Reproductive experience selectively enhances STAT5 (but not ERK1/2) responsiveness to prolactin in the mPOA, PVN, SON, and in arcuate kisspeptin neurons. These neurons may participate in prolactin regulation.
Abstract
Pregnancy and lactation cause long-lasting enhancements in maternal behavior and other physiological functions, along with increased hypothalamic prolactin receptor expression. To directly test whether reproductive experience increases prolactin responsiveness in the arcuate, paraventricular, and supraoptic nuclei and the medial preoptic area, female rats experienced a full pregnancy and lactation or remained as age-matched virgin controls. At 5 wk after weaning, rats received 2.5, 100, or 4000 ng ovine prolactin or vehicle intracerebroventricularly. The brains underwent immunohistochemistry for the phosphorylated forms of signal transducer and activator of transcription 5 (pSTAT5) or ERK1/2 (pERK1/2). There was a marked increase in pSTAT5 and pERK1/2 in response to prolactin in the regions examined in both virgin and primiparous rats. Primiparous rats exhibited approximately double the number of prolactin-induced pSTAT5-immunoreactive cells as virgins, this effect being most apparent at the higher prolactin doses in the medial preoptic area and paraventricular and supraoptic nuclei and at the lowest prolactin dose in the arcuate nucleus. Dual-label immunohistochemistry showed that arcuate kisspeptin (but not oxytocin or dopamine) neurons displayed increased sensitivity to prolactin in reproductively experienced animals; these neurons may contribute to the reduction in prolactin concentration observed after reproductive experience. There was no effect of reproductive experience on prolactin-induced pERK1/2, indicating a selective effect on the STAT5 pathway. These data show that STAT5 responsiveness to prolactin is enhanced by reproductive experience in multiple hypothalamic regions. The findings may have significant implications for understanding postpartum disorders affecting maternal care and other prolactin-associated pathologies.
An abundance of research has shown the integral role that the anterior pituitary hormone prolactin plays in maternal behavior and physiology (1). In particular, newly parturient laboratory rodents demonstrate fully developed maternal behaviors when presented with pups, whereas virgin rats must learn these behaviors (2, 3), and transgenic deletion of prolactin receptors impairs these behavioral responses (4). This suggests that reproductive experience causes a change in central prolactin function to facilitate the maternal behavioral response. Prolactin is known to stimulate maternal care by acting within the medial preoptic area (mPOA) (5), a region that abundantly expresses mRNA (6–8) and protein (9, 10) for the long form of the prolactin receptor. Infusion of prolactin into the mPOA facilitates the onset of maternal care in pup retrieval experiments (11–13), whereas infusion of a prolactin receptor antagonist into this area impedes expression of maternal care (14). Reproductively experienced rats have also been shown to exhibit greater resilience to stress, decreased anxiety, and better memory abilities than female rats that have never experienced motherhood (15), again highlighting the effect of reproductive experience on brain functioning. Somewhat paradoxically, previous studies have revealed that basal serum concentrations of prolactin are significantly decreased in reproductively experienced animals (16, 17). This could imply a heightened sensitivity to prolactin feedback by the arcuate nucleus (ARC) tuberoinfundibular dopamine neurons, which negatively regulate prolactin secretion, or by afferent cells such as kisspeptin and opioid neurons, which have been shown to contribute to this system (18, 19). The same decrease in circulating prolactin concentration is seen in postpartum women, where it is sustained for over 10 yr (20) and thus could play a protective role against prolactin-induced neoplasms.
Recently, we demonstrated an association between reproductive experience and increased prolactin responsiveness in the mPOA and ARC, using induction of suppressors of cytokine signaling (SOCS) mRNA as an indirect index of prolactin signaling (17). SOCS proteins act as feedback inhibitors for a range of cytokines that use Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways (21), including prolactin. Lending further support to the hypothesis that reproductive experience increases hypothalamic prolactin responsiveness, it was also demonstrated that parity markedly up-regulates long-form prolactin receptor mRNA in the mPOA, paraventricular nucleus (PVN, where prolactin may act to modulate stress responses and oxytocin secretion) (22, 23), and ARC (17). The current study aimed to directly elucidate the effect of reproductive experience on prolactin sensitivity at the cell signaling level in various hypothalamic nuclei known to be responsive to prolactin. Activation of two prolactin signaling pathways were examined using immunohistochemistry: the STAT5 (24) and the phosphorylated ERK1/2 (pERK1/2) pathways (25). The STAT5 pathway can be activated by the long form of the prolactin receptor only, whereas the ERK1/2 pathway can potentially be activated by the long and short forms of the receptor (26). Transgenic deletion of the STAT5b isoform has shown that this signaling molecule plays an obligatory and nonredundant role in mediating the negative feedback actions of prolactin on tuberoinfundibular dopamine neurons (27). We also specifically examined the effects of reproductive experience on prolactin responsiveness in oxytocin, kisspeptin, and tuberoinfundibular dopaminergic neurons, using dual-label immunohistochemistry.
