SUMMARY
Costimulatory molecules such as B7-1/2 and PD-L1/2 play an important role in the function of APC. The regulation of the surface levels of costimulatory molecules is one mechanism by which APC maintain the balance between tolerance and immunity. We examined the contributions of B7-1/2 and PD-L1/2 to the function of IL-10-treated, immunosuppressive DC as well as therapeutic exosomes derived from these DC. IL-10 treatment of DC significantly downregulated surface expression of MHC II, B7-1, B7-2, and decreased levels of MHC I and PD-L2. IL-10 treatment of DC resulted in a modified co-stimulatory profile of DC-secreted exosomes with a reduction in B7-1, PD-L1 and PD-L2. We further demonstrate that absence of B7-1 or B7-2 on donor DC results in a loss of ability of IL-10 treated DC and their exosomes to suppress the delayed-type hypersensitivity (DTH) response, whereas IL-10 treated DC deficient in PD-L1/2 as well as their secreted exosomes retained the ability to suppress DTH responses. We conclude that B7-1 and B7-2, but not PD-L1 and PD-L2, on IL-10 treated DC and DC-derived exosomes play a critical role in immunosuppressive functions of both DC and exosomes.
Keywords: Dendritic cells, Exosomes, B7-1/2, PD-L1/2, Delayed-type hypersensitivity
INTRODUCTION
Dendritic cells (DC) are professional APC which play a crucial role in mediating the balance between immunity and tolerance in vivo, and therefore have been increasingly studied for therapeutic applications in allergy, infection, and autoimmunity. Significant efforts in the field of autoimmunity have been made to understand the genesis and function of DC that are immunosuppressive rather than stimulatory, and to understand their underlying phenotype [1].
Costimulatory molecules play a critical role in determining immune system specificity and self-tolerance. The best characterized of the costimulatory molecules are B7-1 (CD80) and B7-2 (CD86), which are primarily expressed on APC and stimulate T cells via interaction with CD28 and antagonize T cell function through interactions with CTLA-4 [2]. B7-1 and B7-2 expression on APC is critical for homeostatic balance in not only effector T cell populations, but also in regulatory T cell populations. In addition, CTLA-4 ligation of B7 signals bidirectionally, inducing expression of IDO in DC, which catabolizes tryptophan and inhibits T cell function [3]. Additional members of the B7 family of costimulatory molecules also provide signals that modify T cell function. Of these, the PD-1:PD-L1/2 pathway plays an important negative regulatory role in T cell function and regulates peripheral T cell tolerance at multiple checkpoints [4]. Programmed Death-1 (PD-1) is upregulated on T cells upon activation, and its ligands, Programmed Death 1-Ligand 1 (PD-L1, also known as B7-H1) and Programmed Death 1-Ligand 2 (PD-L2, also known as B7-H2), have distinct expression patterns, with PD-L1 being expressed much more broadly than PD-L2. Both PD-L1 and PD-L2 are expressed on DC. Of significant interest, recent work indicates that PD-L1 and PD-L2 may not only regulate T cell responses by engaging PD-1 and modify TCR signaling, but also may deliver signals into PD-L1 and PD-L2-expressing cells. Treatment of DC with soluble PD-1Ig inhibited their activation and resulted in increased IL-10 production and a suppressive DC phenotype independent of IDO upregulation. These effects could be prevented by neutralization of PD-1Ig with anti-PD-1, indicating that this phenotype is PD-1 specific and implicating PD-L1 or PD-L2 binding [5]. Because of their demonstrated abilities to confer suppressive properties to DC, we were particularly interested in examining the roles of B7-1/2 and PD-L1/2 costimulatory molecules in the context of immunosuppressive DC therapy.
Exosomes are small (40-100nm) membrane vesicles that originate in the multivesicular endosome of many cell types. Exosomes derived from APC, and in particular DC, have the potential to promote immunity or tolerance in disease models of cancer, transplant or autoimmunity [6]. Exosomes carry molecules important for antigen presentation, such as MHC and B7 family molecules [7, 8] and can regulate antigen specific immune responses. Exosomes are capable of interacting directly with T cells [9, 10], but also can act as a potential source of antigen or MHC complexes for APC presentation [11, 12].
