Abstract
Intracellular microbes have evolved efficient strategies for transitioning from one cell to another in a process termed intercellular transmission. Here we show that host cell transmission of the obligate intracellular parasite Toxoplasma gondii is closely tied to specific cell cycle distributions, with egress and reinvasion occurring most proficiently by parasites in the G1 phase. We also reveal that Toxoplasma undergoes marked changes in mRNA expression when transitioning from the extracellular environment to its intracellular niche. These mRNA level changes reflect a modal switch from expression of proteins involved in invasion, motility, and signal transduction in extracellular parasites to expression of metabolic and DNA replication proteins in intracellular parasites. Host cell binding and signaling associated with the discharge of parasite secretory proteins was not sufficient to induce this switch in gene expression, suggesting that the regulatory mechanisms responsible are tied to the establishment of the intracellular environment. The genes whose expression increased after parasite invasion belong to a progressive cascade known to underlie the parasite division cycle indicating that the unique relationship between the G1 phase and invasion effectively synchronizes short-term population growth. This work provides new insight into how this highly successful parasite competently transits from cell to cell.
Keywords: Egress, Invasion, Gene regulation, Microarray, Promoter, Synchronization
Introduction
Successful infection by intracellular pathogens critically depends on their ability to efficiently transition from one host cell to another in a process termed intercellular transmission (Harth et al., 1993). In preparation for this transition, many intracellular pathogens enter a state of growth arrest accompanied by significant changes in gene expression designed to enhance transmissibility (Hammerschlag, 2002; Samuel et al., 2003; Molofsky et al., 2004). For example, during the late stages of intracellular replication when nutrients become limiting, Legionella pneumophila enters stationary phase and activates genes responsible for cell exit, motility, environmental resilience, and intracellular survival upon infection of a new macrophage (Molofsky et al., 2003). After entry into a new host macrophage, L. pneumophila down-regulates transmissibility genes and activates genes involved in nutrient utilization and bacterial replication (Dalebroux et al., 2010).
Although much is known about bacterial transcriptional reprogramming associated with intercellular transmission, less is known about whether intracellular protozoan parasites also coordinate gene expression with the transition from one host cell to another. Toxoplasma gondii is an obligatory intracellular protozoan parasite that causes life-threatening encephalitis in immuno-compromised individuals such as AIDS patients and persons undergoing immunosuppressive therapy after organ transplantation (Carruthers et al., 2002). The parasite is classified in the phylum Apicomplexa that includes pathogens of medical and veterinary importance such as Plasmodium spp, Cryptosporidium spp, and Eimeria spp (Dubey, 1998; Hill et al., 2002). Apicomplexan parasites divide by unusual processes that often involve multinuclear division where the scale of nuclear reduplication is a species characteristic (Gubbels et al., 2008). Despite a diversity in counting in each replication scheme, daughter formation is held to the end of the division cycle where daughter parasites are formed internally, ultimately consuming the mother cell (Striepen et al., 2007). Apicomplexa daughter cell maturation involves the de novo biosynthesis of a complex invasion apparatus required for motility and egress that replaces the mother cell structures (Nishi et al., 2008). Precisely how this occurs is unknown, although it is reasonable to speculate that parasite invasion efficiency could be affected during the “switch over” from mother to daughter.
Compared to many apicomplexans, Toxoplasma tachyzoite division is quite simple, being composed of single G1, S, mitosis (M)/cytokinesis (C) phases with infectious daughters formed following each nuclear cycle (Radke et al., 2001). Consequently, replicating T. gondii parasites, which can divide 5–6 times in a single host cell, are continuously infective when mechanically liberated from host cells. It is not known if tachyzoites are more or less infective at particular phases of its cell cycle, nor is it known if they preferentially exit host cells during a specific cell cycle stage. Toxoplasma tachyzoites are able to finish budding extracellularly (2N resolves to 1N) (Hu et al., 2004), but are not able to reinitiate a second round of chromosome replication outside the host cell. The development of cellular markers has enabled the timing of specific events in the Toxoplasma tachyzoite cell cycle to be determined. Golgi replication begins in the G1 phase (Pelletier et al., 2002; White et al., 2005) and progresses to late G1 with migration of the centrosome to the basal side of the nucleus (Hartmann et al., 2006). Similar to other eukaryotes, duplication of the centrosomes coincides with S phase entry (Gubbels et al., 2008) and is associated with a basal to apical relocalization of the centrosomes that is functionally not understood (Hartmann et al., 2006). An unusual distribution of late S phase tachyzoites is observed in asynchronous populations with parasites having 1.8N average DNA content just before entering mitosis (Radke et al., 2001; White et al., 2005). This novel cell cycle subpopulation could reflect a parasite checkpoint associated with the initiation of daughter cell budding (Gubbels et al., 2008). During mitosis, T. gondii chromosomes do not substantially condense and the nuclear envelop remains intact as daughter cells form by de novo synthesis of the inner membrane complex (IMC), a laminate of membrane and cytoskeleton that demarcates the new progeny (Gubbels et al., 2004; Gubbels et al., 2006). During budding, organelle partitioning is completed prior to consolidation of the mother cell plasmalemma into the mature daughters (Gubbels et al., 2008; Hu, 2008; Nishi et al., 2008). Though parasites that share a parasitophorous vacuole divide relatively synchronously (Hu et al., 2004) coordination of parasite cell cycles does not extend beyond individual infected host cells. Therefore establishing methods to achieve population synchrony have followed a strategy of reversible inhibition of parasites in specific cell cycle phases (Conde de Felipe et al., 2008; Behnke et al., 2010). While effective, these protocols require in some cases a specialized transgenic strain and suffer from mild drug toxicity.
Here we show that Toxoplasma tachyzoites preferentially egress and invade in the G1 phase of the parasite cell cycle, thus demonstrating functional coordination between the cell cycle and intercellular transmission. We also show that the parasite rapidly alters the expression of hundreds of mRNAs soon after cell invasion, reflecting a modal transition from invasion to intracellular replication. Finally, we establish that it is possible to partially synchronize Toxoplasma cultures in vitro by limiting the time of invasion, thereby providing a new tool to study parasite replication.