Materials and Methods
Animals
Female Sprague Dawley rats were obtained from the University of Otago animal breeding facility. Rats were group housed (except during late pregnancy and when with their litters) under conditions of controlled lighting (lights on from 0500–1900 h) and temperature (22 ± 1 C) and had free access to food and water. Half of the animals were paired with males at 10–12 wk of age; these females were allowed a full gestation and reared their litters (normalized to 10 pups) for 3 wk before weaning. Age-matched control females were never exposed to males. At 19–21 wk of age, all rats were chronically fitted with 22-gauge intracerebroventricular (icv) cannulae (Plastics One Inc., Roanoke, VA) to permit ovine prolactin or vehicle injection into a lateral ventricle (coordinates: 1.3 mm lateral to bregma and 3 mm below the skull surface; the needle of the Hamilton syringe used to deliver the prolactin extended a further 1 mm beyond this). Correct cannula placement was verified visually from the cannula lesion in mounted immunohistochemistry sections. All animal experimental procedures were approved by the University of Otago Animal Ethics Committee.
Prolactin treatment and collection of perfused brains
After icv cannulation, estrous cycles were monitored by daily cytological examination of vaginal smears for at least one cycle. On the day of metestrus, all rats were pretreated with bromocriptine methanesulfonate (200 μg in 200 μl 10% ethanol sc; Sigma Chemical Co., St. Louis, MO) at 1700 h and again at 1000 h on the following (diestrus) day to reduce endogenous circulating prolactin levels to basal levels (17, 24). Four hours later, the rats received a single injection of ovine prolactin (2.5, 100, or 4000 ng icv in 4 μl saline; Sigma) or vehicle. The highest prolactin dose was based on previous experiments (17, 24). The three doses were delivered in three separate vehicle-controlled experiments. Thirty minutes after infusions, animals were deeply anesthetized with 60 mg sodium pentobarbital (containing 1000 IU heparin) and perfused via the ascending aorta with 30 ml physiological saline (containing 120 IU heparin) and then 250 ml of 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). Brains were removed and postfixed in the same fixative overnight and then infiltrated with 30% sucrose for cryoprotection until they sank. Coronal (40 μm thick) sections containing the mPOA, PVN, supraoptic nucleus (SON), and ARC were cut on a sliding microtone with a freezing stage from each brain to provide four similar series of consecutive sections (160 μm apart); these were stored in cryoprotectant at −20 C until required for immunohistochemistry.
pSTAT5, pERK1/2, oxytocin, tyrosine hydroxylase, and kisspeptin immunohistochemistry
All immunohistochemistry steps were separated by three washes in 0.1 m Tris HCl. For pSTAT5 immunohistochemistry, an initial high temperature plus high pH antigen retrieval step (5 min in 0.1 m Tris HCl, pH 10, at 90 C) was required to unmask the antigen as described previously (28). After a 30-min incubation in 1% hydrogen peroxide to quench endogenous peroxides, sections were incubated for 48 h in a solution containing 2% normal goat serum, 0.25% BSA, and 0.1% Triton X-100 of either polyclonal rabbit anti-pSTAT5 (tyr 694, 1:400; Cell Signaling Technology, Beverly, MA) or polyclonal rabbit anti-pERK1/2 (thr202/tyr204, 1:3000; Cell Signaling Technology). The pSTAT5 antibody does not distinguish between phosphorylated STAT5a or STAT5b isoforms. This was followed by a 1-h incubation in biotin-conjugated goat antirabbit IgG antibody (1:500; Vector Laboratories, Burlingame, CA), 1 h of incubation in Vector Elite ABC solution (Vector), and a 3-min incubation in a nickel-enhanced diaminobenzidine (DAB) and hydrogen peroxide solution to visualize the immunoreactivity (blue-black staining).
For dual-labeling studies (pSTAT5 in colocalization with oxytocin or dopamine neurons), pSTAT5-labeled tissues were incubated for 24 h in either rabbit polyclonal anti-oxytocin (1:25,000; Chemicon, Temecula, CA) or mouse monoclonal anti-tyrosine hydroxylase (MAB318, 1:10,000; Chemicon), then horseradish peroxidase-conjugated goat antirabbit or -mouse IgG antibody (1:500; Dako, Glostrup, Denmark) and an unenhanced DAB and hydrogen peroxide solution until sufficient brown cytoplasmic staining was observed. For dual-labeling pSTAT5 and kisspeptin neurons, kisspeptin was first labeled with rabbit polyclonal anti-kisspeptin (1:5000; Chemicon), biotin-conjugated goat antirabbit IgG, ABC solution, and unenhanced DAB as above before directly labeling pSTAT5 with primary antibody, horseradish peroxidase-conjugated goat antirabbit secondary antibody and nickel-enhanced DAB as above. Sections were mounted on (3-aminopropyl)-tirethoxy-silane-coated slides, dehydrated in graded alcohols, and coverslipped. Omission of primary antibodies resulted in a complete absence of staining. Immunoreactive cells from the mPOA, PVN, SON, and ARC were counted by an operator blind to the treatment groups, using a light microscope at ×200 magnification. Oxytocin, dopamine, and kisspeptin neurons were counted only if they showed a cell nucleus (i.e. a white center depicting a pSTAT5-negative nucleus or a blue-black stained nucleus that was darker than the surrounding brown cytoplasmic staining depicting a pSTAT5-positive nucleus). For each animal, the percentage of pSTAT5-colocalized neurons per section was calculated to provide a single data point. For pSTAT5 single-label immunoreactivity, numbers of immunoreactive cells were counted using the image analysis software Image J (National Institutes of Health, Bethesda, MD) to identify and count darkly stained nuclei within standard-sized regions and the Paxinos and Watson (29) brain atlas to define these regions (coordinates anterior to bregma: mPOA, 0.24 to −0.96 mm; PVN and SON, −1.20 to −1.72 mm; ARC, −1.92 to −3.24 mm). Counts are presented as total numbers of identified cells per region per section. For each region, two to three sections were counted and averaged to provide a single data point per region per animal. Representative images were photographed at ×100 and ×200 magnification.
