Abstract
Introduction
Poor hygiene practices and inadequate sanitary conditions play major roles in the increased burden of communicable diseases within developing countries. This study evaluated the knowledge, attitudes, and practices (KAP) of hygiene among rural school children in Ethiopia and assessed the extent to which proper knowledge of hygiene was associated with personal hygiene characteristics.
Methods
This cross-sectional study was comprised of 669 students who were interviewed by trained staff. Participants were in grades 1-6 at Angolela Primary School, located in rural Ethiopia. Data consisted of hygiene and hand washing practices, knowledge about sanitation, personal hygiene characteristics, and presence of gastrointestinal parasitic infection.
Results
Approximately 52% of students were classified as having adequate knowledge of proper hygiene. Most students reported hand washing before meals (99.0%), but only 36.2% reported using soap. Although 76.7% of students reported that washing hands after defecation was important, only 14.8% reported actually following this practice. Students with adequate knowledge of proper hygiene were more likely to have clean clothes (AOR 1.62, CI 1.14-2.29) and to have a lower risk of parasitic infection (AOR 0.78, CI 0.56-1.09) although statistical significance was not achieved for the latter.
Discussion and conclusion
Study findings underscore the need for more hand washing and hygiene education in schools; and provide objective evidence that may guide the development of comprehensive health and hygiene intervention programs in rural Ethiopian schools. Successful implementation of these programs is likely to substantially attenuate the transmissible disease burden borne by school children in rural settings.
Keywords: Hygiene; Hand washing; Angolela; Ethiopia; School Children; Knowledge, Attitudes, Practices
Introduction
A large fraction of the world’s illness and death is attributable to communicable diseases (1). Sixty-two percent and 31% of all deaths in Africa and Southeast Asia, respectively, are caused by infectious disease (2). This trend is especially notable in developing countries where acute respiratory and intestinal infections are the primary causes of morbidity and mortality among young children (3). Inadequate sanitary conditions and poor hygiene practices play major roles in the increased burden of communicable disease within these developing countries.
Previous hand hygiene studies have indicated that children with proper hand washing practices are less likely to report gastrointestinal and respiratory symptoms (4, 5). Hand washing with soap has been reported to reduce diarrheal morbidity by 44% and respiratory infections by 23% (2, 6). However, globally, the rates at which hands are washed with soap range from only 0-34% of the time (7). A study conducted by the Global Public–Private Partnership for Hand Washing (PPPHW) which included several sub-Saharan African countries (i.e. Kenya, Senegal, Tanzania, and Uganda) reported that 17% of participants washed their hands with soap after using the toilet, while 45% used only water (2).
Lack of resources, namely soap and water, as well as inadequate sanitation facilities may be two of the main reasons why children do not wash their hands (8, 9). Overall in rural Ethiopia, only 8% have access to adequate sanitation facilities (10). In the rural Amhara region of the country, only 21% of latrines had hand washing facilities, none of which contained soap, and less than 4% of households had access to adequate sanitation facilities (9).
In addition to having proper resources and facilities, hygiene practices are heavily influenced by students’ knowledge and attitudes towards hygiene. In a study conducted in Senegal, reasons given for not washing hands included stubbornness (not wanting to follow what adults say), laziness, the rush to go to breaks, the time it takes away from playing, and the dirt and smell of the toilets (11). Despite these negative attitudes towards hand washing, many children practice good hand washing behavior (11). Based on the PPPHW study conducted in sub-Saharan Africa, motivating factors behind proper hand washing included avoidance of disgust (i.e. avoid dirt and smell of defecation), nurture (i.e. teach children to wash hands so they stay healthy), status (i.e. clean people are more accepted), affiliation (i.e. cleanliness is associated with better socioeconomic status), attraction (i.e. cleaner people are more attractive), comfort (i.e. hands feel and smell fresh), and fear (i.e. avoid the risk of disease) (12). Furthermore, students did not want to miss school due to illness because they would not be able to spend time with their friends (11). Also, if the children had clean hands, they would have clean books, resulting in better grades (11).