We previously have demonstrated that IL-10 treatment of murine bone-marrow derived DC (BMDC) generates tolerogenic DC and DC-derived exosomes that suppress inflammation in delayed-type hypersensitivity (DTH) and rheumatoid arthritis models [13]. We have also shown that in the DTH model, the in vivo mechanism of action of suppressive exosomes relies on MHC II and FasL, but not MHC I [13, 14]. This led us to hypothesize that direct interaction of exosomes with T-cells may play an important role in immune regulation in this model, and hence costimulatory molecules such as B7-1 and B7-2, as well as PD-L1 and PD-L2 could be required for the in vivo suppressive effects of exosomes. Here we demonstrate that B7-1 and B7-2 are necessary for the suppressive function of both DC and exosomes. In contrast, PD-L1 and PD-L2 on DC and DC-derived exosomes do not play a critical role in their immunosuppressive activity, at least in a DTH model.
RESULTS AND DISCUSSION
IL-10 treatment of BMDC results in tolerogenic phenotype
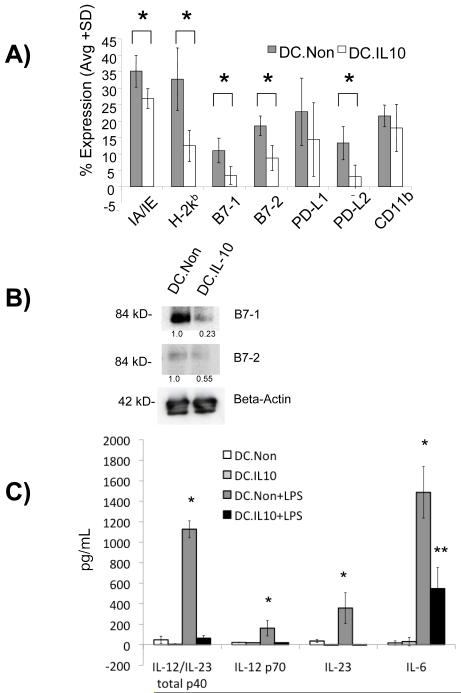
The levels of the B7-1/2 and PD-L1/2 costimulatory molecules on DC before and after 24 hours of treatment with IL-10 were examined. As shown in figure 1A, IL-10 treatment of BMDC resulted in highly decreased levels of MHC I (H-2kb), B7-1, B7-2, as well as lowered levels of MHC II (IA/IE) and PD-L2 after 48 hours. Similarly, Western blotting demonstrated that IL-10 treated DC have decreased overall expression levels of B7-1 and B7-2 (Figure 1B), in addition to decreased surface expression. These observations are consistent with published reports regarding the ability of IL-10 treatment to downregulate levels of costimulatory molecules on DC [15, 16], thereby generating a tolerogenic DC phenotype. We also examined the secreted levels of the proinflammatory cytokines IL-6, IL-23(p19/p40), IL-12p70, and IL-12/IL-23 total p40 by ELISA from supernatants of IL-10 and non-treated DC, plus/minus 24 hours of stimulation with 100ng/mL of LPS. We found that DC treated with IL-10 do not have increased IL-12 or IL-23 production when stimulated with LPS. In addition, although the LPS-stimulated DC.IL10 produce an increased amount of IL-6, it was significantly less than the amount produced by the non-treated control DC in response to LPS stimulation (Figure 1C). Collectively, these results confirm that an immunosuppressive phenotype of DC can be generated after 24 hours of treatment with IL-10.
Figure 1.
