Androgens modulate the impact of serotonin on neuronal activation in regions inhibitory to the hypothalamic-pituitary-adrenal axis.
Abstract
The higher incidence of stress-mediated affective disorders in women may be a function of gonadal hormone influence on complex interactions between serotonin and neural circuits that mediate the hypothalamic-pituitary-adrenal (HPA) stress axis. The paraventricular nucleus of the hypothalamus (PVN) receives serotonergic innervation, and selective serotonin reuptake inhibitors such as citalopram activate the HPA axis independent of stress. We have previously demonstrated that the magnitude of this serotonergic activation was greater in females and was attenuated by testosterone administration; however, the potential central sites of action where androgens reduce these serotonergic effects have not been determined. Therefore, we examined a time course of corticosterone production and used central c-Fos protein levels to assay neuronal activation in stress-related brain regions in female, male, and gonadectomized male mice after an acute citalopram injection (15 mg/kg). In the hippocampus, c-Fos-immunoreactivity was greater in males than in females or gonadectomized males. This same pattern emerged in the lateral septum after vehicle and gonadectomy reversed the effect of citalopram. These regions are important for inhibitory influences on the PVN, and accordingly, hippocampal c-Fos levels were negatively correlated with corticosterone production. No sex differences in c-Fos were detected in the PVN, cingulate cortex, or paraventricular thalamus in response to vehicle or citalopram. These data support brain region-specific regulation of the HPA axis where sex differences may be mediated partly through androgen enhancement of signaling in inhibitory regions.
Affective disorders impact over 20% of the U.S. population, and the onset of these disorders is often associated with stressful life events (1, 2). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and altered levels of corticotropin-releasing factor (CRF) in the cerebrospinal fluid have been reported in patients (reviewed in Refs. 3 and 4). The lifetime prevalence of anxiety and depressive disorders is twice as high in females as males (5, 6), and although the underlying causes of this disparity are not fully understood, gonadal hormones may influence differences in stress responsivity.
Studies in rodents have consistently demonstrated greater HPA stress axis responses in females than in males. Measures of ACTH and corticosterone reveal a higher magnitude response in females after a variety of stressors (7–9). Furthermore, gonadectomy (GDX) leads to a greater, female-like response in males that is attenuated to non-GDX levels by androgen treatment (10–12). Moreover, estrogen treatment of male rats augments ACTH and corticosterone levels after stress through estrogen receptor-α-dependent mechanisms, and application of androgen and its metabolites near the paraventricular nucleus of the hypothalamus (PVN) reduces stress hormone levels through estrogen receptor-β-dependent mechanisms (13, 14). These studies strongly support a role for gonadal hormone modulation of the HPA axis response to stress.
Serotonin (5-HT) neurons project to and activate CRF neurons in the PVN, resulting in increased ACTH and corticosterone release (15–17). In examination of the serotonergic involvement in sex differences in HPA stress axis function, we have previously reported that female mice exhibit greater corticosterone production after acute administration of the selective 5-HT reuptake inhibitor (SSRI) citalopram than males and that testosterone treatment in females attenuates this sex difference (18). Therefore, in the present studies, we compared patterns of neuronal activation after acute citalopram administration in intact male, GDX male, and female mice to determine potential central sites of 5-HT action that may underlie the effects of androgens. More specifically, we counted the number of c-Fos-immunoreactive (c-Fos-IR) cells in brain regions where stress and 5-HT circuits may intersect, including the PVN, cingulate cortex, lateral septum (LS), paraventricular thalamus (PVT), and hippocampus (19–21), and compared these measures with a time course of corticosterone production. These data support previous studies demonstrating sex differences in corticosterone production after acute citalopram treatment (18) and highlight novel brain areas that may play a role in the response. A more complete understanding of stress and 5-HT interactions may help elucidate the heightened stress-related affective disorder predisposition in women.
Materials and Methods
Animals
Six-week-old male (n = 30) and female (n = 16) C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). After 1 wk of habituation, half of the male mice (n = 15) were bilaterally GDX under isoflurane anesthesia. The remaining males (n = 15) underwent a sham surgery in which testes were exposed but not removed. As a control for anesthesia, females were anesthetized for the same amount of time as the males. All mice were group housed under controlled conditions of a 12-h light, 12-h dark cycle with access to food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Corticosterone analysis
At 13 wk, activation of the HPA axis by citalopram (in the absence of any additional stressor) was assessed in all groups (n = 6–8 for vehicle or citalopram treatments). Citalopram (Sigma-Aldrich, St. Louis, MO) was dissolved in water and 0.9% saline (1:4 mixture) and injected ip at 15 mg/kg in a 100-μl volume. Vehicle injections were a 1:4 mixture of water and 0.9% saline. All ip injections occurred between 1 and 5 h after onset of the light cycle. Blood samples were collected from a tail nick upon removal from the cage (time 0) followed immediately by the ip injection of citalopram or vehicle. After the injection, additional blood samples were taken at 30, 70, and 120 min. Samples were collected into EDTA-treated tubes, centrifuged to extract plasma, and stored at −80 C until assayed for corticosterone. Corticosterone concentrations in plasma samples were measured using a commercial 125I RIA kit (MP Biomedicals, Orangeburg, NY). The minimum detection limit of the assay was 7.7 ng/ml, and intraassay coefficient of variation was 7.3%. Initial corticosterone rise was calculated by subtracting the time 0 value from the time 30 value for each mouse. Vaginal smears collected on the day of testing were used to determine cycle stage in females as described previously (22).
