GLUT1 mRNA is the most abundant glucose transporter mRNA in endometrial stroma cells, and decreasing expression by short hairpin RNA leads to suppression of decidualization.
Abstract
Recurrent miscarriages affect about 1–2% of couples trying to conceive; however, mechanisms leading to this complication are largely unknown. Most studies focus on the early embryo, but proper development and implantation of the blastocyst are also dependent on optimal endometrial progression into a receptive state. One of the key steps in the uterine preparation for embryo receptivity, known as decidualization, is the differentiation of endometrial stromal cells (ESCs) into decidual cells. During this transition, the ESCs undergo a drastic change in glucose metabolism. The efficiency of glucose uptake is determined by a family of facilitative glucose transporters (GLUTs), and many have been identified in the stroma. The primary focus of this work was to quantify the absolute amount of GLUT mRNAs in this cell type before and after decidualization. We used primary ESCs isolated from murine and human uteri. We developed and validated cDNA-based calibration curves for each GLUT and used these primers to arrive at absolute mRNA copy numbers. Here, we report all the GLUT mRNAs that are present in the ESCs and their abundance under both conditions, control and decidualized. GLUT1 mRNA is the most abundant and critical transporter in ESCs of both species, because knocking down this GLUT with sort hairpin RNA leads to dramatically reduced decidualization. These findings suggest that GLUT1 mRNA expression is essential for decidualization and we are the first to determine a possible mechanism to explain how maternal conditions of abnormal glucose utilization may impair implantation at the level of the ESCs.
The success of a pregnancy is determined by the completion of a number of sequential processes with proper implantation of the embryo being one of the initial key factors. Failure of the endometrium to properly differentiate toward a receptive state can be the source of early pregnancy loss even when the embryo is properly developing (1–3). One of the essential processes in this preparation for embryo receptivity is the progesterone-mediated differentiation of endometrial stromal cells (ESCs) into decidual cells (4). Decidual cells show evidence of a drastic change in glucose metabolism, which can be evidenced by their high stores of glycogen. The data regarding glucose utilization or its metabolic fate during this differentiation process are very incomplete. The efficiency of glucose uptake is determined by a family of facilitative glucose transporters (GLUTs), and several of them have been identified in the endometrial stroma (5). The expression of at least one of them, GLUT1, increases up to 10-fold in human ESCs in vitro during the process of decidualization and 2-fold in the mouse ESCs in vivo, suggesting GLUT1's critical role in the glucose uptake increase (6). GLUT3 has also been detected in the endometrium (5). Little quantitative or consistent data in regard to the expression and function of these and other GLUTs in the endometrial stroma, however, are available.
In this study, we used primary endometrial cultures from both the mouse and human to show, for the first time, that decidualization in vitro is dependent on sufficient glucose presence in the media. We also quantitatively characterized the mRNA levels of a number of GLUTs before and after decidualization to determine which GLUT transcripts are most abundant in the endometrial stroma. Our data are the first to demonstrate that GLUT1 is the most highly transcribed GLUT in the stroma and that its presence is essential for completion of a full decidual response, the critical step in implantation, in human ESCs.
Materials and Methods
Animal care and use
All mouse studies were approved by the Animal Studies Committee at Washington University School of Medicine and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Female C57BL/6NCr (National Cancer Institute, Bethesda, MD) mice 7–8 wk of age were used for the isolation of primary ESCs.
Isolation of murine ESCs and culture conditions
Isolation of ESCs was described previously (6). ESCs were plated in six-well Costar plates at a concentration of 5 × 105 cells per well, and medium was supplemented with 17β-estradiol-water-soluble at a final estradiol concentration of 10 nm and progesterone-water-soluble at a final progesterone concentration of 1 μm for 72 h (Sigma Chemical Co., St. Louis, MO) to induce decidualization. Control samples received no hormone supplementation.
