Abstract
A group of 48 men (22 aged 65–75 years, 26 aged 80–90 years) and 59 women (32 aged 65–75 years, 27 aged 80–90 years) were enrolled in the Age, Gene/Environment Susceptibility-Reykjavik study and imaged with in-vivo volumetric Quantitative Computed Tomography (QCT) to investigate the effects of age and sex on femoral neck structure and strength. Femoral neck cross-sectional moment of inertia for bending directions near those of standing and walking (IAP), bending strength (My), and axial compressive strength (Fy) were computed at the location of minimum cross-sectional area (minCSA). Local cortical thickness was computed in the inferior femoral neck based on density profiles extending through the cortex of the minCSA femoral neck section. Multivariate models accounting for height, weight, and age group (younger or older) showed that men had a 46% higher My and a 23% higher Fy than women, while women had a 13% thicker inferior cortex than men. Cortical thickness in the inferoposterior region of the femoral neck was significantly related to bending and axial strength after adjusting for overall volumetric bone mineral density. Both minCSA and IAP were higher in the older, gender-pooled age group, but Fy and My did not differ between the two age groups. The results suggest that age-related expansion of the femoral neck primarily occurs in the superior and inferior directions and helps maintain homeostasis of femoral neck stiffness and strength. The higher bending strength of the male femoral neck may partly explain why elderly men have a lower risk of hip fracture than elderly women.
Keywords: bone QCT, biomechanics, bone geometry, aging, osteoporosis
1. Introduction
Approximately half of all osteoporotic hip fractures occur at the femoral neck [1]. The structure of the femoral neck, including its size and geometry, partly determines its physical strength and whether a neck fracture will occur under mechanical loads of sufficient magnitude. To better understand the differential effects of age and sex on the structure of the proximal femur in an aging population, our research group has previously employed quantitative computed tomography (QCT) to analyze the volumetric bone mineral density (vBMD), cortical thickness, bending strength, and compressive strength of the femoral neck and shaft in women [2] and in men and women enrolled in the Age, Gene/Environment Susceptibility-Reykjavik (AGES-REYKJAVIK) study [3]. These previous results revealed important sex-based differences in femoral neck structure and suggested that the increase in cross-sectional area of the femoral neck that occurs with age may have the effect of maintaining homeostasis of femoral neck stiffness as trabecular bone is lost.
The structure of the femoral neck is known to change with age [3–8]. Current understanding of bone functional adaptation suggests that age-related changes in the density and cross-sectional area of the femoral neck are in fact closely related. A loss of trabecular bone with age causes femoral neck stiffness to decrease, leading to elevated strains at the periosteal surface during habitual activities such as walking. Bone cells respond to these increased strains by adding new bone tissue at the periosteal surface, maintaining homeostasis of femoral neck stiffness. The result is an ongoing increase in femoral neck size with declining trabecular vBMD. It is unproven whether periosteal expansion occurs equally around the perimeter of the femoral neck or if bone is added in such a way that strains are reduced for a limited number of loading directions. We hypothesize that bone is primarily added at the superior and inferior femoral neck, where stimulating strains are expected to be highest during habitual activities like standing and walking.
Femoral neck structure also differs between men and women. Elderly male femoral necks have a larger cross-sectional area than those in elderly women, and these disparities increase with age [3, 7]. Body mass and height account for a large part of these sex-based differences, but male bones appear to be larger and stronger for a given body mass or muscle size [9, 10]. Measurements of cortical thickness made using digitized histological samples have also suggested differences between the femoral necks of males and females. In a study of 9 male and 10 female femoral necks, the cortex in the inferior region of the femoral neck was slightly thicker in females than in males, while the cortex in the superior region of the femoral neck was slightly thicker in males than in females [11]. These differences were not statistically significant, most likely because of the small sample size. If the spatial distribution of cortical thickness around the perimeter of the femoral neck does in fact differ between males and females, this fact may have important implications in understanding the different effects of falling direction on femoral neck strength in men and women.
To provide a more detailed understanding of the effects of age and sex on femoral neck size, cortical thickness, bending strength, and axial compressive strength in the elderly population, in the current study we used in vivo QCT imaging of the hip in a sample of men and women enrolled in the AGES-REYKJAVIK study. We sought to determine the magnitude of age and sex-based differences in explicit estimates of femoral neck strength, as opposed to surrogates for bone strength such as BMD or section modulus. The effects of different loading directions on femoral neck bending strength were included in the analysis, and we also examined the effects of age-related periosteal expansion on the bending resistance of the femoral neck in different planes of bending.
2. Material and Methods
2.1 Subjects
A subset of 107 men and women enrolled in the AGES-REYKJAVIK study, an ongoing population-based study of Icelandic men and women [3], were included in the current study. Informed consent was obtained from all participants in the study, which was approved (VSN 00-063) by the National Bioethics Committee in Iceland, the Institutional Review Board of the Intramural Research Program of the National Institute on Aging, and the Committee on Human Research at the University of California, San Francisco. Subjects were randomly selected from the overall AGES-REYKJAVIK cohort to fall within two decadal age groups at the youngest and oldest margins: 65–75 years old and 80–90 years old. The younger group was composed of 26 men and 32 women and the older group composed of 22 men and 27 women. Subjects were selected to exclude those who were currently using medications known to affect bone mineral status, including estrogen replacement therapy, tibolonum, antiepileptics, systemic glucocorticoids and agents for the treatment of osteoporosis (raloxifen, calcitonin or bisphosphonates). No subjects had a history of hip fracture. Subject characteristics are listed in Table 1.