Brain perfusion
Immediately after the final blood collection at time 120, mice were transcardially perfused with 0.1 m PBS followed by ice-cold 4% paraformaldehyde in 0.1 m PBS. This time was selected based on previous studies showing maximal c-Fos induction after SSRI treatment (20). Whole brains were removed from the crania, postfixed for 4 h, and submerged in 30% sucrose in 0.1 m PBS for at least 48 h. Brains were frozen and cut by microtome into three sets of 40-μm coronal sections. Sections were stored in cryoprotectant (23) at −20 C until processed by immunohistochemistry.
c-Fos immunohistochemistry
To assess neuronal activation, immunohistochemistry for c-Fos was performed on one set of free-floating sections from each animal (n = 6 per group). Sections were removed from cryoprotectant, washed in Tris-buffered saline (TBS) (pH 7.4), and incubated for 15 min in TBS containing 0.3% H2O2 before being washed again and incubated overnight at room temperature in rabbit anti-c-Fos (1:5000; Calbiochem, San Diego, CA) diluted in TBS containing 0.2% Triton X-100 and 3% normal donkey serum. Sections then were washed in TBS and incubated for 2 h with biotinylated donkey antirabbit IgG (1:1000; Jackson ImmunoResearch, West Grove, PA). After a brief wash with TBS, sections were incubated for 1 h in an avidin-biotin-peroxidase complex (1:333; Elite kit; Vector Laboratories, Burlingame, CA). Sections were washed again with TBS and then with 50 mm Tris (pH 7.4) before immunoreactivity was visualized by incubation for 20 min with 3,3-diaminobenzidine (0.2 mg/ml) and 0.025% H2O2 in 50 mm Tris with 25 mg/ml nickel sulfate. The reaction was terminated by washing several times with TBS, after which sections were floated onto Superfrost Plus slides (Fisher, Pittsburgh, PA), dehydrated with increasing concentrations of alcohol followed by Hemo-De (Fisher), and coverslipped with Permount (Fisher).
c-Fos analysis
Brain sections were anatomically matched for all mice for each region analyzed by an investigator blinded to sex and treatment groups. Brain regions were identified using the Paxinos and Franklin mouse atlas (24). c-Fos-IR was quantified by counting stained cells within a drawn region of interest using ImageJ software (National Institutes of Health, Bethesda, MD). A threshold was set to delineate Fos-positive stained nuclei from background, and only cells above the threshold were included. For a particular brain region, the area analyzed was of identical size and shape for each mouse. The approximate rostrocaudal levels relative to bregma of the analyzed regions are as follows: cingulate cortex area 2 (1.10 and 0.98 mm), ventral LS (LSv) region (0.74 and 0.62 mm), PVN (−0.70, −0.82, and −0.94 mm, for rostral, middle, and caudal subregions), medial PVT (−1.06 and −1.22 mm), and CA3/dentate gyrus of the hippocampus (−1.70 and −1.82 mm). The number of c-Fos-IR cells were counted and averaged across two to three sections containing the relevant brain areas. To assess total potential activation throughout the PVN, the numbers of c-Fos-IR cells were summed from three tissue sections to form a total count for the PVN. c-Fos-IR as a percentage of vehicle was calculated by dividing the number of c-Fos-IR cells for each citalopram-treated mouse by the mean vehicle amount of the same group.
Statistical analysis
Corticosterone data were analyzed using a three-way ANOVA (time × group × drug) with time as a within-subjects repeated measure. Significant interactions with time were subsequently probed using a two-way ANOVA for group × drug at individual times. The numbers of c-Fos-IR cells were analyzed by two-way ANOVA for group × drug. c-Fos levels expressed as a percentage of vehicle were analyzed for effects of group by one-way ANOVA. Main effects and interactions were further explored with Tukey honestly significant difference post hoc test. Pairwise correlations between corticosterone levels and c-Fos protein were performed using Pearson's coefficients. Significant differences were identified at P < 0.05. Statistical analyses were performed with JMP software (SAS Institute, Cary, NC). All data are reported as mean ± sem.