Characterization of rIL10-treated tolerogenic DC. (A) DC were stained with PE-conjugated mAb specific for IA/IE, H2kb, PD-L1, PD-L2, B7-1, B7-2, and CD11c and analyzed by FACS. Percent of positively stained cells was compared to isotype controls. Results are shown as mean±SD of four independent experiments. * p≤0.05, IL-10-treated DC compared to non-treated controls. (B) 10 μg of DC lysate from rIL-10-treated and control groups were run on SDS-PAGE gels under non-reducing conditions and immunoblotted for B7-1, B7-2 or beta-actin as control. (C) IL-10 treated and non-treated DC were stimulated with 100 ng/mL LPS overnight on day 7 of culture. IL-6, IL-23, IL-12 p70 and IL-12/IL23 total p40 levels in DC culture supernatant were determined on day 8 using ELISA. p≤0.05, DC.Non+LPS group compared to the other three groups. **p≤0.05, DC.IL-10+LPS group compared to the other three groups.
DC-derived exosomes contain costimulatory molecules
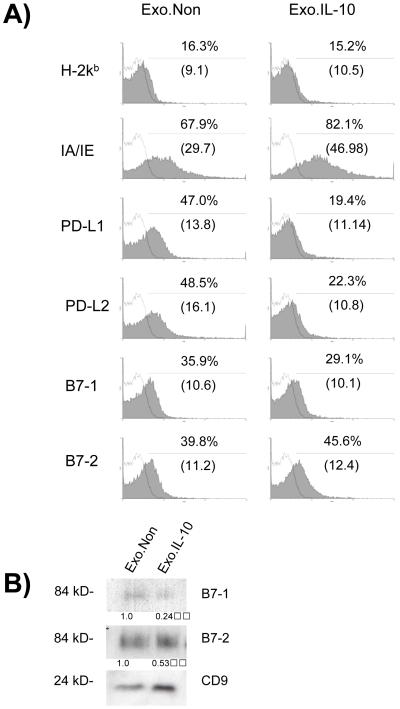
Exosomes were purified from the supernatants of untreated and IL-10-treated DC cultures for analysis of costimulatory marker expression. To analyze by FACS, exosomes were first absorbed onto anti-CD81 coated latex beads, and then stained with labeled antibodies to MHC I (H-2kb), MHC II (IA/IE), B7-1, B7-2, PD-L1, and PD-L2 (Figure 2A). We observe a significant decrease in the levels of PD-L1, PD-L2, and B7-1 on exosomes secreted by IL-10 treated DC. The decrease in the levels of B7-1 was confirmed by Western blot along with a decrease in the levels of B7-2 when compared to the level of the CD9 loading control (Figure 2B). It has been demonstrated previously that exosomes secreted from mature DC are phenotypically and functionally different than those secreted by immature DC [17]. However, this is the first demonstration of phenotypic changes in the exosome population secreted by DC following treatment with cytokines to induce a tolerogenic phenotype. Our data indicate that qualitative changes in costimulatory molecules levels on exosomes secreted by DC occur following treatment with IL-10, suggesting that the phenotypic response of DC to IL-10 treatment is also seen in the exosomal compartment. IL-10 treatment decreased the overall levels of MHCI, MHC II, B7-1, B7-2, and PD-L2 on the surface of the DC whereas the level of MHC II appears to increase on exosomes in contrast to the decrease in the levels of B7-1, B7-2, PD-L1, and PD-L2.
Figure 2.
Characterization of exosomes from tolerogenic DC. Exosomes were purified from supernatant of control or rIL-10-treated DC. (A) Anti-CD81 mAb was preabsorbed onto latex beads which were subsequently incubated with 10 μg of DC-derived exosomes. Samples were stained with PE-conjugated mAb specific for IA/IE, H2kb, PD-L1, PD-L2, B7-1, and B7-2, or isotype control (open black line). Results shown are representative of three independent experiments. Percentage of beads containing positive signal, as well as MFI (in parentheses) are displayed. (B) 5 μg of exosomes were run on SDS-PAGE gels under non-reducing conditions and immunoblotted for B7-1, B7-2 or CD9 as a loading control.