Isolation of human ESCs and culture conditions
Endometrial tissue was obtained from human uteri after hysterectomy conducted for benign disease in premenopausal women. Protocols were approved by the Human Research Protection Office of Washington University (HRPO no. 07-0949). ESCs were isolated as previously described (6). Cells were treated with 1 μm medroxyprogesterone-17-acetate (MPA) (Sigma) and 0.5 mm N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (db-cAMP) (Sigma) for 9 d to induce decidualization. Control samples received 0.1% ethanol vehicle control.
RNA isolation and real-time PCR
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA), and 1 μg RNA was reverse transcribed using the Quantitect reverse transcription kit (QIAGEN, Valencia, CA). Quantitative RT-PCR was performed using SYBR green fluorescence and the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Each reaction was run in triplicate and consisted of 10 ng cDNA, 1× Fast Power SYBR Green PCR System (Applied Biosystems), and 300 nm validated primers listed in Supplemental Tables 1 and 2 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Quantifications were performed with standard curves generated with the pCR 2.1-TOPO plasmids containing specific GLUT cDNA amplicons generated for this study. Although equal amounts of RNA from each sample were reverse transcribed, to account for variations in efficiency during this step, the results were normalized to housekeeping gene β-Actin mRNA for murine ESCs and ribosomal protein 36B4 for human ESCs. To do this, cycle threshold (Ct) values of the housekeeping gene were averaged across all samples, and the ΔΔCt method was used to determine a normalization factor for each sample, with the average Ct value as the baseline. This normalization factor was then applied to all the copy number values to account for the inherent variability in the efficiency of the reverse transcription reactions.
Gene expression knockout
Human primary ESCs were infected with lentiviral particles containing the genomes encoding Mission short hairpin RNA (shRNA) (Sigma-Aldrich, St. Louis, MO), specific to GLUT1 or those having no specific target. Two days later, the cells were treated with puromycin at a concentration of 10 μg/ml for 48 h to eliminate noninfected cells. Cells were further treated for 4 d with MPA and db-cAMP as described above to induce decidualization. Total cellular RNA was isolated as described above. mRNA levels were assessed by real-time PCR as described above, but using the ΔΔCt method to calculate fold change. Protein was also isolated from Trizol following the manufacturer's protocol (Invitrogen), and expression of GLUT1 was confirmed by Western blot as described elsewhere (6). The rabbit anti-GLUT1 antibody (kindly provided by Dr. Michael Mueckler, Washington University) (7) was used at a dilution of 1:2000 to confirm knockdown.
Statistical analyses
Differences between control values and experimental values were compared by Student's t test (PASW Statistics version 17.0).
Results
Decidualization is dependent upon sufficient glucose in vitro
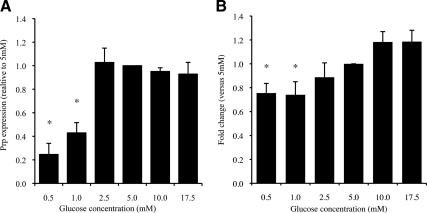
Previous data from our lab have shown that GLUT1 protein expression and glucose uptake increase during decidualization in vitro in both the mouse and human ESCs (6), suggesting that decidualization may be glucose dependent. We therefore used murine ESCs to analyze the efficiency of decidualization under variable glucose concentrations in vitro. Decidualization was monitored via mRNA expression of the mouse decidualization marker Prp and normalized to the level detected in the cells incubated in the presence of 17.5 mm glucose, which is the concentration of glucose used in standard ESC culture conditions (Fig. 1A). Expression of Prp significantly decreased when glucose levels were below 2.5 mm (P < 0.01). PRL mRNA levels were used to monitor ESC decidualization in human primary ESCs and similar results were obtained (Fig. 1B). PRL mRNA was significantly decreased in the human ESCs cultured in the presence of 1.0 and 0.5 mm glucose (P < 0.05).
Fig. 1.
Decidualization of ESCs cultured at different concentrations of glucose. A, mRNA expression of decidualization marker Prp is significantly lower in murine ESCs cultured under low-glucose conditions, 0.5 and 1.0 mm (*, P < 0.01); B, mRNA expression of human decidualization marker PRL is significantly lower in human ESCs cultured under low-glucose conditions, 0.5 and 1.0 mm (*, P < 0.05).
