Abstract
Susceptibility to most autoimmune diseases is dependent on polygenic inheritance, environmental factors, and poorly defined stochastic events. One of the significant challenges facing autoimmune disease research is in identifying the specific events that trigger loss of tolerance and autoimmunity. Although many intrinsic factors, including age, sex, and genetics, contribute to autoimmunity, extrinsic factors such as drugs, chemicals, microbes, or other environmental factors can also act as important initiators. This review explores how certain extrinsic factors, namely drugs and chemicals, can promote the development of autoimmunity, focusing on a few better characterized agents that, in most instances, have been shown to produce autoimmune manifestations in human populations. Mechanisms of autoimmune disease induction are discussed in terms of research obtained using specific animal models. Although a number of different pathways have been delineated for drug/chemical-induced autoimmunity some similarities do exist and a working model is proposed.
1. Introduction
Autoimmunity is the reaction of cells (lymphocytes) or products (antibodies) of the immune system with constituents of the body’s own tissues leading to demonstrable pathology. Autoimmunity can produce a variety of clinical conditions depending upon the target of the attack, with common features including expansion of self-reactive T and B cells, production of autoantibodies and tissue damage. The most baffling and challenging aspect of autoimmunity is identifying the events that contribute to the initiation of the response. While many intrinsic factors including age, sex, and genetics contribute to autoimmunity, it is believed that extrinsic factors such as drugs, chemicals, microbes, and/or the environment can trigger the initiation of an autoimmune response. In this review we will discuss the contribution of extrinsic factors, to autoimmunity, the diseases produced and what has been learned from animal models which use drugs and chemicals to initiate autoimmunity.
2. Types of Autoimmunity
2.1 Systemic Autoimmunity
Systemic autoimmune diseases are a heterogeneous group of diseases in which pathology is evident in a number of organ systems within the body. Systemic autoimmune diseases include connective tissue diseases such as systemic lupus erythematosus (SLE), scleroderma, Sjögren’s syndrome, inflammatory myopathies, and overlap syndromes such as mixed connective tissue disease (MCTD) and undifferentiated (unclassified) connective tissue diseases. Individual diseases often exhibit significant heterogeneity in clinical features, genetics and autoantibodies. In most systemic autoimmune diseases the autoantibody responses can be directed against a number of autoantigens, and the resulting profile of autoantibody specificities may be disease specific [1]. Although of diagnostic importance the contribution of autoantibodies to the initiation, exacerbation or progression of disease remains uncertain but it has been argued that the differences in autoantibody profiles that are associated with systemic autoimmune diseases suggest that they may constitute molecular signatures of the disease process [2].
2.2 Organ Specific Autoimmunity
Organ-specific autoimmune diseases affect specific tissues in which the target auto-antigen is found. Commonly targeted tissues or cells include the thyroid (thyroiditis), the β cells of the islets of Langerhans (diabetes), gastric parietal cells (gastritis), liver (autoimmune hepatitis) and steroid-producing cells in the adrenal and ovary (Addison’s disease) [3]. Susceptibility to these diseases are influenced in large part by genetics, particularly MHC-related genes [4], but they may also be influenced by environmental agents [3]. A number of toxicants have been identified that induce organ-specific autoimmune disease.
3. Toxicants that Induce Autoimmunity
A number of chemicals and drugs have been reported to be associated with features of autoimmunity in human populations (Table 1). In the majority of instances a direct link between exposure and disease manifestations is extremely difficult to establish because of the inherent limitations of epidemiological studies to draw causal conclusions. Additionally human populations are rarely exposed to a single agent over time, there can be a significant delay between exposure and onset of disease, and it is often not possible to identify all the toxicants to which a population may have been exposed. The notable exception to this, however, is exposure to medications because in this situation there is a captive population and the affected individuals can cease use of the suspected agent in order to determine if drug consumption is the cause [5,6]. Indeed induction of autoimmunity following drug exposure has been responsible for acceptance of the possibility that repeated contact with chemicals and toxicants can elicit autoimmunity. In the following sections we will expand upon the roles that drugs, toxicants, and chemicals play in the induction of autoimmunity. Due to space limitations we will focus on a small number of agents, most of which have been shown to produce features of autoimmunity in human populations (Table 1). Where possible, mechanisms of induction are discussed using specific animal models.
Table 1.
Substances associated with autoimmunity in humans and the animal models used to examine disease mechanisms.
| Drugs/Chemicals a | Human disease | Animal model b | References |
|---|---|---|---|
| Drugs (procainamide) | Drug-induced lupus | Mouse | |
| - central tolerance | 16, 17, 18,19 | ||
| - DNA methylation | 10, 11, 12 | ||
| Silica/Asbestos | Lupus, systemic sclerosis, rheumatoid arthritis, vasculitis | Mouse | |
| - C57Bl/6 | 38 | ||
| - lupus-prone | 32, 33, 34 | ||
| Adulterated rapseed oil | Toxic Oil Syndrome | Mouse | |
| - B10.S | 42, 43 | ||
| - lupus-prone | 44 | ||
| Iodine | Thyroiditis | Mouse | |
| - NOD-H-2h4 | 119 | ||
| Trichloroethylene | Hypersensitivity skin disorder, Scleroderma, Hepatitis | Mouse | |
| - lupus prone | 153, 154, 155 | ||
| - lupus prone (prenatal) | 156 | ||
| Metals (Hg, Au, Ag) | Nephropathy, Autoantibodies | Mouse | |
| - B10.S | (Hg) 46, 47 (Ag) 47, 49 (Au) 48 | ||
| - lupus-prone | 76, 108 | ||
| TCDD, dioxinc | Anti-nuclear autoantibodies, | Mouse | |
| - GVHDd | 149 | ||
| - EAEe | 150 | ||
| - neonatal exposure | 152 | ||
| Pesticides/Fungicides (Hexachlorobenzene) | Chronic inflammatory response | Rat | 162 |
| Mouse | |||
| - lupus prone | 164 | ||
| Mineral oil (Pristane, TMPDf) | Chronic inflammatory response (follicular lipidosis) | Mouse | |
| - C57BL/6, BALB/c | 134, 139 | ||
| - lupus prone | 135 | ||
Where multiple examples of a drug or chemical exist only those discussed in the text or cited in the accompanying publications are noted.
In many studies examining the lupus-inducing potential of toxins mouse strains that are prone to develop lupus spontaneously (e.g. NZBWF1, NZM, BXSB, MRL) are used as models of sensitive populations to determine if a specific drug or chemical exposure can affect the natural progression of disease.
