Abstract
While most hematopoietic lineages develop in the bone marrow (BM), T cells uniquely complete their development in the specialized environment of the thymus. Hematopoietic stem cells with long-term self-renewal capacity are not present in the thymus. As a result, continuous T cell development requires that BM-derived progenitors be imported into the thymus throughout adult life. The process of thymic homing begins with the mobilization of progenitors out of the bone marrow, continues with their circulation in the bloodstream, and concludes with their settling in the thymus. This review will discuss each of these steps as they occur in the unirradiated and post-irradiation scenarios, focusing on the molecular mechanisms of regulation. Improved knowledge about these early steps in T cell generation may accelerate the development of new therapeutic options in patients with impaired T cell number or function.
Keywords: thymus, bone marrow, T cells, progenitor, hematopoesis, Hematopoietic stem cells
Introduction
T cells are a lymphocyte lineage critical for defense from many types of pathogens. Several classes of T cells have been described. These include CD8+ T cells, which are effector cells capable of lysing target cells via the release of granules containing perforin, and CD4+ cells, which contribute to immunity primarily by secreting cytokines that control the activity of other immune cells. Work in the last decade has broadened our knowledge of the various types of CD4+ T cells, including Th1, Th2, Th17, T regulatory cells and others. The known roles of these various subsets are numerous and diverse, and ongoing research continues to highlight their immunologic capacities. The HIV/AIDS epidemic has additionally demonstrated the importance of CD4+ cells in immunity; infected individuals with decreased numbers of CD4+ cells are highly susceptible to a range of bacterial, fungal, and viral infections. Yet despite our growing understanding of T cell function in the periphery, major questions remain unanswered regarding their origin and early development.
While T cells complete the majority of their development in the thymus, they ultimately originate from hematopoietic stem cells (HSCs) residing in the bone marrow (BM).1 Derived from these stem cells are various downstream progenitors that first lose the capacity for self-renewal (a defining feature of HSCs) and then progressively become more restricted in their lineage potential. Through a process that remains largely undefined, rare numbers of these progenitors mobilize out of the BM into the circulation, where they gain access to the thymus. These circulating progenitors will then migrate across the thymic endothelium to enter the organ itself. Following thymic entry, the cells will continue to differentiate while proliferating dramatically. Over the course of several weeks, these progenitors will proceed down the well-described intrathymic development stages. Cells begin the process lacking expression of both CD4 and CD8 co-receptors, then undergo a developmental checkpoint that leads to the co-expression of both CD4 and CD8 on so-called CD4+CD8+ double-positive (DP) cells. Following a second checkpoint, they downregulate one of the co-receptors to become either a CD8+ or CD4+ single-positive thymocyte. These cells will subsequently migrate from the thymus to take up residence in the periphery as naïve T cells.
While these major landmarks in this long process are known, many of the mechanistic details remain unclear. This review will address some of the long-standing questions in the field of progenitor migration to the thymus. For example, what is the identity of the cell type (or perhaps multiple cell types) that directly settles the thymus out of the blood? The search for the thymic settlng progenitor (TSP) has been encumbered by their presumed rarity in the thymus perhaps as few as 10 or less progenitors per day entering the organ.2–5 Efforts to identify the TSP have been aided by recent work demonstrating thymic settling to be a highly selective process regulated by a panel of adhesion proteins, chemokines, and cytokines.6–9 The search can now be limited to the subset of BM progenitors expressing molecules required for thymic settling. Yet even this smaller pool of cells remains heterogeneous and further refinement is necessary for precise identification of the TSP. Additionally, what are the precise roles of each of these involved molecules, and how is this process regulated? Are additional yet-undiscovered signals involved? These questions and others will be covered in detail.
An improved understanding of the earliest steps in T cell development could have substantial clinical implications. Loss of T cell production accompanies normal aging, and improving T cell number and function in the elderly population could markedly reduce the immunologic susceptibility of this population.10 Furthermore, patients with HIV/AIDS or those undergoing BM transplantation (most often as a curative therapy for various malignancies and immunodeficient conditions) have vast defects in the number of T cells, and this often leads to serious infections. Improved understanding of the early steps in T cell development, including progenitor migration to the thymus, could lead to efforts to improve T cell counts in these vulnerable patient populations.
This review will describe how rare BM-derived progenitors can mobilize into the circulation and subsequently settle in the thymus to generate downstream T lineage progeny. The many candidates for the physiological thymic settling progenitor will be described. We will consider the several important homing and adhesion molecules that have been implicated in this process. Finally, we will discuss the limited information about how the process of thymic settling and early T lymphoid reconstitution may occur acutely following irradiation and bone marrow transplantation and in other inflammatory conditions.
Pre-thymic hematopoietic development
The thymus does not contain cells with the capacity for long-term self-renewal.11–13 As a result, the production of T cells requires that BM-derived progenitors enter the thymus via the bloodstream (Figure 1). Therefore, any discussion of T cell development must begin by describing the most primitive hematopoietic progenitors in the BM. Through the discovery of an array of cell surface molecules on these cells, various progenitors have been defined with distinct phenotypes and functions. Careful analyses of these BM cells have substantially advanced the search for the TSP. The following section will describe the early steps in pre-thymic T cell development, covering first differentiation, and then mobilization and circulation of BM progenitors.
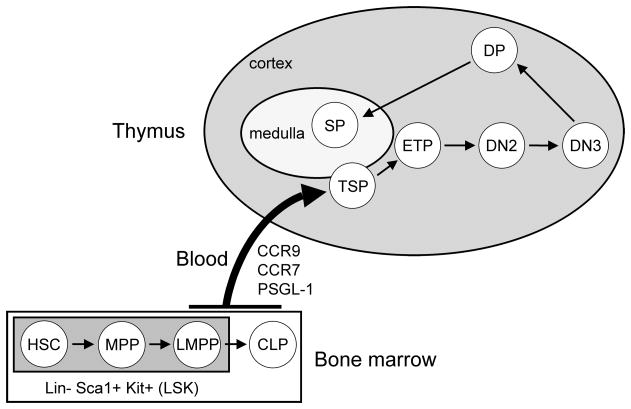
Figure 1. Overview of T cell development from BM to thymus.
The BM contains an array of progenitors with T lineage potential. These include the most primitive progenitor, the hematopoietic stem cells (HSCs), which are multipotent and have the capacity for self-renewal. HSCs give rise to more downstream multipotent progenitors (MPPs), which have lost self-renewal capacity. MPPs in turn produce lymphoid-primed multipotent progenitors (LMPPs) which, while still possessing myeloid potential, express lymphoid-specific genes. These three progenitor types are each negative for lineage markers (Lin−), Sca-1+ and Kithigh; hence they are collectively known as LSK cells. Developing from LMPPs are common lymphoid progenitors (CLPs), which sit outside the LSK pool because they are Kitlow. All of these progenitors are predominantly found in the BM, but each can be detected in blood at low frequencies. From this pool of circulating progenitors, rare numbers are thought to cross the thymic endothelium as thymic settling progenitors (TSPs). The precise identity of these TSPs remains undefined. Once in the thymus, these TSPs differentiate into early thymic progenitors (ETPs), which are the earliest defined intrathymic T cell precursors. ETPs go on to generate CD4/CD8 double-negative 2 cells (DN2) and subsequently DN3 cells. Following a developmental checkpoint at the DN3 stage, CD4/CD8 double-positive (DP) cells are produced. These cells undergo postive selection before differentiating into CD4 or CD8 single-positive (SP) cells. SP cells proceed through negative selection and emigrate from the thymus to populate the peripheral T cell pool.