Facilitative GLUT mRNA levels in ESCs
Having shown that decidualization is dependent upon adequate glucose concentrations and knowing that GLUT1 protein expression increases significantly during this process, we hypothesized that GLUT1 may be the dominant GLUT in these cells. It is possible, however, that other GLUTs may also play a significant role in glucose uptake in ESCs. Therefore, we quantified the absolute levels of GLUT1 and other GLUT mRNA in control vs. decidualized ESCs. Table 1 presents the copy numbers of mRNA per nanogram of total cellular RNA in murine ESCs. Glut1 mRNA demonstrated the highest concentration in the stromal cells. Several other Glut-specific mRNAs were also detectable, but in much lower abundance. Glut2 mRNA was undetectable in all of the samples. Glut4 mRNA, the classical insulin-sensitive GLUT, was also expressed at very low, almost nondetectable, levels.
Table 1.
Glut mRNA quantification in murine ESCs (copy number/ng total RNA)
| Control (C) | Decidualized (D) | P value (C vs. D) | |
|---|---|---|---|
| GLUT1 | 876 ± 117 | 1739 ± 276 | 0.026 |
| GLUT2 | NA | ||
| GLUT3 | 306 ± 26 | 234 ± 40 | 0.16 |
| GLUT4 | 2 ± 0.2 | 2 ± 0.2 | NA |
| GLUT5 | 3 ± 0.1 | 5 ± 0.4 | NA |
| GLUT6 | 67 ± 7 | 134 ± 13 | 0.002 |
| GLUT8 | 57 ± 6 | 108 ± 11 | 0.003 |
| GLUT9 | 30 ± 2 | 27 ± 2 | 0.076 |
| GLUT10 | 58 ± 5 | 65 ± 5 | 0.89 |
| GLUT12 | 154 ± 11 | 140 ± 16 | 0.63 |
NA, Not applicable.
In parallel experiments, we also quantified mRNA levels of GLUTs in primary human ESCs (Table 2). The trend in mRNA copy numbers was similar to that found in the murine cells, although fewer GLUT mRNAs were detected. GLUT1 mRNA demonstrated the highest abundance compared with other transporters. In human ESCs, unlike the murine ESCs, GLUT10 had the second highest mRNA level behind GLUT1; however, this transporter has not been well characterized, and it is not clear whether it transports glucose exclusively or other hexoses and, thus, whether it plays a role in glucose homeostasis.
Table 2.
GLUT mRNA quantification in human ESCs (copy number/ng total RNA)
| Control (C) | Decidualized (D) | P value (C vs. D) | |
|---|---|---|---|
| GLUT1 | 1327 ± 379 | 5257 ± 885 | 0.0002 |
| GLUT2 | NA | ||
| GLUT3 | 207 ± 67 | 325 ± 84 | 0.28 |
| GLUT4 | NA | ||
| GLUT5 | NA | ||
| GLUT6 | 90 ± 13 | 85 ± 14 | 0.49 |
| GLUT8 | 73 ± 9 | 231 ± 24 | <0.0001 |
| GLUT10 | 395 ± 59 | 604 ± 62 | 0.002 |
| GLUT12 | 16 ± 5 | 72 ± 14 | 0.0006 |
NA, Not applicable.