2,3,7,8-Tetrachlorodibenzo-p-dioxin
Graft versus host disease
experimental autoimmune encephalomyelitis
tetramethylpentadecane
3.1. Systemic Autoimmunity
3.1.1. Drug-induced autoimmunity
The chemicals most often associated with development of autoimmunity in humans are medications. Although the manifestations of drug-induced autoimmunity can vary widely, they are most similar to those associated with systemic lupus erythematosus (SLE) [5,7]. Drugs can be considered to either exacerbate pre-existing disease or initiate disease in otherwise previously healthy individuals, with discontinuation of the drug leading to disease abatement in the latter. Other differences in the two types of responses exist [8], including a preponderance of females of child bearing age and greater incidence of autoantibodies to anti-double-stranded DNA in idiopathic lupus, and most notably the relative lack of severe disease features such as major organ involvement (renal and neurologic) in primary “drug-induced” lupus [6]. The latter may be related to a significantly greater presence of complement-fixing antibodies in idiopathic lupus than in patients with drug-induced lupus [9].
A large list of drugs have been shown to induce SLE-like disease [7] and this list continues to grow as new therapeutics are introduced. Recently, biologics such as TNFα antagonists are now identified as inducing more severe forms of autoimmunity [5,6]. Of the implicated drugs only a few, such as procainamide and hydralazine, are considered to be of high risk. Furthermore the immune responses elicited by drugs differ from hypersensitivity responses as neither cellular nor humoral anti-drug responses contribute to the development of autoimmunity [7]. The mechanism by which these two drugs induce an essentially identical autoimmune response has not been completely resolved, but two tentative modes of action have been hypothesized.
The first hypothesis is based on the finding that 4-day pre-culture of cloned T cells with either procainamide or hydralazine resulted in autoreactive T cells that proliferated to autologous antigen presenting cells (APC) alone without antigen [10]. Moreover, adoptive transfer of procainamide pre-treated T cell lines into naïve mice produced autoantibodies and glomerulonephritis [11]. The mechanism driving this response appears to reside, at least in part, in the ability of both procainamide and hydralazine to inhibit DNA methylation. In experimental studies, reduced DNA methylation of alu elements 5′ to the CD11a promoter was argued to lead to an increase in CD11a transcripts and increased expression of the integrin adhesive receptor LFA-1 (CD11a/CD18) [12]. It was postulated that increased LFA-1 stabilizes low affinity interaction between the T cell receptor (TCR) and self class II MHC complexes, leading to autoimmune responses [12], although this is clearly not the sole mechanism leading to autoimmunity. Other studies have found that over expression of CD70, a T cell costimulatory molecule encoded by the TNFSF7 gene, on CD4+ lupus T cells as well as procainamide and hydralazine treated T cells is due to demethylation of a genetic element that suppresses CD70 expression when methylated [13]. These findings suggest that epigenetic modifications following exposure to drugs and chemicals may contribute to the induction of autoimmunity [14].
Another explanation for procainamide-induced autoimmunity has sought to link two common characteristics of drug-induced autoimmunity, namely that different drugs result in clinically similar diseases [7] and that many drugs elicit autoantibodies primarily targeting components of chromatin, specifically the (H2A-H2B)-DNA subnucleosome [15]. In the case of procainamide, the reactive metabolite procainamide-hydroxylamine (PAHA) has been shown to disrupt central immune tolerance [16]. Intrathymic injection of PAHA into young mice produces an anti-chromatin response with reactivity against the (H2A-H2B)-DNA subnucleosome. Adoptive transfer of anti-chromatin-reactive T cells from these mice to naïve mice stimulates B cells to produce the same autoantibody response [17]. Studies with thymus reaggregate cultures show that transgenic T cells specific for a cytochrome c peptide are better able to respond to low affinity analogues if PAHA is present during their development [18]. This mechanism argues that self tolerance (reactivity!!!) is acquired by T cells during positive selection in the thymus due to prevention of the establishment of anergy. It is believed that these cells have a reduced activation threshold as a consequence of exposure to PAHA during their development and that this results in mature autoreactive T cells seeding the peripheral immune system [19]. Although provocative these primarily in vitro observations have yet to demonstrate how drug consumption results in metabolites of procainamide subverting the induction of anergy during positive selection in the thymus.
3.1.2. Silica and Asbestos Induced Autoimmunity
Silica, an oxide of silicon, is the most abundant mineral in the earth’s crust. The association of silica and development of systemic autoimmune diseases such as SLE and scleroderma, stems from exposure during occupations such as mining, construction, and glass and pottery production [20]. Studies have argued that silica has an adjuvant effect [21] mediated in part by activation of alveolar phagocytes following inhalation of silica particles [22,23]. Scavenger receptors, such as MARCO (macrophage receptor with collagenous structure), on alveolar macrophages have been found responsible for the clearance of crystalline silica and the absence of MARCO leads to exacerbations in innate pulmonary immune responses in the presence of silica [24]. Recent studies argue that phagocytosis of asbestos and silica crystals leads to activation of the NLRP3 (NOD-like receptor family, pryin domain containing 3) inflammasome presumably via lysosomal permeabilization [25–28]. The resulting cycle of inflammation is associated with activation of cell signaling pathways, phosphorylation and activation of transcription factor NFκB, increased expression of proinflammatoty cytokines especially IL-1β, generation of reactive oxygen and nitrogen species, and cell death by apoptosis [23,29]. Mouse strains vary in their response to aerosol inhalation of silica [30], including an increase in ovalbumin-specific antibodies following the resulting amplification of the inflammatory response [31].
In autoimmune-prone mice, such as the New Zealand mixed (NZM) strains, silica exposure leads to increased inflammatory infiltrates, fibrotic lesions, collagen deposition, and reduced survival [32]. Although IgG levels are reduced, autoantibodies are increased in silica exposed NZM mice [32]. Following silica exposure numbers of B1a B cells and CD4+ T cells are increased in secondary lymphoid organs and the ratio of CD4+CD25+ T regulatory cells to T cells is decreased, providing possible reasons for the increased autoreactivity [33]. Autoantibodies from silica-exposed NZM mice bind preferentially to apoptotic macrophages, suggesting that silica-induced apoptosis may exacerbate autoimmune responses by exposing autoantigens [34]. Interestingly a defect in the expression of the aforementioned MARCO, that is responsible for the clearance of crystalline silica [24], results in exacerbation of autoimmunity in lupus-prone mice [35]. These findings suggest that modulation of scavenger receptor function by silica can contribute to the severity of systemic autoimmunity.
