Abstract
Adult neurogenesis occurs in two privileged microenvironments, the hippocampal subgranular zone of the dentate gyrus and the subventricular zone (SVZ) along the lateral ventricle. This review focuses on accumulating evidence suggesting that the activity of specific brain regions or bodily states influences SVZ cell proliferation and neurogenesis. Neuromodulators such as dopamine and serotonin have been shown to have long-range effects through neuronal projections into the SVZ. Local GABA and glutamate signaling have demonstrated effects on SVZ proliferation and neurogenesis, but an extra-niche source of these neurotransmitters remains to be explored and options will be discussed. There is also accumulating evidence that diseases and bodily states such as Alzheimer's disease, seizures, sleep, and pregnancy influence SVZ cell proliferation. With such complex behavior and environmentally-driven factors that control subregion-specific activity, it will become necessary to account for overlapping roles of multiple neurotransmitter systems on neurogenesis when developing cell therapies or drug treatments.
Introduction
Neurogenesis exists in two regions of the adult brain: the hippocampal subgranular zone of the dentate gyrus and the subventricular zone (SVZ) along the lateral ventricle. Neural stem cells are found in all mammalian species examined including humans, but the presence of neurogenesis in humans remains controversial (Curtis et al, 2007; Sanai et al, 2004). This review focuses on the SVZ, where neuroblasts are generated and are fate-committed to becoming granule or periglomerular interneurons in the olfactory bulb (OB).
Neurogenesis occurs in a privileged microenvironment called the neurogenic niche. In this niche, neurogenesis has long been thought to be protected from brain activities or states. However, accumulating evidence suggests that the activity of specific brain regions or bodily states can influence neurogenesis. For example, dopamine originating from neurons in the substantia nigra affects SVZ-OB neurogenesis (see references in the dopamine section). Environmental enrichment, which presumably activates several brain regions, also augments SVZ-OB neurogenesis (Rochefort et al, 2002). Environmental enrichment likely targets neurotransmitter systems and other signaling molecules including hormones and growth factors that have been shown to influence SVZ neurogenesis (for reviews see (Grote and Hannan, 2007; Hagg, 2005; Pathania et al, 2010)). In this review, we examine the action of neurotransmitters on neurogenesis and their effects on proliferation, migration, and survival of SVZ cells. We describe their local versus long-range signaling and how their signaling is affected by brain states. For each neurotransmitter system, we discuss evidence and hypotheses that pathological states such as seizures and Parkinson's disease modulate neurogenesis, perhaps through alterations in specific neurotransmitter signaling.
We propose that understanding how the SVZ responds to the discussed signals will help uncover the impact of diseases, treatments, and brain states on cell recruitment and regulation of SVZ cell neurogenic potential. We apologize to the authors of papers that could not be cited here due to space constraints.
Structural organization of the SVZ
The SVZ is made up of three main cell types: neuroblasts, astrocyte-like cells (also called SVZ astrocytes), and intermediate progenitor cells called transit amplifying cells (TACs). Some of the astrocyte-like cells are neural progenitor cells (also called stem cells). They are capable of self-renewal and generate TACs that in turn generate neuroblasts. Neuroblasts are immature neurons that are fate-committed to becoming GABAergic granule or periglomerular interneurons in the OB. The tight association and organization allows for rapid paracrine communication between cells that are within the niche (for review see (Bordey, 2006; Pathania et al, 2010)). Its position near the ventricle and spanning a large portion of the brain allows for the possibility of extra-niche paracrine neurotransmission from nearby neurons and their collaterals. Paracrine neurotransmission has also been referred to as volume or non-synaptic transmission. In addition, the presence of an extensive blood vessel network suggests that hormones could impact neurogenesis in the SVZ (Mirzadeh et al, 2008; Shen et al, 2008; Snapyan et al, 2009; Tavazoie et al, 2008). Such a hormonal control of neurogenesis is not discussed in this review, which instead focuses on neurotransmitter systems.
Neurotransmitter systems directly affecting SVZ neurogenesis
The neurotransmitter systems covered here encompass the ‘traditional’ neurotransmitters, gamma-aminobutyric acid (GABA) and glutamate, normally considered as primarily confined to the synapse and responsible for fast synaptic transmission, and neuromodulatory transmitters or neuromodulators such as dopamine, serotonin, and acetylcholine that are secreted by a small group of neurons and can affect neuronal activity through large brain areas. We first discuss neuromodulators because there are clear examples of long-range paracrine or non-synaptic signaling through neuronal projections into the SVZ. The extra-niche signal from GABA and glutamate is more hypothetical, as discussed below. Figure 1 provides a diagram illustrating the presented findings that are also summarized in Table 1.
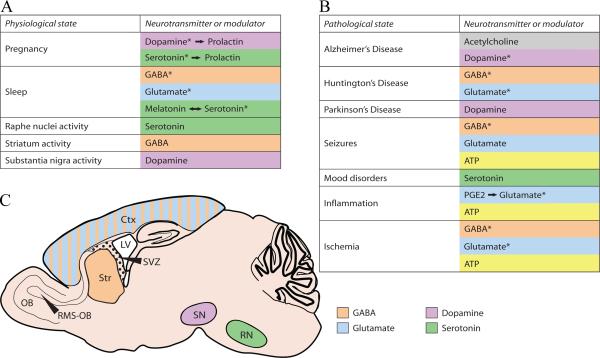
Figure 1.
Neurotransmitters and related neuromodulators modify neurogenesis by relaying signals from other parts of the brain. (A and B) Tables summarizing brain activity (A) and disease or injury states (B) and the corresponding neurotransmitters involved with these states. * denotes postulated role. (C) Sagittal representation of relevant structures in the mouse brain and their associated neurotransmitters. Ctx, cortex; LV, lateral ventricle; OB, olfactory bulb; RMS-OB, rostral migratory stream of the olfactory bulb; RN, raphe nuclei; SN, substantia nigra; Str, striatum; SVZ, subventricular zone.
Table 1.
Summary of discussed findings.
| Neurotransmitter | Receptors in the SVZ | Projections from NT releasing neurons | Effect on SVZ proliferation | Effect on migration | Effect on SVZ cell survival | Associated brain states or pathologies | Effect of pathology on NT signaling |
|---|---|---|---|---|---|---|---|
| Acetylcholine | none; nAChR α7/β2 in OB | ? | ? | ? | Increased in (N) near OB | Alzheimer's Disease | Decreased |
| ATP | P2X2, P2X4 (N*); P2X7 (E*) | SVZ or surrounding astrocyte-like cells? | Increased | ? | ? | Hypoxia/Ischemia | Increased |
|
Seizure |
Increased |
||||||
|
P2Y (N) |
Injury |
Increased |
|||||
| Dopamine | D1-like (TAC cytoplasm) | Substantia nigra (rodents, primates, humans) | Increased | ? | ? | Parkinson's Disease | Decreased |
|
D2-like (TAC, A*) |
|||||||
|
D3 (TACs, but not A or N) |
|||||||
| GABA | α2, 3, 4, β1 and 2, and γ2 (N) | Striatal neurons (?) | Decreased | Decreased | ? | Huntington's Disease | Decreased/altered |
|
GABAA subunits - unknown (A) |
Seizures |
? |
|||||
| Glutamate | AMPA | SVZ or surrounding astrocyte-like cells (rodent) | Increased (in neural progenitor culture) | None | Increased in (N) | Ischemia/Hypoxia | Increases in adult |
|
GluK5 (N) |
Seizure |
Increases |
|||||
|
NMDA (N) | |||||||
|
(Group I) mGluR1 and 5 (N) | |||||||
|
Group 2 mGluRs | |||||||
| Serotonin | 5-HT 1A, 2A, 2 C (may have others) | Raphe nucleus | Increased | ? | ? | Pregnancy | Increased- can regulate prolactin release |
|
Mood disorders |
Decreased/altered |
||||||
|
Sleep |
Melatonin (dependent on serotonin for synthesis)-increases SVZ proliferation |
||||||
A= astrocyte-like cells; N= neuroblast; E= ependymal cell; TAC= transit amplifying cells.