Results
Toxoplasma tachyzoites egress efficiently in G1 phase
To initially determine if Toxoplasma preferentially exits from host cells at a specific stage in the cell cycle, we use flow cytometry to analyze the DNA content of newly egressed tachyzoites. As illustrated in Figure 1A (top panel), asynchronously dividing tachyzoites harvested by forced passage revealed two dominant populations based on genomic DNA content representing G1 and early S phase under a broad 1N average peak and late S and mitotic subpopulations with DNA contents ranging from 1.6–2N in a second peak (Radke et al., 1998; Radke et al., 2001). The 1N population was more abundant in these populations, consistent with the G1 phase comprising at least half the total cell cycle length (Radke et al., 2001). In contrast, newly egressed parasites collected from late stage lytic cultures at one-hour intervals showed a dominant, and very narrow 1N peak devoid of early S phase parasites along with a smaller but similarly well-defined peak centered at ~1.8N (Fig. 1A, middle panels). Mitotic parasites containing a diploid (2N) genomic content were not detected in these egressed populations. Parasites that had not yet egressed in the late stage cultures also showed a DNA content profile strikingly similar to that of newly egressed parasites and distinct from asynchronous cultures harvested mechanically (Fig. 1A, bottom panel). Together, these findings suggest that Toxoplasma egress is not random with respect to the cell cycle, and that the cell cycle distributions of late stage vacuoles may be the source of these unusual 1N/1.8N egressed subpopulations.
Figure 1. Parasites preferentially exit host cells in the G1 phase of their cell cycle.
(A) Newly egressed tachyzoites have 1N or 1.8N nuclear content. Freshly egressed parasites were allowed to invade HFF cells for 4 h and non-invaded parasites were removed by washing. Intracellular parasites were further incubated at 37°C for 44 h when the monolayer was again washed to remove any parasites that had egressed. Newly egressed parasites were collected at 1 h intervals for 2 h (i.e., at 45 h and 46 h post-infection) and intracellular parasites that had not yet egressed at 46 h were mechanically liberated and filter purified. The DNA content was determined flow cytometry using the SYTOX Green dye. The DNA content distribution of asynchronous growing population (Async) harvested from a control culture at 36 h. No gates were used in collecting fluorescent results and the cell size examined (based on forward scatter) exceeds the average tachyzoite size by 20 fold. The data shown are representative of two independent experiments. At least 10,000 events were collected for each sample with all fluorescence results shown in the histograms.
(B) Markers of cell cycle status. The cell cycle stage of parasites was determined by analyzing the status of TgIMC1 and EGFP-TgCentrin2. In early G1, EGFP-TgCentrin2 (a marker for the centrosome) is anterior to the nucleus while in late G1 it migrates to the distal end of the nucleus. Parasites in S phase contain duplicated centrosomes. The M/C phases were identified based on formation of new daughter IMCs, while newly formed daughter cells were identified by phase contrast microscopy. Arrows indicate the centrosome(s).
(C) Toxoplasma tachyzoites are more egressive in G1 phase of the cell cycle. Parasites grown in 8-well chamber slides were synchronized with PDTC treatment for 6 h, resulting in arrest within early G1 phase. The cell cycle block was released by washing cultures with drug-free medium. Intracellular parasites at different points post-drug release and hence in different stages of the cell cycle were assessed for egress efficiency by treating with calcium ionophore A23187 for 2 min at 37°C. The slides were immediately fixed and stained with DAPI and antibodies to TgGRA7. Images of 18 random sites within each well were captured at 600X magnification and the total numbers of intact and ruptured vacuoles were enumerated for each well to determine the percentage of egressed vacuoles. Data are compiled from 3 independent experiments, each with duplicate samples. Error bars represent SEM.
To further investigate the cell cycle dependence of egress, we performed induced egress experiments using synchronized populations of a transgenic strain expressing EGFP-TgCentrin2 (Hu, 2008). TgCentrin2 is an EF hand containing Ca2+-binding protein associated with the juxtanuclear centrosome along with additional apical structures, and hence serves as a excellent marker to assess the occurrence of S phase entry and mitotic progression (Hu, 2008). In early G1, a single centrosome is anterior to the nucleus (Fig. 1B). During mid-to late G1 the centrosome migrates to the posterior side of the nucleus where it duplicates at the onset of S phase and migrates back to an anterior position (Nishi et al., 2008). To visualize budding we also stained parasites with an antibody to inner membrane complex protein 1 (TgIMC1) as an indicator of internal daughter cell formation along with observation of newly emerged daughter cells by phase contrast microscopy. Intracellular parasites were synchronized with PDTC treatment that reversibly growth arrests parasites in early G1 phase (Conde de Felipe et al., 2008). Removal of the drug releases the cell cycle block and the parasites synchronously progress into S phase ~3 h post drug release. Parasites at different times post drug release and hence enriched for different stages of cell cycle were then induced to egress from host cell by treating with the calcium ionophore A23187 (Endo et al., 1982) for 2 min followed by immediate fixation and staining for TgGRA7, a parasitophorous vacuolar membrane protein that serves as a convenient marker for both egressed and intact parasite-containing vacuoles (Sibley et al., 1995). Egress efficiency was determined by enumerating the number of intact and ruptured vacuoles. As shown in Figure 1C, egress was highest soon after PDTC-release when the parasite is still in G1. A significant decline in egress was seen during S and M/C phases before increasing again as the parasite re-entered G1. Analysis of the cell cycle status of parasites that successfully egressed in S and M/C phases indicated that up to half of the egressed parasites were actually in G1 phase due to a window of synchrony that spans the comparatively shorter S and mitotic phases (Conde de Felipe et al., 2008; Behnke et al., 2010). Collectively, our findings suggest that Toxoplasma preferentially egresses in the G1 phase of the cell cycle.
G1 phase tachzyoites are more invasive
To assess the invasion efficiency of tachyzoites in different stages of cell cycle a series of experiments were performed. In the first set of experiments, asynchronously replicating EGFP-Tgcentrin2 parasites were harvested and purified from host cells 24 h post-infection and allowed to invade HFF cells for 30 min before removing noninvaded parasites. The invasion efficiency of different cell cycle phases was assessed by monitoring centrosome duplication and the presence of internal daughters before and after invasion. The results showed that before invasion the percentage of parasites in G1 phase (single centrosome/no internal daughters) of cell cycle is about 40% whereas in the invaded parasites it is 60% (Fig. 2A). Conversely, parasites that are not in G1 (i.e., S or M/C phase parasites) show a diminished capacity for invasion. DNA content analysis further confirmed that the population of newly invaded parasites is almost exclusively comprised of 1N tachyzoites that increases in number but importantly does not diversify in cell cycle distribution over time of invasion (Fig. 2B). The lack of significant 1.6–2N parasites in newly invaded populations compared to the inoculum indicates that parasites undergoing chromosome replication or mitosis are less invasive than G1 parasites.
Figure 2. Parasites are most invasive in G1 phase of their cell cycle.
(A) G1 phase parasites are more invasive than parasites in other stages of the cell cycle. Asynchronously growing intracellular parasites were harvested 24 h post-infection, purified from host cells and added onto HFF cells in 8-well chamber slides. The slides were incubated at 37°C for 30 min, washed three times to remove non-invaded parasites, fixed and stained to determine the invaded parasites per host cell. The cell cycle stage of parasites was assessed by determining IMC and/or centrosome status before and after invasion.
