Using multiple levels of analysis, we describe the brain clock as a novel site of hormone regulated plasticity, with biologically significant ramifications for behavior.
Abstract
Gonadal hormones can modulate circadian rhythms in rodents and humans, and androgen receptors are highly localized within the core region of the mouse suprachiasmatic nucleus (SCN) brain clock. Although androgens are known to modulate neural plasticity in other CNS compartments, the role of androgens and their receptors on plasticity in the SCN is unexplored. In the present study, we ask whether androgens influence the structure and function of the mouse SCN by examining the effects of gonadectomy (GDX) on the structure of the SCN circuit and its responses to light, including induction of clock genes and behavioral phase shifting. We found that after GDX, glial fibrillary acidic protein increased with concomitant decreases in the expression of the synaptic proteins synaptophysin and postsynaptic density 95. We also found that GDX exerts effects on the molecular and behavioral responses to light that are phase dependent. In late night [circadian time (CT)21], GDX increased light-induced mPer1 but not mPer2 expression compared with intact (INT) controls. In contrast, in early night (CT13.5), GDX decreased light induced mPer2 but had no effect on mPer1. At CT13.5, GDX animals also showed larger phase delays than did INT. Treatment of GDX animals with the nonaromatizable androgen dihydrotestosterone restored glial fibrillary acidic protein, postsynaptic density 95, and synaptophysin in the SCN and reinstated the INT pattern of molecular and behavioral responses to light. Together, the results reveal a role for androgens in regulating circuitry in the mouse SCN, with functional consequences for clock gene expression and behavioral responses to photic phase resetting stimuli.
The suprachiasmatic nucleus (SCN) of the hypothalamus coordinates daily rhythms in physiology and behavior, including endocrine rhythms (1, 2). Although neurons of the SCN can act as autonomous oscillators, circadian timing in the SCN tissue is an emergent property of the SCN network (3, 4), underscoring the importance of interneuronal communication in determining circadian function. Thus, factors that modulate central nervous system connectivity may contribute importantly to normal SCN function.
Androgen receptors (ARs) have been described in the SCN of numerous species, including mouse, rat, ferret, and human (5–8). In mouse, SCN ARs are localized primarily in the central retinorecipient “core,” a region that receives dense retinal input but lacks the high amplitude oscillations in clock gene expression that characterize the shell region (9, 10). AR expression overlaps with numerous peptidergic cell types, including gastrin-releasing peptide and vasoactive-intestinal polypeptide (VIP) cells (5). Gonadectomy (GDX) of male mice alters several parameters of circadian rhythmicity known to be SCN dependent, including the period and precision of daily activity onset (5, 11). Importantly, androgen replacement restores these properties to precastration levels.
We hypothesized that these behavioral changes in mice reflect hormone-dependent structural plasticity of the mammalian brain clock, consistent with the effects of hormones in dynamically modulating other neural circuits (12). A role for sex steroid hormones in controlling the structure and function of the SCN could have important implications for the regulation of behavior and physiology, because hormone levels are known to change throughout the day, over the lifespan, and in some species, seasonally. In the present study, we examined the effects of androgens on SCN shortly after GDX and replacement of the nonaromatizable androgen dihydrotestosterone (DHT). Because precision of circadian rhythms is reduced in GDX mice within 7 d (5), we explored whether GDX alters synaptic protein expression and astrocyte morphology within the SCN over the same timescale. Furthermore, to determine whether structural changes in the SCN are accompanied by a change in function, we tested the effect of GDX and DHT replacement on the molecular and behavioral responses to light. The results indicate that GDX and androgen replacement modify the functional anatomy of the SCN network and alter light-induced clock gene expression and phase shifting to a light pulse (LP), underscoring the role of this gonadal hormone in regulating the circuitry of the brain clock.
Materials and Methods
Animals
Male 45-d-old C57Bl/6 mice (n = 103; Charles River, Kingston, NY) were allowed to acclimatize to the animal facility for 7 d, and maintained in a 12-h light, 12-h dark cycle. Animals for gene and protein studies were group housed (two to four per cage) in clear polypropylene cages (29 × 19 × 13 cm). For behavioral studies, animals were individually housed in similar cages (32 × 14 × 13 cm), equipped with a running wheel connected to a computerized data acquisition system (VitalView; Mini Mitter/Respironics, Murrysville, PA). Cages were placed in light-tight chambers with independent lighting control and ventilation. Food and water were available ad libitum, with room temperature maintained at 21 ± 2 C. All animal protocols were approved under the guidelines of the Columbia University Institutional Animal Care and Use Committee.
Gonadectomy
Mice were anesthetized with ketamine (70 mg/kg, ip) and xylazine (5 mg/kg, ip), with buprenorphine (0.5 mg/kg, sc) as an analgesic, and GDX (n = 67) by abdominal incision and removal of both testes. Immediately after GDX, animals were implanted with DHT capsules (n = 31, see below). Animals were allowed to recover for 7 d before experimental manipulation. Control animals (n = 36) were anesthetized but did not receive surgery.
Steroid implants
SILASTIC capsules (inner diameter 0.98 mm, outer diameter 2.16 mm; Dow Corning Corp., Midland, MI) were filled with 10 mm of crystalline DHT (Steraloids, Inc., Newport, RI), as previously described (5). Paired seminal vesicle weights were lower in GDX mice (75 ± 10 mg), compared with intact (INT) and GDX+DHT mice (248 ± 9 and 231 ± 14 mg, respectively), confirming the efficacy of steroid treatment. Our group and others have used similar dosages in mouse to restore physiological levels of androgen in the blood (5, 13).