Early BM hematopoiesis
Hematopoiesis occurs primarily in the liver during fetal life, but relocates to the BM at around the time of birth.14 HSCs in the BM self-renew throughout adult life in order to continuously produce downstream progeny and by definition they are multipotent – they have the capacity to generate all blood lineages.15 Serving as the “roots” of the hematopoietic “tree”, HSCs hold tremendous proliferative potential: the transfer of single HSCs into irradiated hosts can generate detectable progeny in all blood lineages.16,17 HSCs produce terminally differentiated blood cells through a step-wise process of differentiation and proliferation. These steps are considered to be irreversible, such that once a given lineage potential is lost, it cannot be regained by normal physiological mechanisms. There are many signals, both intracellular and extracellular, that govern the process of hematopoietic differentiation. Cytokines, for example, have the capacity to direct lineage specification during at least some stages of hematopoietic differentiation.18 The non-hematopoietic cells found in the BM are involved in early hematopoietic development as well. Endosteal osteoblasts, perivascular cells, and possibly other cell types contribute to the specialized microenvironment of the stem cells niche, wherein HSCs are provided the cytokines, chemokines, and other factors that mediate their survival and development.19,20
The most primitive progenitors in the BM are negative for surface expression of markers found on mature cell types (so-called lineage markers), as well as positive for the molecule Sca-1 and the cytokine receptor Kit; together they are known as LSK cells. HSCs are the most upstream cell within this pool and can be found within the LSK fraction lacking expression of the cytokine receptor Flt3.21 HSC have also been characterized as Thy1low and CD34− (in the case of long-term HSCs).15,16,22 More recently, the SLAM family of markers has been used to identify HSCs in strains of mice lacking Sca-1 expression.20 The direct descendents of HSCs are the multipotent progenitors (MPPs), which retain multilineage reconstituting potential, but only in the short-term.21 MPPs can be distinguished from HSCs by their expression of low levels of the cytokine receptor Flt3.21 These cells in turn give rise to overlapping populations known as early lymphoid progenitors (ELPs) and lymphoid-primed multipotent progenitors (LMPPs), which show expression of lymphoid-specific genes such as Rag and Il7rα.23,24 ELPs were originally defined using a RAG1-GFP knock-in mouse, and LMPPs are characterized by the expression of high levels of Flt3. It is at this stage of development that chemokine receptors critical for thymic settling begin to be expressed, as will be described later.9
Descending from the ELP/LMPP pool are common lymphoid progenitors (CLPs).25 This population was initially identified to possess only lymphoid potential, but several reports have shown some limited myeloid potential both in vitro and in vivo.26–30 One explanation for these contradictory data is that CLPs are a heterogeneous population containing multiple subsets with different lineage potentials. Several recent reports support this hypothesis.29–31 CLPs were divided into two subsets based on surface expression of the marker Ly6D.29 After intravenous transfer into irradiated hosts, the Ly6D− fraction, named the all-lymphoid progenitor (ALP), generated T and B cells with high efficiency, but also low numbers of dendritic cells (DCs), natural killer (NK) cells, and even myeloid cells. Cells expressing Ly6D, however, named B-cell-biased lymphoid progenitors (BLPs), almost exclusively produced B cells. Even after intrathymic transfer, BLPs made mainly B lineage cells. Together these data suggest that the CLP pool is indeed heterogeneous, containing both potent T cell progenitors and cells that are primitive B cell precursors.
More downstream progenitors in the BM with T lineage potential have been described as well. The first of these are known as CLP-2 cells. Identified with the aid of a pre-Tα reporter, they share a common phenotype with traditional CLP cells, except that they are Kit−/lowB220+.32 These cells are generated from more upstream CLP cells, and possess B and T cell potential. An additional lymphoid restricted progenitor with a Kit−Sca-1highFlt3+ surface phenotype has also been described.33 These cells are known as lymphoid-biased progenitors (LBPs) and rapidly produce B and T cells after transfer into irradiated hosts. Furthermore, they are equally efficient as CLPs at generating DP cells in the thymus. Despite their functional similarity with CLPs, LPBs show very little history of RAG-recombinase activity.33 The precise position of LBPs in the hematopoietic hierarchy has yet to be determined.
The generation of T cells in the thymus is critically dependent on signaling by the surface receptor Notch1.34,35 To serve as a TSP, a progenitor must therefore enter the thymus already expressing Notch1 or upregulate it rapidly upon thymic entry. All of the progenitors described above indeed express Notch1 and as a result will generate T cells after direct intrathymic transfer.7,32,36–38 It remains unclear whether extrathymic Notch1 signaling is important for T cell development under physiologic circumstances. Experiments have also utilized a dominant-negative (DN) form of MAML, a key co-activator in the Notch1 signaling pathway, to determine which stage of T cell development first depends on Notch1 signaling. Chimeras employing BM transduced with DN-MAML demonstrated little if any requirement for Notch1 signals in early BM hematopoiesis or mobilization of progenitors; early thymic progenitors (ETPs), however, fail to appear in the absence of functional Notch1 signals.34 Together these data illustrate that Notch1 signaling within adult BM progenitors is unnecessary for T cell production.39
Mobilization of BM progenitors into blood
In order to contribute to T cell development, a progenitor with T lineage potential must find its way to the thymus. This requires that the progenitors mobilize out of the BM into the circulation. HSCs have long been known to be present in peripheral blood; this is most directly demonstrated by the long-term multilineage reconstitution observed in recipients of transferred whole blood.40–43 HSCs are not the sole progenitor in the circulation, as more downstream MPPs and LMPPs can be reliably detected in blood as well, though at very low frequencies.36,38,43 Relatively little is known about the physiologic mechanisms that govern the egress of BM progenitors into the blood. However, numerous factors, including cytokines, chemokines, growth factors, and hormones, can mobilize progenitors after exogenous administration.44
Chemokine receptors have been a particular focus of BM progenitors mobilization studies. These receptors operate through G protein signaling and have established roles in regulating the survival, proliferation, and migration of BM progenitors.45–47 The best studied of these signaling axes is composed of the chemokine SDF-1α (CXCL12) and its receptor CXCR4. This receptor is expressed by early BM progenitors, and these cells will migrate to an SDF-1α gradient in vitro.9 CXCR4 is known to mediate the homing and retention of progenitors to the BM environment.47 SDF-1α is highly expressed by osteoblasts that line the endosteal surface and by BM endothelial cells.48,49 These sources serve to attract circulating progenitors and retain them in the BM. Mice deficient for either the receptor or its ligand demonstrate perinatal lethality concomitant with highly impaired hematopoiesis.50,51 Furthermore, fetal liver progenitors lacking CXCR4 are defective in migrating to the BM.52 Abrogation of this signaling axis through various means leads to the rapid emigration of progenitors out the BM into the blood.44 For example, raising the concentration of plasma SDF-1α abolishes the local gradient normally present in the BM, leading to the rapid mobilization of BM progenitors into the blood.53 Systemic treatment of mice with a pharmacologic inhibitor of CXCR4, AMD3100, recapitulates this result.54
These experiments have established a role for CXCR4 in the retention of progenitors in the BM. Predictably, the abrogation of CXCR4 signals may be a mechanism of physiologic mobilization of progenitors into the circulation. The frequency of HSCs in blood fluctuates with circadian oscillations, with a peak value 5 hours after initiation of light and a minimum 5 hours after darkness.55 This observation suggests the presence of a tightly regulated mechanism of progenitor mobilization. Indeed, neural output derived from the suprachiasmatic nucleus, which controls circadian rhythms, deliver β3-adrenergic signals to BM stromal cells.55 Receptor signaling leads to the downregulation of SDF-1α transcripts in these cells. This reduced expression of CXCR4 ligands may then allow progenitors to escape the retention signals that normally trap them in the BM. Future work is necessary to delineate whether additional stimulican reduce (or raise) BM SDF-1α expression.
Recent investigations have unveiled a physiologic role for the sphingosine-1-phosphate (S1P) receptor 1 in mobilizing immature B cells from the BM into the blood.56 S1P1 has well-established roles in the egress of various cells from several organs, such as thymocytes from the thymus and T cells from lymph nodes.56,57 Using a B cell-specific Cre recombinase, Allende and colleagues found that deletion of the S1P1 receptor led to a defect in the mobilization of immature B cells from the BM. This suggests that S1P1 may regulate physiologic mobilization under steady-state conditions. Furthermore, treatment with the CXCR4 antagonist AMD3100 failed to induce the release of immature B cells from S1P1-deleted mice. The precise mechanism of S1P1 in BM egress remains unclear; a direct chemotactic role appears unlikely, as chemotaxis of immature BM B cells is mediated by a different S1P receptor, S1P3.56,58 Together these findings add S1P1 to the short list of molecules known to regulate mobilization from the BM. It is important to note, however, that these experiments did not address whether S1P1 is involved in regulating BM egress of more primitive progenitors such as HSCs.
