To achieve final adult topology, postnatal GnRH neurons in male rats undergo testosterone-independent remodeling of their dendritic arbors.
Abstract
Adult GnRH neurons exhibit a stereotypic morphology with a small soma, single axon, and single dendrite arising from the soma with little branching. The adult morphology of GnRH neurons in mice reflects an anatomical consolidation of dendrites over postnatal development. We examined this issue in rat GnRH neurons with biocytin filling in live hypothalamic slices from infant males, as adult littermates and in gonad-intact males, castrated males, and in males with one of three levels of testosterone (T) treatment. Somatic area and total dendritic length were significantly greater in infant males than in adults. Moreover, total numbers of dendrite branches were greater in infant males as compared with adults. The number of higher order branches and the lengths of higher order branches were also greater in infant males than in adults. Most interestingly, in adults a single dendrite arose from the somata, consistently at 180° from the axon. In contrast, prepubertal animals had an average of 2.2 ± 0.2 primary dendrites arising from somata (range, one to seven primary dendrites). Angles relative to the axon at which dendrites in prepubertal males emanated from GnRH somata were highly variable. Castration at 25 d of age and castration at 25 d of age with one of three levels of T treatment did not influence morphological parameters when GnRH neurons were examined between 40 d and 48 d of age. Thus, a spatially selective remodeling of primary dendrites and consolidation of distal GnRH dendritic arbors occurs during postnatal development and is largely independent of T.
The postnatal period of development in mammals is characterized by a reproductive quiescence that extends, depending on the species, for several weeks or years. This delay in the onset of gonadal activity reflects a decrement in the release of GnRH, from the GnRH-containing neurons in the hypothalamus (1). The onset of puberty is a consequence of an increase in the secretion of GnRH (1, 2). However, the mechanisms that drive the onset and sustain the progression of the pubertal increase in GnRH release are unknown.
The population of GnRH neurons in adults is generally described as possessing a simple uni- or bipolar morphology and assuming a vertical orientation in the hypothalamus (Ref. 3; see Ref. 4 for review). Developmentally, somata of GnRH neurons can be visualized through immunoreactivity for the GnRH peptide as early as embryonic d 15, when GnRH neurons reside in the olfactory system. Within days, processes of GnRH neurons can also be detected. The prenatal morphology of GnRH neurons has been described as fusiform and with one or two short processes (5, 6).
Transgenic mouse models where GnRH neurons express green fluorescent protein (GFP) have made it possible to fill many hypothalamic GnRH neurons in brain slices with cellular markers such as biocytin (7, 8). This has increased our ability to obtain high-fidelity anatomical reconstructions because the biocytin may reach regions of neurons where peptide may be limited for immunohistochemical-based detection. Transgenic animals have also allowed for highly quantitative morphological assessments because many more GnRH neurons can now be readily identified, filled in thicker, living slices allowing for more optimal preservation of morphology. A direct comparison of the immunohistochemical and biocytin-based approaches indicate that whereas GnRH somata were roughly comparable between immuno- and biocytin detection methods, significantly longer dendrites were identified with biocytin filling (8).
Recent studies have suggested that changes in the morphology of GnRH neurons may be important in the onset of puberty. Prepubertal GnRH neurons in mice exhibit a highly branched dendritic structure before assuming the classic bipolar morphology in adulthood (9). Cottrell et al. (9) reported increases in dendritic spines in mice [similar to the earlier suggestion of Wray and Hoffman (10) in rats] as puberty approached. Interestingly, Cottrell et al. (9) also found 5-fold more branch points in GnRH dendrites from juvenile mice than in adults when studying morphology in live hypothalamic slices using confocal microscopy and cellular filling. These findings indicate GnRH dendrites may undergo a dramatic structural remodeling during the postnatal period.
Changes in global morphology of dendrites likely have functional consequences in the control of GnRH neuronal firing because morphology can substantially influence the electrical filtering properties of dendrites (11). We examined this issue in rats to determine whether the late postnatal remodeling of GnRH neurons is a conserved feature of GnRH neurons during postnatal development. Subsequently, we determined whether the remodeling of GnRH dendrites was dependent on exposure to testosterone (T) during the peripubertal phase of development.
Materials and Methods
Animals
All studies used male rats where GnRH neurons express GFP (12). Animals were maintained on a 12-h light, 12-h dark cycle with ad libitum access to standard rodent chow and water. All procedures performed on animals were reviewed and approved by the IACUC at University of Texas, San Antonio and were in accord with the NIH Guide for Care and Use of Laboratory Animals.
Seven groups of male rats were used. Average ages, number of animals, and number of neurons studied for all groups are shown in Table 1. For the first series of experiments, gonad-intact males were studied either as infants or adults. We selected the age of less than 15 d to study infants because animals were large enough at this time to ensure adequate blood collection for hormone assays but this age was before any developmental shift in synthetic activity of GnRH neurons based pro-GnRH mRNA measurements in rats (13). For T-replacement experiments, GnRH neurons were filled between 40 d and 48 d of age from castrated males, a group of intact males and three groups of males with T-containing implants. The latter groups were bilaterally castrated [using an abdominal approach (14) at 25 d of age under isoflurane anesthesia with aseptic technique. The three T-groups were implanted with SILASTIC capsules 10 mm in length (inner diameter: 0.062 inches, outer diameter: 0.125 inches; Dow Corning, Midland, MI) containing testosterone propionate (Sigma, St. Louis, MO). The number of capsules varied from two to five to generate three levels of circulating T (low, middle, and high). Capsules in castrates were empty. All capsules were sealed with SILASTIC brand adhesive (Dow Corning), allowed to cure for 48 h, incubated for 48 h in three changes of PBS in 0.5% gelatin, and gas sterilized (15).
Table 1.