Dopamine
Dopamine is a catecholamine that is synthesized by neurons mainly in the substantia nigra, ventral tegmental area, and hypothalamus, with neurological functions including roles in punishment and reward, mood, sleep, attention, and learning (Lima et al, 2009). Dopamine receptors are G protein-coupled receptors classified into two groups, D1-like (D1 and D5) and D2-like (D2, D3, and D4). The two groups are distinguished based on structure and interactions with different G proteins. D1-like receptors activate adenylyl cyclase, whereas D2-like receptors inhibit adenylyl cyclase activity (Callier et al, 2003).
Receptors and dopaminergic inputs in the SVZ
Both D1-like and D2-like receptors have been identified in rodent SVZ-derived neurospheres (Coronas et al, 2004; Hoglinger et al, 2004; Winner et al, 2009), and mRNA expression and in vivo studies have implicated the D3 receptor (D2-like) in embryonic and adult SVZ neurogenesis (Diaz et al, 1997; Kim et al, 2010; Van Kampen et al, 2004). D1-like receptors were found in the cytoplasm but not the cell membrane of TACs and in the cell membrane of neuroblasts, whereas D2-like receptors were most abundantly expressed in TAC cell membranes and sparsely in SVZ astrocyte cell membranes (Hoglinger et al, 2004). TACs expressed D3 receptors while neuroblasts and SVZ astrocytes did not (Kim et al, 2010).
Dopaminergic projections to the SVZ from the substantia nigra have been demonstrated in rats (Hoglinger et al, 2004; Winner et al, 2006), mice (Baker et al, 2004) and primates (Freundlieb et al, 2006). In addition, postmortem studies in humans have identified dopaminergic fibers in contact with epidermal growth factor receptor (EGFR)-positive cells in the SVZ, which are presumably TACs (Hoglinger et al, 2004).
Influence of dopaminergic signaling and circuitry on SVZ neurogenesis
Convincing evidence suggests that dopamine has an impact on neurogenesis at several developmental stages and in regions including the adult SVZ. However, some controversy remains regarding the actual effect of dopamine on SVZ cell proliferation and neurogenesis as summarized below (for reviews see (Borta and Hoglinger, 2007; O'Keeffe et al, 2009a)). Two types of manipulations of dopaminergic signaling have been performed to test their effects on SVZ neurogenesis: pharmacological and genetic manipulations targeted to dopamine receptors and ablation of dopaminergic inputs projecting to the striatum and the SVZ. In vivo pharmacological manipulations using agonists or antagonists of selective dopamine receptors led to significant increases or decreases in SVZ neurogenesis in rodents as measured using injections of the S-phase marker bromodeoxyuridine (BrdU) (Kim et al, 2010; Kippin et al, 2005; Van Kampen et al, 2004). However, one study using D3 receptor ligand reported no effect (Baker et al, 2005). Discrepancies have been noted between studies that may be due to the age of the animals, the species, and perhaps the selectivity of the agonists or antagonists. The clinically relevant dopamine precursor, levodopa (L-DOPA), had surprisingly no significant effect on baseline SVZ cell proliferation in rats (O'Keeffe et al, 2009b). Studies using genetic manipulations have been limited. Only one study used transgenic knock-out mice for D2 receptors, but there was no effect on SVZ cell proliferation presumably due to compensatory mechanisms (Kippin et al, 2005). A better approach would be to use the floxed strategy to remove one receptor sub-type at a given time.
Ablation of dopaminergic inputs resulted in decreased proliferation in the SVZ, suggesting that substantia nigra neuron activity controls SVZ cell proliferation and neurogenesis (Baker et al, 2004; Cova et al, 2010; Freundlieb et al, 2006; Hoglinger et al, 2004; O'Keeffe et al, 2009a; 2009b). These studies ablated dopaminergic neurons in the substantia nigra through 6-OHDA or MPTP injection. The lesions resulted in loss of tyrosine hydroxylase (TH, the enzyme responsible from converting amino acid into DOPA, a dopamine precursor) immunoreactivity in the SVZ and a decrease in SVZ proliferation (Baker et al, 2004; Hoglinger et al, 2004; Winner et al, 2006). One study suggested that fiber-released dopamine acted through decreased local EGF production (O'Keeffe et al, 2009b). In addition, L-DOPA injection restored EGF levels to baseline and rescued ablation-induced SVZ cell proliferation (O'Keeffe et al, 2009b). Considering the function of the substantia nigra in reward, addiction, and movement, it is tempting to speculate that SVZ cell proliferation and perhaps neurogenesis are regulated by addictive behaviors and new movement-based learning paradigms.
Pathological brain states
: Loss of dopaminergic neurons in the substantia nigra is the primary cause of Parkinson's disease (PD) and leads to decreased dopamine levels in the striatum (Kadir and Nordberg, 2010). Ablation of dopaminergic neurons in the substantia nigra is a classical method to model PD in animals. In these animal models of PD, SVZ proliferation was decreased suggesting that PD is associated with decreased neurogenesis (Baker et al, 2004; Cova et al, 2010; Hoglinger et al, 2004; O'Keeffe et al, 2009b; Winner et al, 2009). Indeed, postmortem studies of PD patients have demonstrated a decreased number of proliferative cells in the SVZ (Hoglinger et al, 2004).
Serotonin
Serotonin (5-HT) is a monoamine that is synthesized by neurons in the raphe nuclei, which regulates many aspects of behavior including mood, sleep, appetite, reproductive activity, and cognition. Seven families of 5-HT receptors (5-HT1-7) have been classified, and all except 5-HT3 are G protein-coupled receptors. 5-HT3 is a ligand-gated cation channel.