Statistical analysis
All significant treatment effects were identified using two-way ANOVA, with reproductive experience and prolactin treatment as the factors. Where data failed equal variance or normality tests, they were log-transformed (base 10) before ANOVA. When significant main effects or interactions between reproductive experience and prolactin treatment occurred, the Bonferroni t test for post hoc analysis was used to determine where significant effects occurred. Statistical comparison between doses of prolactin was not made because each dose was delivered in separate vehicle-controlled experiments; however, for analysis and presentation of the effects of reproductive experience on basal (vehicle-treated) signaling, results from the three experiments were pooled. Results are presented as mean ± sem, and differences were considered significant at P < 0.05.
Results
All three doses of prolactin markedly increased the number of pSTAT5-immunoreactive cells in the mPOA, PVN, SON, and ARC (main effect, P < 0.001) in a dose-dependant manner. In the mPOA, PVN, and SON, primiparous rats exhibited an approximately 2-fold increase in pSTAT5 responsiveness to prolactin compared with virgins at the higher (≥100 ng/rat) doses (interaction between reproductive experience and prolactin treatment, P < 0.05) (Fig. 1). In the ARC, prolactin-induced pSTAT5 appeared to be maximal at prolactin doses of at least 100 ng/rat, and a heightened pSTAT5 responsiveness to prolactin was seen at the lower (2.5 ng/rat) dose only in reproductively experienced animals (interaction between reproductive experience and prolactin treatment, P < 0.05) (Fig. 1). In all vehicle-treated control rats, pSTAT5 immunoreactivity was very low in all hypothalamic regions. Nevertheless, there was evidence of a basal effect of reproductive experience in the mPOA and PVN but not the SON and ARC, with primiparous rats exhibiting approximately 3-fold higher basal pSTAT5 immunoreactivity than virgins in the former regions (P < 0.05) (Fig. 1). Representative examples of pSTAT5 staining in response to prolactin treatment are shown in Fig. 2.
Fig. 1.
Effect of reproductive experience on numbers of pSTAT5-immunoreactive cells in response to graded doses of prolactin (2.5, 100m or 4000 ng/rat icv) or vehicle in the mPOA, PVN, SON, and ARC (n = 7–9 per group). Each dose was delivered in separate vehicle-controlled experiments; results from all vehicle-treated rats are pooled into groups of 21–24 for presentation. Within bars: **, P < 0.001 vs. corresponding vehicle controls; above bars: *, P < 0.05; **, P < 0.001 between virgin and primiparous rats at the same prolactin dose.
Fig. 2.
Representative examples of pSTAT5 immunoreactivity in vehicle-treated (left panels) or prolactin-treated (right panels; 4000 ng dose is shown) rats. A and B, mPOA; C and D, PVN; E and F, SON; G and H, ARC. Scale bars, 100 μm.
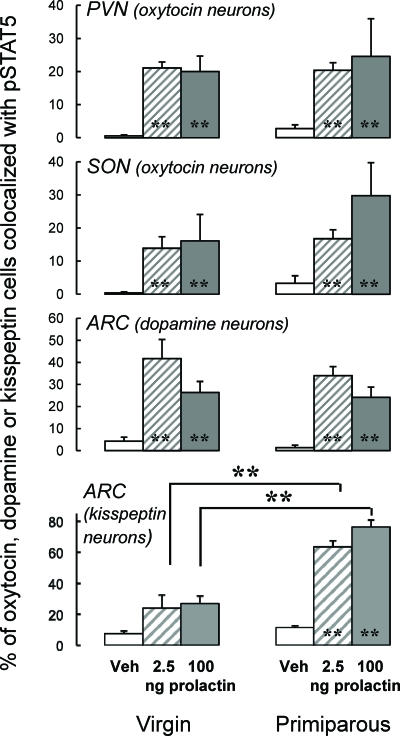
Between 15 and 40% of oxytocin neurons in the PVN and SON and dopaminergic neurons in the ARC coexpressed pSTAT5 after 2.5- or 100-ng prolactin injections (P < 0.001 vs. vehicle treatment) (Fig. 3). The overall effects of reproductive experience on total pSTAT5 immunoreactivity were not reflected in levels of pSTAT5 in oxytocin and dopaminergic neurons, with primiparous and virgin rats exhibiting similar numbers of colocalized cells (Fig. 3). In contrast, prolactin-pSTAT5 signaling in arcuate kisspeptin neurons was not significantly different from basal levels in virgin rats but was pronounced (65–75% of kisspeptin cells colocalized with pSTAT5) in primiparous rats (interaction between reproductive experience and prolactin treatment, P < 0.001 for both prolactin doses) (Fig. 3, lower panel). Despite the possibility that the slightly reduced circulating estradiol concentration that has been noted in reproductively experienced rats compared with virgins (17, 30) might conceivably influence kisspeptin-immunoreactive cell numbers, there was no effect of reproductive experience on this parameter (virgin, 21.0 ± 2.8 cells per section; primiparous, 17.8 ± 2.8 cells per section; P > 0.1). Representative examples of pSTAT5 colocalization in oxytocin, dopamine, and kisspeptin neurons in response to prolactin treatment are shown in Fig. 4.
