Summary
Infantile cholestatic disorders arise in the context of progressively developing intrahepatic bile ducts. Biliary atresia (BA), a progressive fibroinflammatory disorder of extra- and intrahepatic bile ducts, is the most common identifiable cause of infantile cholestasis and the leading indication for liver transplantation in children. The etiology of BA is unclear, and while there is some evidence for viral, toxic, and complex genetic causes, the exclusive occurrence of BA during a period of biliary growth and remodeling suggests an importance of developmental factors. Interestingly, interferon-γ (IFNγ) signaling is activated in patients and in the frequently utilized Rhesus rotavirus mouse model of BA, and is thought to play a key mechanistic role. Here we demonstrate intrahepatic biliary defects and upregulated hepatic expression of IFNγ pathway genes caused by genetic or pharmacological inhibition of DNA methylation in zebrafish larvae. Biliary defects elicited by inhibition of DNA methylation were reversed by treatment with glucocorticoid, suggesting that the activation of inflammatory pathways was critical. DNA methylation was significantly reduced in bile duct cells from BA patients compared to patients with other infantile cholestatic disorders, thereby establishing a possible etiologic link between decreased DNA methylation, activation of IFNγ signaling, and biliary defects in patients. Conclusion: Inhibition of DNA methylation leads to biliary defects and activation of IFNγ-responsive genes, thus sharing features with BA, which we determine to be associated with DNA hypomethylation. We propose epigenetic activation of IFNγ signaling as a common etiologic mechanism of intrahepatic bile duct defects in BA.
Keywords: hepatobiliary development, interferon-γ, epigenetics, infantile cholestasis, microarray
Introduction
Disorders of bile ducts range from infantile disorders such as biliary atresia and ductal plate abnormalities to conditions that affect older individuals such as primary sclerosing cholangitis, primary biliary cirrhosis, and cholangiocarcinoma. Fundamental to understanding all of these conditions is an understanding of the mechanisms of bile duct development. Bile ducts within the liver develop as hepatoblasts differentiate into hepatocytes and bile duct cells. In mammals, ducts develop as bile duct cells along the portal veins initially form plate-like structures that coalesce into individual ducts (1). This process is governed by several transcription factors, including the onecut transcription factors hnf6 and onecut2, the homeodomain factor hnf1β, and members of the jagged/notch signaling pathway (reviewed in (2)).
Biliary atresia (BA) is the most common identifiable cause of biliary disease in infants, but the etiology has remained elusive (3). Although both the Rhesus rotavirus (RRV)-injected mouse model of BA and patients with BA demonstrate activation of IFNγ and other inflammatory pathways (4, 5), efforts to identify associations with viral infections triggering this response in patients have been inconclusive. Interestingly, ewes and cows grazing on a Dysphania species that thrives during drought conditions in New South Wales gave birth to offspring with BA (6), supporting a role of an environmental toxin leading to BA, but no toxic exposures have been demonstrated in patients. There have been several reports of familial BA (7), but twin studies have been inconclusive (8-10), suggesting that a simple genetic cause of BA is unlikely. Genetic mouse models with BA have been described with mutations in laterality genes such as Inversin (11), as occasionally BA is associated with laterality defects (12), but BA patients with mutations in laterality genes have only rarely been identified. Expression microarray studies of patients (13, 14) and mouse models (15, 16) have confirmed the importance of inflammatory response genes and specific developmental pathways. Interestingly, one of these studies identified a small set of genes known to be regulated by DNA methylation as increased in livers from BA patients (14).
Methylation of cytosine at CpG residues leads to repression of gene expression (17), and changes in DNA methylation can be elicited by drugs, toxins, viruses (18, 19), and genetic defects (20). DNA hypomethylation has been implicated as playing a causative role in autoimmune disorders such as systemic lupus erythematosis (21). DNA hypomethylation has also been shown to inhibit neuronal development (22) and lymphocyte differentiation (23). We were intrigued that others had observed upregulation of normally methylated genes in BA, that DNA hypomethylation affects development of specific cell types, and that changes in DNA methylation can be elicited by viruses, toxins, and genetic changes. Thus, we hypothesized that inhibition of DNA methylation may be involved in the pathogenesis of BA.
We have established several models of abnormal intrahepatic biliary development in zebrafish, including morpholino antisense oligonucleotide (MO)-mediated knockdown of Jagged and Notch family members (24) and vps33b (25), which phenocopy biliary defects in patients with Alagille syndrome and arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome, respectively. Additionally, MO-mediated knockdown of the transcription factors hnf6 (26) and oc3 (27) lead to developmental biliary defects, similar to targeted deletions of the orthologous genes in mice (28, 29). The pronounced conservation of molecular pathways regulating vertebrate biliary development led us to use zebrafish to examine whether induced changes in DNA methylation might play a role in mediating developmental biliary defects.
Experimental Procedures
Fish Lines
Procedures for mutagenesis and screening for dtp are reported (30). Wild-type TLF strain fish were used for all morpholino injections and drug treatments. Fish were cared for in accordance with the Institutional Animal Care and Use Committees of both The Children’s Hospital of Philadelphia and the University of Pennsylvania.