Results
HPA axis response to citalopram
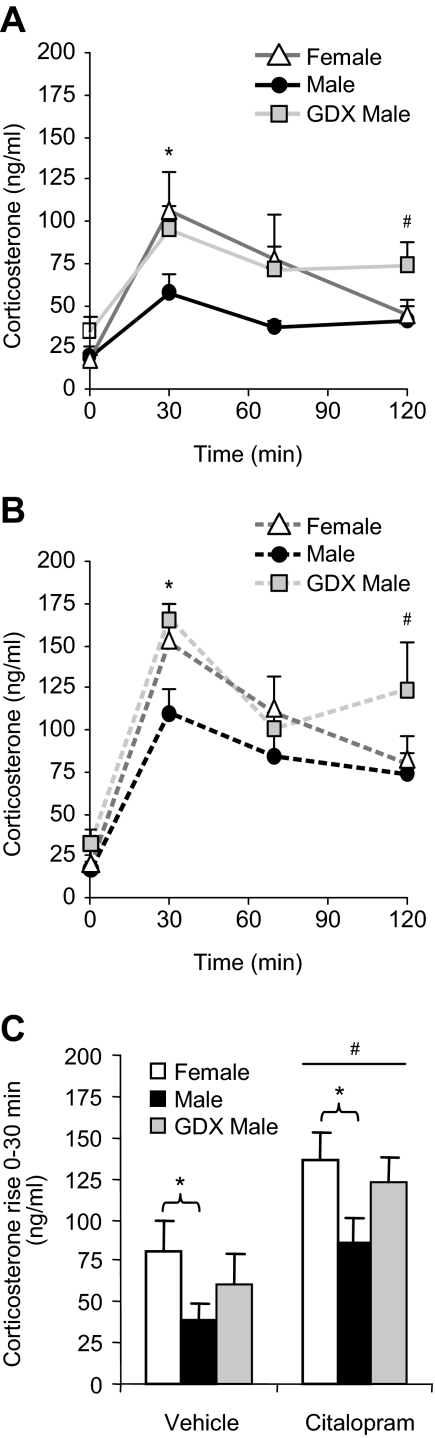
To examine sex differences in 5-HT-mediated HPA axis activity, we administered citalopram (15 mg/kg) to female, intact male, and GDX male mice. Basal values of plasma corticosterone were measured before the injection, and additional measurements occurred 30, 70, and 120 min after the injection (Fig. 1, A and B). Overall, there were significant main effects of group [F(2,26) = 5.99; P < 0.01], drug [F(1,26) = 11.68; P < 0.01], and a time × drug interaction [F(3,24) = 6.32; P < 0.01] by repeated-measures ANOVA. The stress of the vehicle ip injection increased corticosterone in all groups [F(3,12) = 10.87; P < 0.01, main effect of time in vehicle-treated groups by repeated-measures ANOVA]. We further analyzed individual times to determine the effects of group and citalopram. Basally (before the injection), there were no group differences in corticosterone. Citalopram increased corticosterone levels at all times after the injection with a peak response at 30 min. Females and GDX males showed greater levels than intact males at time 30 with both citalopram and vehicle treatment [F(2,36) = 6.25; P < 0.01, main effect of group followed by post hoc tests], indicating a sex difference that is likely to be mediated by the effects of androgens in males. During recovery at time 70 and 120, there were no sex differences. However, GDX males had more circulating corticosterone than intact males at time 120 [F(2,35) = 4.12; P < 0.05, main effect of group followed by post hoc tests], suggesting that the absence of androgens delays recovery of the HPA axis.
Fig. 1.
Corticosterone levels in response to citalopram. Female, male, and GDX males were injected with vehicle (A) or citalopram (B), and plasma corticosterone was measured. Corticosterone levels were lower in males than in females and GDX males in both conditions. The difference was most notable at 30 min (*, P < 0.05), and we observed a delay in the recovery in GDX males (#, P < 0.05). C, Shows the corticosterone rise from 0–30 min, which was greater in females than in males after vehicle or citalopram administration (*, P < 0.05). We also found a main effect of citalopram on the initial rise of corticosterone (#, P < 0.001).
To determine sex differences in the rate of response to citalopram, we analyzed the rise of corticosterone from time 0–30 min (Fig. 1C). Citalopram induced a greater rise in corticosterone than vehicle in all groups [F(1,34) = 17.90; P < 0.001, main effect of drug]. In addition, there was a main effect of group [F(2,33) = 4.44; P < 0.05] where females demonstrated a nearly 2-fold greater response than intact males to both vehicle and citalopram treatment (P < 0.05 by post hoc tests). GDX males showed a response rate between that of intact males and females.
Effects of sex and citalopram on the number of c-Fos-IR cells in stress-related brain regions
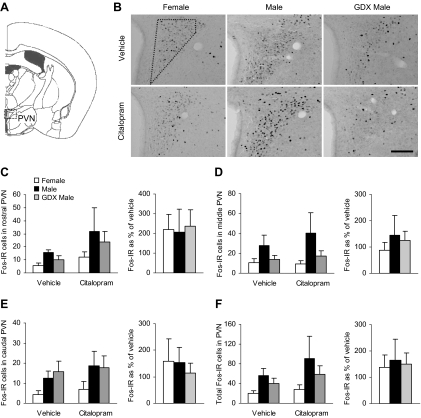
To investigate the effects of sex and gonadal hormones on citalopram-induced neuronal activity, we analyzed the number of c-Fos-positive cells by immunocytochemistry 120 min after the injection. c-Fos-IR was observed in the PVN and in several forebrain regions that are involved in inhibition of the HPA axis and in mediation of stress-related behaviors. We divided the PVN through its rostrocaudal axis and performed analyses on the individual subregions as well as on the combined data. There were no main effects of group or drug on any of the subregions or on total number of c-Fos-IR cells (Fig. 2). The effect of group was not statistically significant in the middle PVN region [F(2,34) = 2.91; P = 0.07].
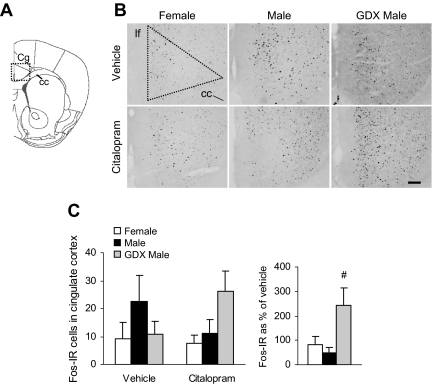
Fig. 2.
