17β-Estradiol regulates VEGF protein in hippocampus, which has important implications for endocrine and vascular effects on cognition and disease.
Abstract
Vascular endothelial growth factor (VEGF) is critical to angiogenesis and vascular permeability. It is also important in the endocrine system, in which VEGF mediates the vascular effects of estrogens in target tissues such as the uterus, a response attributed to an estrogen response element on the VEGF gene. Here we asked whether 17β-estradiol increases VEGF levels in the brain. We focused on the hippocampus, in which 17β-estradiol and VEGF both have important actions, and used immunocytochemistry to evaluate VEGF protein. VEGF immunoreactivity was compared in adult female rats sampled during the estrous cycle when serum levels of 17β-estradiol peak (proestrous morning) as well as when they are low (metestrous morning). In addition, adult rats were ovariectomized and compared after treatment with 17β-estradiol or vehicle. The results demonstrated that VEGF immunoreactivity was increased when serum levels of 17β-estradiol were elevated. Confocal microscopy showed that VEGF immunofluorescence was predominantly nonneuronal, often associated with astrocytes. Glial VEGF labeling was primarily punctate rather than diffuse and labile because glial VEGF immunoreactivity was greatly reduced if tissue sections were left in an aqueous medium overnight. We conclude that VEGF protein in normal female hippocampus is primarily nonneuronal rather than neuronal and suggest that glial VEGF immunoreactivity has been underestimated by past studies with other methods because there is a labile extracellular pool. We suggest that estrogens may exert actions on female hippocampal structure and function by increasing hippocampal VEGF.
Vascular endothelial growth factor (VEGF) is a critical mediator of angiogenesis and vascular permeability (1, 2), which has been implicated in cancer and hypertension. VEGF also is important in reproduction, in which VEGF mediates, for example, estradiol-induced vascularization of the uterus during the ovarian cycle (3, 4). The effects of estradiol may be transcriptional because there is an estrogen response element on the VEGF gene (5).
In the central nervous system (CNS), VEGF mRNA is expressed in many cells including neurons, astrocytes, and cells associated with blood vessels (6–8). Interestingly, VEGF immunoreactivity appears to be relatively weak in most neurons, except for select hypothalamic nuclei (9). In the hippocampus and neocortex, neuronal VEGF immunoreactivity is normally weak (8, 10), but immunoreactivity increases after exercise and learning (11, 12), brain injury (13), hypoxia/ischemia (8, 10), or seizures (14, 15).
In this study, we asked whether 17β-estradiol increases VEGF protein levels in the CNS, similar to the periphery. We focused on hippocampus in which 17β-estradiol has effects that could be mediated by VEGF such as increased postnatal neurogenesis (16–18), which is also increased by VEGF (19, 20). 17β-Estradiol and VEGF also protect vulnerable hippocampal neurons in CA1 and the hilus after ischemia or seizures (15, 21, 22). However, the findings have not been entirely consistent, possibly because of the influence of age and oxidative stress on actions of 17β-estradiol (23–25) and distinct effects of the primary receptors for VEGF, VEGFR1 vs. VEGFR2 (26, 27).
To test whether estradiol might alter VEGF protein in hippocampus, we used adult female rats and asked whether VEGF protein levels change in parallel with circulating levels of 17β-estradiol during the estrous cycle. We also compared VEGF protein levels in female rats after ovariectomy (Ovx) and treatment with 17β-estradiol or vehicle using a protocol designed to simulate the preovulatory surge of estradiol in intact rats (28). The results support the hypothesis that 17β-estradiol increases VEGF in the CNS: hippocampal VEGF immunoreactivity increased in proestrous and 17β-estradiol-treated Ovx rats. In addition, the results show that VEGF immunoreactivity is primarily astrocytic, not neuronal, and could easily have been missed in the past because most hippocampal VEGF immunoreactivity appears to be rapidly lost from tissue sections ex vivo.