Table 1.
Age, height, and weight of subjects included in the study.
| Females (n = 59) |
Males (n = 48) |
65–75 years old (n=58, 32 females) |
80–90 years old (n = 49, 27 females) |
|
|---|---|---|---|---|
| Age (years) | 76 ± 7 | 77 ± 6 | 71 ± 3 | 83 ± 2 |
| Height (cm) | 162 ± 6 | 175 ± 7 | 170 ± 9 | 165 ± 8 |
| Weight (kg) | 71 ± 11 | 80 ± 15 | 79 ± 15 | 70 ± 11 |
2.2 Imaging
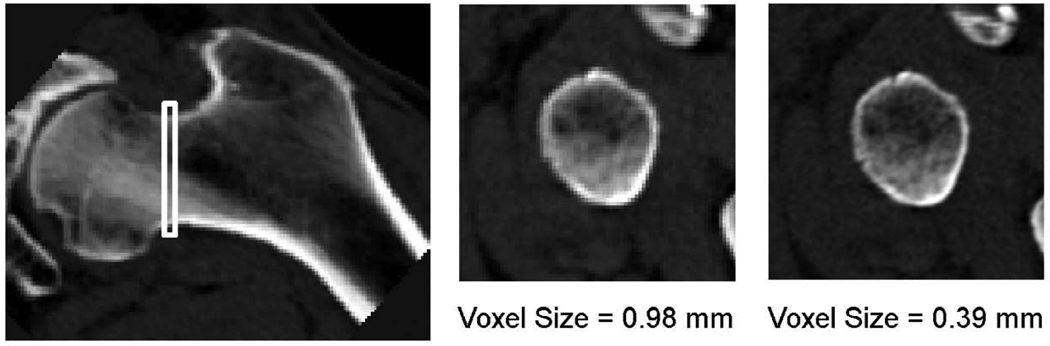
Images of both hips of each subject were obtained on a 4-detector CT system (Sensation, Siemens Medical Systems, Erlangen, Germany). A region extending from a point 1 cm superior to the acetabulum to 3–5 mm distal to the lesser trochanter was imaged using a scanning field of view of 50 cm, 120 kVp, 140 mAs, 1-mm slice thickness, pitch = 1. A solid calcium hydroxyapatite QCT calibration phantom (Image Analysis, Inc., Columbia, KY, USA) was scanned along with each subject and appeared in each image slice. A standard resolution image (0.98-mm in-plane voxel size, 1-mm slice thickness) was reconstructed using the standard kernel with a 50-cm reconstruction field of view (RFOV), and a magnified image (0.39-mm in-plane voxel size, 1-mm slice thickness) centered on the left hip was reconstructed using the standard kernel with a 20-cm RFOV (Figure 1).
Figure 1.
Femoral neck cross-sections at the location of minCSA (indicated by white box on the coronal image on the left) were analyzed. Images were reconstructed with two different RFOV: 50 cm, resulting in 0.98-mm voxels (center); and 20 cm, resulting in 0.39-mm voxels (right).
2.3 Image Analysis
The neck axis of the left femur in each standard-resolution image was defined in the axial and coronal planes using AVS5 image processing software (AVS, Waltham, MA, USA), and the image was then digitally rotated and resliced to obtain cross-sectional slices of the left femoral neck with 0.98-mm isometric voxels [12]. The corresponding magnified image was reformatted using the same set of rotations calculated for the standard resolution image, producing a set of femoral neck cross-sectional slices with 0.39-mm isometric voxels. The proximal femur in both images was segmented from the surrounding tissue using a threshold-driven region growing algorithm. Density and structure measurements were then made using both the standard resolution image and the magnified image.
The cross-sectional area (CSA) of each section was computed by summing the area of all voxels contained within the periosteal margin, and the section of minimum cross-sectional area (minCSA) was isolated for analysis. Voxels containing a large proportion of fatty marrow have negative equivalent hydroxyapatite concentration, and these voxels were excluded from further analyses. The mean vBMD of each minCSA slice was computed by summing the bone mineral content (BMC, equivalent hydroxyapatite concentration multiplied by voxel volume) of each voxel with a positive hydroxyapatite concentration and then dividing by the total volume of those voxels. The vBMD measurement therefore included both trabecular and cortical bone. The cross-sectional moment of inertia about the anterior-posterior axis (IAP) and the cross-sectional moment of inertia about the inferior-superior axis (IIS) were also computed using these voxels [13].
To compute the equivalent compressive strength and bending strength of the minCSA section, first each voxel’s hydroxyapatite concentration was used to compute the equivalent Young’s modulus (material stiffness) using a series of conversions:
| (1) |
| (2) |
| (3) |
and
| (4) |
where ρHA,L = equivalent liquid K2HPO4 concentration, ρHA,S = solid calcium hydroxyapatite concentration, ρ = apparent density, and E = Young’s modulus [14–16]. Using engineering composite beam theory, the yield moment (My, the pure bending moment that would induce irreversible damage in either compression or tension) was computed for 360 different bending directions based on the spatial distribution of E values about the effective centroid of the minCSA section [13]. The average (My,avg), maximum (My,max), and minimum (My,min) values of My over the range of different bending directions were recorded. The compressive yield force (Fy, the magnitude of a force applied to the femoral head along the femoral neck axis that would induce irreversible compressive damage) was computed by multiplying the section’s axial rigidity [13] by 0.0085, the compressive yield strength of trabecular bone in the femoral neck [17].