In contrast to these limited studies on the physiological mechanisms of progenitor mobilization, substantial work has identified several means of pharmacologic mobilization. The chemokine receptor CXCR2 has been a recent focus of such studies. A single injection of the CXCR2 ligand IL-8 (CXCL8) led to the rapid mobilization of progenitors with long-term multilineage repopulating ability.59 IL-8 likely mediates this effect indirectly through the generation and activation of neutrophils.60 The contents of neutrophil granules contain a variety of proteases that cleave various elements that normally act to retain progenitors in the BM, including vascular cell adhesion molecule 1 (VCAM-1)61, CXCR4,62 and SDF-1α.63 Elastase in particular has been proposed to be the critical neutrophil-derived product that mediates the progenitor mobilization activities of IL-8.64 Additional evidence supporting a role for CXCR2 in progenitor mobilization comes from studies using the CXCR2-specific ligand GROβ. A single administration of a truncated form of this chemokine leads to the mobilization of progenitors into the blood within 15 minutes.65 This chemokine mobilizes progenitors synergistically with granulocyte-colony stimulating factor (G-CSF), a cytokine used clinically to mobilize stem cells.66 Somewhat surprisingly, recipients of progenitors collected after GROβ-mediated mobilization show enhanced post-transplantation neutrophil and platelet recoveries.67 The reasons for this improved reconstitution may be due to enhanced or accelerated BM engraftment by the transferred progenitors. Mobilization with GROβ led to greater numbers of circulating CD34− LSK cells as compared to G-CSF treatment, suggesting that GROβ may be more efficient at mobilizing the most primitive progenitors. Furthermore, GROβ-mobilized progenitors showed increased adhesion to endothelial cells in vitro and more efficient BM homing in vivo than G-CSF-mobilized progenitors. These features of GROβ-mobilized progenitors are of great clinical interest; techniques that improve engraftment after SCT could shorten the period of immunologic susceptibility that currently accompanies this treatment.
Pharmacologic mobilization has been used clinically to collect stem cells for use in both autologous and allogeneic transplantation.68 Current treatment protocols primarily utilize G-CSF as the mobilizing agent, a cytokine combining high mobilization efficiency with relatively minor side effects.69 Interestingly, G-CSF does not act directly on stem cells, but rather on neighboring myeloid cells.70 Similar to the proposed mechanism of IL-8, G-CSF activates neutrophils to release their granule contents, which then digest various molecular tethers keeping progenitors retained in the BM, thereby releasing them.71
Different progenitor types may employ distinct mechanisms to mobilize from the BM.72 While treatment with VEGF can increase the number of circulating endothelial progenitor cells, it fails to mobilize either hematopoietic or stromal progenitors.72 Similarly, G-CSF treatment mobilized hematopoietic and endothelial progenitors, but not stromal progenitors.72 Additional work is needed to identify whether more than one mechanism of mobilization is used among the various hematopoietic progenitor types.
A role for pharmacologic BM progenitor mobilization in enhancing T cell development remains unexplored. The number of progenitors in blood is exceedingly small;43 it is not known whether an increased number of circulating progenitors would lead to a corresponding increase in thymic settling and subsequent T cell production. In other words, it is not clear whether thymic settling is normally “saturated” by the physiologic number of circulating progenitors. Furthermore, T cell reconstitution after stem cell transplantation is slow and this delay correlates with an increased risk of infection.73,74 Could increased progenitor mobilization improve the subsequent T cell recovery in the host (similar to how GROβ-mobilized progenitors accelerate post-transplantation neutrophil and platelet recoveries)?67 Additional research is needed to address these questions.
Progenitors in blood
Following egress from the BM, progenitors enter the circulation. It is presumed that the cells circulate freely, but the extent of their path might be restricted by the very limited amount of time they are in the vascular compartment. More than 90% of injected progenitors are cleared from the circulation in 30 seconds, and this increases to greater than 99% by six minutes.42 Hence progenitors are unlikely to actively control their route through the circulation, and their access to sites distal from the BM may be limited. The clearance out of blood may not be permanent, however, as progenitors may egress and re-enter the circulation repeatedly. The relative efficiency of mobilization by different progenitors is not known, or even whether all early progenitor populations mobilize under physiologic conditions.75 Identifying circulating progenitors is hampered by the extreme infrequency of these cells in blood; for example, there are roughly 120–180 LSK progenitor cells per milliliter of blood in the mouse, corresponding to roughly 1 in 100,000 nucleated cells.42,43 Because of their rarity, these circulating progenitors have often been functionally assessed by placing them into irradiated or otherwise lymphodepleted hosts. While convenient for detecting the progeny of rare cells, such assays may produce conclusions not representative of the physiologic scenario. The differences between normal and post-irradiation thymopoiesis will be discussed later.
HSCs have long been known to circulate, and the detection of more downstream progenitors in blood has become possible with improved flow cytometric techniques and reagents.40,42,43 All LSK subsets – HSCs, MPPs, and LMPPs – are found in blood, and it appears that their mobilization is not selective since the relative frequency of each subset among total LSKs is equivalent between BM and blood.43 Though CLPs were initially not found in blood, several independent groups have more recently been able to detect them.36,38,43,76–78 More differentiated CLP-2 cells, however, have not yet been definitively identified in blood.43,79 The failure to detect these cells may represent insufficiently sensitive assays. Ultimately, the blood contains a heterogeneous array of multipotent progenitors that correspond phenotypically and functionally with their BM counterparts.
LSK subsets and CLPs are multipotent cells, each capable of giving rise to T, B, DC, and myeloid cells (the ALP subset of CLPs can produce small numbers of myeloid cells).29 Yet progenitors restricted to the T lineage can also be found in the blood. T-committed progenitors are present in fetal blood at embryonic day 15.5, and possess a Lin−KitlowThy-1+ phenotype.80 After intrathymic transfer, these cells were able to generate DP thymocytes. They were found in athymic mice as well, demonstrating their extrathymic origin. An adult counterpart to this progenitor possessing a similar KitlowThy-1+ phenotype was identified in 2007 through the use of a transgenic pre-Tα reporter.79 These cells are nearly completely T lineage restricted, having only minor B and NK potential in vitro. Like the fetal progenitors, these adult circulating T progenitors (CTPs) arise independently of the thymus. The origin of these cells remains unclear, though similarly T-restricted progenitors have been detected in the BM.81 The physiologic relevance of these T-committed cells in both the fetal and adult scenario has not been determined. As will described later, the vast majority of the most primitive progenitors in the thymus (early thymic progenitors; ETPs) also have myeloid potential, and a small minority also possess B potential.34,82,83 As such, any contribution to this early pool of multipotent ETPs by extrathymic T-committed progenitors is likely to be minor under physiologic conditions. T-committed precursors may, however, bypass the ETP stage and enter the T developmental pathway at a later point when non-T lineage potential can no longer be detected, such as DN3 stage.83 Such “bypass” pathways of thymic settling may take on a larger role under certain circumstances, such as following irradiation and stem cell transplantation.
Thymic settling
Thymic settling signals
Identifying the physiologic TSPs has proven difficult, primarily due to the presumed low frequency of thymic settling. The inability to directly identify TSPs in the thymus has precluded a direct measurement of their number; several independent approaches have estimated this value to be extremely low perhaps less than 10 per day.2–5 Until several years ago, it was not known whether the process of thymic settling was selective. That is, do all circulating progenitors with T lineage potential settle the thymus or do only a subset of these cells have this capacity? Our lab performed experiments in which various early BM progenitors were sorted and transferred either intravenously or intrathymically into unirradiated congenic hosts.84 While LMPPs and CLPs were able to generate donor CD4+CD8+ DP cells in the thymus three weeks after transfer, HSCs failed to do so. Yet all three populations produced DP cells after intrathymic transfer, implying that HSCs are unable to settle the thymus physiologically but are competent to rapidly produce thymocytes if that step is bypassed. We concluded from these experiments that thymic settling is indeed selective towards circulating progenitors. Such selectivity implies that certain signals are required for thymic entry. Characterizing the key homing and adhesion molecules that support the process of thymic settling has been a useful approach towards the identification of TSPs, as these cells can be expected to reside within the BM progenitor populations expressing most, if not all, such molecules. The following section will describe the signals that are utilized by circulating progenitors to settle the thymus, followed by a discussion on the current TSP candidates.
