Abstract
Fetal hearts show a remarkable ability to develop under hypoxic conditions. The metabolic flexibility of fetal hearts allows sustained development under low oxygen conditions. In fact, hypoxia is critical for proper myocardial formation. Particularly, hypoxia inducible factor 1 (HIF-1) and vascular endothelial growth factor play central roles in hypoxia-dependent signaling in fetal heart formation, impacting embryonic outflow track remodeling and coronary vessel growth. Although HIF is not the only gene involved in adaptation to hypoxia, its role places it as a central figure in orchestrating events needed for adaptation to hypoxic stress. Although “normal” hypoxia (lower oxygen tension in the fetus as compared with the adult) is essential in heart formation, further abnormal hypoxia in utero adversely affects cardiogenesis. Prenatal hypoxia alters myocardial structure and causes a decline in cardiac performance. Not only are the effects of hypoxia apparent during the perinatal period, but prolonged hypoxia in utero also causes fetal programming of abnormality in the heart’s development. The altered expression pattern of cardioprotective genes such as protein kinase c epsilon, heat shock protein 70, and endothelial nitric oxide synthase, likely predispose the developing heart to increased vulnerability to ischemia and reperfusion injury later in life. The events underlying the long-term changes in gene expression are not clear, but likely involve variation in epigenetic regulation.
INTRODUCTION
Oxygen is an essential substrate for cell survival. It acts as a final electron acceptor in the electron transport chain (ETC). In humans, oxygen tension varies from 100 mm Hg in alveolar arterioles to between 40 and 20 mm Hg in systemic tissues [1]. When oxygen is scarce, the ETC is compromised. Since the ETC is coupled to oxidative phosphorlyation, ATP (Adensosine Triphosphate) levels drop significantly creating energy disparities within the cell. Hypoxia ensues when the oxygen tension is lower than physiological levels and the demand for oxygen exceeds the supply available. Hypoxia in non-reproductive tissues, as identified by direct measurement of pO2 values and/or significant induction of hypoxia-inducible genes, suggest oxygen tension between ~20 and ~7 mm Hg as physiological hypoxia [2–4]. Researchers commonly use between 1% (~7 mm Hg) and 3% (~21 mm Hg) oxygen in cell culturing to mimic hypoxia [5]. In in vivo studies, animals are typically subjected to between 8% and 12% oxygen in order to significantly reduce arterial pO2 to comparable values [6]. By comparison, fetal arterial blood pO2 ranges from ~30 to ~20 mm Hg [7–9], suggesting normal fetal oxygen is close to physiological hypoxia in adult tissues. This implies that fetal development exists in a state of relative hypoxia, as compared to adult oxygen tension, and that fetal tissues have a lower threshold to reach a state of oxygen insufficiency. Interestingly, short bouts of hypoxia naturally occur during gestation when the uterine artery contracts or becomes compressed, which reduces the amount of oxygen delivered to the placenta. Additionally, since the placenta serves as the major interface between mother and fetus, its development and oxygen consumption also influences fetal oxygen supply. Changes in maternal pO2 and/or abnormal placental development or metabolism may reduce fetal arterial pO2 and result in fetal hypoxia [10, 11]. Under hypoxic conditions, the fetus compensates by altering fetal blood flow away from peripheral tissues to vital organs; shifting from aerobic to greater utilization of anaerobic energy pathways; and the induction of hypoxia-dependent genes necessary for survival in a low oxygen environment.
Cardiogenesis involves the formation of the embryonic heart. Once the heart is formed, it undergoes a stage of rapid growth and maturation during fetal development. The formation and subsequent maturation of the heart are tightly regulated processes in which oxygen tension plays a vital role. Interestingly, the fetal heart is more resistant to hypoxia induced cell death than the adult heart due to its enhanced ability to increase glycolytic flux [12]. There is increasing evidence suggesting that low oxygen tension in the fetus is essential for normal heart formation and maturation. The expressions of hypoxia-induced genes, such as hypoxia inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF), correlate with angiogenesis, vasculogenesis and fetal heart remodeling [13, 14]. Although physiologically “normal” hypoxia (lower oxygen tension in the fetus as compared with the adult) may be beneficial in normal heart development, pathophysiological hypoxia (lower than normal fetal oxygen tension) is associated with significant adverse effects that can lead to changes in structure, function, and gene expression in fetal hearts, which may persist throughout adulthood (See Fig. 1).
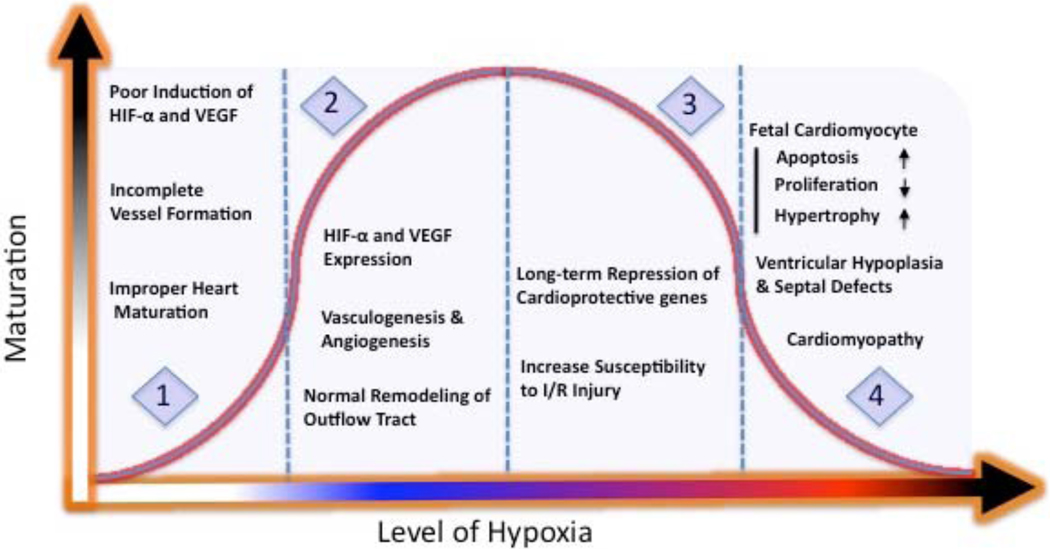
Fig. (1). The effect of hypoxia on fetal heart development.
1. Insufficient exposure to “normal” hypoxia reduces the expression of key genes (i.e. HIF-α, VEGF) needed for heart and vessels formation. 2. Adequate exposure to “normal” hypoxia ensures expression of hypoxia dependent genes needed for vasculogenesis, angiogenesis and fetal heart remodeling. 3. Chronic exposure to moderate “abnormal” hypoxia can lead to programming of cardioprotective genes, which may decrease the ability of heart to adapt to stresses later in life. 4. Exposure to more severe “abnormal” hypoxia can significantly affect fetal cardiomyocytes development, which can lead to cardiomyopathy.
This review focuses on the role of hypoxia in fetal heart development. The initial sections summarize myocardial development and data related to hypoxia and its role in normal heart formation and development. In the following section we examine the role of HIF-1 in cardiogenesis. Next, we discuss hypoxia in the context of abnormal heart formation and development: how oxygen insufficiency may alter the structure and function of fetal hearts. Finally, recent studies linking prenatal hypoxia to increased susceptibility to ischemia and reperfusion injury, as well as the possible mechanisms, are discussed.
FORMATION OF THE MYOCARDIUM
Heart Chambers and Vessels
Our understanding of the events that lead to the formation of a functional heart in vertebrates comes primarily from studies done in chicken and mouse models. Although there exist differences between species, the major events are congruent. This section alone is incapable of covering all points in myocardial formation, but seeks to highlight major events that occur during cardiogenesis. Arguably, the first significant event in myocardial formation involves the formation of the primitive heart tube [15]. Cardiac progenitor cells from the primary (later become left ventricle) and secondary (later become right ventricle, outflow and inflow tracts) heart fields differentiate into myocardial cells and fuse along the midline to form the heart tube [16]. It is during this event that peristalic contractions appear along the heart tube. Once initiated, the heterogeneity of the heart chambers to be formed becomes apparent with regions destined to become atrials and ventricles contracting at different rates. Structurally, the primitive heart tube is composed of a myocardial covering that is one or two cells thick, an acellular cardiac jelly, and endocardium [16]. After the heart tube is formed it undergoes looping and sets the framework in which the chambers can develop. After looping, changes (known as trabeculations) in what will become ventricles emerge within the lumen. Trabeculations are fenestrated sheet-like protrusions into the lumen that initially serve to increase the surface to volume ratio, allowing growth of the myocardium prior to the establishment of the coronary circulation and the separation of blood flow before septation [15]. The heart then undergoes further remodeling with growth primarily from increased cellular proliferation and compaction of trabeculae. At the same time, the coronary vasculature is established to meet the increasing demands brought about by the growing myocardium. Angioblasts form the primitive vascular plexus. The vessels undergo extensive remodeling and patterning and mature into the coronary artery tree. Evidence suggests that hypoxia may be the initial signal mediating angioblast invasion of the embryonic heart and subsequent formation of the coronary vasculature [17, 18]. The establishment of the coronary vessel network ensures adequate nutrients are delivered to the myocardium allowing sustained growth and maturation.