No differences between male and female mice were detected for c-Fos-IR in the PVN after vehicle or citalopram injection. A, Diagram of brain section used for PVN analysis, adapted from the mouse atlas (24). B, Representative images of c-Fos-IR in the middle PVN. Dotted shape indicates area analyzed. 3V, Third ventricle. Scale bar, 100 μm. C–E, c-Fos-IR in the rostral, middle, and caudal subregions of the PVN and the effect of citalopram expressed as a percentage of vehicle. F, Total c-Fos-IR across the rostrocaudal axis of the PVN and the response to citalopram expressed as a percentage of vehicle.
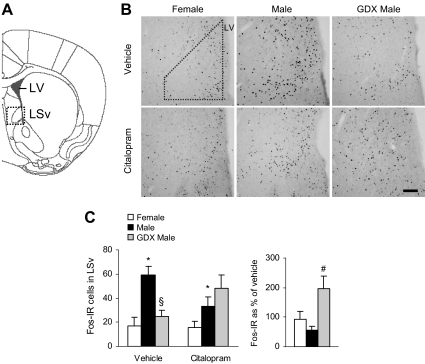
We then analyzed stress-related brain regions that have known inhibitory influences on the HPA axis. The LSv is thought to inhibit HPA axis activity via innervation of the PVN and its surround (25, 26). We observed a significant effect of group [F(2,34) = 7.77; P < 0.01] and a group × drug interaction [F(2,34) = 5.75; P < 0.01; Fig. 3] on the number of c-Fos-IR cells in the LSv. Overall, intact males showed more c-Fos-IR cells than females (P < 0.05 by post hoc tests). With vehicle treatment, GDX males had fewer c-Fos-IR cells than intact males (P < 0.05 by post hoc tests), suggesting that androgens may increase activation in the LSv. Citalopram had differing effects on these two groups, causing the impact of androgens to disappear. When analyzed as a percentage of vehicle, the change in c-Fos levels by citalopram was greater in GDX males than in intact males [F(2,14) = 5.43; P < 0.05, main effect of group, followed by post hoc tests].
Fig. 3.
GDX reversed the effect of citalopram on c-Fos-IR in the LSv. A, Diagram of brain section used for LSv analysis, adapted from the mouse atlas (24). B, Representative images of c-Fos-IR in the LSv. Dotted shape indicates area analyzed. LV, Lateral ventricle. Scale bar, 100 μm. C, Male mice had more c-Fos-IR cells than females after vehicle or citalopram injections (*, P < 0.05). After vehicle treatment, c-Fos levels were lower in GDX males compared with intact males (§, P < 0.05), but this pattern was reversed after citalopram treatment. Citalopram increased c-Fos-IR only in GDX males (#, P < 0.05).
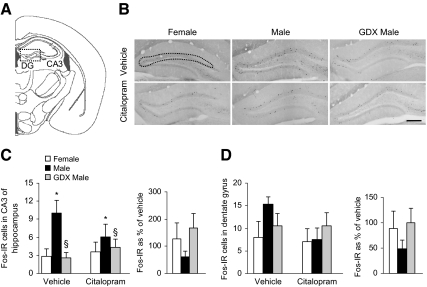
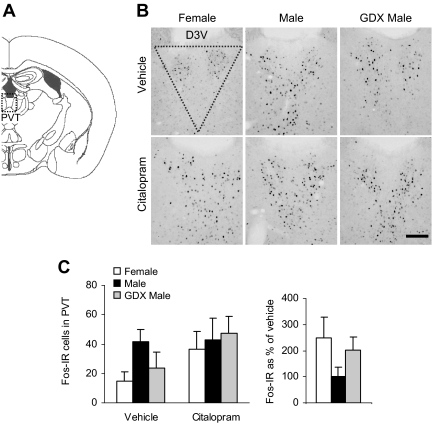
We also analyzed c-Fos protein in two subregions of the hippocampus (Fig. 4, A and B), an area that plays a major role in glucocorticoid-mediated negative feedback of the HPA axis and in anxiety- and depression-like behaviors (27). c-Fos-IR was nearly absent in the CA1 and CA2 subregions. There were few c-Fos-IR cells in hippocampal CA3 subfield (Fig. 4C), but significant group differences were observed [F(2,34) = 5.72; P < 0.01, main effect of group]. c-Fos-IR was overall higher in intact males than in females or GDX males, again suggesting a possible effect of androgens to increase neural activation (P < 0.05 by post hoc tests), but we failed to detect a main effect of citalopram (P = 0.72). There were no main effects of group (P = 0.35) or drug (P = 0.20) on c-Fos in the dentate gyrus (Fig. 4D). Additional stress-related regions that modulate HPA axis activity and behavior were analyzed. In the cingulate cortex (Fig. 5, A–C), there were no significant main effects of group (P = 0.26) or drug (P = 0.88). However, citalopram differentially affected c-Fos levels when analyzed as a percentage of vehicle [F(2,14) = 5.04; P < 0.05, main effect of group]. The change in c-Fos-IR due to citalopram was greater in GDX males than in intact males (P < 0.05 by post hoc tests). Examination of the PVT, a region important in habituation to repeated stressors, failed to detect significant group (P = 0.38) or drug (P = 0.11) effects (Fig. 6, A–C). Sample sizes were too small (estrus = 7, proestrus = 2, and diestrus = 3) to test for the effect of cycle on corticosterone or c-Fos, but our previous studies have suggested that cycle phase and corticosterone are not correlated (18).