Immunohistochemistry (IHC) and image analysis
Seven days after GDX (n = 4) or GDX+DHT (n = 4) treatment, mice were killed at lights off by deep anesthesia and then intracardially perfused with 50 ml saline followed by 100 ml of 4% paraformaldehyde in 0.1 m phosphate buffer (PB) (pH 7.3). Brains were postfixed for 4 h at 4 C and cryoprotected in 20% sucrose in 0.1 m PB overnight. Brains were sliced (35 μm) on a cryostat and placed into PBS. Free-floating sections were first incubated in normal donkey serum for 1 h and then simultaneously in antiarginine vasopressin (AVP) made in guinea pig (1:10,000; Penninsula Laboratories, San Carlos, CA) and antiglial fibrillary acidic protein (GFAP) made in goat (1:250; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 48 h in PB containing 0.3% Triton X. After the primary incubation, sections were washed three times 10 min with PB containing 0.3% Triton X and then placed in the appropriate donkey secondary conjugated to CY2 or CY3 fluorophores (1:200; Jackson ImmunoResearch, West Grove, PA) for 1 h. Sections were washed in PBS, mounted onto gelatin-coated slides, dehydrated in alcohols, and cleared in xylenes. Coverslips were applied with Krystalon (EM Science, Gibbstown, NJ). All experimental groups were run together at the same time to eliminate interrun variability. Controls in which primary antibody was not applied demonstrated no staining.
Expression of GFAP in the mid-SCN was examined by confocal microscopy using a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss, Thornwood, NY) with a LSM 510-META scanning confocal attachment. All images were acquired using the same settings and laser intensity levels, to avoid potential differences in acquisition, and allowing for comparison between groups. Stacked images were collected as 1-μm multitract optical sections. LSM 3.95 software (Carl Zeiss) was used to superimpose red and green images of the sections. Quantification was accomplished using relative optical density (ROD) measures of the immunoreactivity, using ImageJ (NIH, Bethesda, MD). Image stacks were then collapsed along the z-plane for analysis. Measurements were obtained using a circular-sized tool to digitally outline the SCN, based on the extent of AVP expression. Background, measured in areas directly adjacent to the SCN, was subtracted. Animal averages were obtained from at least two bilateral measurements (four per animal). Corrected intensity values were then normalized by dividing by the mean INT value. These numbers were used to compare group averages in the subsequent statistical analyses. Similar approaches have been used in the SCN previously (14) and show congruence with other histological and biochemical techniques. Images were optimized for brightness and contrast to enhance the signal-noise ratio (Photoshop CS3; Adobe Systems, Mountain View, CA), and all images for the same experiment were adjusted equally.
Western blotting
Two groups were run. For GFAP, INT (n = 3), GDX (n = 3), and GDX+DHT (n = 3) were used, whereas for synaptic protein analysis, INT (n = 4), GDX (n = 3), and GDX+DHT (n = 3) animals were used. All mice were euthanized at lights off with CO2 and their brains removed and placed in ice-cold saline. Using a vibratome, a 400-μm section of hypothalamus was collected in ice-cold saline, and the SCN was dissected bilaterally using a scalpel with the aid of a dissecting microscope, as previously reported (14). Tissue samples were stored at −80 C until processed as described previously (15). Lysates (2 or 5 μg total protein per lane) were separated by SDS-PAGE, blotted to a nitrocellulose membrane, and probed with anti-GFAP (1:5000; Santa Cruz Biotechnology, Inc.), antipostsynaptic density 95 (PSD95) (1:20,000), or antisynaptophysin (1:5000) antibodies (both from Sigma-Aldrich, St. Louis, MO). Proteins were visualized by chemiluminescence, according to the manufacturer's instructions (Lumiglo; Cell Signaling, Beverly, MA). ROD of the Western blottings was measured using MCID (St. Catharines, Ontario, Canada).
Light-induced gene expression
To investigate light-induced gene expression in the SCN, INT (n = 12), GDX (n = 12), and GDX+DHT (n = 12), animals were transferred to constant darkness (DD) for 2 d. On the third day in DD, a 30-min LP (800 lux) was delivered from circadian time (CT)13.5 to CT14, or CT21.5 to CT22 (n = 4 per treatment group/time point), after which animals were returned to darkness for 60 min and then killed (16). Non-LP-control animals (n = 2 per treatment group/time point) were not exposed to light and were killed at CT15 or CT23. Animals were euthanized with CO2 and their brains rapidly removed and snap frozen on dry ice. Brains were stored at −80 C until sectioning on a cryostat. Brains were processed for in situ hybridization with probes for mPer1 and mPer2 as previously reported (16, 17). For hybridization, slides were exposed to hybridization buffer (225 μl per slide) with the 35S-labeled (∼1 × 106 cpm per slide) antisense or sense ribonucleotide probes (mPer1 and mPer2) and incubated at 55 C overnight. Slides were then apposed to Kodak BioMax MR film (Sigma, St. Louis, MO) for 2 d. RODs from the autoradiograms were calculated as above using a set circular tool to outline the SCN and to measure the background just adjacent to the SCN in MCID. Individual animal means were computed and then normalized to the mean INT value to compare changes between groups relative to INT.
Light-induced behavioral phase shifting
To determine the effects of GDX and hormone replacement on behavioral phase shifting, INT (n = 13), GDX (n = 14), or GDX+DHT (n = 9) mice were individually housed and treated as for the studies of light-induced gene expression until a 30-min LP at either CT13.5 (n = 18) or CT21 (n = 18). CT on d 2 in DD was determined using the animal's individual free-running rhythm. Phase shifts were calculated as previously described (18). Briefly, activity onsets for d 3 in DD before a manipulation were determined using ClockLab (Actimetrics, Inc., Wilmette, IL) and defined as the first 10-min bin each day to exceed 150 turns without another such bin occurring in the preceding 240 min. After LPs, the activity onsets also were determined for each of the 7 d after the manipulation. Line fits were performed with ClockLab, using linear regression. The difference between these regression lines on the day after the LP was taken as the phase shift and reported as group means ± se.
Statistical analyses
Main effects were probed using one- or two-way ANOVA, as required, with interactions further examined using Newman-Keuls or Bonferroni post hoc tests, respectively (Prism; GraphPad Software, San Diego, CA). Results were considered statistically significant at P < 0.05.