In vivo therapeutic effects of IL-10 treated DC and exosomes require B7-1/2
We previously have demonstrated that gene transfer of IDO to BMDC results in DC and DC-derived exosomes that are therapeutic in DTH and collagen-induced arthritis models [18]. We also demonstrated that exosomes from DC transduced with a CTLA-4-Ig expressing adenoviral vector were suppressive in vivo in an IDO-dependent manner. Interestingly, loss of B7-1 and B7-2 significantly decreased the ability of exosomes from the IDO-transduced DC to suppress inflammation in the DTH model. Therefore, in the following experiments, we set out to determine if the requirement for B7-1 and B7-2 for in vivo efficacy of BMDC and DC-derived exosomes was related to the specific adenoviral therapies used in these previous studies, or represent a broader phenomenon that could be generalized to other populations of suppressive DC and exosomes.
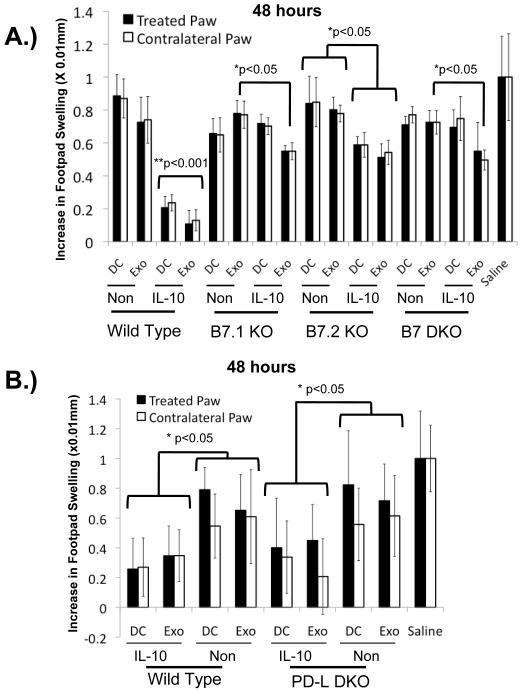
As shown in Figures 1C and 2, DC and DC-derived exosomes normally contain both B7-1 and B7-2. To determine whether these are essential components for tolerogenic DC function, DC were isolated from B7-1−/−, B7-2−/−, or B7-1/B7-2−/− mice and treated with rIL-10 or left untreated. Exosomes were isolated from the enriched media. IL-10 treated donor DC lacking B7-1 or B7-2, as well as the exosomes that they secrete, were significantly less effective at reducing paw swelling than the IL-10 treated wild-type controls (Fig. 3A). Although the therapeutic ability of the knockout exosomes were greatly reduced, we observed some remaining ability of exosomes from IL-10 treated knockout DC to reduce paw swelling by approximately 20%. A 20% reduction in paw swelling was also observed in mice receiving the IL-10 treated B7-2 −/− DC.
Figure 3.
B7-1 and B7-2 are required on DC and DC-derived exosomes for activity whereas PD-L1 and PD-L2 are dispensable. Wild-type C57BL/6 or knockout strain-derived DC were treated with rIL-10 or left untreated, and exosomes were isolated from the enriched media. DC or exosomes were injected into the right hind footpad of KLH-immunized wild-type mice simultaneously with KLH challenge to both hind footpads. Dark bars = swelling post challenge in treated (right) paws, white bars = swelling in contralateral (left) untreated paws. (A) Loss of B7-1 or B7-2 on DC or DC-derived exosomes abrogates ability to suppress inflammation in the DTH model. **p≤0.001 for wild-type IL-10-treated DC and their secreted exosomes compared to all other groups. *p≤0.05, exosomes from IL-10-treated B7-1−/−, B7-2−/−, and double knockout DC conditions as compared to the wild type and their respective knockout untreated controls. B7-2−/− IL-10 treated DC are also significant at p≤0.05 compared to the B7-2−/− non-treated DC as well as the non-treated wild type DC. (B) PD-L1 and PD-L2 are not required on DC and DC-derived exosomes for therapeutic effect in the DTH model. *p≤0.05 for the treated paw of the IL-10-treated wild-type and PD-L DKO samples compared to controls. There are no statistical differences between the wild-type and PD-L DKO IL-10 treated DC and exosome groups.