Fig. 4.
Intact males exhibited greater c-Fos-IR in the hippocampus than females or GDX males in response to vehicle or citalopram. A, Diagram of brain section used for dentate gyrus (DG) and CA3 hippocampal analysis, adapted from the mouse atlas (24). B, Representative images of c-Fos-IR in the DG (CA3 region not shown). Dotted shape indicates area analyzed. Scale bar, 200 μm. C, In the CA3 subfield of the hippocampus, intact males showed greater c-Fos-IR than females (*, P < 0.05) and GDX males (§, P < 0.01). D, c-Fos levels were similar between groups in the dentate gyrus. Citalopram did not significantly alter c-Fos levels compared with vehicle injection in the CA3 or dentate gyrus subregions.
Fig. 5.
c-Fos-IR in the cingulate cortex after vehicle or citalopram injection is similar in males, females, and GDX males. A, Diagram of brain section used for cingulate cortex (Cg) analysis, adapted from the mouse atlas (24). B, Representative images of c-Fos-IR in the Cg. Dotted shape indicates area analyzed. Scale bar, 100 μm. C, There were no group differences in c-Fos levels in the cingulate cortex. Citalopram increased c-Fos-IR only in GDX males (#, P < 0.05). cc, Corpus callosum; lf, longitudinal fissure.
Fig. 6.
No differences between males and females were detected in the c-Fos-IR response to vehicle or citalopram in the PVT. A, Diagram of brain section used for PVT analysis, adapted from the mouse atlas (24). B, Representative images of c-Fos-IR in the PVT. Dotted shape indicates area analyzed. Scale bar, 100 μm. D3V, Dorsal third ventricle. C, c-Fos levels in the PVT were similar across all groups and were not altered by citalopram injection.
Correlational analysis
We hypothesized that corticosterone would be positively correlated to c-Fos induction in the PVN and negatively correlated to c-Fos expression in the brain regions that provide inhibitory feedback to the HPA axis. Pearson's coefficients for pairwise correlations are shown in Table 1. These analyses revealed a significant negative correlation between corticosterone levels 70 min after citalopram or vehicle injection and c-Fos expression in the CA3 subregion of the hippocampus (P < 0.05) and in the dentate gyrus (P < 0.01). There were no significant correlations between corticosterone and c-Fos-IR in the other brain regions.
Table 1.
Corticosterone (Cort) levels are negatively correlated with c-Fos-IR in the hippocampus
| Cingulate cortex | LS | Total PVN | PVT | CA3 | Dentate gyrus | |
|---|---|---|---|---|---|---|
| Cort at 30 | 0.161 | −0.202 | 0.056 | 0.029 | −0.294 | −0.292 |
| Cort at 70 | −0.026 | −0.253 | 0.110 | −0.128 | −0.358a | −0.514b |
P < 0.05.
P < 0.01.
Discussion
Stressful experiences and altered 5-HT function in the brain have independently been implicated as factors contributing to the development and etiology of affective disorders. One of these risk factors alone is unlikely to be responsible for the pathogenesis of a mental illness. However, dysregulation of the interactions between stress and 5-HT pathways can lead to alterations in mood and behavior that are symptomatic of affective disorders (3, 4, 28), and thus, examination of the serotonergic contribution to stress responsivity is critical. The HPA stress axis is regulated by both excitatory and inhibitory inputs and includes serotonergic projections to the PVN. Acute SSRI administration increases corticosterone production independent of stress (16, 17, 30). Sex differences and gonadal regulation of this component of stress responsivity have not been thoroughly examined. Therefore, we have examined sex differences and the possible contribution of androgens in the effects of acute citalopram administration on HPA axis output and neuronal activation.
Consistent with our previous studies, females produced higher corticosterone levels in response to citalopram and vehicle compared with intact males (18). Although there was not a sex difference in the basal state of the HPA axis before the injection, at 30 min, females had elevated corticosterone levels compared with intact males after both vehicle and citalopram injections. The vehicle ip injection raised corticosterone levels in all groups, suggesting that ip injections themselves are stressful. It is also possible that the endogenous serotonergic circuitry plays a role in this response. For instance, the response curve for citalopram-treated intact males was nearly identical to vehicle-treated females. This supports a possible sex difference in the serotonergic tone on the HPA stress axis where males are basally at a lower level than females, and with exogenous serotonergic stimulation, males achieve a greater tone equivalent to that of females given only vehicle. Furthermore, females exhibited a greater corticosterone rise from 0–30 min than intact males with the same pattern of response after both vehicle and citalopram injections, suggesting a greater HPA induction in females.
To determine the possible involvement of androgens in these differences, GDX male mice were also examined. GDX elevated male corticosterone levels to be equivalent to that of intact females, and the rate of corticosterone rise fell between the female and intact male values, suggesting that sex differences in these outcomes are related to hormonal modulation of the HPA stress axis where androgens have an inhibitory effect. Our previous studies have shown a dampening of adult female citalopram-induced corticosterone levels with testosterone treatment (18). The inhibitory effects of testosterone and other androgens such as dihydrotestosterone may be mediated by reductions in hypothalamic CRF levels, ACTH content in the pituitary, or adrenal weights (10, 18, 31). Furthermore, androgens increase glucocorticoid receptor expression in the hippocampus, which may enhance the negative feedback of the HPA axis in males (32). In contrast, in the absence of testosterone in GDX males, we detected increased stimulation of the HPA axis and reduced negative feedback. Corticosterone did not return to basal values likely as a result of repeated blood sampling, but recovery was detected in all groups. Vehicle- and citalopram-injected GDX males showed a delayed recovery at the 120-min time point compared with intact males and females. This may occur as a result of decreased glucocorticoid receptor expression in the hippocampus and other brain regions, such as the bed nucleus of the stria terminalis and the PVN, in addition to increased adrenal weight and CRF expression in the PVN, as mentioned above (18, 25, 31, 32).