Results
Effects of GDX and hormone replacement on GFAP expression
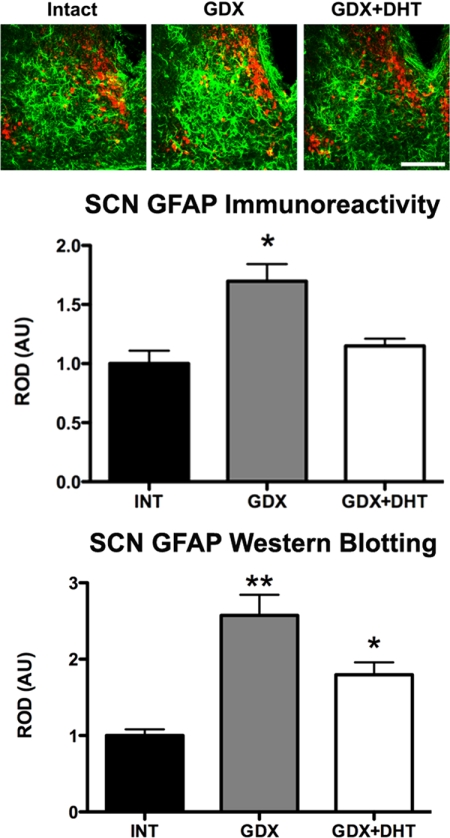
Astrocytes are densely packed in the SCN compared with surrounding hypothalamic areas (19), can directly and indirectly control neuronal connectivity (20), and their prevalence, as measured by GFAP-immunoreactivity (GFAP-ir) is sensitive to androgens (21, 22). To explore the effects of androgens on GFAP-ir in the SCN, brains from INT, GDX, and GDX+DHT animals were collected at the time of lights off. Tissue was processed for double-label IHC using antibodies against GFAP and AVP. The intensity of GFAP-ir was determined using ROD and differed across groups (F2,9 = 11.07) (Fig. 1, top). GDX resulted in increased intensity of GFAP-ir within the SCN (P < 0.05), whereas GDX+DHT restored GFAP-ir to INT levels (P > 0.05). The IHC results were independently confirmed by Western blotting of whole SCN extracts. Total SCN GFAP again varied with hormone group (F2,8 = 17.84, P = 0.001) (Fig. 1, bottom), with more GFAP in GDX animals compared with both INT and GDX+DHT animals (P < 0.05).
Fig. 1.
Androgens modulate SCN GFAP expression. Top, Photomicrographs showing GFAP (green) and AVP (red) staining in the SCN of INT, GDX, and GDX+DHT animals. Scale bar, 150 μm. Middle, Bar graph depicting the intensity of GFAP-ir within the SCN of INT, GDX, and GDX+DHT animals. GDX increases GFAP levels in the SCN, as measured by ROD. *, P < 0.05. Bottom, Bar graphs quantifying Western blottings of SCN cell lysates from INT, GDX, and GDX+DHT mice for GFAP. A statistically significant effect of hormone treatment on GFAP levels in the SCN is observed, with GDX treatment increasing levels relative to both INT and GDX+DHT mice and DHT treatment reducing levels, although they are still elevated over INT. **, P < 0.01; *, P < 0.05. AU, Arbitrary units.
GDX reduces and DHT treatment increases SCN expression of synaptic proteins
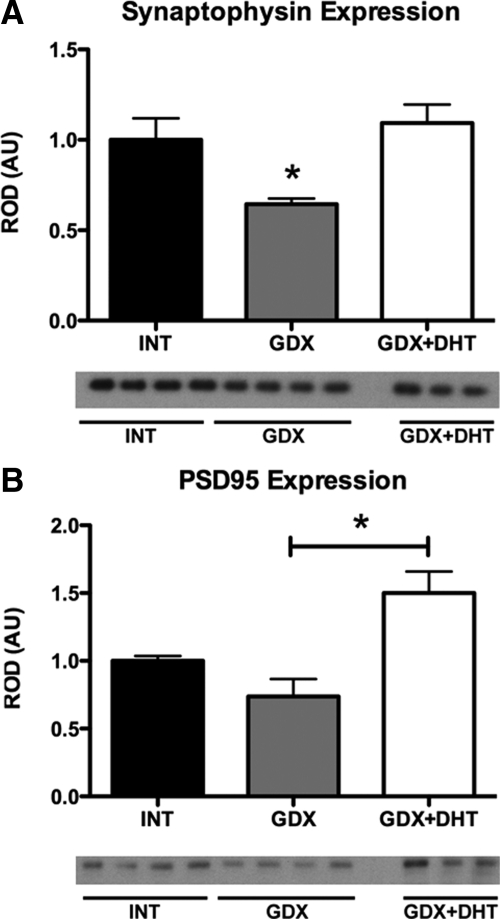
We next tested the hypothesis that changes in GFAP might alter synaptic coupling among neurons by investigating changes in synaptic proteins. For both synaptophysin (presynaptic marker) and PSD95 (a postsynaptic marker), there was a significant main effect of hormone treatment (F2,8 = 10.29, P = 0.006 and F2,8 = 4.91, P = 0.041, respectively) (Fig. 2). Synaptophysin content was significantly reduced in GDX mice compared with both INT and GDX+DHT groups (P < 0.05). A similar pattern was obtained with PSD95. GDX decreased PSD95 compared with INT, although this did not reach statistical significance (P = 0.08); nevertheless, GDX+DHT mice showed increased PSD95 protein levels relative to both INT and GDX (P < 0.05).
Fig. 2.
Androgens alter expression of synaptic proteins in the SCN. Bar graphs quantifying Western blottings of SCN cell lysates from INT, GDX, and GDX+DHT mice for synaptic proteins synaptophysin (A) and PSD95 (B). Synaptophysin levels were decreased after GDX, with DHT treatment blocking the effect. PSD95 levels were affected by hormone treatment, although there was no statistically significant effect of GDX compared with INT. However, GDX+DHT mice had increased synaptophysin levels compared with GDX; *P < 0.05. AU, Arbitrary units.
Androgens modulate light-induced gene expression in the SCN
Light is the principal environmental time cue that sets the phase of the circadian system. To test whether the androgenic effects on SCN structure are related to altered responses to light, we assessed the molecular response of the SCN of INT, GDX, and GDX+DHT mice to a phase delaying (at CT13.5) or phase advancing LP (at CT21) after 2 d of DD, with mRNA for mPer1 and mPer2 then examined by in situ hybridization.
