Kiss1 neurons are present in amygdala of mice and rats of both sexes, and are stimulated there by sex steroids acting via estrogen receptor pathways.
Abstract
Kisspeptin (encoded by the Kiss1 gene) is an important regulator of reproduction. In rodents, Kiss1 is expressed in two hypothalamic regions, the arcuate nucleus and anteroventral periventricular/ periventricular continuum, where it is regulated by sex steroids. However, the distribution, regulation, and functional significance of neural kisspeptin outside of the hypothalamus have not been studied and are poorly understood. Here, we report the expression of Kiss1 in the amygdala, predominantly in the medial nucleus of the amygdala (MeA), a region implicated in social and emotional behaviors as well as various aspects of reproduction. In gonadally intact rats and mice, Kiss1-expressing neurons were identified in the MeA of both sexes, with higher Kiss1 expression levels in adult males than females in diestrus. In rats, Kiss1 expression in the MeA changed as a function of the estrous cycle, with highest levels at proestrus. Next, we tested whether Kiss1 in the MeA is regulated by the circulating sex steroid milieu. Kiss1 levels in the MeA were low in gonadectomized mice and rats of both sexes, and treatment with either testosterone or estradiol amplified Kiss1 expression in this region. Testosterone's inductive effect on Kiss1 expression in the MeA likely occurs via estrogen receptor-dependent pathways, not through the androgen receptor, because dihydrotestosterone (a nonaromatizable androgen) did not affect MeA Kiss1 levels. Thus, in rodents, Kiss1 is expressed and regulated by sex steroids in the MeA of both sexes and may play a role in modulating reproduction or brain functions that extend beyond reproduction.
Kisspeptin, encoded by the Kiss1 gene, and its receptor (Kiss1R) play an essential role in controlling reproduction in mammals, based on several lines of evidence. First, humans and mice with mutations in either the Kiss1R or Kiss1 gene exhibit striking deficits in reproductive function, including impaired pubertal maturation, low levels of sex steroids and gonadotropins, and infertility (1–4). Second, administration of kisspeptin robustly stimulates gonadotropin secretion (5, 6). Lastly, central administration of kisspeptin induces Fos expression in GnRH neurons, evokes depolarization and sustained firing of action potentials in these cells, and stimulates GnRH secretion (5, 6). In rodents, sheep, and primates (including humans), neurons that express Kiss1 mRNA and kisspeptin are located in two discrete regions of the diencephalon: the anteroventral periventricular nucleus-periventricular nucleus continuum (AVPV/PeN; or the preoptic area in sheep and primates) and, more caudally, the arcuate nucleus of the hypothalamus (ARC; the homolog of the primate infundibular nucleus) (7–12).
Although the regulation and function of Kiss1 neurons in the hypothalamus has been well studied, the presence and importance of Kiss1 neurons in other brain regions has been largely ignored. Virtually all roles of neural kisspeptin signaling in the control of puberty and reproduction have been attributed to one or both of the two hypothalamic populations, disregarding the possibility of kisspeptin signaling derived from potential Kiss1 populations residing outside the hypothalamus. However, during the course of our earlier studies of Kiss1 in the hypothalamus, we observed significant Kiss1 expression in the amygdala. The amygdala is a limbic area rich in sex steroid receptors (13), which has previously been implicated in a wide range of sex steroid-dependent behavioral and physiological processes, including anxiety and fear responses, as well as social and sexual behaviors (14–19). Moreover, studies of the amygdala involving lesions, localized hormone treatments, and electrophysiology have implicated this region in the regulation of female reproductive cycles and sexual behavior (20–30). Additionally, the amygdala sends neuronal projections to target sites known to be involved in reproduction, including the preoptic area (where GnRH neurons reside), the AVPV, and ARC (31). There are also numerous documented sex differences in the amygdala, particularly in the medial nucleus, with males having greater medial amygdala nuclear volume, synaptic input, and dendritic spine density than females (32–36) and greater numbers of vasopressin- and substance P-producing neurons (14). Despite ample knowledge of the neuroanatomy and functions of the amygdala, at present, there is a complete paucity of information regarding Kiss1 cells in this region.
In rodents, there are several key differences in the physiology and phenotype of Kiss1 populations in the ARC and AVPV/PeN. First, sex steroids [e.g. estradiol (E2) and testosterone (T)] regulate Kiss1 gene expression in opposite fashions in these two hypothalamic areas. In the ARC, sex steroids inhibit the expression of Kiss1, whereas in the AVPV/PeN, sex steroids increase Kiss1 expression (37–39). Conversely, when sex steroids are low, Kiss1 expression increases in the ARC and decreases in the AVPV/PeN (37–39). Second, in the ARC, sex steroids inhibit Kiss1 expression via either androgen receptors (AR) or estrogen receptors (ER), whereas in the AVPV/PeN, sex steroids induce Kiss1 expression only through ER-dependent pathways (38, 39). Third, the cellular phenotype of Kiss1 neurons differs between the two regions. In the ARC, but not the AVPV/PeN, Kiss1 neurons coexpress dynorphin, neurokinin B, and the neurokinin B receptor NK3R (40, 41). Conversely, in the AVPV/PeN, but not the ARC, Kiss1 neurons coexpress tyrosine hydroxylase (42). Finally, Kiss1 neurons in the AVPV/PeN are sexually dimorphic, with females having more Kiss1 neurons than males, whereas Kiss1 cell number in the ARC is not demonstrably sexually dimorphic in adults under controlled sex steroid conditions (37, 42, 43). Collectively, these findings emphasize that Kiss1 neurons in different brain regions are phenotypically and physiologically distinct, likely relating to differences in function (5, 6). Whether Kiss1 cells elsewhere in the brain, such as the amygdala, share common properties with the AVPV/PeN and/or ARC population is unknown.
Here, we investigated the pattern and regulation of Kiss1 expression in the amygdala of two rodent species, the rat and mouse. We had four main goals: 1) to determine whether Kiss1 is significantly expressed in the amygdala in each species and, if so, precisely where in this region; 2) to assess whether Kiss1 expression in the amygdala of adult animals is sexually dimorphic, as it is in the AVPV/PeN; 3) to determine whether Kiss1 neurons in the amygdala, like those in the hypothalamus, are targets for regulation by sex steroids and, if so, whether Kiss1 expression in the amygdala is stimulated or inhibited by sex steroids; and 4) to elucidate which sex steroid receptor pathway, AR and/or ER, mediates any effects of sex steroids on Kiss1 expression in the amygdala.