To identify the neuroanatomical correlates of gonadal hormone influence on 5-HT-mediated HPA axis activity, we measured c-Fos protein levels in response to citalopram. We began by examining activation of the PVN because this region is directly innervated by serotonergic fibers from the dorsal and median raphe (33, 34) and is a critical component of the HPA axis (35). Surprisingly, we did not observe sex differences or an effect of GDX in the number of c-Fos-IR cells in the PVN at any level of the rostrocaudal axis. Citalopram appeared to increase c-Fos expression in all groups in the rostral PVN, but this effect was not significant. It is important to note that PVN c-Fos protein was examined at a single time point (120 min after the injection), the selection of which was based on previous studies showing that c-Fos-IR after SSRI administration is maximal at this time (20). The duration of neuronal activation as well as the magnitude of response may be an additional marker of PVN function that may correlate with corticosterone production. Evaluation of c-Fos at later time points may provide clues into the integration of inhibitory signals and sex differences in negative feedback. Nevertheless, the lack of a sex difference in our studies is consistent with a previous report that showed an absence of a sex difference in PVN activation of rats after acute fluoxetine treatment (21). Taken together, these findings suggest that PVN activity is not the primary effector for sex or gonadal regulation of 5-HT-induced corticosterone.
The widespread overlap of serotonergic innervation with brain regions involved in HPA axis regulation provides a number of possible targets to examine. Accordingly, we measured c-Fos levels in multiple regions with direct and indirect projections to the PVN. The cingulate cortex is part of an inhibitory relay to the HPA axis and the PVT directly projects to the PVN, which serves to facilitate habituation to repeated stress (26, 36–38). Analysis of c-Fos-IR in these two regions did not reveal an effect of sex within vehicle- or citalopram-treated groups. However, GDX males showed an increase in c-Fos levels in the cingulate cortex in response to citalopram, revealing a possible effect of androgens to modulate 5-HT function. Despite the importance of these regions in stress-related behavior and physiology, in our studies, there was no correlation between neuronal activation in these areas and corticosterone production.
In the LSv and CA3 subfield of the hippocampus, vehicle- and citalopram-treated males had a greater number of c-Fos-IR cells than females. Furthermore, GDX reduced c-Fos levels to female levels after both vehicle and citalopram injections in CA3 and after vehicle administration in the LSv. These data point to a possible effect of androgens to facilitate c-Fos induction in these regions. The sex difference in hippocampal c-Fos levels in our study agrees with previous data showing greater c-Fos mRNA in the CA3 in males than females after restraint stress (39) and may support an important role for 5-HT in this effect. Interestingly, a significant negative correlation was found between corticosterone production 70 min after citalopram or vehicle administration and c-Fos-IR in both the CA3 and dentate gyrus, supporting the inhibitory role of the hippocampus in HPA axis activity. Both the LSv and the hippocampus have dampening effects on HPA axis activity (25, 26), and thus, greater activation of these regions in intact males may explain lower corticosterone levels. Specifically, electrical stimulation of the dorsal hippocampus inhibits PVN cells projecting to the median eminence (40), and stimulation of the dorsal CA3 and dentate gyrus areas evokes decreases in plasma corticosterone (41). Furthermore, we have previously reported that male mice express higher levels of the γ-aminobutyric acid-synthesizing enzyme glutamic acid decarboxylase-65 in the LS compared with females, and that testosterone treatment in females significantly elevates levels of this enzyme, again supporting the potential for increased γ-aminobutyric acid synthesis and inhibitory tone in males (18). Testosterone and dihydrotestosterone have been shown to increase dendritic spine density and N-methyl-d-aspartate receptor binding in the hippocampus (42, 43). These effects are likely mediated through androgen receptors, which are highly expressed in the hippocampus and LSv (44, 45). Thus, increased neuronal excitability in the presence of testosterone in what may potentially be inhibitory neurons could underlie sex differences in c-Fos activation in these regions and downstream corticosterone release.
In addition to limbic regions upstream of the PVN, androgenic modulation of stress-5-HT circuitry may also occur downstream of the hypothalamus. Female pituitary corticotropes are more sensitive to CRF stimulation compared with males (46). The mechanisms underlying this sex difference are unknown but could involve gonadal regulation as well as 5-HT, which has been shown to stimulate ACTH release directly from cultured pituitary cells (29). Sex differences in 5-HT-mediated ACTH secretion have yet to be studied, but female mice exhibit 7-fold greater levels of 5-HT 1A receptor mRNA than males in the pituitary (18). Similarly, females show greater adrenal sensitivity to ACTH compared with males (46), which may also be subject to regulation by androgens and 5-HT. Further studies are needed to elucidate potential gonadal hormone and 5-HT interactions at the level of the pituitary or adrenal that may be involved in greater corticosterone secretion in females.