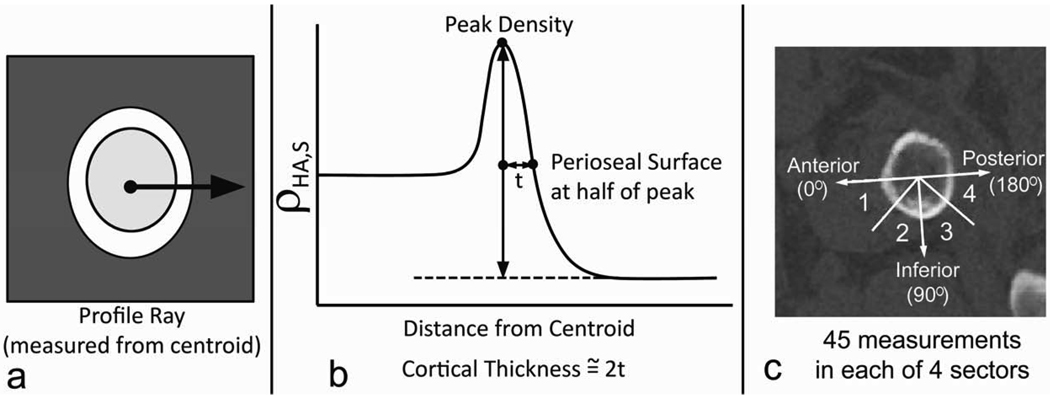
Cortical thickness at 180 different locations around the inferior aspect of the femoral neck cortex was computed using a method based on that of Prevrhal et al. [18] and Yang et al. [19]. We focused our measurements on the inferior aspect of the femoral neck, where the cortical thickness spans a minimum of approximately 3 voxels. Each cortical thickness measurement was made based on the profile of ρHA,S values along a ray extending from the area centroid of the minCSA section through the cortex and into the surrounding tissue (Figure 2a). The periosteal margin of the cortex was identified as the point on the profile halfway between the peak ρHA,S value and the mean ρHA,S value in the soft tissue outside the bone. The cortical thickness was then estimated by doubling the distance between the peak and the periosteal margin (Figure 2b). This measurement assumes that, due to partial volume effects at the periosteal and endosteal surfaces, the peak ρHA,S in the profile will be approximately at the midpoint of the profile extending through the cortex. To summarize the thickness measurements, the inferior half of the minCSA section was divided into 4 sectors evenly spaced within the principal axes of the section, and the mean thickness (t1, t2, t3, and t4) of the 45 measurements in each sector was recorded (Figure 2c).
Figure 2.
Cortical thickness measurement method. a. The ρHA,S profile along a ray extending from the area centroid into the surrounding soft tissue was recorded. b. The distance between the location of the peak ρHA,S value and the location of the half-maximum on the periosteal margin was doubled to arrive at the cortical thickness measurement. c. A total of 45 measurements were made to compute the mean thickness in each of the sectors in the inferior half of the femoral neck section. Sectors were spaced in 45-degree increments starting with the principal axes in the anterior direction. The axes labeled in the figure correspond to anterior (0°) inferior (90°), and posterior (180°) directions.
2.4 Statistical Analysis
Statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC). Least-squares mean values of each measured parameter (vBMD, minCSA, IAP, IIS, My,avg, My,max, My,min, Fy, and t1—t4) were compared between age groups and between men and women using generalized linear models with adjustment of height, weight, age, sex and adjustment of intrasubject correlation between thickness measurements in different sectors using the random effect of subjects. To examine the effect of age group on measured parameters, men and women were grouped together, and the linear model was corrected for sex. To examine the effect of sex on measured parameters, the older and younger age groups were combined, and the generalized linear model was corrected for age. Tukey-Kramer p-values were used to adjust for multiple comparisons. To further investigate the possibility of different effects of cortical thickness in men and women, multiple linear regression models were used to test the effects of vBMD and t1–t8 on My,avg, My,max, My,min, and Fy. A stepwise selection determined the final least parsimonious model. To evaluate the precision of the measurements used in the study, a set of 10 paired repeat QCT hip images obtained in a previous study in our laboratory [20] were analyzed. The short-term precision error of repeat measures was calculated as the root-mean-square average of the standard deviations of the repeat measures, and the coefficients of variation were also calculated as recommended by Glüer et al. [21].