Immunostainings and microscopy
Zebrafish cytokeratin immunostainings were performed as described previously (24, 26). 2F11 (31) stainings were performed on paraformaldehyde-fixed tissue at a dilution of 1:500 and processed as cytokeratin immunostainings. Electron microscopy specimens were prepared and examined as previously (24). Methylcytosine stainings and human cytokeratin stainings were performed using antibodies purchased from Abcam (see below for protocol details).
Injections and drug treatments
Morpholinos were obtained from Gene-Tools and are depicted in Supplemental Table 1. The dnmt1 AUG morpholino is virtually identical to the morpholino used previously (32). The AUG and spice blocking (acceptor junction of exon 25, wherein lie the catalytic residues) morpholinos against dnmt1 were injected together (2 pmol each) at 2 dpf as well; separate injections at 2 dpf had minimal effect, while injection at the 1-cell stage for either morpholino resulted in severe defects consistent with published reports (32).
Injections of azaC (Sigma) into the yolk were performed at 2, 3, and 4 dpf, except as indicated. Initial experiments (not shown) indicated that injected concentrations of 1 mM and (final amount 5 pmol) were most effective and did not appear to adversely affect the larvae. Injections into the yolk at 4 dpf were occasionally technically difficult; in those cases the injection was into the intestine, with identical results. Control larvae were injected with the equivalent volume of vehicle (water). Phenol red was added to all injection solutions, as is standard for zebrafish morpholino injections. For prednisone (Sigma) treatments, larvae were raised in E3 containing 5 μg/ml prednisone starting at 2 dpf.
Methylcytosine staining
For methylcytosine immunostaining, the sheep anti-methylcytosine antibody was used in accordance with standard protocols for treating paraffin-embedded specimens, except that samples were pretreated with HCl (3.5 N) after heating in buffered citric acid. Immunostaining patient samples were obtained as extra unstained slides from samples taken at the time of diagnosis or at portoenterostomy, ranging in age from 2 to 6 months. Samples from patients with Alagille syndrome (AGS) and primary sclerosing cholangitis (PSC) could not be age-matched due to the age of presentation. The general histological appearance of all disease samples appeared similar in terms of severity of fibrosis and inflammation. All patient samples were obtained after approval from the CHOP institutional review board (IRB). For the quantification studies, approximately 10 photomicrographs were obtained per sample, chosen to include at least one duct and neighboring hepatocytes. Quantification of methylcytosine was determined using Adobe Photoshop, by quantifying relative intensity of bile duct cell to hepatocyte nuclear staining, subtracting neighboring background staining. Numbers of cells and bile ducts assayed are listed in Table S2.
For the blinded examination, the sample files used for quantification were randomized and encoded. Bile ducts were outlined based on cytokeratin staining, but only methylcytosine staining was shown in the final samples given to the pathologist, as the cytokeratin staining correlated somewhat with disease. The samples were assigned as “strong”, “weak”, or “ambiguous” methylcytosine staining by a pathologist (P.R.) who was not told diagnoses, and then for analysis the staining was recorded as 1, −1, or 0, respectively. Average staining of each sample was determined, and the means of these averages are depicted below.
Microarray studies
Wild-type 2 dpf larvae were injected with azaC or control as above. At 4 dpf, larvae were immobilized in Tricaine and livers were removed and placed in RNAlater. After RNA isolation, two rounds of amplification were performed. The final RNA was analyzed using an Agilent Bioanalyzer to ensure adequate quality. Labeling was performed using standard reagents to add Cy3, and the labeled RNA was hybridized to Affymetrix zebrafish genome arrays. The raw microarray data were processed by dChip software (biosun1.harvard.edu/complab/dchip) to generate gene level expression measurements.
Annotation beyond that supplied by Affymetrix was performed using information on the Sanger center website (www.sanger.ac.uk). The zebrafish genes were then mapped to corresponding human genes via NCBI HomoloGene database (www.ncbi.nlm.nih.gov/homologene), and mapped human gene symbols were used as inputs of analysis. Important pathways were determined by running the annotated data through Gene Set Enrichment Analysis (www.gsea.com) and Ingenuity Pathway Analysis (www.ipa.com). Statistical cutoffs for pathways identified by GSEA were p<0.05 and FDR<0.10, and p<0.05 for IPA. Because zebrafish platforms are not completely annotated, we probably identified fewer pathways.
Quantitative PCR studies
We isolated RNA from control, azaC-treated, and azaC- and prednisone-treated 5 dpf larvae, similar to previous studies. Following conversion to cDNA, we performed quantitative PCR similar to previous studies, normalizing to hprt. Primers to hprt and vhnf1 have been published previously (26). Primers for irf1, igfr1, psmb9a, irgf1, and tp53 are depicted in Table S1.
Statistical analysis
Statistical analysis for quantification of methylcytosine staining was performed using Student’s t-test on Microsoft Excel. Statistical analysis of microarray data was performed using the analysis within GSEA and IPA. For analysis of PED6 uptake in the prednisone-treated larvae, chi-square analysis was performed (www.graphpad.com).