The settling of rare, rapidly-moving progenitors into the thymus likely requires the coordinated action of several membrane-bound homing and adhesion molecules (Figure 2).84 While direct observation of thymic settling in adult mice has not yet been reported, detailed views of lymph node settling by naïve T cells have been made.85 These studies have revealed that T cells undergo a 3-step process of entry into the lymph nodes.86 A selectin on the T cells interacts with carbohydrate moieties that serve as the selectin ligands. This interaction serves to slow the cell substantially, such that it rolls along the endothelium while being pushed by the force of the bloodflow. Once in close association with the endothelium, the cell has access to chemokines produced and secreted locally by the endothelium. These chemokines signal through their receptors (in the case of naïve T cell, the major chemokine/receptor pair is CCL21/CCR7).87 This signal activates the cell, which in turn leads to the conformation change of integrin dimers on the cell surface from a low- to high-affinity shape and their redistribution on the surface into clusters.86 Once in the high-affinity state, integrins will bind their ligands on the endothelium, firmly arrest the cells, and possibly mediate transmigration through the endothelial wall. It seems likely that circulating progenitors utilize a similar general mechanism to gain thymic entry, although with different molecules.
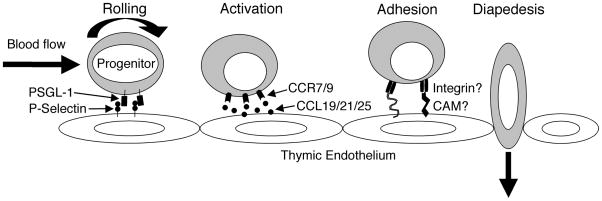
Figure 2. A potential mechanistic model for thymic settling.
TSPs arrive at the thymus via the bloodstream. The interaction between surface PSGL-1 on these progenitors and P-selectin on the thymic endothelial cells slows the progenitors and causes them to roll. By closely associating with the endothelial cells, the progenitors gain access to soluble chemokines available near the vessel wall, including CCL19 and CCL25. These chemokines bind and signal through CCR9 and CCR7 on the progenitors, causing conformational changes in integrin dimers. Once activated, the integrins can now bind their ligands on the endothelial surface with high affinity and mediate firm arrest of the progenitors. This adhesion is followed by the transmigration (diapedesis) of the progenitor through the endothelial layer into the thymic parenchyma where they can proceed down the T cell developmental path. This figure is based on a model described by von Andrian and colleagues.89
As opposed to their naïve T cell progeny which employ L-selectin, TSPs rely on P-selectin for efficient thymic settling.6,88 In this case, P-selectin is expressed by the thymic endothelium and its ligand, PSGL-1, is found on subset of LSKs and CLPs.6 Rossi and colleagues demonstrated the involvement of this signaling axis in thymic settling through elegant parabiosis experiments, in which skin flaps of two mice are sewn together such that they share circulation. These experiments revealed that cells lacking PSGL-1 were highly defective at generating thymocytes in their parabiotic partners. More recent work by this group has reported that only a subset of thymic endothelial cells express P-selectin, and the frequency of these cells is increased in those mice with reduced numbers of early thymic progenitors (ETPs), which are the earliest defined T cell precursors in the thymus.88 In addition to variability across strains, total thymic P-selectin levels oscillated over time with a periodicity of roughly 2 weeks. These data suggest the presence of a feedback mechanism in which the hematpoietic progenitor content of the thymus (and perhaps other factors) may regulate the P-selectin expression on thymic endothelial cells. It remains unclear, however, whether an increased frequency of P-selectin+ thymic endothelial cells actually translates into more efficient thymic setting by circulating progenitors.
P-selectin likely mediates the rolling of progenitors along the thymic endothelium. The described model of leukocyte settling indicates that chemokines are then used to induce firm arrest of the cells. Indeed, two chemokine receptors, CCR7 and CCR9, have demonstrated roles in thymic settling.7–9,36,89–94 The ligand for CCR9, CCL25 (earlier known as thymic-expressed chemokine), is expressed throughout the thymic stroma.95 The absence of CCR9 was first found to confer a defect in thymic reconstitution of Rag1−/− mice.91 Later experiments investigated whether CCR9 is used for recruitment of circulating progenitors or for early intrathymic development of settled progenitors. CCR9−/− BM is defective at generating thymocytes after intravenous but not intrathymic transfer, localizing CCR9 involvement to the thymic settling step.7 Recent evidence suggests that the expression of CCL25, like P-selectin, is increased in some mouse strains with reduced thymic progenitor content and may oscillate over time.88 The precise function of CCR9 in thymic settling has not been determined, but it is reasonable to expect that CCR9 signals convert integrins to their high-affinity state to mediate firm arrest at the endothelium.
Despite the absence of a key molecule in thymic settling, CCR9−/− mice still retain some inefficient thymic settling – demonstrated most simply by the fact that these mice still generate thymocytes.7,91 This observation indicated that additional molecules, perhaps including other chemokine receptors, were likely involved in supporting thymic settling. CCR7 was known to regulate the recruitment of fetal liver progenitors into the thymic enlage, but a role after this developmental period had not been demonstrated.92,94 Our group and another have recently reported that CCR7 supports thymic settling in the adult scenario.8,9 Similar to CCR9−/− BM, CCR7−/− BM is defective at generating donor thymocytes after intravenous, but not intrathymic transfer.8,9 These findings indicate that CCR7 has an independent role in aiding thymic settling, even when CCR9 is present. CCR7/CCR9 double knock-out (DKO) mice were bred to test whether CCR9 and CCR7 each retained thymic settling in the absence of the other. CCR7/CCR9 DKO BM is almost completely restricted from thymic settling when in competition with WT BM.8,9 Consistent with a severe thymic settling defect even under non-competitive conditions, CCR7/CCR9 DKO mice have approximately 100-fold fewer ETPs than WT mice.8,9 Despite this reduction, CCR7/CCR9 DKO mice have thymi of near-normal cellularity; compensatory proliferation by intrathymic progenitors appears to account for this recovery.8,9 Perhaps the most important conclusion from these studies is that under competitive conditions TSPs appear to require expression of CCR7, CCR9, or both in order to contribute to T cell generation. The heterogeneous pool of BM progenitors can now be “filtered” on the basis of CCR7 and CCR9 expression to identify potential TSP populations, which will be discussed later.
Several other molecules have described roles in thymic settling. Limited evidence implicates the integrin dimers α4β1 (VLA-4) and αLβ2 (LFA-1), but additional investigation is needed in this area.89 A more recent report suggested that the glycan polysialic acid may also regulate BM progenitor access to the thymus.96 Mice deficient for sialyltransferase 8Sia IV (ST8Sia IV), which is responsible for polysialic acid expression on hematopoietic cells, possess reduced numbers of thymic progenitors. In competitive mixed BM chimeras, ST8Sia IV−/− cells are defective at generating thymocytes. ST8Sia IV−/− BM produces normal numbers of donor thymocytes after intrathymic injection, however, indicating that the observed defects are prethymic. Future studies are necessary to determine whether the absence of ST8Sia IV confers a defect in BM mobilization or thymic settling.
Identifying TSP candidates
Identifying the specific progenitors that settle the thymus from blood has remained an elusive goal. Two overlapping approaches have been utilized towards this end. The first of these has been to pinpoint the surface phenotype of the TSP with improved resolution. Among the BM progenitors with T lineage potential, the cytokine receptor Flt3 is expressed only on MPPs, LMPPs, CLPs, and CLP-2s.7,21,23,26 This serves as convenient marker with which to classify populations and interrogate their thymic settling capacity. Experiments by three independent groups have localized nearly all thymic settling capacity to within this Flt3+ pool.7,77,78 Such observations predict that HSCs (Flt3−LSK) should not be able to directly settle the thymus. Indeed this is the case; HSCs rapidly generate thymocytes only after intrathymic, but not intravenous, transfer.7 Additional phenotypic “filtering” can rely on the homing molecules integral for thymic settling. PSGL-1 is not particularly useful in identifying likely TSPs, as two-thirds of LSK cells and CLPs have functional PSGL-1; therefore eliminating PSGL-1− cells from consideration fails to significantly shorten the list of candidates.6 Yet as noted earlier, the near-absolute defect of CCR7/CCR9 DKO cells to settle the thymus reveals that the physiologic thymic settling progenitor expresses one or both of these receptors.8,9 Among MPPs, LMPPs, and CLPs, only the latter two populations have expression of CCR7 or CCR9. Specifically, about 40% of LMPPs and 60% of CLPs have detectable surface CCR7.8,9 CCR9 expression is restricted to rare subsets of these CCR7+ LMPPs and CLPs.7–9,36 Thymic settling is most efficient when both CCR7 and CCR9 are present,7–9 so it is reasonable to predict that TSPs reside within the CCR7+CCR9+ LMPP or CLP pool.