(B) Flow cytometry analysis of newly invaded parasites indicates that parasites are most invasive when they have 1N nuclear content (G1). Asynchronously replicating parasites were harvested and added onto HFF cells and the DNA content of the newly invaded parasites was determined by staining with SYSTOX Green dye. For each plot, shown is the time post-addition of parasites and the counts per second (cn/s), reflecting the increase in invaded parasites over time.
(C) Invasion of cell cycle synchronized parasites. Parasite cultures were synchronized with PDTC treatment for 6 h to arrest the population in early G1. The cell cycle block was released by removal of PDTC and intracellular parasites were harvested at different time points post drug release (hence at different stages of cell cycle) and added onto HFF cells in 8-well chamber slides. After incubating at 37° C for 30 min, non-invaded parasites were removed and slides were fixed and stained to determine the invasion efficiency. Data are compiled from 3 independent experiments, each with duplicate samples. Error bars represent SEM.
In the second set of experiments, EGFP-Tgcentrin2 parasites harvested at different time points after release from PDTC-arrest were allowed to invade new host cells for 30 min. As above, the parasite cell cycle status before and after invasion was determined using centrosome and daughter cell markers in addition to assessing their invasion proficiency using a differential staining procedure (Dobrowolski et al., 1997). The results showed that parasites are highly invasive in G1 during the first 2 h following PDTC release before declining 4–5 fold during S and M/C phases 3–6 h post-release (Fig. 2C). Infection proficiency is regained after cytokinesis as new daughter parasites emerge in G1. These results further confirmed that parasites are more efficient at invasion in the G1 phase of the cell cycle.
Tachyzoites undergo rapid changes in gene expression after host cell invasion
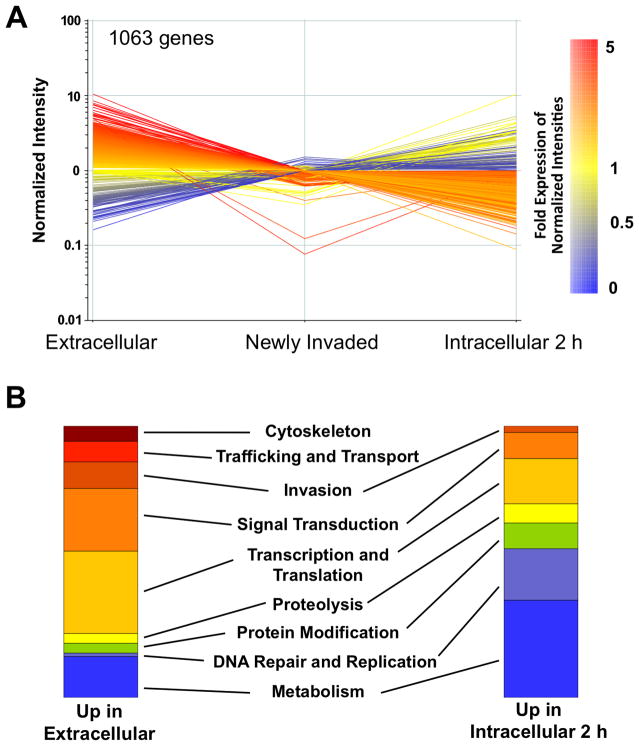
Intracellular pathogens are known to regulate their expression profile based on environmental cues such as nutrients, pH, host defense factors etc. Similarly when Toxoplasma invades a host cell it experiences markedly different environment and presumably has to adjust its complement of expressed genes to reinitiate replication and successfully complete the intracellular cycle. To investigate the dynamic changes in the transcriptome profile of Toxoplasma as it transitions from an extracellular to an intracellular form, we performed microarray analysis of three parasite populations using the Affymetrix ToxoGeneChip: extracellular parasites, intracellular parasites soon after invasion (newly invaded) and intracellular parasites 2 h post-invasion. The most substantial changes in mRNA expression were observed between extracellular and intracellular 2 h parasites where the transcript abundance of 1063 genes changed significantly (ANOVA p<0.05 and >2.5-fold change). These included 952 genes elevated in extracellular parasites compared to intracellular 2 h parasites and 111 genes upregulated in intracellular 2 h parasites compared to extracellular parasites. Interestingly, most of the regulated genes showed intermediate abundance in newly invaded parasites, indicating that invasion represents a node in the transition from high to low expression or low to high expression (Fig. 3A). Some of the elevated mRNAs in extracellular parasites (red or orange lines) showed the steepest decline in newly invaded parasites and leveled off thereafter whereas others continued to decline in intracellular 2 h parasites. Genes that showed moderate mRNA levels in extracellular parasites (yellow lines) showed mixed fates (increase or decrease) in intracellular 2 h parasites. Conversely, low abundant mRNAs in extracellular parasites tended to increase most substantially in newly invaded parasites and remain constant in intracellular 2 h parasites. Overall, these findings suggest that significant changes in gene expression accompany intercellular transmission.
Figure 3. Parasites exhibit marked gene expression changes during intercellular transmission.
(A) Genes that were up- or down-regulated in extracellular parasites compared to intracellular 2 h parasites. (For a list of these genes see Table S1 and S2). Each line represents one gene. Genes that are upregulated in extracellular parasites are colored red to yellow and those upregulated in intracellular 2 h are colored yellow to blue.
(B) Genes upregulated in extracellular or intracellular 2 h parasites were classified into 9 categories based on known or implied function. The data are displayed as stacked column graphs representing the proportion of genes in each category. Category assignments are listed in Table S1 and S2.
Functional categorization of the genes regulated in conjunction with cell invasion revealed a conspicuous trend (Fig. 3B). Genes with elevated mRNA levels in extracellular parasites tended to be either directly involved in cell invasion (invasion proteins, surface proteins, cytoskeletal proteins including the motor system) or indirectly involved in the process (protein trafficking and transport, signal transduction). By contrast, the mRNAs increased in intracellular 2 h parasites encoded genes that were principally devoted to parasite replication (DNA repair and replication, metabolism). Extracellular and intracellular 2 h parasites showed approximately equally representation of genes involved in transcription and translation (i.e., gene expression), reflecting the role of such genes in the expression changes that accompany cell invasion. Comparison of the gene expression changes observed during intercellular transmission with the recently established cell cycle transcriptome for Toxoplasma (Behnke et al., 2010) revealed an alignment between genes elevated in extracellular parasites and the S/M subtranscriptome and the induction of the intracellular mRNA profiles with the late G1 subtranscriptome (see Fig. S2 for details). Altogether, 16% of the ~8,000 protein coding genes coding genes in T. gondii, are significantly regulated during intercellular transmission, indicating that the parasite coordinates a substantial fraction of its mRNA pool with host cell invasion.
In addition to the aforementioned genes that changed significantly between extracellular and intracellular 2 h parasites, 226 genes were either up-regulated (25 genes) or down-regulated (201 genes) in newly invaded parasites compared to extracellular or intracellular 2 h parasites (Fig. S2A). Peak expression of these genes is scattered throughout the cell cycle with a slight enrichment in those that peak in G1 (Fig. S2).
