Abstract
Background & Aims
Studies in patients and chimpanzees that spontaneously cleared hepatitis C virus (HCV) infections demonstrated that natural immunity to the virus is induced during primary infections and that this immunity can be cross protective. These discoveries led to optimism about prophylactic HCV vaccines and several studies were performed in chimpanzees, although most included fewer than 6 animals. To draw meaningful conclusions about the efficacy of HCV vaccines in chimpanzees, we performed statistical analyses of data from previously published studies from different groups.
Methods
We performed a meta-analysis that compared parameters among naïve (n=63), vaccinated (n=53), and rechallenged (n=36) animals, including peak RNA titer post-challenge, timepoints of peak RNA titer, duration of viremia, and proportion of persistent infections.
Results
Each vaccination study induced immune responses that were effective in rapidly controlling HCV replication. Levels of induced T-cell responses did not indicate vaccine success. There was no reduction in the rate of HCV persistence in vaccinated animals, compared with naïve animals, when non-structural proteins were included in the vaccine. Vaccines that contained only structural proteins had clearance rates that were significantly higher than vaccines that contained non-structural components (P=0.015).
Conclusions
The inclusion of non-structural proteins in HCV vaccines might be detrimental to protective immune responses and/or structural proteins might activate T-cell responses that mediate viral clearance.
Keywords: correlates of protection, viral kinetics, Elispot responses
Hepatitis C virus (HCV) is an enveloped virus with a single stranded, plus-sense RNA genome (~9.6 kb) consisting of ~341-base 5’ non-translated region (NTR), a single open reading frame encoding all virus-specific proteins (~3011 amino acids), and a 3’ NTR. The polyprotein is cleaved, co- and post-translationally by host and viral proteases to produce structural proteins (core and envelope glycoproteins (E1,E2)) and nonstructural (NS) components: p7; NS2-3 (protease); NS3 (serine protease and RNA helicase); NS4A; NS4B; NS5A and NS5B (RNA-dependent RNA polymerase, RdRp)1;2.
Transmission of HCV is typically by the parenteral route, persistent infections occur in 70-80% of acutely infected individuals, the majority of which will develop chronic hepatitis and will be at risk for cirrhosis, end-stage liver disease and/or hepatocellular carcinoma3. HCV is associated with 40-60% of chronic liver disease in the U.S. Of these patients, one third goes on to develop progressive fibrosis and cirrhosis4 making hepatitis C the major disease leading to liver transplantation5.
HCV sequences are continually evolving during an infection due to the error-prone NS5B RdRp, which generates an estimated 10-5-10-4 errors/nucleotide/replication cycle6;7, and the high production and clearance rate of the virus, estimated at ~1012 virions/day8. Consequentially, HCV exists as several closely related but distinct viruses within a host; referred to as a quasispecies population. Seven major genotypes (GT) (designated 1-7) have been defined for HCV, differing from each other by ~30-35% over the complete genome9;10. The greatest genetic variability is observed in the E1 and E2 glycoproteins and the NS5A region9. This genetic diversity poses problems for vaccine development from the perspective of target antigens and the potential for escape from vaccine-induced immune responses. Immune escape has been shown directly and indirectly for natural infections in both T-cell11-13 and B-cell14-16 epitopes.
There is no licensed vaccine for HCV and prophylactic vaccine development has been hampered by the fact that the only animal model for pathogenesis or immune control of viral infection is the chimpanzee. This model has been highly important for understanding mechanisms of viral clearance and especially the role of T-cells in control of viral replication17-21. Based on clinical and chimpanzee studies demonstrating the role of T-cells in natural clearance, T-cell-based vaccines have received a great deal of focus, particularly given that these vaccines can target more conserved regions of HCV.
The data generated from chimpanzee vaccine studies is the most comprehensive available to asses the success or shortcomings of HCV vaccine approaches. However, the majority of studies have used small numbers of animals (1 to 6 per study) (Table 1). We have employed statistical methodology to quantitatively examine the published data and compare the course of HCV infection in naïve; vaccinated and rechallenged animals. The results from these analyses have been used to assess how well vaccines against HCV are functioning at controlling viral replication, which areas still require further investigation, and to establish biomarkers and parameters that could be used to assess the success of vaccines in the clinic.
Table 1.