These results demonstrate that both B7-1 and B7-2 are required on DC and DC-derived exosomes for reduction in inflammation in the DTH model. This is consistent with data demonstrating that B7-1 and B7-2 on DC play critical roles in the generation of tolerance by interacting with CD4+CD25+ cells [19], altering effector T cell cytokine profiles [20], and regulating DC function through CTLA-4 signaling. Furthermore, the ability of the B7-2−/− DC to suppress the DTH response 20% more efficiently than DC lacking B7-1 is of interest given that B7-1 and B7-2 are known to play different roles in vivo [21, 22].
Our findings indicate that the majority of in vivo suppressive activity of exosomes is dependent on B7-1 and B7-2. This is consistent with in vitro findings suggesting that both ICAM-1 and CD28/B7 molecules are critical for APC-derived membrane vesicles to directly activate T cells, but contradictory to findings that only ICAM-1, and not B7 molecules are required for exosomes to stimulate T-cell responses when mediated through DC uptake and representation [23, 24]. However, as yet it remains unclear whether the primary in vivo mechanism of exosomes is mediated through APC or involves direct interaction with T cells, and there may be multiple mechanisms of action depending on the particular disease process. While our results demonstrate the importance of both B7-1 and B7-2 molecules on exosomes for in vivo suppression of inflammation in the DTH model, in all three knockout conditions the exosomes from the IL-10 treated knockout DC retain approximately 20% of their suppressive ability. This suggests that while B7 signaling is highly important for the in vivo suppressive activity of exosomes, redundant mechanisms of exosome-host cell communication may be utilized to confer suppressive effects in vivo.
Given the reported suppressive functions of PD-L molecules, and our observation that levels of PD-L1 and PD-L2 on DC and DC-derived exosomes are regulated by IL-10 treatment, we hypothesized that these molecules may also be required for DC and exosome function in the DTH model. BMDC were generated from PD-L1/PD-L2−/− mice, treated with IL-10 or sham treated, and used in the DTH model. Exosomes were isolated from the enriched media and then used for treatment in the DTH model. As shown in Figure 3B, IL-10 treated PD-L1/2-deficient DC and the exosomes they produce were still able to suppress inflammation, and the levels of suppression seen in the IL-10 treated PD-L deficient groups was comparable to DC and exosomes derived from wild-type mice. These results are consistent with data from bone marrow chimera studies that demonstrated expression of PD-L1 and PD-L2 on APC alone was not sufficient to ameliorate early-onset diabetes in PD-L1/PD-L2 −/− NOD mice [25].
CONCLUDING REMARKS
Herein we confirm that IL-10 treatment generates a tolerogenic DC phenotype as well as further demonstrate that IL-10 treated DC secrete a modified population of exosomes that contain decreased levels of B7-1, PD-L1 and PD-L2. This data regarding phenotypic difference between tolerogenic and control exosomes is of particular significance given the growing interest in exosomes for therapeutic applications, as it is critical to understand the characteristics of exosomes that confer their immunomodulatory properties. Our data also demonstrate that the phenotypic changes seen after treatment with IL-10 on the cell surface of DC and on the exosomes secreted by DC are different. This suggests that regulation of protein trafficking in these compartments differs, and may reflect an underlying difference in the physiologic role of DC and exosomes in vivo.
Using the DTH model, we further demonstrate a requirement for B7-1 and B7-2, but not PD-L1 or PD-L2 for in vivo function of IL-10 treated DC and the tolerogenic exosomes they secrete. This is consistent with our previous result demonstrating that B7-1 and B7-2 are partially required for in vivo function when suppressive DC and exosomes were generated using IDO gene transfer. Taken together, these results suggest a critical role for B7-1/2 on DC and DC-derived exosomes in vivo, regardless of the method by which the therapeutic DC or exosomes are generated.