Asbestos is another naturally occurring silicate mineral, composed of long, thin fibrous crystals. Several epidemiological studies have linked exposure to asbestos to autoimmune disease [21]. In Libby, Montana exposure to asbestos-contaminated vermiculite has resulted in significant health problems including reports of an increased prevalence of systemic autoimmune disease [36]. Examination of serum samples from residents of Libby showed positive correlations between autoantibody titers and both lung disease severity and the extent of exposure [36]. A case-control study among the current and former residents of Libby who had worked at the vermiculite mine revealed increased self reporting of autoimmune diseases including systemic lupus erythematosus, scleroderma, and rheumatoid arthritis (odds ratio of 2.14, 95% CI, 0.90–5.10) [37]. Induction of autoimmunity following exposure to the Libby amphibole is also supported by studies showing that exposure of non-autoimmune C57BL/6 mice leads to autoantibodies and immune complex deposits in the kidneys [38]. It is very likely that induction of autoimmunity in mice by asbestos occurs via similar mechanisms to that being uncovered for silica.
3.1.3. Toxic Oil Syndrome
Xenobiotic-induced scleroderma, or pseudoscleroderma, has been associated with occupational exposures, especially occupational exposure to solvents [39]. In the early 1980s in Spain ingestion of adulterated cooking oil lead to an outbreak of pseudoscleroderma [40]. The causative agent was identified as rapeseed oil, originally destined for industrial use but refined to remove the anilide denaturant and then sold as cooking oil for human consumption. Thousands of people were ultimately affected with more than 1,000 deaths being ascribed to what is called Toxic Oil Syndrome (TOS) [40]. The clinical and histopathological features of TOS resemble both eosinophilia myalgia syndrome (EMS), believed to be caused by impure L-tryptophan, and idiopathic diffuse fasciitis with eosinophilia (DFE) [41]. Cases of eosinophilic fasciitis have been described to occur simultaneously with the appearance of EMS associated with L-tryptophan ingestion.
A murine model of TOS developed by continuous exposure of mice to oleic acid anilide revealed strain specific responses. B10.S (H-2s) mice exhibit the more chronic autoimmune form of disease with hypergammaglobulinemia, splenomegaly and polyclonal B cell responses associated with elevation of IL-1β and IL-6. Autoantibodies include predominantly IgM antibodies to histone, denatured DNA, as well as rheumatoid factor [42]. C57BL/6 (H-2b) mice also develop a polyclonal B cell response but without other disease manifestations [43], while A/J (H-2a) mice develop an acute wasting disease more typical of the acute lethal response of humans to the anilide. Cytokine profiles of C57BL/6 mice showed a more Th2-like response whereas the A/J mice had elevated IL- 1α, IL-10, and IFN-γ [43]. Finally, exposure of the mildly autoimmune-prone MRL-+/+ mice to anilide resulted in increased serum immunoglobulins and autoantibodies [44]. While these studies have proven useful the current models do not display the full gamut of disease found in the exposed human population. For example, no model exhibits the vasculitis, eosinophilia, and elevated IgE levels of the acute phase [45]. This suggests that species specific genetic and/or environmental factors are critical for the host response.
3.1.4. Metal Induced Autoimmunity
Mercury [46], silver [47] and gold [48] all produce autoimmunity in mice but the pathological consequences differ, with silver and gold exposure resulting in a less severe response that typically includes the production of anti-nuclear antibodies but lacks glomerular deposits of immunoglobulin and complement [48,49]. The most characteristic aspect of autoimmunity induced by these three metals is an MHC-restricted autoantibody response against fibrillarin, a nucleolar protein component of the box C/D small nucleolar ribonucleoprotein (snoRNP) complexes [50]. Such anti-fibrillarin autoantibodies also occur in a subset of scleroderma patients [51] and in SLE [52]. The features of murine mercury-induced autoimmunity (mHgIA), including lymphadenopathy, hypergammaglogulinemia, humoral autoimmunity, and immune-complex deposits, are consistent with the systemic autoimmunity of SLE [53].
Human exposure to mercury has been implicated as an environmental trigger in the induction of autoimmunity [54–56], although a non-autoimmune-mediated nephropathy is the more common outcome [57]. Few large scale epidemiological studies have addressed the association between mercury exposure and immune dysfunction including autoimmunity. A study of the New Zealand Defense Forces (20,000 individuals, 84% male) found little relationship between adverse health effects and numbers of mercury containing dental amalgam [58]. Whether this study reflects the effects of mercury exposure is debatable as exposure from dental amalgam correlates better with amalgam surfaces than the number of fillings [59]. In addition, this study used broad disease categories from the International Statistical Classification of Diseases and Related Health Problems (ICD-9 codes) and due to the small numbers of patients with individual diseases did not examine individual autoimmune disorders such as SLE. The role of mercury in human autoimmune disease will not be clarified until the level of exposure in large populations of patients has been examined.
Both the immunology of mercury and the mHgIA model have been recently reviewed in great detail [60,61] and so the following discussion is limited to those aspects we believe most relevant to induction and expression of autoimmunity. In animal models mHgIA can be induced by subcutaneous injection [62] or oral ingestion of HgCl2 [63], inhalation of mercury vapor [64], and dental [65] or peritoneal [47] implantation of mercury containing dental amalgam [47]. Depending on the strain disease can range from apparent non-responsiveness to full-blown immune cell activation, lymphadenopathy, elevated immunoglobulin levels, autoantibodies and immune complex glomerulonephritis [46,60,66,67]. The resulting autoantibody responses are primarily against chromatin, and the small nucleolar ribonucleoprotein (snoRNP) component fibrillarin [47,68,69], but can include antibodies to other components of snoRNP complexes [70], as well as to other nuclear and cytoplasmic antigens. Spontaneous human and mercury-induced murine anti-fibrillarin antibodies are both under MHC control [46,67,71] and share recognition of a conserved conformational antigenic determinant [72]. Autoantibodies in scleroderma also recognize other protein components of snoRNP complexes, particularly those of the U3 snoRNP [51] suggesting that RNA/protein complexes may be the source of immunogenic material for the anti-snoRNP response in both mercury-induced and idiopathic responses [51,70]. The mechanism of autoantibody recognition of fibrillarin in spontaneous human autoimmunity or mercury-induced autoimmunity does not involve a mercury-fibrillarin complex [62]. Of potential significance, mercury-induced cell death results in the proteolysis of fibrillarin to a 19kDa fragment which has been shown to elicit an autoantibody response similar to that produced following mercury exposure [73], whereas immunization with native fibrillarin does not elicit an autoantibody response. Thus, proteolysis associated with mercury-induced cell death results in the generation of novel protein fragments that may be sources of cryptic self-antigenic determinants to which B and T lymphocytes have not been tolerized.