Prophylactic HCV vaccine studies in chimpanzees
| Vaccine (Genotype sequence used) (Number of animals vaccinated) | Immunogenicity | Challenge Inoculum* Dose (CID50§) | Outcome | Ref. |
|---|---|---|---|---|
| DNA prime and protein boost (using C, gpE1, gpE2 and NS3) | Induced specific T-cell responses and antibody to E1 and E2. | GT 1b | Modifies infection, protects from chronic infection | 32 |
| (GTs 1a (core, NS3) and 1b (core, E1E2, NS3)) | Homologous | 1 resolved infection | ||
| (N=2) | 25CID50 | 1 developed persistent infection | ||
| Adjuvant: Alum | ||||
| DNA prime and Recombinant Adenovirus expressing core, E1,E2 and NS3 to NS5B | Induced specific T-cell responses and anti-E2 antibody (neutralizing) | GT 1b | Modifies infection, protects from chronic infection | 27 |
| (GT 1b) | Homologous | 1 protected from infection | ||
| (N=6) | 100CID50 | 1 resolved infection | ||
| Adjuvant: Human IL-12-expressing plasmid | 4 developed persistent infection | |||
| DNA prime and Recombinant VV expressing NS3,NS5A,NS5B | Induced specific T-cell responses | GT 1a | Modifies infection | 63 |
| (GT 1a) | Homologous | 1 developed persistent infection | ||
| (N=1) | 100CID50 | |||
| Adjuvant: CpGs and VV expressing co-stimulatory molecules B7.1; ICAM-1; LFA-3.. | ||||
| DNA prime and Recombinant Adenovirus expressing NS3 to NS5B | Induced specific T-cell responses | GT 1a | Modifies infection, protects from chronic infection | 31 |
| (GT 1b) | Heterologous | 4 resolved infection | ||
| (N=5) | 100CID50 | 1 developed persistent infection | ||
| Adjuvant: None | ||||
| DNA prime and rMVA boost (using C, gpE1, gpE2 and NS3) | Induced specific T-cell responses and antibody to E1 and E2. | GT 1b | Modifies infection, protects from chronic infection | 70 |
| (GT 1b) | Homologous | 1 resolved infection | ||
| (N=4) | 25CID50 | 3 developed persistent infection | ||
| Adjuvant: None | ||||
| Recombinant VV core, E1, E2, p7, NS2 and NS3 | Induced specific T-cell responses. Weak anti-E1E2 response. | GT 1b | Modifies infection, protects from chronic infection | 28 |
| (GT 1b) | Homologous | 4 resolved infection | ||
| (N=4) | 2.5 and 24CID50† | |||
| Adjuvant: None | ||||
| DNA prime and Recombinant Adenovirus expressing NS3,NS5A,NS5B | Induced specific T-cell responses | GT 1a | Modifies infection or protects from chronic infection | 64 |
| (GT 1a) | Homologous | 1 resolved infection | ||
| (N=2) | 100CID50 | 1 developed persistent infection | ||
| Adjuvant: Human IL-12-expressing plasmid | ||||
| Recombinant gpE1/gpE2 | Induced antibodies to E1E2 | GT 1a | Protects against infection or chronic infection | 53 |
| (GT 1a) | Homologous | 5 protected from infection | 52 | |
| (N=21) | 10 to 100CID50 | 14 resolved infection | ||
| Adjuvant: MF59 or MF75 oil-water emulsion | 2 developed persistent infection | |||
| Recombinant gpE1/gpE2 + HVR1 peptides | Induced antibodies to E1E2 and HVR1 | GT 2 | Protects from chronic infection | 54 |
| (GT 2) | Homologous | 1 resolved infection | ||
| (N=1) | 10 CID50 | |||
| Adjuvant: Complete and Incomplete Freund’s | ||||
| DNA vaccine expressing E2 protein | Induced antibody and T cell responses to E2. | GT 1a | Modifies infection, protects from chronic infection | 33 |
| (GT 1a) | Homologous | 2 resolved infection | ||
| (N=2) | 100CID50 | |||
| Adjuvant: None | ||||
| Recombinant gpE1/gpE2 | Induced antibodies and cellular responses to E1E2 | GT 1a | Delayed/modified infection | 71 |
| (GT 1a) | Homologous | 1 developed persistent infection | ||
| (N=1) | 100CID50 | |||
| Adjuvant: RIBIs | ||||
| Recombinant VLPs containing C, E1 and E2 | Induced specific T-cell responses. No detectable anti-E1E2 Ab response. | GT 1b | Modifies infection, protects from chronic infection | 34 |
| (GT 1b) | Homologous | 4 resolved infection | ||
| (N=4) | 100CID50 | |||
| Adjuvant: AS01B (N=2) | ||||
Homologous and heterologous refer to the same or different GT, respectively.
CID50=50% chimpanzee infectious doses;
Animals erroneously received 2.5CID50 which did not lead to infection in control animals. Animals were then challenged with 24CID50.
Materials and Methods
Data Search and Inclusion Criteria
Searches of the Scopus database (www.scopus.com) using the terms “hepatitis C virus” and “chimpanzee” or “hepatitis C virus” and “chimpanzee” and “vaccine” resulted in 341 and 93 publications, respectively. These were reviewed and, where available, data was extracted on i) peak HCV RNA titer (the highest titer following infection) (ii) time to peak titer (representing the day post-infection at which viral replication is controlled) (iii) duration of viremia (the time from infection until viremia was consistently undetectable) and (iv) outcome of infection (persistence or clearance) in 152 chimpanzees consisting of 63 naïve; 53 vaccinated and 36 rechallenged animals. The virus titers in the different studies were not all determined using the same quantitative assays. However, studies have used commercial tests (e.g. Amplicor; bDNA assay) or real-time PCR assays that have been shown to be consistent with WHO standards22;23 such that the titers determined can be considered comparable. Data from studies using mutated viruses, such as the deleted HVR1 region24, and cell culture derived HCV25;26 were not included as the viral kinetics were not considered representative of natural infections. Data from studies reporting viral kinetics in naïve17;21;27-47 and rechallenged17;18;48-51 chimpanzees together with unpublished data (A. Prince, M. Major personal communications) were used for comparisons with vaccinated animals. No differentiation was made between homologous and heterologous challenges. Heterologous challenges were considered as GTs different from the primary challenge or immunizing antigens. As shown in Table 1 only one study31 used a different subtype (GT1a) from that represented by the immunizing antigens (GT1b). Some studies used different isolates of the same GT but these were not considered heterologous. The majority of vaccine studies (7/12) (Table 1) used delivery systems designed to induce T-cell responses to NS proteins. Of these 7 studies, 3 targeted NS proteins alone and 4 additionally targeted structural proteins (core and/or E1E2). Structural region proteins alone were targeted by 5/12 studies.
Statistical Approach to Data Analyses
We used non-parametric tests throughout as it was unclear that the data were normally distributed. To compare categorical variables, the two-tailed Fisher exact test was applied. To perform pairwise comparisons the exact Wilcoxon Signed-Rank Test was used. Multiple comparisons were adjusted using the Bonferroni correction; a two-sided P-value of <0.05 was considered significant except where this was corrected for multiple comparisons to a two-sided P-value of ≤0.017. The specific tests used and related P-values (SPSS; V.17, Chicago, IL) are noted in the Figures and Tables.
