Inhibition of Pepck by NF-κB contributes to glyceroneogenesis suppression and adipogenic deficiency.
Abstract
We have reported that the nuclear factor-κB (NF-κB) induces chronic inflammation in the adipose tissue of p65 transgenic (Tg) mice, in which the NF-κB subunit p65 (RelA) is overexpressed from the adipocyte protein 2 (aP2) gene promoter. Tg mice suffer a mild lipodystrophy and exhibit deficiency in adipocyte differentiation. To understand molecular mechanism of the defect in adipocytes, we investigated glyceroneogenesis by examining the activity of cytosolic phosphoenolpyruvate carboxykinase (PEPCK) in adipocytes. In aP2-p65 Tg mice, Pepck expression is inhibited at both the mRNA and protein levels in adipose tissue. The mRNA reduction is a consequence of transcriptional inhibition but not alteration in mRNA stability. The Pepck gene promoter is inhibited by NF-κB, which enhances the corepressor activity through activation of histone deacetylase 3 (HDAC3) in the nucleus. HDAC3 suppresses Pepck transcription by inhibiting the transcriptional activators, peroxisome proliferator-activated receptor-γ, and cAMP response element binding protein. The NF-κB activity is abolished by Hdac3 knockdown or inhibition of HDAC3 catalytic activity. In a chromatin immunoprecipitation assay, HDAC3 interacts with peroxisome proliferator-activated receptor-γ and cAMP response element binding protein in the Pepck promoter when NF-κB is activated by TNF-α. These results suggest that HDAC3 mediates NF-κB activity to repress Pepck transcription. This mechanism is responsible for inhibition of glyceroneogenesis in adipocytes, which contributes to lipodystrophy in the aP2-p65 Tg mice.
Chronic inflammation in adipose tissue is a feedback response in obesity to compensate for adipose growth (1, 2). Adipose tissue growth demands an increase in the blood supply. When the blood supply cannot meet the demands of adipose tissue growth, a hypoxia response will occur in adipose tissue (1). The hypoxia response leads to chronic inflammation and adipocyte dysfunction (3, 4). Adipose tissue hypoxia induces inflammation through multiple channels, such as activation of the transcription factors [nuclear factor-κB (NF-κB), hypoxia-inducible factor-1α, and activator protein 1 (AP-1)] (3) and induction of endoplasmic reticulum stress (5, 6). We have reported that activation of NF-κB leads to adipose tissue growth inhibition and adipogenesis suppression (7). This NF-κB activity provides a molecular mechanism for proinflammatory cytokine TNF-α in the inhibition of adipose tissue function (8–10). We further investigated molecular events at the downstream of NF-κB in adipocytes in this study.
NF-κB is a master transcription factor in the inflammation signaling pathway (11). It is activated by many obesity-associated signals, such as proinflammatory cytokines, reactive oxygen species, hypoxia, endoplasmic reticulum stress, and endotoxin. As a transcriptional activator, NF-κB regulates gene expression through a direct binding to DNA. NF-κB is a heterodimer consisting of two subunits, p65 and p50. The p65 subunit that contains a transactivation domain is responsible for the transcriptional activity of NF-κB. To understand NF-κB activity in the pathogenesis of insulin resistance, we made a line of transgenic mice with overexpression of the p65 subunit in adipose tissue (7). The p65 expression driven by the 5.4 kb adipocyte protein 2 (aP2) gene promoter enhances the NF-κB activity in adipose tissue causing chronic inflammation in a fat-specific manner. The lean body mass is normal in aP2-p65 transgenic (Tg) mice, but fat mass is significantly reduced, which resembles a mild lipodystrophy. We have previously observed that adipogenesis is deficient in aP2-p65 mice, but the mechanism behind the deficiency remains unknown (7).
Glyceroneogenesis is required for triglyceride (TG) biosynthesis and free fatty acid (FFA) recycling in adipocytes (12). The phosphoenolpyruvate carboxykinase (PEPCK) cytosolic form (PEPCK-C) is a key enzyme in the control of glyceroneogenesis. Although glycerol is generated during TG hydrolysis, adipocytes are not able to recycle the glycerol during reesterification of FFA (12). Therefore, de novo glyceroneogenesis is the predominant source of glycerol in TG formation in adipocytes. Inactivation of Pepck gene in adipocytes results in lipodystrophy in mice (13). In contrast, overexpression of Pepck in adipocytes leads to adipose tissue hypertrophy and obesity in mice (14). Overexpression of Pepck enhances FFA reesterification, reducing FFA in the circulation (15). Induction of Pepck expression is a mechanism by which peroxisome proliferator-activated receptor (PPAR)-γ activators reduce plasma FFA (16). This activity of Pepck led us to investigate glyceroneogenesis in the molecular mechanism of lipodystrophy of aP2-p65 mice.
PEPCK-C is extensively investigated in the liver in the study of gluconeogenesis. Pepck transcription is controlled by many transcription factors, including PPARγ, cAMP response element binding protein (CREB), CCAAT/enhancer binding protein (C/EBP)-α and C/EBPβ, glucocorticoid receptor, forkhead box protein O1, sterol regulatory element binding protein, hepatocyte nuclear factor 4 (HNF-4), activating transcription factor 3, and AP-1 (Fos/Jun heterodimer) (17). PEPCK expression is regulated in a tissue-specific manner. Glucocorticoids increase PEPCK expression in the liver and kidney but decrease its expression in white adipose tissue (18). In adipocytes, PPARγ activates Pepck gene through two PPARγ binding sites (13, 19). It is not known how Pepck expression is regulated by NF-κB in adipocytes.
In the present study, we investigated Pepck regulation by NF-κB in adipocytes to understand the molecular mechanism of lipodystrophy of aP2-p65 mice. We report here that PEPCK enzyme activity was lower in aP2-p65 mice relative to the control mice. Pepck gene transcription was inhibited by NF-κB. Our data suggest that NF-κB inhibits Pepck transcription through an epigenetic pathway.
Materials and Methods
Animals
aP2-p65 mice were generated on the C57BL/6J background as described elsewhere (7). All of the mice were housed in the animal facility at the Pennington Biomedical Research Center with a 12-h light, 12-h dark cycle and constant temperature (22–24 C). The mice were ear punched for identification and were housed at four per cage. The mice had free access to water and chow (MF 5015, 11% fat; LabDiet, Richmond, Indiana). All procedures were performed in accordance with the National Institutes of Health guidelines for the care and use of animals and were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center.
Body weight and composition
Body weight and composition were measured after overnight fasting. Body composition was determined using quantitative nuclear magnetic resonance (NMR; Minispec Mn10 NMR scanner) made by Brucker (Milton, Canada) described previously (20).