MATERIALS AND METHODS
Mice
Female C57BL/6 (H-2Kb) and B7 knockout mice (B7-1/B7-2 −/− = B6.129S4-Cd80tm1Shr Cd86tm2Shr/J, B7-1 −/− = B6.129S4-Cd80tm1Shr/J, B7-2 −/− = B6.129S4-Cd86tm1Shr/J) were purchased at 7-8 weeks of age (Jackson Laboratory, Bar Harbor, ME). PD-L1/PD-L2 −/− mice have been previously described [25]. Mice were maintained in a pathogen-free facility according to institutional and National Institutes of Health (NIH) guidelines.
DC Generation
BMDC were prepared following a bulk-culture protocol modified from Son, et al. as previously described [18, 26]. Briefly, bone marrow was collected from tibias and femurs of 6- to 7-week-old mice. Contaminating erythrocytes were lysed with ACK cell lysing buffer (Mediatech, Herdon, VA). Monocytes were collected from the interface after centrifuging on Nycoprep (NycoMed, Roskilde, Denmark) at 600 x g for 20 min at RT. Cells were cultured for 24 h in complete media (RPMI 1640 containing 10% FBS, 50 μM 2-β-Mercaptoethanol, 2 mM glutamine, 0.1 mM nonessential amino acids, 100 IU/mL penicillin/streptomycin) to remove adherent macrophages. Non-adherent cells were placed in fresh growth medial (complete media containing 1000 U/mls of GM-CSF and IL-4) to generate DC. Cells were cultured for 4 days and harvested. For IL-10 treatment, 1 × 107 DC were incubated for 2 hours with 1 μg/mL of the recombinant murine IL-10 (Cell Sciences, Canton, Massachusetts) in total volume of 1 mL serum-free media, then diluted 1:5 in CM for further incubation. After incubation for 22 hr, DC were washed three times in PBS and cultured for 48 h in growth media without rIL-10. On day 8, culture supernatant was collected for exosome purification and DC recovery.
Exosome Isolation
Exosome isolation procedure has previously been described [13]. Briefly, DC culture supernatants were collected and centrifuged at 300 g for 10 min, 1200 g for 20 min, and 10,000 g for 30 min, and then ultra-centrifuged at 100,000 g for 1 h. Exosome pellet was washed in sterile PBS, centrifuged at 100,000 g for 1 h, and resuspended in 120 μl of PBS for further studies. Exosome batch protein content was quantified and standardized by a micro Bradford protein assay (Bio-Rad, CA). 1 μg exosomes were suspended in 20 μl of PBS for in vivo mouse studies.
DTH Model
Female C57BL/6 of 7-8 weeks of age were sensitized by injecting 100 μg of Keyhole Limpet Hemocyanin (KLH) antigen emulsified 1:1 in CFA at a single dorsal site. 14 days later, the right hind footpad of immunized mice were injected with 106 DC or 1 μg of exosomes suspended in 50μL total volume PBS containing 20μg of KLH antigen. Contra-lateral footpad received 50μL total volume PBS containing 20μg of KLH antigen, without any DC or exosomes. Footpad swelling was measured bilaterally using a spring-loaded caliper at 24, 48 and 72 hours after injection. Results are expressed as the normalized difference in swelling (X 0.01 mm), before and after Ag boost injection all groups were normalized to the mean value of the saline-treated group to control for variability between experiments. Experiments were performed with 5 or 6 mice per group and repeated at least twice to ensure reproducibility.
FACS Analysis
Anti-CD81 labeled-latex beads were generated by incubating 250 uL of 4 μm latex beads overnight at 4° C in a solution containing 60 ug anti-CD81 mAb (eBioscience, San Diego, CA) and 250 μL BSA in MES buffer. After washing beads three times in PBS, 10 μg of exosomes were incubated with 15 μL of the anti-CD81 labeled-latex beads in 500 uL of PBS. Samples were centrifuged at 700g for 5 min, resuspended in ice-cold PBS containing goat serum and stained with 1-2 μL of PE-labeled Ab in 100 μL total volume.