Results
Primary and Secondary HCV Infections
We initially analyzed matched data on viral kinetics during primary and secondary infections of recovered chimpanzees. We performed pairwise comparisons of peak RNA titers, time to peak titer and duration of viremia in 11 animals. Figure 1 shows that for each of these parameters the differences between primary and secondary infections are significant (P<0.004). The median peak RNA titer (log10 RNA copies/mL) (Figure 1A) was significantly different between primary and secondary infections (p=0.0038); median difference of 2.0 log10 (Interquartile Range (IQR) =1.2). The median peak RNA titer for primary infections was calculated as 5.9 (IQR=0.5), and for secondary infections as 4.0 (IQR=1.7). The median difference in time post infection that this peak titer was measured (Figure 1B) was also significant (p=0.0037); occurring earlier during secondary infections (13 days; IQR=12) compared to primary infections (56 days; IQR=26); median difference of 42 days (IQR=14). Similarly, the duration of viremia (Figure 1C) was shortened significantly (p=0.0038) during secondary infections from 84 days (IQR=75) to 21 days (IQR=33); median difference 63 days, (IQR=38). Although persistent infections can occur in chimpanzees upon rechallenge27;49;51 this was not observed in any of the animals for which paired data was available.
Figure 1.
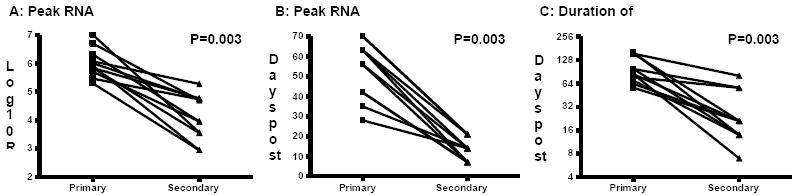
Comparison of HCV kinetics during primary and secondary infections in chimpanzees. A) Maximum RNA titer detected in serum samples. B) Time post-challenge (days) that maximum RNA titer was measured. C) Duration of viremia (days; Log2 scale). P<0.05 indicates a difference between primary and secondary infections (exact Wilcoxon Signed-Rank Test). Data obtained from48-50;69.
Evaluation of Vaccine Efficacy
The type of immune control induced by natural infection with HCV could be taken as the minimum standard expected of any prophylactic vaccine. In order to assess how successful HCV vaccine trials have been in the chimpanzee model we compared peak viral RNA titer; time to peak titer and duration between naïve, rechallenged and vaccinated chimpanzees. Data from all studies listed in Table 1 were included in the statistical analyses. The studies performed by Houghton and co-workers52;53 and Esumi et al54 did not include data for RNA titers therefore these studies were excluded from our statistical comparisons of viral kinetics but were included in analyses of clearance rates.
HCV RNA Kinetics Independent of Outcome
Data obtained comparing peak titer and time to peak titer for the 3 groups of animals is shown in Figure 2. All animals for which kinetics data were available were included in this comparison regardless of infection outcome (persistence or clearance). As expected from the pairwise comparisons performed for Figure 1 there is a significant decrease (P<0.001) for these two parameters in rechallenged animals compared to naïve animals. The median values for each parameter were similar to those seen for paired data. Specifically, 5.8 (IQR=1.1) and 4.6 (IQR=1.1) for peak RNA titer (log10 RNA copies/mL) and 49 days (IQR=42) and 14 days (IQR=14) for peak date in naïve and rechallenged animals, respectively. More encouraging is the fact that we found significant decreases (P<0.001) for each parameter in vaccinated animals compared to naïve animals, median values for peak titer and peak date in vaccinated animals were 4.6 log10 RNA copies/mL (IQR=1.4) and 21days (IQR=21), respectively. There was no significant difference in peak RNA titers (P=0.312; Figure 2A) or control of viral replication (P=0.160; Figure 2B) between rechallenged and vaccinated animals.
Figure 2.
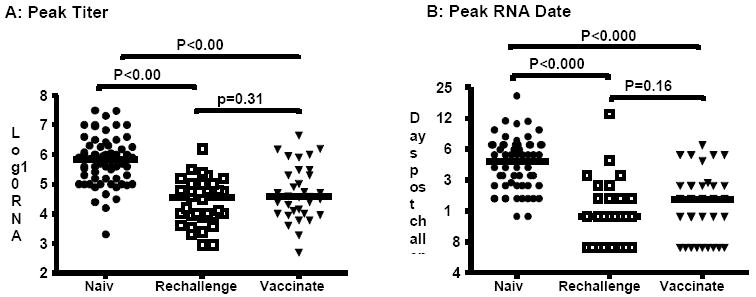
Comparison of HCV kinetics in naïve; rechallenged and vaccinated chimpanzees, regardless of infection outcome. A) Maximum RNA titer detected in serum samples. B) Time post-challenge (days) that maximum RNA titer was measured. Closed circles: naïve; open squares: rechallenged; closed triangles: vaccinated. Horizontal bars represent median values. P<0.017 indicates a difference between groups (Mann-Whitney U Test).
HCV RNA Kinetics Based on Outcome
We performed the same statistical analyses using data only from naïve (n=24); rechallenged (n=28) and vaccinated (n=21) animals that cleared HCV with additional analysis to assess whether vaccination reduces the duration of viremia. Comparisons of peak titer and time to peak titer show significant decreases for rechallenged (P<0.0001) and vaccinated animals (P≤0.005) compared to naïve animals (Figure 3), similar to that seen when all animals are included (Figure 2). The median values for peak titer in naïve, rechallenged and vaccinated animals were calculated as 5.9 (IQR=1.3); 4.1 (IQR=1.1) and 4.6 (IQR=2.0) log10 RNA copies/mL, respectively. The median values for peak date in naïve, rechallenged and vaccinated animals were calculated as 46 (IQR=25); 14 (IQR=14) and 21 (IQR=33) days, respectively. However, there is a trend towards higher (Figure 3A; P=0.112) and later (Figure 3B; P=0.135) peak RNA titers in vaccinated animals that clear compared to rechallenged animals that clear. This data suggests higher levels of viral replication occur in vaccinees prior to clearance. In addition, the duration of viremia in vaccinated animals that clear (median 77 days; IQR=75) is not significantly (P=0.141) shorter than naïve animals (102 days; IQR=51), and is significantly longer (P=0.0001) than that in rechallenged animals (28; IQR=39) (Figure 3C). This further indicates that even when virus is cleared in vaccinees this clearance is not as efficient as in the rechallenged group.