Phase delay point
After a LP at CT13.5, mPer1 was induced in the SCN of all groups (F1,11 = 10.59, P < 0.01), but expression was not altered by any of the experimental treatments (F2,11 = 0.12, P > 0.05), nor were there any interaction effects (F2,11 = 0.02, P > 0.05) (Fig. 3). In contrast, for mPer2, there was a main effect of light (F1,11 = 85.94, P < 0.05) and an interaction between light and hormone treatment (F2,11 = 3.8, P = 0.058) that almost reached the criterion for statistical significance. GDX animals had decreased mPer2 expression when compared with INT or GDX+DHT animals (both P < 0.05); GDX+DHT and INT mice were comparable (P > 0.05) (Fig. 3). In the absence of a LP, expression of both mPer1 and mPer2 was not altered in GDX or GDX+DHT mice (P > 0.05) (Fig. 3).
Fig. 3.
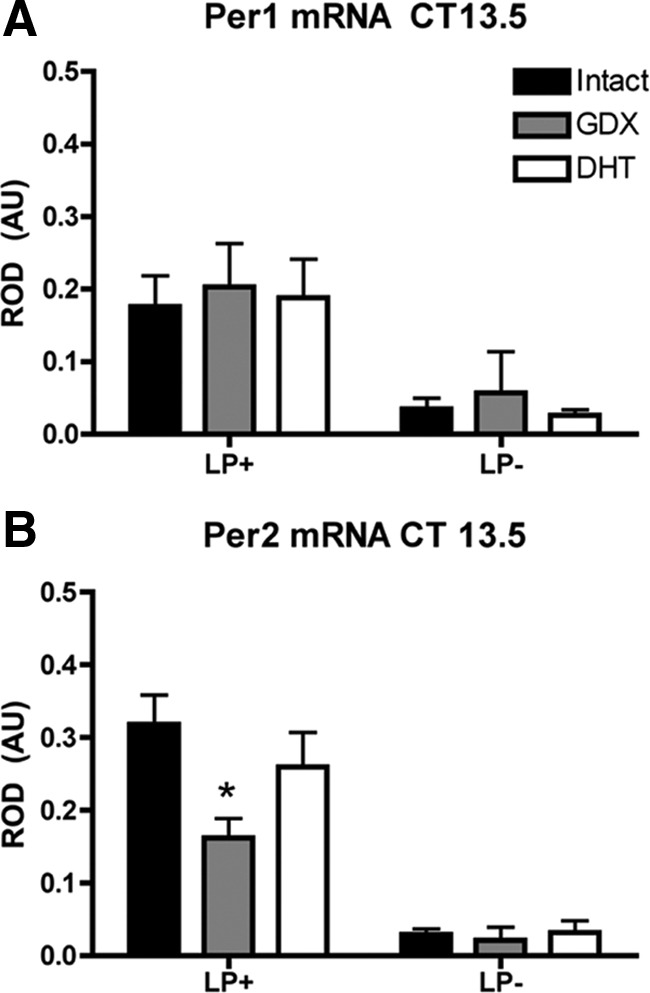
Androgens alter the molecular response to photic stimulation in the delay phase. Bar graphs depict light-induced mPer1 (A) and mPer2 (B) expression in the SCN of INT, GDX, or GDX+DHT mice after a 30-min LP (LP+) at projected CT13.5 and killed 90 min after the start of the LP. LP−, No LP. mPer1 was induced in all groups, but expression was not altered by any of the treatments. In contrast, mPer2 induction was reduced in GDX animals, compared with both INT and GDX+DHT mice. *, P < 0.05. AU, Arbitrary units.
Phase advance point
After a LP at CT21, mPer1 was induced in all experimental groups (F1,11 = 57.52, P < 0.0001), with a main effect of hormone treatment (F2,11 = 8.28, P < 0.001) as well as an interaction of light exposure and hormone treatment (F2,11 = 13.75, P < 0.001). GDX mice showed an enhanced mPer1 response when compared with INT or GDX+DHT mice (P < 0.05). GDX+DHT and INT mice responded identically (P > 0.05) (Fig. 4). In contrast to mPer1, there were no effects of either LP or hormone treatment on mPer2 expression (F1,12 = 2.53 and F2,12 = 1.13, P > 0.05). There were no differences in basal gene expression among mice not exposed to light (P > 0.05) (Fig. 4).
Fig. 4.
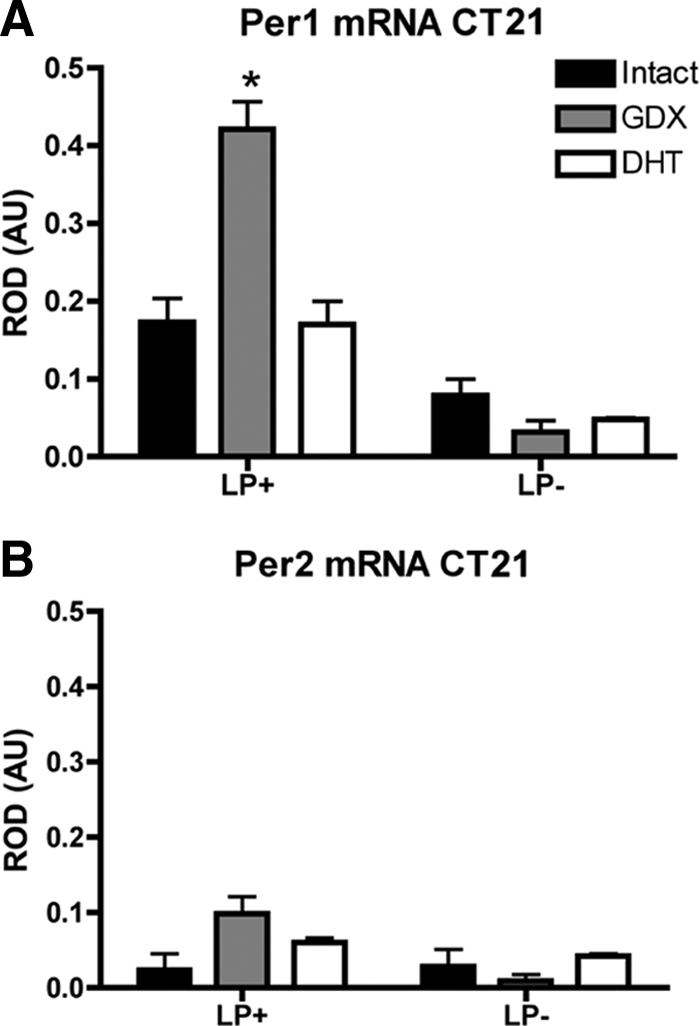
The molecular response to photic stimulation is also altered by GDX and DHT replacement. Bar graphs depict light-induced mPer1 (A) and mPer2 (B) expression in the SCN of INT, GDX, or GDX+DHT mice after a 30-min LP at projected CT21 and killed 90 min after the start of the LP.There was an effect of hormone treatment with GDX animals showing an enhanced mPer1 response when compared with INT or GDX+DHT mice. In contrast to mPer1, there were no effects of either LP or hormone treatment on mPer2 expression. *, P < 0.05. AU, Arbitrary units.