Materials and Methods
Animals
Male and female C57BL6 mice were housed at the University of California, San Diego, and University of Washington on a 12-h light, 12-h dark cycle with food and water available ad libitum. Rat brain tissue was used from adult Wistar rats of both sexes that were used in previous reports by our labs (37, 44). All experiments were conducted in accordance with the National Institutes of Health Animal Care and Use Guidelines and with approval of the local Animal Care and Use Committees of the University of California, San Diego, and the University of Washington.
Surgical treatments and tissue collection
Sex steroids up-regulate Kiss1 gene expression in the AVPV/PeN and down-regulate Kiss1 in the ARC (37, 38) and hence could also augment Kiss1 expression in other brain regions. Thus, in some experiments, circulating sex steroid levels [E2, T, or dihydrotestosterone (DHT)] were equalized among groups using SILASTIC (Dow Corning Corp., Midland, MI) implants. Adults of both sexes were anesthetized and bilaterally gonadectomized (GDX). At the time of gonadectomy, animals received a sc E2-filled, T-filled, or DHT-filled SILASTIC implant, constructed as previously described (37, 39). For rats, the E2, T, and DHT implants have been shown to successfully reduce circulating LH and FSH levels in GDX animals to levels found in intact animals and to also significantly regulate hypothalamic Kiss1 expression (37). For mice, the DHT and T implants have been shown to produce physiological levels of hormone (∼3 and 11 ng/ml, respectively) similar to levels in intact males, and the E2 implants produce slightly elevated levels around 50–88 pg/ml (39, 45); all three implants have been shown to regulate Kiss1 expression in the murine hypothalamus (39). For comparison, some GDX animals of each sex received no hormone implant. One week after surgery, all animals were killed via rapid decapitation. To avoid circadian modulation of Kiss1 expression (46), all animals were killed in the late morning/early afternoon. At the time of killing, brains were collected, immediately frozen on dry ice, and stored at −80 C.
Quantitative (real-time) PCR analysis of Kiss1 gene expression
In one experiment, total RNA from 600-μm-thick frozen micropunches of the medial nucleus of the amygdala (MeA) and AVPV/PeN was extracted using the RNeasy Lipid Tissue Mini kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. RNA was reverse transcribed using the Omniscript RT kit (QIAGEN), and cDNA was stored at −20 C until use in PCR. Quantitative real-time PCR (qPCR) was performed on each cDNA sample using the Bio-Rad iCycler Detection System and Quantitect SYBR Green PCR kit (QIAGEN). To detect Kiss1, Kiss1-specific primers 5′-CAA AAG TGA AGC CTG GAT CC-3′ (forward) and 5′-GTT GTA GGT GGA CAG GTC C-3′ (reverse) were used. Standard curves were generated for each product using cloned cDNAs for Kiss1 and GAPDH to quantify the abundance of cDNA in each sample. For standard curves, a dilution series of cloned Kiss1 and GAPDH templates ranging from 10 to 108 copies were used. The quantitative PCR cycling parameters were one cycle of 95 C for 15 min, followed by 40 cycles of 94 C for 15 sec, 60 C for 30 sec, and 72 C for 30 sec. Data collection was taken at the 72 C extension phase. To ensure the presence of a single product, a dissociation curve was performed after each run. Data were collected from threshold values using the automatic function of the Bio-Rad MyIQ software. All samples were run in duplicate, and Kiss1 values were normalized to GAPDH, whose expression is constant.
Single-label in situ hybridization (ISH)
Single-label ISH for Kiss1 was performed as previously described (11, 37, 39, 46), using a validated Kiss1 riboprobe (11). Briefly, five alternating sets of 20-μm brain sections (i.e. each set contains every fifth section) encompassing the entire forebrain and hypothalamus/amygdala regions were cut and thaw-mounted onto SuperFrost Plus slides and stored at −80 C. One set of sections spanning the entire MeA (or AVPV/PeN or ARC) was fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× sodium citrate, sodium chloride (SSC), delipidated in chloroform, dehydrated in ethanols, and air dried. Radiolabeled (33P) antisense riboprobe (0.05 pmol/ml) was combined with 1/20 volume yeast tRNA (Roche Biochemicals, Indianapolis, IN), heat denatured, added to hybridization buffer, and applied to each slide (100 μl/slide). Slides were coverslipped and put at 55 C for 17 h. The next day, the slides were washed in 4× SSC and placed into ribonuclease (RNase) [37 mg/ml RNase A in 0.15 m sodium chloride, 10 mm Tris, 1 mm EDTA (pH 8.0)] for 30 min at 37 C, then in RNase buffer without RNase at 37 C for 30 min. After washing in 2× SSC at room temperature, slides were washed in 0.1× SSC at 62 C, dehydrated in ethanols, and air dried. Slides were then dipped in Kodak NTB emulsion, air dried, and stored at 4 C for 7–12 d (depending on the assay). Slides were then developed, dehydrated in ethanols, cleared in Citrisolv (Fisher Scientific, Pittsburgh, PA), and coverslipped with Permaslip (Sigma Chemical Co., St. Louis, MO). Slides were analyzed with an automated image processing system and custom grain counting software (Dr. Don Clifton, University of Washington) by a person blind to the treatment group. The software counts the number of silver grain clusters representing Kiss1 cells as well as the number of silver grains in each cell cluster (a semiquantitative index of mRNA content per cell) (38, 47). Cells were considered Kiss1 positive when the number of silver grains in a cluster exceeded that of background by 3-fold.
Statistical analyses
All data are expressed as the mean ± sem for each group. Differences in group means were assessed via overall ANOVA, with post hoc analysis determined by Fisher's least significant difference test. For all comparisons, statistical significance was set at P < 0.05. All analyses were performed with Statview version 5.0.1 (SAS Institute, Cary, NC).
Experiment 1: Kiss1 gene expression in the MeA of intact male and female rats
Experiment 1 mapped the specific neuroanatomical expression of Kiss1 mRNA outside the hypothalamus, particularly in the amygdala region, in gonadally intact adult male and female rats (n = 5 per sex). Adult females were cycled via examination of daily vaginal smears and brains collected at diestrous stage. Single-label ISH was used to identify and compare Kiss1 mRNA expression in specific regions of the amygdala in each sex. The mean number of Kiss1-expressing cells and the relative level of Kiss1 mRNA per cell (as reflected by silver grains per cell) were determined.
