The melanocortin receptors (MCR) are highly unusual, if not unique, among the extensive family of seven transmembrane domain G protein-coupled receptors (GPCR) because their activity is mediated both by endogenous peptide agonists and endogenous antagonists. Classical genetic studies of coat color in the mouse revealed two nonallelic recessive loci, extension (e) and agouti (a), whose loss-of-function mutations result in yellow or black fur, respectively. E encodes the MCR type 1 (MC1R) that is activated by the proopiomelanocortin (POMC)-derived agonist MSH to stimulate the production of black eumelanin by elevation of cAMP (1). A encodes the agouti signaling protein that antagonizes binding of MSH to MC1R and results in the production of yellow/red pheomelanin by reduction of cAMP (2). Experiments using mouse B16 melanoma cells, which express high levels of the MC1R, showed that agouti could inhibit melanogenesis and cell growth even in the absence of MSH in the culture medium, suggesting that agouti can function as a competitive antagonist or an inverse agonist at the MC1R (3) (Fig. 1).
Fig. 1.
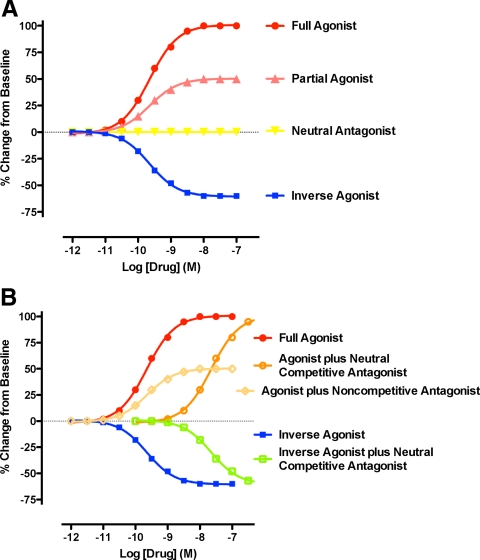
Theoretical dose-response curves for an arbitrary measure of GPCR activation in response to different pharmacological classes of ligands. A, Most GPCR have measureable constitutive activity in the absence of ligand binding. Agonists bind to the receptor and promote the active state. Full agonists (red) exhibit maximum possible efficacy, whereas partial agonists (pink) are less efficacious. Inverse agonists (blue) bind to the receptor and promote an inactive state; i.e. they exhibit negative efficacy. Inverse agonism can be detected only under conditions of constitutive receptor activity. A neutral or competitive antagonist (yellow) binds to the receptor at the same site as endogenous agonists (orthosteric) but has no effect on receptor activity when used alone. B, A neutral competitive antagonist at fixed high dose shifts the dose-response curve of either an agonist (orange) or inverse agonist (green) to the right without altering maximal efficacy. Noncompetitive antagonists (beige) bind to an allosteric site or one different from the orthosteric site and alter the receptor conformation needed for activity. As a result, the dose-response curves of agonists are changed, and efficacy may be reduced.
There has been increasing acceptance of the concepts of GPCR constitutive activity and inverse agonism in the past decade (4), almost to the point of dogma for the melanocortin system in particular (5). Indeed, studies from our laboratory using POMC-deficient mice crossed onto an a/a genetic background confirmed that the wild-type mouse MC1R has sufficient constitutive activity to result in normal black eumelanin pigmentation and, therefore, indicate that agouti can act as an inverse agonist in vivo to cause a shift from black to yellow pigmentation (6).
Analogous to the peripheral MC1R/agouti system and its effects on pigmentation, agouti-related peptide (AgRP), which is expressed predominantly in the brain, can function as a competitive antagonist at central MC4R to produce hyperphagia and obesity (7, 8). Subsequently, two laboratories independently reported that AgRP exhibits inverse agonism at MC4R in transfected heterologous cellular expression systems (9, 10). However, for this latter pharmacological property to be clinically relevant, it is necessary that the MC4R have a physiologically significant degree of constitutive activity in the neural circuits that regulate energy homeostasis.
Srinivasan and colleagues (11) in the Vaisse laboratory postulated that this might indeed be the case based on their analysis of a subset of mutations in the human MC4R (hMC4R) associated with the development of severe obesity. These particular mutations were located in the amino-terminal extracellular domain of hMC4R and did not affect either receptor trafficking to the plasma membrane or the agonist or inverse agonist actions of MSH and AgRP, respectively, in cell-based expression systems. However, the mutations did reduce constitutive receptor activity in vitro, and further studies showed that the amino-terminal peptide of hMC4R acts as a tethered partial agonist that is essential for constitutive receptor activity (11, 12). Therefore, Vaisse predicted that mutant mice deficient in both POMC and AgRP would be less obese than mice deficient only in POMC, providing proof for the hypothesis that constitutive MC4R activity in the brain normally mitigates excessive food intake and weight gain (11). We addressed the same question by the pharmacological administration of AgRP to neuronal-specific POMC-deficient mice and showed a delayed but persistent action on energy balance consisting of small decreases in oxygen consumption and increases in cumulative food intake compared with control mice (13). To further support our interpretation of these results as evidence for a putative inverse agonist action of AgRP in the absence of endogenous MSH agonists, we also suggested the importance of analyzing double-mutant Pomc−/−, Agrp−/− mice (13).