Androgenic modulation of behavioral response to LPs
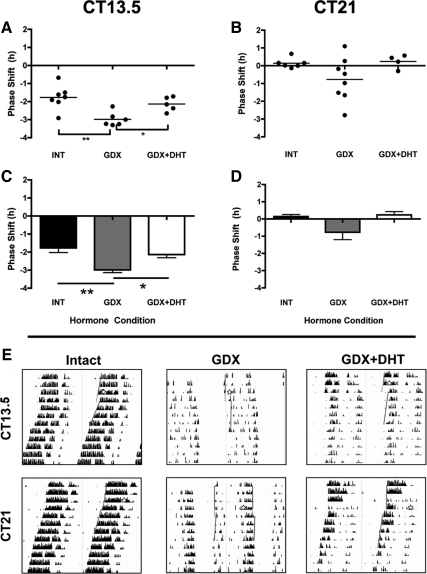
To determine whether the impact of GDX on light-induced clock gene expression is functionally significant, we examined whether phase-shifting behavior was affected after GDX. As above, on the third day of DD, INT, GDX, or GDX+DHT mice were exposed to a 30-min LP at either CT13.5 or CT21 and then left undisturbed while their wheel-running behavior was monitored. There was a significant main effect of hormone treatment on phase shifts after the CT13.5 LP (F2,15 = 8.99, P = 0.0027). GDX mice had significantly larger delays than the INT (−2.98 ± 0.16 h vs. −1.76 ± 0.25 h, P < 0.01) or GDX+DHT (−2.13 ± 0.18 h, P < 0.05). INT and GDX+DHT mice did not differ (P > 0.05) (Fig. 5, A, C, and E).
Fig. 5.
Androgens modulate the behavioral response to photic stimulation. A and B, Scatter plots depict light-induced phase shifts in behavior of INT, GDX, and GDX+DHT mice at either early night (CT13.5) (A) or late night (CT21) (B). Closed circles represent individual animals, and lines represent group means. C and D, Bar graphs replot data from A at CT13.5 (C) and CT21 (D). *, P < 0.05; **, P < 0.01. E, Representative actograms from INT, GDX, and GDX+DHT mice housed in DD, given a LP at either CT13.5 or CT21 (denoted by stars). Lines indicate onset times, with dotted lines representing projected onset on the day after the shift. A second set of lines in the CT21 GDX panel show the phase shift of the offset bout as well, as onsets are weak in GDX animals.
At CT21 (Fig. 5, B, D, and E), there was no main effect of treatment (F2,15 = 2.696, P = 0.099), and there were no significant pair-wise differences between groups (P > 0.05). Although both INT and GDX+DHT groups showed small phase advances (0.14 ± 0.11 and 0.24 ± 0.19 h, respectively) at this time point, most (n = 6/8) GDX mice showed a phase delay with substantial variability in the magnitude and direction of the shift (range, 1.1 h advance to 2.7 h delay; mean delay, −0.77 ± 0.43 h).
Discussion
CNS plasticity enables organisms to adapt to their environments and display dynamic responses in the face of environmental changes. Plasticity in the nervous system can take many forms, from structural to functional, and can involve changes in cell numbers, dendritic arborization, or the number and strength of synapses (23). Importantly, androgens play a key role in the plasticity of neural circuits, both during development and in adulthood (12).
The present study demonstrates a biologically significant effect of androgens on both the structure and function of the SCN. We demonstrate that GDX increased SCN GFAP expression, whereas DHT replacement restores this to the level seen in INT mice. Parallel changes were observed in both pre- and postsynaptic markers, with decreases after GDX and restoration to that seen in INT mice after DHT replacement. Functionally, the consequences of these GDX-induced structural changes were assessed at the molecular level by examining the induction of the canonical clock genes Per1 and Per2 after LPs. In the absence of a LP, there were no differences among INT, GDX, and DHT groups in the expression levels of either gene. In contrast, after a LP, GDX reduced Per2 in the early night and augmented Per1 in the late night. We further probed these altered molecular responses at the level of behavior. During the early night (CT13.5, a time when mice are known to phase delay to a LP), GDX mice had larger phase delays than either INT or DHT groups. In contrast, there were no clear effects of GDX in the late night (CT21), which is expected given that mice have very small phase advances in late night (24). In summary, the androgen-induced changes in glial cells and synaptic markers in the SCN are associated with altered molecular and behavioral responses to light.
The SCN is a functionally and structurally heterogeneous brain nucleus. Although circadian oscillation can be observed in individual SCN neurons, much of the function ascribed to the nucleus arises from the coupled neuronal network (4, 25, 26) and is the product of interactions among its neuronal and glial cellular elements. Thus, any changes in the structure or connectivity of the SCN, such as those observed consequent to GDX, could have significant implications for SCN function.
Steroid hormones, including androgens, are known to contribute to morphological plasticity of astrocytes. Steroid-mediated changes in GFAP expression similar to those we report in SCN are also observed in hippocampus, hypothalamus, and in primary astrocyte cultures (21, 22). Gross morphological steroid-induced changes in glia have been elegantly described in the supraoptic nucleus (27), showing that these changes in astrocyte morphology have clear ramifications for neural function.