The functional unit of the myocardium is the cardiomyocyte. As previously mentioned, cardiomyocytes originate from cardiac progenitor cells in the primary and secondary heart fields. They differ from adult cardiomyocytes in size, organization of myofibrils, and proliferation capacity. Fetal cardiomyocytes are smaller in diameter and are normally mononucleated, whereas adult heart cells tend to be larger and often display polyploidy (two or more nuclei per cell). Fetal cardiomyocytes have fewer and less organized myofibrils with poorly the developed sarcoplasmic reticulum and the T Tubules systems as compared to the numerous and highly organized myofibrils found in adult cardiomyocytes [19]. At early stages of development, the fetal heart grows largely through rapid proliferation of cardiomyocytes [20]. However, towards the end of gestation, cardiac cells become increasingly differentiated and therefore lose the ability to propagate. Immature heart cells lose the ability to proliferate during perinatal periods, with subsequent growth due primarily to enlargement of existing cardiac cells. In comparison, adult mammalian heart cells maintain a permanently differentiated disposition with little or no ability to self-reproduce [21]. The change in the proliferation capacity in cardiomyocytes corresponds to the maturation of contractile machinery and the increase in population of poly-nucleated cells. It is likely the highly ordered structure of mature cardiomyocytes, coupled with the continuous contraction necessary for cardiac output and poly-nucleated disposition, preclude self-propagation. In nature, there exists species capable of regenerating heart cells at an adult stage. The adult newt and zebrafish are both capable of regenerating substantial portions of heart tissue [22, 23]. In particular, the adult newt seems to possess the ability to dedifferentiate cardiac cells and proliferate to replace lost or damaged tissue [24]. Among other important factors, activation of p38 MAP kinase appears to play an important role in inhibiting cardiomyocyte growth. The expression of p38 MAPK expression is inversely correlated with cardiomyocyte proliferation, while inhibition of p38 isoforms promotes increased expression of genes vital for self-propagation [25]. These findings imply that mammalian adult heart cells have the capacity to reproduce. Nonetheless, the factors that give the immature heart cell, adult newt and zebrafish the ability to reproduce may shed light that may promote the development of therapies that can assist in the recovery of heart tissue following a coronary event.
Hemodynamic Stress
Functionally, the embryonic heart at an early stage sets itself apart from most organs in that it is called upon to work almost from its inception. As the vascular system forms, there is an increased stress placed on the fetal heart. However, the plastic nature of the developing myocardium gives it the ability to adjust to hemodynamic load brought upon it by an enlarging peripheral vascular system. An increase in afterload, or elevation in pressure required to eject blood to systemic tissues, requires compensatory growth of the myocardial wall. Laplace’s law explains the relationship between wall thickness and increases in afterload. It states that increases in intraluminal pressure will produce increased wall stress for a given radius. It is compensated by increased wall thickness or decrease internal radius. This phenomenon is seen in individuals with pulmonary hypertension that increases the intraventricular end diastolic pressure resulting in thickening of the right heart wall. In fetal sheep, Barbera et al. [26] demonstrated that right ventricular wall thickness increases in response to pulmonary artery occlusion, which is an increase in systolic pressure load. The findings from this study suggested that the increase in heart size was primarily due to hyperplasia of cardiomyocytes, although some growth could be attributed to hypertrophy. Indeed, arterial hypertension in fetal sheep increases the weight of hearts, stimulates proliferation, size and binucleation of cardiomyocytes resulting in increased growth and accelerated maturation of the myocardium as a compensatory response to increased afterload [27]. Therefore, ventricular wall mass is influenced by the hemodynamic stress under which it develops.
HYPOXIA AND THE DEVELOPING HEART
Methods for Determining Hypoxia in Utero
Many studies have investigated the role of hypoxia in fetal heart development. Hypoxia in vivo is a challenging phenomenon to study; yet, several techniques are used to either measure oxygen tension directly or indirectly in vivo. The surgical implantation of O2 sensitive sensors is a method commonly used on larger animals, such as pregnant sheep, to measure fetal pO2 [28]. Additionally, the unique metabolic plasticity of the fetal heart allows it to rapidly increase glycolysis under conditions of oxygen deprivation [12]. The switch from aerobic to anaerobic respiration corresponds to increases in glycolytic intermediates, such as lactate, that have been used as an indicator of reduced mitochondrial oxidative activity in response to oxygen insufficiency [29]. Beyond surgical implantation and metabolism, Chapman [30] was the first to suggest the use of nitroimadazole compounds (e.g. EF5, pimonidazole) to study hypoxia. In low oxygen conditions, nitroimadazole compounds are chemically reduced by nitroreductases, which permit covalent binding to intracellular proteins to form adducts [31, 32]. Nitroimadazole compounds have been used to study the effects of hypoxia in embryogenesis and tumorgenesis [17, 33].
Furthermore, tissue hypoxia alters gene expression. Hypoxia stabilizes HIF (1, 2 and 3), a family of transcription factors that play a central role in cellular adaptation to insufficient oxygen. The discovery of HIF sheds new light on the role of hypoxia in fetal heart maturation. HIF is comprised of a α and β subunit, of which the former is oxygen-sensitive and affects HIF stability, and the latter is oxygen-insensitive. HIF is widely known to up-regulate numerous genes associated with external and internal cellular adaptation to hypoxia [34–36]. A classic example of HIF associated gene transactivation is the induction of erythropoietin (EPO). Hypoxia promotes HIF-induced up-regulation of EPO [37]. EPO stimulates erythropoiesis, inhibits apoptosis, and mobilizes endothelial progenitors for vessel growth by binding to EPO receptors [38, 39]. HIF and HIF dependent gene expression have become important indicators of tissue hypoxia, and their use has increased our understanding of the role of reduced oxygen in cardiogenesis from a stand point of transcriptional changes. HIF regulation will be discussed in detail later in the review.
Outflow Tract Remodeling
Recent studies in avian and mouse models suggest that hypoxia plays a critical role in the formation and development of the heart. Many of these studies were centered on the remodeling of the embryonic outflow track (OFT) and coronary vessel formation. Embryonic OFT remodeling is necessary for the proper transition from single to dual circulation in mammalian and avian hearts [6]. Apoptosis of OFT cardiomyocytes is vital for proper remodeling of OFT [40, 41]. Programmed cell death of OFT cardiomyocytes brings about the shortening and rotation needed for the aorta to join the left ventricle and pulmonary vessels to connect to the right ventricle. At the height of cardiomyocyte apoptosis in the OFT, researchers have observed increases in EF5 (chemical marker of hypoxia) staining as well as increased nuclear accumulation of HIF-1; suggesting hypoxia via HIF-1 may be involved in OFT remodeling [14, 41]. Druyan el al. [42] demonstrated in chick fetal hearts the upregulation of hypoxia-regulated genes, heme oxygenase, cardiac troponin T and hypoxia up-regulated protein 1 at specific developmental periods (E7 and E19). Heme oxygenase facilitates the degradation of heme and protects against oxidative stress. Cardiac troponin T is involved in calcium handling and contractions of the heart, and hypoxia up-regulated protein 1 protects against cell death during hypoxia. These finding suggests that as the heart develops under hypoxic conditions, cellular defense pathways are employed in order to sustain normal growth, while protecting against oxidative stress and apoptosis. Ironically, cardiomyocytes exhibit increased oxidative stress as a result of hypoxia [43]. Oxidative stress can cause cellular damage, which may lead to cell death. In subsequent experiments, EF5 staining revealed differential levels of oxygen in fetal hearts with areas of low oxygen correlating spatially and temporally with apoptosis-induced myocardial remodeling [14, 44, & 45]. These findings were further substantiated by experiments that demonstrated intense EF5 staining and cell death of OFT cardiomyocytes that were attenuated by hyperoxia resulting in OFT defects. Such defects included the abnormal formation of the right ventricles, which clearly associated oxygen regulation with proper myocardial formation (See Fig. 1) [41]. It appears the timing of the hypoxic insult is tightly regulated for proper heart remodeling. Low oxygen was reported in chick at stages 25–32 and around day 13.5 of gestation in mice [14, 41]. Interestingly, the timing and duration of hypoxia plays an important role in the development of other tissues. In the first trimesters, placenta tissues develop under low pO2 (<20mm Hg), which rises considerably in second trimester (~60mm Hg) then declines (~40mm Hg) by the third trimester [46–48]. Accordingly, studies suggest the fluctuation in pO2 modulates trophoblast differentiation and invasion, affording concomitant growth of the placenta throughout gestation [49]. These findings indicate that hypoxia functions in several tissues to signal changes that promote normal development.
Coronary Vessel Formation
In embryonic tissues, the formation of primary blood vessels from endothelial precursors called angioblasts is known as vasculogenesis. During vasculogenesis, angioblasts combine and form the primary capillary plexus, which serves as the framework from which all subsequent vessel formation occurs. After the establishment of the primary capillary plexus, subsequent budding and sprouting of new vessels from preexisting ones is known as angiogenesis. Though proper vessel development and maturation involves input from multiple signals, VEGF signaling plays a vital role throughout vessel formation and growth. The binding of VEGF to receptor tyrosine kinases (e.g. KDR/Fik-1, Fit-1 in endothelial cells) initiates the expression of genes necessary for the recruitment, proliferation, and differentiation needed for vasculogenesis and angiogenesis [50–53]. For a more thorough review of VEGF signaling consider the following excellent reviews [54–57]. In cardiogenesis, VEGF is essential for vessel growth and the induction of VEGF correlated spatially with coronary vessel patterning [58–60]. In addition, cardiomyocyte-restricted knockout of Vegfα, impaired coronary vessel development, promoted myocardial thinning, depressed basal contractile function, and caused significant dysfunction of beta-adrenergic stimulation [61]. Also, mice deficient of receptor tyrosine kinase showed abnormal heart development, primarily ventricular septal defects [62]. These findings suggest that VEGF-mediated vessel formation promotes adequate growth, remodeling and function of the myocardium.