Results
Global inhibition of methylation leads to biliary defects
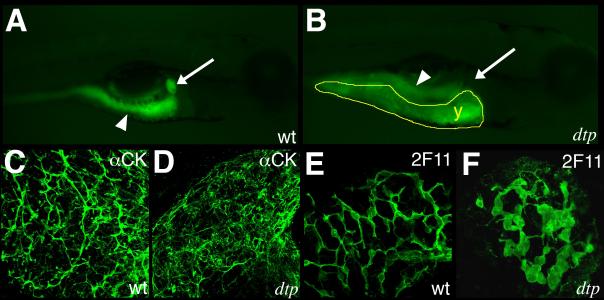
The zebrafish mutant duct-trip (dtp) is caused by mutation in the gene for S-adenosyl homocysteine hydrolase (ahcy), which leads to reduced DNA methylation in dtp due to accumulation of S-adenosyl homocysteine, a potent inhibitor of transmethylation reactions (33). dtp larvae demonstrated hepatic steatosis and progressive liver degeneration (33), but otherwise had normal morphology (30). To examine biliary development in dtp mutants, we examined their ability to process PED6, which we have previously shown serves as a readout of biliary secretion and can be used to indirectly examine biliary anatomy (34). Figure 1 demonstrates reduced processing of PED6 by dtp larvae, suggesting structural biliary defects. Cytokeratin immunostaining of larval dtp livers demonstrated defects in intrahepatic bile ducts (Fig. 1C-D), exhibiting few intact ductular structures. Immunostaining using the bile duct cell marker 2F11 (31) showed larger cell bodies and shortened ductular processes (Fig. 1E-F). These findings suggested to us that inhibition of methylation leads to developmental biliary defects.
Figure 1. Bile duct developmental arrest and degeneration caused by mutation of ahcy.
(A-B) Right-sided images of live 5 dpf (A) wild-type (wt) and (B) duct-trip (dtp) larvae 3 hours after ingestion of PED-6. There is intestinal uptake (white arrowhead) in both (A) and (B), but there is markedly diminished gallbladder uptake only in (B). (C-D) Confocal projections through the liver showing cytokeratin immunostainings from 5 dpf wt (C) and (D) dtp larvae. There is a lattice-like network of cytokeratin-stained bile ducts in wt (C), in contrast to the scant and disorganized cytokeratin staining in dtp (D). Images are representative of >95% of over 30 larvae examined by confocal. (E-F) Confocal projections through the liver of 5 dpf wt (E) and dtp (F) immunostained with 2F11 antibody. Bile duct cell body size is increased in dtp and cells are clustered with reduced development of the ductal network. 2F11 images are representative of 100% of over 10 larvae examined.
Inhibition of DNA methylation leads to biliary defects
To determine whether reduced methylated DNA could account for the dtp biliary phenotype, we treated wild-type larvae with 5-azacytidine (azaC), a DNA methylation inhibitor (35). The larvae were injected with azaC at 2 days post-fertilization (dpf) to avoid toxicity during early development. As depicted in Figure 2, azaC treatment of larvae did not affect liver morphology or overall growth or development, but did lead to reduced gallbladder PED6 uptake (insets). Cytokeratin immunostainings demonstrated that azaC treatment led to a dramatic effect on bile duct development, similar to dtp (Figure 2). Immunostaining with the bile duct cell marker 2F11 demonstrated fewer cells, consistent with the decrease in ducts but distinct from dtp. Unlike in dtp, in which there is global inhibition of methylation, azaC treatment did not lead to hepatic steatosis or degeneration (data not shown).
Figure 2. Phenocopy of dtp bile duct appearance by treatment of wild-type larvae with 5-azacytidine or dnmt1 antisense morpholino oligonucleotides.
Comparison of 5 dpf control larvae (cont, A-A”), 5 dpf larvae treated with 5-azacytidine at 2, 3, and 4 dpf (azaC, B-B”), and larvae injected with antisense morpholino oligonucleotides against zebrafish dnmt1 (dnmt1 MO, C-C”). A right lateral view (A, B, C) demonstrates that azaC-treated and dnmt1 MO-injected larvae are similar in appearance to control. A right-sided view under fluorescence (A, B, C insets), demonstrates gallbladder PED-6 uptake (white arrow) in control (A inset) but not in azaC-treated (B inset) or dnmt1 MO-treated (C inset) larvae. (A’, B’, C’) Comparison of confocal projections of cytokeratin immunostaining of 5 dpf livers. There is an almost complete lack of intrahepatic ducts in azaC-treated (B’) and dnmt1-treated (C’) livers. Images are representative of >95% of over 50 larvae examined. (A”, B”, C”) Comparison of confocal projections of 2F11 immunostaining of 5 dpf livers, showing increased cell size, clustering, and less complex networks in B” and C”, similar to dtp. Images are representative of 100% of over 5 larvae examined per condition.
