Abstract
Anemia is a common finding among patients with chronic heart failure. Although co-morbidities, such as kidney failure, might contribute to the pathogenesis of anemia, many patients with heart failure do not have any other obvious etiology for their anemia. We investigated whether anemia in heart failure is associated with an elevation in hepcidin concentration.
We used time-of-flight mass spectrometry to measure hepcidin concentration in urine and serum samples of patients with heart failure and in control subjects. We found that the concentration of hepcidin was lower in urine samples of patients with heart failure compared to those of control subjects. Serum hepcidin was also reduced in heart failure but was not significantly lower than that in controls. There were no significant differences between hepcidin levels in patients with heart failure and anemia compared to patients with heart failure and normal hemoglobin. We concluded that hepcidin probably does not play a major role in pathogenesis of anemia in patients with chronic heart failure.
Keywords: Anemias, Cytokines, Iron
Between 20-40% of patient with heart failure have anemia 1;2. There are several possible confounding factors that might contribute to the high frequency of anemia among patients with heart failure, including concurrent kidney failure. However, due to an upregulation of several inflammatory cytokines in heart failure 3, it is a reasonable hypothesis that anemia in heart failure can be an anemia of inflammation. Over the past 8 years, hepcidin has been identified as the molecule contributing to anemia of inflammation 4;5. Initially discovered as an antimicrobial molecule 6;7, hepcidin has an important role in regulating iron metabolism, particularly during infection and inflammation 8-10. Hepcidin is mainly synthesized in the liver and released to the plasma. During an acute inflammatory response, hepcidin concentration in plasma increases several folds in a short period of time, which results in a rapid decline in the plasma iron concentration 4. Hepcidin decreases export of iron absorbed from intestinal mucosa to blood and also decreases release of iron from macrophages recycling iron from senescent erythrocytes 9. This results in a deprivation of erythroid progenitor cells from necessary iron for erythropoiesis. If the stimulus for production of hepcidin continues, such as in a chronic inflammatory condition, abnormal erythropoiesis would results in a chronic anemia. We hypothesized that elevation in serum hepcidin mediates anemia in patients with heart failure. In order to study this hypothesis, we measured hepcidin in urine and serum samples of anemic and non-anemic patients with heart failure. Additionally, we measured hepcidin in a control group consisted of individuals without any clinical evidence of heart failure, who has been evaluated in various outpatient clinics.
Baseline characteristics of patients with heart failure and control subjects are summarized in Table 1. We studied 36 patients with heart failure and anemia, 61 patients with heart failure and no anemia, and 38 control subjects. Patients in the anemic group had a lower hemoglobin (11.64 ± 0.19 g/dL) compared to those in the non-anemic group (14.25 ± 0.15 g/dL) or control subjects (14.14 ± 0.27 g/dL). There was no significant difference in the serum concentration of ferritin or creatinine among the three groups.
Table 1.
