Abstract
Mucosal T lymphocyte responses in the female reproductive tract, the primary site of HIV transmission in women, may be critical for initial control of virus infection. In addition, characterization of genital immune responses to HIV will be important for the development of a vaccine capable of preventing infection by this route. We analyzed lymphocytes isolated from vagina and cervix of chronically SIV-infected macaques for the frequency of SIV Gag tetramer-binding cells and expression of chemokine receptors. We found that the frequency of SIV-specific CD8+ T cell responses was 3- to 30-fold higher in genital tissues than in peripheral blood. SIV-specific CD8+ T cells in genital tissues expressed high levels of CXCR3 and CCR5, chemokine receptors normally expressed on memory T cells that home to inflamed tissues. Cells expressing CXCR3 colocalized with its chemokine ligand CXCL9 (MIG, monokine induced by interferon gamma) in the vaginal lamina propria. These results indicate that the frequency of SIV-specific CD8+ T cells in the female genital mucosa is enriched compared with peripheral blood and provide initial information regarding the signals that direct recruitment of T cells to the female reproductive tract.
Introduction
Sexual transmission of HIV infection to women occurs predominantly across cervicovaginal mucosal surfaces. Primate studies have shown that SIV can enter the epithelium of the vaginal mucosa and infect intraepithelial dendritic cells within 60 minutes of exposure to cell free virus, with virus-infected cells appearing in local lymph nodes within 18 hours 1. Virus-specific immune responses in genital mucosa are therefore likely to be critical for initial control of vaginal infection with HIV or SIV.
The presence of HIV- and SIV-specific T cells in the genital mucosa of women and female rhesus macaques has been reported by several groups. Kaul et al demonstrated that HIV-specific CD8+ cytokine responses were lower in lymphocytes isolated from the cervix than in peripheral blood of HIV infected women, while in exposed uninfected subjects these responses were higher in cervix than in blood 2. Virus-specific cytotoxic T cell activity has also been shown following in vitro stimulation of T cells isolated from cervical specimens from HIV-infected women 3 and SIV-infected macaques 4. High frequencies of SIV-specific CD8+ T cell responses were reported in cervicovaginal tissues in SIV-infected macaques 5 and in macaques vaccinated with the live attenuated SHIV 89.6 vaccine 6. While these studies establish the presence of functional cellular immune responses in the female genital mucosa, they have provided only limited information regarding molecules mediating trafficking of virus-specific cells to genital mucosa.
The events that control trafficking of virus-specific lymphocytes into tissue compartments, and particularly genital mucosa, are incompletely understood. Molecules known to participate in this process include chemokines and their receptors, which have been shown to regulate lymphocyte traffic in normal and inflammed tissues 7. Chemokines produced in inflammation induce the migration of lymphocytes expressing CXCR3, CCR5 and other receptors for inflammatory chemokines into the inflamed tissues. This differential expression of chemokines by tissues has been implicated in the control of CTL trafficking to sites of viral replication 8.
In this study of SIV-infected female rhesus macaques, the frequency of CD8+ T cells specific for the immunodominant Mamu-A*01-restricted SIV Gag181–189 epitope 9 was determined in blood, mucosal tissues, and secondary lymphoid organs by flow cytometry using peptide/MHC class I tetramers. SIV-specific CD8+ T cells were analyzed for expression of receptors known to participate in lymphocyte trafficking, including the chemokine receptors CXCR3, CXCR4, CCR5, and CCR7. SIV-specific CD8+ T cells in genital mucosa expressed high levels of CXCR3 and CCR5 relative to expression in peripheral blood. The results presented here demonstrate a significant enrichment of SIV-specific CD8+ T cells in the genital mucosa of infected female macaques and that inflammatory chemokines and their receptors play a role in directing cells to these tissues.
RESULTS
SIV-specific CD8+ T lymphocyte responses are enriched in vaginal and cervical mucosae
SIV-specific CD8+ T cell responses were evaluated in blood, genital mucosa and secondary lymphoid organs of 7 female SIVmac239-infected rhesus macaques at necropsy using techniques similar to those previously published by our group 10–13. All of the monkeys studied were positive for the Mamu-A*01 class I MHC allele, allowing the use of Gag181–189 /Mamu-A*01 tetramers for detection of Gag-specific CD8+ T cells by flow cytometry. SIV-specific CD8+ T cells were detected in lymphocytes isolated from cervical and vaginal mucosae of all 7 monkeys at frequencies between 3 and 30-fold higher than those found in peripheral blood (mean enrichment=12.7 fold for blood vs.vagina or cervix; p=0.018 blood vs. vagina; p<0.028 blood vs. cervix, Wilcoxon Signed Rank Test) (Table 1).