3. Results
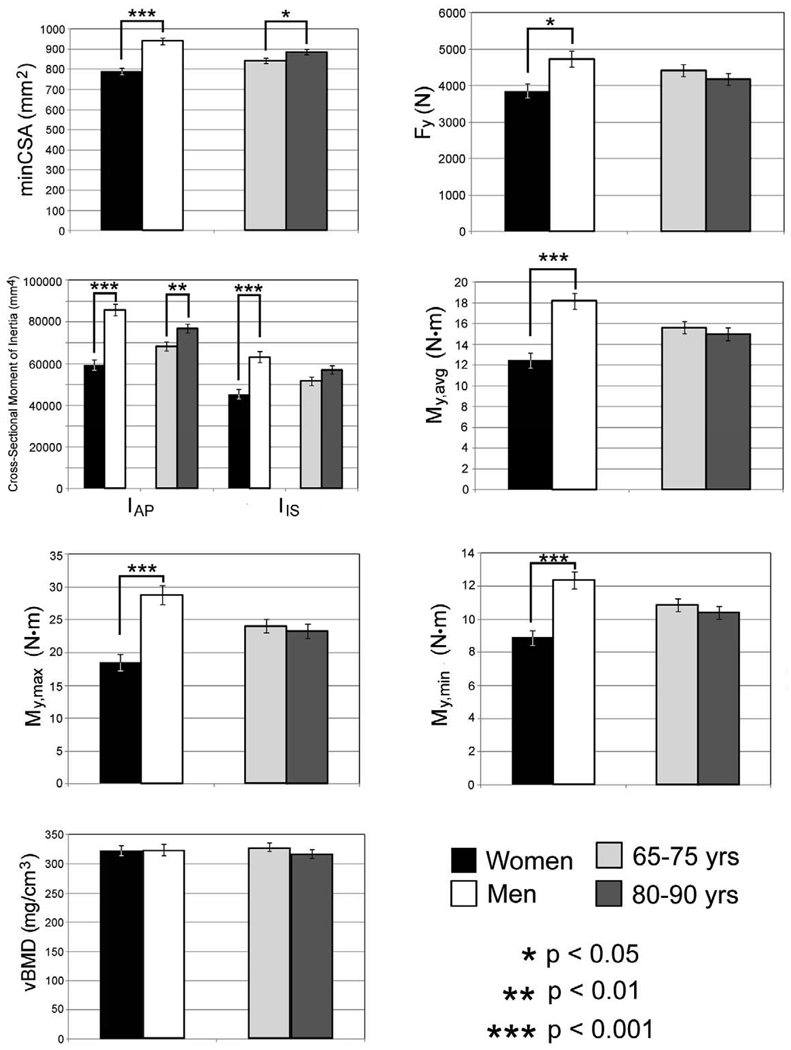
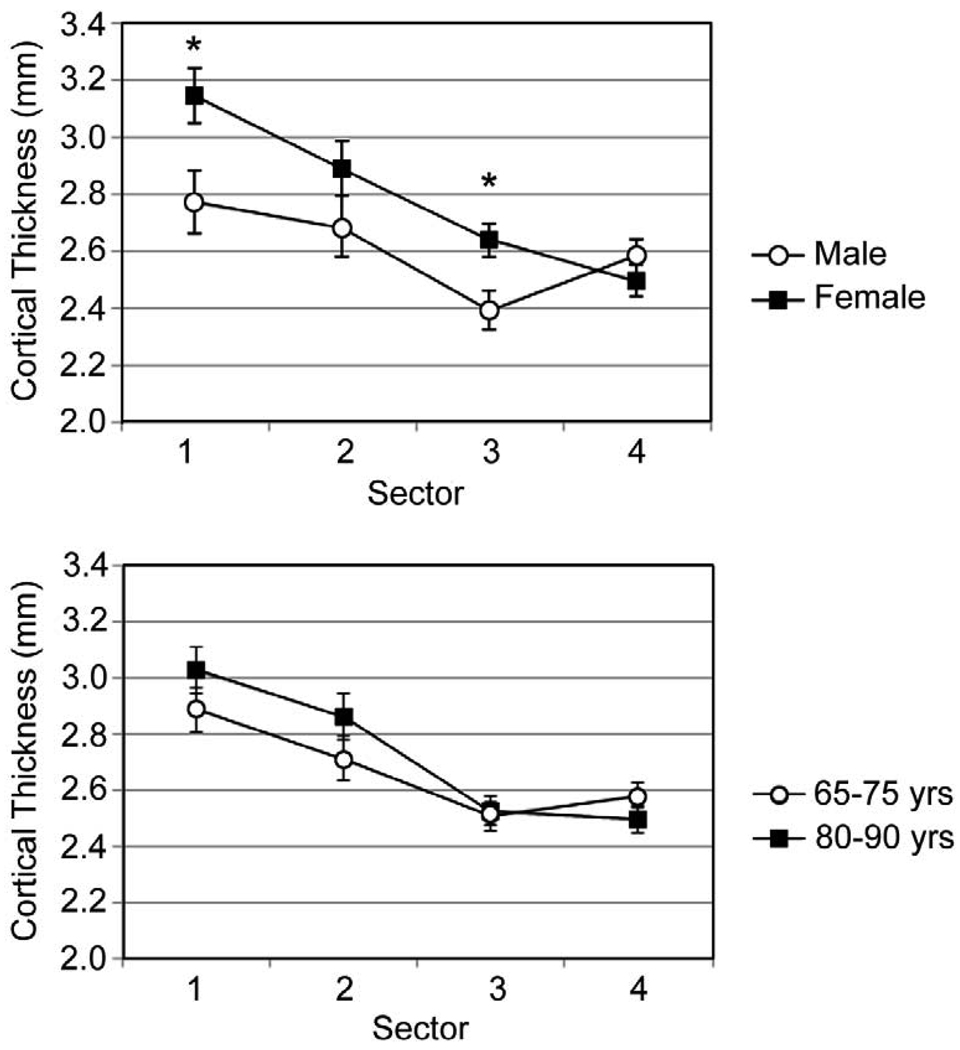
Measurements of vBMD in the minCSA femoral neck slice did not differ significantly (p = 0.96) between men and women, but all other sectional properties had significant sex-based differences (Figure 3). The femoral neck had a larger (p < 0.001) minCSA in men after correction for height, weight, and age, and the men’s larger femoral necks also had higher predicted values of Fy (p < 0.05), My,avg (p < 0.001), My,max (p < 0.001), and My,min (p < 0.001). The femoral neck cortex was found to be significantly (p < 0.05) thicker in women in the inferoanterior (t1) and inferoposterior (t3) compartments (Figure 4).
Figure 3.
Least-squares means and standard errors for femoral neck sectional properties.