Baseline characteristics
| Anemic HF (n=36) |
Non- Anemic HF (n=61) |
Controls (n=38) |
P value |
||
|---|---|---|---|---|---|
| Overall | Anemic vs Non- anemic |
||||
| Male Sex | 34[94.4] | 57[93.4] | 30[78.9] | 0.056 | 1.000 |
|
| |||||
| Race | 0.088 | 0.255 | |||
| Caucasian | 22[61.1] | 39[67.2] | 16[42.1] | ||
| African American | 9[25] | 16[27.6] | 17[44.7] | ||
| Hispanic | 5[13.9] | 2[3.4] | 4[10.5] | ||
| Asian or Pacific | |||||
| Islander | 0[0] | 1[1.7] | 1[2.6] | ||
|
| |||||
| CAD | 28[82.4] | 36[64.3] | 11[28.9] | 0.0001 | 0.093 |
|
| |||||
| CABG | 8[30.8] | 3[10.7] | 1[2.6] | 0.003 | 0.095 |
|
| |||||
| HTN | 32[91.4] | 44[75.9] | 31[81.6] | 0.175 | 0.060 |
|
| |||||
| DM | 19[54.3] | 19[32.8] | 20[52.6] | 0.061 | 0.041 |
|
| |||||
| COPD | 5[14.3] | 10[17.5] | 3[7.9] | 0.398 | 0.681 |
|
| |||||
| NYHA | 0.0001 | 0.646 | |||
| 1 | 0 | 0 | 0 | ||
| 2 | 15[45.5] | 32[55.2] | 0[0] | ||
| 3 | 17[51.5] | 24[41.4] | 0[0] | ||
| 4 | 1[3.0] | 2[3.4] | 0[0] | ||
|
| |||||
|
Ejection Fraction
(%) |
27.6±1.5 | 25.0±1.1 | NA | NA | 0.316 |
|
| |||||
| BNP (pg/ml) | 914.9±184.6 | 288.9±40.8 | 78.8±16.8 | <0.001 | <0.001 |
|
| |||||
| Age | 67.7±1.7 | 60.6±1.2 | 63.6±2.2 | 0.01 | 0.001 |
|
| |||||
| Weight (Kg) | 84.6±4.0 | 89.7±2.9 | 95.9±4.2 | 0.127 | 0.165 |
|
| |||||
| Height (m) | 1.72±0.01 | 1.70±0.03 | 1.74±0.02 | 0.528 | 0.400 |
|
| |||||
| WBC (x103/μl) | 9.9±3.0 | 7.8±0.30 | 8.04±2.82 | 0.558 | 0.083 |
|
| |||||
|
Hemoglobin
(gr/dL) |
11.6±0.2 | 14.3±0.2 | 14.1±0.3 | <0.001 | <0.001 |
|
| |||||
| Platelet (x103/μ) | 234±14 | 248±13 | 261±12 | 0.460 | 0.874 |
|
| |||||
|
Creatinine
(mg/dL) |
1.4±0.1 | 1.3±0.1 | 1.1±0.1 | 0.073 | 0.306 |
|
| |||||
| Iron (μg/dL) | 56.4±4.2 | 77.7±4.0 | 85.1±9.0 | 0.005 | 0.001 |
|
| |||||
| TIBC (μg/dL) | 350.9±15.1 | 355.2±8.2 | 337.2±11.1 | 0.484 | 0.586 |
|
| |||||
| Ferritin (ng/ml) | 160.6±45.4 | 146.1±15.8 | 156.5±38.4 | 0.938 | 0.302 |
Abbreviations: CAD: coronary artery disease, CABG: coronary artery bypass graft surgery, HTN: hypertension, DM: diabetes mellitus, COPD: chronic obstructive pulmonary disease
The values within brackets represent percentages and the rest of the results are as mean ± S.D.
We conducted a multivariate analysis to detect the effect of age, sex, presence of CAD, history of CABG, hypertension, diabetes mellitus, NYHA class, hemoglobin concentration, serum ferritin, left ventricle ejection fraction (LVEF), and the etiology of heart failure (ischemic vs non-ischemic) on serum and urine hepcidin concentration in patients with heart failure. Among these factors only serum ferritin affected hepcidin concentration in both serum (P<0.001) and urine (P<0.002). Importantly, in anemic and non-anemic HF patients, there were no significant independent interactions between hepcidin and hemoglobin, LVEF, or etiology of HF (all p>0.05).
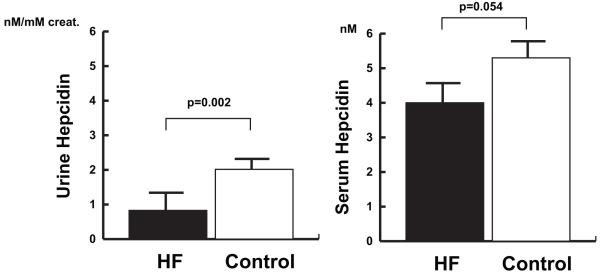
Over all, patients with heart failure had a lower urine hepcidin compared to those of control subjects (0.9 ± 0.2 and 2.0 ± 0.5 respectively, p=0.002) (Figure 1). There was a similar trend in the serum hepcidin concentrations of heart failure patients and controls, which did not reach a statistical significance (4.0 ± 0.4 and 5.3 ± 0.6 respectively, p=0.054).