Materials and Methods
Animals
Animal care and use followed guidelines of the National Institutes of Health. Adult female Sprague Dawley were subjects (for details, see Supplemental Materials, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Estrous cycle stage determination and RIA
Vaginal cytology was used to determine cycle stage, as described elsewhere (29). Animals were used after demonstrating at least two 4-d estrous cycles. Estradiol concentrations were assessed from serum samples, as described previously (29).
Ovx and 17β-estradiol treatment
Rats were ovariectomized under ketamine (80 mg/kg) and xylazine (6 mg/kg) anesthesia as described previously (28). Estradiol treatment followed a previously validated injection protocol, simulating the preovulatory surge (28). Three sequential injections were made starting at 0830–0900 h: 3 μg/kg estradiol benzoate (sc); 11–12 h later, 4 μg/kg estradiol benzoate (sc); and 12 h later, 3 μg/kg 17β-estradiol (sc). Animals were killed approximately 2 h after the final injection, when hormone levels were maximal. Vehicle-treated rats received corn oil instead of 17β-estradiol.
Perfusion and immunocytochemistry
Details of procedures for immunocytochemistry using nonconfocal methods are described elsewhere (30). For confocal microscopy, sections were placed in PBS (3 × 10 min), followed by 1× saline sodium citrate (0.15 m NaCl per 15 mm sodium citrate; 95 C, 5 min), washed in PBS (3 × 5 min), blocked in 5% donkey serum in 0.3% Triton X-100 in PBS (1–2 h), incubated in the same goat polyclonal antibody to VEGF (1:100) as nonconfocal experiments, and a mouse monoclonal antibody to glial-fibrillary acid protein (GFAP; 1:200; Chemicon, Billerica, MA) or mouse monoclonal antibody to a neuronal nuclear antigen (NeuN, 1:100; Chemicon). Incubation in primary antibodies at 4 C overnight was followed by PBS (3 × 10 min) and incubation overnight at 4 C in secondary antibodies (fluorescein isothiocyanate conjugated antigoat for VEGF, 1:200) or rhodamine-conjugated antimouse for GFAP or NeuN (1:200). Immunolabeling was absent when sections were processed without primary antibody. Microscopy used a Zeiss 510 Meta confocal microscope (Carl Zeiss Microimaging GmbH, Jena, Germany).
Data analysis
Quantification of VEGF immunoreactivity was conducted using ImagePro software (Media Cybernetics, Bethesda, MD). Before analysis, sections were digitally photographed using the same microscope (BX-51; Olympus America, Hauppauge, NY), camera (Spot camera model 2.2.1; Diagnostic Instruments, Sterling Heights, MI) and light settings. Because VEGF immunoreactivity was almost exclusively punctate, immunoreactivity was quantified as number of punctae per 100- × 150-μm rectangular viewing frame placed at random within the lamina of interest (DG: hilus, granule cell layer, molecular layer; CA1: stratum lacunosum-moleculare, radiatum, pyramidale, oriens, and oriens/alveus border of subfield CA1b; CA3: stratum pyramidale, lucidum, and radiatum of subfield CA3b). One hundred micrometers was chosen because the thinnest lamina in hippocampus was 100 μm thick in our tissue sections. The 150-μm length was chosen because of the curvature of some layers, making a larger rectangle not feasible to position identically throughout. Pilot studies demonstrated that it was a sufficient size to capture the range of values for that layer (the variance for values using this size frame was < 5%).
Computerized thresholding was used to ensure that VEGF immunoreactivity was included, and background, which was much lighter, was excluded. Gray-scale values computed for each section in its entirety revealed two nonoverlapping Gaussian distributions, one with relatively high values representing dark VEGF immunoreactivity and one with low values representing lighter, background staining. The threshold for quantification was placed between these two distributions. The mean gray-scale values of VEGF-immunoreactive punctae were similar (150–170 with a 0–255 scale) for all sections. In addition, the area of the viewing frame that was suprathreshold was calculated (Supplemental Fig. 1). Septotemporal differences in immunoreactivity were not evident qualitatively, but to ensure that they would not influence the analysis, only dorsal hippocampus was analyzed [corresponding to 2.0–2.5 mm posterior to Bregma (31)].