Figure 4.
Cortical thickness measurements in the inferior femoral neck for male/female grouping (top) and age-based grouping (bottom).
Measurements of vBMD did not differ significantly (p = 0.32) between the two age groups (Figure 3). The geometric sectional properties of the femoral neck differed significantly between the two age groups, with a higher (p < 0.05) minCSA and a higher (p < 0.001) IAP in the older (80–90 years) age group. No significant difference in IIS was detected between the two age groups, although there was a trend toward a larger IIS in the older age group (p = 0.07). The larger size (as indicated by minCSA) and location of more bone tissue further from the AP axis (as indicated by IAP) did not translate into greater strength of the femoral neck, because no age-related differences in Fy (p = 0.46), My,avg (p = 0.47), My,max (p = 0.60), or My,min (p = 0.40) were detected. No age-related difference in cortical thickness was detected in any of the sectors.
Multiple linear regression models showed that My,avg and Fy were chiefly determined by vBMD (Table 3). Cortical thickness in sector 4 (the inferoposterior region) was significantly and positively related to bending and compressive strength after correction for vBMD (Table 3). Measurements of vBMD in women were significantly related to t2 (p < 0.0001), and measurements of vBMD in males were significantly related to t1 (p < 0.02). Results for the regression models suggested that Fy and My,avg were largely determined by sex, vBMD, and t4 (Tables 3 and 4).
Table 3.
Regression model r2 and p-values for models using vBMD and t1–t4 to predict Fy and My,avg.
| Whole Model | p-values for Model Effects | ||||||
|---|---|---|---|---|---|---|---|
| r2 | p | vBMD | t1 | t2 | t3 | t4 | |
| Fy | 0.51 | <0.0001 | <0.0001 | 0.727 | 0.958 | 0.764 | 0.013 |
| My,avg | 0.21 | 0.0002 | <0.0001 | 0.951 | 0.233 | 0.546 | 0.023 |
Table 4.
Regression model r2 and p-value for models using sex, age group, body mass, and height to predict Fy and My,avg.
| Whole Model | p-values for Model Effects | |||||
|---|---|---|---|---|---|---|
| r2 | p | Sex | Age Group | Body Mass | Height | |
| Fy | 0.19 | <0.0001 | 0.013 | 0.338 | 0.655 | 0.802 |
| My,avg | 0.49 | <0.0001 | <0.0001 | 0.467 | 0.601 | 0.138 |
The short-term CV values of sectional properties ranged from 1.8% (for minCSA) to 7.3% (for My,max) (Table 2). The CV values for cortical thickness measurements ranged from 7.9% to 15.3%. The precision error for the thickness measurements was 0.2 mm to 0.6 mm, which amounts to a precision of roughly 1–2 pixels.
Table 2.
Short-term measurement precision errors and coefficients of variation (CV).
| Measurement | Precision Error | CV (%) |
|---|---|---|
| minCSA | 17.2 mm2 | 1.8 |
| vBMD | 6.4 mg/cm3 | 2.2 |
| IAP | 4005 mm4 | 5.0 |
| IIS | 2997 mm4 | 5.0 |
| My,max | 0.5 N·m | 3.3 |
| My,min | 0.6 N·m | 7.3 |
| My,avg | 0.4 N·m | 3.9 |
| t1 | 0.3 mm | 9.6 |
| t2 | 0.6 mm | 13.7 |
| t3 | 0.5 mm | 11.9 |
| t4 | 0.2 mm | 7.9 |
| t5 | 0.5 mm | 15.3 |
| t6 | 0.4 mm | 9.2 |
| t7 | 0.5 mm | 11.5 |
| t8 | 0.4 mm | 12.3 |
4. Discussion
The results support the view that periosteal expansion occurs at the femoral neck with age, as demonstrated by increased minCSA and IAP in our older (80–90 years) age group. The resulting increase in bone size tends to maintain a relatively constant bone stiffness and strength as opposed to increasing them. The results of this study also confirm that men have larger and stronger femoral necks than women, even after correcting for the larger male stature and body mass. However, despite the larger bone size in men, women had thicker cortices than men in the inferior region of the femoral neck.
Age-related differences in our in vivo measurements of minCSA and IAP shed new light on the relationship between periosteal expansion and femoral neck strength. The larger minCSA in the older age group lends further support to the idea suggested in previous studies in our laboratory and others that periosteal expansion occurs with age [2–5]. This result was expected, as it was reported previously for a larger sample from the AGES-REYKJAVIK study [3]. However, when the minCSA and bone material properties were taken into account, larger femoral neck size did not provide an increase in compressive strength in the older age group. The value of IAP, which is related to the resistance to bending about the A-P axis (in the stance phase of gait, for example [22]), was also larger in the older age group, while the value of IIS, which is related to resistance to bending about the I-S axis, was not. This result suggests that, during periosteal expansion of the femoral neck, new bone tissue forms preferentially on the inferior and superior aspects of the femoral neck, providing more bone tissue far from the neutral axis of bending for common daily loads like walking and climbing stairs. The addition of new bone in these locations did not produce an increase in bending strength. Therefore the age-related differences in minCSA and IAP observed in this study have the effect of maintaining a relatively constant compressive strength and bending strength in the femoral neck in the older age group. Without the addition of new bone in these locations, it is likely that the relative strength of the minCSA cross section would decrease with age.