Inhibition of DNA methylation with azaC in the developing liver from 2-4 dpf most likely affects maintenance of methylation via Dnmt1 (36), as biliary cells are highly proliferative at this stage (37). Zebrafish dnmt1 is expressed in a pattern similar to ahcy, and MO-mediated inhibition of dnmt1 has extensive effects on early development (32). To circumvent the early effects of dnmt1 knockdown, we examined 5 dpf larvae injected with dnmt1 MOs at 2 dpf (33, 38), by which point digestive organ anlagen have already formed (39). As noted with azaC treatment, injection of dnmt1 MOs did not affect the overall appearance of the larva, including liver morphology, but did inhibit gallbladder uptake of PED-6, similar to azaC (Figure 2 insets). Intrahepatic cytokeratin stainings in dnmt1-deficient larvae were similar to those seen in dtp and azaC-treated larvae, and 2F11 stainings shared features with both dtp and azaC-treated larvae, with fewer cells in clusters and with shorter ductular processes. Treatment with azaC or injection with MOs against dnmt1 resulted in inhibition of DNA methylation quantitatively similar to dtp (33) (Supplemental Figure 1). Thus, inhibition of DNA methylation disrupts intrahepatic bile duct development in zebrafish, and is likely responsible for the biliary phenotype of dtp.
Elevation of inflammatory genes in azacytidine-treated zebrafish
To identify candidate genes with altered expression in azaC-treated larvae, we performed expression microarray analysis on livers dissected from 4 dpf azaC-treated fish compared to control (see Supplementary Table 3). Many of the upregulated genes were IFNγ-stimulated genes and other inflammatory pathway genes (≥17 of the top 100; see Table S3). This was intriguing, as IFNγ is elevated in patients with BA (4) and is critical in the generation of experimental biliary atresia in mice (5). We examined defined gene sets for differences between control and azaC-treated fish using gene set enrichment analysis (GSEA) and Ingenuity pathway analysis (IPA). As depicted in Table 1, several gene sets were significantly different in azaC-treated larvae, including IFNγ-responsive genes and other inflammatory gene sets. Table 1 also shows that gene sets downstream of several transcription factors were significantly downregulated, including genes regulated by Hnf6, shown previously to be important in biliary development in mammals (29) and zebrafish (26).
Table 1.
Pathways upregulated and downregulated in azaC-treated larvae.
| Upregulated in azaC-treated | Downregulated in azaC-treated |
|---|---|
| Interferon-γ signaling | Hnf6 target genes |
| NF-κB signaling | LHX3 target genes |
| Antigen processing and presentation | FoxF2 target genes |
| Nucleotide metabolism | Hnf3a and 3b target genes |
| Growth hormone (GH)/IGF-1 signaling | Gata1 target genes |
| ER-Golgi intermediate compartment | Other transcription factor target genes |
Listed are pathways that are up- and downregulated by azaC treatment, as determined by GSEA and IPA analysis of microarray results.
The gene profiling data suggested several mechanisms to account for biliary defects associated with inhibition of DNA methylation, including activation of the innate immune response and reduced activation of developmental signaling pathways. As histological analysis revealed no evidence of an inflammatory infiltrate in azaC-treated livers (Figure S2) we hypothesize that activation of IFNγ target genes, normally silenced by DNA methylation, could directly affect developing biliary epithelial cell survival and/or proliferation. Indeed, mammalian biliary cells express several inflammatory mediators, including the IFNγ receptor, which mediate biliary cell proliferation and survival in models of biliary disease (40). The activation of IFNγ-responsive genes was especially intriguing given the importance of IFNγ in mouse models of BA (5) and in patients with BA (4).
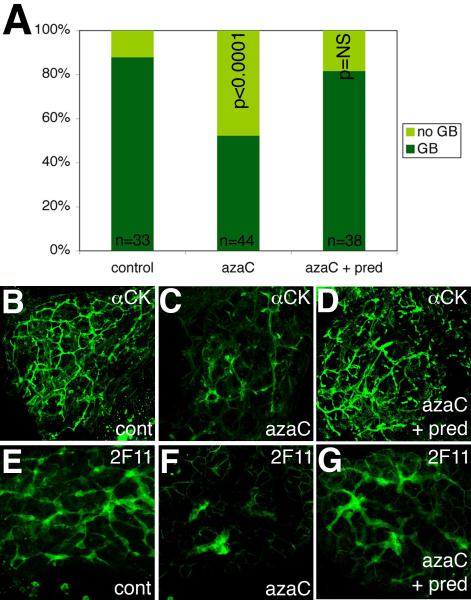
Although extrahepatic defects are the hallmark of BA, progressive destruction of intrahepatic ducts following surgical relief of extrahepatic obstruction is the most important factor determining the eventual need for transplantation. Activation of IFNγ signaling and intrahepatic biliary defects in azaC-treated zebrafish larvae suggests that they may be used to model BA progression. We attempted to rescue the biliary phenotype by treating azaC-injected larvae with the glucocorticoid prednisone, as prednisone has shown promise as a treatment for intrahepatic biliary defects in BA (41), and there is a currently a large national trial examining the effectiveness of prednisone in treating ongoing intrahepatic biliary atresia (NIDDK NCT00294684). As depicted in Figure 3, treatment of azaC-injected larvae with prednisone resulted in normalization of PED-6 gallbladder uptake, suggesting improved bile flow arising from rescue of intrahepatic biliary anatomy. Cytokeratin and 2F11 immunostaining of livers from 5 dpf larvae treated with azaC and prednisone demonstrates rescue of the defects seen in azaC-treated larvae (Figure 3B-G). These results suggest that intrahepatic biliary defects elicited by chemical inhibition of DNA methylation can be prevented by glucocorticoid treatment. Based on well-established models of glucocorticoid mechanisms of action, this is probably not a result of a direct effect of prednisone on DNA methylation, but on gene expression changes elicited by the inhibition of DNA methylation (42).