A study conducted by the United Nations Children’s Fund (UNICEF) and the Ethiopian Ministry of Health found that study participants in rural Ethiopia had poor status regarding knowledge, attitudes, and practices (KAP) of hygiene (13). Approximately 60% of children surveyed did not know about the possible transmission of diseases through human waste (13). Simple hygienic measures such as washing hands with soap were poorly practiced, especially in rural areas (13). Another study conducted by the Research-inspired Policy and Practice Learning in Ethiopia (RiPPLE), a program surveying rural households in the southwest region of Ethiopia, found that hand washing practices were also poor (14). New hand washing facilities, in addition to awareness and knowledge about proper hygiene, have led to some changes in behavior and attitude, yet the prevalence of hand washing remains low in this region (14).
Past reviews about personal hygiene indicate that perception strongly influences one’s hand washing beliefs and practices. Previous studies conducted in Ethiopia provide limited details about the hygiene KAP of populations rural areas. Additionally, few investigators have examined hygiene KAP specifically among rural school children, a population especially susceptible to communicable diseases. The objectives of this study are to evaluate the KAP of hand washing, and to assess the extent to which proper knowledge on hygiene practices is associated with personal hygiene characteristics among rural school children in Angolela, Ethiopia. Information from this study will serve as baseline data for future school-based hygiene intervention programs in rural Ethiopian schools.
MATERIALS AND METHODS
Subjects
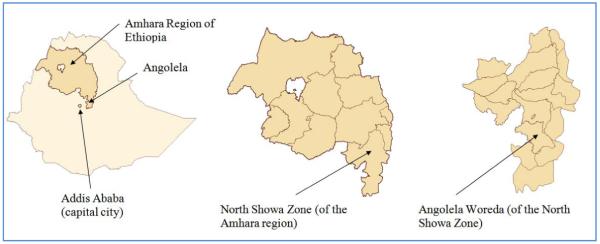
This cross-sectional epidemiologic study was conducted in Angolela, Ethiopia from October 20-25, 2008. Angolela (now known as Basona Werena), located in the North Showa zone, is one of the 105 woredas (a district managed by a local government) in the Amhara region of Ethiopia (Figure 1). It is located about 140 km from Addis Ababa, the capital city of Ethiopia. Angolela has an estimated population of 81,145 and an area of 992 square kilometers with 93% living in rural settings (15). The study was conducted at Angolela Primary School, a government-owned institution which provides free education to children in grades 1-8 who live in the Angolela area.
Figure 1.
Map of Angolela Woreda (now known as Basona Werena Woreda), located in the North Showa zone of the Amhara region of Ethiopia
The study population was comprised of all Angolela Primary School children in grades 1-6. These grades were chosen because infectious diseases most affect younger children. Students absent (N=52) during the survey period were excluded. The final sample size was 669 students (326 girls and 343 boys).
The study staff communicated the objectives of the study to the students and participation was completely voluntary. Study participants provided oral consent prior to participating and there was a 100% participation rate. The Institutional Review Board of Addis Continental Institute of Public Health (Addis Ababa, Ethiopia) and the University of Washington Human Subjects Division (Seattle, WA, USA) granted ethical approval for the study. Approval from the Woreda Health Office and the Woreda Education Office was also granted prior to the commencement of this study.
Instruments and Procedure
Study staff consisted of two supervisors, four ophthalmic nurses, three laboratory technicians, and five research interviewers. Each student was interviewed using a structured questionnaire in a room specifically dedicated for this study. The questionnaire was initially drafted in English, translated to Amharic, and then pre-tested in Dalcha Elementary School in Basona Worena Woreda to assess the suitability with regards to duration, language appropriateness, content validity, and question comprehensibility. All study personnel were trained in interviewing skills, content of the questionnaire, data quality, and ethical conduct of human research.
Variable Specification
The questionnaire consisted of: demographic information (grade, gender); mother and father literacy (no, yes); and frequencies of bathing, washing feet/hair, brushing teeth, and changing clothes (every 1-7 days, 7-14 days, >14 day). Students were queried as to whether their drinking water for the day prior to interview was boiled (no, yes) and about the type of materials used for anal cleansing (paper, leaf, stone, grass, water, nothing, other), bathing (soap, water only, other), and teeth cleaning (twigs, water only, other). Other questions included: if hands were washed during the day prior to interview (no, yes); reasons for washing hands (after defecation, before meals, after meals); materials used for hand washing (soap and water, water only); hand washing preference (before meals, after meals, don’t know); and hand washing importance (after defecation, before meals, after meals). Individual hygiene was also assessed by a trained nurse-interviewer to evaluate the cleanliness of the student. A checklist was used for the following: clean clothing (no, yes); clean fingernails (no, yes); trimmed fingernails (no, yes); clean face (no, yes); eye discharge (no, yes); and clean hair (no, yes). The general appearance of the individual (presence of footwear, condition of clothing) and presence of scabies, cuts, and bruises (present, not present) were also examined. Similar hygiene assessment procedures have been employed by others (16, 17).