Figure 1. Hepcidin and heart failure.
Bar-graphs demonstrate serum and urine hepcidin levels in all patients with HF compared to control subjects. The left-hand panel compares urine hepcidin levels in patients with HF compared to controls. The right-hand panel compares serum hepcidin levels in patients with HF to controls. Error bars represent standard error of the mean.
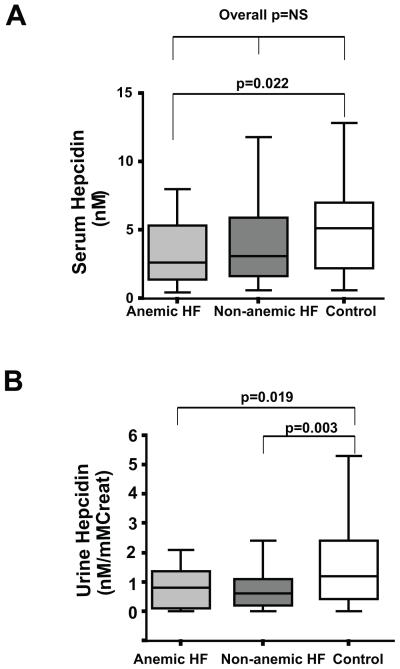
Lower level of hepcidin in heart failure patients was reflected in both anemic and non-anemic subgroups. The serum hepcidin in patients with heart failure and anemia was significantly lower than that in control subjects (p=0.022) (Figure 2A). On the other hand, serum hepcidin in non-anemic heart failure patients was not significantly different from that of control subjects or anemic heart failure patients. (p= 0.251 and p=0.172, respectively). Urine hepcidin in both anemic and non-anemic heart failure patients was lower than that in control subjects (p=0.019 and p=0.003, respectively) (Figure 2B). There was no statistically significant difference between anemic and non-anemic patients regarding their urine or serum hepcidin (p=0.74 and p=0.172, respectively).
Figure 2. Hepcidin and anemia in heart failure.
Bar-and-whisker plots representing hepcidin levels in patients with HF and anemia, patients with HF and no anemia, and control subjects. A) Serum hepcidin levels (nM), and B) Urine hepcidin levels (nM / mM of creatinine).
Hepcidin plays an important role in iron metabolism and in the pathogenesis of anemia of inflammation. Hepcidin mediates anemia of inflammation 8;11;12 by binding to and internalizing ferroportin, a membrane iron transporter responsible for exit of iron from intestine epithelial cells and macrophages, resulting in degradation of ferroportin 13;14. Inflammatory cytokines, such as IL-6, increase synthesis of hepcidin in the liver 15. On the other hand, iron excess, anemia, and hypoxia decrease hepcidin synthesis and its plasma level 16;17. The level of hepcidin increases in various infectious or inflammatory conditions and causes rapid change in plasma iron level.
Progression of heart failure is associated with a sustained elevation of several proinflammatory cytokines, including tumor necrosis factor-α (TNF- α), the interleukin (IL)-1 family, and IL-6 18. Among these cytokines, IL-6 has been shown to increase synthesis of hepcidin in the liver. We studied whether anemia of heart failure is associated with an elevation in hepcidin concentration.
A previous study on anemia in patients with heart failure showed that a relative increase in the plasma volume rather than a decrease in the red blood cell mass was the main finding in these patients 19. However, this study only examined patients with heart failure, and did not include control subjects without heart failure.
Recently, Matsumoto et al. studied serum hepcidin level in patients with chronic heart failure 20. They compared serum hepcidin between 36 patients with heart failure and anemia, 16 patients with heart failure and no anemia, and 16 patients with no heart failure and no anemia. They found that serum hepcidin was lower in patients with heart failure and anemia compared to that of other groups and concluded that anemia of inflammation is a minor cause for anemia of heart failure. Our study confirmed this result in a larger number of patients using both urine and serum hepcidin.