Cells and reagents
Cell lines, including mouse 3T3-L1 (CL-173) and human HEK293 cells (CRL-1573), were purchased from the American Type Culture Collection (Manassas, VA). The supersuppressor inhibitory-κBα (ssIκBα) 3T3-L1 stable cell line was made by expression of Flag-ssIκBα, which was delivered by a pBabe retrovirus that carries the expression cassette (21). The cells were maintained in DMEM supplemented with 10% fetal calf serum. Chemicals and reagents including pCPT-cAMP (N6,2-O-dibutyryl cAMP; C-3912), TNF-α (T-6674), and trichostatin A (TSA; T8552), PD098059 (p-215), and SB203580 (s-8307) were purchased from Sigma (St. Louis, MO). Troglitazone (GR-210) was from Biomol (Plymouth Meeting, PA). c-Jun N-terminal kinase inhibitor SP600125 (EI-305) was purchased from Biomol (Plymouth Meeting, PA). Inhibitory-κB kinase (IKK) inhibitor 15d-PGJ2 (538927) was acquired from Calbiochem (San Diego, CA). Antibodies to SP3 (sc-644x), silencing mediator of retinoid and thyroid receptors (SMRT; sc-1610), PPARγ (sc-7373x), p65 (sc-8008), and polymerase II (Pol II; sc-9001) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to β-actin (ab6276), tubulin (ab7291), histone deacetylase 3 (HDAC3) (ab7030), and PEPCK (ab28455) were obtained from Abcam (Cambridge, UK).
Transfection and reporter assay
Transient transfection was conducted in human embryonic kidney (HEK) 293 cells. The Pepck-luciferase reporter (pA3-Pepck, −1500/+73) was a gift from Dr. Jianhua Shao (the University of Colorado Health Sciences Center, Denver, CO). The plasmid vectors for PPARγ, retinoid X receptor (RXR), HDAC3 interference RNA (RNAi), and P65 were described elsewhere (21). After transfection (48 h), the cells were treated with TNF-α (20 ng/ml) and/or troglitazone (10 μm) and cAMP (200 μm) in serum-free condition as described elsewhere (22). The cells were harvested 18 h later for the luciferase assay. In all of the transient transfection experiments, an internal control reporter (Simian virus 40 renilla luciferase) was used at 0.1 μg/well. The luciferase assay was conducted using a 96-well luminometer with the dual-luciferase substrate system (Promega, Fitchburg, WI). Relative luciferase activity was normalized to the internal control renilla luciferase activity. A mean value of the relative luciferase activity was presented. Each experiment was repeated at least three times.
3T3-L1 adipocyte and triglyceride quantification
3T3-L1 adipocytes were obtained by differentiation of preadipocytes in a standard adipogenic cocktail as described elsewhere (23). In the TG quantification assay, the cells were treated with TNF-α during the differentiation process. TG was extracted and quantified in the differentiated cells. The TG reading (OD) was normalized with cell numbers using the DNA amount (OD) from the same cell preparation.
Quantitative real-time RT-PCR (qRT-PCR)
TaqMan RT-PCR reaction was used to quantify mRNA. The total RNA was prepared from cell lysates or tissues with TRIzol reagent (Sigma, St. Louis, MO). The assay was conducted with on 7900 HT Fast real-time PCR System (Applied Biosystems, Foster City, CA). The target mRNA signal was normalized with ribosome 18S RNA. Pepck (Mm00440636_m1), Tnf-α (Mm00443258_m1), and p65 (Mm00501346_m1) primers and probes were from the Applied Biosystems.
Protein extracts and Western blotting
The protein preparation and the immunoblotting were described elsewhere (21, 24). Briefly, the whole-cell lysate was made in a lysis buffer under sonication, which breaks both cytoplasmic and nuclear membranes. Nuclear and cytoplasmic extracts were made by homogenizing tissue in a 0.25 m sucrose Tris-acetate (1 mm) buffer containing 1 mm dithiothreitol, 1 mm sodium orthovanadate, 1 mm EDTA, and a proteinase inhibitor cocktail (Roche 11697498001, Basel, Switzerland). After centrifugation at 2000 rpm (10 min, 4 C), the supernatant was aspirated and subjected to SDS-PAGE as the cytoplasmic extract, and the pellet was resuspended in sucrose buffer and subjected to SDS-PAGE as the nuclear extract. All of the immunoblotting experiments were conducted at least three times. The intensity of the protein signal was analyzed quantitatively using Image J software (National Institutes of Health, Bethesda, MD).
Lipogenesis
Lipogenesis was tested to indirectly determine PEPCK catalytic activity as described elsewhere (25, 26). In detail, the epididymal adipose tissue (200 mg) was cut into 20 mg/piece and incubated in 2 ml of glucose-free DMEM containing 3% fatty acid (FA)-free BSA and 5 mm pyruvate. After 4 h of TNF-α (20 ng/ml) treatment, the tissue medium was replaced by Krebs buffer containing 3% FA-free BSA and 5 mm pyruvate plus [1-14C]-pyruvate (0.5 μCi). The same assay was also conducted in 3T3-L1 adipocytes. Two hours later, lipid was extracted for quantification of [1-14C]-pyruvate incorporation into TG by radioactivity.
PEPCK enzyme assay
PEPCK-C enzyme activity was measured using a protocol described elsewhere (15). Epididymal adipose tissue (100 mg) was homogenized in a 0.25 m sucrose, 1 mm EDTA buffer (pH 7.4). The homogenate was then centrifuged for 20 min at 10,000 × g. The supernatant was removed and centrifuged for 8 min at 14,000 × g. The resulting supernatant was used in the enzyme assay after assessment of protein content using a standard BCA assay. One hundred microliters of the cell fraction containing PEPCK was added to 0.4 ml of warmed (37 C) assay mixture. The assay mixture contained 100 mm imidizaole-HCL (pH 6.6), 2 mm MnCl2, 15 mm phosphoenolpyruvate, 1.25 mm imidopiphospha, 20 mm potassium bicarbonate, 1 mm dithiothreitol, 2.5 mm NADH, 2 μCi NaH14CO3 per milliliter, and 2 U of malate dehydrogenase per milliliter. Reactions were incubated at 37 C for 10 min. Reactions were stopped by the addition of 40 μl of 50% trichloroacetic acid. Excess 14CO2 was removed by bubbling the reaction for 3 min with CO2. One hundred microliters of the reaction were analyzed for radioactivity.
