Abstract
Purpose of review
The aim of this study is to summarize current advances in research and clinical aspects of cochlear otosclerosis.
Recent Findings
Recent studies have revealed that otosclerosis is a process of bone remodeling that is unique to only the otic capsule. Even though no obvious bone remodeling is seen in the otic capsule under normal conditions, remodeling starts when some molecular factors trigger the capsule in certain patients who have genetic and/or environmental tendencies.
Summary
Cochlear otosclerosis is defined as otosclerosis located in the otic capsule involving the cochlear endosteum and causing sensorineural hearing loss or mixed type hearing loss. It has been clearly shown that when otosclerosis is sufficiently severe to involve the cochlear endosteum, it usually fixes the stapes as well.
Keywords: Cochlear otosclerosis, otic capsule, sensorineural hearing loss, mixed type hearing loss, conductive hearing loss
Introduction
Otosclerosis is a progressive otic capsule disease that affects only human beings through changes in the middle and inner ear. It can occur independently or together at the stapes footplate, and/or the cochlear capsule.
Definition
Histologic otosclerosis is defined as an incidental finding in temporal bone autopsies; however, when otosclerosis fixes the stapes footplate it causes conductive hearing loss and is defined as clinical otosclerosis. On the other hand, the controversial numbers between radiological and histological studies clearly indicate that “cochlear otosclerosis” as a definition is not uniform. The classical description is that cochlear otosclerosis is defined as a focus of otosclerosis located in the otic capsule involving the cochlear endosteum and causing sensorineural hearing loss without any stapes fixation or any conductive component. However, Schucknecht et al. [1] clearly showed that when otosclerosis is sufficiently severe to involve the cochlear endosteum, it usually fixes the stapes as well. If the definition of cochlear otosclerosis is accepted as the involvement of cochlear endosteum without associated stapes fixation, then the incidence among ears with pure progressive sensorineural hearing loss is about 1%[1].
Cochlear otosclerosis can be classified as a mixed type or a sensorineural type according to the clinical appearance: When there is a mixed hearing impairment, the conductive component could be due to clinical otosclerosis where the stapes footplate is fixed with otosclerotic involvement.
The Etiology of cochlear otosclerosis
Otosclerosis is a process of bone remodeling in the otic capsule that has a unique remodeling process different than other parts of the body [2]. Even though little or no bone remodeling is seen in the otic capsule under normal conditions, remodeling may start when certain molecular factors trigger the otic capsule in patients who have genetic and/or environmental tendencies [3].
Even though there is no special report on the genetic component in cochlear otosclerosis, evidence supports the thesis that clinical otosclerosis has an autosomal dominant tendency with incomplete penetrance. Even though the eight loci have been reported so far in patients with otosclerosis, there is still uncertainty about the ratio of cochlear otosclerosis in these groups [4–10]. In addition, the responsible disease related genes in those loci remain unclear. Other genes that have been shown to be involved in the etiopathogenesis of otosclerosis include COL1A1, TGFB1, BMP2, BMP4, ACE, AGT and RELN gene [11–16].
The role of measles virus has been studied by using electron microscopy, immunohistochemistry, and reverse transcriptase polymerase chain reaction for the amplification of the viral RNA in patients with otosclerosis [17–19]. In addition, the presence of measles virus–specific antibodies in perilymph samples from patients with otosclerosis has also been shown [20]. These studies show that the role of the virus in the pathogenesis of disease should be considered, at least in some cases. Even though otosclerosis is reported to deteriorate during periods of intense hormonal activity [21], the association between otosclerosis and pregnancy is still disputed.
Stankovic et al. [22**] investigated the gene expression of the otic capsule and found out that the gene profile of the otic capsule is distinctly different from that of the tibia and parietal bone. The most characteristic genes of the otic capsule are: osteoprotegerin, bone morphogenetic protein receptor 1b and bone morphogenetic protein 3. The authors thought that osteoprotegerin and bone morphogenetic protein receptor 1b can play a role in inhibition of remodeling within the otic capsule.
Histopathology
The histopathologic correlates of the sensorineural hearing loss in patients with cochlear otosclerosis have been the topics of several studies. It has been shown that the sensorineural hearing loss starts to appear when the otosclerotic focus reaches the cochlear endosteum [23]. Later on, atrophy of the spiral ligament and stria vascularis, as well as the formation of hyalinization in the spiral ligament; have been seen to be responsible for the hearing impairment by hindering recycling ions and by reducing the endocochlear potential with subsequent cochlear hair cell dysfunction [24] (Figure 1–2). The recent studies that explored the functions of spiral ligament have supported the findings of histopathologic studies performed in the temporal bones from patients with cochlear otosclerosis [25]. There is increasing evidence that fibrocytes are very specialized cells which maintain the ionic homeostasis of the cochlea that is necessary to function properly. However, limited number of studies with a small number of cases did not give sufficient explanation about the incidence, degree, and the mechanisms of the sensorineural hearing loss found in cochlear otosclerosis. On the other hand, Sato et al. [26*] stated that cochlear otosclerosis adjacent to the round window caused more damage to spiral ganglion cells and outer hair cells than cochlear otosclerosis adjacent to the oval window. On the other hand, cochlear otosclerosis adjacent to the oval window caused more damage in the spiral ligament by ending up with a hyalinized and atrophied spiral ligament. It has been shown that high incidence of fixation of the head of the malleus occurs in otosclerosis.