Astrocytes play both passive and active roles in regulating neurotransmission, from passively restricting the diffusion of neuroactive compounds in the extracellular space to active roles in modulating the strength of neurotransmission, including the secretion of gliotransmitters (28). Compared with nearby hypothalamic regions, the SCN is enriched with astrocytes, and although their specific role in the regulation of circadian rhythms is not known, numerous studies indicate that astrocytes affect SCN function (29). Within the SCN, astrocytes have gap junctions (19) and interdigitate among neurons (30). Importantly, in organotypic SCN cultures, the coordinated rhythms observed in VIP and AVP expression become decoupled after a loss of glial cells (31) and are abolished by treatment with gap junction blockers (32). Furthermore, astrocytes express receptors for many of the neurotransmitters and neuropeptides in the SCN (33). In vivo, GFAP expression changes in the SCN over the course of the day and in response to changes in photic input (34), and rhythmic changes in glial coverage of retinorecipient VIP neurons are thought to be associated with gating of photic cues (35). Thus, there exists ample evidence for a role of SCN glial cells in the regulation of circadian rhythms.
In addition to the changes in GFAP, we demonstrated androgen-induced changes in synaptophysin and PSD95 indicative of changes in the quantity or quality of synaptic connections. The presynaptic marker synaptophysin is a glycoprotein found in synaptic vesicles and interacts with synaptobrevin, an essential synaptic vesicle protein (36). The postsynaptic marker PSD95 is a PDZ-domain protein associated with the PSD and is involved in the anchoring of numerous synaptic components, including NMDA and AMPA receptors and K+ channels (37). Gonadal steroids regulate dendritic spine density throughout development and in adulthood (12), with androgen-induced remodeling of neural circuits observed in the medial prefrontal cortex (38), hippocampus (39–41), and the spinal nucleus of the bulbocavernosus (42). The present results indicate that the SCN is a novel locus of hormone-mediated structural plasticity in the brain.
Changes in astrocytes and synaptic proteins indicate the potential for altered coupling between components of the SCN network. Importantly, the putative change in coupling that we observed is associated with altered behavioral responses. Modeling work has suggested that coupling is important in regulating the precision of the clock, because coupled oscillators are more precise than are individual oscillators (43). Furthermore, such models suggest a relationship between coupling and phase shifting, with faster resynchronization and larger phase shifts after a perturbation in a population of weakly coupled oscillators compared with strongly coupled oscillators (44). Thus, the effects of GDX on the circadian clock are consistent with such a model, because after GDX, there is a marked decrease in precision of the free-running circadian locomotor rhythm (5) and a larger phase delay to a LP. Note that INT mice have very small phase shifts at CT21 (24), and this is the likely explanation for the absence of a clear effect of GDX at this time point.
Behavioral responses to photic stimuli are based on molecular responses at the level of the SCN (16). Light induces phase-dependent changes in both Per1 and Per2 expression within the SCN, and Per gene expression is associated with phase-shifting behavior (45, 46). In the present study, GDX reduced light-induced early night Per2 and increased late night Per1. In the early night, the reduction in Per2 was associated with larger phase delays, whereas the increased Per1 in the late night was not associated with a larger phase shift. These dichotomous results are intriguing, but dissociations between Per gene induction by light and phase shifting have been previously reported (47). It is important to note that the present results present a “snapshot” of mRNA expression after a LP, and it is possible that there are changes in the dynamic aspect of the mRNA signal. For example, it is well known that the spatiotemporal pattern of Per gene expression in the SCN after a LP is related to the direction of the phase shift (16). If the results on GFAP and synaptic proteins that we report here represent altered cellular connectivity in the SCN, then reduced intercellular communication could alter the propagation of photic cues through the extent of the SCN. In the future, it will be of interest to determine the mechanism driving GDX-induced changes in the relationship between Per expression and phase shifts, in both time and space. This is particularly important considering the effects of gonadal hormones on numerous measures of circadian function that have been reported in a number of different species in both adulthood and adolescents, including nocturnal hamsters (Mesocritus auratus) (48–50) and the diurnal rodent Octodon degus (51–54).
The effects of GDX on light-induced molecular and behavioral responses, their rescue by the nonaromatizable hormone, DHT, dense AR expression, and sparse estrogen receptor in the retinorecipient region of the SCN (5, 55) suggest an AR-dependent mechanism. Nevertheless, given the large number of known DHT metabolites, and perhaps other yet to be identified metabolites, hormone actions through other receptors may be involved, including estrogen receptor (56). The 5–7 d required for behavioral changes after GDX or testosterone replacement suggest classical steroid receptor function rather than rapid nongenomic effects (5, 57). An SCN site of action is parsimonious, but regulation by extra-SCN steroid receptors has not been investigated here. This also makes work in females using both androgenic and estrogenic hormone important, because we and others have demonstrated different effects of gonadal hormones on circadian timing in males and females in humans and nonhuman animals (53, 58–60).
Our findings of structural changes in the SCN associated with androgens have both basic and clinical implications, because the SCN regulates almost all physiological rhythms in the body, including the timing of sleep. In both mice and humans, gonadal hormones impact sleep (reviewed in Refs. 61, 62). Sleep patterns change throughout life, and sex differences in these patterns develop around the time of adolescence, remain throughout adulthood, and fade away into old age (63), suggesting that the rise and fall of gonadal hormones throughout the lifespan may influence the timing of sleep-wake cycles. In this context, understanding how hormones affect SCN structure and function is key to unraveling their effects on circadian function and what these changes in the SCN and the circadian timing system mean for physiology and behavior of the organism. Much of the work exploring hormone-brain interactions in circadian timing was undertaken before our full appreciation of the anatomical and functional complexity of the SCN clock, or the greatly varied effects of hormones on neural structure. These developments have allowed neuroscientists to more fully appreciate that the relationship between the brain and the body is bidirectional, making studies that revisit these questions in circadian biology important and timely. The experiments reported here reinforce the idea that hormones can modulate brain and behavior at many different levels and emphasize the importance of understanding how variables, such as age, sex, and time of day (to name a few), interact to modulate neural systems.