Animal groups
| Group | Age (days) | T (ng/ml) | LH (ng/ml) | No. of animals (N) | No. of neurons (n) |
|---|---|---|---|---|---|
| Intact-infant (I-INF) | 12.88 ± 0.26 | 0.40 ± 0.20 | ND | 15 | 76 |
| Intact-juvenile (I-JV) | 42.67 ± 0.56 | 1.94 ± 0.44 | 0.49 ± 0.14 | 6 | 20 |
| Intact-adult (I-AD) | 71.78 ± 2.31 | 2.26 ± 0.32a | 1.23 ± 0.41 | 25 | 37 |
| Castrate | 43.0 ± 0.86 | 0.33 ± 0.25 | 3.66 ± 0.75b | 8 | 35 |
| Castrate-(low-T) | 43.67 ± 0.86 | 1.82 ± 0.31 | 0.81 ± 0.41 | 9 | 30 |
| Castrate- (mid-T) | 41.0 ± 1.32 | 3.40 ± 0.54a | ND | 6 | 27 |
| Castrate-(high-T) | 41.8 ± 0.74 | 7.56 ± 0.95b | ND | 5 | 21 |
ND indicates LH levels below detection in the assay.
Denotes significance when compared with Castrate and I-INF.
Denotes significance when compared with all groups.
Tissue preparation
After decapitation under isoflurane anesthesia, brains were removed and immediately placed in cold (1−2 C), artificial cerebrospinal fluid solution containing (in millimolar concentration): NaCl (124), NaHCO3 (26), NaH2PO4 (1.25), KCl (2.5), CaCl2 (2), MgCl2 (2), and glucose (10), equilibrated with 95% O2/5% CO2, pH 7.3–7.4. Trunk blood was collected from each animal at the time of decapitation. Blood was stored over night at 0 C. Samples were then separated by centrifugation. Serum was collected and stored at −20 C until assay. LH and T levels were assayed by the Ligand Assay and Analysis Core Laboratory at the University of Virginia. The LH assays used the RP-3 standard. Minimum detection was at 0.07 ng/ml with 2.8% and 8.0% intra and interassay coefficients of variation. The T assays were performed using the Siemens total testosterone kit (Siemens Medical Solutions Diagnostics, Los Angeles CA) with minimum detection at 10 ng/dl. Intra and interassay coefficients of variation were 3.5% and 8.3%, respectively.
For all studies, live hypothalamic slices (300 μm) were prepared using a vibrating microtome (HM 650V; Sigmann Elektronik, Hüffenhardt, Germany). All slices were prepared in sagittal orientation to maximally preserve dendrites. After incubation in artificial cerebrospinal fluid for 1–2 h at 32 C, slices were transferred to a recording chamber mounted on the stage of an upright microscope (Axioskop 2 FS plus, Carl Zeiss Microimaging, Inc., Thornwood, NY), and continuously perfused with artificial cerebrospinal fluid (32 C). GnRH neurons were identified through their GFP expression using epifluorescent excitation at 470 nm with a 60 × water immersion Olympus objective (Olympus Corp., Lake Success, NY).
Pipettes (0.86 mm inner diameter, 1.5 mm outer diameter; 9–12 MΩ) were made from borosilicate glass (AM Systems, Carlsborg, WA) using a pipette puller (PC-10; Narishige, Tokyo, Japan) and coated with Sylgard 184 (Dow Corning) to minimize pipette capacitance. Pipettes were filled with (in millimolar concentration): K-chloride (119.5), HEPES (10), EGTA (0.2), NaCl (6), MgCl2 (2), NaATP (4), NaGTP (0.4), spermine (0.05), and glutathione (5) with 0.5% biocytin. Piezoelectric micromanipulators (Luigs and Neumann, Ratingen, Germany) were used to position pipettes.
Live cell filling with biocytin
After achieving the whole-cell recording configuration, GnRH neurons were filled with biocytin via diffusion from the pipette solution for 15–20 min. Pipettes were gently retracted from the somatic surface allowing the plasma membrane to loosen and the ruptured membrane patch to seal. Immediately after cell filling, slices containing biocytin-filled GnRH neurons were placed in 4% paraformaldehyde. Biocytin-containing neurons were identified 24 h later with NeutraAvidin, Cascade Blue Conjugate antibody (A-2663; 1:100; Invitrogen, Carlsbad, CA). Slices were washed three times (10 min each) with PBS on a shaker at room temperature (RT). Slices were then blocked with 600 μl of 5% BSA, 2% Triton-X100, and 6% normal goat serum in PBS for 1.5 h on a shaker at RT, or overnight at 4 C. The slices then were immersed in 250 μl of fresh block and 2.5 μl of the Cascade Blue Conjugate antibody. Slices were then incubated for 5–7 d at 4 C. After incubation, all slices were washed three times (10 min each) with PBS on a shaker at RT and the presence of Cascade Blue-labeled cells, as well as the continued presence of endogenous GFP expression, was verified by epifluorescence microscopy using a standard 4′,6-diamidino-2-phenylindole and fluorescein isothiocyanate filter set, respectively.
Slices containing Cascade Blue-labeled and GFP-expressing cells were then reincubated in 250 μl of the fresh block as described above along with RU11B GnRH antibody (1:12.5) or alternatively, anti-GnRH1 (Sigma, 1:100) for 5–7 d. After incubation, these slices were washed three times (10 min each) with PBS on a shaker at RT and the rhodamine goat-hosted antirabbit secondary antibody (A11036, 1:100; Invitrogen) was applied with fresh, modified blocking solution with 0.2% Triton-X100 replacing the normal 2% Triton-X100 used previously above, and incubated for 2 h at RT on a light-protected shaker.