mHgIA is associated with increases in the activation and proliferation of CD4+ T cells and is dependent on co-stimulatory molecules CD28 and CD40L [74–77]. Thus, mercury does not simply activate T cells directly, but, consistent with the observed increased expression of MHC class II on APCs in mHgIA [78], appears to enhance APC and T cell activity. The mechanism for how mercury induces T cell proliferation in vivo, however, remains incompletely understood [66,79,80], although the time course of the response mimics an antigen-specific response and proliferation of T cell subsets show strain specific differences [81,82]. In vitro proliferation studies have also documented the requirement for T cells, adherent cells [79–83], and IL-1 [81]. T cells also contribute to the suppression of mHgIA as demonstrated by the reduction in disease cause by CD4+CD25+ T regulatory cells [84].
Possible molecular mechanisms responsible for mercury-induced lymphocyte activation and proliferation have been further examined in in vitro studies. Low doses of inorganic mercury perturb CD95 (Fas) mediated apoptotic cell death enhancing survival of Jurkat cells (human T cell line) [85]. This effect appears to be due to disruption of both the death inducing signaling complex (DISC) [86] and death receptor mediated caspase-3 activation [87]. The failure of active DISC formation appears due to the ability of low concentrations of mercury to dissociate preassembled Fas receptor complexes required for DISC formation [88]. Thus mercury exposure would enable T cells, including self reactive T cells, to survive Fas-Fas ligand mediated cell death. However the exacerbation of mHgIA in the absence of functional Fas, as seen in B10.S-Faslpr mice (Pollard and Kono, unpublished), argues that inhibition of Fas-mediated cell signaling is not the only mechanism that can lead to mHgIA.
Another proposed mechanism is the attenuation of T cell receptor (TCR) signaling by mercury which may allow self reactive cells to escape elimination during T cell selection [89]. Experiments with Jurkat cells shows that inorganic mercury inhibits the ability of the TCR to activate Ras and ERK MAP kinase [90] that is associated with negative selection of T cells. The failure of Ras activation appears due to lack of phosphorylation of the upstream component LAT (Linker for Activation of T cells) and the lack of activation of the LAT reactive tyrosine kinase ZAP-70 [91]. The failure of ZAP-70 activation (phosphorylation) is due to the ability of mercury to inhibit phosphorylation of lymphocyte-specific protein tyrosine kinase (Lck) thus impeding early steps in TCR signal transduction [89]. Although these effects highlight the ability of mercury to impact pathways of T cell survival and selection, it must be noted that adult thymectomy does not impact development of mHgIA suggesting that T cell tolerance is broken in the periphery and not during T cell selection in the thymus (Rubin and Pollard, unpublished).
Examination of the spectrum of disease induced by mercury has identified several mouse strains that are resistant to mHgIA [46]. This resistance resides in non-MHC genes because strains with the same MHC can be either sensitive or resistant. Genome-wide scans using F2 intercrosses of the mHgIA resistant DBA/2 (H-2d) strain with the autoimmune-prone NZB (H-2d) and the mHgIA prone SJL (H-2s) identified a single major quantitative trait locus (QTL) on the distal end of chromosome 1, designated Hmr1, as responsible for resistance to development of glomerular immune-complex deposits [92]. This region of chromosome 1 in the mouse overlaps lupus susceptibility loci identified in several other murine studies [93]. The syntenic region of human chromosome 1 lies between 1q31 and 1q42 and is known to carry human lupus susceptibility loci [94–96].
The most significant information on the mechanisms involved in mHgIA have come from animal studies which have shown that individual genes can influence the initiation and development of mHgIA in a variety of ways [61,97,98]. The absence of some genes (e.g. IL-4) appears to have little effect on disease expression, while others influence specific facets of the mHgIA phenotype. Absence of other genes results in suppression of disease (e.g. IFN-γ, CD28, CD40 ligand), while others exacerbate disease (e.g. Daf1). These studies have revealed that mHgIA and idiopathic lupus share a number of effector genes in common including IFN-γ [99], CD28 and CD40 ligand [76], and Daf1 [100], and IgH6 which results in a loss of B cells [97].
Of recent interest, decay accelerating factor 1 (Daf1), has been shown to enhance T cell responses following immunization [101,102] suggesting that Daf1 acts as a negative modulator of T cell immunity. This is supported by studies showing that Daf1 deficiency exacerbates systemic [103] and organ specific [102] autoimmunity. A characteristic of the hyper-T cell responses in Daf1 deficient mice is elevated IFN-γ secretion [101,102]. The dependence of mHgIA on the presence of IFN-γ [99], coupled with the association of reduced Daf1 on activated T cells in mHgIA [100] and the elevated Daf1 levels in mHgIA-resistant DBA/2 [100] argues that Daf1 is a potentially important regulator of chemical-induced autoimmunity. Daf1 lies at the proximal end of the Hmr1 locus and shows greater expression in mHgIA-resistant DBA/2 mice than the autoimmune-prone NZB [100]. Induction of mHgIA leads to a reduction of Daf1 expression on activated/memory CD4+ T cells in mHgIA-sensitive B10.S mice [100]. Mercury exposed DBA/2 mice, which show no change in Daf1 expression following exposure to mercury, fail to accumulate activated/memory CD4+ T cells. Reduction of Daf1 expression in mHgIA was found to require CD4+ T cell co-stimulation as mercury exposure of CD28 deficient mice did not result in an increase of CD44high Daf1low CD4+ T cells [100]. Mercury treatment of C57BL/6 Daf1 deficient mice results in increased levels of serum immunoglobulins, autoantibodies and increased expression of inflammatory cytokines including IFN-γ (Toomey and Pollard, unpublished). Thus Daf1 plays an important role in modulating adaptive immune responses, although the mechanism of action remains unclear.