Receptors and serotonergic inputs in the SVZ
The exact expression pattern of 5-HT receptors in the SVZ remains controversial. RT-PCR from SVZ tissue suggests the expression of a wide array of 5-HT receptors: 1A, 1B, 1D, 2A, 2B, 2C, 3A, and 6 shown by Councill et al, 2006 but only 1A, 2A, and 2C reported by Hitoshi et al, 2007. Dissected SVZ tissue is often contaminated by striatal tissue which may explain some of the discrepancies. Pharmacological experiments implicate the presence and functionality of 5-HT1A, 5-HT1B, and 5-HT2C receptors in the SVZ (Banasr et al, 2004; Soumier et al, 2010). Cell type-specific 5-HT receptor expression using immunohistochemistry remains to be determined. The SVZ in addition to the striatum receives serotonergic inputs from the raphe nuclei (Brezun and Daszuta, 1999; Lorez and Richards, 1982).
Influence of serotoninergic signaling and circuitry on SVZ neurogenesis
It is generally thought that serotonin has a positive effect on SVZ neurogenesis. Indeed, in vivo infusion of serotonin results in increased neurosphere production from the SVZ (Hitoshi et al, 2007). Agonists of 5-HT1A and 5-HT2C receptors increased SVZ proliferation (Banasr et al, 2004; Soumier et al, 2010) although 5-HT1B receptor activation decreased SVZ proliferation in vivo (Banasr et al, 2004). It has not been determined, however, whether these effects are direct through activation of 5-HT receptors on SVZ cells or indirect through receptor activation on cells in other structures such as the choroid plexus, which is adjacent to the SVZ in the lateral ventricle (Soumier et al, 2010).
Ablation of raphe nuclei results in decreased SVZ proliferation (Banasr et al, 2004; Brezun and Daszuta, 1999). Pregnant female mice have higher levels of serotonergic innervation in the SVZ that has been suggested to be responsible for increased SVZ proliferation (Diaz et al, 2009). Considering that serotonin is well-known for its role in mood regulation, it is possible that mood disorders associated with altered serotonin levels have altered SVZ cell proliferation.
GABA
GABA is the main inhibitory neurotransmitter in the adult brain and an excitatory neurotransmitter in the developing brain. It acts through activation of ionotropic ligand-gated GABAA or GABAC receptors and G-protein coupled GABAB receptors.
Receptors and GABAergic inputs in the SVZ
Functional GABAA receptors have been identified in the SVZ/RMS on SVZ astrocytes and neuroblasts (Gascon et al, 2006; Nguyen et al, 2003; Stewart et al, 2002; Wang et al, 2003b). The presence of such receptors on TACs remains unexplored. The exact subunit composition in the SVZ and in the different cell types remains to be examined. RT-PCR from neurospheres or neonatal cultured neuroblasts has yielded the expression of α2, 3, 4, β1 and 2, and γ2 in neuroblasts (Nguyen et al, 2003; Stewart et al, 2002). The expression of GABAB receptors has not been explored.
The SVZ is located along the striatum, which is predominately composed of GABAergic neurons. In addition, nitric oxide-containing GABAergic striatal neurons project into the SVZ (Moreno-Lopez et al, 2000). These neurons may thus provide an activity-dependent GABAergic control of SVZ neurogenesis. In addition, vesicular GABA transporter expression (VGAT, using immunostaining) has been reported in the SVZ in agreement with the presence of GABAergic inputs from the striatum (Platel et al, 2007).
Influence of GABAergic signaling and circuitry on SVZ neurogenesis
The function of GABAergic signaling on SVZ neurogenesis has been previously reviewed (Bordey, 2006; 2007; Platel et al, 2008a). To be brief, GABA has significant impact on several phases of SVZ/OB neurogenesis: proliferation of the astrocyte-like stem cells and neuroblasts (in cultured slices) (Liu et al, 2005; Nguyen et al, 2003), neuroblast migration and differentiation in acute slices (Bolteus and Bordey, 2004a; Gascon et al, 2006). Some of these effects may involve the canonical CREB pathway activation as shown in the SVZ and hippocampal neurogenic zone (Giachino et al, 2005; Herold et al, 2010; Jagasia et al, 2009). Importantly, there are no in vivo data on the function of GABA and its receptors on SVZ neurogenesis.
The proposed source of GABA is intrinsic to the SVZ, i.e. from neuroblasts. Indeed neuroblasts have been shown to synthesize and release GABA in a calcium-dependent but nonvesicular manner (De Marchis et al, 2004; Gascon et al, 2006; Liu et al, 2005; Nguyen et al, 2003; Wang et al, 2003b). However, it remains unclear whether some of the effects on the behavior of SVZ cells could be attributed to an external source, i.e. from striatal GABAergic neurons that are adjacent to SVZ cells.
Pathological brain states
Huntington's Disease (HD) is a neurodegenerative disorder that primarily “attacks” the striatum. HD is generally an inherited neurodegenerative disease caused by the accumulation of CAG-repeats in the huntingtin gene. Disruption of this gene leads to the degeneration and death of medium spiny neurons in the striatum. In human patients, markers of proliferation in the SVZ are increased compared to controls (Curtis et al, 2003; 2005). Similarly, quinolinic acid (QA)-induced models of HD show an increase in both SVZ proliferation and migration to the injury site (Tattersfield et al, 2004). QA is an excitatory amino acid that induces cell death, and injection into the striatum leads to destruction of medium spinal GABAergic neurons while cholinergic neurons remain intact (Beal et al, 1986; Brickell et al, 1999; Nicholson et al, 1995). Interestingly, this increase in SVZ neurogenesis persisted only 14 days post-QA injection perhaps due to repair over time and thus normalization of the injury. Such finding was not observed in a genetic mouse model of HD, the R6/2 mouse line (Phillips et al, 2006; 2005). Nevertheless, there was a decrease in adult born granule cells in these mice perhaps due to the observed re-routing of neuroblasts into the striatum (Kohl et al, 2010). It is important to examine whether GABAergic neurons projecting into the SVZ are spared in the R6/2 mice compared to the QA model. Although these data could be explained by the loss of GABAergic inputs in the SVZ and thus changes in SVZ cell proliferation, factors released due to neuronal cell death could contribute to the changes in SVZ cell proliferation independent of GABA. A more direct genetic manipulation of GABAergic inputs in the SVZ is required to address their function on SVZ proliferation and ultimately neurogenesis.
Glutamate
GABA's counterpart, glutamate, is also known to affect SVZ neurogenesis (for reviews see (Platel et al, 2008a; 2010b)). Glutamate signals through ionotropic AMPA/kainate and NMDA receptors as well as metabotropic mGluR1-8 receptors.
Receptors and glutamatergic inputs in the SVZ
In vitro, Brazel et al. (2005) showed that rat neural progenitor cells express AMPA, kainate, NMDA and group 2 mGluR receptors using calcium imaging and pharmacology (Brazel et al, 2005). In neurospheres, neural progenitor cells were also found to express mGluR1 (Castiglione et al, 2008). We provided evidence that in acute slices SVZ neuroblasts express a mosaic of glutamate receptors including AMPA and kainate (GluK5) receptors (Platel et al, 2007; 2008b), NMDA receptors (Platel et al, 2010a), and mGluR5 (Platel et al, 2008b) based on calcium responses, electrophysiological recordings, and immunohistochemistry. mGluR5 were also shown to be expressed in SVZ cells in vivo (Di Giorgi Gerevini et al, 2004; Gandhi et al, 2008). Astrocyte-like cells do not express functional NMDA or AMPA/kainate receptors (Liu et al, 2005). There are no data on glutamate receptors in TACs. One recently identified source of glutamate is astrocyte-like cells in the SVZ. These cells express vesicular glutamate transporter 1 and release glutamate upon intracellular calcium increases (Platel et al, 2010a). It is unknown whether cortical axons projecting onto striatal neurons invade the SVZ.
