAGCCGGAGCAGAAGATA) All were annealed at 56 °C and run for 40 cycles and normalized to total RNA abundance.
Transmission Electron Microscopy
Cells were fixed and processed directly in the tissue culture dishes. Half-strength Karnovsky fixative (2% paraformaldehyde and 2% glutaraldehyde) in 0.1 M cacodylate buffer was applied for 12 hrs with subsequent overnight washes in the cacodylate buffer. 2% Osmium tetroxide post-fixation in the same buffer for 1 hr was followed by ethanol dehydration series and LR White resin infiltration series with a polymerization at 40°C for 72 hrs. Areas of interest were cut out and mounted on glass rods for ultrathin sectioning. Sections were post-stained with Reynolds lead citrate and 2% uranyl acetate and visualized with JEOL electron microscope.
Supplementary Material
(A): A representative undifferentiated mouse embryonic stem cell exhibits a large euchromatin-rich nucleus and a prominent nucleolus. There is scant cytoplasm that contains few organelles. (B): The undifferentiated cell's cytoplasm is glycogen rich. The occasional mitochondria present have a simple structure with few cristae. Other organelles were infrequently observed. (C): A typical differentiation day 8 cell has a large euchromatin-rich nucleus with a prominent nucleolus. The cytoplasm now comprises a more significant portion of the cross sectional area. (D): The cytoplasm of the differentiation day 8 cell has much more extensive networks of rough endoplasmic reticulum. The mitochondria have a more mature structure and organization than those observed in undifferentiated cells. (E): A differentiation day 14 cell shows more extensive heterochromatin and a dramatic increase in cytoplasmic cross-sectional area and complexity. (F): The cytoplasm of this differentiation day 14 cell exhibit clathrin coated pits and extensive networks of rough endoplasmic reticulum.
Abbreviations: CCP = Clathrin Coated Pit. Gly = Glycogen. Nu = Nucleus. Mt = Mitochondria. RER = Rough Endoplasmic Reticulum.
To validate the antibodies and quality of the immunoprecipitations, precipitated DNA from cells undergoing directed differentiation towards cardiomyocytes were amplified with a primer pair annealing to the ALU repeat or the GAPDH locus. Significant association with ALU is seen with tri-methylated lysine 9 on histone H3 but not trimethylated lysine 27 on histone H3. The increased association at day 7 may reflect the global increase in abundance of this modification.
The constitutively active GAPDH locus showed association with euchromatic acetylated lysine 9 and tri-methylated lysine 4 but not tri-methylated lysine 9 or lysine 27 on histone H3 at all timepoints.
Acknowledgments
The authors thank Julia Reece, Kira Bendixen and James Fugate for general technical assistance, Stephanie Lara and Steve MacFarlane for assistance in electron microscopy, and Joel Nelson and Karol Bomsztyk for assistance in chromatin immunoprecipitation.
Funding Supported in part by NIH grants HL-03174, HL-64387, HL-84682 and GM-69983.
JG was supported by NIH T32 HL-07312, Poncin Foundation, ARCS Foundation and the University of Washington Medical Scientist Training Program.
References
- Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, Fisher AG. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Showell Chris, OBFLC T-box genes in early embryogenesis. Developmental Dynamics. 2004;229:201–218. doi: 10.1002/dvdy.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi N, Era T, Akimaru H, Yasunaga M, Nishikawa S. Dissecting the molecular hierarchy for mesendoderm differentiation through a combination of embryonic stem cell culture and RNA interference. Stem Cells. 2007;25:1664–1674. doi: 10.1634/stemcells.2006-0681. [DOI] [PubMed] [Google Scholar]
- Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of Human Myocardium in the Rat Heart from Human Embryonic Stem Cells. Am J Pathol. 2005;167:663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. Embo J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hart SR, Skalnik DG. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis. 2004;38:32–38. doi: 10.1002/gene.10250. [DOI] [PubMed] [Google Scholar]
- Lei H, Oh SP, Okano M, Juttermann R, Goss KA, Jaenisch R, Li E. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development. 1996;122:3195–3205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. Journal of Molecular and Cellular Cardiology. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Sova P, Bomsztyk K. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34:e2. doi: 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 2007 doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen TP. Embryonic stem cell differentiation: a chromatin perspective. Reprod Biol Endocrinol. 2003;1:100. doi: 10.1186/1477-7827-1-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007;8:263–271. doi: 10.1038/nrg2046. [DOI] [PubMed] [Google Scholar]
- Stewart MD, Li J, Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol Cell Biol. 2005;25:2525–2538. doi: 10.1128/MCB.25.7.2525-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, Fukuda M, Takeda N, Niida H, Kato H, Shinkai Y. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Turner BM. Cellular memory and the histone code. Cell. 2002;111:285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A): A representative undifferentiated mouse embryonic stem cell exhibits a large euchromatin-rich nucleus and a prominent nucleolus. There is scant cytoplasm that contains few organelles. (B): The undifferentiated cell's cytoplasm is glycogen rich. The occasional mitochondria present have a simple structure with few cristae. Other organelles were infrequently observed. (C): A typical differentiation day 8 cell has a large euchromatin-rich nucleus with a prominent nucleolus. The cytoplasm now comprises a more significant portion of the cross sectional area. (D): The cytoplasm of the differentiation day 8 cell has much more extensive networks of rough endoplasmic reticulum. The mitochondria have a more mature structure and organization than those observed in undifferentiated cells. (E): A differentiation day 14 cell shows more extensive heterochromatin and a dramatic increase in cytoplasmic cross-sectional area and complexity. (F): The cytoplasm of this differentiation day 14 cell exhibit clathrin coated pits and extensive networks of rough endoplasmic reticulum.
Abbreviations: CCP = Clathrin Coated Pit. Gly = Glycogen. Nu = Nucleus. Mt = Mitochondria. RER = Rough Endoplasmic Reticulum.
To validate the antibodies and quality of the immunoprecipitations, precipitated DNA from cells undergoing directed differentiation towards cardiomyocytes were amplified with a primer pair annealing to the ALU repeat or the GAPDH locus. Significant association with ALU is seen with tri-methylated lysine 9 on histone H3 but not trimethylated lysine 27 on histone H3. The increased association at day 7 may reflect the global increase in abundance of this modification.
The constitutively active GAPDH locus showed association with euchromatic acetylated lysine 9 and tri-methylated lysine 4 but not tri-methylated lysine 9 or lysine 27 on histone H3 at all timepoints.