Figure 3. Rescue of azacytidine-mediated developmental biliary defects by treatment with glucocorticoid.
(A) Bar graph demonstrating the relative number of normal and faint gallbladders (GB) in control larvae, larvae treated with azaC, and larvae treated with azaC and prednisone. There is a significant decrease in the number of larvae with normal GBs that is reversed by prednisone. The number of larvae per condition is noted. p<0.0001 between control and azaC-treated, p=NS (not significant) between control and azaC + prednisone (chi-square). (B-D) Confocal projections of cytokeratin immunostaining of livers from a 5 dpf control larva (B), larva treated with azaC (C), and (D) larva treated with azaC and prednisone. Panel (D) appears similar to control (B). (E-G) Confocal projections of 2F11 immunostaining of livers from 5 dpf control (E), azaC-treated (F), and azaC and prednisone-treated larvae (G). Panel (G) appears similar to control (E). Samples in (B-G) are representative of a random sampling of 10 larvae from both PED-6 phenotypes within each condition.
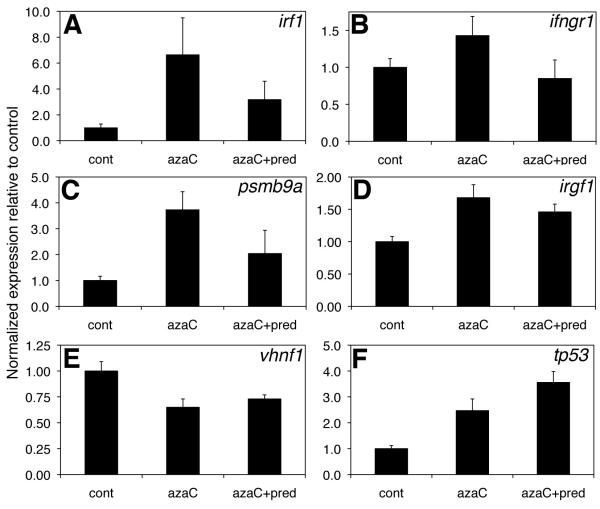
To determine possible mechanisms by which prednisone could be rescuing the azaC-mediated defects in biliary development, we examined gene expression changes in azaC-treated larvae and in larvae treated with azaC and prednisone. The elevated expression of four IFNγ pathway genes was at least partially attenuated by prednisone (Figure 4), which would be consistent with prednisone blunting the stimulation of IFNγ target genes. The genes depicted include the IFNγ pathway members IFNγ regulatory factor 1 (irf1), IFNγ receptor 1 (ifngr1), and IFNγ target genes proteasome subunit beta type 9a (pmsb9a, also known as lmp2) and immunity related GTPase F1 (irgf1) (43). Decreased expression of the Hnf6 target gene vhnf1 was not attenuated by prednisone, and the increase in tp53 expression in azaC-treated larvae was also not rescued by prednisone (Figure 4). Examination of tissue sections from azaC-treated and azaC and prednisone-treated larvae demonstrated no clear difference (Figure S2), supporting our assertion that increased liver expression of IFNγ pathway genes leads to biliary defects without recruitment of inflammatory cells.
Figure 4. Attenuation of IFNγ-responsive gene increases by prednisone.
Real-time quantitative PCR of control (cont) 5 dpf larvae and larvae treated with azaC or azaC and prednisone. Depicted are expression changes of the IFNγ-responsive genes irf1, ifngr1, psmb9a, and irgf1 (A-D), as well as changes in the Hnf6 target gene vhnf1 (E) and tp53 (F). p<0.05 between cont and azaC for all conditions except (A and B), for which p=0.06 and NS, respectively.
Decreased DNA methylation in bile duct cells from patients with biliary atresia
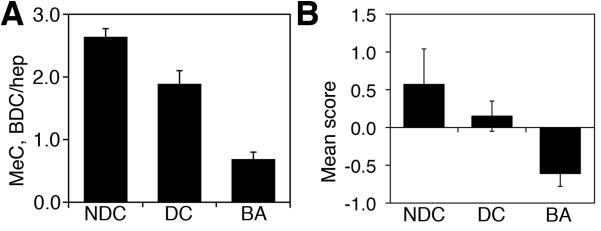
To explore the clinical relevance of DNA methylation in BA, we examined liver samples from BA patients and patients with other pediatric biliary disorders using methylcytosine immunostaining. We examined 3 non-disease controls, 7 disease control patients, and 5 BA patients. As depicted in Figure 5, samples from patients with BA demonstrated weaker methylcytosine staining in the nuclei of bile duct cells compared to control samples, while samples from patients with other liver diseases more closely resembled control specimens. The samples depicted in Figure 4 represent a subset of the total number of specimens examined (n=190; see Figure S3 for additional examples). To quantify this descriptive analysis, we measured intensity of methylcytosine immunostaining in bile duct cells relative to neighboring hepatocytes in tissue specimens from the same patients. These demonstrated a highly significant difference in bile duct cell nuclear methylcytosine levels between controls and BA specimens (Figure 6), examining ~100 bile duct cells and ~100 hepatocytes per patient (see Table S2 for details). Similar results were obtained in a blinded examination of these specimens performed by a pediatric hepatopathologist (Fig. 6). These independent results suggest a correlation between DNA hypomethylation in bile duct cells and BA.