Differences between male and female femoral neck cross-sectional area are well-documented [3, 23]. It is less well understood how these differences affect bone strength and fracture risk in the event of a fall. In vitro mechanical testing and QCT imaging of male and female femora have shown that male femora are stronger than female femora and that proximal femur strength is related (albeit somewhat weakly) to femoral neck CSA [24]. However, because men are taller and heavier than women on average, the force applied to the hip during a fall will be higher [25], and therefore the stronger male femoral neck may not necessarily be protective against fracture. Our in vivo estimates of femoral neck axial strength and bending strength suggest that, even after correcting for height and weight, men have stronger femoral necks than women. Therefore the proportionally higher strength of the femoral neck in men may in fact partly explain why men have a lower risk of hip fracture than women.
No sex-related difference in vBMD was detected in this study. This result was expected, because a previous study using a larger sample (807 men and 908 women) from the AGES-REYKJAVIK cohort also found that integral vBMD of the femoral neck did not differ between men and women [3]. That study also showed that elderly men had a higher vBMD in the trabecular bone compartment than elderly women. The current study shifted focus to the sectional properties and mechanical structure of the femoral neck rather than a detailed analysis of the vBMD of the different bone compartments. It is notable that, in the current study, the male femoral neck was estimated to be significantly stronger than the female femoral neck, even after correction for larger body size, despite their similar vBMD values.
Contrary to our expectations, our relatively small subset of AGES-REYKJAVIK subjects also did not reveal an age-related change in vBMD of the minCSA femoral neck cross-section. The previous, larger study found significant 10-year declines in integral vBMD in volumetric regions of both male and female femoral necks, rather than the single minCSA slice analyzed in the current study [3]. Thus the smaller sample size and more limited analysis region used in the current study likely prevented detection of an age-related decline in vBMD. We also did not analyze the different contributions of cortical and trabecular bone to the overall vBMD value, and it is likely that the two different bone compartments change with age in different ways and at different rates. It is possible that the small sample size also prevented us from detecting age-related changes in the axial compressive strength and bending strength of the femoral neck. A power study based on our results suggests that approximately 250 subjects per age group are needed to detect a difference in Fy with a power of 0.8. Future studies of femoral neck strength with a larger number of subjects are therefore warranted. The cross-sectional nature of this study also produces a number of limitations, including secular differences between the two age groups. Because a longitudinal study will help to address some of these limitations, the AGES-REYKJAVIK subjects are being followed over time, and fracture cases are being recorded. Future studies will therefore be able to test whether our strength and cortical thickness measurements can discriminate between individuals who have and have not suffered a hip fracture.
Our measurements of cortical thickness in the femoral neck showed that women had thicker cortices than men in the inferior region of the femoral neck. This result is consistent with measurements made using histological slides ex vivo [11]. The underlying biological and biomechanical reasons for the sex-based difference in cortical thickness cannot be determined from our study. A combination of factors may interact to produce this difference. Sexual dimorphism in bone size and structure is established early in life, likely due to a combination of genetic differences and differences in body mass [26–28]. As aging continues, the smaller size of the female femoral neck combined with sex-based differences in pelvic and lower limb geometry [29] may require a thicker inferior femoral cortex to maintain bone stresses and strains within normal physiological levels [30]. Thus the differences between men and women seen in our study are most likely due to a combination of processes, including different rates of periosteal expansion and endosteal resorption, that began early in life and continued into old age. More detailed studies combining gait analysis, QCT imaging, and finite element models may help to shed some light on this question.
Estimates of cortical thickness based on dual-energy x-ray absortiometry (DXA), which rely on assumptions about femoral neck geometry and the relative proportions of cortical and trabecular bone in the femoral neck, have suggested that males have thicker cortices than women [9]. Similarly, the “cortical thickness index,” which estimated the average cortical thickness in the whole femoral neck, was larger in men than in women in a previous QCT analysis of subjects in the AGES-REYKJAVIK Study [3]. By revealing a thicker inferior cortex at the minCSA slice in females, our results show the benefit of using 3D QCT to directly analyze in vivo cortical thickness in multiple locations within a given femoral neck section.
We were unable to detect age-related changes in cortical thickness, and this result is likely due to our focus on an older age range (65 to 90 years) in this study. A recent study used QCT to measure age-related changes in female femoral neck structure in females aged 20 to 89 years [8]. In that study the cortical thickness in the thin superoposterior region of the cortex in the oldest decadal age group (80–89 years) was 1.4 mm thinner than in the youngest age group (20–29 years). Limitations in the resolution and precision (8%–15%) of our cortical thickness measurements may have also prevented observation of an age-related difference in the inferior aspect of the cortex in this cross-sectional study. When analyzing phantom images of a 2.4 mm “cortex” using image resolutions comparable to those in this study, the error of the cortical thickness measurement ranged from 3% to 8% (unpublished data). This was the thinnest we were able to machine the cortex, and this appears to approach the limit of our ability to measure cortical thickness under radiation dose considerations and due to the image reformatting necessary to obtain cross-sections of the femoral neck. Our images were reconstructed with an in-plane voxel size of 0.39 mm × 0.39 mm, resulted in blurring of the thin femoral neck cortex, especially in the thin superior regions. Therefore the voxel intensity peak along a ray extending through the cortex (Figure 2) is wider, resulting in larger estimates of thickness. In this study we therefore focused on the inferior cortex, where the cortical thickness spans a minimum of approximately 3 voxels. An alternative method of measuring cortical thickness with QCT is to fit arcs of constant curvature to the inner and outer edges of the thresholded cortex and to estimate cortical thickness as the difference between the radii of curvature of the two arcs [8]. This method has produced thinner estimates of cortical thickness (e.g., 0.5 mm in the superoposterior femoral neck cortex in elderly women) than that measured in our study. Another method recently developed by Treece et al. [31], which accounts for the angle between the cortex of the femoral neck and the axial imaging plane required in whole-body QCT imaging systems, may help to improve the accuracy of our cortical thickness measurements in future studies.