Influence of glutamatergic signaling on SVZ neurogenesis
In cultures from SVZ neurospheres glutamate agonists decreased markers of cell death, and more specifically kainate and group 2 mGluR agonists increased proliferation according to 3H-thymidine incorporation and cell counts (Brazel et al., 2005). There is also evidence that mGluR5 exerts a positive effect on SVZ cell proliferation in vivo and promoted neuroblast survival in cultures (Castiglione et al, 2008; Di Giorgi Gerevini et al, 2004; Gandhi et al, 2008). Antagonists of kainate receptors, but not those of mGluR5, decreased neuroblast migration in whole mount preparations of the SVZ (Platel et al, 2008b). Finally, genetic knockout of NMDA receptors using a floxed strategy in vivo led to neuroblast apoptosis suggesting that NMDA receptors are important for neuroblast survival (Platel et al, 2010a).
Pathological brain states
Signals that increase calcium in astrocyte-like cells through Gq-coupled receptor activation lead to glutamate release and receptor activation in adjacent neuroblasts (Platel et al, 2010a). One remaining question relates to the identity of the signals controlling calcium activity in astrocyte-like cells. One pathological and perhaps physiological signal is prostaglandin E2 (PGE2). Prostaglandins, including PGE2, are potent mediators of inflammation and are produced from arachidonic acid via the action of cyclooxygenases (COX, also known as PGH synthase). PGE2 is synthesized from PGH2 via PGE synthase. Two isoforms of cyclooxygenase, COX-1 (constitutive) and COX-2 (low constitutive and inflammatory induced), have been cloned. COX-2 is expressed in SVZ microglial cells under normal conditions (Goncalves et al, 2010) and the choroid plexus releases significantly more PGE2 than acute SVZ explants (Dave et al, 2010), as shown using immunohistochemistry and ELISA measurements, respectively. As reported in mature astrocytes (Bezzi et al, 1998; Sanzgiri et al, 1999; Zanotti and Charles, 1997), we found that PGE2 induces calcium increase in SVZ astrocytes resulting in glutamate release and NMDA receptor activation in adjacent neuroblasts in acute slices (Dave et al, 2010).
A major pathological condition affecting glutamate levels is ischemia associated with stroke. However, because ischemia also affects other neurotransmitter systems, including GABA, aspartate, and ATP (Andine et al, 1991; Franke et al, 2006b; Vannucci et al, 1999), the role of ischemia on neurogenesis is discussed below.
Adenosine 5′-triphosphate (ATP)
ATP plays an important role in CNS development through activation of selective purinergic receptors such as the P2X family of ligand-gated ion channels (P2X1-7) and the P2Y families of G-protein-coupled receptors (P21-4, 6, 11-14) (for review see (Abbracchio et al, 2009; Zimmermann, 2006)).
Receptors and ATP sources in the SVZ
Astrocyte-like cells of the SVZ express functional ATP hydrolyzing ectonucleotidase in particular NTPDase2 suggesting the presence of ATP signaling in the SVZ (Braun et al, 2003; Lin et al, 2007). Indeed, cultured neurospheres constitutively released episodic bursts of ATP as shown using real-time bioluminescence imaging (Lin et al, 2007). Released ATP activates P2Y1 and P2Y2 receptor in cultured SVZ cells as shown using calcium imaging in vitro and immunohistochemistry in tissue (Lin et al, 2007; Mishra et al, 2006). The identity of the SVZ cell types expressing these receptor remains to be examined.
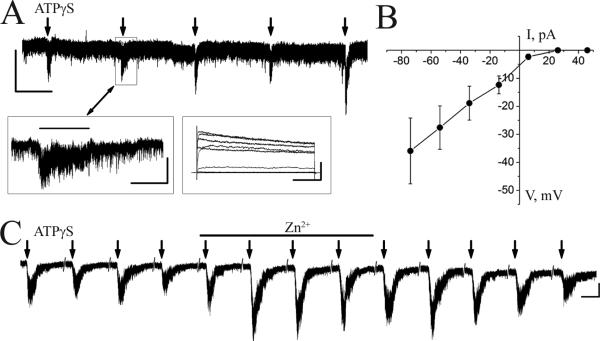
Regarding P2X receptors, ependymal cells that line the lateral ventricle have been shown to express P2X receptors, in particular P2X7 and adenosine A2B receptors (Genzen et al, 2009a; 2009b). There are no data regarding other P2X receptors in the SVZ. However, immunostaining data in the OB show that granule cells express high levels of P2X2 and P2X4 receptors (Kanjhan et al, 1999; Le et al, 1998; Vulchanova et al, 1996), suggesting that neuroblasts acquire P2X receptors along the SVZ-RMS-OB axis. Here, we present data suggesting that neuroblasts acquire functional P2X receptors in the RMS (published with permission from Dr. Anna Bolteus). We used whole-cell patch clamp recording in acute sagittal slices from postnatal day 20-32 mice at room temperature. Experiments were performed in accordance with the Yale Animal Care and Use Committee guidelines. Slice preparation and recording equipment were as previously reported (Bolteus and Bordey, 2004b; Wang et al, 2003a). Recorded cells were identified as neuroblasts as previously reported based on their ionic signature (outwardly rectifying current profile) and biophysical properties (high input resistance) (Bolteus and Bordey, 2004b; Wang et al, 2003a) (see Figure 1A, inset). Pressure application of 100 μM or 1 mM ATPγS did not induce currents in SVZ neuroblasts, but only increased the baseline noise in 1/10 neuroblasts suggesting that SVZ neuroblasts do not express functional P2X receptors. In the RMS-OB, pressure application of 100 μM NaATP or ATPγS and 1 mM ATPγS induced inward currents of -7.9 ± 1.8 pA in 57% (n=12/21) and -13.4 ± 1.6 pA in 84% (n=67/80) neuroblasts of the RMS-OB, respectively (Figure 2A for 1 mM ATP-γS, mean ± SEM). ATPγS-induced currents inwardly rectified and reached a zero-current near +10 mM (n=5, 1 mM, Figure 2B), which is expected for non-selective cationic currents mediated by P2X receptors (North, 2002; Ralevic and Burnstock, 1998). ATPγS-induced currents were not blocked by 100 μM suramin or TNT-ATP (n=3 each, data not shown) suggesting the involvement of P2X4 receptors (Bo et al, 1995; North, 2002; Ralevic and Burnstock, 1998). In addition, ATPγS-induced currents were enhanced by 10 μM zinc (Zn2+) application (n=3, Figure 2C). Collectively, these data suggest that neuroblasts acquire P2X receptors prior to entering the OB synaptic network.