Table 1.
Frequency of SIV-specific CD8+ T cells in blood, lymphoid and mucosal tissues of SIV-infected rhesus macaques.
| % Tetramer+ cells*: |
|||||||
|---|---|---|---|---|---|---|---|
| Macaque | Blood | Vagina | Cervix | Lymph node | Spleen | Jejunum | Colon |
| 97D248 | 0.4 | 6.4 | 13.2 | 2.7 | NT | NT | NT |
| 297.98 | 0.1 | 1.6 | 3.0 | 0.6 | NT | NT | NT |
| 226.88 | 0.1 | 0.3 | NT | 0.5 | 0.6 | 0.5*** | 0.2** |
| 214.87 | 0.5 | 3.4 | 2.0 | 4.9 | 5.6 | 6.7** | 5.1** |
| 252.90 | 0.1 | 1.8 | 2.1 | 1.5 | NT | 1.8 | 2.0 |
| 541.99 | 4.5 | 13 | 16 | 5.3 | 9 | 14 | 12 |
| 430.93 | 0.6 | 4.0 | 2.2 | 1.7 | NT | 2.3*** | 2.9*** |
Percentage of Mamu A*01/SIV Gag181–189 tetramer-binding cells after gating on CD3+CD8+ cells.
Lamina propria lymphocytes (LPL) isolated from jejunum and colon with type II collagenase after removal of intraepithelial lymphocytes (IEL) using EDTA.
Mixed population of IEL and LPL isolated from the jejunum using type II collagenase alone.
To determine whether the observed difference in the frequency of SIV-specific CD8+ T cells in genital mucosa and blood was specific to tissues of the reproductive tract, lymphocytes isolated from intestinal mucosae, spleen and lymph nodes of 5 monkeys infected with wild type or attenuated SIV were analyzed for Gag tetramer-binding cells. The frequency of tetramer+ lymphocytes was found to be up to 20 times higher in secondary lymphoid and mucosal tissues than in peripheral blood of the same animal (Table 1). However, the percentage of SIV-specific cells in these sites were quite similar within each animal, differing by just 1.5 to 3.3-fold. SIV-specific cells were increased relative to blood in lymph nodes of all six monkeys, with an average fold enrichment of 5.6. In summary, all lymphoid and mucosal tissues examined were enriched in SIV-specific CD8+ T cells relative to peripheral blood.
Detection of SIV tetramer+ cells in vaginal biopsies of monkeys immunized with attenuated SIV
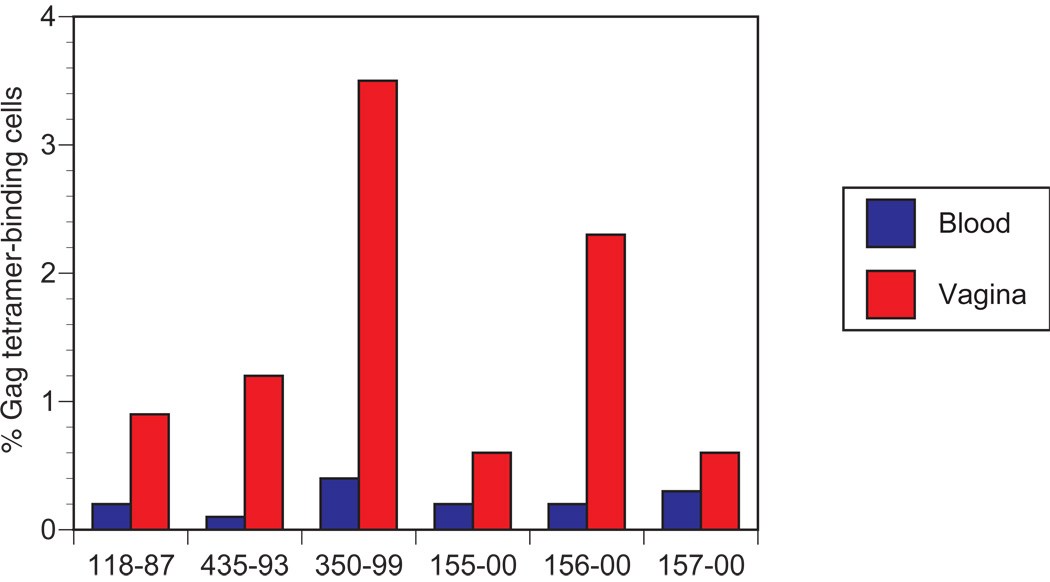
The high frequency of virus-specific CD8+ T cells found in genital mucosal tissues suggested that a method for following these responses over time in living animals would be advantageous for nonhuman primate vaccine studies. We therefore developed a vaginal biopsy technique that permitted us to isolate a sufficient number of cells to perform serial tetramer analyses at 2 to 4 week intervals. Ten to 12 individual pinch biopsies were collected from individual animals at one time, yielding up to 3 million cells. Histological analysis of representative specimens demonstrated that the biopsies included tissue from epithelium and lamina propria with some variation among biopsies (data not shown). Blood and lymphocytes isolated from vaginal biopsies from 6 monkeys immunized with the attenuated SIV vaccines SIVmac239Δ3 or SIVmac239Δnef were analyzed for the frequency of tetramer positive cells. The attenuated SIV-immunized animals exhibited increased frequencies of tetramer positive cells in vaginal mucosa equivalent to those seen in monkeys infected with wild type SIV, with relative enrichment compared with blood ranging from 2 to 11-fold (Figure 1).