Hypoxic stress increases vessel formation in fetal heart tissue, while hyperoxia delays vessel growth [63]. Studies have confirmed that hypoxia is a major stimulus for vessel growth in fetal development and tumorgenesis [63–65]. Hypoxia is known to stabilize HIF-1& 2, which are known to preferentially up-regulate VEGF and its receptor [65, 66]. However, studies conducted by Kotch et al. [67] showed that VEGF mRNA increased modestly compared to controls in HIF-1α null mice, suggesting additional factors may regulate VEGF induction in fetal heart tissue. For example, induction of VEGF in the absences of HIF-1α may be explained by the presence of HIF-2α, which is known to up-regulate VEGF and VEGF receptors, or by increased stability of VEGF mRNA in hypoxic conditions. Furthermore, other growth factors such as platelet-derived growth factor (PDGF) have been shown to induce VEGF expression [68], suggesting VEGF can be induced independent of HIF-1 activity. However, other findings have linked hypoxic stimulus through HIF-1 stabilization with significant induction of VEGF and VEGF receptor in cardiogenesis [44, 69]. The central role of hypoxia-dependent genes during cardiogenesis suggests that oxygen regulation at the molecular level plays a major role in fetal heart formation.
THE ROLE OF HIF IN CARDIAC FORMATION AND MATURATION
Oxygen Regulation
The molecular signals underpinning hypoxia’s role in cardiac formation and development involves numerous genes. By far, the HIF family of genes is the most studied in cardiac development. HIF is a heterodimeric transcription factor that plays a pivotal role in cellular sensing and response to low oxygen tension. HIF belongs to the basic helix-loop-helix (bHLH)/Per-ARNT-Sim (PAS) domain family of transcription factors and is composed of an oxygen-sensitive α subunit and constitutively expressed β subunit [35, 37]. It is known to regulate numerous functions during hypoxia such as energy metabolism, erythropoiesis, cell survival and death, vascularization, angiogenesis, and differentiation [70–74]. Though HIF stability and activity is influenced by a wide range of factors (i.e. Insulin like Growth Factor 1 & 2), the effect of oxygen is the most characterized. Oxygen tension regulates HIF-1 expression via prolyl hydroxylase (PHDs) activity. Under normoxic conditions the HIF-1α subunit is recognized and hydoxylated at proline residues 402 and 564 by PHDs. Hydroxylation allows recruitment and binding of the von Hippel-Lindau protein, an E3 ubiquitin protein ligase, which primes HIF-1α for subsequent proteasome degradation. In addition, the transcriptional activity of HIF-1 is also regulated by hydroxylation. Hydroxylation of arginine 803 by Factor inhibiting HIF-1 (FIH) prevents the association of HIF-1 and CREB-binding protein (CBP)/p300, precluding the transactivation of HIF-1 dependent genes [75]. When cellular oxygen levels fall, the activity of PHDs and FIH are reduced, allowing stability of the HIF-1α subunit. Interestingly, HIF-1 stability is inversely proportional to oxygen concentration within the cell [76]. Stable HIF-1α subunit translocates into the nucleus where it dimerizes with HIF-1β and transactivates HIF-1 genes that possess hypoxic response elements (HRE) (short sequences of DNA that include 5’-CGTGC/T-3’).
HIF and the Developing Heart
Both HIF-1αβ & 2 αβ are expressed in the cardiac tissue [77, 78]. Little is known about the role of HIF-2 in heart formation and development, although HIF-2α knockout impairs vascular development as well as altered cardiac rhythm due to the deregulation of catecholamine release [79]. For this reason, this review focuses mainly on the role of HIF-1α in heart formation and development. Though it is not fully understood how HIF-1 dependent mechanisms coordinate in cardiogenesis, it is known that HIF-1 expression is vital for proper myocardial remodeling and coronary vessel formation. Elevated HIF-1 expression is found in fetal hearts exposed to hypoxia [14, 80]. HIF-1 was also found in the nuclei of OFT cardiomyocytes undergoing apoptosis as well as those that are not [14]. This implies HIF-1 activity is involved in orchestrating OFT cardiomyocyte fate. The importance of hypoxic-dependent signaling in myocardial development is further supported by studies using mice deficient in HIF-1α. Global knockout of HIF-1α resulted in arrested development by day E9 and embryonic lethality by day E11 with significant cardiovascular irregularities including cardiac bifida, abnormal cardiac looping, abnormal remodeling of the aortic outflow tract and cephalic blood vessels, and mesenchymal cell death [13, 34]. When HIF-1α null mice were placed in hyperoxia, partial recovery of the embryos was observed, suggesting that adaptation to hypoxic conditions requires HIF-1 signaling in the developing heart [13]. HIF-1 is known to up-regulate, either directly, through binding to HRE sites, or indirectly, by influencing other transcription factors that promote prosurvival and apoptotic genes. In the fetal heart, HIF-dependent activation of VEGF, stromal cell-derived factor-1 and EPO receptor expression promote the vessel formation [81–83]. In addition, survival signals in the myocardium are augmented by HIF-dependent direct activation of glycolytic genes such as Glucose transporter 1 (Glut1), aldolase A, enolase 1 (EN01), lactate dehydrogcnasc A, and phosphoglyceratc kinase 1(PGK1), which facilitate the shift from aerobic to anaerobic respiration [84, 85]. Paradoxically, HIF-1 has been shown to up-regulate proapoptic genes BNIP3 and Bax in cardiomyocytes [86]. This dual role of HIF is also seen in cerebral ischemia models where HIF-1 up-regulates prosurvival (EPO, GLUT1, VEGF) and death genes (BNIP3, caspase 3, stabilization of p53) depending on the extent and duration of the ischemic insult [87]. It is likely that complex mechanisms contribute to the duality of HIF-1 signaling in cardiogenesis. Presumably the ability of HIF-1 to bind HRE sites and thus influence gene activity changes depending on the timing and duration of hypoxic insult. This twin nature of HIF may influence HIF-dependent cardiomyocyte survival or death, and possibly play a role in long-term programming of fetal cardiomyocytes during chronic hypoxia.
FETAL HYPOXIA AND ABNORMAL HEART DEVELOPMENT
Though fetal hearts show remarkable ability to survive and function under low oxygen, chronically pathophysiological hypoxia is associated with numerous complications that have both short and long-term effects. Some of the most striking data illustrating the effects of hypoxia on fetal development originate from high altitude studies. About 140 million people live in high altitude environment worldwide, of which some 400,000 live in the United States [88]. High altitude is considered to be elevations above 2500 meters (8000ft) [88]. Pregnancies at higher elevations may result in significantly depressed maternal arterial pO2 and changes in placental growth when compared to the sea level [89]. Epidemiological studies have indicated that high altitude pregnancies increase the risk of intrauterine growth restriction (IUGR) and low birth weight [90–92]. These factors are known to cause premature birth, infant mortality, and an increased risk of developing cardiovascular related diseases [93–96]. Other factors that may contribute to hypoxia in utero include pre-existing maternal illness, pre-eclampsia, cord compression, smoking, pollution, hemoglobinopathy, and aberrant placenta development. Lowered maternal arterial pO2 or incomplete delivery of oxygen to fetal tissues induces hypoxemia and sustained tissue hypoxia in utero, resulting in significant changes in fetal development [97, 98].
The body of evidence indicating the adverse effects of prolonged hypoxia in utero is substantial. The developing heart, more than any other organ, is susceptible to hypoxic stress due to its enhanced metabolic demand. Numerous studies, primarily in animals, have shown that hypoxia causes incomplete development of the heart. One of the earliest studies demonstrating the adverse effects of simulated high altitude on pregnant rats found that hypoxia caused ventricle septal defects in rat offspring [99]. More recent studies have found that insufficient oxygen in utero produces myocardial thinning, ventricle dilation, and epicardium detachment. It also slows fetal heart maturation in both chicken and mouse [99, 100]. Other studies demonstrated cardiomyocyte hypertrophy and myocardial hypoplasia in fetal hearts subjected to chronic hypoxia [80, 99, 101 & 102]. It is likely that the increase in size of cardiomyocyte number is compensatory for reduced myocyte. Interestingly, studies have found that prenatal hypoxia increases the heart to body mass ratio [80, 101 & 103], suggesting either reduced growth of nonessential organs or heart enlargement. The reduction in cardiomyocyte number is likely influenced by either increased program cell death and/or reduced cellular proliferation during critical periods of development. Hypoxia-mediated increase in apoptosis is supported by studies that indicate that prenatal hypoxia increased death signaling via elevated caspase 3 activity and Fas mRNA, and suppressed survival pathways via depressed Bcl-2 and Hsp70 expression in fetal hearts [80]. Conversely, the reduction in cardiomyocyte may be attributed to premature exit of cardiomyocytes from the cell cycle. Bae et al. [80] reported that prenatal hypoxia increased the percentage and size of binucleated cardiomyocytes in fetal rat hearts. Binucleated cardiomyocytes are terminally differentiated cells that are no longer capable of division. Taken together, prolonged insufficient oxygen alters fetal heart growth resulting in abnormalities in fetal heart structure. These abnormalities likely involve sustained reduction in cardiomyocyte proliferation and increased apoptosis.