Hematoxylin and eosin staining
Fresh tissues (epididymal fat) were collected at 16 wk of age after 12 wk on the chow diet. Tissue was fixed in 4% formalin solution (HT50-1-2; Sigma). Tissue slides were obtained by serial cross-section at 8 μm thickness and processed with a standard procedure.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was conducted according to the protocol published elsewhere (27). Cells were maintained in a 100-mm cell culture plate and treated with cAMP (200 μm), troglitazone (10 μm), or TNF-α (20 ng/ml) after overnight serum starvation. Chromatin DNA was extracted after the cells were treated with formaldehyde and sonication. Immunoprecipitation (IP) was performed with a specific antibody to HDAC3, SMRT, and Pol II. IgG was used as a negative control for nonspecific signal. DNA in the IP product was quantified using SYBR green real-time PCR with the ChIP assay primers. For the CREB binding site (−91/−84), the primers are −135-GTTCCAAACCGTGCTGACCA-115, and −33-ATATAGAAGGGAGGACAG-51. For the PPARγ binding site [PPARγ response element (PPRE); −999/−987], the primers are −1070AACTCCGACAAGCAAGCT-1052, and −914-AATGCCCAAGTGTCTGGAGAA-935. For the nonspecific control, the primers are −3981-AGTCTGTGGAACTTCTATTCT-3970, and −3847TCCGATCGTCATTGTCTTCTCCA-3870 in the PEPCK promoter DNA. The real-time PCR conditions were as follows: 2× iTaq SYBR Green super mix with ROX buffer (Bio-Rad Laboratories, Hercules, CA; catalog no. 170-8850), 500 nm of each primer, and 5 μl of purified ChIP extract in a 20-μl reaction.
Statistical analysis
Each experiment was conducted at least three times with consistent results. A representative Western blot, transfection results, and gels are presented in this manuscript. Digital data are presented as a mean value with se (sem). The data were analyzed using either Student's t test or one-way ANOVA with significance set at P < 0.05.
Results
Lipodystrophy in aP2-p65 mice
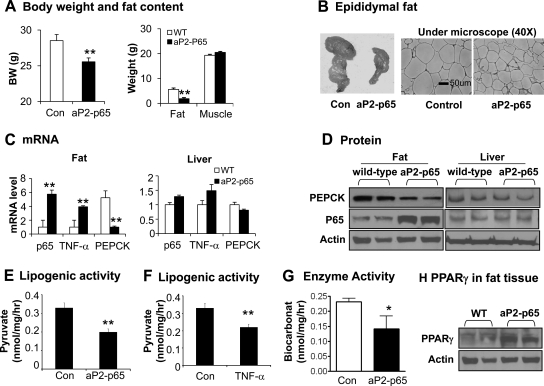
In the aP2-p65 mice, NF-κB activity is enhanced in adipocytes by overexpression of the p65 subunit. Tg mice suffer a mild lipodystrophy as suggested by a loss of white adipose tissue and reduction in adipocyte size (Fig. 1, A and B). These were observed in mice at 12 wk of age on the chow diet. Lipodystrophy led to the lower body weight in Tg mice (Fig. 1A). The lean body mass was unchanged in Tg mice relative to the wild-type control mice (Fig. 1A). Fat loss was not a result of less caloric intake because the food consumption was not decreased in the Tg mice (7). Adipogenic deficiency, reflected by a lower TG content in the differentiated preadipocytes of Tg mice, contributes to the lipodystrophy (7).
Fig. 1.
Lipodystrophy in aP2-p65 mice. A, Body weight (BW), fat, and muscle mass. Fat mass and muscle mass were determined by NMR. B, Epidermal fat pad. The tissue was collected at 12 wk on chow diet (16 wk of age) and used to make the tissue slide. After hematoxylin and eosin staining, the slide was photographed under a microscope at ×40 magnification. C, mRNA in fat and liver tissues. mRNA levels of p65, Pepck, and Tnf-α were determined by qRT-PCR in the epididymal fat and liver. D, p65 and PEPCK proteins in epididymal fat and liver. The protein was determined by Western blot. E, Lipogenesis. Pyruvate incorporation into TG was measured in the adipose tissue in culture and the activity is expressed in unit nanomoles per milligram per hour. F, Lipogenesis. The lipogenic activity was determined in adipose tissue after TNF-α (20 ng/ml) treatment for 4 h. The activity is expressed as nanomoles per milligram per hour. G, PEPCK enzyme activity in adipose tissue of aP2–p65 mice. The activity (nanomoles per milligram per hour) is derived from conversion of C14-labeled sodium bicarbonate into malate in epididymal white adipose tissue extracts. H, PPARγ protein in epididymal fat. In this figure data are represented as mean ± sem (n = 2–8). *, P < 0.05; **, P < 0.001.
To understand the molecular mechanism of adipogenesis deficiency in aP2-p65 mice, we examined representative genes that control TG biosynthesis. We found significant reduction in Pepck mRNA and protein in the white adipose tissue of Tg mice (Fig. 1, C and D). Pepck expression was not altered in the liver of Tg mice (Fig. 1, C and D). We measured TG biosynthesis in adipose tissue using radiolabeled pyruvate and observed a significant reduction in Tg mice (Fig. 1E). A similar reduction was observed in cells treated with TNF-α, which is used to activate NF-κB (Fig. 1F). We measured PEPCK enzyme activity in adipose tissue and found a 39% reduction in Tg mice (Fig. 1G), suggesting that the reduction in PEPCK activity accounts for the reduced TG biosynthesis in adipocytes.
PPARγ is a master transcriptional regulator of Pepck expression in adipocytes (13, 19). Pepck inhibition in the present study may be a result of decreased PPARγ expression or function. To test this possibility, we examined PPARγ protein expression in the adipose tissue by immunoblotting. PPARγ protein was not decreased but rather elevated in the Tg mice (Fig. 1H). Under normal conditions, increased PPARγ protein should lead to an enhanced Pepck expression; however, this was not observed in Tg mice, suggesting that PPARγ function is under inhibition in the adipose tissue of Tg mice.
PEPCK inhibition by NF-κB activator TNF-α
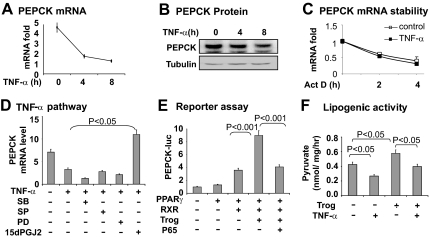
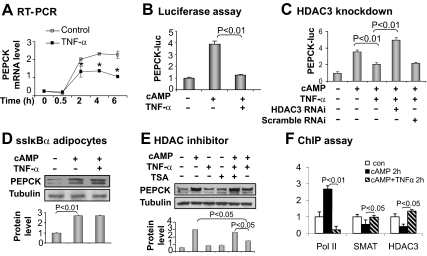
As a representative inflammatory cytokine, TNF-α inhibits Pepck expression in hepatocytes (22, 28, 29). However, it was not known whether TNF-α has the same activity in adipocytes. We tested this possibility using 3T3-L1 adipocytes, in which Pepck mRNA and protein were determined after treatment with TNF-α (Fig. 2, A and B). A time-dependent inhibition was observed. After 4 h of treatment with TNF-α, Pepck mRNA was reduced by 70% and protein by 40%. mRNA level is determined by transcription and mRNA stability. In hepatocytes the Pepck mRNA half-life is less than 1 h (30); however, it is more than 1 h in adipocytes (3T3-F442A) (26). In the present study, we found the Pepck mRNA half-life to be 2 h in 3T3-L1 adipocytes (Fig. 2C). The mRNA half-life was not changed by TNF-α treatment, suggesting that the decrease in Pepck mRNA is a result of transcriptional inhibition rather than a change in mRNA stability.