Promoter activation of genes upregulated after invasion
To validate the expression profiles of parasites obtained by microarray experiments, we used quantitative reverse transcriptase PCR and promoter-based assays to confirm selected changes in gene expression. For qRT-PCR analysis, RNA was purified from extracellular and intracellular 2 h parasites and mRNA levels of four genes each in extracellular (59.m03398, 59.m07778, 80.m02181, and 76.m01659) and intracellular 2 h parasites (55.m10326, 541.m01207, 31.m00940, and 44.m02820) were analyzed. The results of the qRT-PCR revealed similar fold changes as obtained by microarray analysis (Fig. 4).
Figure 4. Validation of the microarray data by qRT-PCR.
Changes in gene expression shown by microarray were confirmed by qRT-PCR analysis for four genes that were upregulated in either extracellular (A) or intracellular parasites
(B) and two genes that were constitutively expressed (α-TUB & EF-1α). n=2 independent experiments, each with triplicate samples. Error bars, SEM.
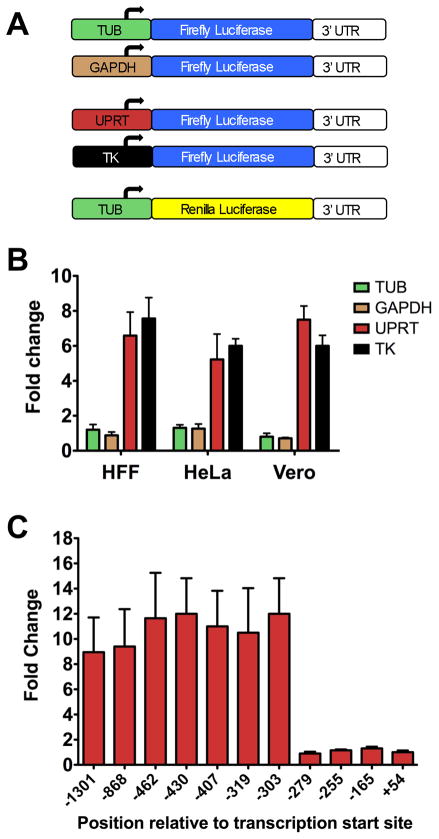
We also used a dual luciferase reporter assay to determine if promoter activation is responsible for the increased expression of mRNAs after cell invasion. We cloned 5′-genomic flanking regions including untranslated regions of two constitutively expressed genes, α-tubulin (583.m000220), GAPDH (80.m000030) and two genes upregulated 2 h after invasion, UPRT (583.m00018) and thymidylate kinase (TK; 542.m0023) upstream of firefly luciferase (Fig. 5A). The reporter constructs were independently co-transfected into parasites with the control vector that expresses renilla luciferase under the control of the constitutive α-tubulin promoter (TUB-REN). Transfected parasites were allowed to invade cells derived from skin (HFF), cervix (HeLa), or kidney (Vero) and were analyzed for luciferase activity. As shown in Figure 5B, UPRT-firefly and TK-firefly expression increased after invasion of all three types of host cells while TUB-firefly and GAPDH-firefly levels did not change. These data indicate that transcriptional mechanisms are likely responsible for the increased mRNA levels during parasite invasion.
Figure 5. Transcriptional control of genes upregulated after invasion.
(A) Schematic representation of reporter constructs used in luciferase reporter assays. Promoter regions (including 5′ untranslated regions) of two constitutively expressed genes (α-TUB & GAPDH) and two genes that are upregulated post-invasion (UPRT & TK) were cloned upstream of the reporter gene firefly luciferase (FLU). The reporter vectors were individually co-transfected with control vector (TUB-RLU) to generate parasite populations stably expressing the dual reporters.
(B) An invasion assay was performed as described previously for the microarray analysis in three different host cell lines. FLU activity was normalized to RLU activity and expressed as the fold change of intracellular 2 h parasites compared to extracellular parasites. The results show that UPRT-FLU & TK-FLU are upregulated 2 h post-invasion in all three types of host cells. n=3 independent experiments, each with triplicate samples. Error bars, SEM.
(C) 5′ serial deletion analysis of UPRT promoter. Promoter deletion constructs were transfected into parasites and transfected parasites were added onto HFF cells. 48 h after transfection, freshly lysed parasites were subjected to a luciferase invasion assay as described in A. FLU activity was normalized to RLU activity and expressed as the fold change of intracellular 2 h parasites compared to extracellular parasites. Results show that 24 bp region between −303 and −279 contains cis-acting element essential for transcriptional activation of UPRT after host cell invasion. n=3 independent experiments, each with triplicate samples. Error bars, SEM.
To confirm that promoter activation is responsible for increased expression after invasion, we created a series of 5′ sequential deletion mutants within the UPRT promoter in the firefly luciferase construct. Activation of the UPRT promoter after invasion was observed in the first 7 deletion constructs up to −303 bp upstream of the transcriptional start site (Fig. 5C). However, deletion of an additional 24 bp to −279 bp abolished UPRT promoter activation to basal levels that were also seen for the subsequent deletion constructs (−255 bp, −165 bp, and +54 bp). Interestingly, the 24 bp segment that is necessary for UPRT promoter activation contains a putative apicomplexan AP2 (ApiAP2) binding site (TGCATGC). ApiAP2 family proteins are DNA binding proteins found in apicomplexan parasites that are related to AP2/ERF (Apetala2/ethylene response factor) transcription factors of plants (Balaji et al., 2005; De Silva et al., 2008).
Changes in mRNA expression profiles appear to occur only after successful host cell invasion
Since mRNAs encoding components of signal transduction pathways were elevated in extracellular parasites, we hypothesized that signal transduction events known to trigger protein secretion from micronemes and rhoptries might also induce changes in mRNA levels reflecting the coupling of immediate invasion mechanisms to gene expression regulation. To test this, we produced a transgenic line stably expressing UPRT-firefly and TUB-renilla (UT-Dual Luc) and pre-treated this strain with mycalolide-B (Myc-B) before washing out the drug and challenging host cells with the treated parasites. Myc-B is an irreversible inhibitor of actin polymerization and hence treated parasites can attach to host cells, secrete micronemes and rhoptries, but cannot invade (Lavine et al., 2008) (Fig. 6A). Results showed that UPRT-firefly is not upregulated in Myc-B treated parasites, indicating that the activation of signaling pathways associated with microneme or rhoptry discharge may not be not sufficient to change mRNA expression profiles (Fig. 6B). To further validate this finding, we also used qRT-PCR to measure the mRNA levels of four genes (55.m10326, 541.m01207, 31.m00940, and 44.m02820) that are normally upregulated 2 h post-invasion. As shown in Fig. 6C, expression levels of these genes in Myc-B treated, invasion arrested parasites were similar to those of extracellular parasites. Myc-B treated parasites also failed to show changes in several mRNAs that are normally downregulated after invasion (data not shown). Collectively, these findings suggest that parasite attachment and activation of signaling pathways associated with invasion is not sufficient to the induce gene expression changes that normally accompany invasion.