IFN-γ is required for mHgIA with even mice heterogeneous for the gene deletion showing dramatically reduced disease [99]. Identification of increased IFN-γ expression in lymphoid organs in mHgIA has proven difficult to demonstrate however [98,99], although a small increase has been noted in the most susceptible mouse strain A.SW [104]. Cytokine expression appears greatest at the site of exposure [98] with significant increases of IFN-γ being observed in a number of cell types (Cauvi and Pollard, unpublished). IL-1β is also significantly elevated as is NLRP3 (Toomey and Pollard, unpublished) suggesting that activation of the inflammasome [25] is an early event in mHgIA. Interestingly absence of IFN-γ reduces both IL-1β and NALP3 (Toomey and Pollard, unpublished) hinting that the ability of IFN-γ to activate the STAT signaling pathway leading to caspase-1 expression and cell death [105,106] may contribute to the role of the inflammasome in mHgIA.
Mercury exposure of lupus-prone mice (NZBWF1, BXSB, MRL-+/+) leads to acceleration of the underlying autoimmune predisposition [107]. Studies in female BXSB mice show that this acceleration is dose dependent and has features of spontaneous disease [75]. Furthermore, tissue mercury levels of mice exposed to lower doses (0.4 μg HgCl2/injection) fell within the range found in non-occupationally exposed humans [59]; yet these mice have accelerated anti-chromatin antibodies and proteinuria. This suggests that environmentally relevant tissue levels of mercury may be associated with exacerbations of autoimmunity in genetically susceptible hosts [75].
3.2. Organ-Specific Autoimmunity
3.2.1. Thyroiditis
Autoimmune thyroiditis, also known as chronic lymphocytic or Hashimoto’s thyroiditis, is characterized by autoantibodies to thyroid-specific antigens such as thyroglobulin and thyroperoxidase that lead to inflammation and eventual impairment of the thyroid gland [108]. Excess iodine ingestion has been identified as a contributing factor in the induction and exacerbation of autoimmune thyroiditis [109,110]. Experimental autoimmune thyroiditis (EAT) is induced in mice by immunization with mouse thyroglobulin and adjuvant [111]. The disease is characterized by infiltration of the thyroid by T cells, B cells and macrophages [112], and is influenced by differences in MHC genotype [113]. EAT does not require IFN-γ [114], its receptor [115] or interferon regulatory factor 1 (IRF-1) [116], which is one of the earliest genes expressed in response to IFN-γ. These observations support earlier studies showing that administration of IFN-γ suppresses EAT [117]. This lack of dependence on IFN-γ contrasts with findings in the NOD.H2h4 mouse, a cross between the non-obese diabetic (NOD) mouse and the B10.A (4R) mouse, which develops spontaneous autoimmune thyroiditis (SAT), but not automimmune insulin dependent diabetes mellitus (IDDM) [118]. Ingestion of excess iodine accelerates the development of thyroid lesions and IgG2a, IgG2b and IgM anti-thyroglobulin antibodies in these mice [118]. SAT requires both CD4+ and CD8+ T cells [119], B cells [120], and IFN-γ [121]. Depletion of CD25+ T regulatory cells in these mice exacerbates thyroiditis, anti-thyroglobulin antibodies and mRNA levels of both IL-4 and IFN-γ [122]. The contrasting role of IFN-γ in these two models of autoimmune thyroiditis remains unexplained but may be influenced by various factors including the amount of IFN-γ produced, site of expression, its presence at various stages of the inflammatory response [123], or the likelihood that EAT, like similarly adjuvant-induced experimental autoimmune encephalomyelitis (EAE) may be also dependent on IL-17 and T cells. It is also possible that the difference in dependence for IFN-γ reflects requirement for this cytokine for antibody production to weakly antigenic self molecules but not for immunization in the presence of adjuvant [99].
3.2.2 Autoimmune liver disease
Various chemicals and drugs have been implicated in autoimmune liver diseases including tienilic acid, dihydralazine, and halothane in autoimmune hepatitis [124]. Moreover, primary biliary cirrhosis (PBC) which is characterized by anti-mitochondrial antibodies (AMA) is also argued to be associated with environmental factors [125]. AMA recognize the inner lipoyl domain of the E2 subunits of 2-oxo-acid dehydrogenase complexes, in particular the E2 component of the pyruvate dehydrogenase complex (PDC-E2) [125]. Synthetic chemical mimics representing a xenobiotically modified lipoyl hapten led to identification of structures that are recognized by AMA with affinities higher than the parent lipoylated-PDC-E2 [125], and such xenobiotically modified antigens can be used to induce PBC in animals. Immunization of rabbits with one of these compounds, 6-bromohexanoate, conjugated to bovine serum albumin (BSA) elicit AMA but no liver pathology, while in contrast, guinea pigs immunized with the same synthetic mimic resulted in both AMA and autoimmune cholangitis [126]. A further study reported two new xenobiotic-induced PBC murine models based on immunization with 2-octynoic acid. NOD.1101 mice developed high titer AMA and liver pathology, including portal infiltrates enriched in CD8+ T cells and liver granulomas [127] while C57BL/6 mice manifested AMA, autoimmune cholangitis, increased liver lymphoid cell numbers, an increase in CD8+ liver infiltrating cells, and elevation of serum TNFα and IFN-γ [128]. However, both models of liver injury failed to progress to cirrhosis [129].
Genetic manipulation has also produced several murine strains that exhibit features of PBC [130]. They include the dnTGFβRII mice which express a dominant negative form of the TGFβ receptor under control of the CD4 promoter resulting in the production of AMA and lymphocytic infiltration in the liver with periportal inflammation. The IL-2Rα knockout, which does not express CD25 and thus lacks T regulatory cells, also develops AMA and portal inflammation, but also biliary ductular damage. Yet another model is the congenic NOD.c3c4 mouse, which has genetic material from B6 and B10 mice introduced into the NOD mouse, a well-characterized spontaneous model of autoimmune type 1 diabetes. The NOD.c3c4 develops biliary lymphocytic infiltrates, autoantibodies, and progressive, often fatal, biliary obstruction. Whether toxicants associated with autoimmune liver diseases exacerbate disease in these spontaneous models has yet to be reported.