Figure 2. Neuroblasts in the RMS-OB express functional P2X receptors.
(A) Pressure application of ATPγS (1 mM, 3 sec) induces reproducible inward currents in a neuroblast recorded at -70 mV in the RMS-OB. The left inset illustrates one of the inward currents at a higher time scale. The cells were identified as neuroblasts based on their outwardly rectifying current profiles (right inset, voltage steps shown in B). The liquid junction potential was corrected off-line. The recording solution contained (in mM): 140 KCl, 1 CaCl2, 10 EGTA, 10 HEPES (pH 7.2). Scales: 10 pA/ 30 s, 5 pA/ 2 s (left inset), 200 pA/ 100 ms (right inset) (B) Current-voltage relationship illustrating that ATPγS-induced currents inwardly rectify (mean ± SEM, n=5). (C) Zn2+ enhanced 1 mM ATPγS-induced current amplitudes (n=3 cells). Scale: 10 pA/ 4 s.
Influence of ATP signaling on SVZ neurogenesis
Activation of the metabotropic P2Y1 and P2Y2 receptors in cultured adult SVZ cells augmented cell proliferation in the presence of mitogenic growth factors (Mishra et al, 2006). Consistent with this result, P2Y receptor antagonists suppressed proliferation and permitted differentiation into neurons and glia in vitro (Lin et al, 2007). The function of P2X7 receptors in ependymal cells and ultimately neurogenesis remains to be examined. Similarly the function of P2X in neuroblasts in the RMS-OB suggests a function on neuroblast differentiation that needs to be explored.
Pathological brain states
Extracellular levels of ATP and its metabolites increase under injurious conditions including hypoxia, ischemia, traumatic insults and epilepsy-associated seizures (for reviews, see (Abbracchio et al, 2009; Franke et al, 2006a; 2006b)). ATP thus plays an important role in the CNS response to inflammation, ischemia, mechanical insult, and seizures. ATP could thus be a signal released during these pathological conditions that will affect neurogenesis.
Indirect action of other neurotransmitter systems and brain states on SVZ neurogenesis
Acetylcholine
Acetylcholine (ACh) was the first neurotransmitter discovered. Although the number of cholinergic neurons in the brain is small, cholinergic afferents are spread throughout the brain. Cholinergic signaling is important in the modulation of various brain states including learning, memory consolidation, attention, and sleep. Its receptors include the ionotropic nicotinic and metabotropic muscarinic ACh receptors.
There is evidence upstream of the SVZ that lesions of cholinergic inputs decrease the number of newly born neurons in the OB using BrdU (Cooper-Kuhn et al, 2004), though no direct cholinergic inputs have been identified in the SVZ. Similarly, enhancing cholinergic signaling with donepezil enhanced the survival of newly born OB neurons although no effect was seen in the proliferation of the SVZ (Kaneko et al, 2006). In contrast, nicotinic beta-2 knockout animals show increased survival of newly born neurons in the OB (Mechawar et al, 2004). ACh has thus a complex effect on neuronal survival in the OB that requires further investigation.
Pathological brain state
ACh is also known to regulate dopamine release in the striatum by regulating the firing of dopaminergic neurons in the substantia nigra (Aosaki et al, 2009; Lester et al, 2010). It is thus highly conceivable that ACh could indirectly regulate SVZ cell proliferation through modulation of dopamine release. ACh levels are altered in certain neurodegenerative disorders, in particular Alzheimer's disease (AD) (Schliebs and Arendt, 2006). Indeed, the severity of AD correlates with a decrease in choline acetyl transferase (ChAT), which is indicative of a decrease in overall cholinergic tone in the brain. Interestingly, in AD patients Ziabreva and colleagues (2006) correlated a decrease in ChAT to a nine-fold decrease in proliferating cells in the SVZ (Ziabreva et al, 2006). Although correlative, it is possible that changes in ACh levels directly or indirectly altered SVZ cell proliferation during the course of AD. This needs to be further investigated.
Ischemia
Even brief insults to blood flow in the brain can have profound effects on SVZ cell proliferation and migration. Middle cerebral artery occlusion increases migration of neuroblasts (i.e. doublecortin-positive cells) from the SVZ to peri-infarct regions (Kojima et al, 2010; Komitova et al, 2005; Ohab et al, 2006) (for review (Ohab and Carmichael, 2008; Zhang et al, 2005)). Stroke models in adult rodents show that ipsilateral to the injury, there is a long-lasting increase in proliferation of SVZ cells and migration toward the injury site (Arvidsson et al, 2002; Komitova et al, 2005; Parent et al, 2002; Zhang et al, 2001). Stroke was shown to increase neurogenesis in the SVZ and shortens the cell cycle of SVZ cells (Zhang et al, 2008b) (for reviews see (Kernie and Parent, 2010; Zhang et al, 2008a)).
In neonates, controversial findings on the effects of ischemia on SVZ neurogenesis have been reported. Using a hypoxia/ischemia model in postnatal day 7-10 rats, increased apoptosis of SVZ cells and more specifically oligodendrocyte progenitors was reported in the SVZ (Brazel et al, 2004; Levison et al, 2001). In contrast, Ong and colleagues (2005) provided evidence that a milder hypoxia/ischemia injury in postnatal day 7 rats increased the size of the SVZ and increased incorporation of BrdU in the SVZ ipsilateral to the injury site as previously reported in adult rats (see above) (Ong et al, 2005). Factors such as severity and site of injury, type of injury, and age or stage of SVZ cells can influence the detection of proliferating or apoptotic cells. In addition, cell type may matter as stem cells may react differently to ischemic conditions compared to neuronal or oligodendrocyte progenitors (Romanko et al, 2004).
As mentioned above, ischemic models lead to alterations in the levels of several neurotransmitters including glutamate, aspartate, GABA and ATP (Andine et al, 1991; Franke et al, 2006b; Vannucci et al, 1999). Previous studies reported glutamate's effects on SVZ cell proliferation and neuroblast survival in vivo through mGluR5 and NMDA receptors (Di Giorgi Gerevini et al, 2004; 2005; Gandhi et al, 2008; Platel et al, 2010a). GABA and ATP also affect neural progenitor cell proliferation and neurogenesis in vitro or in slices (Lin et al, 2007; Liu et al, 2005; Mishra et al, 2006). It is thus possible that part of the ischemic effect on SVZ cell proliferation and survival involve a direct action of GABA, glutamate, and ATP on SVZ cells. The effect on neuroblast migration and attraction to the injury site involves chemokines and other attractant molecules, the synthesis of which may be regulated by neurotransmitters.
Seizures
Seizure activity, in particular pilocarpine-induced status epilepticus, has been shown to increase SVZ cell proliferation and ultimately OB neurogenesis (Kang et al, 2004). The mechanism for this increase remains unclear. Seizures are characterized by abnormal and large amplitude electrical activity. This activity involves an excessive release of glutamate and GABA, among other neurotransmitters, and can also involve hormones over a longer time scale. The neurotransmitter systems discussed above may contribute to the seizure-induced regulations of neurogenesis. In addition, electroconvulsive therapy (ECT) used to treat depression was reported to increase several growth factor genes’ expression in the choroid plexus (Newton et al, 2003). These factors released from the choroid plexus could directly influence SVZ cell proliferation, but the mechanism of seizure-induced neurogenesis remains unknown.