Intrauterine stress via hypoxia induces not only changes in fetal heart morphology, but also in function as well. In humans, changes in fetal heart rate have long been observed in fetuses in response to intrauterine stress [104]. Animal studies have also confirmed the dysfunction of the myocardium in response to hypoxia. Sedmera et al. [105] demonstrated that the rate of recovery from anoxia/reoxygenation declines from the loop tubular heart to the septated trabeculated heart, suggesting oxygen dependence increases with the development. In a study examining the significance of catecholamines in development, researchers reported hypoxia decreased the heart rate of fetal mice by 35–40% in culture and by 20% in utero when compared to wild-type hearts [106]. High altitude Sheep models also present with altered cardiac function in response to prenatal hypoxia as demonstrated by decreased cardiac output and lowered contractility [107–110]. Possible explanations for the depressed cardiac function relate to altered calcium homeostasis and reduced ATP availability due to decreased Mg2+-activated myofibrillar ATPase activity [108, 110 & 111]. In avian, poor cardiac performance was observed as demonstrated by decreased maximum ventricular +dP/dt and peak pressure, increased ventricular end-systolic volume, elevated after-load, and decreased left ventricular ejection fractions [100, 112]. Whether hypoxia directly mediates or indirectly facilitates these changes is not clear. Studies suggest that cardiac defects such as cardiac bifida and looping defects may be mediated via A1 adenosine receptor signaling in chicken hearts [113]. Sarre et al. [114] demonstrated in an in vitro model arrhythmias in 4-day-old isolated embryonic hearts subjected to anoxia/reoxygenation. Graf et al. [115] observed an increased rate of contractions and decreased sensitivity to norepinephrine of cultured rat cardiomyocytes exposed to hypobaric hypoxia during organogenesis. In utero stress may cause an increase in circulating stress hormones, which may explain poor cardiac performance. In addition, structural changes in the myocardium may contribute to depressed cardiac function. It should be noted that many hypoxic models involve maternal exposure to reduced room oxygen. More studies demonstrating the direct effect of hypoxia on fetal hearts are warranted. Taken together, prolong exposure to hypoxia in utero alters heart structure and function and these changes may persist into adulthood.
HYPOXIA AND INTRAUTERINE PROGRAMMING OF THE HEART
Many studies have correlated an adverse intrauterine environment with an increase predisposition for developing cardiovascular-related diseases in adulthood. Most notably, epidemiological studies by Barker and colleagues [93, 116] were the first to correlate undernutrition and low birthweight with increased incidence of coronary heart disease in adulthood. This finding led to what is known as the Fetal Origins hypothesis, which proposes that adaptations made by the fetus in response to undernutrition causes permanent changes in tissue structure and function. This programming in utero predisposes the fetus to cardiovascular disease later in life [94, 95, 117, 118]. Hypoxia in utero is known to cause depressed cardiac performance and cardiomyopathies that persist into adulthood [112]. It is less clear, however, the extent to which hypoxia mediates programming of genes that alter structure and function that are not apparent until later in life.
Recently, researchers have used animal models to elucidate the role of hypoxia in intrauterine programming. Li et al. [119] demonstrated that 6-month-old male rat hearts exposed to prenatal hypoxia responded less favorably than control animals when subjected to simulated ischemia and reperfusion (I/R) injury. The hypoxic animals exhibited a persistent decrease in postischemic recovery, an increase in myocardial infarction (MI), and fewer but larger cardiomyocytes. In addition, the hearts of these animals had elevated caspase 3 activity and decreased levels of Hsp70 and eNOS when compared to control animals. While both Hsp70 and eNOS play important roles in cardioprotection against I/R injury, caspase 3 belongs to a family of proteases that perform critical cellular functions to facilitate programmed cell death [120–123]. This finding was confirmed by Xu et al. [124] who also observed lower levels of metalloproteinase-2 in 4 month old rats. Moreover, prenatal hypoxia abolishes the protective affects afforded by heat stress against I/R injury and significantly reduces HSP70 and PKCε content in the left ventricles [125]. Furthermore, Xue et al. [126] showed that prenatal hypoxia caused a decrease in I/R recovery in 3-month-old male, but not female, rat offspring. When normoxic offspring were exposed to simulated I/R in the presence of PKCε translocation inhibitor peptide, those animals also displayed reduced I/R recovery; suggesting that programming of decreased PKCε gene expression is key to the observed increase vulnerability to I/R injury in males [126]. This finding is supported by studies involving PKCε over-expression, PKCε null animals, and PKCε activating peptide which confirm the pivotal role of PKCε in I/R preconditioning and myocardial protection [127–130].
Conversely, Netuka et al. [131] reported no difference in MI in prenatally hypoxic rats of either sex, but noted that normoxic females had less MI than normoxic males when exposed to I/R. They also noted that prenatal hypoxia protected against ischemic-induced arrhythmias of female rats, but was deleterious in male rats. The disparate results reported by Li [119], Xu [124] & Xue [126] and Netuka’s [131] studies might be explained by differences in strain, age, and experimental models. Whereas the Xue [126], Xu [124] and Li [119] studies used 3, 4 & 6-month-old Sprawley Dawley rats respectively exposed to hypoxia only in utero, Netuka [131] used 3-month-old Wistar rats exposed to intermittent hypobaric hypoxia in utero and 10 days after birth. In addition, Li et al. used simulated I/R insult with hearts removed from the animal, while Netuka et al. performed open chest I/R insult. These reasons may explain the differing results; nonetheless, both groups drew similar conclusions in that prenatal hypoxia causes programming of the fetal heart. It is also clear that prenatal hypoxia has sex dependent effects, as demonstrated by poor recovery of male rats, which likely involves altered programming of cardioprotective genes, namely PKCε.
The long-term effect of hypoxia in utero is not restricted to the cardiovascular system but also produces programming affects in other tissues. Pregnant sheep exposed to chronic high altitude conditions show significant reduction in kidney to body weight size, larger Bowman’s capsule in nephrons and alterations in angiotensin I and II expression [132]. Long-term hypoxia alters the expression of CYP17 and CYP11A1 in fetal sheep adrenal glands [133]. CYP17 and CYP11A1 are two key enzymes involved in steroidogenesis, suggesting high altitude exerts stress on animals such that elevated levels of stress hormone may be measured in fetal tissues. Whether this expression pattern is maintained into adult stages is not known. Furthermore, the direct effects of hypoxia are not clear. The maternal hypoxic model is capable of significantly lowering fetal oxygen status, however maternal hypoxia elicits other systemic effects that may reflect the observed effects. For example, maternal hypoxia increases circulating glucocortoids that may cause abnormal development of the fetus [134]. Identifying the direct effects of hypoxia on fetal heart development is critical to understanding the underlying mechanisms involved in hypoxia-induced programming of fetal hearts.
EPIGENETICS: A PLAUSIBLE MECHANISM
The mechanisms underlying the changes in function and gene expression in fetal hearts are not fully understood and are complex; however, epigenetic modifications are likely present. Conceivably, prolonged exposure to hypoxia in utero may alter gene expression patterns through epigenetic mechanisms. Major epigenetic modifications include methylation of cytosine in CpG dinucleotide, and post-translation modification of histone proteins (e.g. acetylation). Epigenetics has been implicated in fetal development as well as tumorgenesis. There are many excellent reviews in the field [135–137]. Epigenetic modifications alter gene expression pattern in the long-term. In a related model, studies have demonstrated that maternal cocaine injections increases DNA methylation at Sp1 binding sites in PKCε promoter in fetal hearts [138, 139]. Maternal cocaine injections during gestation induce intrauterine stress that results in increased susceptibility to I/R injury and abolition of protection afforded by preconditioning in the hearts of male offspring [140, 141]. In addition, reduced PKCε and phospho-PKCε expression was noted in the left ventricles of male offspring exposed to maternal cocaine, suggesting altered programming of the PKCε gene [141]. Zhang et al. [138] showed that cocaine exposure in utero caused hypermethylation of CpG dinucleotides of Sp1 binding sites for PKCε gene in left ventricles of 3-month-old rats. Supporting this discovery was the finding that Sp1 binding sites on the proximal promoter played a significant role in PKCε gene transcription [138]. Meyer et al. [139] used DNA methylation inhibitors 5-aza-2-deoxycytine and procainamide, to block cocaine-mediated down-regulation of PKCε. These findings clearly link epigenetics via DNA methylation in utero with the increased susceptibility to coronary heart disease later in life. The similarities between prenatal hypoxia and cocaine exposure are striking, and it is possible that the underlying mechanisms for both are concurrent. It is well known that cocaine acts as a potent vasoconstrictor, which may alter blood flow to the uterus resulting in oxygen insufficiency in utero. Furthermore, both hypoxia and cocaine cause an increase in oxidative stress. Whether the observed programming in utero is reversible is not clear and deserves further study. How prenatal hypoxia predisposes fetal hearts to I/R injury, and whether the mechanisms mirrors that of cocaine, are fascinating questions that remain to be elucidated.