Confocal acquisition
Images were collected using a Zeiss LSM 510 Meta laser scanning confocal microscope with Revision 4 of the acquisition software (Carl Zeiss, Heidelberg, Germany). We excited the GFP with the system's Argon Gas Laser equipped with a 488-nm line. To excite the Rhodamine secondary we used a Helium-Neon laser 543-nm line. To excite the Cascade Blue, a 405-nm laser line was used. For visualization of the endogenous GFP expression, an emission filter of 505–560 nm was used. To visualize the Rhodamine secondary an LP 560 emission filter was used. To visualize the Cascade Blue, an LP 420-nm emission filter was used. Biocytin-containing GnRH neurons were then reacted with avidin-biotin-horseradish peroxidase and 3,3′ diaminobenzidine with nickel enhancement. Slices were mounted on glass slides followed by anatomical reconstruction using the Neurolucida System (Microbrightfield, Inc., Williston, VT).
Statistical analysis
Statistical analyses were performed on individual neurons from each animal group throughout the study. Hormone levels and morphological comparisons of soma area, total dendritic length, total branch number, numbers of branch orders, and average lengths of dendrites per branch order were performed using a one-way ANOVA and a post hoc Tukey test. A value of P < 0.05 was considered significant.
Results
Hormone levels
Levels of T confirmed by RIA of serum derived from trunk blood collected at the time of decapitation are shown in Table 1. Circulating levels of LH for each group of animals are also shown in Table 1. There was a progressive increase in both T and LH in intact animals with advancing age. The increase in T and LH reached significance in adulthood. Circulating levels of T also increased based on the number of T-containing implants placed in animals that were castrated at 25 d of age.
Morphological parameters of prepubertal and adult GnRH neurons from rats based on biocytin filling
Pruning of primary dendrites
Figure 1 compares somatic area (Fig. 1A) and total dendritic length (Fig. 1B) between infant (n = 76 neurons) and adult (n = 37 neurons) males based on biocytin filling in live slices. Total dendritic length is the additive sum of all primary dendrites and associated branches for any given neuron. Somatic area (248.5 ± 29.4 μm2 vs. 145.4 ± 9.8; P = 0.01; Fig. 1A) and total dendritic length (523.4 ± 57.7 vs. 320.4 ± 41.6 μm; P = 0.01; Fig. 1B) were both significantly greater in infant males than in adults.
Fig. 1.
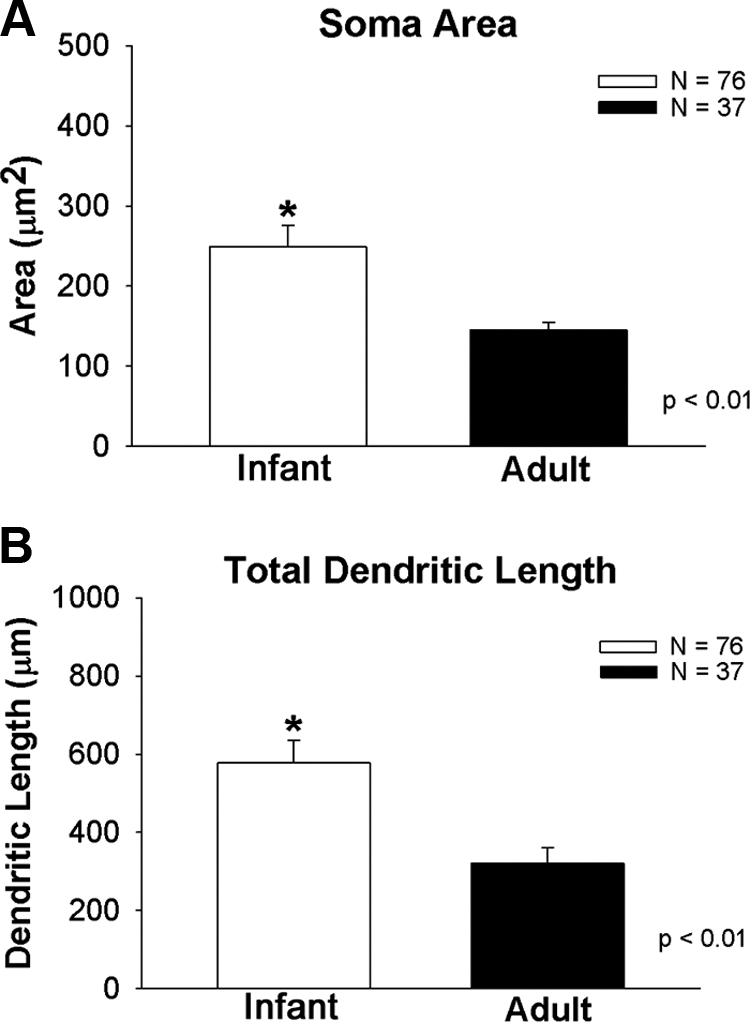
A, Differences in somatic area between GnRH neurons derived from infant and adult males. B, Differences in total dendritic length between GnRH neurons derived from infant and adult males. Total dendritic length is the additive sum of all primary dendrites and associated branches for any given neuron. Asterisks indicate a significant difference between groups.
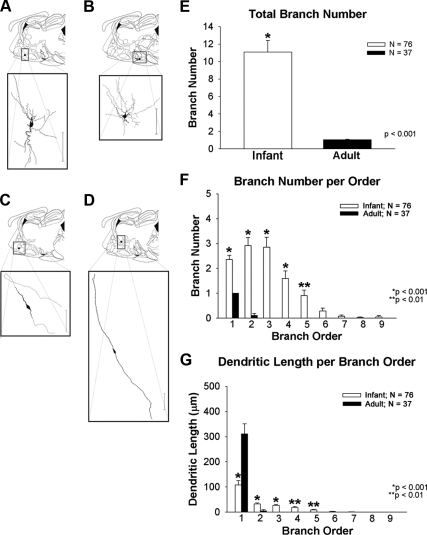
Figure 2, A–D, shows representative topology of infant and adult and GnRH neurons. GnRH neurons from infant males had multiple primary dendrites (Fig. 2A and B). Adult neurons (Fig. 2, C and D) exhibited bipolar morphology. Dendrites in 30% of adults (11/37) turned laterally, arched from the distal pole of somata (relative to the axon), and followed the trajectory of the GnRH axon as previously reported in adult mice (Fig. 2C) (16). Others assumed the classical bipolar topology with the dendrite continuing its course in the opposite direction of the axon (Fig. 2D).