Results are reported as mean ± sem. Statistical analysis of the VEGF data were performed using Bartlett's test for homogeneity of variance, followed by two-way ANOVA. Because significant interaction was observed between the main treatment effects and regional effects (regions reflecting hippocampal layers), comparison of individual group means was carried out using Student's t test (P < 0.05, two tailed).
Results
VEGF immunolabeling is localized primarily to astrocytes in female rat hippocampus
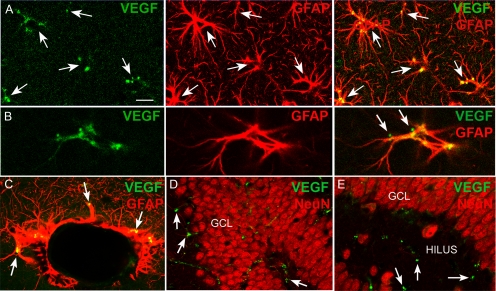
Confocal microscopy demonstrated that VEGF immunofluorescence was primarily associated with GFAP-labeled processes, in which it typically exhibited a punctate appearance, i.e. the strongest immunoreactivity was evident as small, irregular-shaped profiles (Fig. 1A). In addition, some lighter, diffuse VEGF immunolabeling of the cytoplasm of GFAP-immunoreactive cells was present (Fig. 1A). Some of the punctae appeared to be extracellular, at astrocytic plasma membranes (Fig. 1B). VEGF labeling was also in glia surrounding vessels (Fig. 1C) but did not double label with the neuronal marker NeuN (Fig. 1, D and E). This negative result is unlikely to reflect a technical difficulty because double labeling was robust with the same methods after seizures [Supplemental Fig. 2; see also Nicoletti et al. (15)].
Fig. 1.
Hippocampal VEGF immunoreactivity in hippocampus is associated with astrocytes. A, VEGF immunofluorescence (green) and GFAP immunolabeling (red) of the same section is shown separately and merged (yellow; far right panel). VEGF labeling is primarily punctate staining that is localized to astrocytes marked by GFAP. Arrows point to the same areas of each panel. Calibration, 20 μm. B, VEGF immunofluorescence (green) is shown independent (left panel) of GFAP labeling (red; center panel), and a merged image shows double labeling (yellow; far right panel). There are two VEGF punctae (arrows) that appear to be juxtaposed to GFAP-labeled processes, possibly reflecting VEGF that is bound to astrocytic VEGF receptors. Calibration (A), 5 μm. C, VEGF and GFAP double labeling around a cross-section through a vessel shows double labeling (arrows) of glia that are likely to be associated with the blood-brain barrier. Calibration (A), 10 μm. D and E, VEGF immunofluorescence (green, arrows) was not localized to neurons labeled with the neuronal marker NeuN (red). GCL, Dentate gyrus granule cell layer. Calibration (A), 50 μm (D), 20 μm (E).
Within the hilus and stratum lacunosum-moleculare, there was strong VEGF immunofluorescence. This was also observed using nonconfocal microscopy (Fig. 2). In the hilus, GFAP-labeled cells with the morphology of radial glia-like progenitors were double labeled by VEGF (Supplemental Fig. 3).
Fig. 2.