Experiment 2: Kiss1 expression in the MeA of female rats across the estrous cycle
Experiment 1 detected low levels of Kiss1 expression in the MeA of female rats on diestrus. We previously reported that Kiss1 levels in the AVPV/PeN and ARC of rats change with the estrous cycle (44). Thus, this experiment tested whether Kiss1 expression in the MeA also changes over the estrous cycle. Brain tissue was used from a previous study (44) in which female rats were cycled by daily inspection of vaginal smears. After displaying two continuous cycles, females were killed in the morning on either diestrus II, proestrus, or estrus (n = 5 per group). Brains were collected and analyzed via ISH for the number of Kiss1-expressing cells in the MeA as well as the relative amount of Kiss1 mRNA per cell in this region.
Experiment 3: sex steroid regulation of Kiss1 expression in the MeA of male and female rats
In the rodent hypothalamus, sex steroids (T and E2) stimulate Kiss1 levels in the AVPV/PeN and inhibit Kiss1 in the ARC. This experiment tested whether Kiss1 gene expression is also regulated by sex steroids in the MeA and, if so, in which direction (stimulatory as in the AVPV/PeN or inhibitory as in the ARC). Adult male and female rats were GDX and treated for a week with either E2 or nothing (n = 5 per group). To compare the effects of T and E2, an additional cohort of five GDX males received a T implant for 1 wk. After 1 wk, all animals were killed and the brains collected. Sections encompassing the amygdala were processed for Kiss1 ISH to determine the number of Kiss1-expressing cells as well as the relative amount of Kiss1 mRNA per cell.
Experiment 4: Kiss1 expression in the MeA of male and female mice
The previous experiments identified Kiss1 in the MeA of rats, with higher expression levels in adult males than females on diestrus. We next determined whether the presence of Kiss1 in the MeA in rats also extends to other rodent species. In this experiment, we assessed whether Kiss1 is expressed in the MeA of male and female mice and whether there is a sex difference in MeA Kiss1 levels between gonadally intact mice, as there was in rats. Adult male and female (diestrus) mice were killed and the brains collected and processed for Kiss1 ISH, as in experiment 1 (n = 5–6 per sex).
Experiment 5: E2 regulation of Kiss1 expression in the MeA of mice
As in rats, Kiss1 expression in the AVPV/PeN and ARC of mice is strongly regulated by sex steroids, with T and E2 stimulating Kiss1 in the AVPV/PeN and inhibiting Kiss1 in the ARC. Given the robust sex steroid regulation we observed in the MeA of rats (experiment 3), we tested whether Kiss1 in the MeA of mice is also stimulated by sex steroids, particularly E2. Adult male and female mice were GDX and treated for a week with E2 or nothing (n = 5–7 per group). Mice were then killed and the brains collected for ISH of Kiss1 levels in the MeA. To confirm data obtained with ISH, we also conducted a similar experiment in a separate cohort of mice (n = 5 per group) whose brains were analyzed for Kiss1 levels using the sensitive and quantitative method of real-time PCR (qPCR). For each animal, a micropunch was taken from a 600-μm brain section that included the MeA region. For comparison, similar-sized micropunches were also taken from the AVPV/PeN and olfactory bulb regions. Total mRNA was extracted from the micropunches and qPCR for Kiss1 performed on cDNA obtained via reverse transcription.
Experiment 6: effects of androgens on Kiss1 in the MeA of mice
In experiment 3, we determined that both E2 and T can stimulate Kiss1 in the male rat MeA. Here we tested whether T can also stimulate Kiss1 in the MeA in mice, in either males or females. In experiment 6A, adult male and female mice were GDX and treated for a week with T or nothing (n = 4 per group). Animals were then killed and the brains collected for ISH of Kiss1 levels in the MeA. Because T can act via AR or via ER (after aromatization to E2), it is unclear which pathway mediates T's stimulation of Kiss1 in the MeA. Thus, in experiment 6B, we tested whether the Kiss1 gene in the MeA can be regulated selectively via AR pathways. GDX adult male mice were given an implant containing DHT (a nonaromatizable androgen), E2, or nothing (n = 4–6 per group). After 1 wk, mice were killed and the brains collected and processed via ISH to determine the number of Kiss1-expressing cells in the MeA. For comparison of the effects of the different hormones, Kiss1 levels were also determined in the AVPV/PeN and the ARC.
Results
Experiment 1: the Kiss1 gene is expressed in the MeA of gonadally intact male and female rats
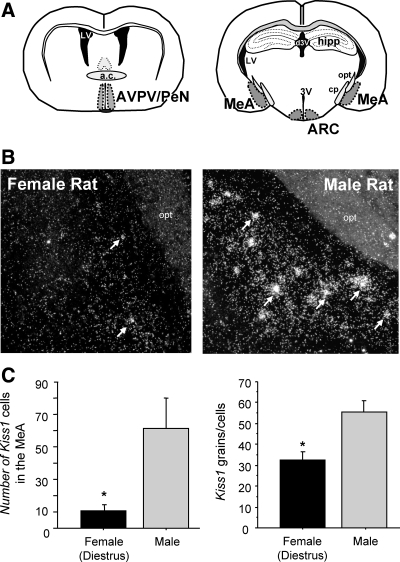
This experiment mapped the expression of Kiss1 mRNA expression in the amygdala of gonadally intact male and female (diestrus) rats. We observed distinct clusters of silver grains (reflecting Kiss1 mRNA staining) scattered in the amygdala of both sexes, although levels were more noticeable in males (Fig. 1). Kiss1 neurons seemed to be restricted to the MeA and were not observed in other amygdaloid regions. Although Kiss1 cells were most prominent in the posterodorsal MeA, there were also occasional cells in more ventral regions of the MeA. Quantitative analysis revealed that male rats had significantly more Kiss1 neurons in the MeA than did diestrous females (P < 0.05, Fig. 1). The relative amount of Kiss1 mRNA per cell was also higher in males than diestrous females (P < 0.05, Fig. 1).
Fig. 1.