Figure 1. Macaques infected with attenuated SIV strains have higher frequencies of SIV-specific CD8+ T cells in vagina than in peripheral blood.
Mononuclear cells isolated from blood and vaginal biopsies were stained with anti-CD3 and anti-CD8 antibodies and Mamu-A*01/SIV Gag181–189 tetramers. The percentage of tetramer-positive cells was determined for CD3+CD8+ cells after gating on lymphocytes. A minimum of 10,000 lymphocyte gated events were analyzed for each specimen.
Chemokine receptor expression on genital CD8+ T cells
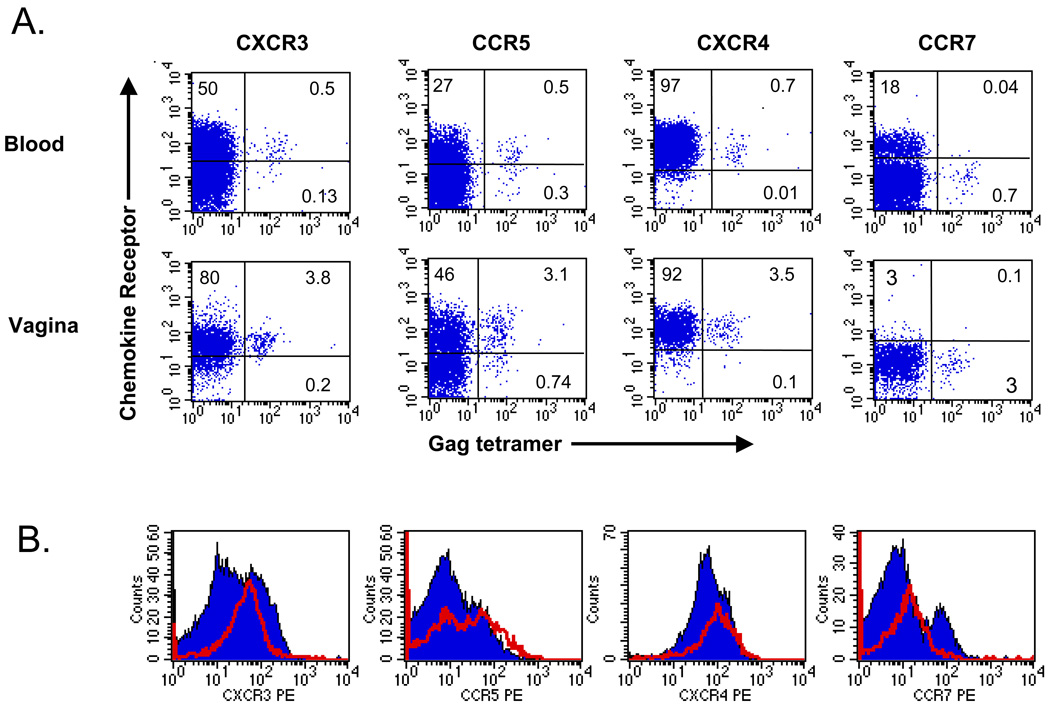
Interactions between chemotactic cytokines and receptors expressed on lymphocytes provide important signals for recruitment of lymphocytes into tissues 7. To investigate the possibility of a role for chemokines in directing genital homing of SIV-specific lymphocytes, we studied expression of CXCR3 and CCR5, receptors for chemokines induced during inflammation, on CD8+ T cells in blood and vagina lymphocytes. CXCR3 was expressed on the majority of CD8+ T cells in both vagina and peripheral blood (representative data are shown in Figure 2). CXCR3 was expressed on a significantly higher percentage of CD8+ T cells in vagina than in blood (86% vs. 51%, p<0.05, Wilcoxon signed rank test). Mean fluorescence intensity was also significantly higher for CXCR3 on CD8+ T cells from the vagina than for CD8+ T cells in blood (p<0.05). While most of the CD8+ T cells in vagina were positive for CXCR3, the frequency was significantly higher for tetramer+ cells than for the total CD8+ T cell population in vagina (91% vs. 86%, p<0.05), and in peripheral blood (71% vs. 51%, p<0.05). CCR5 expression on these cell populations displayed a pattern similar to that of CXCR3 but did not reach statistical significance, a finding that may be related to the fact that fewer animals were included in the analysis (Figure 2). In contrast, expression of CXCR4, a receptor which participates in homeostatic lymphocyte trafficking and is expressed on most circulating CD8+ T cells, was similar on tetramer+ and bulk CD8+ populations in blood and vagina (Figure 2). As expected, expression of CCR7, a chemokine receptor that helps direct migration of central memory T cells into lymph nodes and is low on tissue effector memory cells 14, was largely absent both on bulk CD8+ T cells and SIV tetramer+ cells in vaginal tissue (Figure 2). The expression of receptors specific for inflammatory chemokines on nearly all SIV tetramer+ cells in vaginal tissues suggests that expression of chemokines recognized by these receptors may regulate localization of T cells to the female reproductive tract.