Anemia in patients with heart failure is a multifactorial disease. In this study, we investigated whether anemia in heart failure is associated with an elevated hepcidin concentration. We found that patients with heart failure and anemia had both lower urine and serum hepcidin compared to those in control subjects. It is important to mention that our control subjects are from individuals visiting different outpatient clinics at Baylor College of Medicine and might be different from a group of healthy controls. In our previous study, median of serum hepcidin among healthy individuals were found to be 4.2 nM with a range of 0.5-13.9 nM 21, which is lower than the serum hepcidin of controls in the current study. The main goal of this study was to investigate the role of hepcidin in anemia of heart failure, and the comparison between hepcidin concentration in heart failure patients with and without anemia did not show a significant difference. These findings are not consistent with the presence of a major role for hepcidin in the pathogenesis of anemia in heart failure patients. However, one should be cautious about interpreting our results, because several factors might affect hepcidin level in heart failure, some of them in opposite directions; liver synthetic defect, anemia of dilution, and elevated erythropoietin 20 would decrease hepcidin. On the other hand, inflammatory cytokines can increase the hepcidin level. Chronic heart failure is associated with elevation of several cytokines, among them TNF-α has been shown to decrease iron absorption from intestine and iron release from macrophages 22 and might contribute to anemia of heart failure. Once heart failure patients become anemic, it is likely that their anemia downregulates synthesis of hepcidin and causes lower level of hepcidin. This is a possible explanation for the lower concentration of hepcidin in anemic heart failure patients. The other possible explanation for our results is that in patients with heart failure elevated erythropoietin 20 might decrease hepcidin level and overrides the effect of inflammatory cytokines.
Methods
This study was conducted according to the protocol for human subject study approved by the Institutional Review Board of Baylor College of Medicine. All of the subjects signed a consent form to participate in this study. Ninety seven patients with chronic heart failure (left ventricle ejection fraction less than 40% and New York Heart Association class II-IV symptoms) were recruited from hospitals affiliated with Baylor College of Medicine in Houston, Texas. According to their hemoglobin level, patients with heart failure were divided into anemic (hemoglobin of less than 13 g/dL for men and 12 g/dL for women) and non-anemic subgroups. Thirty eight patients without a history of heart failure were selected during outpatient clinic visits as the control subjects. Exclusion criteria included presence of kidney failure (serum creatinine above 1.5 mg/dL), history of cardiac transplant, and history of gastrointestinal or severe menstrual bleeding.
Hepcidin comprises 3 isoforms that contains 20 (hepcidin-20), 22 (hepcidin-22), or 25 amino acids (hepcidin-25). Hepcidin-25 is the biologically active isoform. We performed urine and serum hepcidin-25 measurements using a combination of weak cation exchange chromatography and surface enhanced laser desorption ionization – time of flight mass spectrometry (TOF MS), as previously reported 23;24. An internal standard (synthetic hepcidin-24; Peptide International Inc.) was used for quantification. Peptide spectra were generated on the TOF MS platform of a PBS IIc mass spectrometer (Purchased from Ciphergen Biosystems). This method reproducibly detect elevated hepcidin levels in inflammatory conditions such as infection and rheumatoid arthritis and decreased hepcidin level in iron deficiency anemia 23;24. Serum hepcidin levels were expressed in nM, and urine levels in nM/ mM creatinine (normalized to urine creatinine concentration). For serum, the intra-run Coefficient of Variation (CV) was 3.9 % at 7.3 nM (n=8) and 3.1 % at 3.4 nM (n=8) and the inter-run CV was 7.5% at 4.0 nM (n=8). Intra-run variation of hepcidin measured in urine is 3.0 % both at 3.3 nM (n=8) and 9.9 nM (n=8). Inter-run variation for urine varies between 12.6% at 1.5 nM (n=8) and 10.2 % at 9.1 nM (n=8). Lower limit of detection for serum was 0.5 nM and for urine was 50 pM.