Kiss1 mRNA expression in the amygdala of rats. A, Schematic of the different brain regions where Kiss1 expression is identified. a.c., Anterior commissure; hipp, hippocampus; LV, lateral ventricle; opt, optic tract; pd, cerebral peduncle; 3V, third ventricle; d3V, dorsal 3rd ventricle. B, Representative photomicrographs of ISH for Kiss1 mRNA in the MeA of gonadally intact male and female rats. White arrows depict sample Kiss1 neurons. C, Mean number of Kiss1 neurons in the MeA of gonadally intact female and male rats. The number of Kiss1 neurons was significantly higher in males than females (diestrus). *, Significantly different (P < 0.05).
Experiment 2: Kiss1 expression in the MeA of female rats changes across the estrous cycle
Kiss1 levels in the AVPV/PeN and the ARC have previously been reported to change with the estrous cycle, and we tested whether the expression of Kiss1 in the MeA also changes with estrous stage. We found that Kiss1 expression was detectable in the MeA of female rats on diestrus, proestrus, and estrus but varied as a function of cycle stage. Specifically, the number of Kiss1 neurons in the MeA was highest on proestrus and lowest on diestrus and estrus (P < 0.05, Fig. 2). There was no significant difference in Kiss1 cell numbers between estrus and diestrus. Correlating with Kiss1 cell numbers in the MeA, mean plasma levels of E2 were highest at proestrus (∼55 pg/ml) and low on diestrus and estrus (∼16–23 pg/ml), as previously reported (44). No significant difference in the content of Kiss1 mRNA per cell (as reflected by grains per cell) was detectable in the MeA during different stages of the estrous cycle (data not shown).
Fig. 2.
Mean number of Kiss1 cells in the MeA of females rats at different stages of the estrous cycle. Kiss1 mRNA was significantly higher in females on proestrus than diestrus or estrus. *, Significantly different from other two groups (P < 0.05).
Experiment 3: sex steroids regulate Kiss1 expression in the MeA of male and female rats
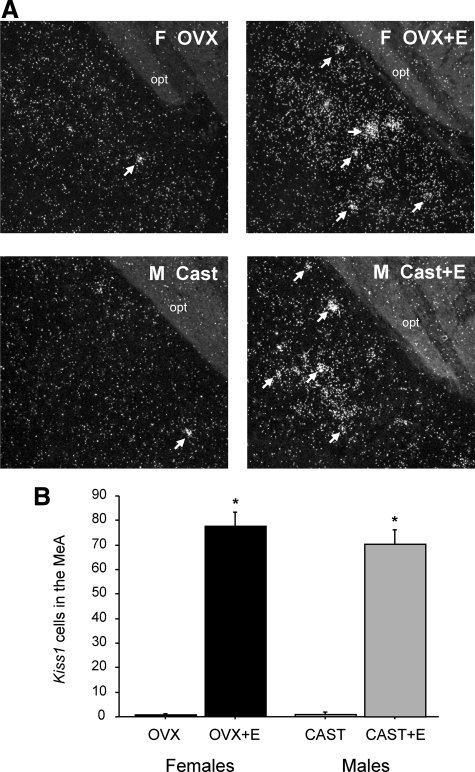
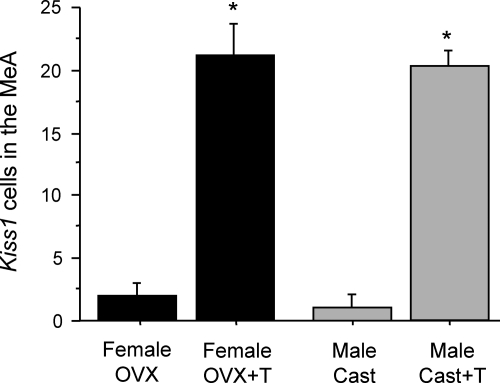
This experiment tested whether the Kiss1 gene in the MeA is regulated by sex steroids and, if so, in which direction (stimulatory as in the AVPV/PeN or inhibitory as in the ARC). We found that sex steroid treatment induced Kiss1 expression in the MeA of rats. GDX male and female rats each had low, sometimes undetectable, levels of Kiss1 in the MeA (Fig. 3). In contrast, females and males given an E2 implant for 1 wk had high Kiss1 levels in the MeA (P < 0.05 vs. GDX animals, Fig. 3). An additional group of GDX males treated with T for 1 wk also had high levels of Kiss1 in the MeA, which were not significantly different from those of E2-treated males or females (Fig. 3).
Fig. 3.
A, Representative photomicrographs of Kiss1 in the MeA of male and female rats treated with sex steroids for 1 wk. White arrows depict examples of Kiss1 neurons. B, Mean number of Kiss1 cells in the MeA of male and female rats that were GDX and treated with T, E2, or nothing. There were more Kiss1 cells in the MeA in T- or E2-treated rats compared with untreated GDX rats. Bars with different letters are significantly different from each other (P < 0.05). Cast, Castrated; OVX, ovariectomized.
Experiment 4: Kiss1 is expressed in a sexually dimorphic pattern in the MeA of gonadally intact mice
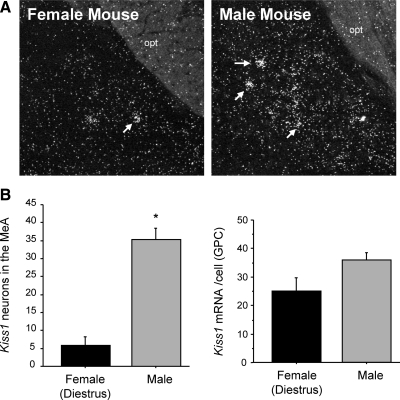
The previous experiments identified Kiss1 in the MeA of rats of both sexes. Here, we examined the presence of Kiss1 in the amygdala in gonadally intact male and female mice. We observed distinct Kiss1-expressing cells scattered loosely in the MeA of both sexes of mice. In mice, as in rats, Kiss1 expression was greater in males than females on diestrus (Fig. 4) and were similarly restricted to the MeA region of the amygdaloid complex. Quantitative analysis determined that male mice had greater numbers of Kiss1 neurons in the MeA than females on diestrus (P < 0.05, Fig. 4). The relative amount of Kiss1 mRNA per cell trended toward being higher in males than diestrus females, but this did not reach statistical significance.
Fig. 4.
