Abstract
High-grade cervical dysplasia caused by human papillomavirus (HPV) type 16 is a lesion that should be susceptible to an HPV-specific immune response; disease initiation and persistence is predicated on expression of two viral Ags, E6 and E7. In immune-competent subjects, at least 25% of HPV16+ high-grade cervical dysplasia lesions undergo complete regression. However, in the peripheral blood, naturally occurring IFN-γ T cell responses to HPV E6 and E7 are weak, requiring ex vivo sensitization to detect, and are not sufficiently sensitive to predict regression. In this study, we present immunologic data directly assessing cervical lymphocytes from this cohort. We found that nearly all cervical tissue T cells express the mucosal homing receptor, α4β7 surface integrin. T cells isolated from dysplastic mucosa were skewed toward a central memory phenotype compared with normal mucosal resident T cells, and dysplastic lesions expressed transcripts for CCL19 and CCL21, raising the possibility that the tissue itself sustains a response that is not detectable in the blood. Moreover, lesion regression in the study window could retrospectively be predicted at study entry by the ability of CD8+ T cells to gain access to lesional epithelium. Vascular endothelial expression of mucosal addressin cell adhesion molecule-1, the ligand that supports entry of α4β7+ T cells into tissues, colocalized tightly with the distribution of CD8 T cells and was not expressed in persistent dysplastic epithelium. These findings suggest that dysregulated expression of vascular adhesion molecules plays a role in immune evasion very early in the course of HPV disease.
Persistent cervical mucosal infection with oncogenic strains of human papillomavirus (HPV), most commonly type 16, is the cause of virtually all squamous cancers of the cervix (1, 2). Current screening strategies to identify preinvasive disease, using pap smears, detection of oncogenic HPV genotypes, or even direct visual inspection, require repeated visits to health care providers as well as the existence of laboratory infrastructure. Although on balance, screening is indeed cost-effective, it is costly. In the United States, annual direct costs associated with HPV-related conditions among insured women have been estimated at $2.25–4.6 billion (2005 United States dollars), which include costs of routine screening, evaluation of false-positive Papanicolau tests, evaluation and care of preinvasive disease, and the care of women with invasive cancer (3–6). Recently available prophylactic vaccines for HPV16, -18, -6, and -11 (Gardasil, Merck, Whitehouse Station, NJ) or HPV16 and -18 (Cervarix, GlaxoSmithKline, Research Triangle Park, NC) are expensive, involve three sequential vaccinations in a 6-mo time frame, and also require intense infrastructure for delivery. In the United States, uptake of prophylactic HPV vaccines in eligible cohorts is low; the Centers for Disease Control reported that in 2009, only 26.7% of eligible girls (age 13–17 y) had completed the three-vaccination series. Among women who initiated vaccination, a substantial proportion (44.3%) did not complete the three-vaccination regimen. Among adolescents below the poverty level, half (51.9%) of those who initiated vaccination failed to complete the series (7). Altogether, because screening and prevention are cumbersome and expensive, cervical cancer remains the second leading cause of cancer death in women worldwide (8). Even in a high-resource setting such as the United States, the burden of disease is not likely to change in our lifetime (9).
High-grade cervical intraepithelial neoplasia (CIN2/3), the dysplastic intraepithelial precursor to invasive disease, is a lesion that should be susceptible to an HPV-specific immune response. The development of cervical cancer and its precursor CIN lesions are associated with integration of the HPV genome into the host genome, with subsequent expression of two HPV gene products, E6 and E7, which inactivate p53 and pRb, respectively. Expression of these viral, nonself proteins is functionally required to initiate and maintain the transformed phenotype, thereby providing true tumor-associated antigenic targets (10, 11).
Whereas all cervical squamous carcinomas arise from untreated CIN2/3, not all CIN2/3 lesions progress to invasive cancer. We and others (12, 13) have reported that across all HPV types, ~35% of CIN2/3 undergo regression in a time frame of 4–6 mo. Lesions associated with HPV16 are less likely to undergo regression than lesions associated with other HPV types; in this time frame, ~25% of HPV16-associated CIN2/3 undergo regression, which is presumably immunologically mediated (13, 14). However, in the peripheral blood of immune-competent subjects with HPV16+ CIN2/3, T cell responses to HPV16 E6 and E7 are only marginal, requiring ex vivo sensitization for detection, both in women whose lesions regress and in those whose lesions do not (15–17). Neither the magnitude nor the breadth of naturally occurring responses in the blood are robust predictors of regression of preinvasive HPV disease of the cervix.
Immunologic data from a recent clinical trial testing therapeutic vaccination targeting HPV16 E6 and E7 in subjects with HPV16-associated vulvar intraepithelial neoplasia (VIN) highlight this conundrum (18). Complete regression occurred postvaccination in 40% of subjects. This outcome is remarkable because clinically, preinvasive HPV-associated disease of the vulva is considerably more recalcitrant than cervical disease; untreated, virtually no VIN lesions undergo regression. However, in complete regressors in this study, the median IFN-γ immune response to HPV16 E6/E7 was in the neighborhood of 50 spot-forming units/105 PBMCs, after 4 d of ex vivo expansion. Moreover, the distribution of blood immune responses in nonegressors significantly overlapped with responses in regressors; in fact, some nonregressors had immune responses of greater breadth and magnitude than regressors.