Fig. 2.
Morphological differences between dendrites of GnRH neurons derived from infant and adult males. Top portion of each panel (A–D) indicates the anatomical location of representative biocytin-filled GnRH neurons shown in the bottom panel. Infant GnRH neurons are shown in A and B. Adult GnRH neurons are shown in C and D. Scale bars, 100 μm. E, Indicates that the total numbers of branches is higher in infant males than adults. There is also more, higher order branching in infant GnRH neurons than adult GnRH neurons (F). Dendritic length per branch order is shown in G. Branch order and length comparisons were only performed between infant and adult groups where branches existed in both groups. Asterisks indicate significant differences as noted by P values.
Consolidation of distal dendrites between prepubertal and adult males
Specifics of the morphological differences between infant and adult male GnRH neurons are shown in Fig. 2, E–G. Total branch number, which is an indicator of the total number of individual dendritic segments of a neuron, was significantly greater in infant males compared with adults (10.3 ± 1.4 vs. 1.06 ± 0.06; P < 0.001; Fig. 2E). The number of dendritic branch orders or the number of proximal and more distal dendritic segments from the soma revealed a greater number of order branches in infant males than adults (2.2 ± 0.2 vs. 1.0 ± 0.0; 2.6 ± 0.3 vs. 0.1 ± 0.07; 2.6 ± 0.4 vs. 0.0; 1.5 ± 0.3 vs. 0.0; 0.8 ± 0.2 vs. 0.0 branches; P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.01; respectively; Fig. 2F). The average lengths of second, third, fourth, and fifth order branches were significantly greater in infants than adults (28.9 ± 4.3 vs. 4.9 ± 4.7; 23.4 ± 3.9 vs. 0.0; 17.5 ± 4.1 vs. 0.0; 8.9 ± 2.4 vs. 0.0 μm; P < 0.001, P < 0.001, P < 0.001, P ≤ 0.01, P = 0.01; respectively; Fig. 2G). However, primary dendrites were longer in adults than in infants.
Spatially selective remodeling of GnRH neurons between prepubertal and adult males
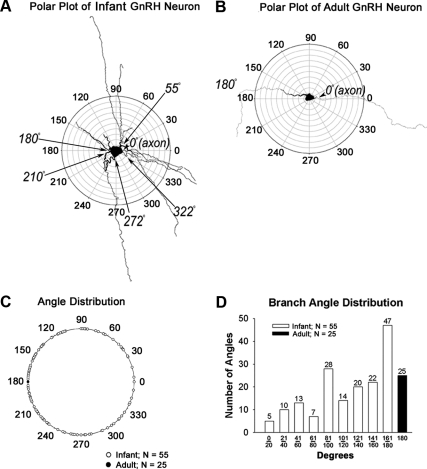
Figure 3 compares topology of GnRH neurons in infant and adult males using a polar plot analysis to quantify differences in locations of primary dendrites. For this analysis, biocytin-filled GnRH neurons were superimposed on a polar plot diagram with the axon oriented at 0° (at arrowhead in Fig. 3A and B). The angles of dendrites were then measured relative to the axon at 0° along radial lines.
Fig. 3.
Polar plot analysis of an infant (A) and an adult GnRH neuron (B). For polar plots, the axon of each GnRH neuron was aligned to 0°. The angles at which dendrites emerged from the soma were then determined and plotted on a single diagram (C) for infant (shown in white circles) and for adult (shown in black circle) males. D, Distribution of dendritic angles for primary dendrites. Only neurons in which clear axons emerged from somata were included in this analysis.
In Fig. 3A and B, biocytin-filled GnRH neurons from an infant (Fig. 3A) and adult (Fig. 3B) animal are shown. Infant GnRH neurons were comprised of one single axon and several primary dendrites (Fig. 3A; also see Fig. 2, A and B). In contrast, adult GnRH neurons were comprised of a single axon and a single dendrite oriented 180° with respect to each other (Fig. 3B; also see Fig. 2, C and D). In Fig. 3C, locations of infant dendrites are shown by open circles on the outer radian. The location of dendrites in adults is shown by the filled circle on the outermost radian. Whereas infant dendrites were distributed throughout 360°, the dendrite of adult GnRH neurons resided exclusively at 180°. The total number and distribution of dendritic angles were then binned in 20° increments for GnRH neurons from infant males and in adults (Fig. 3D) using a 180° axis based on both clockwise and counter clockwise directions in positive angles. Neurons without axons for reference were excluded from this analysis (n = 12 adult neurons). In infant GnRH neurons, angles of orientation varied between 5° and 360° with respect to the axon whereas in adults the axo-dendritic angle was consistently 180°. The most prominent single location for primary dendrites in prepubertal males was between 161° and 180° (n = 47), but the remaining 119 primary dendrites (72%) were in alternative locations. The sole location for the primary dendrites in GnRH neurons with axons remaining for reference (n = 25) was 180°.
Remodeling of GnRH dendrites is independent of T
Pruning of primary dendrites in control and T-treated males
Figure 4 indicates that the changes in area of GnRH somata and in GnRH dendrite length are largely independent of T. The average area of GnRH somata in intact, castrated, and T-treated males was not significantly different (Fig. 4A). Likewise, total dendrite length was comparable between intact, castrated, and T-treated males (Fig. 4B). In intact and castrated males 26.5% of the primary dendrites arched and projected into the axon field whereas in T-treated groups, 29% of primary dendrites followed this course. The total branch number in intact, castrate, and T-treated animals in large part was not different (Fig. 4C). Based on our power analysis, the number of neurons was sufficient to detect differences between groups if differences existed.
Fig. 4.