Fig. 3.
Effect of reproductive experience on the percentage of oxytocin neurons in the PVN and SON and dopamine or kisspeptin neurons in the ARC that colocalized with pSTAT5 immunoreactivity in response to graded doses of prolactin (2.5, 100, or 4000 ng/rat icv) or vehicle (n = 6–8 per group). Each dose was delivered in separate vehicle-controlled experiments; results from all vehicle-treated rats are pooled into groups of 12–14 for presentation. **, P < 0.001 vs. corresponding vehicle controls.
Fig. 4.
Representative examples of pSTAT5 (black nuclear staining) colocalization with oxytocin neurons (A–D), tuberoinfundibular dopaminergic neurons (E and F), and kisspeptin neurons (G and H), shown as brown cytoplasmic staining. Vehicle-treated rats are in the left panels; prolactin-treated rats are in the right panels (4000-ng dose is shown). A and B, PVN; C and D, SON; E–H, ARC. Filled arrows indicate examples of oxytocin, dopamine, or kisspeptin neurons colocalized with pSTAT5; unfilled arrows indicate non-colocalized neurons (boxed insets show 5-fold enlargements of some of these). Scale bars, 100 μm.
Prolactin treatment at 100 and 4000 ng/rat also induced pERK1/2 immunoreactivity in the mPOA, SON, and ARC of virgin and primiparous rats (P < 0.05), although no statistically significant effects of prolactin were seen in the PVN (P = 0.067 at the 4000 ng/rat dose). Furthermore, in contrast to the interactions of prolactin and reproductive experience on pSTAT5 immunoreactivity, there was no increase in pERK1/2 sensitivity to prolactin as a consequence of parity in any of the hypothalamic regions examined; nor were any basal effects of reproductive experience observed in vehicle-treated rats (Fig. 5). Representative examples of pERK1/2 staining in response to prolactin treatment are shown in Fig. 6.
Fig. 5.
Effect of reproductive experience on numbers of pERK1/2-immunoreactive cells in response to graded doses of prolactin (2.5, 100, or 400 ng/rat icv) or vehicle in the mPOA, PVN, SON, and ARC (n = 6–8 per group). Each dose was delivered in separate vehicle-controlled experiments; results from all vehicle-treated rats are pooled into groups of 12–14 for presentation. *, P < 0.05 vs. corresponding vehicle controls.
Fig. 6.
Representative examples of pERK1/2 immunoreactivity in vehicle-treated (left panels) or prolactin-treated (right panels; 4000-ng dose is shown) rats. A and B, mPOA; C and D, PVN; E and F, SON; G and H, ARC. Scale bars, 100 μm.
Discussion
Prolactin is well known to act in the hypothalamus to modulate multiple functions including maternal behavior, stress responses, food intake, and its own release by a negative feedback mechanism. Understanding how physiological situations affect these actions and how hormones of the female reproductive and stress axes interact to influence neuroendocrine functions and mood states is important for identifying the mechanisms underpinning postpartum depression and other prolactin-associated pathologies (31). Here we show that reproductive experience induces enhancements in hypothalamic prolactin responsiveness specifically through the STAT5 pathway in the mPOA, PVN, and SON and in arcuate kisspeptin neurons.
At prolactin doses between 100 and 4000 ng/rat, an effect of reproductive experience was seen in the mPOA, PVN, and SON. There are several possibilities to explain how reproductive experience can cause this increase in prolactin sensitivity. First, increased prolactin sensitivity could be the result of greater accessibility of prolactin to the brain. Prolactin is thought to enter the brain via the choroid plexus by a receptor-mediated mechanism (32), but whether prolactin receptors are up-regulated in the choroid plexus after reproductive experience is unknown. Second, the heightened responsiveness to prolactin after reproductive experience could be as a result of an increase in hypothalamic prolactin receptor expression. Indeed, our lab has shown using quantitative RT-PCR that the level of long-form prolactin receptor mRNA is markedly up-regulated by reproductive experience in the mPOA and ARC (17). The relative levels of long- vs. short-form prolactin receptor induced by reproductive experience would also be of interest, because only the long form is thought to be able to induce JAK/STAT5 signaling (27). Finally, the increase in prolactin sensitivity could be the result of a change in downstream signaling independently of the levels of prolactin and its receptors. Although we have previously shown that prolactin-induced STAT5 signaling in tuberoinfundibular dopamine neurons is reduced in recently postpartum rats (24, 33) to enable lactational hyperprolactinemia, the current and previous (17) results support the notion that hypothalamic STAT5 signaling is increased in long-term primiparous rats. One way in which the tone of JAK/STAT signaling could be increased specifically downstream of the receptor is via a reduction in levels of the inhibitory SOCS proteins (21). However, we have not observed reproductive experience to cause a reduction in gene expression levels of any SOCS proteins under basal conditions, and in response to exogenous prolactin, SOCS mRNA responses tend to be exaggerated rather than diminished (17). In vivo, prolactin has been shown to induce mRNA for SOCS-1, and -3 in ovary, adrenal, and mammary cells (34, 35) as well as in hypothalamic regions (17, 24, 28). STAT5b is translocated to the nucleus in neuroendocrine dopaminergic neurons between 0.5 and 2 h after prolactin treatment in rats (36), whereas induction of SOCS mRNA is maximal about 2 h after treatment (24). Thus, although chronically elevated SOCS protein levels can suppress JAK/STAT signaling (21), we propose that the high SOCS mRNA levels previously observed in reproductively experienced rats after acute exogenous prolactin treatment are indicative of increased prolactin receptor signaling and therefore concur with the current findings.