Is the physiologic TSP exclusively within the LMPP pool, the CLP pool, or both? This question has received some attention in recent years.75,97 LMPPs and CLPs can be distinguished phenotypically by Kit expression, as LMPPs are Kithigh and CLPs are Kitlow. Recent investigations have also gated LMPPs as IL-7Rα− and CLPs as IL-7Rα+,77 although IL-7Rα expression status was not part of the original definition for LMPPs.23 Functionally these two populations differ in their myeloid potential: LMPPs have myeloid generation capacity, while CLPs have very little.23,29 LMPPs and CLPs will both generate donor DP thymocytes three weeks after transfer into unirradiated hosts,7 but at this timepoint it cannot be determined whether the injected cells settled the thymus immediately after injection (direct settling) or whether the injected cells first engrafted the BM and then more downstream progeny settled the thymus (indirect settling).77,98 Yet examining the recipient thymus at earlier timepoints reveals too few donor cells to be a reliable measure of thymic settling ability. To overcome this challenge, two groups have first intravenously injected sorted progenitors, waited a short period of time (five to six days), and then sorted and cultured the rare donor thymocytes using the OP9-DL1 stromal cell system which supports T cell development.77,78,99 Following a week or more of culture, the expanded donor population can be reliably detected. Serwold and colleagues found detected donor thymocytes in 70% of mice receiving CLPs, and only in 10% of mice receiving Flt3+LSKs (including both MPPs and LMPPs).77 They concluded that CLPs were the primary TSP population, and the contribution of LMPPs to T cell development was probably indirect through the generation of daughter CLPs in the BM. Yet more recently a second group found that after depletion of either CLPs or LMPPs, remaining BM progenitors directly settled the thymus after intravenous transfer in both cases.78 These data support a model in which TSPs reside in both the LMPP and the CLP populations. The contradictory results between these two reports may have resulted from different sorting strategies used by the two groups; for example, Serwold and colleagues included some Kithigh cells in their CLP gate that would have been regarded as LMPPs by other investigators, including Saran and colleagues.7,9,77,78
The second major approach towards identification of TSPs has been to clarify the lineage potential of the earliest progenitors in the thymus. Obviously these cells carry T lineage potential, but it has been appreciated for more than a decade that ETPs are a multipotent population, holding B, NK, DC, and myeloid potential.5,34,100–104 According to the hierarchical model of hematopoiesis, progenitors lose lineage potentials in a sequential and irreversible manner. The possession of non-T potential among ETPs predicts that the more upstream TSP population must also possess these same potentials. This offers a convenient method with which to “screen” TSP candidates. We recently found that the vast majority of ETPs possess myeloid potential and produce granulocytes in vivo, indicating they originated from myelolyphoid progenitors.82,83 Recent work with human cells has also established the existence of analogous lymphoid progenitors with myeloid potential.105 This stands to effectively eliminate lymphoid restricted cells as substantial contributors to the ETP pool; however, they may yet contribute to T cell development if they bypass the ETP stage.106 CLPs were initially proposed to be devoid of myeloid potential,25,107 but more recently they have been shown to possess some myeloid potential in vitro.26–30 Furthermore, the more primitive Ly6D− CLP subset (also called ALPs) have been proposed to generate small numbers of myeloid cells in vivo; however, this population included some Kithi cells which may have been responsible for the observed myeloid potential.29 This demonstration of myeloid potential within the ersthwhile CLP population has blurred the line between LMPPs and some CLPs. As a result, these Ly6D− progenitors are potential TSPs. Additional segmentation of the heterogenous LMPP and CLP populations seems likely and could serve to clarify the identity of the physiologic TSP.
Thymopoiesis following stem cell transplantation
Physiologic mechanisms of T cell recovery following SCT
Gamma irradiation has been used for decades to ablate the hematopoietic system in the course of treatment for cancer and other diseases. Hematopoietic reconstitution depends on the transfer of HSCs, which have long-term repopulating capacity, into the irradiated hosts. Stem cell transplantion (SCT) has been used successfully as a curative therapy for many illnesses, particulary hematologic malignancies and severe immunodeficiencies. This therapy has significant risks, one of which is the period following transplantation during which the immune system is not yet reconstituted. T cell recovery following SCT can be particularly slow and limiting towards immune function, often taking years for T cell numbers to return to normal levels.73,74,108 Indeed, the T lineage is the slowest hematopoietic lineage to recover following SCT in humans.108 This delay in T lineage restoration is not insignificant; the risk of transplant related mortality and of developing life-threatening infections are inversely correlated CD4+ T recovery.109,110 Improved knowledge about T cell reconstitution could have far-reaching implications towards developing new protocols or therapies that would moderate the immunodeficiency that currently follows SCT.
Why is T cell mediated immunity so poor after transplantation? Following transplantation, T cell numbers recover through two distinct mechanisms.111 The first is the homeostatic expansion of both the rare radio-resistant host T cells and donor T cells provided in the transferred graft.111–114 These mature cells, particularly CD8+ cells, proliferate in response to cytokines Il–7 and Il-15.111 This cytokine-driven expansion provides the first wave of T cells following transplantation, but this initial partial recovery of T cell numbers fails to fully restore T-mediated immune protection because these cells are functionally deficient.111,112,115–117 Such peripheral expansion of mature T cells is not unique to the post-transplantion scenario; indeed, such mechanisms are thought to occur in lymphopenic situations and with normal aging.118 This process maintains normal numbers of T cells with age, but at the expense of function. Aged cells derived through peripheral homeostatic mechanisms demonstrate defects in antigen-induced division and IL-2 production.119 It is not surprising, therefore, that the T cells detected early after transplant are similarly functionally deficient.115–117 The basis for their impaired function has been proposed to include the limited diversity of T-cell receptors within this population, susceptibilty to replicative senescence, and the low expression of homing molecules that recruit T cells into lymph nodes where they can organize immune responses.111,120–122
The second pathway of T cell reconstitution is the de novo generation of naïve T cells in the thymus. Restoration of full T-mediated immunity following SCT relies on this thymic output of donor-derived T cells, which are self-tolerant and clonally diverse.122–126 Despite the immunologic importance of generating thymus-derived T cells following SCT, the process by which the donor graft generates such cells in the recipient is poorly understood. Thymus-derived T cells are very slow to appear after SCT, particularly in adults, and the delay in the generation of thymus-derived cells can contribute to post-SCT morbidity and mortality.73,126 The reasons for this delay are not fully understood, but may reflect defects of both the thymic stromal compartment and hematopoietic progenitors. Most transplants are performed on adults with thymi that are already substantially involuted even before transplantation.127 The minimal residual thymic tissue is further damaged by both the pre-transplant conditioning regimens and graft-versus-host disease after transplant.128,129 This combination of involution and damage thus leaves the thymus unable to generate substantial numbers of large numbers of new T cells.130 This observation has led to efforts to protect the thymus from the deleterious effects of conditioning using keratinocyte growth factor (KGF). While KGF is not required to achieve normal thymic size, KGF-deficient thymi are more susceptible to radiation-induced damage.131 As a result, KGF has been used successfully to protect the thymus from conditioning-induced damage and thereby enhance T lineage reconstitution following transplantation, as will be discussed below.132
Other factors outside the thymus may also impair T lineage recovery after SCT. Myeloablative conditioning destroys the host hematopoietic progenitors, and a full recovery of these cells after transplant likely takes months. The number of progenitors available to settle the thymus and give rise to T cells is thus reduced early after transplantation. Furthermore, the BM is nearly acellular in the early weeks after transplantation in mice. Till and McCulloch’s classic experiments demonstrated that hematopoesis can occur outside the BM shortly after SCT.133 It remains unclear whether the spleen environment is capable of generating TSPs with comparable efficiency as the BM.