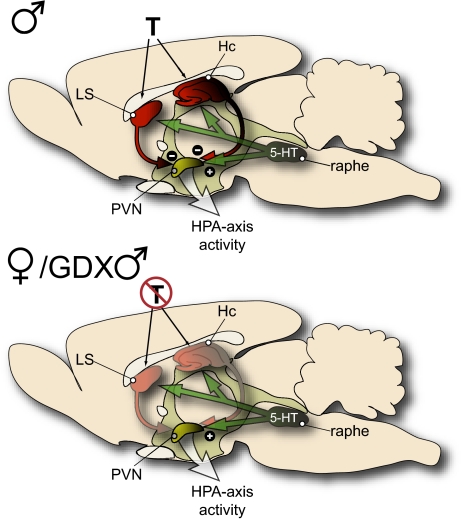
In summary, androgens are an important factor in regulating many facets of stress-related neurocircuitry, including inhibitory and serotonergic inputs. Our data demonstrate sex differences in select brain regions that have hierarchical control over the HPA axis. Specifically, the number of c-Fos-IR cells was greater in the hippocampal CA3 subregion and the LSv in males than it was in females or GDX males. Taken together with the higher corticosterone levels observed in female and GDX male mice, these data are consistent with a role of these structures in inhibition of the HPA axis and suggest that androgens facilitate this inhibition. Accordingly, we hypothesize that observable sex differences in the regulation of the HPA axis may not be a function of the stimulation of the PVN but may instead relate to the enhancement of inhibitory signals by androgens (see Fig. 7). This requires further testing, but androgenic regulation as hypothesized would provide males with a greater ability to dampen and modulate stress pathway activation, thereby contributing to sex differences in stress sensitivity and perhaps vulnerability to stress-related affective disorders.
Fig. 7.
Schematic image illustrating a hypothesized model explaining androgen-mediated differences in activation of the HPA stress axis. Equivalent levels of c-Fos-IR in the PVN of male, female, and GDX males suggest that neural activity in the PVN did not account for the observed differences in corticosterone levels after vehicle or 5-HT stimulation. Instead, the present data suggest that regions known to provide negative feedback to the HPA axis, such as the LS and hippocampus (Hc), are more active in the presence of androgen (T) but not in its absence. Accordingly, we hypothesize that androgens modulate the stress circuitry by enhancing inhibitory signaling to the PVN.
Acknowledgments
We thank N. McKay, Dr. R. Banno, D. Zimmer, and Dr. K. Bence for their assistance.
Funding was provided by National Institutes of Health (NIH) Grant MH073030 (to T.L.B.) and NIH Grant DK073800 (to D.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- c-Fos-IR
- c-Fos immunoreactive
- CRF
- corticotropin-releasing factor
- GDX
- gonadectomy
- HPA
- hypothalamic-pituitary-adrenal
- 5-HT
- serotonin
- LS
- lateral septum
- LSv
- ventral lateral septum
- PVN
- paraventricular nucleus of the hypothalamus
- PVT
- paraventricular thalamus
- SSRI
- selective 5-HT reuptake inhibitor
- TBS
- Tris-buffered saline.
References
- 1. Kendler KS, Karkowski LM, Prescott CA. 1999. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841 [DOI] [PubMed] [Google Scholar]
- 2. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602 [DOI] [PubMed] [Google Scholar]
- 3. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. 1999. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12 [DOI] [PubMed] [Google Scholar]
- 4. Bale TL. 2005. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav 48:1–10 [DOI] [PubMed] [Google Scholar]
- 5. Holden C. 2005. Sex and the suffering brain. Science 308:1574. [DOI] [PubMed] [Google Scholar]
- 6. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. 1994. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19 [DOI] [PubMed] [Google Scholar]
- 7. Goel N, Bale TL. 2008. Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology 149:6399–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. 1994. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28:464–476 [DOI] [PubMed] [Google Scholar]
- 9. Viau V, Bingham B, Davis J, Lee P, Wong M. 2005. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology 146:137–146 [DOI] [PubMed] [Google Scholar]
- 10. Kitay JI. 1963. Pituitary-adrenal function in the rat after gonadectomy and gonadal hormone replacement. Endocrinology 73:253–260 [DOI] [PubMed] [Google Scholar]
- 11. Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. 2004. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol 16:989–998 [DOI] [PubMed] [Google Scholar]
- 12. Viau V, Meaney MJ. 1996. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci 16:1866–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lund TD, Hinds LR, Handa RJ. 2006. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. Journal of Neuroscience 26:1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Handa RJ, Weiser MJ, Zuloaga DG. 2009. A role for the androgen metabolite, 5α-androstane-3β,17β-diol, in modulating oestrogen receptor β-mediated regulation of hormonal stress reactivity. J Neuroendocrinol 21:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen JB, Jessop DS, Harbuz MS, Mørk A, Sánchez C, Mikkelsen JD. 1999. Acute and long-term treatments with the selective serotonin reuptake inhibitor citalopram modulate the HPA axis activity at different levels in male rats. J Neuroendocrinol 11:465–471 [DOI] [PubMed] [Google Scholar]
- 16. McEuen JG, Semsar KA, Lim MA, Bale TL. 2009. Influence of sex and corticotropin-releasing factor pathways as determinants in serotonin sensitivity. Endocrinology 150:3709–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drapier D, Bentué-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann JM. 2007. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res 176:202–209 [DOI] [PubMed] [Google Scholar]