Kiss1 mRNA expression in the medial amygdala of male and female mice. A, Representative photomicrographs of ISH for Kiss1 mRNA in the MeA of gonadally intact male and female (on diestrus) mice. White arrows depict examples of Kiss1 neurons. opt, Optic tract. B, Mean number of Kiss1 neurons in the MeA of gonadally intact female and male mice. The number of Kiss1 neurons was significantly higher in males than females (on diestrus). GPC, Grains per cell. *, Significantly different (P < 0.05).
Experiment 5: E2 up-regulates Kiss1 expression levels in the MeA of mice
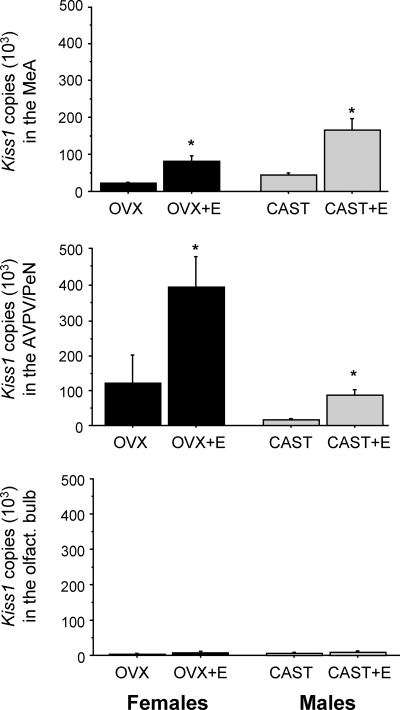
In mice, like rats, sex steroids stimulate the expression of Kiss1 in the AVPV/PeN and inhibit Kiss1 in the ARC. This experiment tested whether Kiss1 in the MeA of mice is also regulated by sex steroids, specifically E2. First, using ISH, we determined that E2 stimulates Kiss1 levels in the murine MeA. Male and female mice that were GDX and given E2 for a week had significantly more Kiss1-expressing cells in the MeA than untreated GDX mice (P < 0.05 for each sex, Fig. 5). The number of Kiss1 cells in the MeA was not different between E2-treated males and E2-treated females. Although there was also a trend toward higher Kiss1 mRNA content per cell in the MeA in E2-treated animals vs. GDX animals, this did not reach statistical significance (data not shown). To confirm the ISH findings, we repeated the experiment in a separate cohort of mice and used the sensitive method of qPCR to measure Kiss1 mRNA levels in the MeA. We detected Kiss1 mRNA in the MeA of all animals. In corroboration of the ISH data, we found that animals of each sex given E2 had significantly more Kiss1 mRNA in the MeA than GDX animals without E2 (Fig. 6). A similar pattern of enhanced Kiss1 expression in E2-treated animals was observed in the AVPV/PeN, where sex steroids have previously been shown to increase Kiss1 levels (Fig. 6). Virtually no Kiss1 mRNA was detected in the olfactory bulb (Fig. 6).
Fig. 5.
A, Representative photomicrographs of Kiss1 in the MeA of male and female mice treated with E2 for 1 wk. B, Mean number of Kiss1 cells in the MeA of male and female mice that were GDX and treated with E2. In each sex, there were more Kiss1 cells in the MeA in E2-treated mice compared with untreated GDX mice. *, Significantly different (P < 0.05) from GDX control in same sex. Cast, Castrated; opt, optic tract; OVX, ovariectomized.
Fig. 6.
Mean Kiss1 mRNA copy number, as determined via qPCR, in the MeA and AVPV of male and female mice that were GDX and treated with E2 or nothing. In both regions, there were significantly more Kiss1 mRNA-expressing cells in E2-treated mice compared with untreated GDX mice. *, Significantly different (P < 0.05) from GDX mice in same region. Cast, Castrated; OVX, ovariectomized.
Experiment 6: T, but not a nonaromatizable androgen, stimulates Kiss1 expression in the MeA of mice
In experiment 3, we determined that T can stimulate Kiss1 in the MeA of male rats. Here we tested whether T can also regulate Kiss1 in the MeA of mice of either sex. We determined that T significantly up-regulates Kiss1 expression in the murine MeA in both sexes; GDX male and female mice given T implants had significantly more Kiss1 neurons in the MeA than untreated GDX mice (P < 0.05, Fig. 7). This stimulatory effect of T was similar between the sexes.
Fig. 7.
Mean number of Kiss1 cells in the MeA of male and female mice that were GDX and treated with T. In both sexes, there were more Kiss1 cells in the MeA in T-treated mice compared with untreated GDX mice. *, Significantly different (P < 0.05) from GDX control in same sex. Cast, Castrated; OVX, ovariectomized.
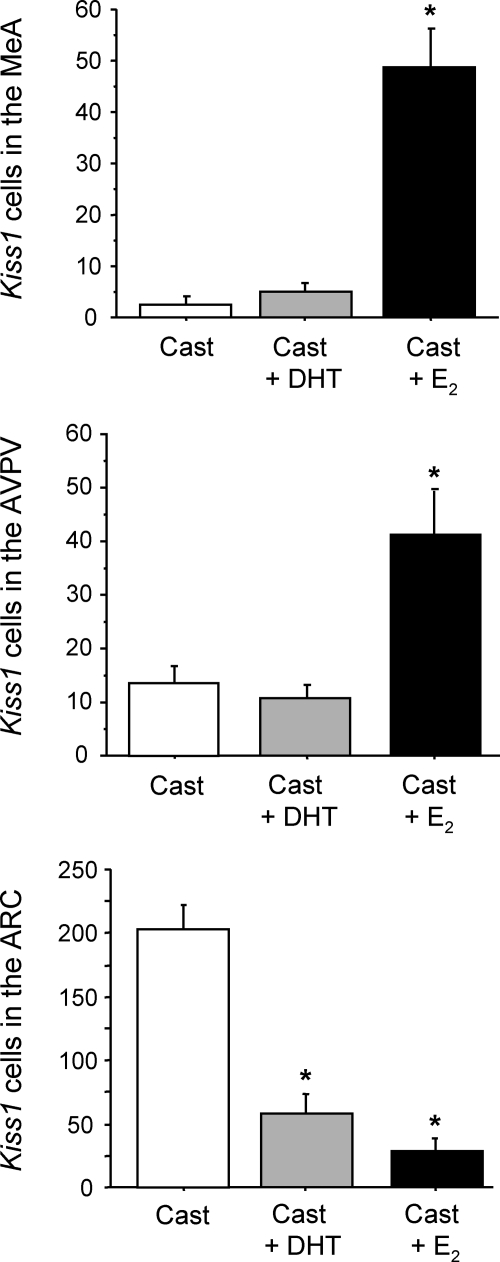
Because T can act via either AR or ER (after aromatization to E2), we tested whether MeA Kiss1 expression can be induced by a nonaromatizable androgen (DHT), which can act only via AR. Although we found that GDX mice treated with E2 had significantly elevated MeA Kiss1 levels relative to untreated GDX mice (P < 0.05), DHT-treated mice did not have increased Kiss1 expression in the MeA (Fig. 8). Levels of Kiss1 in the MeA were not significantly different between untreated and DHT-treated mice and were low in each group (Fig. 8). A similar pattern was found in the AVPV/PeN, where E2 but not DHT stimulated Kiss1 levels (P < 0.05, Fig. 8). In contrast, both E2 and DHT treatment significantly reduced Kiss1 levels in the ARC relative to the high expression levels in untreated GDX mice in this region (P < 0.05 for both hormones, Fig. 8).