Figure 6. Successful invasion is required for upregulation of UPRT promoter activity.
(A) Myc-B treated parasites attach to host cells and secrete micronemes and rhoptries but do not invade. A parasite clone stably expressing UPRT-FLU and TUB-RLU (UT-DualLuc) was treated with Myc-B and added onto HFF cells in an 8 well chamber slide. After 30 min, slides were fixed and stained for M2AP without permeabilization. The slides were then treated with saponin, which permeabilizes the host cell plasma membrane but not the parasite cell membrane, and then stained for ROP1.
(B) UT-DualLuc parasites were treated with DMSO (vehicle control) or Myc-B and added to HFF cells for 30 min. Non-attached parasites were gently washed and the cultures were incubated at 37°C for 2 h followed by assessment of luciferase activity as described in experimental procedures. UPRT-FLU is not upregulated in Myc-B treated parasites indicating that the activation of signaling pathways associated with microneme or rhoptry discharge are not sufficient to induce expression reprogramming. n=3 independent experiments, each with triplicate samples. Error bars, SEM.
(C) Fresh naturally egressed RH parasites were purified, treated with DMSO (vehicle control) or Myc-B and added onto HFF cells. After incubation at 37°C for 30 min, Myc-B treated parasites were gently washed once to remove unattached parasites while DMSO treated parasites were vigorously washed thrice to remove non-attached and non-invaded parasites and the cultures were further incubated at 37°C for 2 h. DMSO treated parasites were incubated without HFF cells for 2 h and 30 min to serve as extracellular parasites. RNA was purified from pellets of extracellular parasites, HFF cells with only attached parasites (Myc-B treated), and 2 h intracellular parasites (DMSO treated) and analyzed by qRT-PCR. n=3 independent experiments. Error bars, SEM.
We used UT-Dual Luc parasites to investigate the kinetics of altered gene expression after invasion. Human fibroblasts were infected with UT-Dual Luc parasites cells for 30 min followed by removal of non-invaded parasites. UPRT and α-tubulin promoter activity was determined by assessing firefly and renilla luminescence, respectively, from extracellular parasites and intracellular parasites at different times post-invasion. As shown in Figure 7A, firefly luciferase activity under control of the UPRT promoter increased steadily after invasion, reaching a plateau 5 h post-invasion. Interestingly, α-tubulin promoter activity changed minimally during the first few hours after invasion but showed a marked increase 6–7 h post-invasion consistent with the cyclical pattern of this mRNA with peak timing in the S/M periods (Behnke et al., 2010).
Figure 7. Parasite cell division is synchronous following cell invasion.
(A) UT-DualLuc parasites were allowed to invade HFF cells for 30 min followed by removal of non-invaded parasites. UPRT-FLU and TUB-RLU activity was determined for extracellular parasites (parasites incubated without HFF cells for 30 min) and intracellular parasites at different time points 0 h to 10 h post-invasion. UPRT-FLU and TUB-RLU activity for intracellular parasites at different points post-invasion was normalized to the respective UPRT-FLU and TUB-RLU values of extracellular parasites to determine the fold changes. n=3 independent experiments, each with triplicate samples. Error bars, SEM.
(B) UT-DualLuc parasites were allowed to invade HFF cells for 30 min and non-invaded parasites were removed. Extracellular and intracellular parasites were fixed and stained for TgIMC1 at 2 h intervals post-invasion. n=3 independent experiments, each with single samples. Error bars, SEM.
(C) Parasites stably expressing EGFP-TgCentrin2 were allowed to invade HFF cells for 30 min and non-invaded parasites were removed. Extracellular and intracellular parasites were then fixed and examined for location and duplication status of centrosome at various time points before and after invasion. n=3 independent experiments, each with single samples. Error bars, SEM.
Population growth is synchronous following limited invasion
The increased UPRT-firefly and TUB-renilla expression revealed a sequential pattern more consistent with synchronous than asynchronous population growth (Behnke et al., 2010). To further investigate the timing of the first cell division after invasion, parasites were allowed to invade human fibroblasts using the limited invasion timeframe as above. Samples were fixed at 2 h intervals post-invasion and parasites were stained for TgIMC1 to visualize daughter cell formation. Results showed that daughter cell formation initiates at 6 h post-invasion and peaks at 8 h when 40% of the parasites show daughter parasites developing therein (Fig. 7B). Within 10 h post-invasion, ~70% of the intracellular parasites completed their first cell division. This level of population synchrony is comparable to that achieved with either the previously reported synchrony methods (Radke et al., 1998; Conde de Felipe et al., 2008).
In cultures of EGFP-TgCentrin2 parasites subjected to limited invasion we examined the location and duplication status of the centrosome as extracellular parasites and at various time points after invasion. Results showed that extracellular and intracellular parasites at 0 h post-invasion have similar centrosome status i.e., that of G1 (Fig. 7C). At 6 h post-invasion, ~55% parasites have duplicated their centrosome coinciding with initiation of DNA replication (S phase) and hence these findings are in agreement with α-tubulin kinetics and IMC formation post-invasion. Altogether these results demonstrate that when tachyzoites are provided a short window of time for invasion the effective selection of G1 parasites leads to an intracellular population that replicates synchronously for at least one cell division.
Discussion
In this study we showed that the intercellular transmission of Toxoplasma is closely linked to its cell cycle, with parasite egress and invasion principally occurring when parasites are in G1 phase. We also found that parasite transition from the extracellular to intracellular niche involves marked changes in gene expression, and that completion of invasion is necessary to activate the regulatory mechanisms involved. Finally, we established that the cell cycle bias in invasion might be exploited to experimentally achieve growth synchrony in potentially any parasite strain.
Several factors probably dictate the high efficiency of parasite egress and invasion in G1 phase. First, invasion secretory organelles including the micronemes and rhoptries are formed late in the parasite cell cycle and are ready for use in G1 phase parasites. In contrast, these organelles are being dismantled in the mother cell during the early stages of daughter cell formation (M/C phase) creating a vulnerable time during the functional switch from mother to daughter (Nishi et al., 2008). Similarly, the mother cell IMC and motility apparatus, the glideosome, is disassembled during M/C phase, thereby compromising its ability to actively depart an expiring cell and enter a new target cell. Second, egress and invasion are both energy dependent events that require ATP. G1 phase parasites can direct ATP pools to motility for invasion and egress, while parasites in S, M, or C phases may have limited energy reserves due to consumption for anabolic processes associated with daughter cell formation. Finally, the parasite has a small girth during G1 phase (Conde de Felipe et al., 2008), particularly as they emerge from the previous cell cycle. Manifested by a waist-like constriction around its girth, the parasite appears to squeeze through host cell membranes and cytoskeletal structures during egress and invasion; hence narrow parasites probably have a selective advantage in completing these events.