Treatments for seizures often involve altering and enhancing GABAergic neurotransmission to counteract the excessive excitatory activity induced by a seizure. Such seizure treatment can potentially alter neurogenesis directly through GABAA receptors or indirectly by modulating other neurotransmitter systems. Supporting such an effect although it has not been examined in the SVZ, the seizure medication levetiracetam inhibits the ectopic neurogenesis in the hippocampus that occurs with kindled seizures (Sugaya et al, 2010).
Sleep
Almost all animals go through periods of rest that can be referred to as sleep. Sleep itself is a complex process controlled by various neurotransmitter systems and is modulated by circadian rhythms. There are also two main types of categorized sleep that have been studied in relation to neurogenesis: rapid eye movement (REM) and non-REM sleep. Changes in neurogenesis due to circadian rhythms have mostly been examined in the hippocampus, while literature surrounding sleep and SVZ neurogenesis appears to be more limited. In the rat SGZ of the hippocampus, prolonged, but not short-term, sleep deprivation appears to decrease neurogenesis (for reviews see (Guzman-Marin et al, 2007; Meerlo et al, 2009)). Sleep deprivation has been known to affect the function of various neurotransmitter systems including glutamatergic, GABAergic, cholinergic, and serotonergic systems, so it is surprising that there have not been reported effects of sleep deprivation on SVZ cell proliferation. Effects, however, may be missed if not assessed at the peak of proliferation during circadian rhythms, and changes can depend on the duration or type of sleep deprivation or species (for review see (Longordo et al, 2009)).
Melatonin, a hormone that is regulated by circadian rhythms involved in sleep, can also affect other neurotransmitter systems such as the production of serotonin (Sun et al, 2002). Cultured SVZ cells were shown to express melatonin receptors, the inhibition of which drove cells toward neuronal differentiation (Sotthibundhu et al, 2010). In addition, melatonin increased the proliferation of adult SVZ-derived cells in culture (Sotthibundhu et al, 2010). Given this recent evidence, it would be interesting to examine the effects of melatonin on SVZ cell proliferation and differentiation in vivo, and whether this action is direct or indirect through serotonin.
Pregnancy
Prolactin contributes to increases in SVZ neurogenesis during pregnancy (Shingo et al, 2003). Prolactin levels cycle and change during pregnancy, and the spikes in serum levels have an impact on maternal behavior post-partum. Larsen and Gratten (2010) show that blocking prolactin spikes during pregnancy not only decreases SVZ neurogenesis, but concurrently increases anxiety in new mothers and inhibits maternal behavior such as pup retrieval (Larsen and Grattan, 2010). To show that the inhibition of post-partum maternal behavior is not just due to the lack of prolactin, but also due to the decrease in neurogenesis, Larsen and Grattan (2010) injected the mitotic inhibitor methylazoxymethanol treatment (MAM). MAM is a mitotic inhibitor and decreases SVZ neurogenesis. These authors also reported that MAM-treated mice exhibited similar post-partum behavioral deficits as those mice without prolactin surges during pregnancy. In this case, the observed behavior is presumably driven by SVZ neurogenic defects that in turn may be controlled by prolactin. More importantly, prolactin release appears to be under the control of both serotonin and dopamine (Clemens and Shaar, 1980).
Conclusion
It is evident that many neurotransmitter systems impinge on the SVZ, creating a complex picture of the various signals influencing the production of new neurons. These systems provide a link between our brain activity or emotional and bodily states and SVZ neurogenesis. As discussed, activity in the subtantia nigra (dopamine) or the raphe nucleus (serotonin) can directly influence SVZ cell proliferation. These findings emphasize or re-emphasize two important limitations in our studies of neurogenesis. First, captive mice reared in small cages may display lack of such system-based control of neurogenesis or altered control in a low stimuli environment. Second, enervation by neurotransmitter systems such as dopamine and serotonin as well as receptor expression may differ in the rostral/caudal and dorsal/ventral regions of the SVZ. It is well-known that the SVZ is not homogeneous in its architecture and fate of neural stem cells (Merkle et al, 2007). Preferential control by neurotransmitter systems of SVZ sub-regions is thus expected. This obviously can lead to significant errors in analyzing global proliferation all along the SVZ using the mitotic marker bromodeoxyuridine. Such a heterogeneity calls for a live imaging approach such as functional MRI to examine which subregions of the SVZ are activated by certain brain activity and neurotransmitter systems to spatially target cell proliferation analysis. Finally, attempts to use neurogenic regions to produce new neurons to regenerate lost nuclei in neurodegenerative disease will have to take into account the potentially overlapping role of multiple neurotransmitter systems on neurogenesis.
Acknowledgments
This work was supported by grants from the NIH R01 DC007681 and NS062731 (A.B.) and NRSA F31 NS063758 (S.Z.Y). We would like to thank Dr. Anna Bolteus for data included in this manuscript.