To further examine the advantage of using a reduced RFOV for examining the femoral neck, we analyzed the standard resolution images (0.98-mm voxels) using the same methods used for the enhanced-resolution (0.39-mm voxels) images used in this study. The age-related difference in minCSA bordered on statistical significance (p = 0.07) for the standard-resolution images. No sex-based difference in t5 (p = 0.17) was detected using the standard-resolution images, while the difference in t7 bordered on significance (p = 0.07). Thus the enhanced-resolution images offered a greater ability to detect subtle differences in femoral neck size and structure. These results suggest that performing CT image reconstruction with a reduced RFOV centered on the proximal femur can enhance the ability to detect sex- and age-related differences in femoral neck minCSA and cortical thickness without increasing radiation dose to patients.
Our estimates of axial and bending strength were largely determined by vBMD. Because bone material properties were computed based on vBMD, this result was expected. We also expected that cortical thickness in the femoral neck would be an independent predictor of bending and axial strength, and this was the case for t4 in the inferoposterior region of the femoral neck. Our vBMD and cortical thickness measurements from QCT images were closely tied, because cortical thickness was computed from the ρHA,S profiles and because a thicker cortex for a given cross-sectional size will produce a higher vBMD. Our results showed that, particularly in the thick inferoanterior cortex (t2 in women and t1 in men), cortical thickness is predictive of vBMD.
5. Conclusions
The age-related differences in minCSA and IAP observed in this study had the effect of maintaining a relatively constant compressive strength and bending strength of the femoral neck in the older age group. The age-based difference in IAP indicates a direction of periosteal expansion that results in homeostasis of bending resistance in the direction of habitual loading. The increase in bone size did not produce an increase in bone strength. Our results also support the view that the decreased hip fracture risk in males as compared to females is determined in part by higher femoral neck strength for a given body size. Both axial compressive strength and bending strength at the most narrow region of the femoral neck were higher in males even after correction for height and weight. Cortical thickness in the inferoposterior region was related to femoral neck strength after adjustment for vBMD. Finally, our results showed that women have a thicker inferoanterior cortex in the femoral neck than men and that using a reduced RFOV can help to measure this difference without increasing radiation dose.
Research Highlights
The cross-sectional area of the femoral neck increases with age.
New bone is added to the inferior and superior aspects of the femoral neck with age.
The age-related increase in femoral neck size does not increase bone strength.
Women have thicker inferior femoral neck cortices than men.
Acknowledgements
The authors would like to thank A. Gudmundsdottir, A. Reynisdottir, A. Sigmardsottir, B. Oskarsdottir, D. Vilmundarson, G. Karlsdottir, and G. Johannsdottir for their aid in acquiring and processing QCT images. Thank you to Gary S. Beaupré for the use of his femoral neck phantom for accuracy analysis. This study was funded by the National Institute on Aging (grant 5R01AG028832 and contract N01-AG-1-2100), in part by the Intramural Research Program of the National Institute on Aging, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament).
Abbreviations
- QCT
quantitative computed tomography
- vBMD
volumetric bone mineral density (mg/cm3)
- RFOV
reconstruction field of view (cm)
- CSA
cross-sectional area (mm2)
- minCSA
minimum cross-sectional area (mm2)
- BMC
bone mineral content (mg)
- IAP
second area moment of inertia about the anterior-posterior axis (mm4)
- IIS
second area moment of inertia about the inferior-superior axis (mm4)
- ρHA,L
liquid K2HPO4 concentration (mg/cm3)
- ρHA,S
solid calcium hydroxyapatite concentration (g/cm3)
- ρ
apparent density (g/cm3)
- E
Young’s modulus (MPa)
- My
yield moment (N·m)
- My,avg
average yield moment over 360 different bending directions (N·m)
- My,max
maximum yield moment over 360 different bending directions (N·m)
- My,min
minimum yield moment over 360 different bending directions (N·m)
- Fy
compressive yield force (N)
- t1–t4
cortical thickness in sectors 1 through 4 (mm)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors have no conflict of interest.