Transcriptional profiling revealed that Toxoplasma parasites undergo significant changes in gene expression as they gain entry into host cells. In extracellular parasites, mRNAs encoding invasion and motility genes are elevated and hence the parasite appears to be optimally primed for cell invasion. Conversely, DNA replication and metabolic genes are increased after entry, indicating a switch to replication mode. It is interesting to note that among top 20 categorized mRNAs that are most elevated in extracellular parasites, 6 encode invasion and cytoskeleton proteins, and another 6 are related to transcription and translation. This suggests that the extracellular parasites are utilizing the transcription and translation machinery to actively replenishing the proteins involved in cell invasion and gliding. Indeed, extracellular parasites readily incorporate 35S methionine and cysteine into invasion proteins (Parussini et al., 2010), indicating ongoing synthesis of such proteins in G1 phase despite having peak synthesis normally in late S and M/C phases (Behnke et al., 2010). In intracellular parasites, 13 of the top 20 mRNAs increased are related to metabolic processes including proteolysis. Interestingly two of the proteases that are upregulated after invasion are uncharacterized subtilisin-like serine proteases. Their increased expression after invasion could indicate that they play novel roles in metabolism and/or replication.
Recent studies have revealed that gene regulation in apicomplexan parasites such as Plasmodium falciparum is mediated by stage specific expression of ApiAP2 family transcription factors (De Silva et al., 2008). These groups of transcription factors are also conserved in other members of the phylum including Toxoplasma (Balaji et al., 2005; De Silva et al., 2008; Behnke et al., 2010). The presence of the TGCATGC putative ApiAP2 DNA binding motif within the 24 bp region of UPRT promoter essential for transcriptional activation suggests that cell cycle specific expression may also involve ApiAP2 proteins in Toxoplasma. This motif is found in hundreds of T. gondii promoters (van Poppel et al., 2006) and is particularly enriched in the promoters of genes activated in G1 phase (Behnke et al., 2010). The TGCATGC motif is also recognized by a conserved G1-specific ApiAP2 factor in P. falciparum (De Silva et al., 2008). We used the FIRE (Finding Informative Regulatory Elements) algorithm (Elemento et al., 2007) to determine if promoter regions (2 kb upstream) of genes with regulated expression during invasion are enriched in putative regulatory elements. This analysis revealed that promoters of genes that are elevated in extracellular parasites are enriched in a motif (GCGTCNC) that was previously described as being essential for expression of microneme genes in Toxoplasma (Mullapudi et al., 2009). Interestingly, FIRE analysis also revealed that the TGCATGC G1-specific motif is under-represented in genes that are up-regulated in extracellular parasites, supporting the notion that the transcriptional profile of extracellular parasites reflects the cell cycle at or prior to the G1 phase checkpoint when late G1 genes are activated (Behnke et al., 2010).
During transmission from one cell to another, intracellular microbes experience radical environmental changes that they often use as cues to trigger developmental changes for departure, re-entry, and initial survival in a new cell. For example, in response to waning amino acid availability inside infected macrophages, Legionella pneumophila undergoes marked gene expression changes to induce phagocyte necrosis, bacterial motility, environmental resilience, and intracellular survival upon re-entry. After invasion, Mycobacterium, Salmonella, and Legionella use intracellular environmental cues such as pH, nutrients, and host defense factors to trigger expression of genes involved in intracellular replication (Eriksson et al., 2003; Molofsky et al., 2004; Rohde et al., 2007). It is unknown to what extent Toxoplasma is sensitive to environmental cues that could beneficially adapt their transcriptome to changing extracellular conditions. Since resumption of vegetative growth after entry is a key event, Toxoplasma may integrate multiple intracellular cues to initiate replication and further expand the infection. With the principal gene expression transitions now identified it may be possible to exploit this information to explore axenic conditions for parasite growth.
Experimental Procedures
Parasite cultures
T. gondii tachyzoites were maintained by passage through human foreskin fibroblasts (HFF) in a humidified incubator at 37°C with 5% CO2. The normal growth medium consisted of DMEM supplemented with 10% fetal bovine serum, 10 mM HEPES, 2 mM L-glutamine and 50 μg/ml penicillin streptomycin. EGFP-TgCentrin2 parasites (kindly provided by Dr. Ke Hu, Indiana University, Bloomington) were propagated without drug selection. Purification of parasites was performed according as described previously (Harper et al., 2006).
Cell cycle synchronization
Parasite cultures were synchronized with PDTC (ammonium pyrrolidine dithiocarbamate, Sigma–Aldrich St. Louis, MO) treatment as described previously (Conde de Felipe et al., 2008). Briefly, parasites were serially passaged twice every 24 h to obtain mid-log cultures (4–8 parasites per vacuole). Infected host cells were then scraped, purified, inoculated onto HFF monolayers and incubated at 37°C for 1 h to allow invasion. Non-invaded parasites were removed by washing thrice with growth medium followed by incubation at 37°C for 10 h. Intracellular parasites were then treated with 60 μM PDTC for 6 h to arrest parasites in early G1 stage of cell cycle. The cell cycle block was released by washing cultures three times with pre-warmed growth medium.
Invasion Assays for asynchronous and synchronized parasites
Invasion assays were performed in 8-well chambered slides as described previously (Dobrowolski et al., 1997) with the following modifications. EGFP-Tgcentrin2 parasites were added onto HFF monolayers (1×107 parasites/well) and incubated at 37°C for 30 min. Slides were then washed three times to remove non-invaded parasites, fixed, blocked and stained with monoclonal antibody 11–132 (Argene) against SAG1 without permeabilization. After 1 h, slides were washed and secondary antibody, Alexa Fluor-594-conjugated goat anti-mouse (Molecular Probes) and DAPI (1 μg/ml; Sigma) were added. After 1 h, slides were washed and mounted using Mowiol (Sigma-Aldrich). Parasites that were both GFP- and Alexa Fluor 594-positive were identified as extracellular whereas those that were GFP-positive but not Alexa Fluor 594-positive were identified as intracellular. Images of 18 random sites within each well were captured at 600X magnification and total number of intracellular parasites and host cell nuclei were enumerated.