Figure 5. DNA hypomethylation in bile duct cells from patients with biliary atresia.

Immunostaining of liver sections from patient samples, using anti-methylcytosine (red) and anti-cytokeratin 19 (green). Bile ducts from non-disease control (A, cont), Alagille syndrome (B, AGS), cystic fibrosis liver disease (C, CF), and biliary atresia (D-F, BA), showing methylcytosine staining in nuclei (arrowhead). Methylcytosine staining pattern in a neighboring hepatocyte is depicted in the insets. The methylcytosine staining of the bile duct cells is more diffuse and homogeneous in the BA samples, while the control staining pattern demonstrates strong perinuclear and punctate intranuclear staining. In general, this latter pattern is also seen in the hepatocyte nuclei.
Figure 6. Quantification of bile duct cell methylcytosine staining from patients.
(A) Relative methylcytosine staining of bile duct cells normalized to hepatocytes in non-disease controls (NDC), disease controls (DC), and patients with biliary atresia (BA). Columns represent average relative methylcytosine staining from several patients (see Table S2 for details). Staining in BA patients is significantly lower than both control groups, p<0.00003 for NDC and p<0.001 for DC. (B) Identical samples assayed in a blinded fashion for intensity of methylcytosine staining, rated as “strong”, “weak”, or “ambiguous”. The assessment was then scored as +1, −1, or 0, respectively; samples from the same case were pooled, and the mean pooled scores are depicted, ±S.E.M. p<0.03 for BA vs. both NDC and DC.
Discussion
Here we have demonstrated that inhibition of DNA methylation leads to defects in intrahepatic bile duct formation. We also demonstrate that DNA hypomethylation leads to activation of inflammatory genes, including IFNγ-responsive genes, without evidence of cellular inflammation. Treatment with the anti-inflammatory prednisone leads to reversal of the biliary defects, suggesting that the inflammatory gene changes may be causative. We also show that bile duct cells in patients with BA demonstrate a decrease in DNA methylation. Taken together, these results suggest a possible novel mechanism for BA, in which inhibition of DNA methylation leads to activation of IFNγ and defects in the biliary tree.
There is considerable evidence that activation of inflammation targeting the biliary system plays an important role in both extrahepatic and intrahepatic aspects of BA (44). Studies examining the importance of the inflammatory process have strengthened the argument for an infectious pathogenesis to BA, but there is evidence from other diseases that non-infectious etiologies may lead to inflammatory activation, including activation of IFNγ (45). Here we demonstrate that developmental defects in biliary anatomy and activation of IFNγ-stimulated genes can be elicited by genetic and pharmacologic inhibition of DNA methylation.
Interferon-γ activation in biliary cells may lead to cell damage via activation of IFNγ downstream pathways, or potentially by inhibition of TGFβ. Activation of IFNγ inhibits TGFβ signaling in several model systems (46). TGFβ exerts a positive effect on the development of bile duct cells (28), and thus inhibition of TGFβ would be expected to have a negative effect on biliary development. Such a mechanism is attractive in the developing liver, as the differentiation of hepatoblasts into bile duct cells is probably not present in the healthy mature liver. Thus, the specificity of this mechanism would be due to pathways that are developmentally limited.
While there are similarities between our zebrafish with inhibition of DNA methylation and BA, there are also key differences. We did not observe extrahepatic biliary defects in dtp, azaC-treated larvae, or dnmt1 morphants, while extrahepatic biliary abnormalities are clearly important in BA. Of note, we have not observed extrahepatic defects in any of our models of abnormal biliary development in zebrafish, including hnf6 morphants, while targeted deletion of Hnf6 in mice clearly leads to extrahepatic biliary defects (29). This discrepancy may be due to a lack of evolutionary conservation in development of the extrahepatic biliary tree, or may be due to other factors such as timing of knockdown with respect to development or technical difficulties in observing the extrahepatic biliary tree in developing zebrafish.
We also did not observe inflammatory infiltration of the liver or biliary tree in our models, although we did observe activation of inflammatory genes. This activation of IFNγ responsive genes in particular was attenuated by prednisone, which also led to rescue of the biliary defects in our fish and has been shown to be of some benefit for patients with BA post portoenterostomy (47, 48). These results suggest that the gene expression changes elicited by prednisone may be responsible for the rescue of biliary defects, but other possible mechanisms, such as altered expression of non-IFNγ pathway genes that lead to biliary growth and development, may be functioning as well.
In addition to our findings in zebrafish, we report pronounced decreased nuclear methylcytosine staining in bile duct cells from patients with BA, compared to patients with other hepatobiliary disorders, demonstrating an association between DNA hypomethylation and BA. Potential causes of BA such as drugs, toxins, viruses and genetic defects can induce changes in DNA methylation, and there may be differential effects of environmental factors on different epigenetic backgrounds. Moreover, epigenetic alterations of DNA methylation may demonstrate non-Mendelian inheritance (49), thus accounting for occasional familial cases and non-concordance in monozygotic twins. Our results suggest the possibility of a unifying etiology to BA, in which multiple possible primary insults lead to a common epigenetic effect in biliary cells, resulting in a chronic destructive inflammatory process targeting the biliary system.