Increased hippocampal VEGF immunoreactivity on proestrous morning and after estradiol treatment. A, A Nissl-stained section showing the areas of the hippocampus in which micrographs in B and C were taken. Calibration, 200 μm. B, 1, VEGF immunoreactivity (arrows) in stratum lacunosum-moleculare (SLM) and the molecular layer (MOL) from an animal that was perfused midmorning of proestrus (Pro). 2, Relatively weak VEGF immunoreactivity in an animal that was perfused midmorning of metestrus (Met) and processed concurrently with the sections from the proestrous rat (B1). V, Vessel. C, 1, In the same animal as B1, VEGF immunoreactivity (arrows) is shown in the hilus and granule cell layer (GCL). 2) Relatively weak VEGF immunoreactivity in the hilus and granule cell layer in the metestrous animal (B2). Calibration (A), 25 μm (B and C). D, A Nissl-stained section is used to illustrate the locations of hippocampal areas in the bar graphs on the right, which are analyses of different strata of the hippocampus in intact or Ovx rats. A, Alveus; O, stratum oriens; R, stratum radiatum; LM, stratum lacunosum-moleculare; P, stratum pyramidale; G, stratum granulosum; H, hilus; M, stratum-moleculare; DG, dentate gyrus. E, Quantification of VEGF-immunoreactive punctae for comparisons of proestrous (white, n = 7) and metestrous rats (black, n = 7). Two-way ANOVA: effect of cycle stage, F = 73.457 (df 1,132), P < 0.0001; hippocampal region effect, F = 38.765 (df 10,132), P < 0.0001; interaction between cycle stage and region effects, F = 5.209 (df 10,132), P < 0.0001. Asterisks indicate proestrus significantly greater than metestrus (P < 0.05). F, Quantification for 17β-estradiol-treated (white, n = 9) vs. vehicle-treated rats (black, n = 7). Two-way ANOVA: effect of 17β-estradiol treatment, F = 83.458 (df 1,153), P < 0.0001; region effect, F = 46.511 (df 10,153), P < 0.0001; interaction between estradiol treatment and hippocampal region effects, F = 7.800 (df 10,153), P < 0.0001. Asterisks indicate a significant effect of estradiol treatment (P < 0.05).
Estradiol increases VEGF protein immunoreactivity in hippocampus
Quantitative comparisons of VEGF immunoreactivity were made in rats that were euthanized midmorning (0930–1130 h) on proestrus (aged 127.0 ± 7.4 d, range 90–143; n = 7) or midmorning of metestrus (aged 99.9 ± 21.8 d, range 70–188; n = 7), which were not different ages (Student's t test, P = 0.5406). Ovx rats treated with 17β-estradiol (76.0 ± 2.4 d old, range 64–86; n = 9) or vehicle (aged 74.7 ± 4.5 d old, range 60–86; n = 7) were also not different in age (Student's t test, P = 0.7812). There was no difference in delays between Ovx and treatment (Ovx + 17β-estradiol, 16.4 ± 0.7 d, n = 9; Ovx + vehicle, 15.8 ± 0.9 d, n = 6; Student's t test, P = 0.6598).
Serum concentrations of 17β-estradiol immediately before perfusion fixation confirmed low levels in Ovx rats treated with vehicle (3.88 ± 0.944 pg/ml, n = 4) and proestrous levels in 17β-estradiol-treated rats (51.38 ± 5.83 pg/ml, range 38.0–65.7; n = 4; Student's t test, P = 0.000197 (29, 32).
VEGF immunoreactivity was increased in intact rats on proestrous morning compared with intact rats on metestrous morning; there also was greater immunoreactivity in Ovx rats treated with 17β-estradiol relative to vehicle (Fig. 2). Notably, processing sections after leaving them in buffer overnight, rather than processing immediately, led to greatly reduced immunoreactivity (Supplemental Fig. 4). Prolonging the reaction did not restore immunoreactivity but led to increased background, and cell layers appeared to develop reactivity also (Supplemental Fig. 4), which is important because it could lead to the impression that neuronal expression is robust. Therefore, when delays occur or washing tissue is part of the procedure (e.g. for Western blot and ELISA), glial VEGF may be underestimated. These data, in addition to those described above showing VEGF-labeled punctae were often juxtaposed to GFAP-labeled processes, and the identification of glial VEGFRs (26, 27), suggest that the punctate VEGF immunoreactivity was extracellular VEGF bound to glial VEGF receptors.
Discussion
The results show that estradiol increases VEGF protein immunolabeling in the hippocampus of the adult female rat. VEGF protein appeared to be primarily astrocytic and relatively labile.