Summary
Virtually every physiological process is regulated on a circadian basis, including the production and secretion of hormones (2). Such signals can in turn feedback to influence circadian timing (64). In male mice, AR is expressed in the SCN, and removal of androgens is associated with changes in numerous aspects of circadian rhythms, including changes in period and precision (5) and (as the present study demonstrates) phase shifting. Our current results provide potential mechanisms by which androgens can influence circadian behavior by altering the structure and function of the SCN. Together, the changes that we observed in GFAP expression and synaptic protein levels, in the context of known effects of androgens in remodeling circuits in other CNS regions, indicate a novel hormonally regulated effect on the structural plasticity of the SCN, with a significant effect on behavior. This work adds to the growing literature on the effects of gonadal hormones on circadian timing and highlights hormone-dependent plasticity in the SCN that may contribute to behavioral changes.
Acknowledgments
We thank Dr. Lance Kriegsfeld for helpful comments on a previous version of this manuscript.
Present address for I.N.K.: Laboratory of Neuroendocrinology, The Rockefeller University, 1230 York Avenue, Box 165, New York, New York 10065.
This work was supported by a Natural Sciences and Engineering Research Council of Canada Post-Graduate Scholarship grant (I.N.K.) and by National Institutes of Health Grants T32DK07328 (to M.P.B.) and NS37919 (to R.S.).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 1727
- AR
- Androgen receptor
- AVP
- arginine vasopressin
- CT
- circadian time
- DD
- constant darkness
- DHT
- dihydrotestosterone
- GDX
- gonadectomy
- GFAP
- glial fibrillary acidic protein
- GFAP-ir
- GFAP-immunoreactivity
- IHC
- immunohistochemistry
- INT
- intact
- LP
- light pulse
- PB
- phosphate buffer
- PSD95
- postsynaptic density 95
- ROD
- relative optical density
- SCN
- suprachiasmatic nucleus
- VIP
- vasoactive-intestinal polypeptide.
References
- 1. Dibner C, Schibler U, Albrecht U. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549 [DOI] [PubMed] [Google Scholar]
- 2. Butler MP, Kriegsfeld LJ, Silver R. 2009. Circadian regulation of endocrine functions. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R. eds. Hormones, brain and behavior. 2nd ed. San Diego: Academic Press; 473–505 [Google Scholar]
- 3. Yan L, Karatsoreos IN, LeSauter J, Welsh DK, Kay S, Foley DK, Silver R. 2007. Exploring spatiotemporal organization of SCN circuits. Cold Spring Harb Symp Quant Biol 72:527–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Welsh DK, Takahashi JS, Kay SA. 2010. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72:551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karatsoreos IN, Wang A, Sasanian J, Silver R. 2007. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology 148:5487–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou L, Blaustein JD, De Vries GJ. 1994. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology 134:2622–2627 [DOI] [PubMed] [Google Scholar]
- 7. Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF. 2000. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 425:422–435 [DOI] [PubMed] [Google Scholar]
- 8. Kashon ML, Arbogast JA, Sisk CL. 1996. Distribution and hormonal regulation of androgen receptor immunoreactivity in the forebrain of the male European ferret. J Comp Neurol 376:567–586 [DOI] [PubMed] [Google Scholar]
- 9. Abrahamson EE, Moore RY. 2001. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916:172–191 [DOI] [PubMed] [Google Scholar]
- 10. Antle MC, Silver R. 2005. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci 28:145–151 [DOI] [PubMed] [Google Scholar]
- 11. Daan S, Damassa D, Pittendrigh CS, Smith ER. 1975. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci USA 72:3744–3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McEwen BS, Coirini H, Westlind-Danielsson A, Frankfurt M, Gould E, Schumacher M, Woolley C. 1991. Steroid hormones as mediators of neural plasticity. J Steroid Biochem Mol Biol 39:223–232 [DOI] [PubMed] [Google Scholar]
- 13. Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. 1998. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-α knockout mice. Endocrinology 139:4092–4101 [DOI] [PubMed] [Google Scholar]
- 14. Rieux C, Carney R, Lupi D, Dkhissi-Benyahya O, Jansen K, Chounlamountri N, Foster RG, Cooper HM. 2002. Analysis of immunohistochemical label of Fos protein in the suprachiasmatic nucleus: comparison of different methods of quantification. J Biol Rhythms 17:121–136 [DOI] [PubMed] [Google Scholar]
- 15. Karatsoreos IN, Romeo RD, McEwen BS, Silver R. 2006. Diurnal regulation of the gastrin-releasing peptide receptor in the mouse circadian clock. Eur J Neurosci 23:1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan L, Silver R. 2002. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci 16:1531–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romeo RD, Karatsoreos IN, Jasnow AM, McEwen BS. 2007. Age- and stress-induced changes in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Neuroendocrinology 85:199–206 [DOI] [PubMed] [Google Scholar]
- 18. Antle MC, Kriegsfeld LJ, Silver R. 2005. Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J Neurosci 25:2447–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van den Pol AN. 1980. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol 191:661–702 [DOI] [PubMed] [Google Scholar]
- 20. Oliet SH, Piet R, Poulain DA, Theodosis DT. 2004. Glial modulation of synaptic transmission: insights from the supraoptic nucleus of the hypothalamus. Glia 47:258–267 [DOI] [PubMed] [Google Scholar]
- 21. Melcangi RC, Riva MA, Fumagalli F, Magnaghi V, Racagni G, Martini L. 1996. Effect of progesterone, testosterone and their 5 α-reduced metabolites on GFAP gene expression in type 1 astrocytes. Brain Res 711:10–15 [DOI] [PubMed] [Google Scholar]
- 22. Day JR, Laping NJ, McNeill TH, Schreiber SS, Pasinetti G, Finch CE. 1990. Castration enhances expression of glial fibrillary acidic protein and sulfated glycoprotein-2 in the intact and lesion-altered hippocampus of the adult male rat. Mol Endocrinol 4:1995–2002 [DOI] [PubMed] [Google Scholar]
- 23. Butz M, Wörgötter F, van Ooyen A. 2009. Activity-dependent structural plasticity. Brain Res Rev 60:287–305 [DOI] [PubMed] [Google Scholar]