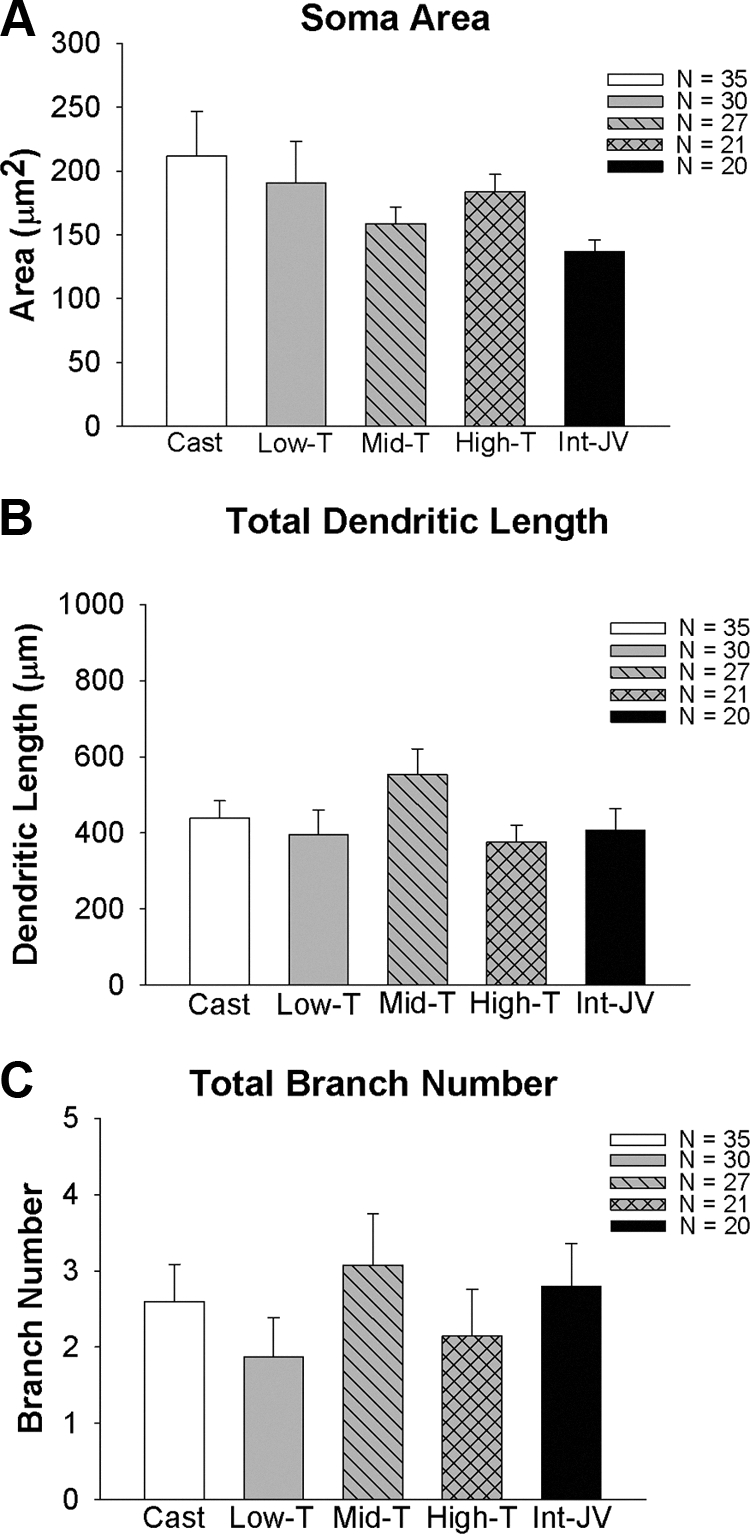
Somatic area (A), total dendritic length (B), and total branch number (C) in GnRH neurons derived from juvenile males that were castrated at 25 d of age and were either implanted with empty implants (castrates) or implants containing one of three levels of T. A group of intact males was also studied. T levels are shown in Table 1.
Consolidation of distal dendrites in control and T-treated males
Figure 5 indicates that the distal dendritic consolidation is also independent of T. Branch numbers per order (Fig. 5A) and dendrite length per branch order (Fig. 5B) in general did not differ between intact, castrated, and T-treated animals. The only significant difference between groups was between low and middle T treatment, which resulted in more branching in first-order branches than low T. By 40–48 d of age, the number of primary dendrites was reduced in both control and T-treated animals relative to infants (Fig. 2F). The length of dendrites in lower order branches was not influenced by T (Fig. 5B).
Fig. 5.