Generation of donor-derived T cells following SCT improves overall T mediated immunity, but relatively little is known about how intrathymic T cell development following SCT may differ from the normal unirradiated scenario.125,126 Irradiation destroys most hematopoietic cells in the host, but has many effects on non-hematopoetic tissue as well. The thymic environment, and particularly the cytokine environment, undergoes acute changes following irradiation.7,134 Transcripts for IL-7, a key cytokine for lymphoid development, are upregulated 5-fold among the epithelial compartment (CD45−MHC II+) 3 days after sublethal irradiation.135 The chemokines CCL21 (ligand for CCR7) and SDF-1α (CXCL12; ligand for CXCR4) are upregulated as well.135 Flt3 ligand (FL) is also upregulated in a dose-dependent manner in the days following irradiation on thymic fibroblasts and stromal cells, but not epithelial cells.136 This upregulation peaks around day five following treatment and then returns to normal levels. Availability of FL following lethal irradiation is beneficial towards thymic reconstitution;136 administration of exogenous FL enhanced improved T lineage recovery, indicating that FL may be limiting physiologically.137
In addition to these non-hematopoietic changes following irradiation, the hematopoietic compartment of the thymus is dramatically altered in the acute period following irradiation. Of particular interest is that fact that donor ETPs and DN2 cells are nearly absent for the first 3–4 weeks following irradiation and BMT.138 Donor DN3 cells, however, can be readily detected at two weeks; the source of these DN3 cells remains a mystery. A first hypothesis is that the numbers of ETPs and DN2s are reduced so far as to be nearly undetectable, but are in fact still present and give rise to the downstream DN3 cells. An alternative possibility is that TSPs after irradiation feed directly into the DN3 pool, thus bypassing the ETP and DN2 stages. This second hypothesis has gained strength through the identification of candidate progenitors in the periphery with a DN3-like phenotype. Ezine’s group first identified this population of T-restricted splenic cells present 12 days after irradiation with a Lin−CD44−/lowThy1+ phenotype.139 The generation of these cells is not dependent on the thymus, and they rapidly produce DP thymocytes after intrathymic or intravenous transfer into sublethally irradiated mice.139 Additional studies showed that the number of splenic pre-T (SpT) cells peaks two weeks after transplantation and subsequently returns to basal levels by week four.138 The generation of SpT is completely dependent on extrathymic Notch signals, and these cells generate T lineage progeny in vitro with similar kinetics as DN3 cells.138 Despite these findings, the in vivo relevance of these cells towards generating T cells after irradiation remains unclear.
The mechanisms of thymic settling following irradiation are poorly understood. Do the same TSPs that normally settle the thymus continue to do so after irradiation? Are the same signals – PSGL-1, CCR7, and CCR9 – used? Is thymic settling efficiency altered following irradiation? It is unclear even whether post-SCT thymic settling remains an active, selective process, as in the unirradiated scenario. If indeed the process continues to be selective, the identification of the critical homing signals could lead to efforts to increase the efficiency of thymic settling following SCT and thereby accelerate and enhance T lineage reconstitution.
Strategies to enhance T lineage reconstitution after BM transplantation
Given the clinical importance of T cell-mediated immunity after SCT, substantial work has been aimed towards enhancing the recovery of the T cell compartment following transplantation. These attempts can generally be grouped into two categories: the alteration of the cytokine and hormone milieu, and the adoptive transfer of T cells or their precursors into transplanted hosts. Within the first category, sex steroid ablation has been shown to improve T lineage reconstitution after transplantation in mice.140,141 Additionally, chemical castration in humans also leads to enhanced T lineage recovery.142,143 The administration of exogenous hormones and cytokines can improve T cell reconstitution as well. Treatment with growth hormone (GH) or its downstream mediator insulin-like growth factor 1 (IGF-1) can each enhance T lineage recovery, perhaps by increasing the de novo generation of T cells from the thymus rather than simply enhancing peripheral expansion of mature cells.144–146 Additionally, the cytokines IL-7, keratinocyte growth factor (KGF), and Flt3 ligand (FL) have each been used successfully to improve T cell reconstitution.132 Moreover, at least part of the therapeautic mechanism of these cytokines appears to be increased thymic output of new donor T cells.132,147
In addition to these efforts at enhancing the physiologic mechanisms of post-SCT T lineage recovery, the second major strategy has been the direct transfer of T cells or their precursors into the hosts. The direct transfer of mature T cells can increase T cell numbers in the months following transplant, but the clinical advantage of such increases remains unclear.148,149 This approach has several intrinsic difficulties, including the risk of graft-versus-host disease (GVHD), the limited number of cells that can be practically obtained, and the absence of ex vivo culture methods to activate these cells prior to transfer.148 To circumvent these challenges, T lineage progenitors, rather than mature T cells, can be transferred into hosts as a means of enhancing reconstituion. The co-transfer of CLPs alongside HSCs into irradiated hosts can improve early T lineage recovery.150 Van den Brink’s group has successfully used the OP9-DL1 culture system to generate large numbers of T-committed precursors which improve peripheral T cell numbers after transfer into irradiated mice.151 These precursor-derived T cells are host-tolerant, and importantly have normal TCR repertoires, cytokine production, and antigen-driven proliferation. The quantitative increase in donor T cells has clinical impact as well, including improved resistance to Listeria infection and enhanced graft-verus-tumor responses.151 The generation of T cell precursors form human CD34+ cord blood cells using Notch signaling in vitro has also been demonstrated.152 We can therefore be optimistic that the co-transfer of T lineage progenitors, whether derived from donor BM or in vitro culture systems, may soon offer enhanced and accelerated T cell reconstitution following SCT.
CONCLUSION
T cells uniquely complete their development in an organ physically separate from the BM. This requires that the progenitors that ultimately contribute to T lymphopoiesis depart the BM, travel through the circulation, and finally settle in the thymus. A variety of primitive progenitors with T lineage potential reside in the BM. It remains unclear how these cells are mobilized into the peripheral blood, and whether this process acts on all of these progenitors or only a subset. Whatever the case, rare LSKs and even more downstream CLPs can be found in the blood.36,38,43,76–78 Considering the remarkable selectivity displayed in LN settling, perhaps it is not surprising that circulating progenitors employ critical surface molecules for thymic entry.7,86 Despite growing knowledge about thymic settling mechanisms, the identity of the physiologically relevant TSPs has not yet been determined.
Why is it important to discern the nature of the TSP? T cells are critically important immune mediators; in their absence, immunity to a variety of pathogens, particularly some viruses and fungi, is greatly diminished. Certain populations suffer from reduced numbers of T cells, particularly HIV/AIDS patients, those recovering from irradiation and SCT, and the elderly.73,153 Improving T cell mediated immunity would provide greater protection from infections that cause significant morbidity and mortality in these groups. This review has discussed how the rate of thymic settling is likely to be very low. Determining the signals used to recruit progenitors into the thymus could lead to efforts to increase the rate of thymic settling. This might be accomplished pharmacologically or via cell-based protocols. Such efforts could potentially increase T cell production, particularly in patients recovering from irradiation-induced cytoablation. Ongoing work on the cells and signals involved in thymic settling, both in the physiologic and post-SCT scenario, could bring about significant public health benefits in the near future.
Acknowledgments
We thank Jeremiah Bell for his comments. This work was supported by NIH grants HL099271 (D.A.Z), AI059621, HL086900, and HL099758 (A.B.), and a Scholar Award from the Leukemia and Lymphoma Society (A.B.).