HIF and Epigenetics
To date, little is known about HIF-1 in epigenetics and whether it plays a role in long-term gene silencing in immature cardiomyocytes. Since HIF activity is central to cellular adaptation to hypoxic stress, it is plausible HIF may play a role in hypoxia-induced fetal programming of cardiomyocytes. Hypoxia through HIF plays an important role in influencing transcription of multiple genes in embryogenesis and tumorgenesis. Epigenetic mechanisms play an important role in the process of both cases. In embryogenesis, developmental changes involve a progressive specialization of gene expression patterns that incorporate epigenetic mechanisms such as DNA methylation, chromatin remodeling, and/or histone posttranslational modification [142]. An example of the influence of hypoxia in embryogenesis is seen in stem cell differentiation. Studies have demonstrated that normal placental development requires HIF-1 induction for proper trophoblast differentiation [72, 73 & 143]. One fascinating study demonstrated that HIF-2 preferentially up-regulated Oct-4 [77]. Oct-4 plays an important role in maintaining a dedifferentiated cell disposition in embryonic stem cells, primordial germ cells and embryonic epiblast [144–146]. Furthermore, reports have shown the involvement of HIF-1 in tumor formation and irregular gene expression silencing via DNA methylation [147, 148]. HIF-1 has also been shown to interact directly with epigenetic modulators. Studies done by Beyer [149] and Wellmann et al. [150] identified a HRE site in the proximal promoter of the histone demethylase Jumonji domain containing 1A and 2B (JMJD1A, JMJD2B). JMJD1A and JMJD2b demethylate H3K9 residues of histones, which is usually associated with reduced acetylation and gene repression [149, 151]. In renal cell carcinoma, von Hippel Lindau activity is lost leading to aberrant expression of HIF-1 & 2, as well as increased expression and activity of histone demethylases JMJD1A and JMJD2b [147, 148 & 152]. In addition, studies suggest that HIF-1 interacts directly with histone deacetylases 7 (HDAC7) [143, 153]. The interaction seems to highlight HDAC7 ability to enhance HIF activity by forming a complex with HIF and p300 and influencing gene transcription. Histone deacetylases remove acetyl groups from histone residues, causing structural changes that preclude the binding of transcription factors and RNA polymerase II to gene targets. HIF-1 is known to influence cellular adaptation in response to acute and chronic hypoxia; yet HIF-1 may also mediate long-term changes in gene expression through direct modulation of epigenetic effectors.
Recently, studies have linked c-myc activity with HIF-1 and HIF-2. C-myc is a proto-oncogene involved in cell proliferation and enhancement of cellular metabolism. There is evidence that c-myc can modulate epigenetic mechanisms. Work done by He et al. [154] demonstrated a link between overexpression of c-myc and hypermethylation of CpG sites in the tumor suppressor gene’s retinoic acid receptor beta (RARbeta) and PDLIM4 in a prostate cancer cell line. Brenner et al, [155] found that c-myc is able to direct DNA methyltransferase 3a (DNMT3a) to the p21Cip1 promoter through direct binding with DNMT3a and Miz-1, identifying a potential mechanism for epigenetic silencing in cancer. In cancer cell models, HIF-1 actively competes with c-myc causing inhibition of gene targets, while HIF-2 promotes c-myc activity [148, 156]. These findings suggest HIF may also indirectly influence epigenetic patterns through interaction with c-myc or other transcription factors. Undoubtedly, HIF-1 plays an important role in normal fetal heart development, and may also play a key role in fetal programming of the myocardium through modulation of epigenetic effectors (See Fig. 2).
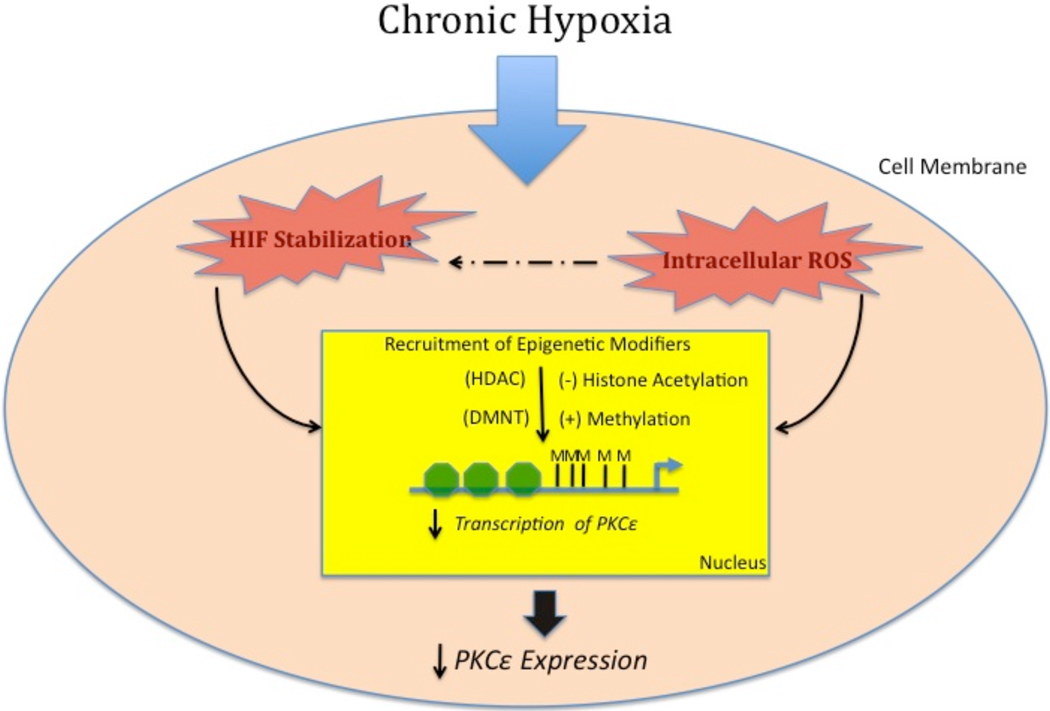
Fig. (2). Plausible mechanisms for hypoxia-induce down-regulation of cardioprotective genes in hearts.
Hypoxia causes the stabilization of HIF-1α and increased ROS production in fetal hearts. These events either act in collusion or independently to cause the recruitment of epigenetic modifiers, i.e. DNA methyltransferase (DNMT) or histone deacetylases (HDAC). These modifiers increase methylation of promoter at transcription factor binding sites and deacetylate histone residues resulting in the decreased transcription of cardioprotective genes (ex. PKCε, HSP70) and decreased cardioprotection in the long-term.
Oxidative Stress and Fetal Programming
Hypoxia is known to increase reactive oxygen species (ROS) and alter cellular oxidative homeostasis [157]. The predominant source of hypoxia-induce ROS comes from the electron transport system of the mitochondria. Studies have identified complex I and III as the main producers of mitochondrial ROS. The rate at which electrons flow through the mitochondria impacts the likelihood of ROS generation. Under normal conditions electrons flow freely, reducing the time at which free radicals such as ubisemiquinone can interact with molecular oxygen to produce superoxide anion. During hypoxia, the flow rate of electrons through the electron transport system slows, increasing the chance of molecular oxygen gaining an unpaired electron to produce superoxide ion [158]. Cells possess endogenous mechanisms to deal with ROS (i.e. superoxide dismutase, catalase, glutathione reductase), however, when those systems are overwhelm the cell experiences a state of oxidative stress. Oxidative stress can damage structures and alter function substantially, which may lead to cell death. Sustain ROS can trigger significant changes in gene expression patterns. ROS production has been shown to initiate the Integrated Stress Response (ISR), which invokes PERK activation, eIF2α phosphorylation and ATF4-mediated stress gene induction that promotes global alterations in transcription and translation [159]. Hypoxia-derived ROS via mitochondrial complex III also increases HIF-1α expression [158]. In cardiomyogenesis, oxidative stress plays an important role in signaling events that regulate cardiomyocyte differentiation [160]. These finding suggests hypoxia can alter redox status, which may lead to significant changes in cellular homeostasis and subsequent changes in gene expression.
Although it is unclear what signals mediate the hypoxia-induced repression of cardioprotective genes in utero, induction of stress pathways via oxidative stress may be involved. A recent study in cancer cells demonstrated a link between prolonged oxidative stress and repression of tumor suppressor gene E-cadherin [161]. It was shown that oxidative stress increases Snail expression, which recruits DNA methyltransferease 1 (DNMT1) and histone deacetylase 1 (HDAC1) to methylate E-cadherin promoter thereby resulting in reduce E-cadherin expression. Cardiomyocytes are major producers of ROS due to their high metabolic demand. It has been demonstrated that cardiomyocytes are subjected to increased oxidative stress when exposed to hypoxia [162]. These findings are intriguing, suggesting stress pathways may underline the hypoxia-induced repression of cardioprotective genes (i.e. PKC epsilon) in fetal cardiomyocytes (See Fig. 2).