Figure 3.
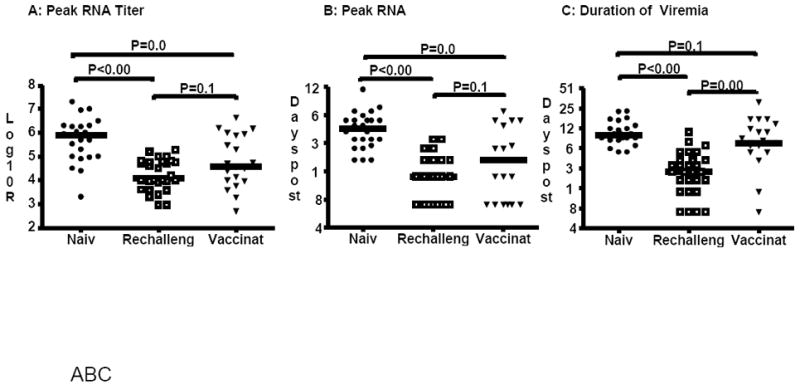
Comparison of HCV kinetics in naïve; rechallenged and vaccinated chimpanzees that cleared HCV. A) Maximum RNA titer detected in serum samples. B) Time post-challenge (days) that maximum RNA titer was measured. C) Duration of viremia (days; Log2 scale). Closed circles: naïve; open squares: rechallenged; closed triangles: vaccinated. Horizontal bars represent median values. P<0.017 indicates a difference between groups (Mann-Whitney U Test).
Vaccination and Outcome of Infection
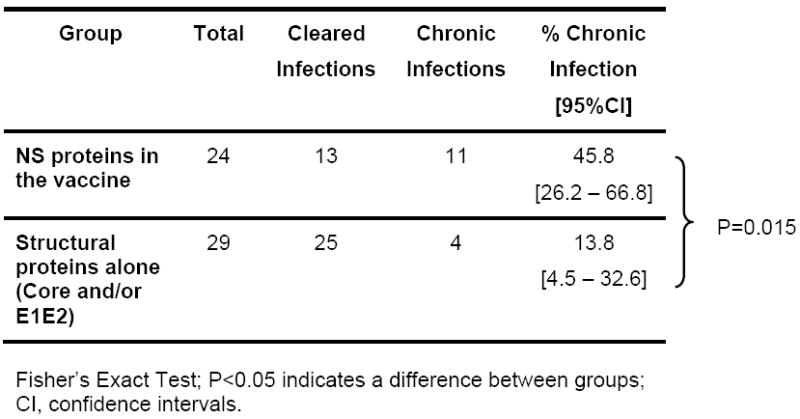
Despite the encouraging data showing control of viral replication in vaccinated animals (Figures 2 and 3) the crucial question is whether the rate of clearance decreases as a result of vaccination. Comparing all vaccinated animals independent of vaccine antigen there is a significant (P<0.0001) decrease in the proportion of animals that develop persistent infections in vaccinees (28.3%) compared to naïve animals (61.9%) (Table 2). The data also shows the rate of clearance in vaccinees is similar to rechallenged animals (16.7%) (P=0.214). However, if the proportion of persistent infections in vaccinated animals is analyzed with respect to the regions targeted, structural region alone compared to NS region alone or in addition to structural proteins, a different picture emerges. We find that a significantly (P=0.015) higher proportion of persistent infections (45.8%) are found when antigens to NS proteins are included in the vaccine (Table 3). The proportion of chronic infections in animals that receive vaccines targeting only the structural region (13.8%) is similar to that seen in rechallenged animals (16.7%, Table 2) while the proportion of chronic infections in animals that received vaccine containing NS components (Table 3;45.8%) is not significantly different (P=0.227) from naïve animals (Table 2;61.9%).
Table 2.
Outcome and rates of clearance in naïve; rechallenged and vaccinated animals.
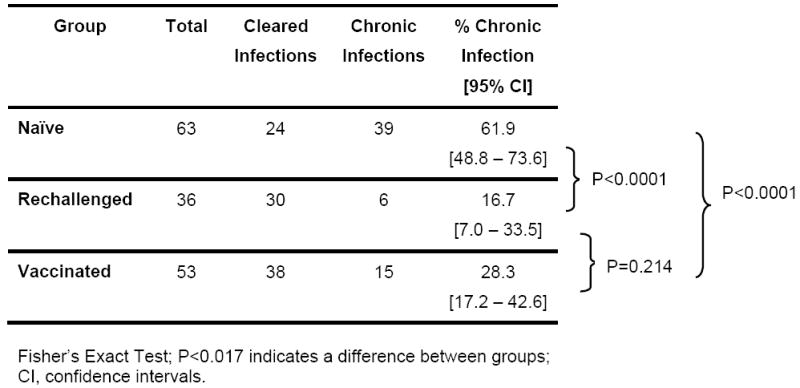 |
Table 3.
Outcome and rates of clearance in vaccinated animals based upon the genome regions included in the vaccine.
 |
T-cell Responses at Challenge do not Predict Outcome
The analyses above suggest that T-cell responses induced by vaccination are in some way inferior to those induced by natural infection; possibly at the level of T-cell function or phenotype. This inferiority of the vaccine-induced T-cell response does not appear to be a question of magnitude. Independent of infection outcome Elispot responses (expressed as spot forming units/106 peripheral blood mononuclear cells) were not significantly different (P=0.38) between rechallenged and vaccinated animals at challenge (Figure 4A). The log10 median values at challenge were calculated as 2.5 (IQR=0.48) (rechallenged) and 2.7 (IQR=0.9) (vaccinated). At 2-4 weeks post challenge the Elispot values are also not statistically different (P=0.44) (Figure 4B); 3.1 (IQR=1.3) (rechallenged) and 2.9 (IQR=0.7) (vaccinated). Despite a lack of statistical difference the numbers suggest there is a better recall of the T-cell responses in the rechallenged group than in the vaccinated group. The statistical comparisons for immune responses are less powerful than the comparisons of RNA titers due to few results available for rechallenged animals (n=5) and due to the variation of antigens and immune response study techniques between laboratories. Nonetheless, these results indicate that vaccination induces comparable levels of HCV-specific T-cells, relative to natural infection, which are recalled upon challenge.