Figure 1.
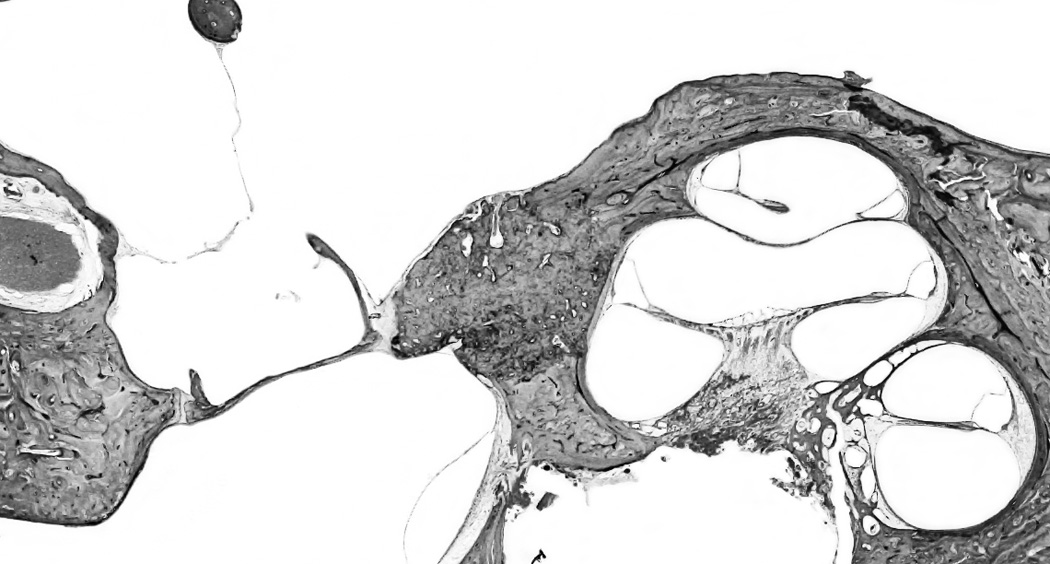
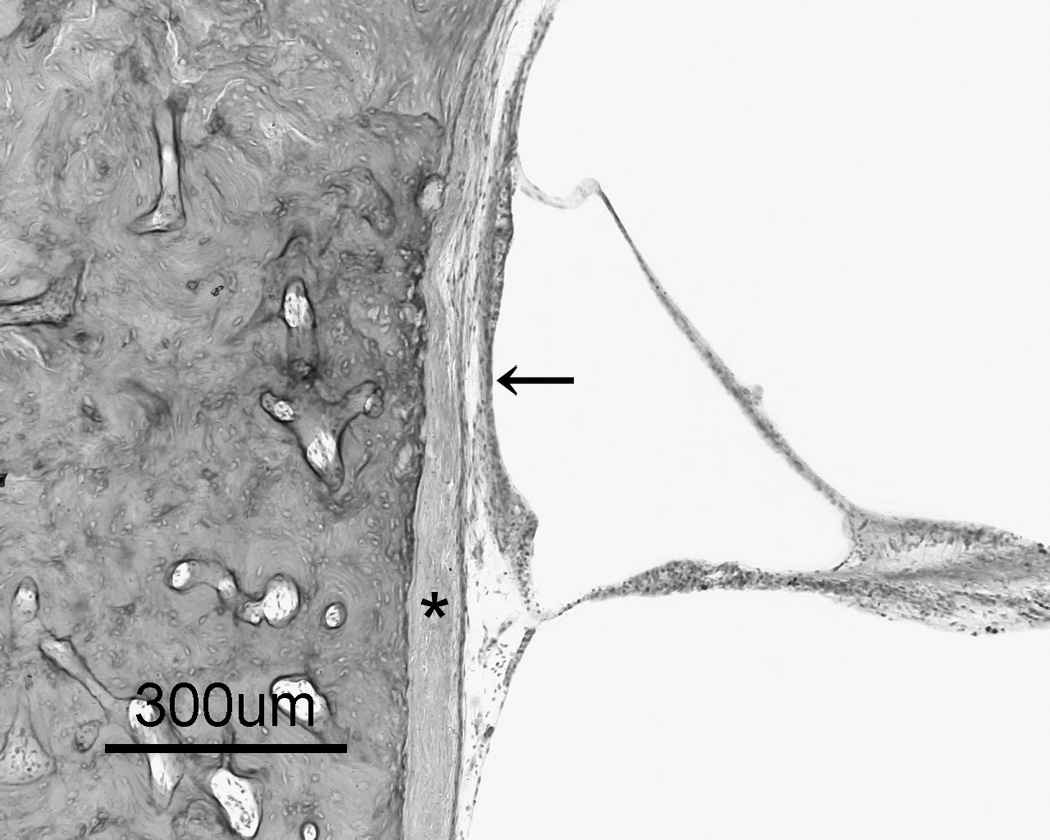
a: Otosclerosis focus is involving cochlear endosteum and extending to the stapes footplate. 1b: A high magnification of the cochlear endosteum shows the hyalinization of the spiral ligament (*); and atrophy of the stria vascularis (arrow) (H&E staining).