In this issue of Endocrinology, Corander et al. (14) studied precisely the proposed compound mutant mice, and surprisingly, their data do not support the predicted result. The investigators performed a comprehensive and carefully controlled analysis of energy homeostasis that compared three mutant strains: Pomc−/−, Agrp−/−, and double Pomc−/−, Agrp−/− mice with and without corticosterone replacement supplied in the drinking water. The researchers found no differences between Pomc−/− and double Pomc−/−, AgRP−/− mutant mice in any of the parameters measured pertaining to their obesity phenotype, including body weight growth curves, body composition, body length, home cage food intake, oxygen consumption, ambulatory activity, metabolic responses to a 24-h fast followed by ad libitum refeeding, metabolic responses to corticosterone supplementation, metabolic responses to the pharmacological administration of AgRP or the MCR agonist 4-norleucine, 7-D-phenylalanine MSH, and glucose homeostasis (14).
The authors conclude that AgRP functions in vivo predominantly as a competitive antagonist to MSH peptides and not as an inverse agonist. Furthermore, Coll and his colleagues (14) provide a number of well-reasoned explanations for the apparent contradictions between the results of their elegantly performed experiments and past predictions based on less direct methods (14). These possibilities include a ceiling effect due to the already extreme hyperphagia exhibited by Pomc−/− mice after corticosterone replacement that precludes any further increase by exogenous AgRP, background strain differences in mutant mice used by different laboratories, developmental adaptations in response to the life-long AgRP deficiency, and region-specific effects of AgRP on MCR or possibly other unrecognized targets.
How can the data and theories be reconciled? Examination of another mouse model focused on the peripheral melanocortin system and pigmentation may be instructive. As noted earlier in this narrative, loss of POMC function on an a/a genetic background does not result in yellow fur pigmentation, consistent with constitutive activity of the mouse MC1R (6). However, this finding is at odds with the reports of red hair pigmentation in humans with complete POMC deficiency (15). To explain this discrepancy, Jackson et al. (16) systematically studied a series of mutant mice that carried a humanized MC1R BAC transgene in combination with null alleles for the endogenous mouse Mcr1, varying gene dosage at the A and a alleles of the agouti locus and wild-type or null Pomc alleles. The human MC1R was expressed from its own regulatory elements at levels approximately 10-fold less than that of mouse MC1R, and as a result, the mice were significantly pheomelanic in the absence of POMC and agouti. A normal black coat color was established on the Pomc+/+, a/a background and normal agouti banding on the Pomc+/+, A/a background, whereas the mice were yellow on the Pomc+/+, Ay-Jkn/a background. Therefore, differences in available levels of human or mouse MC1R, despite identical in vitro constitutive activity and differing intrinsic sensitivity of the receptors to MSH, produces functional consequences that are species specific in the physiological context of hair follicles (16). A similar situation may apply to the human and mouse MC4R in their natural cellular environments.
Contemporary biophysical models of the interaction between a GPCR, its ligand, and a G protein emphasize a dynamic equilibrium between at least two thermodynamic receptor states, inactive (R) and active (R*) (17). All GPCR have a finite probability of entering the R* state, and agonist binding at its orthosteric site increases this probability. Constitutive activity of a GPCR simply is an expression of the R* state in the absence of agonist ligand binding. The efficacy of different ligands, whether to increase or decrease GPCR activity around this basal level depends on numerous other factors including receptor number, the presence of allosteric modulators, the local accessibility of different G proteins, and factors controlling individual GPCR translocation and internalization (18). AgRP, for example, can modulate MC4R signaling by the induction of receptor endocytosis as well as by altering the R to R* equilibrium (19). In addition, different intracellular signaling pathways may be engaged in a ligand-specific manner to determine whether a compound acts as a neutral antagonist or an inverse agonist (20). A monoclonal antibody directed at an epitope in the amino-terminal domain of the hMC4R was recently shown to function as a noncompetitive antagonist for MSH but to synergize with AgRP in mediating inverse agonism (21). The intact antibody or its recombinantly produced single-chain variable fragment reduced food intake in rats, suggesting that it may serve as a new lead compound in the development of compounds with high specificity to the MC4R for the treatment of cachexia (21). However, until it is possible to study more directly the human MC4R in its native neuronal context, it seems wise to remain agnostic on the questions of whether AgRP is a physiologically relevant inverse agonist in humans or whether novel synthetic MC4R ligands, such as the monoclonal antibody just described, will demonstrate clinical efficacy by antagonism, inverse agonism, or some combination of the two mechanisms.