Fig. 2.
Pepck inhibition by TNF-α in adipocytes. A, Inhibition of Pepck mRNA expression by TNF-α in 3T3-L1 adipocytes. The cells were serum starved overnight and treated with TNF-α (20 ng/ml) for different times as indicated. The total RNA was extracted and subjected to qRT-PCR analysis for Pepck mRNA. B, Inhibition of PEPCK protein expression by TNF-α. The cells were serum starved overnight and treated with TNF-α (20 ng/ml). The PEPCK protein was determined in the whole-cell lysate in a Western blot with anti-PEPCK antibody. C, Influence of TNF-α on the Pepck mRNA stability. De novo mRNA expression was inhibited in 3T3-L1 adipocytes by actinomycin D (5 μg/ml). Then the cells were treated with TNF-α (20 ng/ml, filled square) for different times as indicated. The Pepck mRNA was quantified by qRT-PCR. D, TNF-α activity in 3T3-L1 adipocytes. The 3T3-L1 adipocytes were pretreated with the pharmacological inhibitors for 30 min. The inhibitors are SB203580 (SB; 10 μm), SP600125 (SP; 25 μm), PD98059 (PD; 40 μm), 15dPGJ2 (15 μm). PEPCK mRNA was determined 6 h later after addition of TNF-α. E, Blocking of PPARγ activity by p65. In this experiment, PPARγ and RXR were cotransfected with a Pepck -luciferase reporter. F, Lipogenesis for PEPCK activity in 3T3-L1 adipocytes. The pyruvate incorporation assay was performed in cells after treatment with TNF-α (20 ng/ml) or troglitazone (Trog; 10 μm) for 4 h. In this figure each bar represents mean ± sem (n = 3–8).
Inhibition of the Pepck gene promoter by NF-κB
To understand the transcriptional inhibition of Pepck, we examined the Pepck gene promoter activity using a luciferase reporter in transient transfection of HEK 293 cells. TNF-α reduced the luciferase activity, and the activity was blocked by pretreatment of cells with an IKK/NF-κB inhibitor (15dPGJ2) (Fig. 2D). The TNF-α activity was not affected by inhibitors to other serine kinases, such as p38, ERK, and c-Jun N-terminal kinase (Fig. 2D). The data suggest a role for NF-κB in the inhibition of Pepck transcription. NF-κB is a DNA-binding transcription factor that regulates gene transcription through a DNA-binding site in the target gene promoter. We analyzed the Pepck promoter DNA but did not identify a NF-κB site in the promoter region. This result suggests that NF-κB may inhibit Pepck expression through a nonconventional mechanism. Then we examined interaction between NF-κB and PPARγ for the inhibition. The Pepck promoter was induced by overexpression of PPARγ in a cotransfection assay (Fig. 2E). Cotransfection of NF-κB p65 led to a decrease in the PPARγ activity, suggesting that NF-κB antagonizes PPARγ activity in the Pepck promoter. The NF-κB activity led to inhibition of TG biosynthesis in 3T3-L1 cells (Fig. 2F).
HDAC3 in transcriptional inhibition of Pepck
Pepck transcription is regulated by cofactors (such as PPARγ coactivator 1α and transducer of regulated cAMP response element-binding protein) (31, 32) and corepressors [such as HDAC3 and sirtuin (SIRT)-1] (22, 33). Although HDAC3 inhibits Pepck transcription in hepatocytes (22), this activity of HDAC3 was not tested in adipocytes. We investigated the role of HDAC3 in adipocytes to understand the PPARγ inhibition by NF-κB. PPARγ activation led to Pepck mRNA expression in a time-dependent manner (Fig. 3A). In response to NF-κB activation, the PPARγ activity was completely inhibited as indicated by Pepck mRNA (Fig. 3A), confirming that PPARγ activity is inhibited by NF-κB.
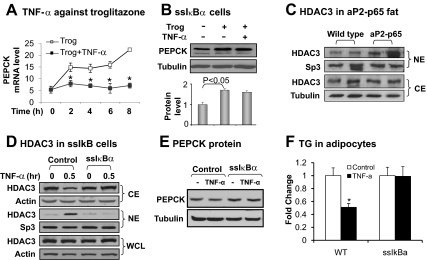
Fig. 3.
Inhibition of TNF-α activity in ssIκBα 3T3-L1 cells. A, Inhibition of troglitazone activity by TNF-α. The cells were treated with troglitazone (Trog; 10 μm) with or without TNF-α (20 ng/ml) for different times as indicated. Pepck mRNA was determined by qRT-PCR. B, Inhibition of TNF activity in ssIκBα-3T3-L1. The cells were treated with troglitazone (10 μm) with or without TNF-α (20 ng/ml) for 6 h. PEPCK protein was determined in the whole-cell lysate by Western blot. The PEPCK protein signal was determined by densitometry and normalized with tubulin protein level. C, HDAC3 in cytoplasm (CE) and nuclear (NE) fractions of epididymal white adipose tissue of control wild-type and aP2-p65 mice. HDAC3 protein was determined in the CE or NE by Western blot. Transcription factor Sp3 was a loading control for NE and tubulin was the loading control for CE. D, HDAC3 nucleus translocation. The CE and NE were made from ssIκBα or control adipocytes after TNF-α treatment for 30 min. HDAC3 protein was determined in the CE, NE, and whole-cell lysate (WCL) in an immunoblot. Sp3 was a loading control for NE and actin was the loading control for CE and the whole-cell lysate. E, PEPCK protein. In differentiated 3T3-L1 adipocytes, the PEPCK protein was examined after TNF-α treatment for 4 h. F, TG in differentiated 3T3-L1. Triglyceride was extracted and quantified in differentiated 3T3-L1 adipocytes, which were treated with TNF-α during differentiation. TG was expressed in fold change with activity in the vehicle control for 1. In this figure each bar represents mean ± sem (n = 3). *, P < 0.05.