Figure 2.
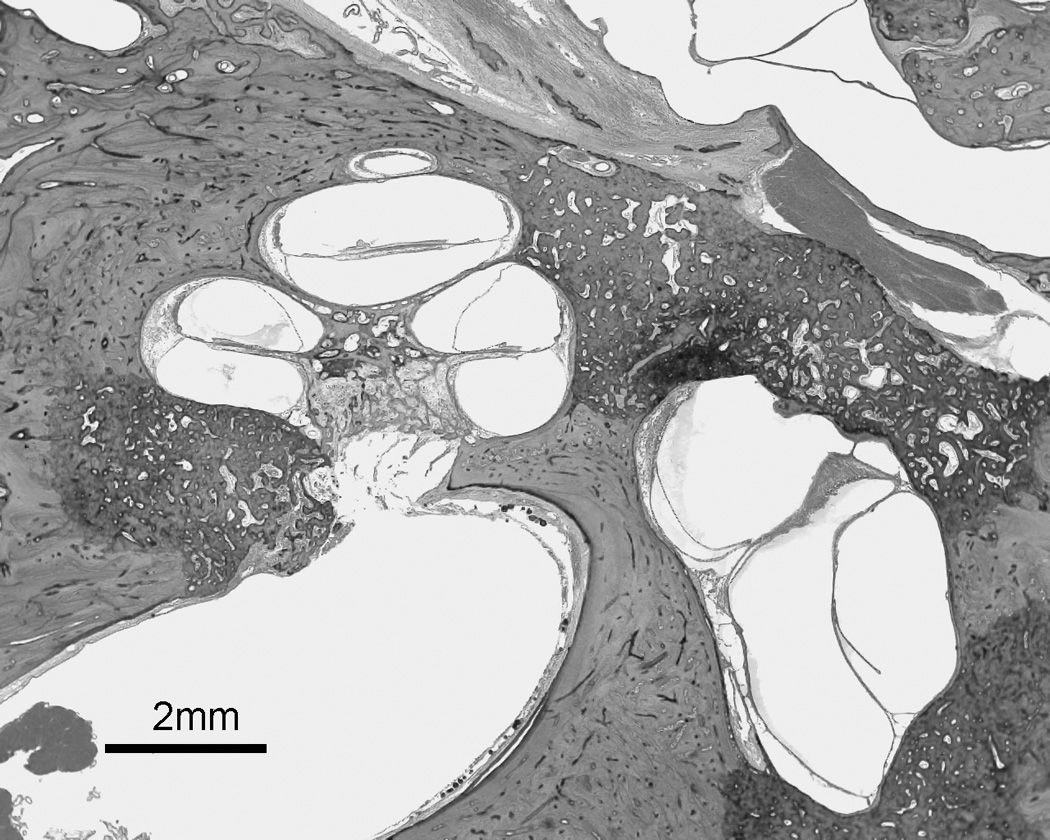
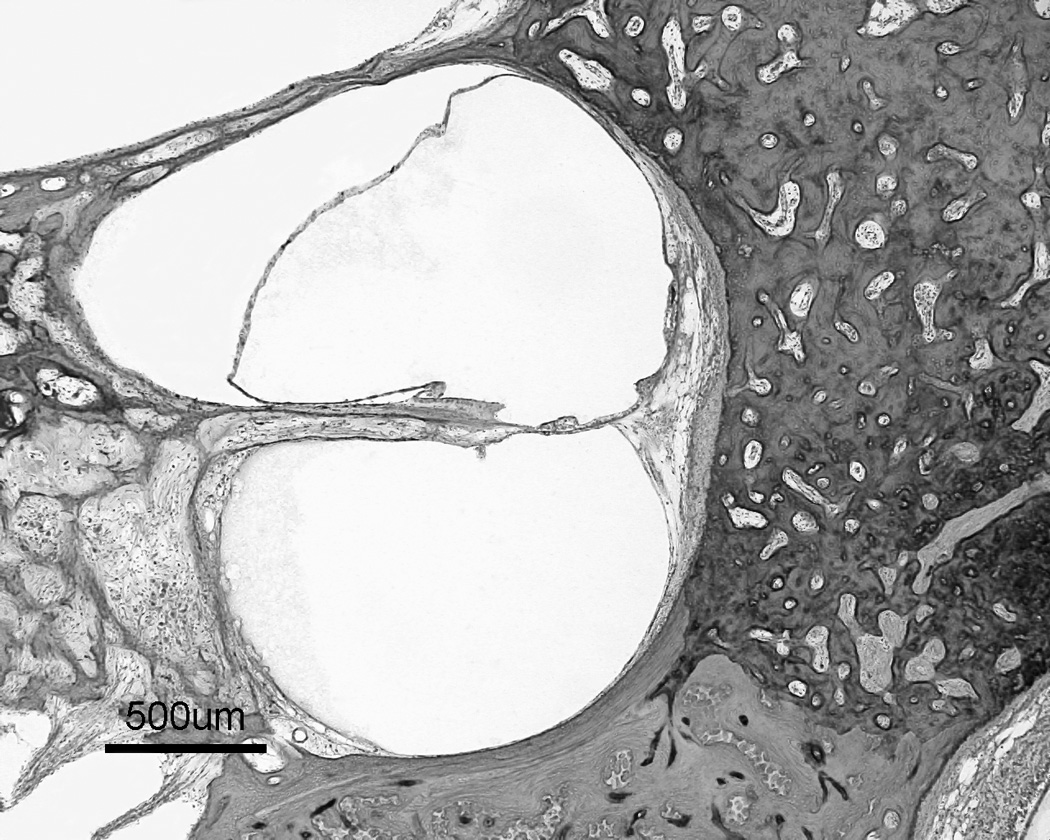
a: Multiple otosclerotic foci involving stapes footplate and cochlear endosteum as well as blocking the vestibular aqueduct. 2b: a higher magnification of the rectangular area from figure 2a shows involvement of cochlear endosteum, hyalinization of the spiral ligament, and endolymphatic hydrops (H&E staining).
Clinical manifestations of cochlear otosclerosis
The clinical course of the disease depends on the progression of the cochlear otosclerosis and its otic capsule involvement. In addition to sensorineural or mixed type hearing loss, tinnitus (common) and vertigo (less common) can also occur in patients with cochlear otosclerosis. If there is no stapes fixation, there is expected to be a positive stapes reflex. Linthicum [27] indicated that the presence of a small depression of the preoperative bone conduction threshold in a patient with clinical otosclerosis shows the presence of otic capsule involvement and the possibility of hearing deterioration in the postoperative years. Makarem and Linthicum [28] described cavity formation in cochlear otosclerosis surrounding the middle and apical turns of the cochlea. They thought the cavitating otosclerosis may be a cause of “third window” lesions, and it is a potential cause for cerebrospinal fluid (CSF) gushing and electrode misplacement during cochlear implantation.
Otosclerosis can be associated with an increased incidence of vestibular symptoms [29–31]. The otosclerotic involvement of the vestibular organs has been thought to be the cause of the vestibular symptoms [30,32]. It has been assumed that the dizziness could be due to the changes in the biochemical composition of the perilymph [33,34]. Vestibular