H9c2 Cell Line
Questions remain as to how prenatal hypoxia can alter the expression of cardioprotective genes (PKCε, HSP70, eNOS etc.) resulting in an increase in susceptibility to I/R injury. The mechanisms behind hypoxia-induced gene silencing in utero are not fully understood and likely involve complex mechanisms. Whether hypoxia, and/or a secondary stress such as increase maternal or fetal glucocortcoids causes long-term gene programming is not known. Elucidating the various molecular mechanism underlying the structure and functional changes in myocardial formation is an area of ongoing research. Since the fetal heart cell plays a central role in cardiogenesis, both structurally and functionally, there is a need for a model that can be manipulated to explore the various molecular events that occur in response to local and systemic changes in the fetal heart. A viable model to study myocardial formation and the effect of stresses (i.e. hypoxia) on embryonic cardiomyocytes is cell culturing using the H9c2 cell line. The clonal cell line H9c2 was derived from embryonic rat heart tissue [163]. It possesses similar shape and structure to immature embryonic heart cells and it retains the electrical and hormone phenotype consistent with adult cardiomyocytes [163]. The H9c2 cell line has been widely used to study a variety of processes in heart tissue including apoptosis, differentiation, and I/R injury [115, 164, 165]. For example, Chong et al. was able to overexpressed Hsp70 in the H9c2 cell line conferring added resistance to thermal killing, hydrogen peroxide, menadione, hydroxyl radical and hypoxia/reoxygenation [166]. Furthermore, cell culturing provides a large number of cells in a relatively short period of time while allowing easier manipulation of genes using techniques that allow gene overexpression or knockdown. Although electrophysically, H9c2 cells and primary culture cardiomyocytes are similar, differences exist phenotypically. Freshly isolated cardiomyocytes display a contractile phenotype and finite level of cell division, whereas H9c2 cells are not contractile and are capable of continuous cell division. In addition, H9c2 cells change phenotype when full confluence is reached in culture. Despite these discrepancies, the H9c2 cell line remains a viable model for studying fetal cardiomyocytes. Taking together, where suitable it is important to verify findings in this cell line with primary cultures from fetal heart cells. The use of the cell line may potentially elucidate mechanisms involved in a wide range of related areas, including the role of hypoxia in fetal heart programming.
CONCLUSION
Until recently, the essential role of low oxygen tension in the developing heart was not well known. Researchers have undoubtedly linked hypoxia and hypoxia-dependent gene expression with proper OFT remodeling and vessel formation. HIF-1 expression is vital for cardiogenesis, but its function is dual in nature in that HIF-1 is capable of upregulating both prosurvival and death genes. It is quite clear, however, that chronically pathophysiological hypoxia causes abnormalities in fetal heart morphology and function. Some aberrations in fetal heart development are not always apparent until the heart is challenged later in life. This proverbial ticking time bomb is programmed in utero as the fetus experiences prolong oxygen insufficiency. Hypoxia-dependent intrauterine programming may involve epigenetic mechanisms that predispose offspring to coronary heart disease. Since HIF-2 plays an important role in stem cell differentiation, and HIF-1 interacts with epigenetic modulators, it is likely that the HIF family of genes is involved in mechanisms that alter myocardial gene expression in long-term. Furthermore, the role of hypoxia induce oxidative stress is intriguing since its can alter genes in the long-term. Identifying the underlying mechanism is an area that warrants further study.
ACKNOWLEDGEMENTS
Studies from the authors’ laboratory presented in this article were supported in part by the United States National Institutes of Health (NIH) grants HL57787, HL67745, HL82779, HL83966, HL89012, HD31226, and by Loma Linda University School of Medicine. Andrew J. Patterson was supported by NIH grant 5R25GM060507. We apologize to all authors whose work could not be cited due to the space limitation.
REFERENCES
- 1.Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res C Embryo Today. 2007;81:215–228. doi: 10.1002/bdrc.20102. [DOI] [PubMed] [Google Scholar]
- 2.Gross MW, Karbach U, Groebe K, Franko AJ, Mueller-Klieser W. Calibration of misonidazole labeling by simultaneous measurement of oxygen tension and labeling density in multicellular spheroids. Int J Cancer. 1995;61:567–573. doi: 10.1002/ijc.2910610422. [DOI] [PubMed] [Google Scholar]
- 3.Raleigh JA, Calkin-Adams DP, Rinker LH, et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765–3768. [PubMed] [Google Scholar]
- 4.Flueck M. Plasticity of the muscle proteome to exercise at altitude. High Alt Med Biol. 2009;10:183–193. doi: 10.1089/ham.2008.1104. [DOI] [PubMed] [Google Scholar]
- 5.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 6.Fisher SA, Burggren WW. Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid Redox Signal. 2007;9:1339–1352. doi: 10.1089/ars.2007.1704. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther. 2008;324:331–341. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds JD, Penning DH, Dexter F, et al. Ethanol increases uterine blood flow and fetal arterial blood oxygen tension in the near-term pregnant ewe. Alcohol. 1996;13:251–256. doi: 10.1016/0741-8329(95)02051-9. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JA, Van Kainen BR. Effects of alcohol on intrauterine oxygen tension in the rat. Alcohol Clin Exp Res. 1992;16:308–310. doi: 10.1111/j.1530-0277.1992.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 10.Jensen A, Garnier Y, Berger R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. Eur J Obstet Gynecol Reprod Biol. 1999;84:155–172. doi: 10.1016/s0301-2115(98)00325-x. [DOI] [PubMed] [Google Scholar]
- 11.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ascuitto RJ, Ross-Ascuitto NT. Substrate metabolism in the developing heart. Semin Perinatol. 1996;20:542–563. doi: 10.1016/s0146-0005(96)80068-1. [DOI] [PubMed] [Google Scholar]
- 13.Compernolle V, Brusselmans K, Franco D, et al. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc Res. 2003;60:569–579. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Sugishita Y, Leifer DW, Agani F, Watanabe M, Fisher SA. Hypoxia-responsive signaling regulates the apoptosis-dependent remodeling of the embryonic avian cardiac outflow tract. Dev Biol. 2004;273:285–296. doi: 10.1016/j.ydbio.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 16.Fishman MC, Chien KR. Fashioning the vertebrate heart: earliest embryonic decisions. Development. 1997;124:2099–2117. doi: 10.1242/dev.124.11.2099. [DOI] [PubMed] [Google Scholar]
- 17.Wikenheiser J, Doughman YQ, Fisher SA, Watanabe M. Differential levels of tissue hypoxia in the developing chicken heart. Dev Dyn. 2006;235:115–123. doi: 10.1002/dvdy.20499. [DOI] [PubMed] [Google Scholar]
- 18.Nanka O, Valasek P, Dvorakova M, Grim M. Experimental hypoxia and embryonic angiogenesis. Dev Dyn. 2006;235:723–733. doi: 10.1002/dvdy.20689. [DOI] [PubMed] [Google Scholar]
- 19.Hoerter JA, Vassort G. Participation of the sarcolemma in the control of relaxation of the mammalian heart during perinatal development. Adv Myocardiol. 1982;3:373–380. doi: 10.1007/978-1-4899-5561-6_37. [DOI] [PubMed] [Google Scholar]
- 20.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol. 2007;102:1130–1142. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph AM. Myocardial growth before and after birth: clinical implications. Acta Paediatr. 2000;89:129–133. doi: 10.1080/080352500750028681. [DOI] [PubMed] [Google Scholar]
- 22.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 23.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 24.Bettencourt-Dias M, Mittnacht S, Brockes JP. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J Cell Sci. 2003;116:4001–4009. doi: 10.1242/jcs.00698. [DOI] [PubMed] [Google Scholar]
- 25.Engel FB, Schebesta M, Duong MT, et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbera A, Giraud GD, Reller MD, et al. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1157–R1164. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- 27.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;292:R913–R919. doi: 10.1152/ajpregu.00484.2006. [DOI] [PubMed] [Google Scholar]
- 28.Kamitomo M, Longo LD, Gilbert RD. Cardiac function in fetal sheep during two weeks of hypoxemia. Am J Physiol. 1994;266:R1778–R1785. doi: 10.1152/ajpregu.1994.266.6.R1778. [DOI] [PubMed] [Google Scholar]
- 29.Breuer E, Barta E, Zlatos L, Pappova E. Developmental changes of myocardial metabolism. II. Myocardial metabolism of fatty acids in the early postnatal period in dogs. Biol Neonat. 1968;12:54–64. [PubMed] [Google Scholar]
- 30.Chapman JD. Hypoxic sensitizers--implications for radiation therapy. N Engl J Med. 1979;301:1429–1432. doi: 10.1056/NEJM197912273012606. [DOI] [PubMed] [Google Scholar]
- 31.Evans SM, Du KL, Chalian AA, et al. Patterns and levels of hypoxia in head and neck squamous cell carcinomas and their relationship to patient outcome. Int J Radiat Oncol Biol Phys. 2007;69:1024–1031. doi: 10.1016/j.ijrobp.2007.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans SM, Jenkins KW, Jenkins WT, et al. Imaging and analytical methods as applied to the evaluation of vasculature and hypoxia in human brain tumors. Radiat Res. 2008;170:677–690. doi: 10.1667/RR1207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koch CJ, Evans SM. Non-invasive PET and SPECT imaging of tissue hypoxia using isotopically labeled 2-nitroimidazoles. Adv Exp Med Biol. 2003;510:285–292. doi: 10.1007/978-1-4615-0205-0_47. [DOI] [PubMed] [Google Scholar]