Dendritic cells were washed and stained with 1-2 μL of PE-labeled mAb (B7.1 (16-10A1), B7.2 (PO3.1), PD-L1 (MIH5), PD-L2 (TY25), IA-IE (M5/114.15.2), H2-kb (AF6-88.5), all from eBioscience, San Diego, CA) in 100 μL total volume of ice-cold PBS containing goat serum. Exosome-coated beads and DC were washed twice in FACS buffer, resuspended in 400 μL FACS buffer, and examined by FACS (FACScan, BD Biosciences, San Jose, CA). Results were analyzed using WinMDI 2.9.
Western Blot Analysis
Cellular (10 ug) and exosomal proteins (5 μg) were separated under non-reducing conditions by 12% or 15% SDS-PAGE, and semi-dry transferred onto PVDF. Bands were detected using primary mAb to B7-1 (clone 16-10A1), CD9 (clone KCM9), (both from BD Pharmigen, San Jose, California), B7-2 (clone GL1, R&D systems, Minneapolis, Minnesota), and beta-actin (Abcam, Cambridge Massachussets) and an enhanced chemiluminescence detection kit. Semi-quantitative analysis of the protein density bands was performed using the NIH Image J program.
ELISA
Supernatants from LPS- and mock-stimulated BMDC were collected and analyzed using a sandwich ELISA to detect IL-23 (R&D systems, Minneapolis, Minnesota), IL-6, IL-12/IL-23 total p40, and IL-12p70 (all from eBioscience, San Diego, CA) according to the manufacturer’s protocol. The limits of detection were 2 pg/mL for IL-12/IL-23 total p40, 1pg/mL for IL-23, and 4 pg/mL for IL-12p70 and IL-6.
Statistics
Results were compared by ANOVA with Fisher’s Least Significant Difference post-hoc test. P values ≤ 0.05 were considered statistically significant, and all tests were conducted using SPSS statistical software (SPSS, Chicago IL).
ACKNOWLEDGEMENTS
This work was supported by NIH grant AI56374 and JDRF grant 7-2005-1154 to PDR, NIH grant DK082217-01 to MAR, and NIH grant PO1 AI056299 to AHS.
List of Abbreviations Used
- B7-1
CD80
- B7-2
CD86
- PD-1
Programmed Death-1
- PD-L1
Programmed Death 1-Ligand 1, B7-H1
- PD-L2
Programmed Death 1-Ligand 2, B7-DC
- BMDC
Bone marrow-derived DC
- DTH
Delayed-type Hypersensitivity
- KLH
Keyhole Limpet Hemocyanin
Footnotes
CONFLICT OF INTEREST:
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Keir ME, Sharpe AH. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128–143. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 3.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 4.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36:2472–2482. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 6.Hegmans JP, Gerber PJ, Lambrecht BN. Exosomes. Methods Mol Biol. 2008;484:97–109. doi: 10.1007/978-1-59745-398-1_7. [DOI] [PubMed] [Google Scholar]
- 7.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 8.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 9.Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 10.Sprent J. Direct stimulation of naive T cells by antigen-presenting cell vesicles. Blood Cells Mol Dis. 2005;35:17–20. doi: 10.1016/j.bcmd.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503–1510. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 12.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J Immunol. 2007;179:2235–2241. doi: 10.4049/jimmunol.179.4.2235. [DOI] [PubMed] [Google Scholar]
- 15.Faulkner L, Buchan G, Baird M. Interleukin-10 does not affect phagocytosis of particulate antigen by bone marrow-derived dendritic cells but does impair antigen presentation. Immunology. 2000;99:523–531. doi: 10.1046/j.1365-2567.2000.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buelens C, Willems F, Delvaux A, Pierard G, Delville JP, Velu T, Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–2672. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 17.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Bianco NR, Kim SH, Ruffner MA, Robbins PD. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009;60:380–389. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 20.Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke XY, Rennert PD, Gray GS, Gribben JG, Nadler LM. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 21.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang J, Gu X, Qian S, Chen Z. Graded function of CD80 and CD86 in initiation of T-cell immune response and cardiac allograft survival. Transpl Int. 2008;21:163–168. doi: 10.1111/j.1432-2277.2007.00590.x. [DOI] [PubMed] [Google Scholar]
- 23.Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci U S A. 2003;100:6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 25.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr., Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–157. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]