symptoms associated with otosclerosis can be recurrent, positional, or spontaneous. Benign paroxysmal positional vertigo is seen in patients with extensive otosclerosis [35]. On the other hand, though Hayashi et al. [36] found a significant increase in cupular deposits in otosclerosis, they did not find any association between vestibular symptoms or endosteal involvement and cupular deposits. The association of endolymphatic hydrops with or without vertigo and extensive otosclerosis can occur [37]. The mechanisms suggested include physical and physiological obstruction of the vestibular aqueduct [38], biochemical changes in the content of the perilymph by involving the otic capsule [30], and neural damage to the vestibular organ [39]. On the other hand, Morales-Garcia [29] indicated that vestibular symptoms can occur without cochlear involvement as well. If there is an active otosclerosis focus located in the promontory, there could be a reddish appearance upon examination of the tympanic membrane that is known as Schwartze’s sign.
In the clinical diagnosis of cochlear otosclerosis, there are some criteria suggested in the literature, such as sensorineural hearing loss with good speech discrimination [40], a family history of otosclerosis in a patient with symmetrical sensorineural hearing loss accompanied by stapes fixation [41], and a positive Schwartze’s sign [41]. Cause et al. [42] stated that the sensorineural hearing loss in cochlear otosclerosis is suddenly aggravated at puberty or at periods of endocrine activity such as pregnancy, menopause, or treatment with estrogen. The presence of a depression in bone conduction threshold in a patient with otosclerosis may indicate cochlear otosclerosis even if the acoustic reflex is normal [27].