- 34.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 36.Ward J. Oxygen sensors in context. Biochim Biophys Acta. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 38.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 39.Marzo F, Lavorgna A, Coluzzi G, et al. Erythropoietin in heart and vessels: focus on transcription and signalling pathways. J Thromb Thrombolysis. 2008;26:183–187. doi: 10.1007/s11239-008-0212-3. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe M, Jafri A, Fisher SA. Apoptosis is required for the proper formation of the ventriculo-arterial connections. Dev Biol. 2001;240:274–288. doi: 10.1006/dbio.2001.0466. [DOI] [PubMed] [Google Scholar]
- 41.Barbosky L, Lawrence DK, Karunamuni G, et al. Apoptosis in the developing mouse heart. Dev Dyn. 2006;235:2592–2602. doi: 10.1002/dvdy.20885. [DOI] [PubMed] [Google Scholar]
- 42.Druyan S, Cahaner A, Ashwell CM. The expression patterns of hypoxia-inducing factor subunit alpha-1, heme oxygenase, hypoxia upregulated protein 1, and cardiac troponin T during development of the chicken heart. Poult Sci. 2007;86:2384–2389. doi: 10.3382/ps.2007-00152. [DOI] [PubMed] [Google Scholar]
- 43.Duranteau J, Chandel NS, Kulisz A, et al. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 44.Lee YM, Jeong CH, Koo SY, et al. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 45.Sugishita Y, Watanabe M, Fisher SA. Role of myocardial hypoxia in the remodeling of the embryonic avian cardiac outflow tract. Dev Biol. 2004;267:294–308. doi: 10.1016/j.ydbio.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 47.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks' gestation. Am J Obstet Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 48.Soothill PW, Nicolaides KH, Rodeck CH, Campbell S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther. 1986;1:168–175. doi: 10.1159/000262264. [DOI] [PubMed] [Google Scholar]
- 49.Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod. 2010;16:415–431. doi: 10.1093/humupd/dmp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martyn U, Schulte-Merker S. Zebrafish neuropilins are differentially expressed and interact with vascular endothelial growth factor during embryonic vascular development. Dev Dyn. 2004;231:33–42. doi: 10.1002/dvdy.20048. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein BM, Lawson ND. Arteries, veins, Notch, and VEGF. Cold Spring Harb Symp Quant Biol. 2002;67:155–162. doi: 10.1101/sqb.2002.67.155. [DOI] [PubMed] [Google Scholar]
- 54.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 55.Josko J, Gwozdz B, Jedrzejowska-Szypulka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6:1047–1052. [PubMed] [Google Scholar]
- 56.Roskoski R., Jr VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375:287–291. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 57.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 58.Tomanek RJ, Lotun K, Clark EB, Suvarna PR, Hu N. VEGF and bFGF stimulate myocardial vascularization in embryonic chick. Am J Physiol. 1998;274:H1620–H1626. doi: 10.1152/ajpheart.1998.274.5.H1620. [DOI] [PubMed] [Google Scholar]
- 59.Tomanek RJ, Ratajska A, Kitten GT, Yue X, Sandra A. Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev Dyn. 1999;215:54–61. doi: 10.1002/(SICI)1097-0177(199905)215:1<54::AID-DVDY6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 60.Tomanek RJ, Lund DD, Yue X. Hypoxic induction of myocardial vascularization during development. Adv Exp Med Biol. 2003;543:139–149. doi: 10.1007/978-1-4419-8997-0_10. [DOI] [PubMed] [Google Scholar]
- 61.Giordano FJ, Gerber HP, Williams SP, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci USA. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeuchi S, Takeda K, Oishi I, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 63.Yue X, Tomanek RJ. Stimulation of coronary vasculogenesis/angiogenesis by hypoxia in cultured embryonic hearts. Dev Dyn. 1999;216:28–36. doi: 10.1002/(SICI)1097-0177(199909)216:1<28::AID-DVDY5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 64.Lainakis G, Bamias A. Targeting angiogenesis in renal cell carcinoma. Curr Cancer Drug Targets. 2008;8:349–358. doi: 10.2174/156800908785133132. [DOI] [PubMed] [Google Scholar]
- 65.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 68.Edelberg JM, Aird WC, Wu W, et al. PDGF mediates cardiac microvascular communication. J Clin Invest. 1998;102:837–843. doi: 10.1172/JCI3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H, Fisher SA. Hypoxia-inducible transcription factor-1alpha triggers an autocrine survival pathway during embryonic cardiac outflow tract remodeling. Circ Res. 2008;102:1331–1339. doi: 10.1161/CIRCRESAHA.107.167858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semenza GL, Agani F, Iyer N, et al. Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann N Y Acad Sci. 1999;874:262–268. doi: 10.1111/j.1749-6632.1999.tb09241.x. [DOI] [PubMed] [Google Scholar]
- 71.Galanis A, Pappa A, Giannakakis A, Lanitis E, Dangaj D, Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008;266:12–20. doi: 10.1016/j.canlet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 72.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cowden Dahl KD, Fryer BH, Mack FA, et al. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Covello KL, Kehler J, Yu H, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 77.Wiesener MS, Jurgensen JS, Rosenberger C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 78.Stroka DM, Burkhardt T, Desbaillets I, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 79.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc Natl Acad Sci. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- 81.Ladoux A, Frelin C. Cardiac expressions of HIF-1 alpha and HLF/EPAS, two basic loop helix/PAS domain transcription factors involved in adaptative responses to hypoxic stresses. Biochem Biophys Res Commun. 1997;240:552–556. doi: 10.1006/bbrc.1997.7708. [DOI] [PubMed] [Google Scholar]
- 82.Tillmanns J, Rota M, Hosoda T, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1alpha-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics. 2005;23:79–88. doi: 10.1152/physiolgenomics.00279.2004. [DOI] [PubMed] [Google Scholar]
- 84.Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 85.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Graham RM, Frazier DP, Thompson JW, et al. A unique pathway of cardiac myocyte death caused by hypoxia-acidosis. J Exp Biol. 2004;207:3189–3200. doi: 10.1242/jeb.01109. [DOI] [PubMed] [Google Scholar]
- 87.Chen W, Ostrowski RP, Obenaus A, Zhang JH. Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury. Exp Neurol. 2009;216:7–15. doi: 10.1016/j.expneurol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998 Suppl 27:25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 89.Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 90.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public health. 1997;87:1003–1007. doi: 10.2105/ajph.87.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Oxygen transport in tibetan women during pregnancy at 3,658 m. Am J Phys Anthropol. 2001;114:42–53. doi: 10.1002/1096-8644(200101)114:1<42::AID-AJPA1004>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 92.Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol. 2003;4:141–156. doi: 10.1089/152702903322022767. [DOI] [PubMed] [Google Scholar]
- 93.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barker DJ. Outcome of low birthweight. Horm Res. 1994;42:223–230. doi: 10.1159/000184197. [DOI] [PubMed] [Google Scholar]
- 95.Barker DJ. Fetal nutrition and cardiovascular disease in later life. Br Med Bull. 1997;53:96–108. doi: 10.1093/oxfordjournals.bmb.a011609. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Blackburn ST. Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective. 3rd ed. Maryland Heights, MO: Elsevier Science; 2007. [Google Scholar]
- 98.Lueder FL, Kim SB, Buroker CA, Bangalore SA, Ogata ES. Chronic maternal hypoxia retards fetal growth and increases glucose utilization of select fetal tissues in the rat. Metabolism. 1995;44:532–537. doi: 10.1016/0026-0495(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 99.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol. 2008;292:583–595. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma SK, Lucitti JL, Nordman C, Tinney JP, Tobita K, Keller BB. Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr Res. 2006;59:116–120. doi: 10.1203/01.pdr.0000191579.63339.90. [DOI] [PubMed] [Google Scholar]
- 101.Martin C, Yu AY, Jiang BH, et al. Cardiac hypertrophy in chronically anemic fetal sheep: Increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. Am J Obstet Gynecol. 1998;178:527–534. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- 102.Val'kovich EI, Molchanova VV, Davydova MK, Davydova OK. [Changes in the myocardium of fetuses and newborn infants as a result of hypoxia] Arkh Anat Gistol Embriol. 1986;90:35–39. [PubMed] [Google Scholar]
- 103.Xiao D, Ducsay CA, Zhang L. Chronic hypoxia and developmental regulation of cytochrome c expression in rats. J Soc Gynecol Investig. 2000;7:279–283. [PubMed] [Google Scholar]
- 104.Thornburg KL. Fetal response to intrauterine stress. Ciba Found Symp. 1991;156:17–29. doi: 10.1002/9780470514047.ch3. discussion 29–37. [DOI] [PubMed] [Google Scholar]