GLUT1 is essential for decidualization of ESCs in vitro
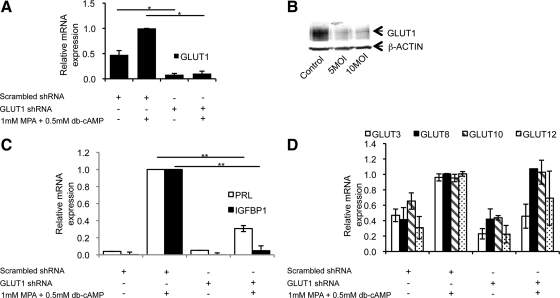
To further investigate the role of GLUT1, the most abundant transporter, in ESC decidualization, we employed RNA interference technology to knock out GLUT1 expression in human ESCs. Murine ESCs could not be used for these experiments due to their short life in cell culture once they are isolated from the gravid uteri. The human cells, however, can be cultured for a longer duration because they are extracted from a nongravid uterus and yet retain the ability to decidualize upon hormone stimulation in vitro. Human ESCs were infected with lentiviruses encoding shRNA specific to human GLUT1 or a scrambled shRNA control. A greater than 90% reduction in GLUT1 mRNA expression was achieved (Fig. 2A), and GLUT1 protein was also reduced by greater than 5-fold (Fig. 2B). Analysis of the functional consequences of GLUT1 knockdown revealed that after 4 d in culture with MPA and db-cAMP, the knockdown of GLUT1 leads to a significant (2-fold, P < 0.01) reduction in the expression levels of two widely used human decidualization markers, PRL and IGFBP1 (Fig. 2C).
Fig. 2.
Effects of GLUT1 knockdown on decidualization of human ESCs in vitro. Human ESCs were infected with lentivirus expressing GLUT1-specific shRNA or a scramble control at an multiplicity of infection (MOI) of 5 inf. u per cell. Two days later, ESCs were exposed to 10 μm puromycin for 48 h to eliminate noninfected cells. Four days after infection, the cells were treated with 1 μm MPA and 0.5 mm db-cAMP to induce decidualization. A, GLUT1 mRNA expression decreases in ESCs receiving GLUT1-specific shRNA vs. those receiving the scrambled shRNA control. *, P < 0.005 relative to the uninfected ESCs treated with MPA and db-cAMP. B, Protein levels of GLUT1 decrease with MOI of 5 and 10 infectious units per cell equally. C, mRNA expression of decidualization markers PRL and IGFBP1 are used to quantify decidualization of human ESCs in vitro. ESCs infected with lentivirus expressing GLUT1-specific shRNA show a decrease in PRL and IGFBP1 induction when they are exposed to MPA and db-cAMP relative to the ESCs receiving control scrambled shRNA. **, P < 0.01 relative to the uninfected ESCs treated with MPA and db-cAMP. D, Expression of other GLUTs does not increase in compensation for GLUT1 loss. Values are means of three independent experiments ± sem.
To exclude the possibilities of shRNA cross-reaction or compensatory up-regulation of other GLUTs, we analyzed the mRNA levels of several other high-abundance GLUTs. Under the conditions of GLUT1 knockdown, none of the other GLUTs demonstrated a significant increase in mRNA levels found in the cells infected with control lentivirus and not decidualized (Fig. 2D).
Discussion
Our experiments demonstrate that proper glucose uptake is essential for decidualization of ESCs in preparation for embryo implantation. Specifically, we show that GLUT1 mRNA is the most abundant GLUT transcript in the endometrial stroma of both the human and mouse, and its expression is crucial for proper decidualization. Strong similarities in GLUT1 transcription regulation in the mouse and human ESCs also suggest that the mouse is an adequate model for studying glucose utilization in the endometrium.
In a previous study, we showed that GLUT1 protein expression is up-regulated and glucose uptake parallels this increase in ESCs (6), suggesting that GLUT1 is responsible for the regulation of glucose uptake into the stroma. A number of other GLUTs were detected by Western blot as well, GLUT4, -8, -9b, and -12, but none showed an increase in protein expression during decidualization. Additionally, we were not able to determine the abundance of these GLUTs to rule out which GLUTs are too scarce to play a significant role in glucose homeostasis in ESCs. The current study presents a method of determining the abundance of each GLUT transcript in a way that allows for quantitative cross-comparison of the gene transcription. Using this method, we were able to determine that GLUT1 mRNA is the most abundant among the GLUTs transcribed in ESCs and to confirm that it is a major contributor to glucose homeostasis, as was hypothesized based on our earlier study. These data also unambiguously demonstrate that decreased GLUT1 expression leads to less efficient ESC decidualization and that the increase in glucose uptake detected during ESC decidualization is most likely determined by GLUT1, but not other GLUTs.