Carhart [43] reported a reduction in bone conduction sensitivity with a peak at 2.000 Hz, which is an audiological marker for otosclerosis. Tonndorf [44] indicated three pathways of bone conduction as bone to cochlea, bone to middle ear then cochlea, and bone to external auditory canal to middle ear and then cochlea. Because the middle ear pathway is blocked to a certain extent in otosclerosis due to stapedial fixation, bone conduction threshold is elevated in some frequencies with a peak at 2.000 Hz. It is thought that the 2.000 Hz frequency is the resonant frequency of the human ossicular chain for bone conducted signals; therefore the effect is mostly pronounced at this frequency. Since this is not an indicator of cochlear hair cell loss or cochlear sensitivity, it may be corrected by surgical intervention. On the other hand, in cochlear otosclerosis, the hearing loss has a persistent sensorineural component that is totally different from Carhart’s notch.
On computerized tomography (CT), a distinctive pericochlear hypodense double ring appearance due to demineralization of the bone around the cochlea is seen in patients with cochlear otosclerosis [45]. Since active otosclerosis will appear as a hypodense ring within the otic capsule, CT of the temporal bone will only detect active otosclerosis. Even though the temporal bone and otosclerosis is best characterized on high resolution CT, magnetic resonance imaging (MRI) may suggest central causes in patients with sensorineural hearing loss. MRI demonstrates a ring of intermediate signal in the pericochlear and perilabyrinthine regions on T1 weighted images, and demonstrates mild to moderate enhancement after gadolinium administration. MRI shows perilabyrinthine and pericochlear soft tissue with contrast enhancement. T2 weighted images also show increased signals.
Treatment
Sodium Fluoride that is very safe in therapeutic doses may be effective if there is active otosclerosis focus. The idea came from comparable studies indicating the high incidence of clinical otosclerosis in low-fluoride areas [46]. The mechanisms postulated regarding fluoride’s activity included antienzymatic activity against proteolytic enzymes [41–48], reduction of bone resorption [49], changing otospongiotic active lesions to more dense inactive lesions [50] and an increase of new bone formation in the healing process of bone structures.
The adverse reactions reported during fluoride treatment included synovitis, plantar faciitis, gastrointestinal side effects such as recurrent vomiting, peptic ulcer, anemia, and increased skeletal fragility [51,52].
The dosage can be adjusted starting with 60 mg and continuing with the maintenance dosage of 20–25 mg if clinical proof of effectiveness of the treatment is seen [50–54]. Derks et al. [55] found out that fluoride treatment is more effective for the higher frequencies in cases with cochlear otosclerosis and an initial sensorineural hearing loss of less than 50dB.
Biphosphonates also have a potential use in the treatment of cochlear otosclerosis by inhibiting the activation of osteoclasts [56]. Blookler and Tanyeri [57] reported 70% improvement in their hearing, 54% in dizziness, and 52% in tinnitus in patients with otosclerosis by assessing the efficacy of etidronate, a biphosphonate. Even though the adverse effects are rare, those effects are nausea, ulcers of upper gastrointestinal tract, and similar findings of osteomalacia due to impaired mineralization. In addition, acute renal failure was also reported with IV infusion of etidronate in three patients with malignancies [58].
Since patients with cochlear otosclerosis have a sensorineural hearing loss usually with adequate speech discrimination, hearing can be amplified with hearing aids or in the case of deafness and lack of hearing aid assistance, a cochlear implant. It has been reported that there is a significant risk of cochlear ossification and facial nerve stimulation after cochlear implantation in patients with cochlear otosclerosis and profound sensorineural hearing loss [59].
Conclusion
The fact that there are some clinical studies with imaging techniques which report patients with a pure cochlear sensorineural type of otosclerosis, while otopathologic studies indicate that this type of ear pathology is very rare; points to the need for more clinical control studies in large series. In this regard, future human temporal bone studies with audiological and clinical correlation are indispensable. Cochlear otosclerosis is defined as an otosclerosis foci located in the otic capsule involving the cochlear endosteum and causing sensorineural hearing loss or a mixed type of hearing loss with reasonable speech discrimination. On CT, a distinctive pericochlear hypodense double ring appearance due to demineralization of the bone around the cochlea is seen in patients with cochlear otosclerosis. Sodium fluoride treatment can be tried to arrest the disease progress but amplification of hearing with hearing aids should be considered.