- 18. Goel N, Bale TL. 2010. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology 151:1784–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. 1995. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64:477–505 [DOI] [PubMed] [Google Scholar]
- 20. Beck CH. 1995. Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiatry Neurosci 20:25–32 [PMC free article] [PubMed] [Google Scholar]
- 21. Torres G, Horowitz JM, Laflamme N, Rivest S. 1998. Fluoxetine induces the transcription of genes encoding c-fos, corticotropin-releasing factor and its type 1 receptor in rat brain. Neuroscience 87:463–477 [DOI] [PubMed] [Google Scholar]
- 22. Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. 1982. A longitudinal study of estrous cyclicity in aging C57BL/6J mice. I. Cycle frequency, length and vaginal cytology. Biol Reprod 27:327–339 [DOI] [PubMed] [Google Scholar]
- 23. Watson RE, Wiegand SJ, Clough RW, Hoffman GE. 1986. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- 24. Paxinos G, Franklin KBJ. 2001. The mouse brain atlas in stereotaxic coordinates. 2nd ed. San Diego: Academic Press [Google Scholar]
- 25. Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180 [DOI] [PubMed] [Google Scholar]
- 26. Silverman AJ, Hoffman DL, Zimmerman EA. 1981. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN). Brain Res Bull 6:47–61 [DOI] [PubMed] [Google Scholar]
- 27. Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. 2002. Neurobiology of depression. Neuron 34:13–25 [DOI] [PubMed] [Google Scholar]
- 28. Owens MJ, Nemeroff CB. 1994. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 40:288–295 [PubMed] [Google Scholar]
- 29. Calogero AE, Bagdy G, Moncada ML, D'Agata R. 1993. Effect of selective serotonin agonists on basal, corticotrophin-releasing hormone- and vasopressin-induced ACTH release in vitro from rat pituitary cells. J Endocrinol 136:381–387 [DOI] [PubMed] [Google Scholar]
- 30. Kulkarni SK, Dhir A. 2007. Effect of various classes of antidepressants in behavioral paradigms of despair. Prog Neuropsychopharmacol Biol Psychiatry 31:1248–1254 [DOI] [PubMed] [Google Scholar]
- 31. Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. 1994. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology 59:228–234 [DOI] [PubMed] [Google Scholar]
- 32. Ahima RS, Harlan RE. 1992. Regulation of glucocorticoid receptor immunoreactivity in the rat hippocampus by androgenic-anabolic steroids. Brain Res 585:311–314 [DOI] [PubMed] [Google Scholar]
- 33. Larsen PJ, Hay-Schmidt A, Vrang N, Mikkelsen JD. 1996. Origin of projections from the midbrain raphe nuclei to the hypothalamic paraventricular nucleus in the rat: a combined retrograde and anterograde tracing study. Neuroscience 70:963–988 [DOI] [PubMed] [Google Scholar]
- 34. Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. 1983. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res 277:355–360 [DOI] [PubMed] [Google Scholar]
- 35. Bruhn TO, Plotsky PM, Vale WW. 1984. Effect of paraventricular lesions on corticotropin-releasing factor (CRF)-like immunoreactivity in the stalk-median eminence: studies on the adrenocorticotropin response to ether stress and exogenous CRF. Endocrinology 114:57–62 [DOI] [PubMed] [Google Scholar]
- 36. Bhatnagar S, Huber R, Nowak N, Trotter P. 2002. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 14:403–410 [DOI] [PubMed] [Google Scholar]
- 37. Diorio D, Viau V, Meaney MJ. 1993. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13:3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radley JJ, Gosselink KL, Sawchenko PE. 2009. A discrete GABAergic relay mediates prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci 29:7330–7340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Figueiredo HF, Dolgas CM, Herman JP. 2002. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology 143:2534–2540 [DOI] [PubMed] [Google Scholar]
- 40. Saphier D, Feldman S. 1987. Effects of septal and hippocampal stimuli on paraventricular nucleus neurons. Neuroscience 20:749–755 [DOI] [PubMed] [Google Scholar]
- 41. Dunn JD, Orr SE. 1984. Differential plasma corticosterone responses to hippocampal stimulation. Exp Brain Res 54:1–6 [DOI] [PubMed] [Google Scholar]
- 42. Leranth C, Petnehazy O, MacLusky NJ. 2003. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci 23:1588–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romeo RD, Staub D, Jasnow AM, Karatsoreos IN, Thornton JE, McEwen BS. 2005. Dihydrotestosterone increases hippocampal N-methyl-d-aspartate binding but does not affect choline acetyltransferase cell number in the forebrain or choline transporter levels in the CA1 region of adult male rats. Endocrinology 146:2091–2097 [DOI] [PubMed] [Google Scholar]
- 44. Simerly RB, Chang C, Muramatsu M, Swanson LW. 1990. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95 [DOI] [PubMed] [Google Scholar]
- 45. Risold PY, Swanson LW. 1997. Chemoarchitecture of the rat lateral septal nucleus. Brain Res Brain Res Rev 24:91–113 [DOI] [PubMed] [Google Scholar]
- 46. Osborn JA, Yu C, Stelzl GE, Weinberg J. 2000. Effects of fetal ethanol exposure on pituitary-adrenal sensitivity to secretagogues. Alcohol Clin Exp Res 24:1110–1119 [PubMed] [Google Scholar]