- 24. Pendergast JS, Friday RC, Yamazaki S. 2010. Photic entrainment of period mutant mice is predicted from their phase response curves. J Neurosci 30:12179–12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butler MP, Silver R. 2009. Basis of robustness and resilience in the suprachiasmatic nucleus: individual neurons form nodes in circuits that cycle daily. J Biol Rhythms 24:340–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miche S, Colwell CS. 2001. Cellular communication and coupling within the suprachiasmatic nucleus. Chronobiol Int 18:579–600 [DOI] [PubMed] [Google Scholar]
- 27. Theodosis DT, Poulain DA, Oliet SH. 2008. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88:983–1008 [DOI] [PubMed] [Google Scholar]
- 28. Newman EA. 2003. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci 26:536–542 [DOI] [PubMed] [Google Scholar]
- 29. Servière J, Lavialle M. 1996. Astrocytes in the mammalian circadian clock: putative roles. Prog Brain Res 111:57–73 [DOI] [PubMed] [Google Scholar]
- 30. van den Pol AN, Finkbeiner SM, Cornell-Bell AH. 1992. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci 12:2648–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. 1995. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA 92:7396–7400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shinohara K, Funabashi T, Mitushima D, Kimura F. 2000. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci Res 38:43–47 [DOI] [PubMed] [Google Scholar]
- 33. Hösli E, Hösli L. 1993. Receptors for neurotransmitters on astrocytes in the mammalian central nervous system. Prog Neurobiol 40:477–506 [DOI] [PubMed] [Google Scholar]
- 34. Lavialle M, Begue A, Papillon C, Vilaplana J. 2001. Modifications of retinal afferent activity induce changes in astroglial plasticity in the hamster circadian clock. Glia 34:88–100 [DOI] [PubMed] [Google Scholar]
- 35. Girardet C, Blanchard MP, Ferracci G, Lévêque C, Moreno M, François-Bellan AM, Becquet D, Bosler O. 2010. Daily changes in synaptic innervation of VIP neurons in the rat suprachiasmatic nucleus: contribution of glutamatergic afferents. Eur J Neurosci 31:359–370 [DOI] [PubMed] [Google Scholar]
- 36. Lu B, Chow A. 1999. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res 58:76–87 [PubMed] [Google Scholar]
- 37. Keith D, El-Husseini A. 2008. Excitation control: balancing PSD-95 function at the synapse. Front Mol Neurosci 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. 2007. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology 148:1963–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leranth C, Hajszan T, MacLusky NJ. 2004. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci 24:495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacLusky NJ, Hajszan T, Leranth C. 2004. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology 145:4154–4161 [DOI] [PubMed] [Google Scholar]
- 41. MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. 2006. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology 147:2392–2398 [DOI] [PubMed] [Google Scholar]
- 42. Kurz EM, Sengelaub DR, Arnold AP. 1986. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science 232:395–398 [DOI] [PubMed] [Google Scholar]
- 43. Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. 2004. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms 19:35–46 [DOI] [PubMed] [Google Scholar]
- 44. Achermann P, Kunz H. 1999. Modeling circadian rhythm generation in the suprachiasmatic nucleus with locally coupled self-sustained oscillators: phase shifts and phase response curves. J Biol Rhythms 14:460–468 [DOI] [PubMed] [Google Scholar]
- 45. Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. 1999. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci 19:1115–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. 1997. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91:1043–1053 [DOI] [PubMed] [Google Scholar]
- 47. Hannibal J, Jamen F, Nielsen HS, Journot L, Brabet P, Fahrenkrug J. 2001. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J Neurosci 21:4883–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morin LP. 1980. Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav 24:741–749 [DOI] [PubMed] [Google Scholar]
- 49. Morin LP, Cummings LA. 1981. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav 26:825–838 [DOI] [PubMed] [Google Scholar]
- 50. Morin LP, Fitzgerald KM, Zucker I. 1977. Estradiol shortens the period of hamster circadian rhythms. Science 196:305–307 [DOI] [PubMed] [Google Scholar]
- 51. Jechura TJ, Walsh JM, Lee TM. 2000. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav 38:243–249 [DOI] [PubMed] [Google Scholar]
- 52. Jechura TJ, Walsh JM, Lee TM. 2003. Testosterone suppresses circadian responsiveness to social cues in the diurnal rodent Octodon degus. J Biol Rhythms 18:43–50 [DOI] [PubMed] [Google Scholar]
- 53. Lee TM, Hummer DL, Jechura TJ, Mahoney MM. 2004. Pubertal development of sex differences in circadian function: an animal model. Ann NY Acad Sci 1021:262–275 [DOI] [PubMed] [Google Scholar]
- 54. Hummer DL, Jechura TJ, Mahoney MM, Lee TM. 2007. Gonadal hormone effects on entrained and free-running circadian activity rhythms in the developing diurnal rodent Octodon degus. Am J Physiol Regul Integr Comp Physiol 292:R586–R597 [DOI] [PubMed] [Google Scholar]
- 55. Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kalló I. 2008. Oestrogen receptor α and β immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol 20:1270–1277 [DOI] [PubMed] [Google Scholar]
- 56. Pak TR, Chung WC, Hinds LR, Handa RJ. 2009. Arginine vasopressin regulation in pre- and postpubertal male rats by the androgen metabolite 3β-diol. Am J Physiol Endocrinol Metab 296:E1409–E1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Foradori CD, Weiser MJ, Handa RJ. 2008. Non-genomic actions of androgens. Front Neuroendocrinol 29:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iwahana E, Karatsoreos I, Shibata S, Silver R. 2008. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav 53:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paul KN, Dugovic C, Turek FW, Laposky AD. 2006. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep 29:1211–1223 [DOI] [PubMed] [Google Scholar]
- 60. Wever RA. 1984. Sex differences in human circadian rhythms: intrinsic periods and sleep fractions. Experientia 40:1226–1234 [DOI] [PubMed] [Google Scholar]
- 61. Empson JA, Purdie DW. 1999. Effects of sex steroids on sleep. Ann Med 31:141–145 [DOI] [PubMed] [Google Scholar]
- 62. Paul KN, Turek FW, Kryger MH. 2008. Influence of sex on sleep regulatory mechanisms. J Womens Health 17:1201–1208 [DOI] [PubMed] [Google Scholar]
- 63. Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. 2004. A marker for the end of adolescence. Curr Biol 14:R1038–R1039 [DOI] [PubMed] [Google Scholar]
- 64. Karatsoreos IN, Silver R. 2007. Minireview: the neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology 148:5640–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]