When NF-κB activity is inhibited, the PPARγ activity was no longer suppressed. This relationship was observed in a 3T3-L1 stable cell line, in which NF-κB activation is prevented by ssIκBα (21). Inhibitory-κBα (IκBα) is a cytoplasmic protein that inhibits NF-κB activation by blocking its nuclear translocation. ssIκBα is a nondegradable form of IκBα generated through DNA mutation. The ssIκBα-expressing cells were responsive to the PPARγ activator for Pepck expression (Fig. 3B), but the induction was not inhibited by TNF-α (Fig. 3B). The data suggest that NF-κB activation is required for PPARγ suppression in adipocytes.
HDAC3 is a component of the nuclear corepressor, which represses transcriptional activities of both NF-κB and PPARγ (21, 27). HDAC3 is distributed in the nucleus and cytoplasm (34). Cytoplasmic HDAC3 enters the nucleus to enhance the corepressor function upon NF-κB activation (21). This HDAC3 activity provides a molecular mechanism for PPARγ inhibition by NF-κB. If this hypothesis is correct, HDAC3 activity should be elevated in the nucleus in Tg mice. To test this possibility, we compared HDAC3 in the adipose tissue of Tg mice and wild-type mice. Nuclear extract and cytoplasmic extract were made from epididymal fat and used in HDAC3 quantification in a Western blot. Tg mice had an increased abundance in nuclear HDAC3 (Fig. 3C), suggesting HDAC3 nuclear translocation in response to p65 overexpression. To test this possibility, we examined HDAC3 in the ssIκBα-stable cell line (Fig. 3D). The nuclear translocation was blocked by NF-κB inactivation in this cell line. HDAC3 protein expression was not changed in the whole-cell lysate (Fig. 3D). These data suggest that NF-κB induces HDAC3 nucleus translocation in adipocytes, and HDAC3 acts downstream of NF-κB to inhibit Pepck expression in adipocytes.
We examined PEPCK expression and triglyceride accumulation in the adipocytes. In the control 3T3-L1 adipocytes, PEPCK protein was reduced significantly by NF-κB activation from TNF-α treatment (Fig. 3E). In the ssIκBα adipocytes, the PEPCK reduction was completely blocked. Our hypothesis suggests that if NF-κB is activated during the process of adipocyte differentiation, TG accumulation should be inhibited in 3T3-L1 adipocyte from the glyceroneogenesis inhibition. The ssIκBα adipocytes should be protected from the inhibition. To tests this possibility, TG was quantified in differentiated 3T3-L1 cells that were treated with TNF-α during the differentiation process. A 50% reduction was observed in the control cells (Fig. 3F). In the ssIκBa cell, no reduction was observed. Those data suggest that inhibition of NF-κB protects adipocytes from glyceroneogenesis inhibition and lipid disorder.
HDAC3 inhibits Pepck transcription
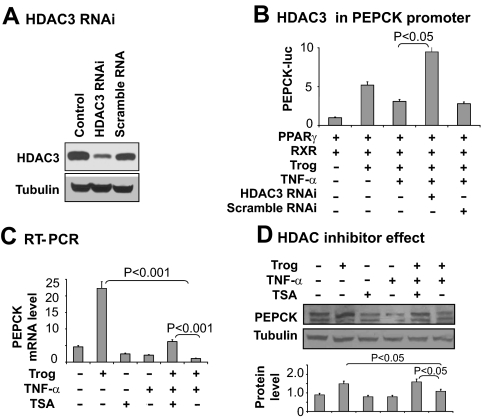
To determine the role of HDAC3 in Pepck inhibition, we used Hdac3 knockdown and a chemical inhibitor (TSA) of HDAC. Knockdown of Hdac3 was conducted with a vector-based RNAi expression vector. The HDAC3 protein was reduced by 90% in cells after the gene knockdown (Fig. 4A). The knockdown restored the Pepck promoter activity in the presence of NF-κB activation (Fig. 4B). Similarly, the HDAC inhibitor restored Pepck mRNA and protein in cells treated with TNF-α (Fig. 4, C and D). In these two models of HDAC3 inhibition, NF-κB remains activated in response to TNF-α (data not shown), but NF-κB failed to suppress Pepck expression. The data suggest that HDAC3 is required for Pepck inhibition by NF-κB.
Fig. 4.
Inhibition of TNF-α activity by HDAC knockdown or chemical inhibitor. A, HDAC3 protein in knockdown cells. HDAC3 protein was determined in whole-cell lysates of HEK 293 cells by Western blot 48 h after transfection with the HDAC3 RNAi plasmid. B, Restoration of Pepck promoter by HDAC3 knockdown. The assay was conducted in 293 cells. C, Restoration of Pepck mRNA by TSA. mRNA was determined using qRT-PCR after 4 h of treatment. D, Restoration of PEPCK protein expression by TSA. The mature 3T3-L1 cells were pretreated with TSA (50 nm) before troglitazone and TNF-α treatment. PEPCK protein was examined in whole-cell lysates by Western blot. The PEPCK protein signal was quantified and presented in the bar graph. In this figure each bar represents mean ± sem (n = 3).
HDAC3 interaction with the Pepck gene promoter
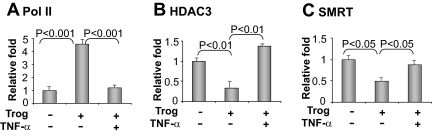
To understand the mechanism of HDAC3 action, we examined HDAC3-PPARγ interaction in the Pepck gene promoter in a ChIP assay. The assay was performed using a pair of PCR primers flanking the PPRE (−999/−987) in the Pepck promoter DNA. RNA Pol II was determined as a signal for transcriptional activation. When the gene promoter is inhibited, Pol II recruitment is reduced in the gene promoter. In the ChIP assay, the Pol II signal was enhanced by the PPARγ activator in the gene promoter (Fig. 5A). The corepressor signals for HDAC3 and SMRT were decreased at the same time (Fig. 5, B and C). In the presence of NF-κB activation, these responses were all inhibited (Fig. 5, A–C). Given the role of HDAC3 and SMRT in suppression of PPARγ function (21, 35–37), the data further support that HDAC3 mediates the NF-κB activity in inhibition of Pepck gene transcription.
Fig. 5.
ChIP assay for HDAC3-PPARγ interaction. Mature 3T3-L1 cells were treated with troglitazone (Trog) and TNF-α for 2 h. After cross-linking, the chromatin DNA was prepared for ChIP assay. Antibodies to Pol II, HDAC3, and SMRT were used in the immunoprecipitation. Rabbit IgG was used as a negative control for the antibodies. Chromatin DNA in the IP product was quantified by SYBR green quantitative PCR with primers for the PPRE element in the mouse Pepck gene promoter. The specific signal was normalized with the IgG signal. The signal change over untreated control was used to represent the fold change in ChIP signal. A mean value of three tests is presented: Pol II (A); HDAC3 (B); SMRT (C).