RNA isolation and microarray analysis
A number of precautionary measures were exercised in obtaining fresh naturally egressed parasite populations. The medium was changed 40 h post-infection to remove any extracellular parasites. When the parasites were harvested 8 h later, some host cell debris was still visible but there were no intact vacuoles. These fresh, naturally egressed RH parasites were gently pipetted, sequentially syringed with 20 and 23 gauge needles, filtered (3.0 μM, Whatman) and resuspended in invasion medium (DMEM containing 4.5 g/L glucose, L-glutamine, sodium pyruvate supplemented with 20 mM HEPES and 3% FBS) and were either incubated without HFF cells at 37° C for 2 h 30 min (extracellular parasites) or allowed to invade HFF cells for 30 min. After 30 min, non-invaded parasites were removed by washing thrice with warm medium and were either incubated at 37° C for 2h (intracellular 2 h parasites) or subjected to RNA isolation (newly invaded parasites). Total RNA was purified from pellets of extracellular parasites, HFF cells with newly invaded parasites and HFF cells with intracellular 2 h parasites using Qiagen RNeasy kit according to manufacturer’s instructions (Qiagen, Valencia, CA). RNA quality was assessed using Agilent Bioanalyzer 2100 (Santa Clara, CA). A total of 3 μg RNA was used to make cRNA with an Affymetrix One-Cycle kit (Affymetrix, Santa Clara, CA). Fragmented cRNA (5 μg) was hybridized to the Toxoplasma gondii Affymetrix microarray (http://ancillary.toxodb.org/docs/Array-Tutorial.html) according to previously described protocols (Behnke et al., 2008). Two samples harvested from independent experiments were hybridized for each sample type. Hybridization data was preprocessed with Robust Multi-array Average (RMA), normalized using per chip and per gene median polishing and analyzed using software package Gene Spring 7.2 (Agilent Technologies, Santa Clara, CA). A total of 1289 genes were found to be differentially regulated between the three sample types using the combination of two filtering criteria; ANOVA p<0.05 and genes with >2.5 fold expression difference in at least one sample. Expression data has been submitted to the Gene Expression Omnibus (GEO) database under series GSE20480.
Quantitative reverse transcriptase PCR
Total RNA purified from extracellular, newly invaded and intracellular 2 h parasites was transcribed into cDNA using Superscript First-Strand Synthesis System (Invitrogen) according to the manufacturer’s protocol. qRT-PCR was performed in 25 μl volume reaction mixture containing 2X SYBR Green PCR master mix (Stratagene), 10 μM gene specific primer and 0.1 μg cDNA. Target genes were amplified using an Mx3000P thermal cycler (Stratagene). Melting curve analysis was used to confirm a lack of primer dimers or genomic DNA contamination of reagents. The relative gene expression levels were determined using 2−ΔΔCT method (Livak et al., 2001). α-tubulin (583.m00022) and EF1-α (76.m00016) genes were used as controls. Primers used for qRT-PCR are listed in Table S6.
Plasmid constructs
To generate firefly luciferase expression constructs, promoter regions of Toxoplasma genes: α-tubulin (583.m00022), GAPDH (80.m000030), UPRT (583.m00018) and TK (542.m00223) were amplified by PCR using primers (Table S7) and cloned into DEST-p-firefly (Behnke et al., 2008) as described previously. Briefly the promoter fragments were first cloned into entry vector pDONR221 (Invitrogen) via the BP reaction (Invitrogen) and confirmed by DNA sequencing. The promoter fragments were then moved into the destination vector DEST-p-firefly via the LR reaction (Invitrogen) that resulted in insertion of promoter fragment upstream of the reporter gene, firefly luciferase. UPRT promoter deletion constructs were also generated using BP and LR cloning techniques as described above using primers listed in Table S8. The transfection control plasmid DEST-p-renilla-α-tubulin that contains α-tubulin promoter upstream of reporter gene renilla luciferase has been described previously (Behnke et al., 2008).
Transfection and selection
Toxoplasma tachyzoites were electroporated according to established protocols (Soldati et al., 1993)with a combination of DEST-p-firefly promoter construct of choice (50 μg), control vector DEST-p-renilla-α-tubulin (50 μg) and plasmid TUB-DHFR (10 μg) and added onto HFF monolayer. Parasite populations stably expressing both reporter genes firefly and renilla luciferase were selected by culturing transfected parasites in presence of 1 μM pyrimethamine. A single parasite clone stably expressing UPRT-firefly and TUB-renilla was obtained by limiting dilution.
Luciferase assays
Luciferase reporter assays were performed in 96-well plates using Dual-Glo™ Luciferase Assay System (Promega) with following modifications. Freshly egressed tachyzoites were harvested, filter purified and resuspended in growth medium without phenol red. Parasites were added onto HFF monolayers and incubated at 37°C for 30 min to allow invasion. After 30 min, non-invaded parasites were removed by washing thrice with growth medium and further incubated for 2 h at 37°C (For extracellular parasites, filter purified tachyzoites in invasion medium were incubated without host cells for 2 h, 30 min). Dual-Glo Luciferase Reagent (Promega) equal to the culture medium was then added to each well, mixed and incubated at room temperature for 10 min. Firefly luminescence was determined using a plate luminometer. Thereafter, Stop & Glo® reagent (Promega) equal to the original culture volume was added to each well, mixed and incubated at room temperature for 10 min before measuring luminescence. Firefly luminescence readings were normalized using TUB-renilla luminescence readings and the normalized firefly luciferase reporter activity was compared between extracellular parasites and intracellular parasites to determine the fold changes.
Immunofluorescence
Fluorescent staining was performed according to previously described protocols (Harper et al., 2006; Brydges et al., 2008) using primary antibodies: rabbit anti-TgGRA1, rabbit anti-TgIMC1, rabbit anti-TgM2AP, and mouse anti-TgROP1 (monoclonal Tg49). Secondary antibodies were: Alexa Fluor-594- or Alexa Fluor-488-conjugated goat anti-mouse or goat anti-rabbit (Molecular Probes). Slides were viewed using an Axio observer Z1 (Zeiss) inverted wide field fluorescent microscope, and digital images were captured and analyzed using an AxioCam MRm (Zeiss) charge-coupled device camera and Axiovision software (Zeiss).
Supplementary Material
Acknowledgments
We thank Tracey Schultz and Kate McInnerney for expert technical assistance, Ke Hu, Con Beckers, and Joseph Schwartzman for providing key reagents, and My-Hang Huynh for proof reading the manuscript prior to submission. This work was supported by National Institutes of Health grants AI077662 and AI076078 (M.W.W) and AI046675 (V.B.C.).