3.3. Other Examples
There are a number of other agents associated with perturbation of the human immune system and autoimmunity, a few of which have been tested for their ability to induce autoimmune disease in animals. The adjuvant properties of certain hydrocarbons can precipitate inflammatory or autoimmune disease in humans and animals and this topic has been recently reviewed in detail [131]. Tetramethylpentadecane (TMPD) is a common constituent of mineral oil and is found in a number of processed foods made for human consumption. Exposure can elicit “lipogranulomas” (follicular lipidosis), a chronic inflammatory response, in a number of organs (e.g. liver, spleen, lymph nodes) [132]. Adjuvant oils, especially TMPD (commonly known as pristane) [131], elicit systemic autoimmunity in otherwise healthy mice [133], and exacerbates autoimmunity in lupus-prone NZBWF1 mice [134]. Induction of autoimmunity is promoted by both type I (IFN-α and -β) and type II (IFN-γ) interferons and IL-12 [131]. Absence of IL-6 reduces anti-chromatin and anti-DNA antibodies but not anti-RNP, anti-Sm, or anti-Su [135], suggesting that induction of these autoantibody specificities can occur by different pathways, or differ in their susceptibility to TMPD. Exposure of mice to TMPD results in many features of SLE including female preponderance, arthritis, and glomerulonephritis [131]. TMPD exposed mice also possess the “interferon signature” associated with human SLE [136]; the lupus-interferon signature consists of the over expression of interferon-induced genes in the peripheral blood cells of SLE patients [137]. TMPD-induced disease requires Fas, a member of the TNF-receptor superfamily that plays a central role in the physiological regulation of cell death, as C57BL/6 mice lacking either Fas or Fas ligand do not develop autoimmunity [138]; this contrasts with the mHgIA model (Pollard and Kono, unpublished), procainamide-induced lupus [17], and lupus-prone MRL-Faslpr mice [139] in which absence of Fas exacerbates autoimmunity. Another difference between pristane-induced autoimmunity and both mHgIA (Kono and Pollard, unpublished) and lupus-prone MRL-Faslpr mice [140] is the reduced role that type I interferons plays in the latter two models. Another unique feature of pristane-induced autoimmunity is the spectrum of autoantibodies produced (anti-DNA, anti-ribosomal P, anti-Su, anti-chromatin and anti-anti-RNP/Sm) which mimics the diversity of autoantibody responses in SLE [131].
The halogenated aromatic hydrocarbon 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most potent of the dioxins and is an unintentional by-product of many industrial processes. TCDD is also a contaminant of the herbicide Agent Orange [141]. The role of TCDD in autoimmune responses is controversial. Seventeen years after exposure to TCDD eighteen workers were in good health although they did have significantly greater frequency of anti-nuclear antibodies and immune complexes in their serum, and increased numbers of Leu-7+ (CD57) natural killer cells compared to matched controls [142]. Korean veterans of the Vietnam War, who did military service from 1964 to 1973 and were exposed to TCDD contaminated Agent Orange, had reduced IgG levels especially IgG1 and reduced IFN-γ, but increased IgE, IL-4 and IL-10 suggesting disturbed immune-homeostasis [143]. Lymphocytes from a group of German industrial workers showed reduced proliferative responses in the mixed lymphocyte reaction or with IL-2 suggesting mild immunosuppression 20 years after TCDD exposure [144]. Reduced IgG levels were also found in individuals exposed to TCDD following an industrial accident in Seveso, Italy [145]. Moreover, children exposed to TCDD in the same accident had initially reduced total lymphocyte numbers and total serum complement activity that returned to normal within ten years [146]. In contrast, examination of 995 individuals involved in aerial spraying of herbicides in Vietnam reported similar health problems to 1299 controls [147], however the TCDD exposure of these two groups was not quantitatively determined. These epidemiological studies suggest that while TCDD does influence the human immune system there is little evidence of autoimmunity.
In contrast, animal studies of TCDD have revealed novel and intriguing affects on the immune system. The immunosuppressive effects of TCDD have been argued due to activation of the aryl hydrocarbon receptor (Ahr) by TCDD and the generation of CD4+ CD25+ T regulatory cells [148]. Moreover Ahr activation by TCDD can induce T regulatory cells that suppress experimental autoimmune encephalomyelitis (EAE) while another Ahr ligand, 6-formylindolo[3,2-b]carbazole (FICZ-carbazole), interfers with T regulatory cell development, boosting Th17 cell development and increasing the severity of EAE [149]. Thus modulation of the Ahr influences both suppression and development of autoimmunity [150]. Neonatal exposure of NFS/sld mice to TCDD elicited a phenotype consistent with the human autoimmune disease Sjögren’s Syndrome with increased anti-SS-A/Ro and anti-SS-b/LA autoantibodies and elevated IL-2 and IFN-γ [151]. In this model TCDD exposure was associated with reduced AIRE, a protein that has been found to be important for the maintenance of self tolerance [152], suggesting that thymocyte differentiation and central tolerance may be disrupted by TCDD [151].
Trichloroethylene (TCE) is a chlorinated hydrocarbon commonly used as an industrial solvent. Exposure of moderately lupus-prone MRL-+/+ mice to TCE exacerbates features of autoimmunity in adult mice [153–155] as well mice exposed from conception [156], and recent studies have suggested that TCE-induced protein oxidation (carbonylation and nitration) may be responsible [157]. Although these studies provide compelling evidence that TCE is capable of exacerbating autoimmunity in mice, occupational cohorts with exposures to TCE or other specific solvents lack well-characterized exposure histories that would support its role as a causative agent in human autoimmunity [157,158].
Exposure to the fungicide hexachlorobenzene (HCB) is associated with a form of hepatic porphyria called porphyria turcica, which resembles porphyria cutanea tarda, a disease caused by altered porphyrin metabolism. Porphyria turcica is associated with bullous skin lesions, mainly in sun-exposed skin areas, that heal with severe scars, and primarily affects children 6–16 years of age [159]. Other reported clinical manifestations include hepatomegaly, enlarged thyroid, splenomegaly, hyperpigmentation, hirsutism, enlarged lymph nodes and painless arthritic changes of the hands, with the arthritis increasing in prevalence 3–5 years after exposure [159]. Exposure to HCB can also produce neurological, dermatological, and orthopaedic abnormalities that may persist for decades [160]. Elevated levels of HCB in the blood, however, correlate with reduced IFN-γ, but only weakly with other immune abnormalities [161]. Studies in Brown Norway rats suggests that HCB may act as an adjuvant, activating macrophages and generating pro-inflammatory cytokines and polyclonal activation of T and B cells leading to visible clinical sign such as skin lesions and lung eosinophilia [162].
As described above, a common approach in testing the effects of chemicals on autoimmunity is to expose autoimmune-prone strains and determine if the chemical in question alters the natural history of the disease. Other chemicals examined in this way include the synthetic steroid diethylstilbesterol [163], organochlorine pesticides [164], and the metals cadmium [165] and lead [166].