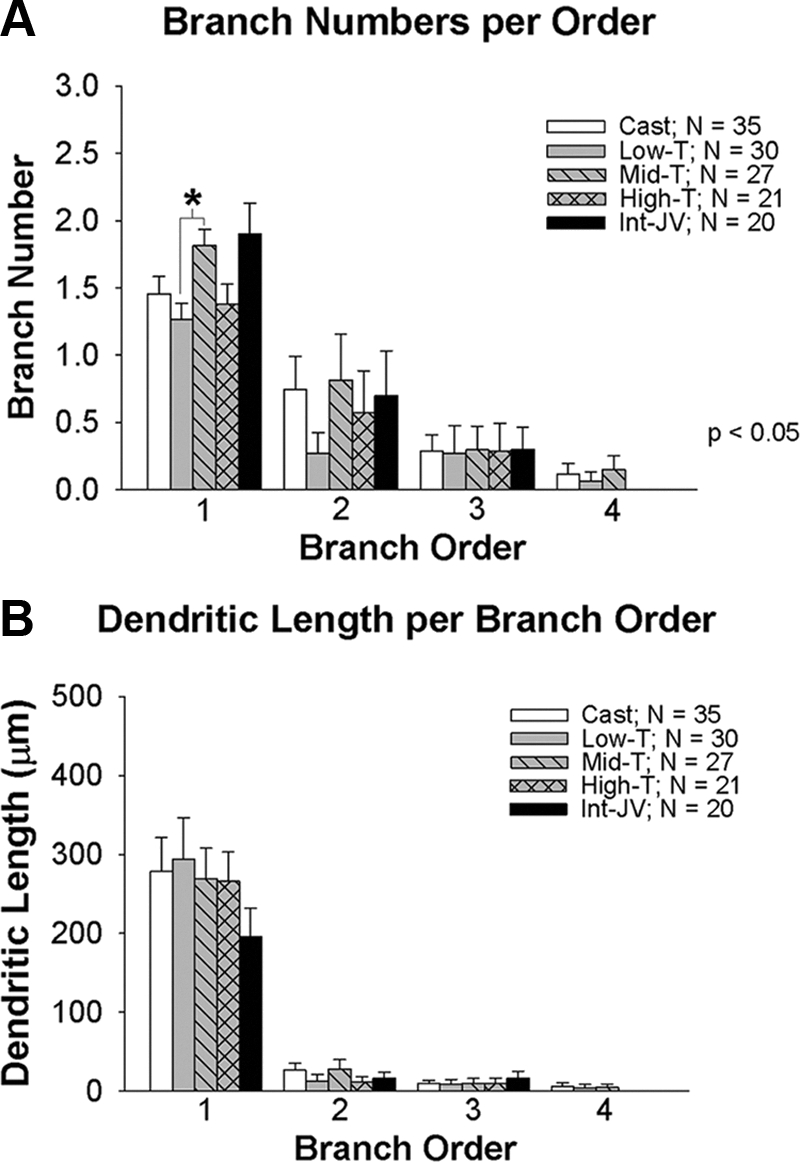
Total numbers of branches per order (A) and dendritic length per branch order (B) of dendrites in GnRH neurons from juvenile males that were castrated at 25 d of age and were either implanted with empty implants (Cast) or implants containing one of three levels of T (Low-T, Mid-T, High-T). A group of intact juvenile males (Int-JV) was also studied. T levels are shown in Table 1. Asterisks indicate a significant difference between groups.
Spatially selective pruning in control and T-treated males
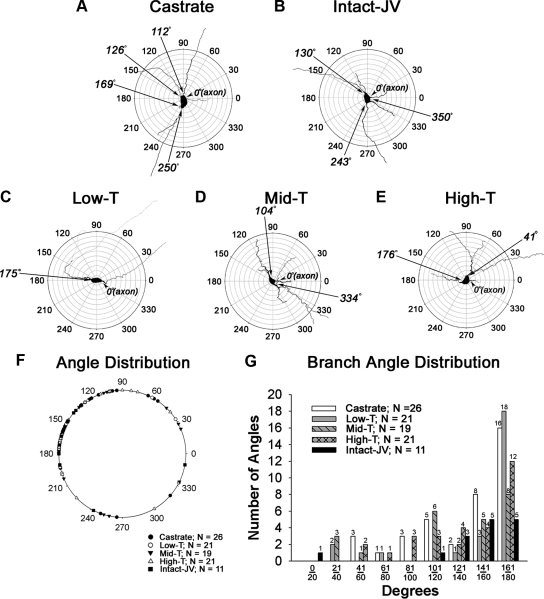
Representative biocytin-filled GnRH neurons from control and T-treated males are shown in Fig. 6, A–D. Topology had not yet reached the adult configuration in any group. The location of dendrites at 40–48 d of age remained highly variable (Fig. 6, F and G). The most prominent single location for dendrites was between 161° and 180° for most groups. In castrated animals, 76% of dendrites were located between 161° and 180°. In males treated with low T, 86% of primary dendrites resided between 161° and 180°. In middle T, high T, and intact juveniles, 42%, 57%, and 45% of primary dendrites were located between 161° and 180°.
Fig. 6.
Polar plot analysis of GnRH neurons derived from juvenile males that were castrated at 25 d of age were implanted with empty implants (A), left intact and not treated (B), or castrated and treated with implants containing one of three levels of T (C–E). For polar plots, the axon of each GnRH neuron was aligned to 0°. The angle at which dendrites emerged from the soma was then determined and plotted on a single diagram (F). G, Shows the distribution of dendritic angles. Only neurons in which clear axons emerged from somata were included in this analysis. JV, Juvenile.
Discussion
Initial studies with GFP-identified biocytin-filled GnRH neurons from adult mice concluded that primary dendrites were about 125 μm and exhibited modest branching (8). The later studies of Campbell et al. (17) and Roberts et al. (16) provided additional insight regarding the morphology of GnRH neurons. Campbell et al. (17) studied biocytin-filled GnRH neurons and found lengths of about 300 μm. In our earlier study of adult GnRH dendrites in mice, we found average dendrite lengths of 500 μm (16). In both studies, some biocytin-filled dendrites exceeded 1200 μm in length, and some dendrites exited the plane of slices, thereby preventing measurement of their total length. Thus, dendrites of GnRH neurons in adult mice are much longer than previously appreciated. The second important observation regarding morphology of GnRH neurons from the above studies was that the dendrite often branches (16, 17). Moreover, often dendritic branches did not assume the classical vertical orientation in the hypothalamus but rather arched and project back toward GnRH somata and closely follow the trajectory of the GnRH axon toward the median eminence (16).
Our findings indicate the dendrites of GnRH neurons in rats are equally complex, particularly in prepubertal animals. First, the above dendritic course toward the median eminence observed in mice also occurs in dendrites of GnRH neurons from adult rats. This finding renews the potential that regulation of activity of GnRH neurons could occur via exposure of dendrites to substances in the median eminence. Thus, although axons of GnRH neurons have been proposed as a target of modulation at the level of the median eminence (18), our findings indicate that GnRH dendrites are an equally plausible substrate. Second, it appears based on the present data, that GnRH dendrites assume their course toward the median eminence at sometime during pubertal maturation. This finding is quite different than the establishment of the axon terminals in the median eminence that occurs about 2 d after positioning of GnRH neurons in the preoptic area after migration from the olfactory system (19). Therefore, the mechanisms that direct GnRH nerve terminals to the median eminence may persist and then direct dendrites along this course later in development. Alternatively, a distinct set of guidance cues may be expressed during postnatal development and direct GnRH dendrites to the median eminence.