The data in both unvaccinated and vaccinated subjects demonstrate that weak blood responses to viral Ags can nonetheless be associated with disease regression and raise the question of whether it is possible to identify immune responses in the target tissue that can predict either disease outcome or the likelihood of a clinical response to immune manipulation. In this paper, we present our findings that dysplastic epithelium excludes recruited Ag-experienced T cells, that cervical tissue T cells express the α4β7 surface integrin, and that lesional epithelial vascular expression of mucosal addressin cell adhesion molecule (MAdCAM) correlates directly with T cell infiltration. These data provide evidence that one mechanism that may permit preinvasive HPV lesions to elude immune-mediated clearance is by preventing egress of CD8 T cells into lesional epithelium. Furthermore, our data suggest that clinical trials testing immune-based therapies for HPV-associated disease should include, in addition to eliciting CD8+ T cell responses that are capable of homing to the cervical mucosa, local manipulation to activate lesion-associated vascular endothelium to enhance extravasation of effector cells to the lesional epithelial compartment.
Materials and Methods
Study subjects and cell samples
The prospective observational cohort study was a single-institution protocol that was reviewed and approved by the Johns Hopkins Institutional Review Board (Baltimore, MD), and all subjects enrolled provided written informed consent. All subjects were HIV-seronegative and had no evidence of tri-chomonas or any other sexually transmitted disease. Subjects with colposcopically directed, biopsy-confirmed CIN2/3 underwent surveillance for a period of 15 wk prior to standard therapeutic resection of the cervical squamocolumnar junction at week 15 (conization or loop electrosurgical excision procedure). Data reported in this analysis include subjects whose lesions were HPV16+ by PCR. All histological slides underwent two independent histologic reviews. For study protocol eligibility, they were reviewed by the Johns Hopkins Medical Institutions institutional gynecologic pathology service as part of standard medical procedures, blinded as to study. Subsequently, all cases were reviewed by study pathologists. Regression was defined as absence of CIN2/3 in the resection specimen at week 15. Immunohistochemical analysis was done on paraffin-fixed, formalin-embedded archival tissue that was residual after routine diagnostic sections had been cut, from the diagnostic biopsy (study entry), and from the excision at week 15 (conization). Frozen tissue from the excision was used for determining MAdCAM-1 expression.
Isolation of cervical lymphocytes
So as not to compromise the ability to make a definitive diagnosis on the surgical resection specimen, a frozen section was performed on a radial segment of the cervical tissue. Only if there was no suspicion of invasive disease in the frozen block, a 2-mm3 section from the cut face of the tissue immediately adjacent to the frozen section was reserved for short-term explant culture as previously described (19). T cells isolated from cervical tissue were analyzed by flow cytometry phenotyping. The amount of fresh tissue reserved for research purposes from the cervical conizations was intentionally small, as our primary intent was to not compromise the ability to make an accurate histologic diagnosis.
HPV typing
HPV testing was performed by the Hopkins Molecular Pathology Core Lab, using the HPV16-specific TaqMan real-time PCR method developed by Gravitt et al. (20).
Flow cytometry
Flow cytometry analysis of cervical T cells was performed using directly conjugated mAbs obtained from: BD Biosciences (San Jose, CA; CD3, CD4, CD8, CD45RO, CD45RA); BD Pharmingen (San Diego, CA; cutaneous lymphocyte-associated Ag [CLA], integrin α4/CD49d, integrin β7); Beckman Coulter (Miami, FL; L-selectin/CD62L); and R&D Systems (Minneapolis, MN; CCR7). Analysis of flow cytometry samples was performed on BD Biosciences FACScan or FACSCanto instruments, and data were analyzed using FACSDiva software (version 5.1, BD Biosciences).
Immunohistochemical staining
Immunohistochemistry studies were restricted to tissue that was residual after standard diagnostic decisions had been made. Immunohistochemistry was performed on flash-frozen as well as on formalin-fixed, paraffin-embedded tissue. Five-micron sections were applied to coated slides (Superfrost Plus, Menzel, Braunschweig, Germany). Frozen sections were fixed in cold (4°C) acetone for 10 min, and primary Abs were applied for 1 h at room temperature. Endogeneous peroxidase was blocked with a solution of 0.1% Na3 in 0.3% H2O2. For paraffin sections, heat-based Ag retrieval was performed for 30 min, followed by incubation with primary Abs. Incubation with biotinylated horse–anti-mouse secondary Ab (Vector Laboratories, Burlingame, CA) was followed by an avidin-biotin complex (ABC-Elite, Vector Laboratories). Diaminobenzidine served as a chromogen, and Gill’s hematoxylin was used as a counterstain. The following primary Abs were used: CD8 (clone C8/144B, DakoCytomation, Carpinteria, CA), L-selectin/CD62L (clone 9H6, Leica Microsystems, Deerfield, IL), peripheral lymph node addressin (PNAd; clone MECA-79, BD Biosciences), and MAdCAM-1 (clone 314G8, Santa Cruz Biotechnology, Santa Cruz, CA).
Immune cell quantitation
Images were captured using a Nikon E600 microscope/Plan fluor ×20/0.50 ocular and a DXM1200f Nikon digital camera (Nikon, Melville, NY). Regions of interest (ROIs) were delineated in normal epithelium, stroma immediately beneath normal epithelium, CIN2/3 epithelium, and stroma immediately beneath CIN2/3 using NIS Elements AR3 imaging software (Nikon). Chromagen was quantitated by normalization against the area of the ROI (μm2). At least 3 and up to 10 discrete ROIs were quantified in each compartment in each case. The mean density of chromagen staining for each compartment for each case were calculated and used for statistical comparisons between groups.