Knowledge about sanitation was also assessed. Students who answered ‘yes’ to all the following questions were classified as having adequate knowledge of proper hygiene : if boiling water kills germs, if water containers need cleaning and covering, and if human feces contains germs.
In addition to hygiene assessments, students were also evaluated for the presence of parasitic infection. A total of 644 stool samples were collected in labeled plastic cups and preserved with 10% formalin and 0.85% saline. The samples were then processed at Debre Birhan Hospital (10 km from the school). Parasitological examination of feces was conducted according to World Health Organization (WHO) operating procedures (18). Random reprocessing of 5% of the stool samples was performed for accuracy purposes. Presence of any parasitic infection was defined by a positive laboratory result of any of the following parasites: Ascaris lumbricoides, hookworm, Hymenolopis dimunita, Trichuris trichiura, Giardia lamblia, Entamoeba histolytica, Hymenolopis nana, and Enterobius vermicularis.
Data Analysis
Data were entered into EPI INFO (Version 3.3.2), a public access software package made available by the U.S. Centers for Disease Control and Prevention. Statistical analysis was done using SPSS (Version 17.0, SPSS Inc. Chicago, IL, USA). Frequency tables were used to quantify the occurrence of hygiene practices. Bivariate methods were used to classify frequency distributions of students’ attitudes and practices according to appropriate knowledge. Odds ratios (OR) and 95% confidence intervals (CI) were calculated by logistic regression in order to determine the association of adequate knowledge of proper hygiene with hygiene practices and parasitic infection after adjusting for students’ age. All reported p-values are two-tailed and statistical significance was set at 0.05.
RESULTS
Table 1 shows the sociodemographic and personal hygiene practices of Angolela school children. From a total of 669 students (mean age= 10.8 years old), approximately 49% of them were girls (mean age=10.7 years old) and 51% of them were boys (mean age=10.9 years old). Estimates of maternal and paternal literacy were 39.7% (N=263) and 67.5% (N=437), respectively. Approximately, one-third of the students reported not bathing for at least 14 days (i.e. poor hygiene practice). Similarly, approximately 9% (N=41), 2% (N=16), 12% (N=79), and 21% (N=135) reported not brushing their teeth, washing their feet, washing or changing their clothes, and washing their hair for at least 14 days, respectively. Taking baths and washing hair were the least common hygiene practices.
Table 1.
Sociodemographic and personal hygiene practices of primary school children, Angolela, Ethiopia, October 2008
| Characteristics | N( Total=669) | % |
|---|---|---|
| Grade | ||
| 1 | 110 | 16.4 |
| 2 | 188 | 28.1 |
| 3 | 114 | 17.0 |
| 4 | 70 | 10.5 |
| 5 | 109 | 16.3 |
| 6 | 78 | 11.7 |
| Gender | ||
| Female | 326 | 48.7 |
| Male | 343 | 51.3 |
| Mother Literate * | ||
| No | 399 | 60.3 |
| Yes | 263 | 39.7 |
| Father Literate * | ||
| No | 210 | 32.5 |
| Yes | 437 | 67.5 |
| Hygiene Practices | ||
| Taking bath * | ||
| 1-7 days | 364 | 58.7 |
| 7-14 days | 45 | 7.3 |
| >14 days | 211 | 34.0 |
| Brushing teeth * | ||
| 1-7 days | 414 | 89.2 |
| 7-14 days | 9 | 1.9 |
| >14 days | 41 | 8.8 |
| Feet washing * | ||
| 1-7 days | 645 | 97.4 |
| 7-14 days | 1 | 0.2 |
| >14 days | 16 | 2.4 |
| Wash or change cloth * | ||
| 1-7 days | 557 | 84.9 |
| 7-14 days | 20 | 3.0 |
| >14 days | 79 | 12.0 |
| Wash hair * | ||
| 1-7 days | 502 | 76.2 |
| 7-14 days | 22 | 3.3 |
| >14 days | 135 | 20.5 |
Numbers/percentages may not add up to the total number due to missing data
Approximately, 61% (N=411), 94% (N=631), and 73% (N=488) of students answered ‘yes’ to whether boiling water kills germs, if a water container needs cleaning and covering, and if human feces contain germs, respectively (Table 2). Fourteen percent of students reported that they boiled their drinking water the day prior to the interview. The most common materials used for anal cleansing were paper (32.2%), leaves (31.3%), and stones (28.9%). When bathing, 94.3% used soap, while 4.5% used only water. The vast majority of children (92%) reported using twigs to clean their teeth.