Our quantification also identified GLUT3 as a moderately abundant transporter in the stroma. Previous studies have been conflicting in their assessment of GLUT3 localization in the endometrium and its abundance in the stroma specifically (5, 8, 9). Our data are the first to quantify the mRNA and put the results in the context of other GLUTs. We can therefore conclude that the GLUT3 transcript is the second (mouse) or third (human) most abundant in the endometrial stroma and, thus, may be responsible for a large portion of the glucose uptake. GLUT3 is expressed primarily in tissues with high metabolic needs such as the brain because it has both a higher affinity and transport capacity for glucose than GLUT1 (10). Most interesting is the fact that GLUT3 can be found in the first-trimester trophoblast cells and the extraembryonic membranes, which are responsible for the uptake of maternal glucose (11). Because the decidua is responsible for fulfilling the metabolic needs of the embryo at the early stages of development, it is not surprising that GLUT3 would be an abundant transporter here as well, allowing for efficient glucose transfer and storage. Although our data do not show a significant change in the GLUT3 mRNA levels, additional studies need to be done to confirm that protein content does not change.
Another important contribution of this study is the finding that GLUT4 mRNA levels were very low in the murine stroma and below detection in the human ESCs. The current study presents a technique that is more specific and quantitative than immunohistochemistry, which was used to detect GLUT4 protein in the rat (8). Moreover, this study focuses specifically on stromal cells vs. whole endometrial biopsies, which can include other cell populations such as the epithelium (12). Effects of insulin and glucose on fertility are of great interest due to the rise of insulin resistance and type 2 diabetes prevalence among women of reproductive age. Insulin acts through the insulin receptor and IGF-I receptor, which have been identified in the endometrial stroma (13), to signal for GLUT4 translocation to the cell surface in insulin-sensitive tissues such as muscle and fat (14). Our results, although confirming a lack of GLUT4 (5), show that levels of GLUT8 and GLUT12 mRNAs are abundant and even increase upon decidualization. These two transporters have been shown to be insulin sensitive in other tissues (15, 16) and may be the effectors of insulin signaling rather than GLUT4 in the endometrial stroma.
The correlation between abnormal pregnancy outcomes and obesity and diabetes has been well documented and discussed in the literature (17–21). The link between obesity and GLUT expression has also been studied, but primarily in the skeletal muscle and adipose tissue, where high-fat diets in mice have tissue-specific effects on GLUT1 and GLUT4 expression (22, 23). No studies investigating GLUT expression specifically in the uteri of obese or diabetic subjects have been published. However, adequate transfer of maternal glucose to the developing embryo is essential for embryo survival (10). The quantitative approach presented in this work to characterize GLUT mRNA in the endometrial stroma is not only novel but also narrows the focus to the most abundant GLUTs. Moreover, this study is the first to demonstrate the functional consequences of knocking down expression of one of these transporters, GLUT1, in this critical cell type. In summary, this work establishes GLUT1 as a necessary transporter required for differentiation of ESCs to decidual cells in preparation for successful embryo implantation as well as identifies other abundant GLUTs for future studies of glucose utilization in this tissue.
Acknowledgments
We thank Dr. Kate O'Neill for her assistance in the collection and preparation of the human ESCs.
This work was supported by grants from the National Institutes of Health F30DK083224 (to A.I.F.) and R01HD06543 (to K.H.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ct
- Cycle threshold
- db-cAMP
- N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt
- ESC
- endometrial stromal cell
- GLUT
- glucose transporter
- MPA
- medroxyprogesterone-17-acetate
- shRNA
- short hairpin RNA.