Real-time quantitative PCR for tissue expression of CCL19 and CCL21
Mucosal specimens were flash-frozen from fresh tissue obtained at the time of therapeutic surgical excision of HPV16+CIN2/3 lesions and from cervixes from hysterectomies performed for nonneoplastic indications (e.g., uterine fibroids). Total RNA was extracted using RNeasy columns (Qiagen, Valencia, CA). cDNA was synthesized using random primers and Ready-To-go beads (GE Healthcare, Buckinghamshire, U.K.). Multiplexed quantitative determination of CCL19 and CCL21 was performed in triplicate wells using ~1:30 of the cDNA per well and primer/probe sets for target genes (FAM-labeled) and GADPH (VIC-labeled) with standard ABI chemistry and reagents (Applied Biosystems, Foster City, CA). Expression relative to GADPH was determined by comparing the difference in cycle threshold values.
Statistical analysis
Two-tailed t tests were used to compare the intensity of tissue diamino-benizidine-positive infiltrates and the frequency of immune cell subset populations in lesional and normal cervical mucosa.
Results
CD8 T cell infiltration in lesional epithelium correlates with subsequent regression
To gain insight into cervical tissue T cell populations that could play a role in the elimination of dysplastic lesions, we determined the density and localization of CD8+ T cells in subject-matched diagnostic biopsy and subsequent conization specimens. Tissues were obtained from immune-competent subjects with biopsy-proven HPV16+ CIN2/3 enrolled in a 15-wk observational protocol prior to standard surgical excision (cervical conization).
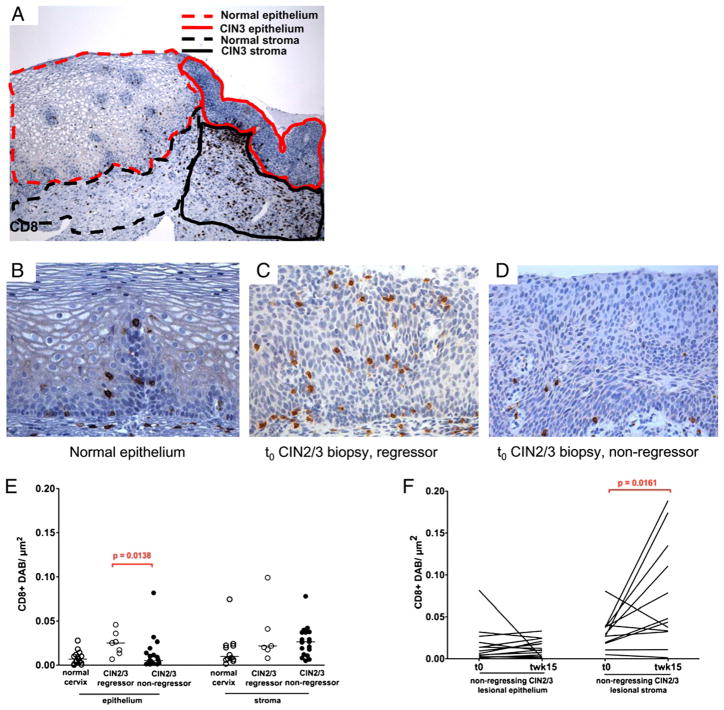
Despite barely detectable blood responses to HPV16 E6 and E7 in these subjects, we found dense immune cell infiltrates in dysplastic mucosa, which were significantly greater than in immediately adjacent normal tissue. The density of CD8 T cell infiltrates in tissue sections was assessed quantitatively. Using laser capture microdissection imaging software, ROIs were delineated in normal epithelium, stroma immediately beneath normal epithelium, CIN2/3 epithelium, and CIN2/3 stroma (Fig. 1A). Image analysis software was then used to quantitate the total CD8+ area in each ROI, which was normalized against the total area of the demarcated region. This method was used to provide a quantitative measurement of CD8 infiltrates.
FIGURE 1.
CD8+ infiltrates localize to dysplastic mucosal. A, Representative CD8 immunohistochemical staining of persistent CIN2/3 adjacent to normal mucosa. CD8+ cells localize to dysplastic stroma compared with immediately adjacent normal tissue. ROIs are demarcated as indicated to illustrate the method used to quantitate the intensity of immunostaining (original magnification ×100). B, Representative section depicting CD8+ cells in normal cervical squamous mucosa distributed along the basal epithelial layer and clustered around a vessel extending into the squamous compartment (immunohistochemical staining, original magnification ×400). C, Representative section depicting CD8+ cell penetration into lesional epithelium in t0 biopsy specimen, lesion regressor (immunohistochemical staining, original magnification ×400). D, Representative example of lack of CD8+ cells in lesional epithelium at t0, nonregressor (immunohistochemical staining, original magnification ×400). E, Quantification of CD8+ infiltrates in cervical mucosa in normal (control) mucosa (n = 15) and from lesion sites at t0 in lesions that regressed (n = 7) and lesions that did not regress (n = 20). At least 3 and up to 10 fields from each section were counted to define the density of infiltrates. F, Quantification of stromal and epithelial CD8+ in nonregressing lesions (n = 20), at study entry (t0), and at the time of excision (twk15).