Table 2.
Knowledge, Attitudes, and Practices (KAP) towards sanitation of primary school children, Angolela, Ethiopia, October 2008
| Characteristics | N(Total=669) | % |
|---|---|---|
| Boiling water kills germs | ||
| No | 25 | 3.7 |
| Yes | 411 | 61.4 |
| Don’t know | 233 | 34.8 |
| Boiled drinking water yesterday | ||
| No | 575 | 85.9 |
| Yes | 94 | 14.1 |
|
Water container needs cleaning
and covering | ||
| No | 17 | 2.5 |
| Yes | 631 | 94.3 |
| Don’t know | 21 | 3.1 |
| Human feces contain germs | ||
| No | 65 | 9.7 |
| Yes | 488 | 72.9 |
| Don’t know | 116 | 17.3 |
| Materials used for anal cleaning * | ||
| Paper | 215 | 32.2 |
| Leaf | 209 | 31.3 |
| Stone | 193 | 28.9 |
| Grass | 16 | 2.4 |
| Water | 12 | 1.8 |
| Nothing | 15 | 2.2 |
| Other | 8 | 1.2 |
| Materials used for bathing * | ||
| Soap | 630 | 94.3 |
| Water only without soap | 30 | 4.5 |
| Other | 8 | 1.2 |
| Materials used for cleaning teeth | ||
| Twigs | 612 | 91.5 |
| Water only | 10 | 1.5 |
| Other | 47 | 7.0 |
Numbers/percentages may not add up to the total number due to missing data
As shown in Table 3, nearly all participants reported washing their hands the day before the interview (N=662, 99.7%), but only 36.2% (N=242) of children reported using soap. The day prior to the interview, 99% (N=655) of students washed their hands before meals and 46% (N=304) washed after meals, but only 15% (N=96) washed after defecation. The majority of participants reported usually washing hands before and after meals (N=658, 99.4% and N=620, 93.9%, respectively) and overall, the preference for hand washing was 98.8% (N=649) before meals and 53.1% (N=351) after meals. Approximately, 75% of students felt hand washing after defecation was important, while the majority of the participants reported that hand washing before and after meals was important (N=660, 99.7% and N=569, 85.7%, respectively).
Table 3.
Hand washing practices among primary school children, Angolela, Ethiopia, October 2008
| Characteristics | N( Total=669) | % |
|---|---|---|
| Washed hands day prior to interview | ||
| No | 2 | 0.3 |
| Yes | 662 | 99.7 |
| Reasons for washing hand ** | ||
| After defecation | 99 | 14.8 |
| Before meals | 661 | 99.0 |
| After meals | 306 | 45.8 |
| Materials used for hand washing | ||
| Soap and water | 242 | 36.2 |
| Water only | 426 | 63.8 |
| Usually wash hands before meal? | ||
| No | 4 | 0.6 |
| Yes | 665 | 99.4 |
| Usually wash hands after meal? | ||
| No | 41 | 6.1 |
| Yes | 627 | 93.9 |
| When do you prefer to wash hands? ** | ||
| Before eating meals | 656 | 98.8 |
| After eating meals | 353 | 53.1 |
| Don’t know | 3 | 0.5 |
| Which do you think is important? ** | ||
| Washing after defecation | 513 | 76.7 |
| Washing before eating meals | 667 | 99.7 |
| Washing after eating meals | 573 | 85.7 |
Numbers/percentages may not add up to the total number due to missing data
Not mutually exclusive
Nearly half of the students (48%) were classified as not having adequate knowledge of proper hygiene (Table 4). Those with adequate knowledge of proper hygiene were more likely to have been assessed as having clean clothes (1.62 AOR, CI 1.14-2.29) on the day of evaluation. Associations of knowledge and hygiene with other hygiene characteristics were not evident. As shown in Table 5, the prevalence of parasitic infections was lower among students with adequate knowledge of proper hygiene (33.2%) than those without adequate knowledge (40.0%), though the association did not reach statistical significance (AOR 0.78, CI 0.56-1.09).