The results are consistent with the ability of estrogens to increase VEGF synthesis (5). However, one cannot exclude the possibility that the increase in hippocampal VEGF immunolabeling by estradiol was due to an indirect effect via increased neuronal activity, which has been argued for other effects of estradiol (33, 34) and is important to consider here because VEGF synthesis is activity dependent (11, 14, 15). In addition, estrogens can increase the permeability of the blood-brain barrier (35–37), making it possible that peripheral VEGF could be redistributing within the brain. VEGF itself could also facilitate entry of VEGF into the brain, because it increases vascular permeability.
It is interesting that VEGF immunoreactivity was robust in stratum lacunosum-moleculare because it is a target zone of several important afferent inputs to hippocampus. One is the temporoammonic pathway from entorhinal cortex, which mediates entorhinal input to area CA1 (38). Therefore, actions of estrogens may regulate hippocampal area CA1 via effects of VEGF on glia in this location. Stratum lacunosum-moleculare also is the site of terminals from nucleus reuniens of the thalamus, which has been shown to exert effects on CA1 neurons (39, 40). Interneurons are also located in stratum lacunosum-moleculare, which are important in regulating area CA1 pyramidal cell activity (41). Interestingly, stratum lacunosum-moleculare is a site of cholinergic regulation, which has been linked to the effects of estrogens (42).
The results have functional implications because of the known effects of estrogens and VEGF in hippocampus, such as stimulation of postnatal neurogenesis in the dentate gyrus (16, 18, 43). The results of the present study suggest that the effects of estrogens on neurogenesis may be mediated, at least in part, by their ability to increase VEGF in the neurogenic niche. This idea is further supported by the observation that estradiol had a robust effect on VEGF immunolabeling in the hilus. As mentioned above, the border of the granule cell layer and the hilus was often studded by punctate VEGF staining on GFAP-labeled cells. Some of these cells could be radial glia-like precursors because of their immunoreactivity for GFAP, a radial glial marker, and their morphology, which is distinct from typical hippocampal astrocytes (Supplemental Fig. 3).
In light of the evidence that estradiol and VEGF protect hippocampal neurons from insult or injury (15, 21, 22, 44–48), the results of the present study suggest that one of the reasons estradiol is protective is that it increases VEGF protein. An interesting example to consider is stroke, in which female rodents appear to be protected relative to males in experimental models of stroke, and adult women appear to be protected compared with men (48–52). One of the reasons for the reduced risk of stroke in females could be that estrogens maintain a higher level of VEGF protein in areas of the brain such as the hippocampus. Increased VEGF may also improve the ability to recover from injury because increased VEGF levels would be likely to increase angiogenesis, which would potentially improve recovery (1, 53, 54). Indeed, estradiol has been shown to prevent the down-regulation of VEGF in the brain caused by ischemic injury in rodents (55). It is notable that the primary site of VEGF labeling was not neuronal. This finding is consistent with a previous study showing that hippocampal VEGF mRNA in the principal cell layers of the hippocampus does not appear to change in response to 17β-estradiol in the rat (56, 57). Taken together, it appears that both VEGF mRNA and protein expression in neurons of the normal adult hippocampus are not affected by 17β-estradiol. Instead, another pool of VEGF, which appears to be associated with astrocytes, is increased by 17β-estradiol. In contrast, after insult or injury, VEGF protein expression is robust in the hippocampal neurons (15, 43, 58), and an extracellular pool of VEGF develops adjacent to the neuronal processes (Supplemental Fig. 4).
Other conditions that are known to be influenced by estrogens and have a vascular component, such as migraine, could also involve VEGF regulation. In migraine, many women are known to have cyclical changes in symptoms (59, 60), and both estrogens and the vasculature have been implicated (61–64). Cyclical changes in VEGF protein induced by estradiol could potentially contribute to cyclical variations in migraine (65).
In conclusion, our results suggest that 17β-estradiol increases VEGF protein in the brain, analogous to actions of 17β-estradiol in the female reproductive tract. However, in brain, astrocytes are a primary target. These findings have implications for normal brain function because of the diverse roles of glia, in particular for neuroendocrine regulation because of the contribution of astrocytes to estrogen action (66, 67), as well as for clinical conditions in which both estrogens and VEGF contribute.