We wished to determine, albeit retrospectively, whether we could identify lesions that were likely to regress. Indeed, the tissue distribution of CD8 T cells at t0 was strikingly different in lesions that subsequently regressed compared with lesions that failed to regress. As a baseline for comparison, we used the density and distribution of CD8+ cells in normal cervical mucosa, where CD8 T cells were sparsely distributed, predominantly perivascularly and along the epithelial–stromal junction (Fig. 1B). In diagnostic biopsies (t0), lesional intraepithelial CD8 infiltrates were significantly higher in lesions that regressed (Fig. 1C) compared with lesions that did not (Fig. 1D) (p = 0.0138). Lesions in which t0 CD8+ epithelial infiltrates were greater than in normal cervical epithelium were >20-fold more likely to regress than lesions in which CD8 infiltrates were less than or equal to the median value in normal cervical epithelium (odds ratio 22.06, 95% confidence interval 1.79–272; p = 0.0081). Stromal CD8 infiltrates at t0, although greater in dysplastic mucosa than in normal, did not predict regression (Fig. 1E). Nonregressing lesions at the time of resection were associated with significant increases of CD8+ infiltrates in dysplastic stroma compared with study entry, suggesting either recruitment or expansion of T cells over the study window (Fig. 1F). Together, these findings suggested that recruitment of CD8+ T cells to dysplastic mucosa is not sufficient for lesion regression; intraepithelial access is required.
CIN2/3 mucosa recruits activated memory T cells
Tissue T cells in other human nonsterile barrier epithelia including the skin and the gut are comprised of Ag-experienced effector memory cells that express tissue-specific homing addressins and have pronounced tropism for the tissue in which they first encountered their cognate Ag (21–27). To gain insight into mechanisms of immune cell recruitment to the genital mucosa, we determined the subset distribution, tissue localization, and surface integrins expressed on cervical tissue T cells in normal and dysplastic mucosa. T cells were isolated from from histologically normal cervical tissue obtained from hysterectomies performed for nonneoplastic indications and from CIN2/3 mucosal explants using 7-d explant cultures as previously described (19). Surface phenotyping of cervical tissue lymphocytes was performed by flow cytometry. Representative FACS plots are shown in Fig. 2A, 2C, and 2E.
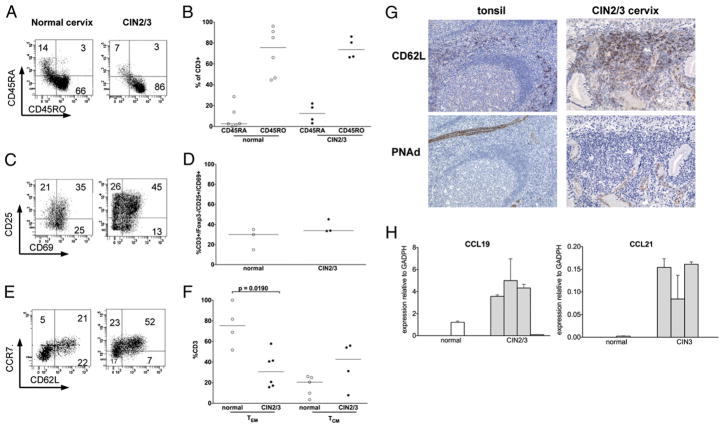
FIGURE 2.
Dysplastic mucosa recruits activated memory T cells. A, Representative flow cytometry analysis of the percentage of CD3+CD45RO+ and CD3+CD45RA+ T cells in normal and CIN2/3 cervix. B, Quantification of CD45RA+ and CD45RO+CD3+ T cells in normal (n = 6) and CIN2/3 cervix (n = 4). C, Representative flow cytometry analysis of the percentage of CD3+ T cells expressing activation markers CD25 and CD69 in normal and CIN2/3 cervixes. D, Quantification of CD3+CD45RO+ T cells expressing CD25 and CD69 in normal (n = 3) and CIN2/3 (n = 3) cervixes. E, Representative flow cytometry analysis of CD3+CD45RO+ T cells expressing surface phenotype of effector memory T cells (CCR7−CD62L−) versus TCM (CCR7+CD62L+) in normal and CIN2/3 cervixes. F, Quantification of effector memory T cells and TCM in normal (n = 6) and CIN2/3 (n = 6) cervixes. G, Representative histologic sections of tonsil (left panels) and CIN2/3 cervix (right panels), immunostained for CD62L (top panels) and PNAd (bottom panels) demonstrate colocalization of cells expressing CD62L, and endothelial vasculature expressing PNAd (original magnification ×200). H, CCL19 and CCL21 mRNA transcripts relative to GADPH were quantified by quantitative real-time RT-PCR in normal (n = 3) and CIN2/3 (n = 4) flash-frozen cervical tissue samples.
Similar to other tissue-resident T cell populations (23, 28–30), the subset composition of cervical tissue T cells differed markedly from blood T cells of healthy normal subjects. In normal cervical mucosa, 72% of T cells had an Ag-experienced phenotype expressing CD45RO (n = 6, SD 22.6) (Fig. 2A, 2B). The majority (75%) of CIN2/3 T cells were also Ag-experienced (n = 4, SD 9.8). In contrast, in the peripheral blood of healthy normal subjects, Ag-experienced cells comprise in the range of 25% of CD4+ T cells and ~10% of CD8+ T cells (31). Although the frequency of CD45RA+ T cells was low in both normal cervix (median 2.7%, range 0–13.8%) and in CIN2/3 cervix (median 12.5%, range 3.1–22%), the majority (81%, SD 8.9) were CD8 T cells (data not shown). Cervical T cells also differed from PBLs in that a significantly higher proportion displayed an activated phenotype (CD25+/CD69+) in both normal (26.7%, SD 10.5) and lesional (37.6%, SD 6.5) mucosa (Fig. 2C, 2D). In the systemic circulation, normally <5% of T cells are activated (31, 32).