Acknowledgments
Funding/Support: This study was supported by NIDCD 3U24DC008559-03S109, the International Hearing Foundation, and the Starkey Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schuknecht HF, Kirchner JC. Cochlear otosclerosis: fact or fantasy. Laryngoscope. 1974;84:766–782. doi: 10.1288/00005537-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Frisch T, Sørensen MS, Overgaard S, Bretlau P. Estimation of volume referent bone turnover in the otic capsule after sequential point labeling. Ann Otol Rhinol Laryngol. 2000;109:33–39. doi: 10.1177/000348940010900106. [DOI] [PubMed] [Google Scholar]
- 3.McKenna MJ, Kristiansen AG. Molecular biology of otosclerosis. Adv Otorhinolaryngol. 2007;65:68–74. doi: 10.1159/000098674. [DOI] [PubMed] [Google Scholar]
- 4.Tomek MS, Brown MR, Mani SR, et al. Localization of a gene for otosclerosis to chromosome 15q25–q26. Hum Mol Genet. 1998;7:285–290. doi: 10.1093/hmg/7.2.285. [DOI] [PubMed] [Google Scholar]
- 5.Van Den Bogaert K, Govaerts PJ, Schatteman I, et al. A second gene for otosclerosis, OTSC2, maps to chromosome 7q34–36. Am J Hum. Genet. 2001;68:495–500. doi: 10.1086/318185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Campbell CA, Green GE, et al. Linkage of otosclerosis to a third locus (OTSC3) on human chromosome 6p21.3–22.3. J Med Genet. 2002;39:473–477. doi: 10.1136/jmg.39.7.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownstein Z, Goldfarb A, Levi H, et al. Chromosomal mapping and phenotypic characterization of hereditary otosclerosis linked to the OTSC4 locus. Arch Otolaryngol Head Neck Surg. 2006;132:416–424. doi: 10.1001/archotol.132.4.416. [DOI] [PubMed] [Google Scholar]
- 8.Van Den Bogaert K, De Leenheer EM, Chen W, et al. A fifth locus for otosclerosis, OTSC5, maps to chromosome 3q22–24. J Med Genet. 2004;41:450–453. doi: 10.1136/jmg.2004.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thys M, Van Den Bogaert K, Iliadou V, et al. A seventh locus for otosclerosis, OTSC7, maps to chromosome 6q13–16.1. Eur J Hum Genet. 2007a;15:362–368. doi: 10.1038/sj.ejhg.5201761. [DOI] [PubMed] [Google Scholar]
- 10.Bel Hadj Ali I, Thys M, Beltaief N, et al. A new locus for otosclerosis, OTSC8, maps to the pericentromeric region of chromosome 9. Hum Genet. 2008;123:267–272. doi: 10.1007/s00439-008-0470-3. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Meyer NC, McKenna MJ, et al. Single-nucleotide polymorphisms in the COL1A1 regulatory regions are associated with otosclerosis. Clin Genet. 2007;71:406–414. doi: 10.1111/j.1399-0004.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 12.Imauchi Y, Jeunemaître X, Boussion M, et al. Relation between renin-angiotensin-aldosterone system and otosclerosis: a genetic association and in vitro study. Otol Neurotol. 2008;29:295–301. doi: 10.1097/mao.0b013e318164d12c. [DOI] [PubMed] [Google Scholar]
- 13.McKenna MJ, Kristiansen AG, Bartley ML, et al. Association of COL1A1 and otosclerosis: evidence for a shared genetic etiology with mild osteogenesis imperfecta. Am J Otol. 1998;19:604–610. [PubMed] [Google Scholar]
- 14.Schrauwen I, Thys M, Vanderstraeten K, et al. Association of bone morphogenetic proteins with otosclerosis. J Bone Miner Res. 2008;23:507–516. doi: 10.1359/JBMR.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thys M, Van Den Bogaert K, Iliadou V, et al. A seventh locus for otosclerosis, OTSC7, maps to chromosome 6q13–16.1. Eur J Hum Genet. 2007;15:362–368. doi: 10.1038/sj.ejhg.5201761. [DOI] [PubMed] [Google Scholar]
- 16.Schrauwen I, Ealy M, Huentelman MJ, et al. A genome-wide analysis identifies genetic variants in the RELN gene associated with otosclerosis. Am J Hum Genet. 2009;84:328–338. doi: 10.1016/j.ajhg.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenna MJ, Mills BG. Immunohistochemical evidence of measles virus antigens in active otosclerosis. Otolaryngol Head Neck Surg. 1989;101:415–421. doi: 10.1177/019459988910100401. [DOI] [PubMed] [Google Scholar]
- 18.McKenna MJ, Mills BG. Ultrastructural and immunohistochemical evidence of measles virus in active otosclerosis. Acta Otolaryngol Suppl. 1990;470:130–139. [PubMed] [Google Scholar]
- 19.Niedermeyer H, Arnold W, Neubert WJ, et al. Evidence of measles virus RNA in otosclerotic tissue. ORL J Otorhinolaryngol Relat Spec. 1994;56:130–132. doi: 10.1159/000276627. [DOI] [PubMed] [Google Scholar]
- 20.Arnold W, Niedermeyer HP, Lehn N, et al. Measles virus in otosclerosis and the specific immune response of the inner ear. Acta Otolaryngol. 1996;116:705–709. doi: 10.3109/00016489609137910. [DOI] [PubMed] [Google Scholar]
- 21.Markou K, Goudakos J. An overview of the etiology of otosclerosis. Eur Arch Otorhinolaryngol. 2009;266:25–35. doi: 10.1007/s00405-008-0790-x. [DOI] [PubMed] [Google Scholar]
- 22. Stankovic KM, Adachi O, Tsuji K, et al. Differences in gene expression between the otic capsule and other bones. Hear Res. 2010 Feb 8; doi: 10.1016/j.heares.2010.02.006. (Epub ahead of print) This is the first article of gene expression in the otic capsule, emphasizing the difference of the otic capsule from other bones. The unique expression of genes in the otic capsule may play a role in remodeling.