Fig. 8.
Mean number of Kiss1 cells in the MeA, AVPV/PeN, and ARC of mice that were GDX and treated with DHT, E2, or nothing. There were significantly more Kiss1 cells in the MeA of E2-treated mice compared with either DHT-treated or untreated GDX mice. *, Significantly different (P < 0.05) from untreated, GDX control. Cast, Castrated.
Discussion
After the discovery in 2003 that humans and mice lacking KISS1R exhibit hypogonadotropic hypogonadism and impaired puberty onset (1, 4), many reports testified to the functional significance of kisspeptin signaling in the brain to regulate mammalian reproduction. In virtually every instance, attention has been focused exclusively on Kiss1 or kisspeptin neurons in the hypothalamus, specifically in the AVPV/PeN (or preoptic area in some species) and the ARC. However, we show here in the mouse and rat that Kiss1 is also expressed outside the hypothalamus in an important limbic region, the amygdala, and more specifically, the MeA. Moreover, we demonstrate that Kiss1 neurons in the MeA are regulated by the circulating levels of sex steroids in both sexes and that this hormonal regulation likely occurs via ER-dependent mechanisms rather than AR-dependent pathways. We also show that Kiss1 expression in the MeA is lower in gonadally intact females than males but that Kiss1 levels change as a function of the estrous cycle in females, peaking at proestrus, suggesting a function relating to ovulation or sexual behavior. Collectively, these findings implicate a novel population of extrahypothalamic Kiss1-expressing neurons in participating in sex-steroid-regulated physiology and/or behavior.
In gonadally intact adult mice and rats of both sexes, we identified Kiss1 mRNA-expressing cells in the amygdala, in addition to previously well-recognized areas of expression (AVPV/PeN and ARC) (37) (this report). In the amygdala, Kiss1 neurons were primarily restricted to the MeA, most prominently the posterodorsal MeA and were not readily observed in other amygdaloid regions. Interestingly, in both mice and rats, Kiss1-expressing cells in the MeA were more readily detectable in gonadally intact males than gonadally intact females on diestrus. Thus, like the Kiss1 population in the AVPV/PeN, Kiss1 expression in the MeA is sexually dimorphic in gonadally intact adult rodents, at least when females are in diestrus. Notably, the direction of the gender bias differs between the two regions: in the AVPV/PeN, diestrous females have more Kiss1 neurons than males (37, 42, 43), whereas in the MeA, males have more Kiss1-expressing neurons than diestrous females. Whether MeA Kiss1 expression is also sexually dimorphic between intact males and proestrous females remains to be determined. Previous studies have found a similar male-biased sex difference in other aspects of the amygdala, including the MeA. For example, in the rodent MeA, the expression of vasopressin, cholecystokinin, and substance P are all greater in intact males than intact females (48–50). Moreover, the overall size of the MeA region is larger in male rats than female rats (35). Thus, Kiss1 gene expression follows the general pattern of male bias that appears to predominate in the MeA. However, at present, the functional significance of such a male-biased sex difference in MeA Kiss1 levels is unknown.
Although we found that Kiss1 expression is sexually dimorphic in both the AVPV/PeN and MeA of intact rodents, the mechanisms generating these Kiss1 sex differences appear to differ between the two regions. In the AVPV/PeN, we previously determined that circulating sex steroid levels in adulthood are not responsible for the sex difference in the number of Kiss1 neurons, because females have many more Kiss1 cells in this region than males, even when adult males are treated with E2 to simulate levels found in females (37). Rather, it appears that the sex difference in Kiss1 expression in the AVPV/PeN is permanently organized by the prevailing sex steroid milieu during the perinatal critical period (37, 51). In contrast, our present results show that adult male and female mice and rats had the same number of Kiss1 neurons in the MeA when the circulating sex steroid milieu was equalized between sexes. These findings indicate that the sex difference in the MeA Kiss1 population reflects activational effects of the prevailing sex steroid milieu in the adult animal instead of a permanent developmental organization induced during the perinatal critical period (as with the AVPV/PeN). This is in agreement with findings that the sex difference in the overall size of the MeA is due to activational effects of sex steroids because equilibration of the sex steroid environment in adulthood eliminates the sex difference in the size of the MeA (35), analogous to the situation with Kiss1 in the MeA.
We and others have previously shown that Kiss1 expression in the rodent AVPV/PeN and ARC is differentially regulated by sex steroids, with T and E2 stimulating Kiss1 in the AVPV/PeN and inhibiting Kiss1 in the ARC (6, 52). We now report that the Kiss1 gene in the MeA is also regulated by sex steroids. Specifically, using both ISH and qPCR, we show that sex steroid treatment significantly up-regulates MeA Kiss1 expression in both sexes, whereas GDX males and females not treated with sex steroids have low levels of Kiss1 in the MeA. Thus, sex steroid regulation of Kiss1 expression in the MeA mirrors that of Kiss1 in the AVPV/PeN, being stimulatory in both regions. In concordance with this observation, we found that Kiss1 levels in the MeA of females varies across the estrous cycle, peaking at proestrus when gonadal sex steroids are maximal and being much lower in diestrus and estrus when E2 levels are reduced. The ability of sex steroids to up-regulate Kiss1 in the MeA is similar to several other neuropeptides in this nucleus (e.g. vasopressin and substance P in the MeA are up-regulated by T or E2 and, conversely, decreased by GDX) (reviewed in Ref. 14).