In normal cervical mucosa, the majority of T cells had an effector memory phenotype (CD3+CD45RO+CCR7−CD62L−). The subset distribution in persistent CIN2/3 mucosa was markedly shifted, containing a higher proportion of Ag-experienced cells expressing CD62L and CCR7, suggesting either recruitment or expansion of a central memory T cell (TCM) population (Fig. 2E, 2F).
As central memory cells are found predominantly in the blood or in peripheral lymph nodes, but not in peripheral tissues, we studied normal and dysplastic mucosa for evidence of mechanisms that mediate localization and retention of TCM in lymph nodes. In peripheral lymph nodes, endothelial expression of PNAd in high endothelial venules mediates tethering of CD62L-expressing cells. Therefore, in persistent CIN2/3 lesions, we determined the colocalization of cells expressing CD62L and vascular endothelial expression of PNAd. To query whether dysplastic cervical tissue might actively recruit TCM, we quantitated mRNA transcripts for CCL19 and CCL21, both of which are chemoattractants for cells expressing CCR7.
In persistent CIN2/3 lesions, the tissue localization of CD62L+ cells was restricted to stromal inflammatory infiltrates subjacent to dysplastic epithelium (Fig. 2G). Expression of PNAd corresponded directly with the tissue distribution of CD62L+ cells; expression was confined to vascular endothelial cells in inflamed stroma immediately subjacent to dysplastic epithelium and was not detected either on vessels that extended into the epithelial compartment or on vessels in uninflamed stroma. Gene expression of CCL19 and CCL21 was upregulated in CIN2/3 and not detected in normal cervical tissue (Fig. 2H). The fact that nonregressing lesions were associated with recruitment of activated memory T cells that nonetheless failed to access lesional epithelium prompted the question of whether dysregulated expression of adhesion molecules could play a role in immune evasion.
Cervical T cells express α4β7, and vascular expression of MAdCAM-1 correlates with CD8+ T cell access to cervical tissue
Decreased expression of adhesion molecules on vascular endothelium associated with invasive solid tumors is a common mechanism by which human malignancies evade infiltration by effector immune cells (33–35). Neovascularization occurs early in HPV disease. The colposcopic detection of CIN2/3 involves identification of characteristic patterns of neovasculature in lesional mucosa, namely punctation and mosaicism (Fig. 3). Therefore, we examined the expression of homing addressins in cervical lymphocytes and the expression of adhesion molecules in cervical vascular endothelium. Over 90% of cervical dysplastic lesions occur at the cervical squamocolumnar junction, an abrupt transition from stratified squamous epithelium to single-layer, mucin-producing columnar epithelium. Therefore, we assessed cervical lymphocytes for expression of CLA and CCR4, which are found on memory T cells with tropism for cutaneous squamous epithelium, and for expression of the α4β7 surface integrin, which mediates homing to the small intestinal mucosa (36).
FIGURE 3.
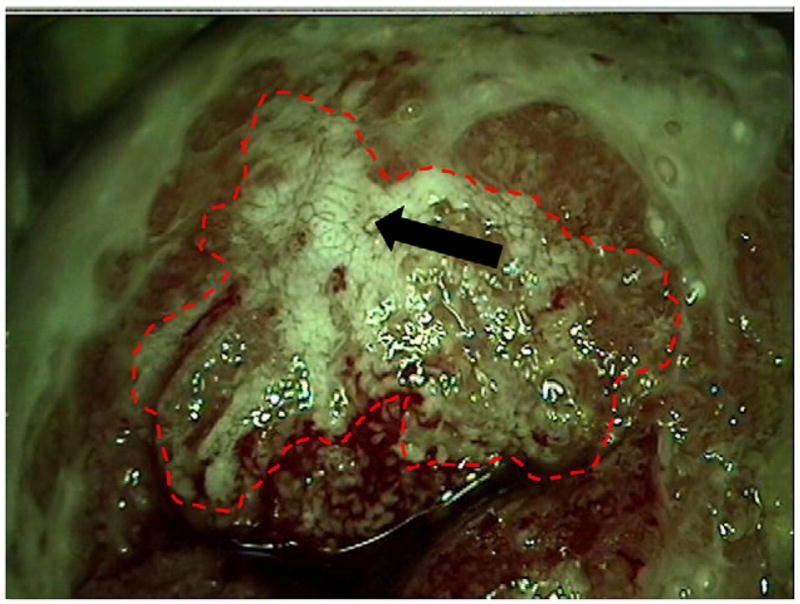
Colpograph of CIN2/3: acetowhite epithelium (dashed red line) with characteristic patterns of neovascularization, including punctation, and mosaicism (bold arrow). Original magnification ×10.