References
- 1.Aguila HL, et al. From stem cells to lymphocytes: biology and transplantation. Immunol Rev. 1997;157:13–40. doi: 10.1111/j.1600-065x.1997.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 2.Wallis VJ, Leuchars E, Chwalinski S, Davies AJ. On the sparse seeding of bone marrow and thymus in radiation chimaeras. Transplantation. 1975;19:2–11. doi: 10.1097/00007890-197501000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kadish JL, Basch RS. Hematopoietic thymocyte precursors. I. Assay and kinetics of the appearance of progeny. J Exp Med. 1976;143:1082–1099. doi: 10.1084/jem.143.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–3668. [PubMed] [Google Scholar]
- 5.Ceredig R, Bosco N, Rolink AG. The B lineage potential of thymus settling progenitors is critically dependent on mouse age. Eur J Immunol. 2007;37:830–837. doi: 10.1002/eji.200636728. [DOI] [PubMed] [Google Scholar]
- 6.Rossi FM, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz BA, et al. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 8.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 9.Zlotoff DA, et al. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zediak VP, Maillard I, Bhandoola A. Multiple prethymic defects underlie age-related loss of T progenitor competence. Blood. 2007;110:1161–1167. doi: 10.1182/blood-2007-01-071605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scollay R, Smith J, Stauffer V. Dynamics of early T cells: prothymocyte migration and proliferation in the adult mouse thymus. Immunol Rev. 1986;91:129–157. doi: 10.1111/j.1600-065x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 12.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- 13.Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 16.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 17.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 18.Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 19.Lymperi S, Ferraro F, Scadden DT. The HSC niche concept has turned 31. Has our knowledge matured? Ann N Y Acad Sci. 2010;1192:12–18. doi: 10.1111/j.1749-6632.2009.05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 21.Adolfsson J, et al. Upregulation of Flt3 Expression within the Bone Marrow Lin(−)Sca1(+)c- kit(+) Stem Cell Compartment Is Accompanied by Loss of Self-Renewal Capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 22.Smith LG, Weissman IL, Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991;88:2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Igarashi H, Gregory S, Yokota T, Sakaguchi N, Kincade P. Transcription from the RAG1 Locus Marks the Earliest Lymphocyte Progenitors in Bone Marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 25.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 26.Balciunaite G, Ceredig R, Massa S, Rolink AG. A B220+ CD117+ CD19-hematopoietic progenitor with potent lymphoid and myeloid developmental potential. Eur J Immunol. 2005;35:2019–2030. doi: 10.1002/eji.200526318. [DOI] [PubMed] [Google Scholar]
- 27.Rumfelt LL, Zhou Y, Rowley BM, Shinton SA, Hardy RR. Lineage specification and plasticity in CD19- early B cell precursors. J Exp Med. 2006;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu M, et al. The earliest thymic progenitors in adults are restricted to T, NK, and dendritic cell lineage and have a potential to form more diverse TCRbeta chains than fetal progenitors. J Immunol. 2005;175:5848–5856. doi: 10.4049/jimmunol.175.9.5848. [DOI] [PubMed] [Google Scholar]
- 29.Inlay MA, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansson R, et al. Single cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2009 doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 31.Welner RS, et al. Asynchronous RAG-1 expression during B lymphopoiesis. J Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin CH, et al. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- 33.Harman BC, Northrup DL, Allman D. Resolution of unique Sca-1highc-Kit-lymphoid-biased progenitors in adult bone marrow. J Immunol. 2008;181:7514–7524. doi: 10.4049/jimmunol.181.11.7514. [DOI] [PubMed] [Google Scholar]
- 34.Sambandam A, et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 35.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 36.Lai AY, Kondo M. Identification of a bone marrow precursor of the earliest thymocytes in adult mouse. Proc Natl Acad Sci U S A. 2007;104:6311–6316. doi: 10.1073/pnas.0609608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 38.Perry SS, Welner RS, Kouro T, Kincade PW, Sun XH. Primitive lymphoid progenitors in bone marrow with T lineage reconstituting potential. J Immunol. 2006;177:2880–2887. doi: 10.4049/jimmunol.177.5.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan JB, Visan I, Yuan JS, Guidos CJ. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat Immunol. 2005;6:671–679. doi: 10.1038/ni1217. [DOI] [PubMed] [Google Scholar]
- 40.Goodman JW, Hodgson GS. Evidence for Stem Cells in the Peripheral Blood of Mice. Blood. 1962;19:702–714. [PubMed] [Google Scholar]
- 41.Dorie MJ, Maloney MA, Patt HM. Turnover of circulating hematopoietic stem cells. Exp Hematol. 1979;7:483–489. [PubMed] [Google Scholar]
- 42.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 44.Schulz C, von Andrian UH, Massberg S. Hematopoietic stem and progenitor cells: their mobilization and homing to bone marrow and peripheral tissue. Immunol Res. 2009;44:160–168. doi: 10.1007/s12026-009-8109-6. [DOI] [PubMed] [Google Scholar]
- 45.Lee Y, et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood. 2002;99:4307–4317. doi: 10.1182/blood.v99.12.4307. [DOI] [PubMed] [Google Scholar]
- 46.Broxmeyer HE, Cooper S, Hangoc G, Kim CH. Stromal cell-derived factor-1/CXCL12 selectively counteracts inhibitory effects of myelosuppressive chemokines on hematopoietic progenitor cell proliferation in vitro. Stem Cells Dev. 2005;14:199–203. doi: 10.1089/scd.2005.14.199. [DOI] [PubMed] [Google Scholar]
- 47.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 48.Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 49.Ponomaryov T, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 51.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 52.Ara T, et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 53.Hattori K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 54.Broxmeyer HE, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 56.Allende ML, et al. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiba K, Matsuyuki H, Maeda Y, Sugahara K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol. 2006;3:11–19. [PubMed] [Google Scholar]
- 58.Donovan EE, Pelanda R, Torres RM. S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur J Immunol. 2010;40:688–698. doi: 10.1002/eji.200939858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 1995;85:2269–2275. [PubMed] [Google Scholar]
- 60.Pruijt JF, et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci U S A. 2002;99:6228–6233. doi: 10.1073/pnas.092112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 62.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 64.van Pel M, et al. Serpina1 is a potent inhibitor of IL-8-induced hematopoietic stem cell mobilization. Proc Natl Acad Sci U S A. 2006;103:1469–1474. doi: 10.1073/pnas.0510192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.King AG, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001;97:1534–1542. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- 66.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 67.Fukuda S, Bian H, King AG, Pelus LM. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 2007;110:860–869. doi: 10.1182/blood-2006-06-031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levesque JP, Winkler IG. Mobilization of hematopoietic stem cells: state of the art. Curr Opin Organ Transplant. 2008;13:53–58. doi: 10.1097/MOT.0b013e3282f42473. [DOI] [PubMed] [Google Scholar]
- 69.Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr Opin Hematol. 2002;9:183–189. doi: 10.1097/00062752-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95:3025–3031. [PubMed] [Google Scholar]
- 71.Levesque JP, et al. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 72.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 73.Mackall CL, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 74.Hakim FT, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Umland O, Mwangi WN, Anderson BM, Walker JC, Petrie HT. The blood contains multiple distinct progenitor populations with clonogenic B and T lineage potential. J Immunol. 2007;178:4147–4152. doi: 10.4049/jimmunol.178.7.4147. [DOI] [PubMed] [Google Scholar]
- 77.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saran N, et al. Multiple extrathymic precursors contribute to T-cell development with different kinetics. Blood. 2010;115:1137–1144. doi: 10.1182/blood-2009-07-230821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krueger A, von Boehmer H. Identification of a T lineage-committed progenitor in adult blood. Immunity. 2007;26:105–116. doi: 10.1016/j.immuni.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodewald HR, Kretzschmar K, Takeda S, Hohl C, Dessing M. Identification of pro-thymocytes in murine fetal blood: T lineage commitment can precede thymus colonization. Embo J. 1994;13:4229–4240. doi: 10.1002/j.1460-2075.1994.tb06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dejbakhsh-Jones S, Garcia-Ojeda ME, Chatterjea-Matthes D, Zeng D, Strober S. Clonable progenitors committed to the T lymphocyte lineage in the mouse bone marrow; use of an extrathymic pathway. Proc Natl Acad Sci U S A. 2001;98:7455–7460. doi: 10.1073/pnas.131559798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wada H, et al. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 83.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 84.Schwarz BA, Bhandoola A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunol Rev. 2006;209:47–57. doi: 10.1111/j.0105-2896.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 85.Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 86.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 87.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 88.Gossens K, et al. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206:761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wurbel MA, Malissen B, Campbell JJ. Complex regulation of CCR9 at multiple discrete stages of T cell development. Eur J Immunol. 2006;36:73–81. doi: 10.1002/eji.200535203. [DOI] [PubMed] [Google Scholar]