References
- 1. Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. 2000. Embryo implantation. Dev Biol 223:217–237 [DOI] [PubMed] [Google Scholar]
- 2. Gellersen B, Brosens IA, Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 3. Giudice LC. 1999. Potential biochemical markers of uterine receptivity. Hum Reprod 14(Suppl 2):3–16 [DOI] [PubMed] [Google Scholar]
- 4. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. 2010. Endometrial decidualization: of mice and men. Semin Reprod Med 28:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Wolff M, Ursel S, Hahn U, Steldinger R, Strowitzki T. 2003. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab 88:3885–3892 [DOI] [PubMed] [Google Scholar]
- 6. Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. 2009. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 150:1512–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marshall BA, Ren JM, Johnson DW, Gibbs EM, Lillquist JS, Soeller WC, Holloszy JO, Mueckler M. 1993. Germline manipulation of glucose homeostasis via alteration of glucose transporter levels in skeletal muscle. J Biol Chem 268:18442–18445 [PubMed] [Google Scholar]
- 8. Korgun ET, Demir R, Hammer A, Dohr G, Desoye G, Skofitsch G, Hahn T. 2001. Glucose transporter expression in rat embryo and uterus during decidualization, implantation, and early postimplantation. Biol Reprod 65:1364–1370 [DOI] [PubMed] [Google Scholar]
- 9. Zhou J, Bondy CA. 1993. Placental glucose transporter gene expression and metabolism in the rat. J Clin Invest 91:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. 2008. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab 295:E242–E253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hahn D, Blaschitz A, Korgun ET, Lang I, Desoye G, Skofitsch G, Dohr G. 2001. From maternal glucose to fetal glycogen: expression of key regulators in the human placenta. Mol Hum Reprod 7:1173–1178 [DOI] [PubMed] [Google Scholar]
- 12. Mozzanega B, Mioni R, Granzotto M, Chiarelli S, Xamin N, Zuliani L, Sicolo N, Marchesoni D, Vettor R. 2004. Obesity reduces the expression of GLUT4 in the endometrium of normoinsulinemic women affected by the polycystic ovary syndrome. Ann NY Acad Sci 1034:364–374 [DOI] [PubMed] [Google Scholar]
- 13. Strowitzki T, von Eye HC, Kellerer M, Häring HU. 1993. Tyrosine kinase activity of insulin-like growth factor I and insulin receptors in human endometrium during the menstrual cycle: cyclic variation of insulin receptor expression. Fertil Steril 59:315–322 [DOI] [PubMed] [Google Scholar]
- 14. Huang S, Czech MP. 2007. The GLUT4 glucose transporter. Cell Metab 5:237–252 [DOI] [PubMed] [Google Scholar]
- 15. Stuart CA, Howell ME, Zhang Y, Yin D. 2009. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. J Clin Endocrinol Metab 94:3535–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. 2000. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci USA 97:7313–7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sørensen TI, Olsen J. 2007. Subfecundity in overweight and obese couples. Hum Reprod 22:1634–1637 [DOI] [PubMed] [Google Scholar]
- 18. Gesink Law DC, Maclehose RF, Longnecker MP. 2007. Obesity and time to pregnancy. Hum Reprod 22:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Metwally M, Ong KJ, Ledger WL, Li TC. 2008. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril 90:714–726 [DOI] [PubMed] [Google Scholar]
- 20. Lashen H, Fear K, Sturdee DW. 2004. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod 19:1644–1646 [DOI] [PubMed] [Google Scholar]
- 21. Jungheim ES, Moley KH. 2010. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol 203:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sevilla L, Gumà A, Enrique-Tarancón G, Mora S, Muñoz P, Palacín M, Testar X, Zorzano A. 1997. Chronic high-fat feeding and middle-aging reduce in an additive fashion Glut4 expression in skeletal muscle and adipose tissue. Biochem Biophys Res Commun 235:89–93 [DOI] [PubMed] [Google Scholar]
- 23. Kahn BB. 1994. Dietary regulation of glucose transporter gene expression: tissue specific effects in adipose cells and muscle. J Nutr 124:1289S–1295S [DOI] [PubMed] [Google Scholar]