Acknowledgments
This work was supported by National Institutes of Health Grant DK066604.
Disclosure Summary: The author has nothing to disclose.
For article see page 1819
- AgRP
- Agouti-related peptide
- GPCR
- G protein-coupled receptor
- hMC4R
- human MC4R
- MCR
- melanocortin receptor
- MC1R
- MCR type 1
- POMC
- proopiomelanocortin.
References
- 1. Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. 1993. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72:827–834 [DOI] [PubMed] [Google Scholar]
- 2. Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD. 1994. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371:799–802 [DOI] [PubMed] [Google Scholar]
- 3. Siegrist W, Drozdz R, Cotti R, Willard DH, Wilkison WO, Eberle AN. 1997. Interactions of α-melanotropin and agouti on B16 melanoma cells: evidence for inverse agonism of agouti. J Recept Signal Transduct Res 17:75–98 [DOI] [PubMed] [Google Scholar]
- 4. Parra S, Bond RA. 2007. Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant? Curr Opin Pharmacol 7:146–150 [DOI] [PubMed] [Google Scholar]
- 5. Adan RA. 2006. Constitutive receptor activity series: endogenous inverse agonists and constitutive receptor activity in the melanocortin system. Trends Pharmacol Sci 27:183–186 [DOI] [PubMed] [Google Scholar]
- 6. Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ. 2005. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology 146:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. 1997. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 [DOI] [PubMed] [Google Scholar]
- 8. Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. 1997. Overexpression of Agrt leads to obesity in transgenic mice. Nat Genet 17:273–274 [DOI] [PubMed] [Google Scholar]
- 9. Nijenhuis WA, Oosterom J, Adan RA. 2001. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol 15:164–171 [DOI] [PubMed] [Google Scholar]
- 10. Haskell-Luevano C, Monck EK. 2001. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Pept 99:1–7 [DOI] [PubMed] [Google Scholar]
- 11. Srinivasan S, Lubrano-Berthelier C, Govaerts C, Picard F, Santiago P, Conklin BR, Vaisse C. 2004. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest 114:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen M, Celik A, Georgeson KE, Harmon CM, Yang Y. 2006. Molecular basis of melanocortin-4 receptor for AGRP inverse agonism. Regul Pept 136:40–49 [DOI] [PubMed] [Google Scholar]
- 13. Tolle V, Low MJ. 2008. In vivo evidence for inverse agonism of agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes 57:86–94 [DOI] [PubMed] [Google Scholar]
- 14. Corander MP, Rimmington D, Challis BG, O'Rahilly S, Coll AP. 2011. Loss of AgRP does not significantly impact the phenotype of murine POMC deficiency. Endocrinology 152:1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, Grüters A. 2003. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4–10. J Clin Endocrinol Metab 88:4633–4640 [DOI] [PubMed] [Google Scholar]
- 16. Jackson IJ, Budd PS, Keighren M, McKie L. 2007. Humanized MC1R transgenic mice reveal human specific receptor function. Hum Mol Genet 16:2341–2348 [DOI] [PubMed] [Google Scholar]
- 17. Bridges TM, Lindsley CW. 2008. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol 3:530–541 [DOI] [PubMed] [Google Scholar]
- 18. Gilchrist A. 2007. Modulating G-protein-coupled receptors: from traditional pharmacology to allosterics. Trends Pharmacol Sci 28:431–437 [DOI] [PubMed] [Google Scholar]
- 19. Breit A, Wolff K, Kalwa H, Jarry H, Büch T, Gudermann T. 2006. The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin-3 and -4 receptors. J Biol Chem 281:37447–37456 [DOI] [PubMed] [Google Scholar]
- 20. Galandrin S, Oligny-Longpré G, Bouvier M. 2007. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28:423–430 [DOI] [PubMed] [Google Scholar]
- 21. Peter JC, Lecourt AC, Weckering M, Zipfel G, Niehoff ML, Banks WA, Hofbauer KG. 2010. A pharmacologically active monoclonal antibody against the human melanocortin-4 receptor: effectiveness after peripheral and central administration. J Pharmacol Exp Ther 333:478–490 [DOI] [PMC free article] [PubMed] [Google Scholar]