- 105.Sedmera D, Kucera P, Raddatz E. Developmental changes in cardiac recovery from anoxia-reoxygenation. Am J Physiol Regul Integr Comp Physiol. 2002;283:R379–R388. doi: 10.1152/ajpregu.00534.2001. [DOI] [PubMed] [Google Scholar]
- 106.Portbury AL, Chandra R, Groelle M, et al. Catecholamines act via a beta-adrenergic receptor to maintain fetal heart rate and survival. Am J Physiol Heart Circ Physiol. 2003;284:2069–2077. doi: 10.1152/ajpheart.00588.2002. [DOI] [PubMed] [Google Scholar]
- 107.Kamitomo M, Longo LD, Gilbert RD. Cardiac function in fetal sheep during two weeks of hypoxemia. Am J Physiol. 1994;266:R1778–R1785. doi: 10.1152/ajpregu.1994.266.6.R1778. [DOI] [PubMed] [Google Scholar]
- 108.Kamitomo M, Onishi J, Gutierrez I, Stiffel VM, Gilbert RD. Effects of long-term hypoxia and development on cardiac contractile proteins in fetal and adult sheep. J Soc Gynecol Investig. 2002;9:335–341. [PubMed] [Google Scholar]
- 109.Gilbert RD. Fetal myocardial responses to long-term hypoxemia. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:669–674. doi: 10.1016/s1095-6433(98)01003-4. [DOI] [PubMed] [Google Scholar]
- 110.Browne VA, Stiffel VM, Pearce WJ, Longo LD, Gilbert RD. Activator calcium and myocardial contractility in fetal sheep exposed to long-term high-altitude hypoxia. Am J Physiol. 1997;272:H1196–H1204. doi: 10.1152/ajpheart.1997.272.3.H1196. [DOI] [PubMed] [Google Scholar]
- 111.Onishi J, Browne VA, Kono S, Stiffel VM, Gilbert RD. Effects of long-term high-altitude hypoxia and troponin I phosphorylation on cardiac myofilament calcium responses in fetal and nonpregnant sheep. J Soc Gynecol Investig. 2004;11:1–8. doi: 10.1016/j.jsgi.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 112.Tintu A, Rouwet E, Verlohren S, et al. Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long-term consequences. PLoS ONE. 2009;4:e5155. doi: 10.1371/journal.pone.0005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghatpande SK, Billington CJ, Jr, Rivkees SA, Wendler CC. Hypoxia induces cardiac malformations via A1 adenosine receptor activation in chicken embryos. Birth Defects Res A Clin Mol Teratol. 2008;82:121–130. doi: 10.1002/bdra.20438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sarre A, Maury P, Kucera P, Kappenberger L, Raddatz E. Arrhythmogenesis in the developing heart during anoxia-reoxygenation and hypothermia-rewarming: an in vitro model. J Cardiovasc Electrophysiol. 2006;17:1350–1359. doi: 10.1111/j.1540-8167.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 115.Graf AV, Maslova MV, Maklakova AS, et al. Effect of hypoxia during early organogenesis on cardiac activity and noradrenergic regulation in the postnatal period. Bull Exp Biol Med. 2006;142:543–545. doi: 10.1007/s10517-006-0412-9. [DOI] [PubMed] [Google Scholar]
- 116.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 117.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–50. discussion 50-35. [PubMed] [Google Scholar]
- 119.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 120.Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001;81:1461–1497. doi: 10.1152/physrev.2001.81.4.1461. [DOI] [PubMed] [Google Scholar]
- 121.Sharp BR, Jones SP, Rimmer DM, Lefer DJ. Differential response to myocardial reperfusion injury in eNOS-deficient mice. Am J Physiol Heart Circ Physiol. 2002;282:H2422–H2426. doi: 10.1152/ajpheart.00855.2001. [DOI] [PubMed] [Google Scholar]
- 122.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 123.Okamura T, Miura T, Takemura G, et al. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res. 2000;45:642–650. doi: 10.1016/s0008-6363(99)00271-0. [DOI] [PubMed] [Google Scholar]
- 124.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 125.Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol. 2004;286:1717–1719. doi: 10.1152/ajpheart.00898.2003. [DOI] [PubMed] [Google Scholar]
- 126.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: Role of PKC{epsilon} J Pharmacol Exp Ther. 2009;330:624–632. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS. Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovasc Res. 2002;55:672–680. doi: 10.1016/s0008-6363(02)00325-5. [DOI] [PubMed] [Google Scholar]
- 128.Ping P, Song C, Zhang J, et al. Formation of protein kinase C(epsilon)-Lck signaling modules confers cardioprotection. J Clin Invest. 2002;109:499–507. doi: 10.1172/JCI13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 130.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70:222–230. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 131.Netuka I, Szarszoi O, Maly J, et al. Effect of perinatal hypoxia on cardiac tolerance to acute ischaemia in adult male and female rats. Clin Exp Pharmacol Physiol. 2006;33:714–719. doi: 10.1111/j.1440-1681.2006.04423.x. [DOI] [PubMed] [Google Scholar]
- 132.Mao C, Hou J, Ge J, et al. Changes of Renal AT(1)/AT(2) Receptors and Structures in Ovine Fetuses following Exposure to Long-Term Hypoxia. Am J Nephrol. 2009;31:141–150. doi: 10.1159/000259901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1707–R1714. doi: 10.1152/ajpregu.00343.2005. [DOI] [PubMed] [Google Scholar]
- 134.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 135.McKenna ES, Roberts CW. Epigenetics and cancer without genomic instability. Cell Cycle. 2009;8:23–26. doi: 10.4161/cc.8.1.7290. [DOI] [PubMed] [Google Scholar]
- 136.Hirst M, Marra MA. Epigenetics and human disease. Int J Biochem Cell Biol. 2009;41:136–146. doi: 10.1016/j.biocel.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 137.Delcuve GP, Rastegar M, Davie JR. Epigenetic control. J Cell Physiol. 2009;219:243–250. doi: 10.1002/jcp.21678. [DOI] [PubMed] [Google Scholar]
- 138.Zhang H, Meyer KD, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod. 2009;80:440–448. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCvarepsilon gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47:352–358. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bae S, Gilbert RD, Ducsay CA, Zhang L. Prenatal cocaine exposure increases heart susceptibility to ischaemia-reperfusion injury in adult male but not female rats. J Physiol. 2005;565:149–158. doi: 10.1113/jphysiol.2005.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meyer KD, Zhang H, Zhang L. Prenatal cocaine exposure abolished ischemic preconditioning-induced protection in adult male rat hearts: role of PKC{varepsilon} Am J Physiol Heart Circ Physiol. 2009;296:H1566–H1576. doi: 10.1152/ajpheart.00898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu H, Sun YE. Epigenetic regulation of stem cell differentiation. Pediatr Res. 2006;59:21R–25R. doi: 10.1203/01.pdr.0000203565.76028.2a. [DOI] [PubMed] [Google Scholar]
- 143.Maltepe E, Krampitz GW, Okazaki KM, et al. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- 144.Scholer HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki N, Rohdewohld H, Neuman T, Gruss P, Scholer HR. Oct-6: a POU transcription factor expressed in embryonal stem cells and in the developing brain. EMBO J. 1990;9:3723–3732. doi: 10.1002/j.1460-2075.1990.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 147.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 148.Gordan JD, Lal P, Dondeti VR, et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem. 2008;283:36542–36552. doi: 10.1074/jbc.M804578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wellmann S, Bettkober M, Zelmer A, et al. Hypoxia upregulates the histone demethylase JMJD1A via HIF-1. Biochem Biophys Res Commun. 2008;372:892–897. doi: 10.1016/j.bbrc.2008.05.150. [DOI] [PubMed] [Google Scholar]
- 151.Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res. 2006;66:9009–9016. doi: 10.1158/0008-5472.CAN-06-0101. [DOI] [PubMed] [Google Scholar]
- 152.Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 153.Granger A, Abdullah I, Huebner F, et al. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He M, Vanaja DK, Karnes RJ, Young CY. Epigenetic regulation of Myc on retinoic acid receptor beta and PDLIM4 in RWPE1 cells. Prostate. 2009;69:1643–1650. doi: 10.1002/pros.21013. [DOI] [PubMed] [Google Scholar]
- 155.Brenner C, Deplus R, Didelot C, et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005;24:336–346. doi: 10.1038/sj.emboj.7600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 158.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 159.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283:31153–31162. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sauer H, Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423–1434. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- 161.Lim SO, Gu JM, Kim MS, et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology. 2008;135:2128–2140. doi: 10.1053/j.gastro.2008.07.027. 2140.e1–8. [DOI] [PubMed] [Google Scholar]
- 162.Mansfield KD, Simon MC, Keith B. Hypoxic reduction in cellular glutathione levels requires mitochondrial reactive oxygen species. J Appl Physiol. 2004;97:1358–1366. doi: 10.1152/japplphysiol.00449.2004. [DOI] [PubMed] [Google Scholar]
- 163.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 164.Coles JG, Boscarino C, Takahashi M, et al. Cardioprotective stress response in the human fetal heart. J Thorac Cardiovasc Surg. 2005;129:1128–1136. doi: 10.1016/j.jtcvs.2004.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hwang JM, Weng YJ, Lin JA, et al. Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol Cell Biochem. 2008;311:179–187. doi: 10.1007/s11010-008-9708-6. [DOI] [PubMed] [Google Scholar]
- 166.Chong KY, Lai CC, Lille S, Chang C, Su CY. Stable overexpression of the constitutive form of heat shock protein 70 confers oxidative protection. J Mol Cell Cardiol. 1998;30:599–608. doi: 10.1006/jmcc.1997.0623. [DOI] [PubMed] [Google Scholar]