Supplementary Material
Acknowledgements
We thank Weilong Gong, Liyuan Ma, Mani Methamani, Louis Capecci, and Erin Smith for expert technical assistance. We also thank Dr. Eric Rappaport and other members of the Nucleic Acid/Protein Core at CHOP. Thanks to Dr. Barbara Haber, Dr. Joshua Friedman, and Dr. David Piccoli for critical reviews of the manuscript.
Financial Support: This work was supported by K08 DK68009 (R.P.M) and R01 DK61142 (M.P.), as well as institutional support (R.P.M.) and support from the Fred and Suzanne Biesecker Pediatric Liver Center at The Children’s Hospital of Philadelphia, and from the Center for Molecular Studies in Digestive and Liver Disease at the University of Pennsylvania, under P30 DK50306.
Abbreviations used
- BA
biliary atresia
- IFNγ
interferon-γ
- RRV
rhesus rotavirus
- MO
morpholino oligonucleotide
- azaC
5-azacytidine
- ARC
arthrogryposis-renal dysfunction-cholestasis
- AGS
Alagille syndrome
- CF
cystic fibrosis
- TGFβ
transforming growth factor β
References
- 1.Lemaigre FP. Development of the biliary tract. Mech Dev. 2003;120:81–87. doi: 10.1016/s0925-4773(02)00334-9. [DOI] [PubMed] [Google Scholar]
- 2.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Haber BA, Russo P. Biliary atresia. Gastroenterol Clin North Am. 2003;32:891–911. doi: 10.1016/s0889-8553(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 4.Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653–1659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 5.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper P, Plant JW, Unger DB. Congenital biliary atresia and jaundice in lambs and calves. Aust Vet J. 1990;67:18–22. doi: 10.1111/j.1751-0813.1990.tb07385.x. [DOI] [PubMed] [Google Scholar]
- 7.Lachaux A, Descos B, Plauchu H, Wright C, Louis D, Raveau J, Hermier M. Familial extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr. 1988;7:280–283. doi: 10.1097/00005176-198803000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Poovorawan Y, Chongsrisawat V, Tanunytthawongse C, Norapaksunthorn T, Mutirangura A, Chandrakamol B. Extrahepatic biliary atresia in twins: zygosity determination by short tandem repeat loci. J Med Assoc Thai. 1996;79(Suppl 1):S119–124. [PubMed] [Google Scholar]
- 9.Silveira TR, Salzano FM, Howard ER, Mowat AP. Extrahepatic biliary atresia and twinning. Braz J Med Biol Res. 1991;24:67–71. [PubMed] [Google Scholar]
- 10.Smith BM, Laberge JM, Schreiber R, Weber AM, Blanchard H. Familial biliary atresia in three siblings including twins. J Pediatr Surg. 1991;26:1331–1333. doi: 10.1016/0022-3468(91)90613-x. [DOI] [PubMed] [Google Scholar]
- 11.Mazziotti MV, Willis LK, Heuckeroth RO, LaRegina MC, Swanson PE, Overbeek PA, Perlmutter DH. Anomalous development of the hepatobiliary system in the Inv mouse. Hepatology. 1999;30:372–378. doi: 10.1002/hep.510300223. [DOI] [PubMed] [Google Scholar]
- 12.Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr. 2006;149:393–400. doi: 10.1016/j.jpeds.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Goryachev A, Sun J, Kim P, Zhang H, Phillips MJ, Macgregor P, et al. Altered expression of genes involved in hepatic morphogenesis and fibrogenesis are identified by cDNA microarray analysis in biliary atresia. Hepatology. 2003;38:567–576. doi: 10.1053/jhep.2003.50363. [DOI] [PubMed] [Google Scholar]
- 14.Zhang DY, Sabla G, Shivakumar P, Tiao G, Sokol RJ, Mack C, Shneider BL, et al. Coordinate expression of regulatory genes differentiates embryonic and perinatal forms of biliary atresia. Hepatology. 2004;39:954–962. doi: 10.1002/hep.20135. [DOI] [PubMed] [Google Scholar]
- 15.Ader T, Norel R, Levoci L, Rogler LE. Transcriptional profiling implicates TGFbeta/BMP and Notch signaling pathways in ductular differentiation of fetal murine hepatoblasts. Mech Dev. 2006;123:177–194. doi: 10.1016/j.mod.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho E, Liu C, Shivakumar P, Sabla G, Aronow B, Bezerra JA. Analysis of the biliary transcriptome in experimental biliary atresia. Gastroenterology. 2005;129:713–717. doi: 10.1016/j.gastro.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Burgers WA, Blanchon L, Pradhan S, Launoit YD, Kouzarides T, Fuks F. Viral oncoproteins target the DNA methyltransferases. Oncogene. 2006 doi: 10.1038/sj.onc.1209950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruger DH, Schroeder C, Santibanez-Koref M, Reuter M. Avoidance of DNA methylation. A virus-encoded methylase inhibitor and evidence for counterselection of methylase recognition sites in viral genomes. Cell Biophys. 1989;15:87–95. doi: 10.1007/BF02991582. [DOI] [PubMed] [Google Scholar]
- 20.Glenn CC, Nicholls RD, Robinson WP, Saitoh S, Niikawa N, Schinzel A, Horsthemke B, et al. Modification of 15q11-q13 DNA methylation imprints in unique Angelman and Prader-Willi patients. Hum Mol Genet. 1993;2:1377–1382. doi: 10.1093/hmg/2.9.1377. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara H, Okada M, Kaneko H, Hishikawa T, Sekigawa I, Hashimoto H. Possible role of DNA hypomethylation in the induction of SLE: relationship to the transcription of human endogenous retroviruses. Clin Exp Rheumatol. 2003;21:733–738. [PubMed] [Google Scholar]