A significant problem demonstrated by a number of the agents described above, including mercury, TMPD, TCDD, TCE and HBC, is the difficulty in determining the relevance of exposure to human autoimmune diseases. While each of these toxicants does elicit immunological responses, evidence for a significant role in human autoimmunity is lacking for the most part because adequate studies are difficult and have not been done. However many of these agents do induce autoimmunity in animal models with significant similarities to human disease [131].
4. Mechanisms of Toxicant Induced Autoimmunity
Experimental data from animal models suggest that induction of autoimmunity by drugs and chemicals can occur through different mechanisms. Drugs such as procainamide and contaminants like TCDD can influence central tolerance mechanisms, solvents such as TCE can perturb the development of the immune system in utero, and a lowered threshold of activation for T cells appears to contribute to autoimmunity induced by mercury and procainamide. Autoimmunity induced by silica, asbestos, and heavy metals such as mercury are also associated with chronic inflammatory processes, cell death, suppression of immunoregulation, and perturbation of self/non-self discrimination. It is also important to consider that extrinsic agents may interact with self-molecules and that such interactions may affect development of autoimmunity [167]; however in the majority of situations in which chemicals, particularly drugs, induce autoimmunity it is clear that a chemical–self-antigen conjugate is not the target of an autoantibody response [7,62]. Additionally, most extrinsic agents can exacerbate autoimmunity in the presence of a lupus-prone genotype indicating a role for genetic susceptibility in toxicant-induced autoimmunity. While it is unlikely that toxicant-induced autoimmunity can be explained by a single mechanism, the development of disease following exposure to individual agents can share certain aspects with the pathogenesis of spontaneous autoimmunity (Table 2).
Table 2.
Features of autoimmunity shared by different autoimmunity-inducing drugs and chemicals.
| Drugs/Chemicals | Mechanism | References |
|---|---|---|
| Silica/Asbestos | Adjuvant effect | 21 |
| T regulatory cells | 33 | |
| Inflammasome (NLRP3a, IL-1β) | 28 | |
| Exacerbates lupus-prone genotype | 32 | |
| Heavy Metals (Hg, Ag, Au) | T cell activation threshold | 86 |
| T regulatory cells | 80 | |
| Inflammasome (NLRP3a, IL-1β) | Toomey and Pollard (unpublished) | |
| Exacerbates lupus-prone genotype | 76, 108 | |
| Drugs (procainamide) | T cell activation threshold | 12 |
| Central tolerance | 18 | |
| TCDD, dioxinb | Central tolerance | 152 |
| Aryl hydrocarbon receptor | 150 | |
| T regulatory cells | 150 | |
| Adjuvant oils (pristane) | Adjuvant effect | 134 |
| Exacerbates lupus-prone genotype | 135 | |
| Pesticides/Fungicides (Hexachlorobenzene) | Adjuvant effect | 162 |
| Exacerbates lupus-prone genotype | 164 | |
| Adulterated rapseed oil | Exacerbates lupus-prone genotype | 44 |
| Iodine | T regulatory cells | 110 |
| Trichloroethylene (TCE) | Exacerbates lupus-prone genotype | 153 |
NOD-like receptor family, pryin domain containing 3
2,3,7,8-Tetrachlorodibenzo-p-dioxin
Induction of systemic autoimmunity by drugs and chemicals requires a source of self antigens. However an unanswered question, especially for intracellular antigens, is how and in what form the inciting self antigens are made available to the immune system. For some toxicants this is very likely via mechanisms of cell death that may be reflective of the toxicant rather than an established biological process such as classical apoptosis. For example, in the case of mHgIA it appears that an aberrant form of cell death results in altered proteolysis of cellular material [73], while in the case of silica it has been suggested that engulfment of silica by alveolar macrophages leads to disruption of the normal internalization process of endocytosis, leading to cytokine release and cell death [23]. Whatever the process, these mechanisms most likely lead to activation of pattern recognition receptors, such as Toll-like receptors (TLR) and Nod-like receptors (NLR) (Figure 1), that are essential elements in the activation of the innate immune system. The resulting response includes both inflammation following proinflammatory cytokine production via the inflammasome and NLRP3 [25,28] and activation of the TLR system though recognition of complexes of RNA and DNA bound to nuclear proteins. In the latter case it is the specificity of TLRs for their cognate (nuclear) ligands that is required for the production of the anti-nuclear autoantibodies that characterize many forms of idiopathic [168] and chemical-induced autoimmunity (Kono and Pollard, unpublished).
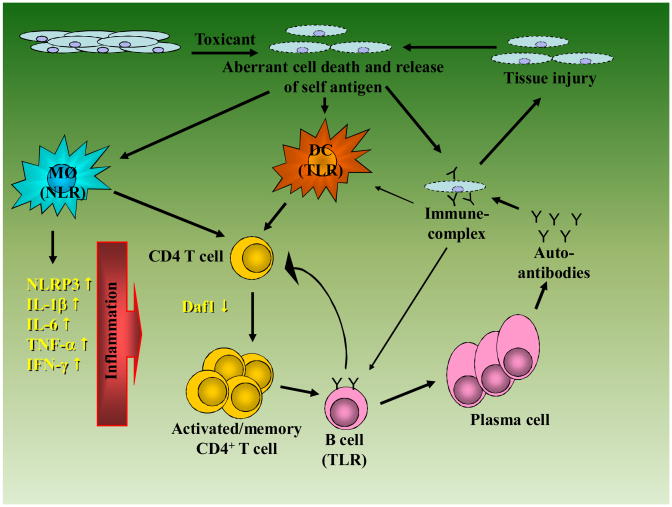
Figure 1. Putative mechanism of drug/chemical-induced autoimmunity.
Exposure to a toxicant results in aberrant cell death making available cellular material which activates Nod-like receptors (NLR) and Toll-like receptors (TLR). In the case of NLR activation this leads to NLRP3-inflammasome activation and proinflammatory events including production of proinflammatory cytokines such as IL-1β, IL-6, and IFN-γ. For TLR-mediated response, activation of TLR by nuclear material is essential for autoantibody responses to nuclear antigens such as chromatin and RNA/protein complexes. Activation of self-reactive T cells proceeds via breaking of self-tolerance which may be mediated by a number of mechanisms (see text). Reduction in the expression of regulators of T cell activation, such as Daf1, enhances T cell responses and promotes the activation of autoantibody producing B cells. The binding of autoantibodies to self-antigen leads to immune-complex formation and tissue injury which in turn can release cellular material to amplify the response. Self-antigen containing immune complexes can also be taken up by B cells and other antigen presenting cells (e.g. dendritic cells) and amplify activation of autoreactive T cells.