- 23.Hueb MM, Goycoolea MV, Paparella MM, Oliveira JA. Otosclerosis: the University of Minnesota temporal bone collection. Otolaryngol Head Neck Surg. 1991 Sep;105(3):396–405. doi: 10.1177/019459989110500308. [DOI] [PubMed] [Google Scholar]
- 24.Doherty JK, Linthicum FH., Jr Spiral ligament and stria vascularis changes in cochlear otosclerosis: effect on hearing level. Otol Neurotol. 2004 Jul;25(4):457–464. doi: 10.1097/00129492-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Adams JC. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otol Neurotol. 2002 May;23(3):316–322. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 26. Sato T, Morita N, Cureoglu S, et al. Cochlear otosclerosis adjacent to round window and oval window: A Histopathological temporal bone study. Otol Neurotol. 2010 doi: 10.1097/MAO.0b013e3181d8d73b. in press. The authors describe the histopathologic changes in two different locations of cochlear otosclerosis.
- 27.Linthicum F., Jr Post-stapedectomy cochlear otosclerosis. Ear Nose Throat J. 2009;88:872. [PMC free article] [PubMed] [Google Scholar]
- 28.Makarem A, Linthicum FH. Cavitating otosclerosis. Otol Neurotol. 2008;29:730–731. doi: 10.1097/MAO.0b013e3181799763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales-Garcia C. Cochleo-vestibular involvement in otosclerosis. Acta Otolaryngol. 1972;73:484–492. doi: 10.3109/00016487209138969. [DOI] [PubMed] [Google Scholar]
- 30.Ghorayeb BY, Linthicum FH., Jr Otosclerotic inner ear syndrome. Ann Otol Rhinol Laryngol. 1978;87:85–90. doi: 10.1177/000348947808700115. [DOI] [PubMed] [Google Scholar]
- 31.Cody DT, Baker HL., Jr Otosclerosis: vestibular symptoms and sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1978;87(6 Pt 1):778–796. doi: 10.1177/000348947808700605. [DOI] [PubMed] [Google Scholar]
- 32.Sando I, Hemenway WG, Miller DR, et al. Vestibular pathology in otosclerosis temporal bone histopathological report. Laryngoscope. 1974;84:593–605. doi: 10.1288/00005537-197404000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence M. Possible influence of cochlear otosclerosis on inner ear fluids. Ann Otol. 1966;75:553–558. [Google Scholar]
- 34.Paparella MM, Mancini F, Liston SL. Otosclerosis and Meniere’s syndrome: diagnosis and treatment. Laryngoscope. 1984;94:1414–1417. [PubMed] [Google Scholar]
- 35.Crossland G, De R, Axon P. Far advanced otosclerosis and intractable benign paroxysmal positional vertigo treated with combined cochlear implantation and posterior semicircular canal occlusion. J Laryngol Otol. 2004;118:302–304. doi: 10.1258/002221504323012076. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi H, Cureoglu S, Schachern PA, et al. Association between cupular deposits and otosclerosis. Arch Otolaryngol Head Neck Surg. 2006;132:1331–1334. doi: 10.1001/archotol.132.12.1331. [DOI] [PubMed] [Google Scholar]
- 37.Paparella MM, Cureoglu S, Shao W, Schachern PA. Otosclerosis and associated otopathologic conditions. Adv Otorhinolaryngol. 20047;65:31–44. doi: 10.1159/000098666. [DOI] [PubMed] [Google Scholar]
- 38.Yoon TH, Paparella MM, Schachern PA. Otosclerosis involving the vestibular aqueduct and Menière's disease. Otolaryngol Head Neck Surg. 1990;103:107–112. doi: 10.1177/019459989010300116. [DOI] [PubMed] [Google Scholar]
- 39.Saim L, Nadol JB., Jr Vestibular symptoms in otosclerosis--correlation of otosclerotic involvement of vestibular apparatus and Scarpa's ganglion cell count. Am J Otol. 1996;17:263–270. [PubMed] [Google Scholar]