Figure 4.
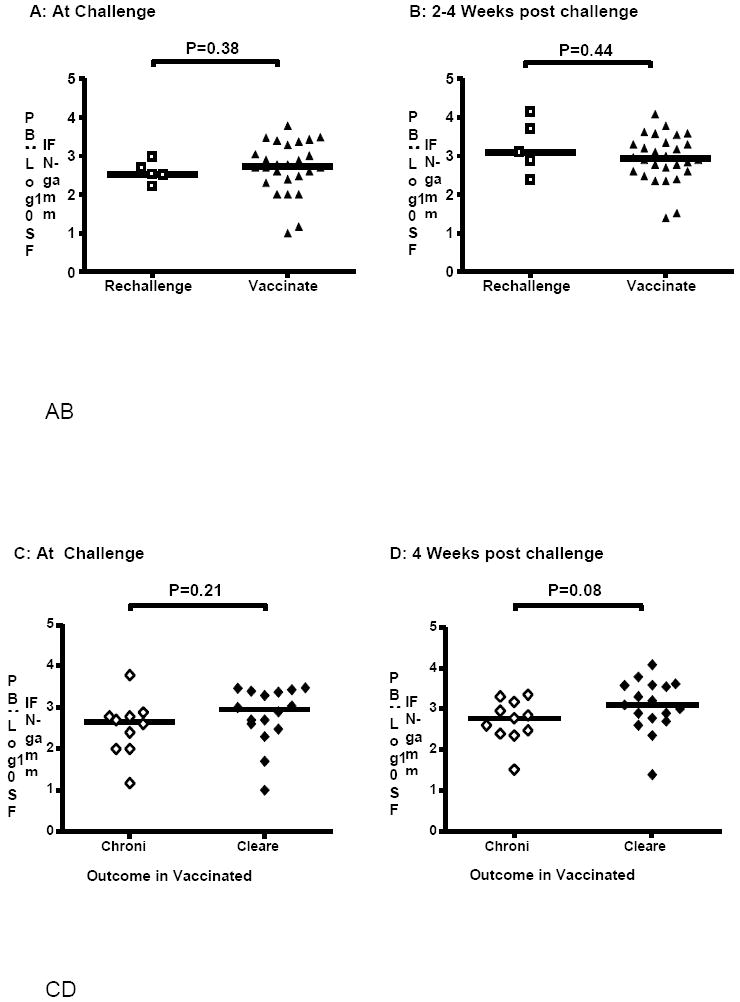
Comparison of Elispot responses. A) Rechallenged and vaccinated at challenge. B) Rechallenged and vaccinated 2-4 weeks post challenge. C) Vaccinated animals at challenge. D) Vaccinated animals 4 weeks post challenge. Data is shown as IFN-γ spot forming units (SFU) per 106 peripheral blood mononuclear cells (PBMCs). (A) and (B): Open squares: rechallenged; closed triangles: vaccinated. (C) and (D): Open diamonds: persistent infections; closed diamonds: cleared infections. Horizontal lines represent median values. P<0.05 indicates a difference between groups (Mann-Whitney U Test).
If Elispot responses between vaccinated animals are compared based upon the outcome of infection we found that these were not significantly different (P=0.21) at challenge between vaccinated animals that cleared (n=16) and those that developed persistent infections (n=10) (Figure 4C). The median log10 responses were 2.95 (IQR=0.8) and 2.7 (IQR=0.8) for cleared and chronic animals, respectively. However, there is a trend (P=0.08) for the recall response in vaccinated animals that cleared HCV (3.1; IQR=0.9) to be higher than that in vaccinated animals that developed persistent infections (2.8; IQR=0.8) (Figure 4D). These data further suggest that the magnitude of the vaccine-induced T-cell response cannot be used to predict success of a vaccine and that within vaccinees the quality of the response possibly accounts for more efficient proliferation of cells upon exposure to virus that then leads to clearance.
Discussion
This meta-analysis provides important data for future development, expectations and assessment of HCV prophylactic vaccines. We show statistically significant differences in viral kinetics between primary and secondary infections in recovered chimpanzees. This statistical analysis is consistent with previous observations demonstrating that although infection can occur upon re-exposure following clearance, viral kinetics are very different with rapid clearance in the majority of cases18;48;55;56. Similar significant decreases in maximum HCV RNA titer and duration of infection for secondary infections in intravenous drug users were recently reported57. Interestingly, in both chimpanzees (Table 2) and humans57 the HCV clearance rate was 83% of reinfected subjects, this compares to ~25% in patients57 and ~38% in chimpanzees (Table 2) with primary infections. These data together with studies on immune correlates of clearance support the argument that memory immune responses are induced during primary infections, that these responses are recalled following secondary infections and that the responses are primarily T-cell based (reviewed in ref 58).
All of the chimpanzee vaccine approaches listed in Table 1 induced HCV-specific immune responses and HCV kinetics were significantly impacted in vaccinees compared to naïve animals. The peak viral titers were significantly decreased (P<0.001) and viral replication was controlled earlier (P<0.0001) regardless of the final outcome of infection. This shows that vaccination against HCV induces memory immune responses that are recalled upon exposure to virus and are effective at controlling viral replication. However, the duration of viremia in vaccinated animals was not significantly improved over naïve animals; suggesting vaccination was not able to consistently produce immune responses qualitatively similar to those induced during spontaneous clearance. Thus, not only does previous infection with HCV induce immune responses that protect from secondary infections but this natural immunity is superior to vaccine-induced immunity.