In both rats and mice, E2 and T induced Kiss1 expression in the MeA. Because T can act either via AR or ER (after aromatization to E2), it was unclear which receptor pathways mediated the effect of T on Kiss1 expression in the MeA. The MeA shows high expression of both AR and ER (13, 53, 54), suggesting that either receptor pathway could conceivably mediate T's stimulation of Kiss1 in this region. To address this issue, we evaluated whether DHT, a nonaromatizable androgen that can act via AR but not via ER, could stimulate Kiss1 expression in the MeA. Despite the known presence of AR in the MeA, we found that DHT had no inductive effect on Kiss1 expression in this region (or in the AVPV/PeN), unlike E2 treatment, which robustly increased Kiss1 levels in both areas. In contrast, DHT significantly inhibited the expression of Kiss1 in the ARC, as did E2. These data suggest that T up-regulates Kiss1 in the MeA and AVPV/PeN after first being aromatized to E2 and then acting through ER-dependent pathways. This mechanism is corroborated by the fact that both aromatase and ERs are expressed in the MeA (13, 54–58). However, it remains to be determined whether the effects of E2 on Kiss1 expression occur directly in MeA Kiss1 neurons or indirectly via afferent circuits. Current studies in our lab are pursuing this important question.
The function of Kiss1 neurons in the MeA is unknown. The amygdala has been implicated in numerous physiological and behavioral processes, including those relating to fear and anxiety, arousal and reward, stress, and social behavior. However, given the known roles of kisspeptin and its receptor in governing puberty onset and fertility, kisspeptin cells in the MeA may also play important modulatory functions in some aspects of reproduction. Indeed, the amygdala, including the MeA, has been implicated in modulating several facets of reproductive physiology, including sexual behaviors, ejaculation, gonadotropin secretion, ovulation, and pubertal maturation. For example, Fos is induced in the MeA of rats during sexual behavior (29), and electrical stimulation of the MeA results in elevated LH secretion (31, 59). Moreover, in rodents, pheromones and olfactory cues regulate reproductive function, and the MeA is a primary relay station linking the olfactory systems with reproductive nuclei, including to the preoptic area, AVPV, and ARC. The prominent location of the MeA in this olfactory-hypothalamic circuit indicates that the MeA is poised to play a role in olfactory or pheromone-induced changes in reproductive function; however, many of the specific cellular and molecular players in the MeA that play a role in this process are poorly characterized. Whether the newly identified MeA Kiss1 neurons are in fact involved in the olfactory pathway or play a role in sexual behavior or gonadotropin secretion in rodents remains to be established.
In summary, we report that in addition to its previously recognized expression in the hypothalamus, Kiss1 is also expressed in the amygdala, specifically, the MeA. We provide evidence in two rodent species that the expression of Kiss1 in the MeA is robustly regulated by sex steroids in both sexes and that this hormonal regulation occurs through ER-dependent pathways. We also show that levels of Kiss1 change as a function of the estrous cycle, peaking at proestrus, and implying that MeA Kiss1 signaling may play a role in ovulation or sexual behavior. Collectively, these findings identify a previously unrecognized population of extrahypothalamic Kiss1-expressing neurons that may play an important role in sex-steroid-regulated physiology and/or behavior. Future studies assessing the actions of kisspeptin signaling in the brain should consider the possible involvement of amygdala Kiss1 neurons.
Acknowledgments
This research was supported by National Science Foundation Grant IOS-1025893 (to A.S.K.) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants R00 HD056157 (to A.S.K.), F32 HD066849 (to S.J.S.), R01 HD27142 (to R.A.S.), and R01 HD065856 (to A.S.K.). Additional support was provided by the NICHD/National Institutes of Health through cooperative agreements U54 HD012303 and U54 HD12629, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- ARC
- arcuate nucleus of the hypothalamus
- AVPV/PeN
- anteroventral periventricular nucleus-periventricular nucleus continuum
- DHT
- dihydrotestosterone
- E2
- estradiol
- ER
- estrogen receptor
- GDX
- gonadectomized
- ISH
- in situ hybridization
- Kiss1R
- Kiss1 receptor
- MeA
- medial nucleus of the amygdala
- qPCR
- quantitative real-time PCR
- RNase
- ribonuclease
- SSC
- sodium citrate, sodium chloride
- T
- testosterone.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. 2007. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 27:8826–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 4. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 5. Kauffman AS. 2010. Coming of age in the Kisspeptin Era: Sex differences, development, and puberty. Mol Cell Endocrinol 324:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin Signaling in the Brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. 2009. Distribution of kisspeptin neurons in the adult female mouse brain. J Neuroendocrinol 21:673–682 [DOI] [PubMed] [Google Scholar]
- 8. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. 2007. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- 9. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. 2007. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 10. Smith JT. 2009. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: Comparative aspects. Peptides 30:94–102 [DOI] [PubMed] [Google Scholar]
- 11. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 12. Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. 2010. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod 83:568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simerly RB, Chang C, Muramatsu M, Swanson LW. 1990. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95 [DOI] [PubMed] [Google Scholar]
- 14. Cooke BM. 2006. Steroid-dependent plasticity in the medial amygdala. Neuroscience 138:997–1005 [DOI] [PubMed] [Google Scholar]
- 15. McKenna KE. 2001. Neural circuitry involved in sexual function. J Spinal Cord Med 24:148–154 [DOI] [PubMed] [Google Scholar]
- 16. Dobson H, Ghuman S, Prabhakar S, Smith R. 2003. A conceptual model of the influence of stress on female reproduction. Reproduction 125:151–163 [DOI] [PubMed] [Google Scholar]
- 17. Chudasama Y, Izquierdo A, Murray EA. 2009. Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci 30:2327–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray EA. 2007. The amygdala, reward and emotion. Trends Cogn Sci 11:489–497 [DOI] [PubMed] [Google Scholar]