References
- 1.Fox KM, Cummings SR, Williams E, Stone K. Femoral neck and intertrochanteric fractures have different risk factors: a prospective study. Osteoporos Int. 2000;11:1018–1023. doi: 10.1007/s001980070022. [DOI] [PubMed] [Google Scholar]
- 2.Meta M, Lu Y, Keyak JH, Lang T. Young-elderly differences in bone density, geometry and strength indices depend on proximal femur sub-region: a cross sectional study in Caucasian-American women. Bone. 2006;39:152–158. doi: 10.1016/j.bone.2005.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK) Bone. 2006;39:644–651. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC, Genant HK, Cummings SR. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. J Bone Miner Res. 2001;16:1108–1119. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 5.Ruff CB, Hayes WC. Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science. 1982;217:945–948. doi: 10.1126/science.7112107. [DOI] [PubMed] [Google Scholar]
- 6.Havill LM, Mahaney MC, T LB, Specker BL. Effects of genes, sex, age, and activity on BMC, bone size, and areal and volumetric BMD. J Bone Miner Res. 2007;22:737–746. doi: 10.1359/jbmr.070213. [DOI] [PubMed] [Google Scholar]
- 7.Yates LB, Karasik D, Beck TJ, Cupples LA, Kiel DP. Hip structural geometry in old and old-old age: similarities and differences between men and women. Bone. 2007;41:722–732. doi: 10.1016/j.bone.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole KE, Mayhew PM, Rose CM, Brown JK, Bearcroft PJ, Loveridge N, Reeve J. Changing Structure of the Femoral Neck Across the Adult Female Lifespan. J Bone Miner Res. 2010;25:482–491. doi: 10.1359/jbmr.090734. [DOI] [PubMed] [Google Scholar]
- 9.Looker AC, Beck TJ, Orwoll ES. Does body size account for gender differences in femur bone density and geometry? J Bone Miner Res. 2001;16:1291–1299. doi: 10.1359/jbmr.2001.16.7.1291. [DOI] [PubMed] [Google Scholar]
- 10.Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437–444. doi: 10.1016/s8756-3282(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 11.Bell KL, Loveridge N, Power J, Garrahan N, Stanton M, Lunt M, Meggitt BF, Reeve J. Structure of the femoral neck in hip fracture: cortical bone loss in the inferoanterior to superoposterior axis. J Bone Miner Res. 1999;14:111–119. doi: 10.1359/jbmr.1999.14.1.111. [DOI] [PubMed] [Google Scholar]
- 12.Lang TF, Keyak JH, Heitz MW, Augat P, Lu Y, Mathur A, Genant HK. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21:101–108. doi: 10.1016/s8756-3282(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter RD, Beaupre GS, Lang TF, Orwoll ES, Carter DR. New QCT analysis approach shows the importance of fall orientation on femoral neck strength. J Bone Miner Res. 2005;20:1533–1542. doi: 10.1359/JBMR.050510. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner KG, Gluer CC, Grampp S, Genant HK. Cross-calibration of liquid and solid QCT calibration standards: corrections to the UCSF normative data. Osteoporos Int. 1993;3:36–42. doi: 10.1007/BF01623175. [DOI] [PubMed] [Google Scholar]
- 15.Lotz JC, Gerhart TN, Hayes WC. Mechanical properties of trabecular bone from the proximal femur: a quantitative CT study. J Comput Assist Tomogr. 1990;14:107–114. doi: 10.1097/00004728-199001000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Orr TE, Beaupre GS, Carter DR, Schurman DJ. Computer predictions of bone remodeling around porous-coated implants. J Arthroplasty. 1990;5:191–200. doi: 10.1016/s0883-5403(08)80074-5. [DOI] [PubMed] [Google Scholar]
- 17.Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. J Biomech. 2001;34:569–577. doi: 10.1016/s0021-9290(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 18.Prevrhal S, Engelke K, Kalender WA. Accuracy limits for the determination of cortical width and density: the influence of object size and CT imaging parameters. Phys Med Biol. 1999;44:751–764. doi: 10.1088/0031-9155/44/3/017. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Maric I, McCloskey EV, Eastell R. Shape, structural properties, and cortical stability along the femoral neck: a study using clinical QCT. J Clin Densitom. 2008;11:373–382. doi: 10.1016/j.jocd.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Sode M, Saeed I, Lang T. Automated registration of hip and spine for longitudinal QCT studies: integration with 3D densitometric and structural analysis. Bone. 2006;38:273–279. doi: 10.1016/j.bone.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5:262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 22.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34:859–871. doi: 10.1016/s0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 23.Riggs BL, Melton Iii LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 24.Cheng XG, Lowet G, Boonen S, Nicholson PH, Brys P, Nijs J, Dequeker J. Assessment of the strength of proximal femur in vitro: relationship to femoral bone mineral density and femoral geometry. Bone. 1997;20:213–218. doi: 10.1016/s8756-3282(96)00383-3. [DOI] [PubMed] [Google Scholar]
- 25.Robinovitch SN, Hayes WC, McMahon TA. Prediction of femoral impact forces in falls on the hip. J Biomech Eng. 1991;113:366–374. doi: 10.1115/1.2895414. [DOI] [PubMed] [Google Scholar]
- 26.Moro M, van der Meulen MC, Kiratli BJ, Marcus R, Bachrach LK, Carter DR. Body mass is the primary determinant of midfemoral bone acquisition during adolescent growth. Bone. 1996;19:519–526. doi: 10.1016/s8756-3282(96)00263-3. [DOI] [PubMed] [Google Scholar]
- 27.Forwood MR, Bailey DA, Beck TJ, Mirwald RL, Baxter-Jones AD, Uusi-Rasi K. Sexual dimorphism of the femoral neck during the adolescent growth spurt: a structural analysis. Bone. 2004;35:973–981. doi: 10.1016/j.bone.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Tommasini SM, Nasser P, Jepsen KJ. Sexual dimorphism affects tibia size and shape but not tissue-level mechanical properties. Bone. 2007;40:498–505. doi: 10.1016/j.bone.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen AD, Boling MC, Levine B, Shultz SJ. Relationships between lower extremity alignment and the quadriceps angle. Clin J Sport Med. 2009;19:201–206. doi: 10.1097/JSM.0b013e3181a38fb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter DR. Mechanical loading histories and cortical bone remodeling. Calcif Tissue Int. 1984;36 Suppl 1:S19–S24. doi: 10.1007/BF02406129. [DOI] [PubMed] [Google Scholar]
- 31.Treece GM, Gee AH, Mayhew PM, Poole KE. High resolution cortical bone thickness measurement from clinical CT data. Med Image Anal. 2010;14:276–290. doi: 10.1016/j.media.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]