Our meta-analysis indicates that the rate of viral persistence is not increased following vaccination. However, the T-cell vaccine approach that includes NS proteins does not significantly (P=0.227) decrease the rate of persistence (45.8%) compared to naïve animals (61.9%) and results in a significantly higher rate compared to vaccines that target the structural region alone (P=0.015). Vaccines with the greatest success at leading to resolved infections included all or part of the HCV envelope region inducing either neutralizing antibody33;52-54, E1E2 T-cell responses34 or both28, suggesting that neutralizing antibodies can play a role in protection but also that this region may contain T-cell epitopes that are important for clearance. However, the induction of neutralizing antibodies or T-cell responses to the envelope region does not necessarily guarantee success of a vaccine and absence of these antigens from a vaccine was not always associated with persistence.
There are a number of mechanisms that may account for our observed difference in outcome based upon the vaccine antigen. The choice or use of adjuvants may have influenced the quality of the immune response and memory cells induced. Most of the vaccines targeting structural proteins used adjuvants (Table 1) but a number of vaccines targeting the NS proteins also included adjuvants e.g. CpGs or recombinant viruses expressing co-stimulatory molecules. An analysis of animals vaccinated with adjuvants compared to those vaccinated without adjuvants showed no significant difference (p=0.51) in the rate of persistence. The type of recombinant virus used for the T-cell based vaccines also did not impact the outcome of infection after challenge. The rate of persistence in animals immunized with Vaccinia virus vectors compared to those immunized with Adenovirus vectors was not significantly different (p=0.64). It is more difficult to analyze the use of viral vectors on the outcome of infection as the vaccines designed to induce T-cells mainly used recombinant viruses (Table 1). This approach is unlikely to change unless significant drawbacks can be associated with viral vectors as these are one of the most efficient means of inducing T-cell responses. It is also possible that antibody plays a role in successful clearance through antibody-dependent cell-mediated cytotoxicity (ADCC). This has been shown to be important in a number of viral infections59;60 and also to be induced by a recent HIV vaccine that showed a reduced risk of HIV infection in vaccinees61;62.
It is not possible to predict from this meta-analysis which vaccines should be developed for future clinical trials, but vaccines with a T-cell component targeting NS proteins should not be dismissed. It should be remembered that all the vaccines trials induced immune responses that modified viral replication and in order to protect against heterologous viruses responses to the more conserved NS proteins will almost certainly be required. However, more extensive studies need to be performed at the level of T-cell function in order to obtain a better understanding of the types of T-cells induced and provide reliable biomarkers to predict vaccine success.
Vaccine failure may also be caused by immune escape from the induced response. The persistence of HCV in some vaccine studies has been associated with immune escape from CD4+63 or CD8+31 T-cells and with higher viral mutation rates64 although immune escape from neutralizing antibody has not yet been demonstrated in vaccine studies. These data suggest that in cases where the immune response cannot rapidly clear the virus there remains an environment for immune pressure.
Ideally, the goal of prophylactic vaccines is to prevent infection upon exposure. However, from our data analysis sterilizing immunity seems to be unrealistic for hepatitis C. Most efficacy trials use reduction of clinical disease as the endpoint and not elimination of all evidence of infection. With the highly sensitive assays such as PCR, it is likely that low level replication of the infectious agent may be detected in successfully vaccinated individuals. HCV differs from many viruses in that it causes persistent infection which leads to chronic liver disease. A vaccine that leads to low-level HCV titers long-term would probably not be considered successful. Although low-level HCV titers increases the chances of virus elimination under the current standard of care (SOC)65 the risk to benefit ratio in vaccinees would be considered too high and a clinical trial demonstrating a significant beneficial effect in vaccinees when combined with treatment would also be difficult. However, eliminating persistent infections while reducing acute phase viral titers would eventually prevent the major disease burden and most transmissions66;67 such that any prophylactic hepatitis C vaccine could target prevention of chronic infections as the primary endpoint.
Clinical development of HCV vaccine candidates is challenging. It is now known that treatment of acute HCV infections has a high probability of success. At present, the SOC recommends monitoring patients for ~12 weeks after diagnosis of acute HCV infection prior to initiating treatment with pegylated interferon-α, usually in combination with ribavirin, if the individual has not cleared the virus68. In clinical trials there is active monitoring; if treatment is initiated at the time of diagnosis it would be difficult to determine if the vaccine can prevent chronic infections. Our meta-analysis suggests that this difficult problem is not unsolvable. In clinical vaccine trials, the subjects would be monitored periodically after the last dose of vaccine, typically at 6 month intervals. Given our data analysis, the median duration of viremia in vaccinees that clear HCV is 77 days (IQR=75); for rechallenged animals it is 28 days (IQR=39) and naive animals it is 102 days (IQR=51). These data indicate that if vaccine-induced immune responses are as efficient at clearing HCV as the immunity induced by natural infection there is a high probability that vaccinees will have cleared HCV within 3 months of exposure and significant differences would be seen between vaccine and placebo groups. If vaccines are developed that do not decrease the duration of viremia but decrease the rate of virus persistence differences could still be seen between vaccine and placebo groups if sufficient numbers were included in the studies.
This meta-analysis provides values for viral kinetic parameters that can be used to judge the success of future vaccine studies. However, it also demonstrates that there are scientific questions that still need to be resolved in HCV vaccine development, specifically correlates of protection. However, substantial progress has been made: vaccines can induce immune responses that impact viral replication; clinical studies are feasible and we have the means to judge efficacy. The development of effective HCV vaccines is a very real possibility.
Acknowledgments
This work was supported by intramural funds from the Food and Drug Administration, by NIH grant number P20 RR018754 and by the University of Illinois Walter Payton Liver Center GUILD (H.D.). The authors wish to thank Dr. Fred Prince for providing unpublished data on HCV kinetics in chimpanzees.