Although hypothalamic prolactin signaling via the JAK/STAT5 pathway is well described (e.g. 24, 27, 28), prolactin-induced ERK phosphorylation in the brain has only recently been reported, and only within the PVN and SON (25). In the current study, these two prolactin signaling pathways showed a marked divergence in their sensitivities to prolactin after reproductive experience. Although the PVN, SON, and mPOA showed increased prolactin sensitivity via the STAT5 pathway, no significant change in prolactin sensitivity via the ERK1/2 pathway was observed in these areas and the ARC after reproductive experience. It is possible that this divergence in sensitivity of these two major prolactin signaling pathways is the result of modification of the ratio of long and short prolactin receptor isoforms. Although activation of the STAT5 pathway is dependant on the long form of the receptor, the ERK1/2 pathway can be activated by both the long and short forms (26). The distribution of prolactin receptor isoforms has been carried out in the pregnant and lactating rat by quantitative RT-PCR and in situ hybridization (6, 37–39), but the reproductively experienced animal is yet to be characterized in regard to the short form of the receptor.
The mPOA expresses mRNA (6–8) and protein (9, 10) for the long form of the prolactin receptor and is of particular importance for both the onset and maintenance of maternal behavior (40) as well as inhibiting aversive behaviors toward pups (41). Infusion of prolactin or the closely related rat placental lactogens into the mPOA facilitates the onset of maternal care in pup retrieval experiments (11–13), whereas infusion of the prolactin receptor antagonist S179DPRL into this area impedes expression of maternal care (14). It is of great interest that the mPOA showed an increased sensitivity to prolactin after reproductive experience, because behavioral studies have previously shown that reproductively experienced animals respond more readily to foster pups compared with nulliparous rats (3). These data extend our previous report that prolactin-induced SOCS-1 and -3 are increased in the mPOA primiparous rats (17) by demonstrating that it is specifically JAK/STAT5 signaling that is enhanced. This is supported by the fact that both basal and prolactin-induced long-form prolactin receptor mRNA are also up-regulated by reproductive experience in the mPOA (17). Although these data together provide an intracellular mechanism for enhancement of maternal care in parous animals, further work is required to characterize the neuronal cell populations responsible for the altered sensitivity to prolactin in the mPOA.
In the single-label pSTAT5 studies, enhanced STAT5 signaling was evident in the PVN and SON in reproductive experienced animals. Colocalization studies were conducted to identify whether oxytocin neurons were the cell type affected by reproductive experience, because prolactin may modulate oxytocin secretion (23). In contrast to this hypothesis, however, the percentage of oxytocin neurons colocalized with pSTAT5 in the PVN did not change in response to reproductive experience, implying that the cells that increase in prolactin sensitivity after reproductive experience are nonoxytocin cells. The PVN contains a wide range of neuronal cell types including oxytocin, vasopressin, TRH, and CRH neurons, many of which may be prolactin responsive (23, 25, 42–44). It is plausible that CRH neurons may be involved in the increased prolactin sensitivity after reproductive experience. Support for these neurons as the neuronal population responsible for increased prolactin responsiveness in the PVN comes from behavioral studies in which reproductively experienced animals have been shown to exhibit long-term attenuated stress responses compared with female rats that have never experienced motherhood (15, 22, 45). Because prolactin has also been shown to be involved in stress hyporesponsiveness (46), it may be that an increase in prolactin sensitivity in CRH neurons would result in attenuated stress responses after reproductive experience. In the SON, a relatively small nucleus that contains mainly magnocellular neurons, it seems more likely that either oxytocin or vasopressin neurons are responsible for the effect of increased prolactin sensitivity after reproductive experience. More than 80% of prolactin receptor long-form mRNA has been shown to be colocalized with oxytocin neurons, whereas fewer than 10% of vasopressin neurons have been shown to express the long form of the prolactin receptor (23). Consistent with this, during analysis of pSTAT5 and oxytocin colocalization in the current study, relatively few pSTAT5-immunoreactive cells not associated with oxytocin cells were seen. Thus, it was somewhat surprising that we found no evidence for enhanced oxytocin cell responsiveness to prolactin in the SON, and further work is required to resolve this issue.