Figure 2. Differential expression of CXCR3 and CCR5 on SIV-specific CD8+ T cells in blood and vagina.
A. Expression of chemokine receptors on CD8+ T cells in the blood and vagina of a SIV-infected macaque. The percentage of all CD8+ T cells expressing either the indicated chemokine receptor, Gag tetramer, or both is indicated in the appropriate quadrant. B. Histograms of expression of chemokine receptors on total CD8+ T cells (solid blue) or Gag tetramer+ cells (red line).
Expression of the CXCR3 ligand, CXCL9, in vaginal mucosa
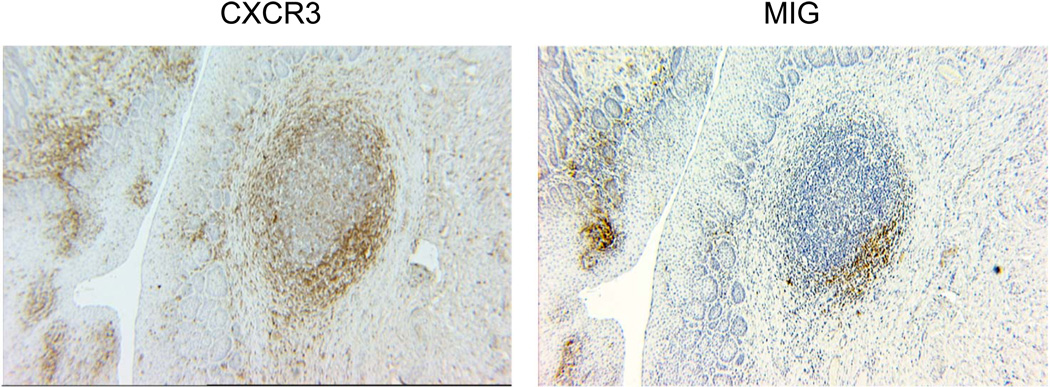
To investigate whether the inflammatory chemokines that recognized the receptors expressed on CD8+ T cells tracking to vaginal tissues are produced in situ, vaginal tissues from SIV-infected macaques were stained with antibodies against CXCR3 and one of its ligands, CXCL9 (MIG). Large numbers of CXCR3+ cells were detected in the vaginal lamina propria, with high concentrations of positive cells localized to lymphoid aggregates (Figure 3). Staining of adjacent tissue sections for CXCL9+ cells in the lamina propria, which colocalized with CXCR3-positive cells within the aggregates (Figure 3). This colocalization of CXCL9- and CXCR3-expressing cells in the vagina suggests a role for this chemokine in regulation of lymphocytes trafficking to genital tissues.
Figure 3. The chemokine receptor CXCR3 and chemokine CXCL9 (MIG) are expressed by cells in vaginal epithelium and in a lymphoid aggregate in the lamina propria of a SIV-infected macaque.
Immunohistochemical staining was performed on paraffin-fixed tissues.
Discussion
Accumulating evidence indicates that induction of HIV-specific CTL responses in genital mucosa may be critical for initial control of vaginal infection with HIV or SIV. This study demonstrates that SIV-specific CD8+ T cells are significantly enriched in the genital tract of SIV-infected female macaques relative to peripheral blood, and provides evidence for a role for receptors for inflammatory chemokines in directing the trafficking of these cells to genital tissues.