- 40.Beales PH. Diagnosis of sensorineural deafness. In: Beales PH, editor. otosclerosis. Bristol: Stone Bridge Press; 1981. pp. 68–75. [Google Scholar]
- 41.Schambaugh G., JR The therapy of cochlear otosclerosis. Ann Otol. 1966;75:579. [Google Scholar]
- 42.Causse B, Causse JR. Cochlear otosclerosis. J laryngol Otol Suppl. 1983;8:84. [PubMed] [Google Scholar]
- 43.Carhart R. Clinical applications of bone conduction audiometry. Arch Otolaryngol. 1950;51:798–808. doi: 10.1001/archotol.1950.00700020824003. [DOI] [PubMed] [Google Scholar]
- 44.Tondorf J. A new concept of bone conduction. Arch Otolaryngol. 1968;87:595–600. doi: 10.1001/archotol.1968.00760060597008. [DOI] [PubMed] [Google Scholar]
- 45.Diaz RC. Cochlear otosclerosis. Otol Neurotol. 2008;29:723–724. doi: 10.1097/mao.0b013e31816021cf. [DOI] [PubMed] [Google Scholar]
- 46.Daniel HJ, 3rd, Shambaugh GE, Jr, Fisch U. Fluoride and clinical otosclerosis. Arch Otolaryngol. 1973;98:327–329. doi: 10.1001/archotol.1973.00780020339012. [DOI] [PubMed] [Google Scholar]
- 47.Parahy C, Linthicum FH., Jr Otosclerosis and otospongiosis: clinical and histological comparisons. Laryngoscope. 1984;94:508–512. doi: 10.1288/00005537-198404000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Causse JR, Uriel J, Berges J, et al. The enzymatic mechanism of the otospongiotic disease and NaF action on the enzymatic balance. Am J Otol. 1982;3:297–314. [PubMed] [Google Scholar]
- 49.Causse JR, Causse JB, Uriel J, et al. Sodium fluoride therapy. Am J Otol. 1993;14:482–490. doi: 10.1097/00129492-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Bretlau P, Causse J, Causse JB, et al. Otospongiosis and sodium fluoride. A blind experimental and clinical evaluation of the effect of sodium fluoride treatment in patients with otospongiosis. Ann Otol Rhinol Laryngol. 1985;94:103–107. doi: 10.1177/000348948509400201. [DOI] [PubMed] [Google Scholar]
- 51.Riggs BL, Hodgson SF, Hoffman DL, et al. Treatment of primary osteoporosis with fluoride and calcium. Clinical tolerance and fracture occurrence. JAMA. 1980;243:446–449. [PubMed] [Google Scholar]
- 52.Riggs BL, Hodgson SF, O'Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med. 1990;322:802–809. doi: 10.1056/NEJM199003223221203. [DOI] [PubMed] [Google Scholar]
- 53.Cole JM, Funkhouser G. Meniere's disease and otosclerosis (without oval window involvement) Laryngoscope. 1972;82:1027–1034. doi: 10.1288/00005537-197206000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Forquer BD, Linthicum FH, Bennett C. Sodium fluoride: effectiveness of treatment for cochlear otosclerosis. Am J Otol. 1986;7:121–125. [PubMed] [Google Scholar]
- 55.Derks W, De Groot JA, Raymakers JA, Veldman JE. Fluoride therapy for cochlear otosclerosis? an audiometric and computerized tomography evaluation. Acta Otolaryngol. 2001;121:174–177. doi: 10.1080/000164801300043361. [DOI] [PubMed] [Google Scholar]
- 56.Brookler K. Medical treatment of otosclerosis: rationale for use of bisphosphonates. Int Tinnitus J. 2008;14:92–96. [PubMed] [Google Scholar]
- 57.Brookler KH, Tanyeri H. Etidronate for the the neurotologic symptoms of otosclerosis: preliminary study. Ear Nose Throat J. 1997;76:371–376. 379–381. [PubMed] [Google Scholar]
- 58.Bounameaux HM, Schifferli J, Montani JP, et al. Renal failure associated with intravenous diphosphonates. Lancet. 1983;1:471. doi: 10.1016/s0140-6736(83)91465-4. [DOI] [PubMed] [Google Scholar]
- 59.Quaranta N, Bartoli R, Lopriore A, et al. Cochlear implantation in otosclerosis. Otol Neurotol. 2005;26:983–987. doi: 10.1097/01.mao.0000185047.77017.31. [DOI] [PubMed] [Google Scholar]