Inhibition of CREB activity by NF-κB
In adipocytes, CREB is another activator of Pepck in addition to PPARγ (17, 38). CREB induces Pepck transcription in response to glucagon, which activates CREB through the cAMP pathway (39). HDAC3 inhibits the CREB function in a study of gluconeogenesis in hepatocytes (22). In adipocytes, it is not known whether the HDAC3-CREB relationship plays a role in Pepck inhibition by NF-κB. This possibility was tested in 3T3-L1 adipocytes. CREB activity was induced by cAMP, which induced a time-dependent expression of Pepck mRNA in a 6-h study (Fig. 6A). When the HDAC3 activity was enhanced by TNF-α, the Pepck mRNA expression was inhibited in the time-course study (Fig. 6A), suggesting CREB inhibition by HDAC3.
Fig. 6.
CREB as a HDAC3 target in adipocytes. A, Inhibition of cAMP activity by TNF-α. Pepck mRNA was determined with qRT-PCR in 3T3-L1 adipocytes after treatment with cAMP (200 μm) with or without TNF-α (20 ng/ml). B, Inhibition of cAMP-induced Pepck promoter activity by TNF-α. The Pepck-luc reporter was inhibited in HEK 293 cells in the presence of TNF-α. C, Restoration of CREB activity by Hdac3 knockdown. The Pepck gene promoter was cotransfected with the HDAC RNAi expression vector at a 1:2 ratio in HEK 293 cells. Twenty-four hours after transfection, the cells were treated with cAMP and TNF-α overnight. D, Inhibition of TNF-activity in ssIκBα-3T3-L1. The cells were treated with cAMP (200 μm) with or without TNF-α (20 ng/ml) for 6 h. PEPCK protein was determined in whole-cell lysates by Western blot. E, Restoration of PEPCK protein expression by TSA. The mature 3T3-L1 adipocytes were pretreated with TSA (50 nm) before cAMP and TNF-α treatment. PEPCK protein was examined in whole-cell lysates by Western blot. F, ChIP assay of cAMP response element. ChIP assay was performed in 3T3-L1 adipocytes that were treated with cAMP and TNF-α for 2 h.
The HDAC3-CREB pathway was further tested using the Pepck luciferase reporter. The Pepck promoter was activated by the cAMP treatment of cells. In the presence of TNF-α, the cAMP-induced promoter activity was completely inhibited (Fig. 6B). In this system, the reporter activity was restored by HDAC3 knockdown (Fig. 6C) or NF-κB inhibition (Fig. 6D). A similar result was observed in this system when the HDAC inhibitor (TSA) was used (Fig. 6E).
In the ChIP assay, we observed HDAC3-CREB interaction at the cAMP response element (−91/−84) in the mouse Pepck gene promoter. In response to cAMP, the Pol II signal was increased, and the corepressor HDAC3/SMRT signals were decreased (Fig. 6F). The cAMP activity was blocked by TNF-α (Fig. 6F). These data suggest that HDAC3 inhibits CREB at the cAMP response element in the Pepck gene promoter in adipocytes.
Discussion
This study helps us to understand the cellular mechanism of adipose tissue dysfunction in obesity. Adipose tissue dysfunction contributes to metabolic disorders in obesity. Inflammation is a key factor in the pathogenesis of adipose tissue dysfunction. However, it is not clear how Pepck gene plays a role in the inflammation pathway. Pepck gene is important in the control of fat storage in adipose tissue as shown in several excellent transgenic studies (13–15). Overexpression of Pepck in adipose tissues induces TG accumulation in adipocytes and promotes adipose tissue hypertrophy in mice (14, 15). Inhibition of Pepck expression in adipocytes reduces adipocyte size and decreases fat mass in mice (13). Those studies suggest that inhibition of Pepck activity may contribute to adipose tissue dysfunction in obesity.
In an early study, we reported that PEPCK expression is inhibited by inflammation in hepatocytes (22). In this study, we tested this possibility in adipocytes to understand the adipogenic deficiency in the aP2-p65 Tg mice, which suffer chronic inflammation in adipose tissue (7). The PEPCK activity was reduced in adipose tissue of this Tg mice. The data consistently suggest that the reduction occurs at the transcriptional level in adipocytes, which leads to the decrease in protein and enzyme activity of PEPCK. These alterations were observed with glyceroneogenic deficiency, which explains the adipogenic deficiency in the phenotype of aP2-p65 mice (7). The deficiency in glyceroneogenesis and adipogenesis provide a cellular mechanism for lipodystrophy in the Tg mice. Energy expenditure may play a role in the reduced fat mass in the Tg mice (7). It may not influence preadipocyte differentiation in vitro. In the aP2-p65 mice, NF-κB activation in nonadipocytes may contribute to the elevated energy expenditure. In addition to adipocytes, macrophages and epithelial cells also express aP2 gene activities (40, 41).
In the Pepck gene regulation, there is abundant literature about the transcriptional activators and coactivators (17, 38). However, there is little information regarding the transcriptional repressors and corepressors of Pepck (22, 33). PEPCK is predominantly expressed in hepatocytes, adipocytes, and renal cells. The expression is regulated both transcriptionally and posttranscriptionally (42). The transcription is induced by hormones (glucocorticoid and glucagon) and metabolites (FA) in a tissue-specific manner. In hepatocytes, the transcriptional activators include CREB (for glucagon), glucocorticoid receptor (for glucocorticoid), C/EBPα (for FFA), HNF-4, and forkhead box protein O1. In adipocytes, PPARγ and CREB are the major transcriptional activators. The transcriptional coactivators for Pepck include cAMP response element-binding protein/P300 (43), transducer of regulated cAMP response element-binding protein 2 (44–46) and PPARγ coactivator-1α (31, 32), which promote acetylation of histone proteins in the transcriptional activation of Pepck. The nuclear corepressors include HDAC3 and SIRT1, which inhibit CREB (22) and HNF-4, respectively (33). Currently all of the information on corepressors is derived from hepatocytes, and it is not clear whether the corepressors act in the same way in adipocytes. This issue is addressed in the current study. Our data suggest that HDAC3, in concert with SMRT, inhibits Pepck expression in adipocytes through suppression of the transcriptional activities of PPARγ and CREB.