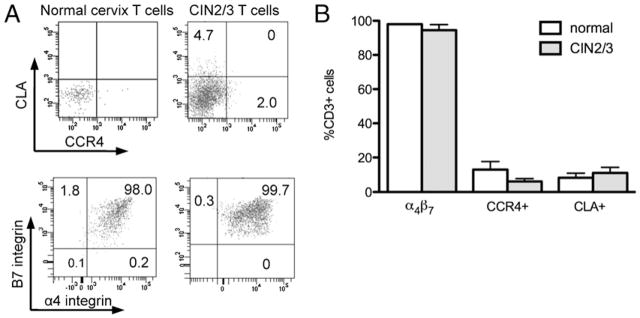
Relatively few T cells isolated from either normal cervical mucosa or from CIN2/3 expressed either CLA or CCR4 (Fig. 4, Table I). However, nearly all T cells isolated from both normal cervical mucosa and CIN2/3 expressed α4β7. Very few cervical T cells expressed CCR9, which, in concert with α4β7, mediates homing and egress of memory T cells in the small intestine. These data suggest that although memory T cells that home to the cervical mucosa express α4β7, chemokines other than CCR9 are likely to play a role in recruiting and sustaining T cells in cervical tissue.
FIGURE 4.
Cervical tissue T cells express the α4β7 surface integrin. A, Representative flow cytometry surface phenotyping analyses of T cells isolated from normal cervixes (left panels) and CIN2/3 cervixes (right panels). B, Quantification of the percent of cervical CD3+ T cells expressing α4β7, CCR4, and CLA in normal (n = 5) and CIN2/3 (n = 4) cervixes.
Table I.
Frequency of chemokine receptors expressed on normal and CIN2/3 cervix T cells
| Normal Cervix | n | CIN2/3 | n | p Value | |
|---|---|---|---|---|---|
| CLA | 14.0 (4.8–26.5) | 5 | 10.9 (8.9–34.4) | 3 | NS |
| CCR4 | 15.2 (4.1–18.2) | 5 | 8.7 (4.9–9.1) | 3 | NS |
| CCR9 | 2.3 (1.1–3.2) | 4 | 1.5 (1.2–3.9) | 3 | NS |
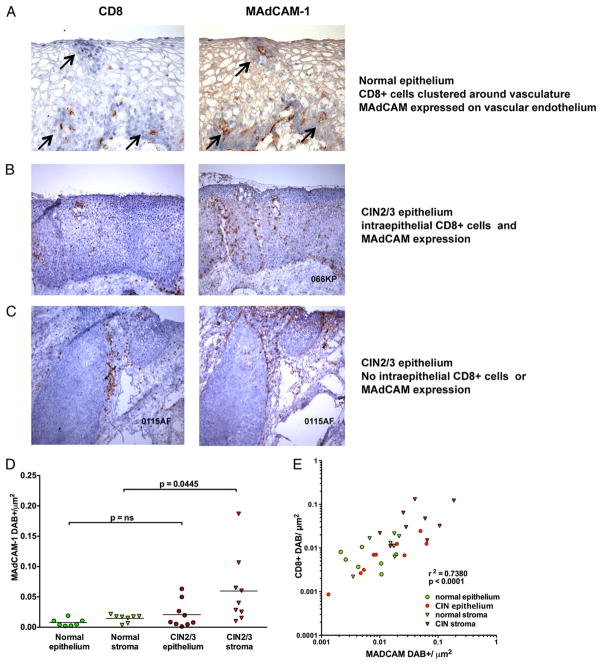
MAdCAM-1 is an adhesion molecule that mediates homing of T cells expressing α4β7 in the small intestine. Therefore, we quantitated the expression and colocalization of MAdCAM-1 and CD8 in normal and dysplastic mucosa. Sequential tissue sections were analyzed from 10 cervical conization specimens with persistent disease at week 15. Overall, the density of cells expressing CD8 and MAdCAM-1 was greater in diseased tissue than in normal mucosa. In normal mucosa, MAdCAM-1 was expressed on vascular endothelium of small stromal vessels as well as in vessels extending into stromal papillae of the squamous compartment. This anatomic distribution colocalized with CD8+ cells, which were clustered around vessels (Fig. 5A).
FIGURE 5.
Tissue expression of MAdCAM colocalizes with CD8+ infiltrates in CIN2/3 epithelium. A, Representative serial tissue sections of normal cervical epithelium, immunostained for CD8 (left panel) and MAdCAM-1 (right panel). Arrows identify perivascular CD8+ cells (left panel) and MAdCAM-1 expression in corresponding vessels (right panel) (original magnification ×400). B, Representative CIN2/3 epithelium containing CD8+ cell infiltrates (left panel), with MAdCAM-1 expression in a serial section, same field (right panel) (original magnification ×100). C, Representative histologic sections of CIN2/3 epithelium with very sparse CD8 infiltrates (left panel) and MAdCAM-1 expression in a serial section, same field (right panel) (original magnification ×100). D, Quantification of MAdCAM-1 immunostaining in normal cervical epithelium (n = 7), normal cervical stroma (n = 8), CIN2/3 epithelium (n = 9), and CIN2/3 stroma (n = 9). E, Within-specimen correlation of the intensity of MAdCAM-1 and CD8 expression in normal and CIN2/3 mucosa. Each symbol represents the density of MAdCAM-1 (x coordinate) and CD8 (y coordinate) in the same field. The overall correlation is 0.738; correlation in CIN2/3 epithelium is 0.8833. p = 0.0031.