In the present study, we focused on the postnatal remodeling of GnRH dendrites and its relationship to the timing of the pubertal increase in hormone secretion. We found that the number of primary dendrites emanating from somata and branch order of dendrites in infant rats was significantly greater than those in adult GnRH neurons, indicating both a proximal remodeling and distal consolidation of dendrites during the peripubertal period. The finding of multiple primary dendrites in GnRH neurons of infant males is intriguing, particularly because our preliminary data indicate that these multipolar putative GnRH-GFP neurons are immune positive for the GnRH peptide. Neurons from the amygdala of intact Syrian hamsters undergo pruning of primary dendrites (i.e. those emanating from somata) during puberty but branch orders did not differ (20). However, neurons in the amygdala lack the strict topology of GnRH neurons. In adults of multiple species including rats, the primary dendrite of GnRH neurons consistently resides at a 180° angle from the GnRH axon whereas dendrites in infant males and males of older prepubertal ages arise at multiple angles. Thus, it seems GnRH neurons undergo both an anatomical consolidation of dendritic branching in distal dendrites [as also seen in mice (9)] in addition to a spatially specific process of primary dendritic pruning (at the level of somata) during postnatal development to achieve their final adult morphology.
Perhaps the most interesting finding of the present study is the positioning of a remaining primary GnRH dendrite at 180° relative to the axon. This may occur through simple pruning (e.g. removal of all dendrites not residing at 180° from the axon). Earlier work in cortical neurons indicated that dendrites can move about in the space surrounding the cell body (21). Thus, GnRH neurons may undergo a process of dendritic selection from the initial pool of their primary GnRH dendrites, and this single, selected dendrite could relocate to the final position at 180° from the axon. A final possibility is that the reduction in size of somatic area between infants and adults (which has not been previously reported and thus, may reflect a species difference between rats and mice) might contribute to the alignment of the primary dendrite at 180°.
In either regard, the findings of a more complex dendritic arbor in infants (in which GnRH neurons presumably have a lower level of activity based on hormone secretion) might seem contradictory. The importance of dendritic branching was demonstrated in very early compartmental models of neurons, namely branch points shunt passive currents (22, 23). In our earlier study using multicompartmental models of GnRH neurons in adults, the impact of structure on repetitive firing in adults was exerted in models with both active and passive dendrites (11). Thus, although dendrites of adult GnRH neurons exhibit active properties (24), structural differences alone can make a defining contribution to neuronal function through their impact on passive electrical properties. The branching patterns at the level of GnRH somata may exert additional complex effects on neuronal activity that are only partially accounted for by distal branching in dendrites. Therefore, structural remodeling of the GnRH neuron may facilitate the shift in neuronal behavior at the time of puberty.
The question then becomes what drives the pubertal consolidation of GnRH neurons? Gonadal steroids have profound effects on neuronal morphology (25). During the period of late postnatal development in males, there is a conspicuous rise in T. Taken together, this suggested that rising levels of T might drive these structural alterations. This would indicate that the changes in morphology are a postpubertal event (26) because the pubertal rise in T occurs in response to activation of GnRH neurons at the time of puberty. In medial amygdala neurons, an earlier study indicated that synaptic innervation increases in intact male rats between pre- and postpuberty but dendrite morphology was not examined (27). In a second study, prepubertal gonadectomy reduced dendritic spines. However, gonadectomy did not alter total dendrite length or branching (28). Therefore, we tested the hypothesis that pre- and peripubertal T exposure was responsible for alterations in the morphology of GnRH neurons, and this occurred during the immediate postpubertal period.
Surprisingly, our findings indicate that a majority of the morphological changes in GnRH neurons were largely independent of T. The structural changes detailed in the present study were underway before puberty would have been anticipated had the animals remained gonadally intact (e.g. between 40 d and 45 d of age). Moreover, alterations in the proximal and distal dendrites of GnRH neurons were similar independent of the level of T replacement. Therefore, the pubertal increase in T probably does not lead to either the reduction in primary dendrites or the consolidation of distal dendritic arbors that occurs in postnatal GnRH neurons.
In rodents, the pubertal increase in GnRH release appears to reflect, in part, a developmental decrease in the sensitivity to negative feedback by gonadal hormones because castration during the prepubertal period results in an increase in circulating levels of gonadotropins (29, 30) but not to adult levels. This indicates that the prepubertal restraint on GnRH secretion in rodents is at least somewhat dependent on the gonad. Relatively little is known regarding control of the secondary rise in gonadotropin and presumably GnRH secretion that occurs in castrated rodents (and therefore is steroid independent) at the time of puberty. In male Syrian hamsters, control of GnRH mRNA is independent of T during the prepubertal period, making it a candidate for the nonsteroidal control of rodent puberty (31). Our findings indicate that the consolidation of distal GnRH dendrites and spatially selective remodeling of primary dendrites may also be part of the long repudiated nongonadal component of the control of the onset of puberty in rodents.
Acknowledgements
We thank M. Kato for providing founders from their group's GnRH-GFP rat line. We thank H. Urbanski for generous provisions of HU 11B (GnRH primary antibody). We also thank Drs. Charles Wilson (University of Texas San Antonio, San Antonio, TX) and Vernon Gay (University of Pittsburgh, Pittsburgh, PA) for insightful discussions. Assays for LH and T were performed at University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core National Institute of Child Health and Human Development (SCCPRR) Grant U54-HD28934.
This work was supported by Grant HD-060818. Assays for LH and T were performed at University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core National Institute of Child Health and Human Development (SCCPRR) Grant U54-HD28934. A preliminary report of these data was presented at The 92nd Annual Meeting of The Endocrine Society, San Diego, CA, and The 40th Annual Meeting of The Society for Neuroscience, San Diego, CA.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- GFP
- Green fluorescent protein
- RT
- room temperature
- T
- testosterone.