Considering that reproductive experience has been shown to cause a long-term decrease in basal (20) and dopamine antagonist-induced prolactin secretion in women (47) as well as in rats (48), it was hypothesized that the ARC would show a markedly increased sensitivity to prolactin feedback after reproductive experience, especially because we have previously demonstrated that prolactin-induced SOCS mRNA levels are increased in the ARC of primiparous rats (17). However, although the heightened STAT5 responsiveness to exogenous prolactin was apparent at the higher (100 and 4000 ng/rat) ovine prolactin doses in the mPOA, PVN, and SON in primiparous animals, enhanced prolactin-induced STAT5 signaling in the ARC in response to reproductive experience was seen only at the lowest dose (2.5 ng/rat). These data are supported by our recent findings that confirm that cells within the ARC are able to respond to prolactin at doses 50 times lower than that required to stimulate STAT5 signaling in the POA and PVN and is apparently maximally activated at these low doses (Sapsford, T., I. C. Kokay, L. Östberg, R. S. Bridges, and D. R. Grattan, submitted for publication). Furthermore, no increase in the number of tuberoinfundibular dopaminergic neurons responding to the prolactin challenge was observed at any dose in experienced rats. Very recently, it has been revealed that arcuate kisspeptin neurons, best known for their pronounced influence on the reproductive axis, are able to stimulate prolactin release through inhibition of the tuberoinfundibular dopamine neurons (18). Moreover, we recently demonstrated that these neurons express prolactin receptor mRNA (49). In the absence of a direct effect of reproductive experience on dopaminergic sensitivity to prolactin, this effect might be relayed indirectly via the kisspeptin neurons. Consistent with this hypothesis, arcuate kisspeptin neurons displayed pronounced prolactin-induced pSTAT5 in primiparous rats, whereas in virgin controls, prolactin-induced signaling was not detected above basal levels (Fig. 3). These data lead us to propose the existence of a novel prolactin-kisspeptin-dopamine relay in the negative feedback control of prolactin, the tone of which appears to be exquisitely enhanced by reproductive experience. Alternatively, it may be that the key neurotransmitters responding to prolactin in this system are in fact opioid peptides, because these have been shown to be highly colocalized with kisspeptin neurons in the ARC (50) and are also known to suppress tuberoinfundibular dopamine function (19).
In the mPOA and PVN, primiparous rats exhibited approximately 3-fold higher basal pSTAT5 immunoreactivity than virgins. Even allowing for enhanced prolactin responsiveness after reproductive experience, this is intriguing given that circulating prolactin secretion was clamped with bromocriptine pretreatment. Furthermore, in non-bromocriptine-treated rats, circulating prolactin concentrations are known to be decreased in primiparous rats (16, 17). There is some evidence that the mPOA and PVN can themselves produce prolactin (51, 52) and that pregnancy and lactation are associated with an increase in hypothalamic prolactin gene expression (51). It is possible that the higher basal pSTAT5 levels in reproductively experienced animals in the current study could have been caused by an increase in brain-derived prolactin production that is maintained after pregnancy and lactation. If a source of brain-derived prolactin does exist, it would be of interest to determine whether it is increased in reproductively experienced animals, because a small amount of brain-derived prolactin from outside the negative feedback loop of prolactin regulation could lead to decreased pituitary prolactin secretion by stimulation of the tuberoinfundibular dopamine neurons (53).
In conclusion, these results show that reproductive experience induces region-specific enhancements in prolactin responsiveness specifically through the STAT5 pathway, whereas the ERK1/2 pathway was unaffected by reproductive experience. Considering that prolactin actions in these hypothalamic regions are implicated in maternal behavior and stress responses, these data may have significant implications for understanding the mechanisms underlying disorders affecting maternal care such as postpartum depression. Finally, these data raise the novel possibility that the neuronal cell population responsible for the decrease in circulating prolactin concentration after reproductive experience is the arcuate kisspeptin group, in which markedly enhanced prolactin-pSTAT5 signaling was observed after parity.
Acknowledgments
This work was supported by Royal Society of New Zealand Marsden Fund and National Institutes of Health Grant HD39895 (to R.S.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- DAB
- diaminobenzidine
- icv
- intracerebroventricular
- JAK
- Janus kinase
- mPOA
- medial preoptic area
- pERK1/2
- phosphorylated ERK1/2
- PVN
- paraventricular nucleus
- SOCS
- suppressors of cytokine signaling
- SON
- supraoptic nucleus
- STAT
- signal transducer and activator of transcription.