Abbreviations
- CID50
chimpanzee infectious doses 50
- GT
Genotype
- IFN
Interferon
- IQR
Interquartile Range
- NS
Non-structural
- NTR
Non-translated region
- PBMCs
Peripheral blood mononuclear cells
- SFU
Spot forming units
Footnotes
Author Contributions: Harel Dahari: Acquisition of data; study design; interpretation of data; drafting of the manuscript; critical revision of manuscript; statistical analyses
Stephen Feinstone: Drafting of the manuscript; revision of manuscript for content; analysis of data
Marian Major: Acquisition of data; study design and concept; interpretation of data; drafting manuscript; critical revision of manuscript; statistical analyses; study supervision.
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Bartenschlager R, Frese M, Pietschmann T. Novel insights into hepatitis C virus replication and persistence. Adv Virus Res. 2004;63:71–180. doi: 10.1016/S0065-3527(04)63002-8. [DOI] [PubMed] [Google Scholar]
- 2.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Liang TJ, Rehermann B, Seeff LB, et al. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 6.Stumpf MP, Pybus OG. Genetic diversity and models of viral evolution for the hepatitis C virus. FEMS Microbiol Lett. 2002;214:143–152. doi: 10.1111/j.1574-6968.2002.tb11338.x. [DOI] [PubMed] [Google Scholar]
- 7.Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet. 2008;9:267–276. doi: 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
- 8.Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 9.Simmonds P, Bukh J, Combet C, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 10.Murphy D, Chamberland J, Dandavino R, et al. A new genotype of hepatitis C virus originating from central Africa. Hepatology. 2007;46:623A. doi: 10.1128/JCM.02831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson AL, Kimura Y, Igarashi S, et al. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity. 2001;15:883–895. doi: 10.1016/s1074-7613(01)00245-x. [DOI] [PubMed] [Google Scholar]
- 12.Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med. 2005;201:1709–1714. doi: 10.1084/jem.20050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uebelhoer L, Han JH, Callendret B, et al. Stable cytotoxic T cell escape mutation in hepatitis C virus is linked to maintenance of viral fitness. PLoS Pathog. 2008;4:1–15. doi: 10.1371/journal.ppat.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner AJ, Geysen HM, Christopherson C, et al. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: Potential role in chronic HCV infections. Proc Natl Acad Sci U S A. 1992;89:3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu YK, Hijikata M, Iwamoto A, et al. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 18.Major ME, Mihalik K, Puig M, et al. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586–6595. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nascimbeni M, Mizukoshi E, Bosmann M, et al. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thimme R, Bukh J, Spangenberg HC, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puig M, Mihalik K, Yu MY, et al. Sensitivity and reproducibility of HCV quantitation in chimpanzee sera using TaqMan real-time PCR assay. J Virol Methods. 2002;105:253–263. doi: 10.1016/s0166-0934(02)00119-2. [DOI] [PubMed] [Google Scholar]
- 23.Engle RE, Russell RS, Purcell RH, et al. Development of a TaqMan assay for the six major genotypes of hepatitis C virus: comparison with commercial assays. J Med Virol. 2008;80:72–79. doi: 10.1002/jmv.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forns X, Thimme R, Govindarajan S, et al. Hepatitis C virus lacking the hypervariable region 1 of the second envelope protein is infectious and causes acute resolving or persistent infection in chimpanzees. Proc Natl Acad Sci U S A. 2000;97:13318–13323. doi: 10.1073/pnas.230453597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindenbach BD, Meuleman P, Ploss A, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci U S A. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakita T, Pietschmann T, Kato T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youn JW, Park SH, Lavillette D, et al. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology. 2005;42:1429–1436. doi: 10.1002/hep.20934. [DOI] [PubMed] [Google Scholar]
- 28.Youn JW, Hu YW, Tricoche N, et al. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J Virol. 2008;82:10896–10905. doi: 10.1128/JVI.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Major ME, Dahari H, Mihalik K, et al. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology. 2004;39:1709–1720. doi: 10.1002/hep.20239. [DOI] [PubMed] [Google Scholar]
- 30.Woollard DJ, Grakoui A, Shoukry NH, et al. Characterization of HCV-specific Patr class II restricted CD4+ T cell responses in an acutely infected chimpanzee. Hepatology. 2003;38:1297–1306. doi: 10.1053/jhep.2003.50478. [DOI] [PubMed] [Google Scholar]
- 31.Folgori A, Capone S, Ruggeri L, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 32.Rollier C, Depla E, Drexhage JA, et al. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J Virol. 2004;78:187–196. doi: 10.1128/JVI.78.1.187-196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forns X, Payette PJ, Ma X, et al. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618–625. doi: 10.1053/jhep.2000.9877. [DOI] [PubMed] [Google Scholar]
- 34.Elmowalid GA, Qiao M, Jeong SH, et al. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci U S A. 2007;104:8427–8432. doi: 10.1073/pnas.0702162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bukh J, Apgar CL, Engle R, et al. Experimental infection of chimpanzees with hepatitis C virus of genotype 5a: genetic analysis of the virus and generation of a standardized challenge pool. J Infect Dis. 1998;178:1193–1197. doi: 10.1086/515683. [DOI] [PubMed] [Google Scholar]
- 36.Hong Z, Beaudet-Miller M, Lanford RE, et al. Generation of transmissible hepatitis C virions from a molecular clone in chimpanzees. Virology. 1999;256:36–44. doi: 10.1006/viro.1999.9603. [DOI] [PubMed] [Google Scholar]
- 37.Yanagi M, St, Shapiro M, et al. Transcripts of a chimeric cDNA clone of hepatitis C virus genotype 1b are infectious in vivo. Virology. 1998;244:161–172. doi: 10.1006/viro.1998.9092. [DOI] [PubMed] [Google Scholar]