Table 4.
Objectively observed personal hygiene characteristics according to adequate knowledge of proper hygiene practices among primary school children, Angolela, Ethiopia, October 2008
| Characteristics | Distribution of characteristics Total N=669 |
Presence of adequate knowledge of hygene N=349 |
Unadjusted Odds Ratio (95%CI) |
*Adjusted Odds Ratio (95%CI) |
||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Clean clothes | ||||||
| No | 432 | 64.6 | 200 | 58.1 | 1.00(Reference) | 1.00(Reference) |
| Yes | 228 | 34.1 | 144 | 41.9 | 1.99 (1.43-2.76) | 1.62 (1.14-2.29) |
| Fingernails clean | ||||||
| No | 496 | 74.1 | 250 | 72.5 | 1.00(Reference) | 1.00(Reference) |
| Yes | 166 | 24.8 | 95 | 27.5 | 1.32 (0.93-1.88) | 1.11 (0.76-1.61) |
| Fingernails trimmed | ||||||
| No | 404 | 60.4 | 196 | 56.8 | 1.00(Reference) | 1.00(Reference) |
| Yes | 258 | 38.6 | 149 | 43.2 | 1.45 (1.06-1.99) | 1.24 (0.89-1.74) |
| Clean face | ||||||
| No | 191 | 28.6 | 78 | 22.6 | 1.00(Reference) | 1.00(Reference) |
| Yes | 471 | 70.4 | 267 | 77.4 | 1.90 (1.35-2.67) | 1.23 (0.85-1.79) |
|
Eye discharge
present | ||||||
| No | 341 | 51.0 | 298 | 86.4 | 1.00(Reference) | 1.00(Reference) |
| Yes | 320 | 47.8 | 47 | 13.6 | 0.68 (0.45-1.03) | 0.97 (0.62-1.52) |
| Clean hair | ||||||
| No | 341 | 51.0 | 160 | 46.5 | 1.00(Reference) | 1.00(Reference) |
| Yes | 320 | 47.8 | 184 | 53.5 | 1.53 (1.13-2.08) | 1.15 (0.83-1.60) |
Adjusted for age (continuous)
Numbers/percentages may not add up to the total number due to missing data
Table 5.
Presence of any intestinal parasitic infections according to adequate knowledge of proper hygiene practices among primary school children, Angolela, Ethiopia, October 2008
| Presence of Parasitic Infection |
Adequate Knowledge | Unadjusted OR (95%CI) |
*Adjusted OR (95%CI) |
|||
|---|---|---|---|---|---|---|
| No (N=320) | Yes (N=349) | |||||
| N | % | N | % | |||
| No | 192 | 60.0 | 232 | 66.8 | 1.00 (Reference) | 1.00(Reference) |
| Yes | 128 | 40.0 | 116 | 33.2 | 0.75(0.55-1.02) | 0.78(0.56-1.09) |
Adjusted for age (continuous)
DISCUSSION
In this study of school children grades 1-6, we assessed the knowledge, attitudes and practices of hygiene. Of the students surveyed, 52% were classified as having proper knowledge of hygiene. This knowledge is necessary for the practice of proper hygiene in the school environment. Only 14.8% of the students washed hands after defecation the day prior to the interview. We also found that out of the personal hygiene characteristics assessed, students having proper knowledge of hygiene were more likely to have clean clothes (AOR 1.62, CI 1.14-2.29). Overall our findings are consistent with previous studies that have documented knowledge and practices of hygiene among school children in developing countries (16, 19-21).
Overall, the majority of students reported washing hands before meals. The percentages of children who reported the importance of and the preference for hand washing before eating were 99.7% and 98.8%, respectively. These high proportions are consistent with the high proportion of children who reported actually washing their hands before meals (99.0%). Notably, the self-reported frequency of hand washing before meals among children in our study is substantially higher than frequencies reported from studies of children in other countries. For instance, studies from the Philippines and Colombia indicated that 75.9% and 46.9% of students, respectively, reported washing hands before meals. The considerably higher frequency of hand washing before meals among Ethiopian children may be due, in part, to the Ethiopian cultural tradition and ceremonial practice of washing hands before meals (22) or the desire for clean, fresh hands before eating (12). However, only 36.2% of students who washed their hands reported using soap. This is similar to the Philippines and Turkey studies where an average of 37.7% and 42.4% of children, respectively, washed their hands with soap (19, 20).