- 19. Newman SW. 1999. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci 877:242–257 [DOI] [PubMed] [Google Scholar]
- 20. Bagga N, Chhina GS, Kumar VM, Singh B. 1984. Cholinergic activation of medial preoptic area by amygdala for ovulation in rat. Physiol Behav 32:45–48 [DOI] [PubMed] [Google Scholar]
- 21. Baum MJ. 2009. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Horm Behav 55:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beltramino C, Taleisnik S. 1978. Facilitatory and inhibitory effects of electrochemical stimulation of the amygdala on the release of luteinizing hormone. Brain Res 144:95–107 [DOI] [PubMed] [Google Scholar]
- 23. Döcke F, Lemke M, Okrasa R. 1976. Studies on the puberty-controlling function of the mediocortical amygdala in the immature female rat. Neuroendocrinology 20:166–175 [DOI] [PubMed] [Google Scholar]
- 24. Döcke F, Smollich A, Rhode W, Okrasa R, Dörner G. 1975. Studies on extrahypophyseal sites of estrogen action in the induction of ovulation in rats. Endokrinologie 65:274–287 [PubMed] [Google Scholar]
- 25. Dudley CA, Rajendren G, Moss RL. 1996. Signal processing in the vomeronasal system: modulation of sexual behavior in the female rat. Crit Rev Neurobiol 10:265–290 [DOI] [PubMed] [Google Scholar]
- 26. Kang N, Janes A, Baum MJ, Cherry JA. 2006. Sex difference in Fos induced by male urine in medial amygdala-projecting accessory olfactory bulb mitral cells of mice. Neurosci Lett 398:59–62 [DOI] [PubMed] [Google Scholar]
- 27. Norman RL, Spies HG. 1981. Brain lesions in infant female rhesus monkeys: effects on menarche and first ovulation and on diurnal rhythms of prolactin and cortisol. Endocrinology 108:1723–1729 [DOI] [PubMed] [Google Scholar]
- 28. Sherwood NM. 1977. Estrous cycles after electrical stimulation of the brain in conscious rats: effect of current strength, estradiol benzoate and progesterone. Endocrinology 100:18–29 [DOI] [PubMed] [Google Scholar]
- 29. Velasco ME, Taleisnik S. 1969. Release of gonadotropins induced by amygdaloid stimulation in the rat. Endocrinology 84:132–139 [DOI] [PubMed] [Google Scholar]
- 30. Velasco ME, Taleisnik S. 1971. Effects of the interruption of amygdaloid and hippocampal afferents to the medial hypothalamus on gonadotrophin release. J Endocrinol 51:41–55 [DOI] [PubMed] [Google Scholar]
- 31. Canteras NS, Simerly RB, Swanson LW. 1995. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol 360:213–245 [DOI] [PubMed] [Google Scholar]
- 32. Mizukami S, Nishizuka M, Arai Y. 1983. Sexual difference in nuclear volume and its ontogeny in the rat amygdala. Exp Neurol 79:569–575 [DOI] [PubMed] [Google Scholar]
- 33. Nishizuka M, Arai Y. 1981. Organizational action of estrogen on synaptic pattern in the amygdala: implications for sexual differentiation of the brain. Brain Res 213:422–426 [DOI] [PubMed] [Google Scholar]
- 34. Nishizuka M, Arai Y. 1981. Sexual dimorphism in synaptic organization in the amygdala and its dependence on neonatal hormone environment. Brain Res 212:31–38 [DOI] [PubMed] [Google Scholar]
- 35. Cooke BM, Tabibnia G, Breedlove SM. 1999. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci USA 96:7538–7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johansen JA, Jordan CL, Breedlove SM. 2004. Steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav 83:271–277 [DOI] [PubMed] [Google Scholar]
- 37. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 38. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 39. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 40. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of GnRH secretion by Kiss1/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. 2010. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151:5807–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. 2006. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pak TR, Lynch GR, Ziegler DM, Lunden JB, Tsai PS. 2003. Disruption of pubertal onset by exogenous testosterone and estrogen in two species of rodents. Am J Physiol Endocrinol Metab 284:E206–E212 [DOI] [PubMed] [Google Scholar]
- 46. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. 2009. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory GnRH/LH surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. 1990. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology 52:581–588 [DOI] [PubMed] [Google Scholar]
- 48. De Vries GJ, Buijs RM, Van Leeuwen FW. 1984. Sex differences in vasopressin and other neurotransmitter systems in the brain. Prog Brain Res 61:185–203 [DOI] [PubMed] [Google Scholar]
- 49. Micevych P, Akesson T, Elde R. 1988. Distribution of cholecystokinin-immunoreactive cell bodies in the male and female rat. II. Bed nucleus of the stria terminalis and amygdala. J Comp Neurol 269:381–391 [DOI] [PubMed] [Google Scholar]
- 50. Malsbury CW, McKay K. 1989. Sex difference in the substance P-immunoreactive innervation of the medial nucleus of the amygdala. Brain Res Bull 23:561–567 [DOI] [PubMed] [Google Scholar]
- 51. Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. 2009. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 81:1216–1225 [DOI] [PubMed] [Google Scholar]
- 52. Kauffman AS. 2010. Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol 22:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gréco B, Allegretto EA, Tetel MJ, Blaustein JD. 2001. Coexpression of ERβ with ERα and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology 142:5172–5181 [DOI] [PubMed] [Google Scholar]
- 54. Wood RI, Newman SW. 1995. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology 62:487–497 [DOI] [PubMed] [Google Scholar]
- 55. Roselli CE, Abdelgadir SE, Resko JA. 1997. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull 44:351–357 [DOI] [PubMed] [Google Scholar]
- 56. Roselli CE, Abdelgadir SE, Rønnekleiv OK, Klosterman SA. 1998. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod 58:79–87 [DOI] [PubMed] [Google Scholar]
- 57. Sinchak K, Roselli CE, Clemens LG. 1996. Levels of serum steroids, aromatase activity, and estrogen receptors in preoptic area, hypothalamus, and amygdala of B6D2F1 male house mice that differ in the display of copulatory behavior after castration. Behav Neurosci 110:593–602 [DOI] [PubMed] [Google Scholar]
- 58. Kelliher KR, Liu YC, Baum MJ, Sachs BD. 1999. Neuronal Fos activation in olfactory bulb and forebrain of male rats having erections in the presence of inaccessible estrous females. Neuroscience 92:1025–1033 [DOI] [PubMed] [Google Scholar]
- 59. Simerly RB. 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]