HDACs are divided into three classes: class I HDACs (HDAC1, 2, 3, and 8), class II HDACs (HDAC4, 5, 6, 7, 9, and 10), and class III (SIRT1-7). In class I, HDAC3 is a 50-kDa protein (428 amino acids) with both a nucleus localization signal (313/428 amino acids) and a nucleus export signal (180/313 amino acids) (34). HDAC3 usually forms a corepressor complex with SMRT (21, 35–37). HDAC3 activity has been studied in knockout (KO) mice. Global Hdac3 KO is embryonic lethal at embryonic d 9.5 (47, 48). Liver-specific Hdac3 KO leads to fatty liver and small body size in KO mice (49). Heart-specific Hdac3 KO leads to heart hypertrophy with lipid accumulation (47). The molecular mechanism of these phenotypes is not known. However, an increase in PPARγ activity may play a role in organ-specific lipid accumulation in the mice. Pparγ mRNA is increased in the KO organs, and this change may promote TG accumulation by stimulating Pepck expression. Hdac3 inactivation also promotes the ligand-dependent activity of PPARγ. The current study suggests that HDAC3 is able to regulate glyceroneogenesis through epigenetic inhibition of PPARγ and CREB. We provide a molecular mechanism by which Hdac3 regulates lipid metabolism. HDAC3 also inhibits other transcription factors, such as AP-1 (50, 51), myocyte enhancer factor 2 (52), thyroid hormone receptor (53), and signal transducers and activators of transcription (54). Interaction of HDAC3 with those transcription factors may contribute to the Pepck inhibition by NF-κB. This possibility remains to be tested.
The HDAC3 activity provides a new mechanism for the inhibition of TG biosynthesis by TNF-α in adipocytes (55). The TNF-α activity is dependent on IKK2/NF-κB, and the NF-κB activity is demonstrated in aP2-p65 mice (7). Although NF-κB inhibits PPARγ activity, this activity was not reported previously in the inhibition of glyceroneogenesis. We report here that NF-κB inhibits glyceroneogenesis through suppression of PPARγ. Although NF-κB is a DNA-binding protein, NF-κB is able to regulate Pepck through a DNA-independent mechanism as shown in current study. This conclusion is consistent with our previous study in which NF-κB does not change DNA-binding activity of PPARγ (21). PPARγ function is regulated at multiple levels, such as expression, phosphorylation, and cofactors (55). Here PPARγ regulation by corepressors is investigated in the control of Pepck transcription in adipocytes.
In summary, our data suggest that inhibition of Pepck by NF-κB contributes to glyceroneogenesis suppression and adipogenic deficiency. The underlying mechanism of NF-κB action involves HDAC3 activation and suppression of the transcriptional activities of PPARγ and CREB at the Pepck gene promoter. HDAC3 interacts with SMRT in the regulation of the Pepck gene promoter. The study suggests that Pepck inhibition by inflammation is a mechanism for adipose tissue dysfunction and lipid disorders in obesity. This concept may apply to the pathogenesis of several inflammation-associated diseases, such as metabolic syndrome, type 2 diabetes, cardiovascular disease, and atherosclerosis.
Acknowledgments
We highly appreciate technical support from Ms. Xin Ye and Ms. Tianyi Tang.
This work was supported by National Institutes of Health (NIH) Grants R56DK068036 and DK085495 and American Diabetes Association Research Award 7-07-RA-189 (to J.Y.), American Diabetes Association Award 1-09-JF-17 (to Z.G.), and NIH T32 Award 5T32DK064584-08 (to T.M.H.). The qRT-PCR test, metabolic phenotyping, and imaging studies were conducted in the genomic core, phenotyping core, and imaging core that are supported by the NIH Grants 1P30 DK072476 and P20-RR021945.
Disclosure Summary: The authors have no conflict of interest to declare.
Footnotes
- AP-1
- Activator protein 1
- aP2
- adipocyte protein 2
- C/EBP
- CCAAT/enhancer binding protein
- ChIP
- chromatin immunoprecipitation
- CREB
- cAMP response element binding protein
- FA
- fatty acid
- FFA
- free fatty acid
- HDAC3
- histone deacetylase 3
- HEK
- human embryonic kidney
- HNF-4
- hepatocyte nuclear factor 4
- IκBα
- inhibitory-κBα
- IKK
- inhibitory-κB kinase
- IP
- immunoprecipitation
- KO
- knockout
- NF-κB
- nuclear factor-κB
- NMR
- nuclear magnetic resonance
- PEPCK
- phosphoenolpyruvate carboxykinase
- PEPCK-C
- cytosolic form of PEPCK
- Pol II
- polymerase II
- PPAR
- peroxisome proliferator-activated receptor
- PPRE
- PPARγ response element
- qRT-PCR
- quantitative real-time RT-PCR
- RNAi
- interference RNA
- RXR
- retinoid X receptor
- SMRT
- silencing mediator of retinoid and thyroid receptors
- ssIκBα
- supersuppressor inhibitory-κBα
- Tg
- transgenic
- TG
- triglyceride
- TSA
- trichostatin A.
References
- 1. Ye J. 2009. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33:54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 3. Ye J, Gao Z, Yin J, He Q. 2007. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293:E1118–E1128 [DOI] [PubMed] [Google Scholar]
- 4. Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. 2009. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab 296:E333–E342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. 2002. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2α. Mol Cell Biol 22:7405–7416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. 2007. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911 [DOI] [PubMed] [Google Scholar]
- 7. Tang T, Zhang J, Yin J, Staszkiewicz J, Gawronska-Kozak B, Jung DY, Ko HJ, Ong H, Kim JK, Mynatt R, Martin RJ, Keenan M, Gao Z, Ye J. 2010. Uncoupling of inflammation and insulin resistance by NF-κB in transgenic mice through induction of energy expenditure. J Biol Chem 285:4637–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torti FM, Torti SV, Larrick JW, Ringold GM. 1989. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor β. J Cell Biol 108:1105–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ron D, Brasier AR, McGehee RE, Jr, Habener JF. 1992. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP). J Clin Invest 89:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, Vidal-Puig A, Jones R, Considine RV. 2005. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor α. Obes Res 13:662–669 [DOI] [PubMed] [Google Scholar]
- 11. Häcker H, Karin M. 2006. Regulation and function of IKK and IKK-related kinases. Sci STKE 2006:re13. [DOI] [PubMed] [Google Scholar]
- 12. Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. 2003. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem 278:30413–30416 [DOI] [PubMed] [Google Scholar]
- 13. Olswang Y, Cohen H, Papo O, Cassuto H, Croniger CM, Hakimi P, Tilghman SM, Hanson RW, Reshef L. 2002. A mutation in the peroxisome proliferator-activated receptor γ-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci USA 99:625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franckhauser S, Muñoz S, Elias I, Ferre T, Bosch F. 2006. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes 55:273–280 [DOI] [PubMed] [Google Scholar]
- 15. Franckhauser S, Muñoz S, Pujol A, Casellas A, Riu E, Otaegui P, Su B, Bosch F. 2002. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 51:624–630 [DOI] [PubMed] [Google Scholar]
- 16. Saltiel AR, Olefsky JM. 1996. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 45:1661–1669 [DOI] [PubMed] [Google Scholar]
- 17. Hanson RW, Reshef L. 1997. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66:581–611 [DOI] [PubMed] [Google Scholar]