Recruitment of specific lymphocyte subset into tissue compartments can be regulated by the differential expression of chemokines in tissues 7. These chemotactic signals attract lymphocytes expressing the appropriate receptors for the chemokines produced in the target tissues. The selective expression of the chemokine receptors CXCR3 and CCR5 on the majority of SIV tetramer-binding cells in the vagina suggests that these chemokines may play a key role in the recruitment of T cells to the genital mucosa. The frequency of cells expressing CXCR3 was highest among vaginal tetramer+ cells, and it was significantly higher than total vaginal CD8+ T cells, blood tetramer+ cells, and total blood CD8+ T cells. Our demonstration that cells producing CXCL9, one of three chemokines recognized by CXCR3, localized in proximity to CXCR3+ cells in the vaginal lamina propria, further supports the role of CXCR3 and its ligands in the recruitment of cells to tissues in the female reproductive tract.
The enrichment of virus-specific cells in genital mucosae suggests that factors related to infection with SIV can influence the migration patterns of these cells. Effects of several viral proteins on chemokine production have been reported, including induction by HIV Nef of MIP-1α and MIP-1β, chemokines ligands for CCR5, by macrophages 15. Expression of the CXCR3 ligand IP-10 CXCL10) can also be induced in dendritic cells in vitro by HIV Tat 16. These findings suggest a scenario in which SIV infection of cells in vaginal mucosa may induces chemokine production and recruitment of CD8+ T cells expressing the appropriate chemokine receptors.
Acknowledgements
The authors thank John Altman (Emory University) for providing the Mamu A*01 Gag tetramers and Andrew Luster (Massachusetts General Hospital) for helpful discussions. This work was supported through NIH grants AI062412, AI071306, and RR00168.
References
- 1.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, Njagi E, MacDonald KS, Bwayo JJ, McMichael AJ, Rowland-Jones SL. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1- resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 3.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohman BL, Miller CJ, McChesney MB. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 5.Stevceva L, Kelsall B, Nacsa J, Moniuszko M, Hel Z, Tryniszewska E, Franchini G. Cervicovaginal Lamina Propria Lymphocytes: Phenotypic Characterization and Their Importance in Cytotoxic T-Lymphocyte Responses to Simian Immunodeficiency Virus SIV(mac251) J Virol. 2002;76:9–18. doi: 10.1128/JVI.76.1.9-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genesca M, Skinner PJ, Bost KM, Lu D, Wang Y, Rourke TL, Haase AT, McChesney MB, Miller CJ. Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys. Mucosal Immunol. 2008;1:219–228. doi: 10.1038/mi.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 8.Cerwenka A, Morgan TM, Harmsen AG, Dutton RW. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J Exp Med. 1999;189:423–434. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen TM, Sidney J, delGuercio M-F, Glickman RL, Lensmeyer GL, Wiebe DA, Pauza CD, Johnson RP, Sette A, Watkins DI. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 10.Cromwell MA, Veazey RS, Altman JD, Mansfield KG, Glickman R, Allen TM, Watkins DI, Lackner AA, Johnson RP. Induction of mucosal homing virus-specific CD8(+) T lymphocytes by attenuated simian immunodeficiency virus. J Virol. 2000;74:8762–8766. doi: 10.1128/jvi.74.18.8762-8766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veazey RS, Gauduin MC, Mansfield KG, Tham IC, Altman JD, Lifson JD, Lackner AA, Johnson RP. Emergence and kinetics of simian immunodeficiency virus-specific CD8(+) T cells in the intestines of macaques during primary infection. J Virol. 2001;75:10515–10519. doi: 10.1128/JVI.75.21.10515-10519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Motal UM, Gillis J, Manson K, Wyand M, Montefiori D, Stefano-Cole K, Montelaro RC, Altman JD, Johnson RP. Kinetics of expansion of SIV Gag-specific CD8+ T lymphocytes following challenge of vaccinated macaques. Virology. 2005;333:226–238. doi: 10.1016/j.virol.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 15.Swingler S, Mann A, Jacque J, Brichacek B, Sasseville VG, Williams K, Lackner AA, Janoff EN, Wang R, Fisher D, Stevenson M. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1995;5:997–103. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izmailova E, Bertley FM, Huang Q, Makori N, Miller CJ, Young RA, Aldovini A. HIV-1 Tat reprograms immature dendritic cells to express chemoattractants for activated T cells and macrophages. Nat Med. 2003;9:191–197. doi: 10.1038/nm822. [DOI] [PubMed] [Google Scholar]