- 38.Sakai A, Takikawa S, Thimme R, et al. In vivo study of the HC-TN strain of hepatitis C virus recovered from a patient with fulminant hepatitis: RNA transcripts of a molecular clone (pHC-TN) are infectious in chimpanzees but not in Huh7.5 cells. J Virol. 2007;81:7208–7219. doi: 10.1128/JVI.01774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meunier JC, Engle RE, Faulk K, et al. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci U S A. 2005;102:4560–4565. doi: 10.1073/pnas.0501275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato T, Choi Y, Elmowalid G, et al. Hepatitis C virus JFH-1 strain infection in chimpanzees is associated with low pathogenicity and emergence of an adaptive mutation. Hepatology. 2008;48:732–740. doi: 10.1002/hep.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamili S, Krawczynski K, McCaustland K, et al. Infectivity of hepatitis C virus in plasma after drying and storing at room temperature. Infect Control Hosp Epidemiol. 2007;28:519–524. doi: 10.1086/513727. [DOI] [PubMed] [Google Scholar]
- 42.Prince AM, Pawlotsky JM, Soulier A, et al. Hepatitis C virus replication kinetics in chimpanzees with self-limited and chronic infections. J Viral Hepat. 2004;11:236–242. doi: 10.1111/j.1365-2893.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 43.Katayama K, Kumagai J, Komiya Y, et al. Titration of hepatitis C virus in chimpanzees for determining the copy number required for transmission. Intervirology. 2004;47:57–64. doi: 10.1159/000076643. [DOI] [PubMed] [Google Scholar]
- 44.Shata MT, Tricoche N, Perkus M, et al. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–616. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomson M, Nascimbeni M, Havert MB, et al. The clearance of hepatitis C virus infection in chimpanzees may not necessarily correlate with the appearance of acquired immunity. J Virol. 2003;77:862–870. doi: 10.1128/JVI.77.2.862-870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomson M, Nascimbeni M, Gonzales S, et al. Emergence of a distinct pattern of viral mutations in chimpanzees infected with a homogeneous inoculum of hepatitis C virus. Gastroenterology. 2001;121:1226–1233. doi: 10.1053/gast.2001.28669. [DOI] [PubMed] [Google Scholar]
- 47.Bukh J, Pietschmann T, Lohmann V, et al. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc Natl Acad Sci U S A. 2002;99:14416–14421. doi: 10.1073/pnas.212532699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassett SE, Guerra B, Brasky K, et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 49.Bukh J, Thimme R, Meunier JC, et al. Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J Virol. 2008;82:8183–8195. doi: 10.1128/JVI.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanford RE, Guerra B, Chavez D, et al. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575–1581. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prince AM, Brotman B, Lee DH, et al. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis. 2005;192:1701–1709. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- 52.Coates S, Choo QL, Kuo G, et al. Protection of Chimpanzees against Heterologous 1a Viral Challenge using a gpE1/gpE2 Heterodimer Vaccine. In: Jilbert AR, Grgacic E, Vickery K, Burrell CJ, Cossart YE, editors. Proc 11th International Symposium on Viral Hepatitis and Liver Disease. Australian Center for Hepatitis Virology; 2004. pp. 118–123. [Google Scholar]
- 53.Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci U S A. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esumi M, Rikihisa T, Nishimura S, et al. Experimental vaccine activities of recombinant E1 and E2 glycoproteins and hypervariable region 1 peptides of hepatitis C virus in chimpanzees. Arch Virol. 1999;144:973–980. doi: 10.1007/s007050050559. [DOI] [PubMed] [Google Scholar]
- 55.Dahari H, Major M, Zhang X, et al. Mathematical modeling of primary hepatitis C infection: noncytolytic clearance and early blockage of virion production. Gastroenterology. 2005;128:1056–1066. doi: 10.1053/j.gastro.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 56.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 57.Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous Control of Primary Hepatitis C Virus Infection and Immunity against Persistent Reinfection. Gastroenterology. 2009;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 59.Duerst RJ, Morrison LA. Innate immunity to herpes simplex virus type 2. Viral Immunol. 2003;16:475–490. doi: 10.1089/088282403771926300. [DOI] [PubMed] [Google Scholar]
- 60.Forthal DN, Landucci G, Haubrich R, et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 61.Karnasuta C, Paris RM, Cox JH, et al. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine. 2005;23:2522–2529. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 62.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 63.Puig M, Mihalik K, Tilton JC, et al. CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology. 2006;44:736–745. doi: 10.1002/hep.21319. [DOI] [PubMed] [Google Scholar]
- 64.Zubkova I, Choi YH, Chang E, et al. T-cell vaccines that elicit effective immune responses against HCV in chimpanzees may create greater immune pressure for viral mutation. Vaccine. 2009;27:2594–2602. doi: 10.1016/j.vaccine.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 65.Dahari H, Layden-Almer JE, Kallwitz E, et al. A mathematical model of hepatitis C virus dynamics in patients with high baseline viral loads or advanced liver disease. Gastroenterology. 2009;136:1402–1409. doi: 10.1053/j.gastro.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 67.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 68.Corey KE, Mendez-Navarro J, Gorospe EC, et al. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2009 doi: 10.1111/j.1365-2893.2009.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Major ME, Mihalik K, Fernandez J, et al. Long-term follow-up of chimpanzees inoculated with the first infectious clone for hepatitis C virus. J Virol. 1999;73:3317–3325. doi: 10.1128/jvi.73.4.3317-3325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rollier CS, Paranhos-Baccala G, Verschoor EJ, et al. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology. 2007;45:602–613. doi: 10.1002/hep.21573. [DOI] [PubMed] [Google Scholar]
- 71.Puig M, Major ME, Mihalik K, et al. Immunization of chimpanzees with an envelope protein-based vaccine enhances specific humoral and cellular immune responses that delay hepatitis C virus infection. Vaccine. 2004;22:991–1000. doi: 10.1016/j.vaccine.2003.09.010. [DOI] [PubMed] [Google Scholar]