- 18. Chakravarty K, Cassuto H, Reshef L, Hanson RW. 2005. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol 40:129–154 [DOI] [PubMed] [Google Scholar]
- 19. Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. 1995. PPARγ2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol 15:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao Z, Wang Z, Zhang X, Butler AA, Zuberi A, Gawronska-Kozak B, Lefevre M, York D, Ravussin E, Berthoud HR, McGuinness O, Cefalu WT, Ye J. 2007. Inactivation of PKCθ leads to increased susceptibility to obesity and dietary insulin resistance in mice Am J Physiol Endocrinol Metab 292:E84–E91 [DOI] [PubMed] [Google Scholar]
- 21. Gao Z, He Q, Peng B, Chiao PJ, Ye J. 2006. Regulation of nuclear translocation of HDAC3 by IκBα is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor γ function. J Biol Chem 281:4540–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan J, Gao Z, Yu G, He Q, Weng J, Ye J. 2007. Nuclear corepressor is required for inhibition of phosphoenolpyruvate carboxykinase expression by tumor necrosis factor-α. Mol Endocrinol 21:1630–1641 [DOI] [PubMed] [Google Scholar]
- 23. Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. 2004. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18:2024–2034 [DOI] [PubMed] [Google Scholar]
- 24. Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tordjman J, Khazen W, Antoine B, Chauvet G, Quette J, Fouque F, Beale EG, Benelli C, Forest C. 2003. Regulation of glyceroneogenesis and phosphoenolpyruvate carboxykinase by fatty acids, retinoic acids and thiazolidinediones: potential relevance to type 2 diabetes. Biochimie (Paris) 85:1213–1218 [DOI] [PubMed] [Google Scholar]
- 26. Khazen W, Distel E, Collinet M, Chaves VE, M'Bika JP, Chany C, Achour A, Benelli C, Forest C. 2007. Acute and selective inhibition of adipocyte glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase by interferon γ. Endocrinology 148:4007–4014 [DOI] [PubMed] [Google Scholar]
- 27. Gao Z, Chiao P, Zhang X, Zhang X, Lazar MA, Seto E, Young HA, Ye J. 2005. Coactivators and corepressors of NF-κB in IκBα gene promoter J Biol Chem 280:21091–21098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill MR, McCallum RE. 1992. Identification of tumor necrosis factor as a transcriptional regulator of the phosphoenolpyruvate carboxykinase gene following endotoxin treatment of mice. Infect Immun 60:4040–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang CK, Gatan M, Schumer W. 1996. Efficacy of anti-tumor necrosis factor polyclonal antibody on phosphoenolpyruvate carboxykinase expression in septic and endotoxemic rats. Shock 6:57–60 [DOI] [PubMed] [Google Scholar]
- 30. Nelson K, Cimbala MA, Hanson RW. 1980. Regulation of phosphoenolpyruvate carboxykinase (GTP) mRNA turnover in rat liver. J Biol Chem 255:8509–8515 [PubMed] [Google Scholar]
- 31. Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. 2001. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 [DOI] [PubMed] [Google Scholar]
- 32. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. 2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 [DOI] [PubMed] [Google Scholar]
- 33. Yang J, Kong X, Martins-Santos ME, Aleman G, Chaco E, Liu GE, Wu SY, Samols D, Hakimi P, Chiang CM, Hanson RW. 2009. Activation of SIRT1 by resveratrol represses transcription of the gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) by deacetylating hepatic nuclear factor 4α. J Biol Chem 284:27042–27053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang WM, Tsai SC, Wen YD, Fejer G, Seto E. 2002. Functional domains of histone deacetylase-3. J Biol Chem 277:9447–9454 [DOI] [PubMed] [Google Scholar]
- 35. Guenther MG, Barak O, Lazar MA. 2001. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol 21:6091–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J. 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wen YD, Perissi V, Staszewski LM, Yang WM, Krones A, Glass CK, Rosenfeld MG, Seto E. 2000. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc Natl Acad Sci USA 97:7202–7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanson RW. 2005. Metabolism in the era of molecular biology. J Biol Chem 280:1705–1715 [DOI] [PubMed] [Google Scholar]
- 39. Roesler WJ, Vandenbark GR, Hanson RW. 1989. Identification of multiple protein binding domains in the promoter-regulatory region of the phosphoenolpyruvate carboxykinase (GTP) gene. J Biol Chem 264:9657–9664 [PubMed] [Google Scholar]
- 40. Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. 2001. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 7:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shum BO, Mackay CR, Gorgun CZ, Frost MJ, Kumar RK, Hotamisligil GS, Rolph MS. 2006. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest 116:2183–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang J, Reshef L, Cassuto H, Aleman G, Hanson RW. 2009. Aspects of the control of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 284:27031–27035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duong DT, Waltner-Law ME, Sears R, Sealy L, Granner DK. 2002. Insulin inhibits hepatocellular glucose production by utilizing liver-enriched transcriptional inhibitory protein to disrupt the association of CREB-binding protein and RNA polymerase II with the phosphoenolpyruvate carboxykinase gene promoter. J Biol Chem 277:32234–32242 [DOI] [PubMed] [Google Scholar]
- 44. Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. 2005. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437:1109–1111 [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Vera L, Fischer WH, Montminy M. 2009. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460:534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. 2008. Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319:1402–1405 [DOI] [PubMed] [Google Scholar]
- 47. Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, Richardson JA, Bassel-Duby R, Olson EN. 2008. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest 118:3588–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, Hiebert SW. 2008. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell 30:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW. 2008. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J 27:1017–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xia Y, Wang J, Liu TJ, Yung WK, Hunter T, Lu Z. 2007. c-Jun downregulation by HDAC3-dependent transcriptional repression promotes osmotic stress-induced cell apoptosis. Mol Cell 25:219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weiss C, Schneider S, Wagner EF, Zhang X, Seto E, Bohmann D. 2003. JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. EMBO J 22:3686–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grégoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, Wong J, Seto E, Yang XJ. 2007. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol Cell Biol 27:1280–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ishizuka T, Lazar MA. 2003. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23:5122–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klampfer L, Huang J, Swaby LA, Augenlicht L. 2004. Requirement of histone deacetylase activity for signaling by STAT1. J Biol Chem 279:30358–30368 [DOI] [PubMed] [Google Scholar]
- 55. Ye J. 2008. Regulation of PPARγ function by TNF-α. Biochem Biophys Res Commun 374 405–408 [DOI] [PMC free article] [PubMed] [Google Scholar]