Acknowledgments
We thank Dr. Vicky Luine for providing equipment for the RIA and Drs. Joseph Pierce and Alejandra Poveda for assistance with image analysis.
This work was supported by National Institutes of Health Grant NS 37562, the New York State Department of Health, and the Office of Mental Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CNS
- Central nervous system
- GFAP
- glial-fibrillary acid protein
- Ovx
- ovariectomy
- VEGF
- vascular endothelial growth factor
- VEGFR
- VEGF receptor.
References
- 1. Ribatti D. 2005. The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. Br J Haematol 128:303–309 [DOI] [PubMed] [Google Scholar]
- 2. Breen EC. 2007. VEGF in biological control. J Cell Biochem 102:1358–1367 [DOI] [PubMed] [Google Scholar]
- 3. Cullinan-Bove K, Koos RD. 1993. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 133:829–837 [DOI] [PubMed] [Google Scholar]
- 4. Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH. 1998. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 4:336–340 [DOI] [PubMed] [Google Scholar]
- 5. Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. 2000. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors α and β. Proc Natl Acad Sci USA 97:10972–10977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoehn BD, Harik SI, Hudetz AG. 2002. VEGF mRNA expressed in microvessels of neonatal and adult rat cerebral cortex. Mol Brain Res 101:103–108 [DOI] [PubMed] [Google Scholar]
- 7. Rajah TT, Grammas P. 2002. VEGF and VEGF receptor levels in retinal and brain-derived endothelial cells. Biochem Biophys Res Commun 293:710–713 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, Hermann DM. 2005. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain 128:52–63 [DOI] [PubMed] [Google Scholar]
- 9. Alonso G, Galibert E, Duvoid-Guillou A, Vincent A. 2005. Hyperosmotic stimulus induces reversible angiogenesis within the hypothalamic magnocellular nuclei of the adult rat: a potential role for neuronal vascular endothelial growth factor. BMC Neurosci 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA. 2000. Induction of vascular endothelial growth factor and hypoxia-inducible factor-1α by global ischemia in rat brain. Neuroscience 99:577–585 [DOI] [PubMed] [Google Scholar]
- 11. Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. 2004. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835 [DOI] [PubMed] [Google Scholar]
- 12. During MJ, Cao L. 2006. VEGF a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res 3:29–33 [DOI] [PubMed] [Google Scholar]
- 13. Sköld MK, von Gertten C, Sandberg-Nordqvist AC, Mathiesen T, Holmin S. 2005. VEGF and VEGF receptor expression after experimental brain contusion in rat. J Neurotrauma 22:353–367 [DOI] [PubMed] [Google Scholar]
- 14. Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. 2003. Gene profile of electroconvulsive seizures: Induction of neurotrophic and angiogenic factors. J Neurosci 23:10841–10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicoletti JN, Shah SK, McCloskey DP, Goodman JH, Elkady A, Atassi H, Hylton D, Rudge JS, Scharfman HE, Croll SD. 2008. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience 151:232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanapat P, Hastings NB, Reeves AJ, Gould E. 1999. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19:5792–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang JM, Konkle AT, Zup SL, McCarthy MM. 2008. Impact of sex and hormones on new cells in the developing rat hippocampus: A novel source of sex dimorphism? Eur J Neurosci 27:791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalla C, Papachristos EB, Whetstone AS, Shors TJ. 2009. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proc Natl Acad Sci USA 106:2927–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18:2803–2812 [DOI] [PubMed] [Google Scholar]
- 20. Warner-Schmidt JL, Duman RS. 2007. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA 104:4647–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. 2000. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia 41(Suppl 6):S30–S35 [DOI] [PubMed] [Google Scholar]