- 91.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 92.Liu C, et al. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood. 2005;105:31–39. doi: 10.1182/blood-2004-04-1369. [DOI] [PubMed] [Google Scholar]
- 93.Bajoghli B, et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 94.Liu C, et al. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 95.Misslitz A, et al. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drake PM, et al. Polysialic acid governs T-cell development by regulating progenitor access to the thymus. Proc Natl Acad Sci U S A. 2009;106:11995–12000. doi: 10.1073/pnas.0905188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Izon DJ. T-cell development: thymus-settling progenitors: settled? Immunol Cell Biol. 2008;86:552–553. doi: 10.1038/icb.2008.50. [DOI] [PubMed] [Google Scholar]
- 98.Boehm T, Bleul CC. Thymus-homing precursors and the thymic microenvironment. Trends Immunol. 2006;27:477–484. doi: 10.1016/j.it.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 100.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 101.Balciunaite G, Ceredig R, Rolink AG. The earliest subpopulation of mouse thymocytes contains potent T, significant macrophage, and natural killer cell but no B-lymphocyte potential. Blood. 2005;105:1930–1936. doi: 10.1182/blood-2004-08-3087. [DOI] [PubMed] [Google Scholar]
- 102.Allman D, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 103.Wu L, Antica M, Johnson GR, Scollay R, Shortman K. Developmental potential of the earliest precursor cells from the adult mouse thymus. J Exp Med. 1991;174:1617–1627. doi: 10.1084/jem.174.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hao QL, et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7- lympho-myeloid thymic progenitors. Blood. 2008;111:1318–1326. doi: 10.1182/blood-2007-08-106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doulatov S, et al. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 106.Krueger A, Garbe AI, von Boehmer H. Phenotypic plasticity of T cell progenitors upon exposure to Notch ligands. J Exp Med. 2006;203:1977–1984. doi: 10.1084/jem.20060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–5570. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Storek J, et al. Recovery from and consequences of severe iatrogenic lymphopenia (induced to treat autoimmune diseases) Clin Immunol. 2004;113:285–298. doi: 10.1016/j.clim.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berger M, et al. Lymphocyte subsets recovery following allogeneic bone marrow transplantation (BMT): CD4+ cell count and transplant-related mortality. Bone Marrow Transplant. 2008;41:55–62. doi: 10.1038/sj.bmt.1705870. [DOI] [PubMed] [Google Scholar]
- 110.Small TN, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 111.Storek J, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 112.Aqui NA, June CH. Post-transplant adoptive T-cell immunotherapy. Best Pract Res Clin Haematol. 2008;21:503–519. doi: 10.1016/j.beha.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roux E, et al. Analysis of T-cell repopulation after allogeneic bone marrow transplantation: significant differences between recipients of T-cell depleted and unmanipulated grafts. Blood. 1996;87:3984–3992. [PubMed] [Google Scholar]
- 114.Alpdogan O, et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beatty GL, et al. Functional unresponsiveness and replicative senescence of myeloid leukemia antigen-specific CD8+ T cells after allogeneic stem cell transplantation. Clin Cancer Res. 2009;15:4944–4953. doi: 10.1158/1078-0432.CCR-08-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Witherspoon RP, Lum LG, Storb R, Thomas ED. In vitro regulation of immunoglobulin synthesis after human marrow transplantation. II. Deficient T and non-T lymphocyte function within 3–4 months of allogeneic, syngeneic, or autologous marrow grafting for hematologic malignancy. Blood. 1982;59:844–850. [PubMed] [Google Scholar]
- 117.Ferrara JL, Daley JP, Burakoff SJ, Miller RA. Functional T cell deficits after bone marrow transplantation across minor histocompatibility barriers: effects of graft-vs-host disease on precursor frequency of reactive cells. J Immunol. 1987;138:3598–3603. [PubMed] [Google Scholar]
- 118.Swain S, Clise-Dwyer K, Haynes L. Homeostasis and the age-associated defect of CD4 T cells. Semin Immunol. 2005;17:370–377. doi: 10.1016/j.smim.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsukamoto H, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 121.Mackall CL, et al. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 122.Roux E, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96:2299–2303. [PubMed] [Google Scholar]
- 123.Dumont-Girard F, et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood. 1998;92:4464–4471. [PubMed] [Google Scholar]
- 124.Gandhi MK, et al. Late diversification in the clonal composition of human cytomegalovirus-specific CD8+ T cells following allogeneic hemopoietic stem cell transplantation. Blood. 2003;102:3427–3438. doi: 10.1182/blood-2002-12-3689. [DOI] [PubMed] [Google Scholar]
- 125.Heitger A, et al. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90:850–857. [PubMed] [Google Scholar]
- 126.Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–78. doi: 10.1111/j.1600-065x.1997.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 127.Zediak VP, Bhandoola A. Aging and T cell development: Interplay between progenitors and their environment. Semin Immunol. 2005;17:337–346. doi: 10.1016/j.smim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 128.Douek DC, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 129.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19:318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krenger W, Hollander GA. The immunopathology of thymic GVHD. Semin Immunopathol. 2008;30:439–456. doi: 10.1007/s00281-008-0131-6. [DOI] [PubMed] [Google Scholar]
- 131.Alpdogan O, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chidgey AP, Seach N, Dudakov J, Hammett MV, Boyd RL. Strategies for reconstituting and boosting T cell-based immunity following haematopoietic stem cell transplantation: pre-clinical and clinical approaches. Semin Immunopathol. 2008;30:457–477. doi: 10.1007/s00281-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 133.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 134.Chung B, Barbara-Burnham L, Barsky L, Weinberg K. Radiosensitivity of thymic interleukin-7 production and thymopoiesis after bone marrow transplantation. Blood. 2001;98:1601–1606. doi: 10.1182/blood.v98.5.1601. [DOI] [PubMed] [Google Scholar]
- 135.Zubkova I, Mostowski H, Zaitseva M. Up-regulation of IL-7, stromal-derived factor-1alpha, thymus-expressed chemokine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J Immunol. 2005;175:2321–2330. doi: 10.4049/jimmunol.175.4.2321. [DOI] [PubMed] [Google Scholar]
- 136.Kenins L, Gill JW, Boyd RL, Hollander GA, Wodnar-Filipowicz A. Intrathymic expression of Flt3 ligand enhances thymic recovery after irradiation. J Exp Med. 2008;205:523–531. doi: 10.1084/jem.20072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wils EJ, et al. Flt3 ligand expands lymphoid progenitors prior to recovery of thymopoiesis and accelerates T cell reconstitution after bone marrow transplantation. J Immunol. 2007;178:3551–3557. doi: 10.4049/jimmunol.178.6.3551. [DOI] [PubMed] [Google Scholar]
- 138.Maillard I, et al. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–3519. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lancrin C, et al. Major T cell progenitor activity in bone marrow-derived spleen colonies. J Exp Med. 2002;195:919–929. doi: 10.1084/jem.20011475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goldberg GL, et al. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- 141.Goldberg GL, et al. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- 142.Sutherland JS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 143.Sutherland JS, et al. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res. 2008;14:1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]
- 144.Jardieu P, Clark R, Mortensen D, Dorshkind K. In vivo administration of insulin-like growth factor-I stimulates primary B lymphopoiesis and enhances lymphocyte recovery after bone marrow transplantation. J Immunol. 1994;152:4320–4327. [PubMed] [Google Scholar]
- 145.Alpdogan O, et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 146.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31:953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 147.Zakrzewski JL, Goldberg GL, Smith OM, van den Brink MR. Enhancing T cell reconstitution after hematopoietic stem cell transplantation: a brief update of the latest trends. Blood Cells Mol Dis. 2008;40:44–47. doi: 10.1016/j.bcmd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Porter DL, June CH. T-cell reconstitution and expansion after hematopoietic stem cell transplantation: ‘T’ it up! Bone Marrow Transplant. 2005;35:935–942. doi: 10.1038/sj.bmt.1704953. [DOI] [PubMed] [Google Scholar]
- 149.Amrolia PJ, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–1808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arber C, et al. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 151.Zakrzewski JL, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 152.Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(−) cord blood cells. J Clin Invest. 2002;110:1165–1174. doi: 10.1172/JCI16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jenkins M, Hanley MB, Moreno MB, Wieder E, McCune JM. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multilineage hematopoiesis in vivo. Blood. 1998;91:2672–2678. [PubMed] [Google Scholar]