In dysplastic mucosa, the density and distribution of MAdCAM-1 expression correlated tightly with the distribution of CD8+ cells. In dysplastic epithelium that contained CD8+ cell infiltrates, MAdCAM-1 expression was also increased compared with normal mucosa (Fig. 5B). In contrast, in dysplastic lesions that did not have intraepithelial CD8+ infiltrates, MAdCAM-1 expression was restricted to the lesional stroma (Fig. 5C). The correlation between the intensity of MAdCAM-1 and CD8 infiltrates was strongest in CIN epithelium (r2 = 0.8833; p = 0.0031) (Table II). Correlation was weakest in normal epithelium. This finding suggests that the interaction of α4β7 on tissue-localized T cells and tissue vascular endothelial expression of MAdCAM-1 plays a more critical role in dysplastic mucosa than in normal mucosa. Indeed, the near lack of correlation in normal epithelium suggests that other mechanisms are likely to be responsible for immune surveillance in normal, quiescent mucosa.
Table II.
Density of CD8+ and MAdCAM-1 infiltrates in case-matched tissue compartments
| Tissue Compartment | r2 Value | p Value (Two-Tailed) | n |
|---|---|---|---|
| Normal epithelium | −0.2857 | 0.5560 (NS) | 7 |
| Normal stroma | 0.3571 | 0.3894 (NS) | 8 |
| CIN epithelium | 0.8833 | 0.0031** | 9 |
| CIN stroma | 0.4000 | 0.2912 (NS) | 9 |
Statistically significant.
Discussion
To our knowledge, this study is the first to suggest that exclusion of recruited CD8 T cells may play a role in the persistence of intraepithelial HPV disease, well before the development of overt malignancy. The finding of nearly universal expression of α4β7 on cervical lymphocytes and the colocalization of MAdCAM-1 expression with the distribution of CD8 cells in dysplastic epithelium suggest that this homing integrin–adhesion molecule pair plays a central role in effector T cell homing in the cervix.
It is possible that the increased tissue aggregates of activated T cells expressing α4β7 could increase susceptibility to HIV-1 transmission in women with preinvasive HPV disease. Previous work studying tissue-resident T cells in genital HSV-2 infections showed that persisting CD4+ T cells at the site of healed lesions reacted to HSV-2 Ag. These CD4+ T cells also expressed the HIV coreceptors CCR5 and CXCR4, and in ex vivo experiments, HIV-1 infectivity was greater in tissue from healed lesion sites than tissue from unaffected genital tissue from the same subjects (37). HIV-1 gp120 binds to α4β7, and α4β7highCD4+ T cells have been shown to be more susceptible to productive infection than CD4+ T cells that are α4β7low (38). Studies of cervical tissue explant susceptibility in the setting of persistent HPV infection remain to be done.
The question of whether the Ag-experienced T cell aggregates in CIN2/3 lesions arise from expansion of tissue-resident memory T cells or are derived from recruited circulating memory cells remains to be addressed and has implications for monitoring the effect of systemic vaccination. Recruitment of activated memory T cells to dysplastic mucosa would not be unexpected in the context of a chronic, tissue-sequestered viral infection; memory T cells are recruited to sites of inflammation regardless of Ag specificity or where they initially encountered their cognate Ag (39). Indeed, the observation of increased numbers of TCM in lesions that failed to regress in the study window is consistent with non-Ag–specific recruitment. However, the colocalization of cells expressing CD62L with vessels expressing PNAd, in concert with expression of CCL19 and CCL21, suggests that dysplastic mucosa could serve as an extralymphoid site for conditioning or sustaining T cell responses. In theory, manipulation of the mucosal microenvironment could potentially have therapeutic effect if tissue-resident effector memory cells that recognized lesional Ags could be activated and expanded. Therapeutic effect could also be enhanced if appropriate signals could be delivered to elicit expression of adhesion molecules on lesion-associated vascular endothelium to enhance egress of locally accumulated effector cells. For example, effector T cells isolated from human cutaneous squamous carcinomas that had been treated with imiquimod, a topically applied TLR7 agonist, produced more IFN-γ, granzyme, and perforin and less IL-10 and TGF-β than T cells from carcinomas that had not been treated with imiquimod pre-excision (40). Moreover, imiquimod application induced vascular endothelial expression of E-selectin in tumor vessels, with subsequent egress of T cells expressing CLA, and tumor destruction (41).
A subset of preinvasive HPV lesions does appear to be susceptible to local immune manipulation with imiquimod; although to date, the mechanisms of therapeutic effect are incompletely understood. Much of the literature on the effect of TLR7 stimulation has focused on immune cell subsets, particularly on maturation and migration of plasmacytoid dendritic cells. However, two recent clinical studies have assessed the use of imiquimod in the treatment of HPV16-associated VIN (42, 43). Both trials reported a significantly higher rate of complete regression of lesions that were treated with imiquimod compared with lesions that were not (30% versus 0%). Neither study was able to identify robust predictors of response. However, in lesions that regressed, intraepithelial CD8+ infiltrates increased after imiquimod application. A detectable endogenous systemic T cell response to HPV Ags prior to imiquimod application was also associated with clinical response, although the magnitude of the systemic immune response did not reliably identify subjects whose lesions regressed. The fact that systemic responses to HPV Ags became detectable only after several days of ex vivo stimulation is consistent with the kinetics of a central memory response with a very low frequency of precursors, as opposed to what would be predicted in an effector memory population with a high precursor frequency. Tissue-resident memory T cells in other systems appear to be relatively sessile and capable of mounting a functional recall response (44, 45). It may be that in preinvasive HPV disease, tissue-resident effector memory cells could eliminate disease if the mucosal microenvironment could be manipulated appropriately.