References
- 1.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Andine P, Orwar O, Jacobson I, Sandberg M, Hagberg H. Changes in extracellular amino acids and spontaneous neuronal activity during ischemia and extended reflow in the CA1 of the rat hippocampus. J.Neurochem. 1991;57:222–229. doi: 10.1111/j.1471-4159.1991.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 3.Aosaki T, Miura M, Masuda M. [Physiological interaction between acetylcholine and dopamine in the striatum]. Brain Nerve. 2009;61:373–380. [PubMed] [Google Scholar]
- 4.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat.Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 5.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur.J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 6.Baker SA, Baker KA, Hagg T. D3 dopamine receptors do not regulate neurogenesis in the subventricular zone of adult mice. Neurobiol.Dis. 2005;18:523–527. doi: 10.1016/j.nbd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 8.Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- 9.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 10.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- 11.Bolteus A, Bordey A. Local GABA signaling regulates neuronal precursor migration in the postnatal subventricular zone. Soc.Neurosci.Abstr. 2004a doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolteus AJ, Bordey A. GABA Release and Uptake Regulate Neuronal Precursor Migration in the Postnatal Subventricular Zone. J.Neurosci. 2004b;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordey A. Adult Neurogenesis: Basic Concepts of Signaling. Cell Cycle. 2006;5:722–728. doi: 10.4161/cc.5.7.2614. [DOI] [PubMed] [Google Scholar]
- 14.Bordey A. Enigmatic GABAergic networks in adult neurogenic zones. Brain Res.Brain Res.Rev. 2007;53:124–134. doi: 10.1016/j.brainresrev.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- 16.Braun N, Sevigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur.J.Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- 17.Brazel CY, Nunez JL, Yang Z, Levison SW. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience. 2005;131:55–65. doi: 10.1016/j.neuroscience.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Brazel CY, Rosti RT, III, Boyce S, Rothstein RP, Levison SW. Perinatal hypoxia/ischemia damages and depletes progenitors from the mouse subventricular zone. Dev.Neurosci. 2004;26:266–274. doi: 10.1159/000082143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 20.Brickell KL, Nicholson LF, Waldvogel HJ, Faull RL. Chemical and anatomical changes in the striatum and substantia nigra following quinolinic acid lesions in the striatum of the rat: a detailed time course of the cellular and GABA(A) receptor changes. J.Chem.Neuroanat. 1999;17:75–97. doi: 10.1016/s0891-0618(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 21.Callier S, Snapyan M, Le Crom S, Prou D, Vincent JD, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol.Cell. 2003;95:489–502. doi: 10.1016/s0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 22.Castiglione M, Calafiore M, Costa L, Sortino MA, Nicoletti F, Copani A. Group I metabotropic glutamate receptors control proliferation, survival and differentiation of cultured neural progenitor cells isolated from the subventricular zone of adult mice. Neuropharmacology. 2008;55:560–567. doi: 10.1016/j.neuropharm.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Clemens JA, Shaar CJ. Control of prolactin secretion in mammals. Fed.Proc. 1980;39:2588–2592. [PubMed] [Google Scholar]
- 24.Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J.Neurosci.Res. 2004;77:155–165. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- 25.Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91:1292–1301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- 26.Councill JH, Tucker ES, Haskell GT, Maynard TM, Meechan DW, Hamer RM, Lieberman JA, LaMantia AS. Limited influence of olanzapine on adult forebrain neural precursors in vitro. Neuroscience. 2006;140:111–122. doi: 10.1016/j.neuroscience.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi DG, Polli E, Nappi G, Silani V, Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 29.Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc.Natl.Acad.Sci.U.S.A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis MA, Waldvogel HJ, Synek B, Faull RL. A histochemical and immunohistochemical analysis of the subependymal layer in the normal and Huntington's disease brain. J.Chem.Neuroanat. 2005;30:55–66. doi: 10.1016/j.jchemneu.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Dave KA, Platel J, Huang F, Tian D, Bordey A. Prostaglandin E2 induces glutamate release from subventricular zone astrocytes. Neuron Glia Biol. 2010 doi: 10.1017/S1740925X10000244. In Press. [DOI] [PubMed] [Google Scholar]
- 32.De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, Szabo G, Puche AC. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur.J.Neurosci. 2004;20:1307–1317. doi: 10.1111/j.1460-9568.2004.03584.x. [DOI] [PubMed] [Google Scholar]
- 33.Di Giorgi Gerevini V, Caruso A, Cappuccio I, Ricci Vitiani L, Romeo S, Della Rocca C, Gradini R, Melchiorri D, Nicoletti F. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res.Dev.Brain Res. 2004;150:17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Di Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, Biagioni F, Iacovelli L, Canudas AM, Parati E, De Maria R, Nicoletti F. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death.Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- 35.Diaz D, Valero J, Airado C, Baltanas FC, Weruaga E, Alonso JR. Sexual dimorphic stages affect both proliferation and serotonergic innervation in the adult rostral migratory stream. Exp.Neurol. 2009;216:357–364. doi: 10.1016/j.expneurol.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Diaz J, Ridray S, Mignon V, Griffon N, Schwartz JC, Sokoloff P. Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain. J.Neurosci. 1997;17:4282–4292. doi: 10.1523/JNEUROSCI.17-11-04282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franke H, Grummich B, Hartig W, Grosche J, Regenthal R, Edwards RH, Illes P, Krugel U. Changes in purinergic signaling after cerebral injury -- involvement of glutamatergic mechanisms? Int.J.Dev.Neurosci. 2006a;24:123–132. doi: 10.1016/j.ijdevneu.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006b;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 39.Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandhi R, Luk KC, Rymar VV, Sadikot AF. Group I mGluR5 metabotropic glutamate receptors regulate proliferation of neuronal progenitors in specific forebrain developmental domains. J Neurochem. 2008;104:155–172. doi: 10.1111/j.1471-4159.2007.04955.x. [DOI] [PubMed] [Google Scholar]
- 41.Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, De Roo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J.Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genzen JR, Platel JC, Rubio ME, Bordey A. Ependymal cells along the lateral ventricle express functional P2X(7) receptors. Purinergic.Signal. 2009a;5:299–307. doi: 10.1007/s11302-009-9143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genzen JR, Yang D, Ravid K, Bordey A. Activation of adenosine A2B receptors enhances ciliary beat frequency in mouse lateral ventricle ependymal cells. Cerebrospinal.Fluid Res. 2009b;6:15. doi: 10.1186/1743-8454-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J.Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goncalves MB, Williams EJ, Yip P, Yanez-Munoz RJ, Williams G, Doherty P. The COX-2 inhibitors, meloxicam and nimesulide, suppress neurogenesis in the adult mouse brain. Br.J.Pharmacol. 2010;159:1118–1125. doi: 10.1111/j.1476-5381.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grote HE, Hannan AJ. Regulators of adult neurogenesis in the healthy and diseased brain. Clin.Exp.Pharmacol.Physiol. 2007;34:533–545. doi: 10.1111/j.1440-1681.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- 47.Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol.Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. J.Neurosci.Res. 2007;85:3574–3585. doi: 10.1002/jnr.21455. [DOI] [PubMed] [Google Scholar]
- 51.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat.Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 52.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J.Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadir A, Nordberg A. Target-specific PET probes for neurodegenerative disorders related to dementia. J.Nucl.Med. 2010;51:1418–1430. doi: 10.2967/jnumed.110.077164. [DOI] [PubMed] [Google Scholar]
- 54.Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11:1145–1159. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 55.Kang D, Choe C, Kim D. Functional expression of TREK-2 in insulin-secreting MIN6 cells. Biochem.Biophys.Res.Commun. 2004;323:323–331. doi: 10.1016/j.bbrc.2004.08.089. [DOI] [PubMed] [Google Scholar]
- 56.Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J.Comp Neurol. 1999;407:11–32. [PubMed] [Google Scholar]