- 22.Fan G, Beard C, Chen RZ, Csankovszki G, Sun Y, Siniaia M, Biniszkiewicz D, et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J Neurosci. 2001;21:788–797. doi: 10.1523/JNEUROSCI.21-03-00788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 24.Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131:5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 25.Matthews RP, Plumb-Rudewiez N, Lorent K, Gissen P, Johnson CA, Lemaigre F, Pack M. Zebrafish vps33b, an ortholog of the gene responsible for human arthrogryposis-renal dysfunction-cholestasis syndrome, regulates biliary development downstream of the onecut transcription factor hnf6. Development. 2005;132:5295–5306. doi: 10.1242/dev.02140. [DOI] [PubMed] [Google Scholar]
- 26.Matthews RP, Lorent K, Russo P, Pack M. The zebrafish onecut gene hnf-6 functions in an evolutionarily conserved genetic pathway that regulates vertebrate biliary development. Dev Biol. 2004;274:245–259. doi: 10.1016/j.ydbio.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Matthews RP, Lorent K, Pack M. Transcription factor onecut3 regulates intrahepatic biliary development in zebrafish. Dev Dyn. 2008;237:124–131. doi: 10.1002/dvdy.21407. [DOI] [PubMed] [Google Scholar]
- 28.Clotman F, Jacquemin P, Plumb-Rudewiez N, Pierreux CE, Van der Smissen P, Dietz HC, Courtoy PJ, et al. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev. 2005;19:1849–1854. doi: 10.1101/gad.340305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, Roskams T, et al. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819–1828. doi: 10.1242/dev.129.8.1819. [DOI] [PubMed] [Google Scholar]
- 30.Yee NS, Lorent K, Pack M. Exocrine pancreas development in zebrafish. Dev Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 31.Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- 32.Rai K, Nadauld LD, Chidester S, Manos EJ, James SR, Karpf AR, Cairns BR, et al. Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development. Mol Cell Biol. 2006;26:7077–7085. doi: 10.1128/MCB.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews RP, Lorent K, Manoral-Mobias R, Huang Y, Gong W, Murray IV, Blair IA, et al. TNF{alpha}-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish s-adenosylhomocysteine hydrolase. Development. 2009;136:865–875. doi: 10.1242/dev.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, et al. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- 35.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 36.Tucker KL, Beard C, Dausmann J, Jackson-Grusby L, Laird PW, Lei H, Li E, et al. Germ-line passage is required for establishment of methylation and expression patterns of imprinted but not of nonimprinted genes. Genes Dev. 1996;10:1008–1020. doi: 10.1101/gad.10.8.1008. [DOI] [PubMed] [Google Scholar]
- 37.Lorent K, Moore JC, Siekmann AF, Lawson N, Pack M. Reiterative use of the notch signal during zebrafish intrahepatic biliary development. Dev Dyn. 2010;239:855–864. doi: 10.1002/dvdy.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev Biol. 2003;258:349–363. doi: 10.1016/s0012-1606(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 39.Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255:12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 40.Chen XM, O’Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol. 2008;86:497–505. doi: 10.1038/icb.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davenport M, Stringer MD, Tizzard SA, McClean P, Mieli-Vergani G, Hadzic N. Randomized, double-blind, placebo-controlled trial of corticosteroids after Kasai portoenterostomy for biliary atresia. Hepatology. 2007;46:1821–1827. doi: 10.1002/hep.21873. [DOI] [PubMed] [Google Scholar]
- 42.Beck IM, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30:830–882. doi: 10.1210/er.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieger D, Stein C, Neifer D, van der Sar AM, Leptin M. The role of gamma interferon in innate immunity in the zebrafish embryo. Dis Model Mech. 2009;2:571–581. doi: 10.1242/dmm.003509. [DOI] [PubMed] [Google Scholar]
- 44.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115:200–209. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16:801–807. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muraji T, Nio M, Ohhama Y, Hashimoto T, Iwanaka T, Takamatsu H, Ohnuma N, et al. Postoperative corticosteroid therapy for bile drainage in biliary atresia--a nationwide survey. J Pediatr Surg. 2004;39:1803–1805. doi: 10.1016/j.jpedsurg.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Lao OB, Larison C, Garrison M, Healey PJ, Goldin AB. Steroid use after the Kasai procedure for biliary atresia. Am J Surg. 199:680–684. doi: 10.1016/j.amjsurg.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gopalakrishnan S, Van Emburgh BO, Robertson KD. DNA methylation in development and human disease. Mutat Res. 2008;647:30–38. doi: 10.1016/j.mrfmmm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.