References
- 1. Urbanski HF, Ojeda SR. 1987. Activation of luteinizing hormone-releasing hormone release advances the onset of female puberty. Neuroendocrinology 46:273–276 [DOI] [PubMed] [Google Scholar]
- 2. Harris GC, Levine JE. 2003. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology 144:163–171 [DOI] [PubMed] [Google Scholar]
- 3. Merchenthaler I, Görcs T, Sétáló G, Petrusz P, Flerkó B. 1984. Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res 237:15–29 [DOI] [PubMed] [Google Scholar]
- 4. Silverman A-J, Livne I, Witkin JW. 1994. The gonadotropin releasing-hormone (GnRH) neuronal systems: immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD. eds. The physiology of reproduction. New York: Raven Press; 1683–1710 [Google Scholar]
- 5. Jennes L. 1989. Prenatal development of the gonadotropin-releasing hormone-containing systems in rat brain. Brain Res 482:97–108 [DOI] [PubMed] [Google Scholar]
- 6. Tobet SA, Crandall JE, Schwarting GA. 1993. Relationship of migrating luteinizing hormone-releasing hormone neurons to unique olfactory system glycoconjugates in embryonic rats. Dev Biol 155:471–482 [DOI] [PubMed] [Google Scholar]
- 7. Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. 1999. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. 2000. Genetic targeting of green fluorescent protein to GnRH neurons: Characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- 9. Cottrell EC, Campbell RE, Han SK, Herbison AE. 2006. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147:3652–3661 [DOI] [PubMed] [Google Scholar]
- 10. Wray S, Hoffman G. 1986. Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology 43:93–97 [DOI] [PubMed] [Google Scholar]
- 11. Roberts CB, O'Boyle MP, Suter KJ. 2008. Dendrites determine the contribution of after depolarization potentials (ADPs) to generation of repetitive action potentials in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. J Comput Neurosci 26:39–53 [DOI] [PubMed] [Google Scholar]
- 12. Kato M, Ui-Tei K, Watanabe M., Sakuma Y. 2003. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology 144:5118–5125 [DOI] [PubMed] [Google Scholar]
- 13. Jakubowski M, Blum M, Roberts JL. 1991. Postnatal developmental of gonadotropin-releasing hormone and cyclophilin gene expression in the female and male rat brain. Endocrinology 128:2702–2708 [DOI] [PubMed] [Google Scholar]
- 14. Brown C. 2008. Intra-abdominal castration in the rat. Lab Animal 37:73–74 [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto AM, Karpas AE, Southworth MB, Dorsa DM, Bremner WJ. 1986. Evidence for activation of the central nervous system-pituitary mechanism for gonadotropin secretion at the time of puberty in the male rat. Endocrinology 119:362–369 [DOI] [PubMed] [Google Scholar]
- 16. Roberts CB, Best JA, Suter KJ. 2006. Dendritic processing of excitatory synaptic input in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. Endocrinology 147:1545–1555 [DOI] [PubMed] [Google Scholar]
- 17. Campbell RE, Han SK, Herbison AE. 2005. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology 146:1163–1169 [DOI] [PubMed] [Google Scholar]
- 18. Yin W, Mendenhall JM, Bratton SB, Oung T, Janssen WG, Morrison JH, Gore AC. 2007. Novel localization of NMDA receptors within neuroendocrine gonadotropin-releasing hormone terminals. Exp Biol Med 232:662–673 [PubMed] [Google Scholar]
- 19. Wu TJ, Gibson MJ, Rogers MC, Silverman AJ. 1997. New observations on the development of the gonadotropin-releasing hormone system in the mouse. J Neurobiol 33:983–998 [DOI] [PubMed] [Google Scholar]
- 20. Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. 2006. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol 66:578–590 [DOI] [PubMed] [Google Scholar]
- 21. Horch HW, Krüttgen A, Portbury SD, Katz LC. 1999. Destabilization of cortical dendrites and spines by BDNF. Neuron 23:353–364 [DOI] [PubMed] [Google Scholar]
- 22. Rall W, Rinzel J. 1973. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys J 13:648–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rinzel J, Rall W. 1974. Transient response in a dendritic neuron model for current injected at one branch. Biophys J 14:759–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts CB, Campbell RE, Herbison AE, Suter KJ. 2008. Dendritic action potential initiation in hypothalamic gonadotropin releasing hormone (GnRH) neurons. Endocrinology 149:3355–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cooke BM, Woolley CS. 2005. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol 64:34–46 [DOI] [PubMed] [Google Scholar]
- 26. Plant TM. 2007. Gonadotropin-releasing hormone neuron remodeling: causal for puberty onset? Trends Endocrinol Metab 18:50–51 [DOI] [PubMed] [Google Scholar]
- 27. Cooke BM, Woolley CS. 2009. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev Neurobiol 69:141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Witkin JW, Romero MT. 1995. Comparison of ultrastructural characteristics of gonadotropin-releasing hormone neurons in prepubertal and adult male rats. Neuroscience 64:1145–1151 [DOI] [PubMed] [Google Scholar]
- 29. Goldman BD, Grazia YR, Kamberi IA, Porter JC. 1971. Serum gonadotropin concentrations in intact and castrated neonatal rats. Endocrinology 88:771–776 [DOI] [PubMed] [Google Scholar]
- 30. Eldridge JC, Dmowski WP, Mahesh VB. 1974. Effects of castration of immature rats on serum FSH and LH, and of various steroid treatments after castration. Biol Reprod 10:438–446 [DOI] [PubMed] [Google Scholar]
- 31. Richardson HN, Gore AC, Venier J, Romeo RD, Sisk CL. 2004. Increased expression of forebrain GnRH mRNA and changes in T negative feedback following pubertal maturation. Mol Cell Endocrinol 214:63–70 [DOI] [PubMed] [Google Scholar]