- 57.Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol.Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y, Wang WZ, Comte I, Pastrana E, Tran PB, Brown J, Miller RJ, Doetsch F, Molnar Z, Szele FG. Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J.Neurochem. 2010;114:750–760. doi: 10.1111/j.1471-4159.2010.06799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J.Neurosci. 2005;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohl Z, Regensburger M, Aigner R, Kandasamy M, Winner B, Aigner L, Winkler J. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington's disease. BMC.Neurosci. 2010;11:114. doi: 10.1186/1471-2202-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- 62.Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- 63.Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- 64.Le KT, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Seguela P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–190. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- 65.Lester DB, Rogers TD, Blaha CD. Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS.Neurosci.Ther. 2010;16:137–162. doi: 10.1111/j.1755-5949.2010.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev.Neurosci. 2001;23:234–247. doi: 10.1159/000046149. [DOI] [PubMed] [Google Scholar]
- 67.Lima MM, Reksidler AB, Vital MA. The neurobiology of the substantia nigra pars compacta: from motor to sleep regulation. J.Neural Transm.Suppl. 2009:135–145. doi: 10.1007/978-3-211-92660-4_11. [DOI] [PubMed] [Google Scholar]
- 68.Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev.Biol. 2007;302:356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat.Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Longordo F, Kopp C, Luthi A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur.J.Neurosci. 2009;29:1810–1819. doi: 10.1111/j.1460-9568.2009.06719.x. [DOI] [PubMed] [Google Scholar]
- 71.Lorez HP, Richards JG. Supra-ependymal serotoninergic nerves in mammalian brain: morphological, pharmacological and functional studies. Brain Res.Bull. 1982;9:727–741. doi: 10.1016/0361-9230(82)90179-4. [DOI] [PubMed] [Google Scholar]
- 72.Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc.Natl.Acad.Sci.U.S.A. 2004;101:9822–9826. doi: 10.1073/pnas.0403361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med.Rev. 2009;13:187–194. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 75.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mishra SK, Braun N, Shukla V, Fullgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sevigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- 77.Moreno-Lopez B, Noval JA, Gonzalez-Bonet LG, Estrada C. Morphological bases for a role of nitric oxide in adult neurogenesis. Brain Res. 2000;869:244–250. doi: 10.1016/s0006-8993(00)02474-4. [DOI] [PubMed] [Google Scholar]
- 78.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J.Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, Rigo JM. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J.Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicholson LF, Faull RL, Waldvogel HJ, Dragunow M. GABA and GABAA receptor changes in the substantia nigra of the rat following quinolinic acid lesions in the striatum closely resemble Huntington's disease. Neuroscience. 1995;66:507–521. doi: 10.1016/0306-4522(94)00607-7. [DOI] [PubMed] [Google Scholar]
- 81.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 82.O'Keeffe GC, Barker RA, Caldwell MA. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle. 2009a;8:2888–2894. doi: 10.4161/cc.8.18.9512. [DOI] [PubMed] [Google Scholar]
- 83.O'Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc.Natl.Acad.Sci.U.S.A. 2009b;106:8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14:369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- 85.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J.Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ong J, Plane JM, Parent JM, Silverstein FS. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatr.Res. 2005;58:600–606. doi: 10.1203/01.PDR.0000179381.86809.02. [DOI] [PubMed] [Google Scholar]
- 87.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann.Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 88.Pathania M, Yan LD, Bordey A. A symphony of signals conduct early and late stages of adult neurogenesis. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phillips W, Jennifer MA, Barker RA. Limbic neurogenesis/plasticity in the R6/2 mouse model of Huntington's disease. Neuroreport. 2006;17:1623–1627. doi: 10.1097/01.wnr.0000236855.85962.f6. [DOI] [PubMed] [Google Scholar]
- 90.Phillips W, Morton AJ, Barker RA. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington's disease are attributable to the in vivo microenvironment. J.Neurosci. 2005;25:11564–11576. doi: 10.1523/JNEUROSCI.3796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J.Physiol.(Lond) 2008a;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010a;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J.Mol.Histol. 2007;38:602–610. doi: 10.1007/s10735-007-9153-y. [DOI] [PubMed] [Google Scholar]
- 94.Platel JC, Stamboulian S, Nguyen I, Bordey A. Neurotransmitter signaling in postnatal neurogenesis: The first leg. Brain Res.Rev. 2010b;63:60–71. doi: 10.1016/j.brainresrev.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Platel J, Heintz T, Young S, Gordon V, Bordey A. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in a whole mount preparation of the subventricular zone. J.Physiol.(Lond) 2008b;586:3783–3793. doi: 10.1113/jphysiol.2008.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol.Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 97.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J.Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romanko MJ, Rothstein RP, Levison SW. Neural stem cells in the subventricular zone are resilient to hypoxia/ischemia whereas progenitors are vulnerable. J.Cereb.Blood Flow Metab. 2004;24:814–825. doi: 10.1097/01.WCB.0000123906.17746.00. [DOI] [PubMed] [Google Scholar]
- 99.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia VJ, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 100.Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J.Neurobiol. 1999;41:221–229. [PubMed] [Google Scholar]
- 101.Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer's disease. J.Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- 102.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 104.Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P. Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J.Pineal Res. 2010 doi: 10.1111/j.1600-079X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 106.Soumier A, Banasr M, Goff LK, Daszuta A. Region- and phase-dependent effects of 5-HT(1A) and 5-HT(2C) receptor activation on adult neurogenesis. Eur.Neuropsychopharmacol. 2010;20:336–345. doi: 10.1016/j.euroneuro.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 107.Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABA(A) receptors. J.Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- 108.Sugaya Y, Maru E, Kudo K, Shibasaki T, Kato N. Levetiracetam suppresses development of spontaneous EEG seizures and aberrant neurogenesis following kainate-induced status epilepticus. Brain Res. 2010;1352:187–199. doi: 10.1016/j.brainres.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 109.Sun X, Deng J, Liu T, Borjigin J. Circadian 5-HT production regulated by adrenergic signaling. Proc.Natl.Acad.Sci.U.S.A. 2002;99:4686–4691. doi: 10.1073/pnas.062585499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tattersfield AS, Croon RJ, Liu YW, Kells AP, Faull RL, Connor B. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington's disease. Neuroscience. 2004;127:319–332. doi: 10.1016/j.neuroscience.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 111.Tavazoie M, Van d., V, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A Specialized Vascular Niche for Adult Neural Stem Cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D receptor stimulation. Eur.J.Neurosci. 2004;19:2377–2387. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- 113.Vannucci RC, Brucklacher RM, Vannucci SJ. CSF glutamate during hypoxiaischemia in the immature rat. Brain Res.Dev.Brain Res. 1999;118:147–151. doi: 10.1016/s0165-3806(99)00142-x. [DOI] [PubMed] [Google Scholar]
- 114.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc.Natl.Acad.Sci.U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang DD, Krueger DD, Bordey A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J.Neurophysiol. 2003a;90:2291–2302. doi: 10.1152/jn.01116.2002. [DOI] [PubMed] [Google Scholar]
- 116.Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J.Physiol (Lond) 2003b;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winner B, Desplats P, Hagl C, Klucken J, Aigner R, Ploetz S, Laemke J, Karl A, Aigner L, Masliah E, Buerger E, Winkler J. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp.Neurol. 2009;219:543–552. doi: 10.1016/j.expneurol.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, Kuhn G, Winkler J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp.Neurol. 2006;197:113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 119.Zanotti S, Charles A. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J.Neurochem. 1997;69:594–602. doi: 10.1046/j.1471-4159.1997.69020594.x. [DOI] [PubMed] [Google Scholar]
- 120.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 121.Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008a;55:345–352. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang RL, Zhang ZG, Roberts C, LeTourneau Y, Lu M, Zhang L, Wang Y, Chopp M. Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J.Cereb.Blood Flow Metab. 2008b;28:602–611. doi: 10.1038/sj.jcbfm.9600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 124.Ziabreva I, Perry E, Perry R, Minger SL, Ekonomou A, Przyborski S, Ballard C. Altered neurogenesis in Alzheimer's disease. J.Psychosom.Res. 2006;61:311–316. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 125.Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452:573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]