Statistical Analysis
All values are expressed as mean ± SEM. Differences in baseline characteristics between the three groups (anemic heart failure, non-anemic heart failure, and control) were tested using the Chi-Square test or Fisher Exact test for categorical variables and ANOVA for continuous variables. Overall differences in biomarker levels were tested using one-way ANOVA or Kruskal-Wallis test (for non-gaussian variables). Tukey’s test was used for post-hoc testing where appropriate. Heart failure patients as a group were compared to control subjects using the Student’s t-test or Mann-Whitney U test. Multivariate regression analysis was performed on serum and urine hepcidin using the following predictors: age, sex, presence of CAD, history of CABG, hypertension, diabetes mellitus, NYHA class, left ventricular ejection fraction, ferritin, hemoglobin, and etiology of heart failure (ischemic vs. non-ischemic). All data analysis was performed using SPSS 13 (SPSS, Chicago, IL, USA). A p-value <0.05 was considered statistically significant.
Acknowledgments
This research is supported by grants from National Institute of Health (RO1 HL58081, RO1 HL61543, RO1 HL42250) to D.L.M.
Reference List
- 1.Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 2.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 3.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 4.Ganz T. Hepcidin in iron metabolism. Curr Opin Hematol. 2004;11:251–254. doi: 10.1097/00062752-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Fleming RE, Sly WS. Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc Natl Acad Sci U S A. 2001;98:8160–8162. doi: 10.1073/pnas.161296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 7.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemna E, Pickkers P, Nemeth E, van der HH, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 11.Maes K, Nemeth E, Roodman GD, Huston A, Esteve F, Freytes C, Callander N, Katodritou E, Tussing-Humphreys L, Rivera S, Vanderkerken K, Lichtenstein A, Ganz T. In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2. Blood. 2010 doi: 10.1182/blood-2010-03-274571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 13.Yeh KY, Yeh M, Glass J. Hepcidin regulation of ferroportin 1 expression in the liver and intestine of the rat. Am J Physiol Gastrointest Liver Physiol. 2004;286:G385–G394. doi: 10.1152/ajpgi.00246.2003. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 17.Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93:90–97. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 18.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3:161–182. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 19.Adlbrecht C, Kommata S, Hulsmann M, Szekeres T, Bieglmayer C, Strunk G, Karanikas G, Berger R, Mortl D, Kletter K, Maurer G, Lang IM, Pacher R. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body’s red cell volume. Eur Heart J. 2008;29:2343–2350. doi: 10.1093/eurheartj/ehn359. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto M, Tsujino T, Lee-Kawabata M, Naito Y, Akahori H, Sakoda T, Ohyanagi M, Tomosugi N, Masuyama T. Iron regulatory hormone hepcidin decreases in chronic heart failure patients with anemia. Circ J. 2010;74:301–306. doi: 10.1253/circj.cj-09-0663. [DOI] [PubMed] [Google Scholar]
- 21.Kroot JJ, Hendriks JC, Laarakkers CM, Klaver SM, Kemna EH, Tjalsma H, Swinkels DW. (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: implications for clinical studies. Anal Biochem. 2009;389:124–129. doi: 10.1016/j.ab.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006;397:61–67. doi: 10.1042/BJ20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swinkels DW, Girelli D, Laarakkers C, Kroot J, Campostrini N, Kemna EH, Tjalsma H. Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS One. 2008;3:e2706. doi: 10.1371/journal.pone.0002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroot JJ, Laarakkers CM, Geurts-Moespot AJ, Grebenchtchikov N, Pickkers P, van Ede AE, Peters HP, van Dongen-Lases E, Wetzels JF, Sweep FC, Tjalsma H, Swinkels DW. Immunochemical and Mass-Spectrometry-Based Serum Hepcidin Assays for Iron Metabolism Disorders. Clin Chem. 2010 doi: 10.1373/clinchem.2010.149187. [DOI] [PubMed] [Google Scholar]