Fecal-oral contamination is a major cause of transmissible diseases such as gastrointestinal infections. Although not statistically significant, it was found that students with adequate knowledge of proper hygiene were 22% less likely to have prevalent parasitic infections (AOR 0.78, CI 0.56-1.09) than their less knowledgeable peers.
Washing hands after defecation is one of the most effective ways to prevent gastrointestinal parasitic infections (2, 6). While 76.7% of students reported that washing hands after defecation is important, only 14.8% reported actually following this practice. This may be due, in part, to the attitudes of the school children. Although the students know that washing hands after defecation is important, they may be negatively influenced by factors such as laziness, the rush to play with friends, or even the lack of hand washing facilities close to the latrines (11). In contrast, studies conducted in Colombia and India reported that 82.5% and 86.4% of students, respectively, wash their hands after using the toilet (16, 21).
The most common hygiene practices, in order of rank, were washing feet (97.4%), brushing teeth (89.2%), and changing clothes (84.9%). Bathing and hair washing received the lowest ranks. Approximately 34% of the students reported poor bathing practices and 21% reported poor hair washing practices. These findings are in concurrence with a study conducted in the Philippines which found that 35% of students reported poor bathing (19). Based on these results, it appears that the hygiene practices which require the greatest amount of water result in lower rates of practice. Both bathing and hair washing require relatively larger volumes of water. Given the high proportion of students in homes with limited access to piped water and the burden of carrying water from long distances, it is likely that many of the students forgo these two practices. Since obtaining water is a challenge in rural settings, it is a common practice for families to ration and re-use their supply (8). Thus, personal hygiene becomes a low priority when water is scarce. Rather than use water for personal cleanliness, families prioritize their use of available water for drinking, cooking, washing clothes, and household cleaning (8).
The low frequencies of hand washing with soap (36.2%) may be attributed to the lack of soap in school and at home. Soap, water, and latrines are essential for proper hygiene practice in schools, (23) but previous studies have cited inadequate resources (8, 16). A study conducted among Colombian school children reported that only 7% of students reported having clean water and soap regularly available at school (16). Those that had water and soap were three times more likely to wash their hands before eating or after using the toilet (16). Even if knowledge of hygiene exists, lack of appropriate resources may negatively affect proper hand washing practices. Though data about the availability of resources in the student’s households were not collected in our study, the resources available in rural communities are generally lacking (24). A UNICEF study conducted in Ethiopia found that less than one-third of schools had water points and only 5% had hand washing facilities, none of which had soap (24).
Another reason that can influence hygiene practice among school children is the low level of parental literacy. In this study, the mother’s literacy rate was lower than the father (39.7% and 67.5%, respectively). In Ethiopia, the mother is typically the primary caretaker of the family and is thus charged with teaching her children proper health and hygiene practices (25). An illiterate or uneducated mother may be less knowledgeable about teaching her children proper hygiene practices, subsequently leading to increased rates of infection and disease amongst her children (26). In light of these observations, future school-based health and hygiene education programs should include strategies to involve family members, particularly mothers and siblings.
Several limitations must be considered when interpreting our results. First, students’ self-reported behaviors may have resulted in over-reporting of proper hygiene practices. We attempted to mitigate this bias by including objective measures of students’ personal hygiene characteristics. Namely, trained research-nurse interviewers assessed each student and these assessments were made without knowledge of students’ reported hygiene behaviors. Data from observations were generally consistent with students’ self-reported practices. Second, our study was limited to students in grades 1-6 and those who were present in school. Children absent due to illness or other circumstances were not included, thus results may not be generalized to all school children. Third, the cross-sectional study design makes determining causality impossible. Lastly, there was no significant association between knowledge of proper hygiene and overall reported hygiene which may be attributed to the small sample size and possible over reporting of hygiene practices.
Previous studies have indicated that personal hygiene in Ethiopia is very low,(13) however, to the best of our knowledge, no published reports have assessed KAP of hygiene among rural schools in Ethiopia. Our findings underscore the need for integrating hand washing and hygiene education programs in schools. Successful implementation of such programs is likely to contribute to reductions in morbidity and mortality associated with communicable diseases.