References
- 1. Grattan DR, Kokay IC. 2008. Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol 20:752–763 [DOI] [PubMed] [Google Scholar]
- 2. Sugiyama T, Minoura H, Toyoda N, Sakaguchi K, Tanaka M, Sudo S, Nakashima K. 1996. Pup contact induces the expression of long form prolactin receptor mRNA in the brain of female rats: effects of ovariectomy and hypophysectomy on receptor gene expression. J Endocrinol 149:335–340 [DOI] [PubMed] [Google Scholar]
- 3. Mann PE, Bridges RS. 2001. Lactogenic hormone regulation of maternal behavior. Prog Brain Res 133:251–262 [DOI] [PubMed] [Google Scholar]
- 4. Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. 1998. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139:4102–4107 [DOI] [PubMed] [Google Scholar]
- 5. Numan M. 1974. Medial preoptic area and maternal behavior in the female rat. J Comp Physiol Psychol 87:746–759 [DOI] [PubMed] [Google Scholar]
- 6. Bakowska JC, Morrell JI. 1997. Atlas of the neurons that express mRNA for the long form of the prolactin receptor in the forebrain of the female rat. J Comp Neurol 386:161–177 [DOI] [PubMed] [Google Scholar]
- 7. Pi X, Grattan DR. 1999. Expression of prolactin receptor mRNA is increased in the preoptic area of lactating rats. Endocrine 11:91–98 [DOI] [PubMed] [Google Scholar]
- 8. Bridges RS, Hays LE. 2005. Steroid-induced alterations in mRNA expression of the long form of the prolactin receptor in the medial preoptic area of female rats: Effects of exposure to a pregnancy-like regimen of progesterone and estradiol. Mol Brain Res 140:10–16 [DOI] [PubMed] [Google Scholar]
- 9. Pi XJ, Grattan DR. 1998. Distribution of prolactin receptor immunoreactivity in the brain of estrogen-treated, ovariectomized rats. J Comp Neurol 394:462–474 [DOI] [PubMed] [Google Scholar]
- 10. Pi XJ, Grattan DR. 1999. Increased prolactin receptor immunoreactivity in the hypothalamus of lactating rats. J Neuroendocrinol 11:693–705 [DOI] [PubMed] [Google Scholar]
- 11. Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. 1990. Central prolactin infusions stimulate maternal behavior in steroid-treated nulliparous female rats. Proc Natl Acad Sci USA 87:8003–8007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bridges RS, Robertson MC, Shiu RP, Friesen HG, Stuer AM, Mann PE. 1996. Endocrine communication between conceptus and mother: placental lactogen stimulation of maternal behavior. Neuroendocrinology 64:57–64 [DOI] [PubMed] [Google Scholar]
- 13. Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE. 1997. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat placental lactogen I. Endocrinology 138:756–763 [DOI] [PubMed] [Google Scholar]
- 14. Bridges R, Rigero B, Byrnes E, Yang L, Walker A. 2001. Central infusions of the recombinant human prolactin receptor antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed, nulliparous female rats. Endocrinology 142:730–739 [DOI] [PubMed] [Google Scholar]
- 15. Macbeth AH, Luine VN. 2010. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev 34:452–467 [DOI] [PubMed] [Google Scholar]
- 16. Byrnes EM, Bridges RS. 2007. Reproductive experience and expression of dopamine D(2) receptor mRNA: a possible mechanism for reduced prolactin secretion in primiparous rats. J Neuroendocrinol 19:773–778 [DOI] [PubMed] [Google Scholar]
- 17. Anderson GM, Grattan DR, van den Ancker W, Bridges RS. 2006. Reproductive experience increases prolactin responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology 147:4688–4694 [DOI] [PubMed] [Google Scholar]
- 18. Szawka RE, Ribeiro AB, Leite CM, Helena CV, Franci CR, Anderson GM, Hoffman GE, Anselmo-Franci JA. 2010. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology 151:3247–3257 [DOI] [PubMed] [Google Scholar]
- 19. Andrews ZB, Grattan DR. 2003. Opioid receptor subtypes involved in the regulation of prolactin secretion during pregnancy and lactation. J Neuroendocrinol 15:227–236 [DOI] [PubMed] [Google Scholar]
- 20. Musey VC, Collins DC, Musey PI, Martino-Saltzman D, Preedy JR. 1987. Long-term effect of a first pregnancy on the secretion of prolactin. N Engl J Med 316:229–234 [DOI] [PubMed] [Google Scholar]
- 21. Starr R, Hilton DJ. 1999. Negative regulation of the JAK/STAT pathway. Bioessays 21:47–52 [DOI] [PubMed] [Google Scholar]
- 22. Donner N, Bredewold R, Maloumby R, Neumann ID. 2007. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur J Neurosci 25:1804–1814 [DOI] [PubMed] [Google Scholar]
- 23. Kokay IC, Bull PMD, Davis RL, Ludwig M, Grattan DR. 2006. Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol Regul Integr Comp Physiol 290:1216–1225 [DOI] [PubMed] [Google Scholar]
- 24. Anderson GM, Beijer P, Bang AS, Fenwick MA, Bunn SJ, Grattan DR. 2006. Suppression of prolactin-induced signal transducer and activator of transcription 5b signaling and induction of suppressors of cytokine signaling messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat during late pregnancy and lactation. Endocrinology 147:4996–5005 [DOI] [PubMed] [Google Scholar]
- 25. Blume A, Torner L, Liu Y, Subburaju S, Aguilera G, Neuman ID. 2009. Prolactin activates mitogen-activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinology 150:1841–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. 1998. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268 [DOI] [PubMed] [Google Scholar]