In toto, this constellation of findings suggests that in the setting of preinvasive HPV disease, weak peripheral blood responses to HPV Ags may not reflect tissue-localized immune responses. Because HPV-associated intraepithelial neoplasia of the cervix is accessible and clinically indolent, these lesions provide an opportunity to study proof-of-principle immune therapeutic interventions, including the use of vaccine vectors with tropism for genital tissue or adjuvants that elicit expression of α4β7 integrins. The 15-wk window of observation prior to excisional therapy is well within the standard of care; it is worth stating that in our cohort, no subjects had progression of disease in the study window. Our study supports the safety of using this time frame, or even longer, with adequate monitoring, in the design of clinical trials testing immune therapeutic interventions in healthy subjects with CIN2/3. Therapeutic vaccination should be carried out in concert with strategies to activate lesional vascular endothelium to allow egress of recruited effector T cells to dysplastic epithelium.
Acknowledgments
This work was supported by National Institutes of Health Grants 1K23CA85437 (to C.L.T.) and 1K08AI060890 (to R.A.C.), the Dana Foundation (to C.L.T.), and the Damon Runyon Cancer Research Foundation (to R.A.C.).
Abbreviations used in this paper
- CIN2/3
high-grade cervical intraepithelial neoplasia
- CLA
cutaneous lymphocyte-associated Ag
- HPV
human papillomavirus
- MAdCAM
mucosal addressin cell adhesion molecule
- PNAd
peripheral lymph node addressin
- ROI
region of interest
- TCM
central memory T cell
- VIN
vulvar intra-epithelial neoplasia
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: Prospects and challenges for the 21st century. Eur J Immunol. 2007;37(Suppl 1):S148–S155. doi: 10.1002/eji.200737820. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Fleurence RL, Dixon JM, Milanova TF, Beusterien KM. Review of the economic and quality-of-life burden of cervical human papillomavirus disease. Am J Obstet Gynecol. 2007;196:206–212. doi: 10.1016/j.ajog.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Monk BJ, Herzog TJ. The evolution of cost-effective screening and prevention of cervical carcinoma: implications of the 2006 consensus guidelines and human papillomavirus vaccination. Am J Obstet Gynecol. 2007;197:337–339. doi: 10.1016/j.ajog.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol. 2004;191:114–120. doi: 10.1016/j.ajog.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107–1122. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 7.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach PB. Gardasil: from bench, to bedside, to blunder. Lancet. 2010;375:963–964. doi: 10.1016/S0140-6736(09)62029-8. [DOI] [PubMed] [Google Scholar]
- 10.Hudson JB, Bedell MA, McCance DJ, Laiminis LA. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990;64:519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 12.Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–735. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 13.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, Wilgus B, Yutzy W, Daniel R, Shah K, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11:4717–4723. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlecht NF, Platt RW, Duarte-Franco E, Costa MC, Sobrinho JP, Prado JC, Ferenczy A, Rohan TE, Villa LL, Franco EL. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95:1336–1343. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, Palefsky JM, Moscicki AB. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J Infect Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 16.Welters MJ, de Jong A, van den Eeden SJ, van der Hulst JM, Kwappenberg KM, Hassane S, Franken KL, Drijfhout JW, Fleuren GJ, Kenter G, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–641. [PubMed] [Google Scholar]
- 17.Trimble CL, Peng S, Thoburn C, Kos F, Wu TC. Naturally occurring systemic immune responses to HPV antigens do not predict regression of CIN2/3. Cancer Immunol Immunother. 2010;59:799–803. doi: 10.1007/s00262-009-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 19.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 20.Gravitt PE, Peyton C, Wheeler C, Apple R, Higuchi R, Shah KV. Reproducibility of HPV 16 and HPV 18 viral load quantitation using TaqMan real-time PCR assays. J Virol Methods. 2003;112:23–33. doi: 10.1016/s0166-0934(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, Moser B. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199:1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 26.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bos JD, Hagenaars C, Das PK, Krieg SR, Voorn WJ, Kapsenberg ML. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24–30. doi: 10.1007/BF00424268. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- 30.Hladik F, Lentz G, Delpit E, McElroy A, McElrath MJ. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol. 1999;163:2306–2313. [PubMed] [Google Scholar]
- 31.Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72:203–212. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- 32.Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, Campbell JJ. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002;160:347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 34.Madhavan M, Srinivas P, Abraham E, Ahmed I, Vijayalekshmi NR, Balaram P. Down regulation of endothelial adhesion molecules in node positive breast cancer: possible failure of host defence mechanism. Pathol Oncol Res. 2002;8:125–128. doi: 10.1007/BF03033721. [DOI] [PubMed] [Google Scholar]
- 35.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner N, Löhler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Müller W. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O’Shea A, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci USA. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrançois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 40.Huang SJ, Hijnen D, Murphy GF, Kupper TS, Calarese AW, Mollet IG, Schanbacher CF, Miller DM, Schmults CD, Clark RA. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol. 2009;129:2676–2685. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, Kagie MJ, Meijer CJ, Aaronson NK, Kleinjan A, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358:1465–1473. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 43.Winters U, Daayana S, Lear JT, Tomlinson AE, Elkord E, Stern PL, Kitchener HC. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res. 2008;14:5292–5299. doi: 10.1158/1078-0432.CCR-07-4760. [DOI] [PubMed] [Google Scholar]
- 44.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 45.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]