Importantly, Ethiopian and foreign global public health agencies have been taking steps towards enhancing access to resources and to increase health literacy particularly concerning sanitation and hygiene. In 2007, UNICEF launched the Water, Sanitation, and Hygiene (WASH) Program which is designed to promote hand washing and sanitation practices in low income countries including Ethiopia (24). Additionally, the Ethiopian Ministry of Health recently implemented a National Millennium Hygiene and Sanitation Movement Program with the aim of cleaning up all homes, kebeles, and towns for the new millennium (14). These initiatives, coupled with well developed school-based health and hygiene curricula that promote improved personal hygiene at home and at school should contribute to better health and hygiene conditions among school children (24).
This study targets two key issues that must be addressed when creating health and hygiene promotion programs. First, only half of students (52%) were classified as having proper hygiene knowledge. To increase this rate, health clubs can be formed to teach students about disease causation and transmission, demonstrate proper hand washing and hygiene practices, and provide incentives for good hygiene. Education has the potential to significantly alter the behavior patterns of students and can thereby lead to improved outlooks on hygiene. Second, much of hygiene practices are contingent upon availability of sufficient resources. Well-designed and well-located hand washing facilities and latrines that include adequate amounts of soap and water, are essential in promoting hygiene. If hygiene intervention programs implement these two important factors—education and resources—the needs of students can be better met and can thereby result in decreased risk of disease.
In conclusion, school-based hygiene education is vital in order to decrease the rates of transmissible diseases (16). Children are more receptive to learning and are very likely to adopt healthy behaviors at a younger age. They can also be agents of change by spreading what they have learned in school to their family and community members. Future studies regarding KAP should specifically assess the attitudes that students have towards hygiene, availability of water and sanitation facilities at home and at school, and the reasons behind hand washing. Enhanced, comprehensive knowledge about these issues should be used to improve low-cost but highly effective programs that will meaningfully attenuate the burden of transmissible disease among students in rural settings.
ACKNOWLEDGEMENTS
This research was completed while Ms. Alyssa Vivas was a research training fellow with the Multidisciplinary International Research Training (MIRT) Program of the University of Washington, School of Public Health (Seattle, WA, USA). The MIRT Program is supported by an award from the National Institutes of Health, National Center on Minority Health and Health Disparities (T37-MD001449). The authors wish to thank Feed the Children Ethiopia, Angolela Primary School, and Addis Continental Institute of Public Health for providing facilities and logistics support throughout the research process. The authors would also like to thank Debre Birhan Hospital, the Woreda Health Office, and the Woreda Education Office for providing testing facilities and granting access to conduct the study.
REFERENCES
- 1.World Health Organization [Accessed August 4, 2009];Better Health for Poor Children. Available at: http://www.who.int/child_adolescent_health/documents/a91061/en/index.html.
- 2.Curtis VA, Danquah LO, Aunger RV. Planned, motivated and habitual hygiene behaviour: an eleven country review. Health Educ Res. 2009;4:655–673. doi: 10.1093/her/cyp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization [Accessed August 5, 2009];Hand-washing could save the lives of millions of children. Available at: http://www.scielosp.org/scielo.php?lng=en.
- 4.Ejemot RI, Ehiri JE, Meremikwu MM, Critchley JA. Hand washing for preventing diarrhoea. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD004265.pub2. CD004265. [DOI] [PubMed] [Google Scholar]
- 5.Snow M, White GL, Jr., Kim HS. Inexpensive and time-efficient hand hygiene interventions increase elementary school children’s hand hygiene rates. J Sch Health. 2008;78:230–233. doi: 10.1111/j.1746-1561.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 6.United Nations Children’s Fund [Accessed August 5, 2009];Soap, Toilets, and Taps. A Foundation for Healthy Children. Available at: www.unicef.org/wash/files/FINAL.
- 7.Global Handwashing Day [Accessed August 5, 2009];Global Public-Private Partnership for Hand Washing. Available at: www.globalhandwashingday.org.