References
- Balaji S, Babu MM, Iyer LM, Aravind L. Discovery of the principal specific transcription factors of apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 2005;33:3994–4006. doi: 10.1093/nar/gki709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Radke JB, Smith AT, Sullivan WJ, Jr, White MW. The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol Microbiol. 2008;68:1502–1518. doi: 10.1111/j.1365-2958.2008.06249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, Nawas J, et al. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PloS One. 2010;5:e12354. doi: 10.1371/journal.pone.0012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges SD, Harper JM, Parussini F, Coppens I, Carruthers VB. A transient forward-targeting element for microneme-regulated secretion in Toxoplasma gondii. Biol Cell. 2008;100:253–264. doi: 10.1042/BC20070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde de Felipe MM, Lehmann MM, Jerome ME, White MW. Inhibition of Toxoplasma gondii growth by pyrrolidine dithiocarbamate is cell cycle specific and leads to population synchronization. Mol Biochem Parasitol. 2008;157:22–31. doi: 10.1016/j.molbiopara.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalebroux ZD, Yagi BF, Sahr T, Buchrieser C, Swanson MS. Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol Microbiol. 2010;76:200–219. doi: 10.1111/j.1365-2958.2010.07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva EK, Gehrke AR, Olszewski K, Leon I, Chahal JS, Bulyk ML, Llinas M. Specific DNA-binding by apicomplexan AP2 transcription factors. Proc Natl Acad Sci U S A. 2008;105:8393–8398. doi: 10.1073/pnas.0801993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski JM, VC, Sibley LD. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 1997;26:163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28:1019–24. doi: 10.1016/s0020-7519(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Elemento O, Slonim N, Tavazoie S. A universal framework for regulatory element discovery across all genomes and data types. Mol Cell. 2007;28:337–350. doi: 10.1016/j.molcel.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Sethi KK, Piekarski G. Toxoplasma gondii: Calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp Parasitol. 1982;53:179–188. doi: 10.1016/0014-4894(82)90059-5. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unraveling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, White M, Szatanek T. The cell cycle and Toxoplasma gondii cell division: Tightly knit or loosely stitched? Int J Parasitol. 2008;38:1343–1358. doi: 10.1016/j.ijpara.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, Wieffer M, Striepen B. Fluorescent protein tagging in Toxoplasma gondii: Identification of a novel inner membrane complex component conserved among apicomplexa. Mol Biochem Parasitol. 2004;137:99–110. doi: 10.1016/j.molbiopara.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J Cell Sci. 2006;119:2236–2245. doi: 10.1242/jcs.02949. [DOI] [PubMed] [Google Scholar]
- Hammerschlag MR. The intracellular life of Chlamydiae. Semin Pediatr Infect Dis. 2002;13:239–248. doi: 10.1053/spid.2002.127201. [DOI] [PubMed] [Google Scholar]
- Harper JM, Huynh MH, Coppens I, Parussini F, Moreno S, Carruthers VB. A cleavable propeptide influences Toxoplasma infection by facilitating the trafficking and secretion of the TgMIC2-M2AP invasion complex. Mol Biol Cell. 2006;17:4551–4563. doi: 10.1091/mbc.E06-01-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harth G, Andrews N, Mills AA, Engel JC, Smith R, McKerrow JH. Peptide-fluoromethyl ketones arrest intracellular replication and intercellular transmission of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;58:17–24. doi: 10.1016/0166-6851(93)90086-d. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Hu K, He CY, Pelletier L, Roos DS, Warren G. Golgi and centrosome cycles in Toxoplasma gondii. Mol Biochem Parasitol. 2006;145:125–127. doi: 10.1016/j.molbiopara.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Hill D, Dubey JP. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8:634–40. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Hu K. Organizational changes of the daughter basal complex during the parasite replication of Toxoplasma gondii. PLoS Pathog. 2008;4:e10. doi: 10.1371/journal.ppat.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Roos DS, Angel SO, Murray JM. Variability and heritability of cell division pathways in Toxoplasma gondii. J Cell Sci. 2004;117:5697–5705. doi: 10.1242/jcs.01494. [DOI] [PubMed] [Google Scholar]
- Lavine MD, Arrizabalaga G. Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility. Eukaryot Cell. 2008;7:131–140. doi: 10.1128/EC.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Swanson MS. Differentiate to thrive: Lessons from the Legionella pneumophila life cycle. Mol Microbiol. 2004;53:29–40. doi: 10.1111/j.1365-2958.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Swanson MS. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol Microbiol. 2003;50:445–461. doi: 10.1046/j.1365-2958.2003.03706.x. [DOI] [PubMed] [Google Scholar]
- Mullapudi N, Joseph SJ, Kissinger JC. Identification and functional characterization of cis-regulatory elements in the apicomplexan parasite Toxoplasma gondii. Genome Biol. 2009;10:R34. doi: 10.1186/gb-2009-10-4-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Hu K, Murray JM, Roos DS. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci. 2008;121:1559–1568. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parussini F, Coppens I, Shah PP, Diamond SL, Carruthers VB. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Mol Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07181.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L, Stern CA, Pypaert M, Sheff D, Ngo HM, Roper N, et al. Golgi biogenesis in Toxoplasma gondii. Nature. 2002;418:548–52. doi: 10.1038/nature00946. [DOI] [PubMed] [Google Scholar]
- Radke JR, White MW. A cell cycle model for the tachyzoite of Toxoplasma gondii using the herpes simplex virus thymidine kinase. Mol Biochem Parasitol. 1998;94:237–247. doi: 10.1016/s0166-6851(98)00074-7. [DOI] [PubMed] [Google Scholar]
- Radke JR, Striepen B, Guerini MN, Jerome ME, Roos DS, White MW. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:165–175. doi: 10.1016/s0166-6851(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Rohde KH, Abramovitch RB, Russell DG. Mycobacterium tuberculosis invasion of macrophages: Linking bacterial gene expression to environmental cues. Cell Host Microbe. 2007;2:352–364. doi: 10.1016/j.chom.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Samuel JE, Kiss K, Varghees S. Molecular pathogenesis of Coxiella burnetii in a genomics era. Ann N Y Acad Sci. 2003;990:653–663. doi: 10.1111/j.1749-6632.2003.tb07440.x. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108 ( Pt 4):1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite, Toxoplasma gondii. Science. 1993;260:349–351. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- Striepen B, Jordan CN, Reiff S, van Dooren GG. Building the perfect parasite: Cell division in Apicomplexa. PLoS Pathog. 2007;3:e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VC Host cell invasion by the opportunistic pathogen Toxoplasma gondii. Acta Trop. 2002;81:111–122. doi: 10.1016/s0001-706x(01)00201-7. [DOI] [PubMed] [Google Scholar]
- van Poppel NF, Welagen J, Duisters RF, Vermeulen AN, Schaap D. Tight control of transcription in Toxoplasma gondii using an alternative tet repressor. Int J Parasitol. 2006;36:443–452. doi: 10.1016/j.ijpara.2006.01.005. [DOI] [PubMed] [Google Scholar]
- White MW, Jerome ME, Vaishnava S, Guerini M, Behnke M, Striepen B. Genetic rescue of a Toxoplasma gondii conditional cell cycle mutant. Mol Microbiol. 2005;55:1060–1071. doi: 10.1111/j.1365-2958.2004.04471.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.