- 22. Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. 2009. Estradiol facilitates the release of neuropeptide y to suppress hippocampus-dependent seizures. J Neurosci 29:1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bake S, Ma L, Sohrabji F. 2008. Estrogen receptor-α overexpression suppresses 17β-estradiol-mediated vascular endothelial growth factor expression and activation of survival kinases. Endocrinology 149:3881–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brinton RD. 2008. Estrogen regulation of glucose metabolism and mitochondrial function: Therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev 60:1504–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brinton RD. 2008. The healthy cell bias of estrogen action: Mitochondrial bioenergetics and neurological implications. Trends Neurosci 31:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Croll SD, Goodman JH, Scharfman HE. 2004. Vascular endothelial growth factor (VEGF) in seizures: a double-edged sword. Adv Exp Med Biol 548:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee C, Agoston DV. 2009. Inhibition of VEGF receptor 2 increased cell death of dentate hilar neurons after traumatic brain injury. Exp Neurol 220:400–403 [DOI] [PubMed] [Google Scholar]
- 28. Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. 2007. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci 26:2595–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. 2003. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci 23:11641–11652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scharfman HE, Sollas AL, Smith KL, Jackson MB, Goodman JH. 2002. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J Comp Neurol 454:424–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paxinos G, Watson C. 1986. The rat brain in stereotaxic coordinates. New York: Academic Press; [DOI] [PubMed] [Google Scholar]
- 32. Freeman ME. 2006. Neuroendocrine control of the ovarian cycle in the rat. In: Knobil E, Neill JD. eds. Knobil and Neill's physiology of reproduction. New York: Academic Press; 2327–2388 [Google Scholar]
- 33. Blurton-Jones M, Kuan PN, Tuszynski MH. 2004. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol 468:347–360 [DOI] [PubMed] [Google Scholar]
- 34. Blurton-Jones M, Tuszynski MH. 2006. Estradiol-induced modulation of estrogen receptor-β and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol 499:603–612 [DOI] [PubMed] [Google Scholar]
- 35. Reid AC, Teasdale GM, McCulloch J. 1983. Hormonal influence on water permeability across the blood-brain barrier. Clin Exp Neurol 19:50–53 [PubMed] [Google Scholar]
- 36. Ziylan YZ, Lefauconnier JM, Bernard G, Bourre JM. 1990. Blood-brain barrier permeability: regional alterations after acute and chronic administration of ethinyl estradiol. Neurosci Lett 118:181–184 [DOI] [PubMed] [Google Scholar]
- 37. Bake S, Sohrabji F. 2004. 17β-Estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology 145:5471–5475 [DOI] [PubMed] [Google Scholar]
- 38. Moser EI, Kropff E, Moser MB. 2008. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci 31:69–89 [DOI] [PubMed] [Google Scholar]
- 39. Wouterlood FG, Saldana E, Witter MP. 1990. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer phaseolus vulgaris-leucoagglutinin. J Comp Neurol 296:179–203 [DOI] [PubMed] [Google Scholar]
- 40. Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. 1997. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci 17:5640–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chapman CA, Lacaille JC. 1999. Cholinergic induction of theta-frequency oscillations in hippocampal inhibitory interneurons and pacing of pyramidal cell firing. J Neurosci 19:8637–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gibbs RB. 2000. Oestrogen and the cholinergic hypothesis: Implications for oestrogen replacement therapy in postmenopausal women. Novartis Found Symp 230:94–107; discussion 107–111 [DOI] [PubMed] [Google Scholar]
- 43. Segi-Nishida E, Warner-Schmidt JL, Duman RS. 2008. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci USA 105:11352–11357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin KL, Mao XO, Greenberg DA. 2000. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA 97:10242–10247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dumitriu D, Rapp PR, McEwen BS, Morrison JH. 2010. Estrogen and the aging brain: an elixir for the weary cortical network. Ann NY Acad Sci 1204:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Merchenthaler I, Dellovade TL, Shughrue PJ. 2003. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann NY Acad Sci 1007:89–100 [DOI] [PubMed] [Google Scholar]