- 8.Oswald WE, Hunter GC, Lescano AG, Cabrera L, Leontsini E, Pan WK, et al. Direct observation of hygiene in a Peruvian shantytown: not enough handwashing and too little water. Trop Med Int Health. 2008;13:1421–1428. doi: 10.1111/j.1365-3156.2008.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Loughlin R. Follow-up of a low cost latrine promotion programme in one district of Amhara, Ethiopia: characteristics of early adopters and non-adopters. Tropical Medicine and International Health. 2006;11:1406–15. doi: 10.1111/j.1365-3156.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization [Accessed August 5, 2009];World Health Statistics 2009. Available at: www.who.int/entity/whosis/whostat/2009.
- 11.Water and Sanitation Program [Accessed July 10, 2009];Can hygiene be cool and fun: Insights from School Children in Senegal. Available at: http://www.comminit.com/en/node/264152/38.
- 12.Scott B, Curtis C, Rabie T, N G-A. Health in our hands but not in our heads: understanding hygiene motivation in Ghana. Health Policy and Planning. 2007;22:225–233. doi: 10.1093/heapol/czm016. [DOI] [PubMed] [Google Scholar]
- 13.Kumie A, Ali A. An overview of environmental health status in Ethiopia with particular emphasis to its organization, drinking water and sanitation: A literature survey. Ethiopian Journal of Health Development. 2005;19:89–103. [Google Scholar]
- 14.Research-inspired Policy and Practice Learning in Ethiopia [Accessed July 13, 2009];Promoting Sanitation and Hygiene to Rural Households: the experience of the Southern Nations region of Ethiopia. Available at: http://www.rippleethiopia.org/documents/stream/20081208-synthesissanitation-hygiene.
- 15.Central Statistical Agency of Ethiopia [Accessed July 13, 2009];Summary and Statistical Report of the 2007 Population and Housing Census. Available at: http://www.csa.gov.et/pdf/Cen2007_firstdraft.pdf.
- 16.Lopez-Quintero C, Freeman P, Neumark Y. Hand washing among school children in Bogota, Colombia. Am J Public Health. 2009;99:94–101. doi: 10.2105/AJPH.2007.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauhanen LA, Lynch JW, Lakka HM, Kauhanen J, Davey Smith G. Association of diarrhoea, poor hygiene and poor social conditions in childhood with blood pressure in adulthood. J Epidemiol Community Health. 2009 Sep 8; doi: 10.1136/jech.2008.083402. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Basic Laboratory Methods in Medical Parasitology. Geneva: 1991. [Google Scholar]
- 19.Department of Health [Accessed July 10, 2009];Republic of the Philippines. Personal Hygiene. Available at: http://www.doh.gov.ph/h1n1/images/dm2009-0113.pdf.
- 20.Yalcin SS, Yalcin S, Altin S. Hand washing and adolescents. A study from seven schools in Konya, Turkey. Int J Adolesc Med Health. 2004;16:371–6. [PubMed] [Google Scholar]
- 21.Banda K, Sarkar R, Gopal S, Govindarajan J, Harijan BB, Jeyakumar MB, et al. Water handling, sanitation and defecation practices in rural southern India: a knowledge, attitudes and practices study. Trans R Soc Trop Med Hyg. 2007;101:1124–30. doi: 10.1016/j.trstmh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Intercultural Communication Specialists [Accessed July 28, 2009];Ethiopia: Language, Culture, Customs and Etiquette. Available at: http://www.kwintessential.co.uk/resources/global-etiquette/ethiopia.html.
- 23.Gorter AC, Sandiford P, Pauw J, Morales P, Perez RM, Alberts H. Hygiene behaviour in rural Nicaragua in relation to diarrhoea. Int J Epidemiol. 1998;27:1090–100. doi: 10.1093/ije/27.6.1090. [DOI] [PubMed] [Google Scholar]
- 24.United Nations Children’s Fund [Accessed August 5, 2009];Water, Sanitation, and Hygiene Annual Report. Available at: www.unicef.org/wash.
- 25.Huq MN, Tasnim T. Maternal education and child healthcare in Bangladesh. Matern Child Health J. 2008;12:43–51. doi: 10.1007/s10995-007-0303-3. [DOI] [PubMed] [Google Scholar]
- 26.Nematian J, Nematian E, Gholamrezanezhad A, Asgari AA. Prevalence of intestinal parasitic infections and their relation with socio-economic factors and hygienic habits in Tehran primary school students. Acta Trop. 2004;92:179–86. doi: 10.1016/j.actatropica.2004.06.010. [DOI] [PubMed] [Google Scholar]