- 27. Grattan DR, Xu J, McLachlan MJ, Kokay IC, Bunn SJ, Hovey RC, Davey HW. 2001. Feedback regulation of PRL secretion is mediated by the transcription factor, signal transducer, and activator of transcription 5b. Endocrinology 142:3935–3940 [DOI] [PubMed] [Google Scholar]
- 28. Anderson GM, Kieser DC, Steyn FJ, Grattan DR. 2008. Hypothalamic prolactin receptor messenger ribonucleic acid levels, prolactin signaling, and hyperprolactinemic inhibition of pulsatile luteinizing hormone secretion are dependent on estradiol. Endocrinology 149:1562–1570 [DOI] [PubMed] [Google Scholar]
- 29. Paxinos G, Watson C. 2005. The rat brain atlas in sterotaxic coordinates. 5th ed. Sydney: Elsevier [Google Scholar]
- 30. Bridges RS, Byrnes EM. 2006. Reproductive experience reduces circulating 17β-estradiol and prolactin levels during proestrus and alters estrogen sensitivity in female rats. Endocrinology 147:2575–2582 [DOI] [PubMed] [Google Scholar]
- 31. Russell JA, Douglas AJ, Ingram CD. 2001. Brain preparations for maternity: adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res 133:1–38 [DOI] [PubMed] [Google Scholar]
- 32. Walsh RJ, Slaby FJ, Posner BI. 1987. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 120:1846–1950 [DOI] [PubMed] [Google Scholar]
- 33. Grattan DR, Steyn FJ, Kokay IC, Anderson GM, Bunn SJ. 2008. Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol 20:497–507 [DOI] [PubMed] [Google Scholar]
- 34. Tam SP, Lau P, Djiane J, Hilton DJ, Waters MJ. 2001. Tissue-specific induction of SOCS gene expression by PRL. Endocrinology 142:5015–5026 [DOI] [PubMed] [Google Scholar]
- 35. Motta M, Accornero P, Baratta M. 2004. Leptin and prolactin modulate the expression of SOCS-1 in association with interleukin-6 and tumor necrosis factor-α in mammary cells: a role in differentiated secretory epithelium. Regul Pept 121:163–170 [DOI] [PubMed] [Google Scholar]
- 36. Ma FY, Anderson GM, Gunn TD, Goffin V, Grattan DR, Bunn SJ. 2005. Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic neurons. Endocrinology 146:5112–5119 [DOI] [PubMed] [Google Scholar]
- 37. Bakowska JC, Morrell JI. 2003. The distribution of mRNA for the short form of the prolactin receptor in the forebrain of the female rat. Brain Res Mol Brain Res 116:50–58 [DOI] [PubMed] [Google Scholar]
- 38. Augustine RA, Kokay IC, Andrews ZB, Ladyman SR, Grattan DR. 2003. Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. J Mol Endocrinol 31:221–232 [DOI] [PubMed] [Google Scholar]
- 39. Kokay IC, Grattan DR. 2005. Expression of mRNA for prolactin receptor (long form) in dopamine and pro-opiomelanocortin neurones in the arcuate nucleus of non-pregnant and lactating rats. J Neuroendocrinol 17:827–835 [DOI] [PubMed] [Google Scholar]
- 40. Bridges RS, Freemark MS. 1995. Human placental pactogen infusions into the medial preoptic area stimulate maternal behavior in steroid-primed, nulliparous rats. Horm Behav 29:216–226 [DOI] [PubMed] [Google Scholar]
- 41. Numan M. 1994. A neural circuitry analysis of maternal behavior in the rat. Acta Paediatrica Suppl 397:19–28 [DOI] [PubMed] [Google Scholar]
- 42. Grattan DR, Pi XJ, Andrews ZB, Augustine RA, Kokay IC, Summerfield MR, Todd B, Bunn SJ. 2001. Prolactin receptors in the brain during pregnancy and lactation: implications for behavior. Horm Behav 40:115–124 [DOI] [PubMed] [Google Scholar]
- 43. Mejía S, Torner LM, Jeziorski MC, Gonzalez C, Morales MA, de la Escalera GM, Clapp C. 2003. Prolactin and 16K prolactin stimulate release of vasopressin by a direct effect on hypothalamo-neurohypophyseal system. Endocrine 20:155–162 [DOI] [PubMed] [Google Scholar]
- 44. Donner N, Neumann ID. 2009. Effects of chronic intracerebral prolactin on the oxytocinergic and vasopressinergic system of virgin ovariectomized rats. Neuroendocrinology 90:315–322 [DOI] [PubMed] [Google Scholar]
- 45. Byrnes EM, Bridges RS. 2006. Reproductive experience alters anxiety-like behavior in the female rat. Horm Behav 50:70–76 [DOI] [PubMed] [Google Scholar]
- 46. Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. 2001. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci 21:3207–3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Espinosa de los Monteros A, Cornejo J, Parra A. 1991. Differential prolactin response to oral metoclopramide in nulliparous versus parous women throughout the menstrual cycle. Fertil Steril 55:885–889 [DOI] [PubMed] [Google Scholar]
- 48. Bridges RS, Henriquez BM, Sturgis JD, Mann PE. 1997. Reproductive experience reduces haloperidol-induced prolactin secretion in female rats. Neuroendocrinology 66:321–326 [DOI] [PubMed] [Google Scholar]
- 49. Kokay IC, Petersen SL, Grattan DR. 2011. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology 152:526–535 [DOI] [PubMed] [Google Scholar]
- 50. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torner L, Neumann ID. 2002. The brain prolactin system: involvement in stress response adaptations in lactation. Stress 5:249–257 [DOI] [PubMed] [Google Scholar]
- 52. Suzuki S, Handa RJ. 2005. Estrogen receptor-β, but not estrogen receptor-α, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol 484:28–42 [DOI] [PubMed] [Google Scholar]
- 53. Grattan DR, Averill RL. 1991. Intrahypothalamic pituitary grafts elevate prolactin in the cerebrospinal fluid and attenuate prolactin release following ether stress. Proc Soc Exp Biol Med 196:42–46 [DOI] [PubMed] [Google Scholar]