- 47. Svensson B, Peters M, König HG, Poppe M, Levkau B, Rothermundt M, Arolt V, Kögel D, Prehn JH. 2002. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab 22:1170–1175 [DOI] [PubMed] [Google Scholar]
- 48. Hoffman GE, Merchenthaler I, Zup SL. 2006. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine 29:217–231 [DOI] [PubMed] [Google Scholar]
- 49. Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MS, Stern B, Herrington D, Ford-Lynch G, Gorelick P, James A, Brown CM, Choi E, Bray P, Newby LK, Goldstein LB, Simpkins J. 2006. Advancing the study of stroke in women: summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke 37:2387–2399 [DOI] [PubMed] [Google Scholar]
- 50. Hyvärinen M, Qiao Q, Tuomilehto J, Söderberg S, Eliasson M, Stehouwer CD. 2010. The difference between acute coronary heart disease and ischaemic stroke risk with regard to gender and age in Finnish and Swedish populations. Int J Stroke 5:152–156 [DOI] [PubMed] [Google Scholar]
- 51. Vagnerova K, Koerner IP, Hurn PD. 2008. Gender and the injured brain. Anesth Analg 107:201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herson PS, Koerner IP, Hurn PD. 2009. Sex, sex steroids, and brain injury. Semin Reprod Med 27:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hansen TM, Moss AJ, Brindle NP. 2008. Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr Neurovasc Res 5:236–245 [DOI] [PubMed] [Google Scholar]
- 54. Krum JM, Mani N, Rosenstein JM. 2008. Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp Neurol 212:108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. 2007. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci USA 104:6013–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sibug RM, Helmerhorst FM, Tijssen AM, de Kloet ER, de Koning J. 2002. Estrogen reduces vascular endothelial growth factor(164) expression in the mouse nucleus paraventricularis of the hypothalamus. Neurosci Lett 333:199–202 [DOI] [PubMed] [Google Scholar]
- 57. Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hum PD. 2005. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-α in a rodent experimental stroke model. Stroke 36:337–341 [DOI] [PubMed] [Google Scholar]
- 58. Newton SS, Duman RS. 2004. Regulation of neurogenesis and angiogenesis in depression. Curr Neurovasc Res 1:261–267 [DOI] [PubMed] [Google Scholar]
- 59. MacGregor EA. 2005. Female sex hormones and migraine. Rev Neurol (Paris) 161:677–678 [DOI] [PubMed] [Google Scholar]
- 60. MacGregor EA. 2008. Menstrual migraine. Curr Opin Neurol 21:309–315 [DOI] [PubMed] [Google Scholar]
- 61. MacGregor EA. 2004. Oestrogen and attacks of migraine with and without aura. Lancet Neurol 3:354–361 [DOI] [PubMed] [Google Scholar]
- 62. Krause DN, Duckles SP, Pelligrino DA. 2006. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol 101:1252–1261 [DOI] [PubMed] [Google Scholar]
- 63. Puri V, Puri S, Svojanovsky SR, Mathur S, Macgregor RR, Klein RM, Welch KM, Berman NE. 2006. Effects of oestrogen on trigeminal ganglia in culture: Implications for hormonal effects on migraine. Cephalalgia 26:33–42 [DOI] [PubMed] [Google Scholar]
- 64. Welch KM, Brandes JL, Berman NE. 2006. Mismatch in how oestrogen modulates molecular and neuronal function may explain menstrual migraine. Neurol Sci 27(Suppl 2):S190–S192 [DOI] [PubMed] [Google Scholar]
- 65. Scharfman HE, MacLusky NJ. 2008. Estrogen-growth factor interactions and their contributions to neurological disorders. Headache 48(Suppl 2):S77–S89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garcia-Segura LM, McCarthy MM. 2004. Minireview: role of glia in neuroendocrine function. Endocrinology 145:1082–1086 [DOI] [PubMed] [Google Scholar]
- 67. Mong JA, Blutstein T. 2006. Estradiol modulation of astrocytic form and function: Implications for hormonal control of synaptic communication. Neuroscience 138:967–975 [DOI] [PubMed] [Google Scholar]