The activation of elements of the innate immune system, dendritic cells and macrophages, by cellular material is accompanied by antigen presentation to T cells. Activation of self-reactive T cells proceeds via breaking of self-tolerance which may be mediated by a number of mechanisms including inhibition of Fas-mediated cell death as suggested in the case of mercury exposure [85,88]. Defects in expression of regulators of T cell function such as Daf1 [100] can enhance T cell responses which in turn leads to activation of autoantibody producing B cells. The formation of immune complexes of self-antigen and autoantibodies provides material that can be bound by antigen presenting cells such as B cells and macrophages leading to further rounds of T cell activation and expansion. Deposition of immune-complexes in tissues such as the kidney can lead to tissue injury which can provide additional cellular material for the activation of innate immune cells. Although this appears to be a vicious cycle it is easy to see how, in the absence of a lupus-prone genotype, removal of the toxicant would result in a significant attenuation of the process.
5. Conclusion
There is ample evidence that exposure to a variety of drugs and chemicals can lead to autoimmunity. In many situations the drug or chemical-induced disease resembles idiopathic disease although the presence of disease is dependent upon exposure to the drug or chemical. Animal model studies have revealed a number of possible mechanisms, some of which are shared by different drug and chemical agents. Intriguingly exposure of autoimmune-prone mice to many autoimmune-inducing chemicals and drugs leads to earlier onset and exacerbation of disease arguing that exposure in the presence of a susceptible genotype likely increases the risk of developing human autoimmunity.
Acknowledgments
This work was supported by National Institutes of Health grants ES007511 and ES014847 to KMP and ES08666, AR053731, and AR42242 to DHK. This is manuscript number 20422 from the Scripps Research Institute.
References
- 1.Tan EM, Chan EK, Sullivan KF, Rubin RL. Antinuclear antibodies (ANAs): diagnostically specific immune markers and clues toward the understanding of systemic autoimmunity. Clin Immunol Immunopathol. 1988;47:121–41. doi: 10.1016/0090-1229(88)90066-9. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 3.Lam-Tse WK, Lernmark A, Drexhage HA. Animal models of endocrine/organ-specific autoimmune diseases: do they really help us to understand human autoimmunity? Springer Semin Immunopathol. 2002;24:297–321. doi: 10.1007/s00281-002-0110-2. [DOI] [PubMed] [Google Scholar]
- 4.Badenhoop K, Boehm BO. Genetic susceptibility and immunological synapse in type 1 diabetes and thyroid autoimmune disease. Exp Clin Endocrinol Diabetes. 2004;112:407–15. doi: 10.1055/s-2004-821206. [DOI] [PubMed] [Google Scholar]
- 5.Dedeoglu F. Drug-induced autoimmunity. Curr Opin Rheumatol. 2009 doi: 10.1097/BOR.0b013e32832f13db. [DOI] [PubMed] [Google Scholar]
- 6.Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res. 2009;301:99–105. doi: 10.1007/s00403-008-0895-5. [DOI] [PubMed] [Google Scholar]
- 7.Rubin RL. Drug-induced lupus. Toxicology. 2005;209:135–47. doi: 10.1016/j.tox.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Totoritis MC, Rubin RL. Drug-induced lupus. Genetic, clinical, and laboratory features. Postgrad Med. 1985;78:149–52. 155–61. doi: 10.1080/00325481.1985.11699121. [DOI] [PubMed] [Google Scholar]
- 9.Rubin RL, Teodorescu M, Beutner EH, Plunkett RW. Complement-fixing properties of antinuclear antibodies distinguish drug-induced lupus from systemic lupus erythematosus. Lupus. 2004;13:249–56. doi: 10.1191/0961203304lu1007oa. [DOI] [PubMed] [Google Scholar]
- 10.Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, Richardson B. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol. 1988;140:2197–200. [PubMed] [Google Scholar]
- 11.Yung RL, Quddus J, Chrisp CE, Johnson KJ, Richardson BC. Mechanism of drug-induced lupus. I. Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025–35. [PubMed] [Google Scholar]
- 12.Richardson BC. Epigenetics and autoimmunity. Overview. Autoimmunity. 2008;41:243–4. doi: 10.1080/08916930802024129. [DOI] [PubMed] [Google Scholar]
- 13.Lu Q, Wu A, Richardson BC. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. J Immunol. 2005;174:6212–9. doi: 10.4049/jimmunol.174.10.6212. [DOI] [PubMed] [Google Scholar]
- 14.Ballestar E, Esteller M, Richardson BC. The epigenetic face of systemic lupus erythematosus. J Immunol. 2006;176:7143–7. doi: 10.4049/jimmunol.176.12.7143. [DOI] [PubMed] [Google Scholar]
- 15.Rubin RL, Bell SA, Burlingame RW. Autoantibodies associated with lupus induced by diverse drugs target a similar epitope in the (H2A-H2B)-DNA complex. J Clin Invest. 1992;90:165–73. doi: 10.1172/JCI115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin RL, Kretz-Rommel A. Initiation of autoimmunity by a reactive metabolite of a lupus-inducing drug in the thymus. Environ Health Perspect. 1999;107(Suppl 5):803–6. doi: 10.1289/ehp.99107s5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kretz-Rommel A, Rubin RL. Persistence of autoreactive T cell drive is required to elicit anti-chromatin antibodies in a murine model of drug-induced lupus. J Immunol. 1999;162:813–20. [PubMed] [Google Scholar]
- 18.Kretz-Rommel A, Rubin RL. Disruption of positive selection of thymocytes causes autoimmunity. Nat Med. 2000;6:298–305. doi: 10.1038/73152. [DOI] [PubMed] [Google Scholar]
- 19.Rubin RL, Kretz-Rommel A. A nondeletional mechanism for central T-cell tolerance. Crit Rev Immunol. 2001;21:29–40. [PubMed] [Google Scholar]
- 20.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107(Suppl 5):793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otsuki T, Maeda M, Murakami S, Hayashi H, Miura Y, Kusaka M, Nakano T, Fukuoka K, Kishimoto T, Hyodoh F, Ueki A, Nishimura Y. Immunological effects of silica and asbestos. Cell Mol Immunol. 2007;4:261–8. [PubMed] [Google Scholar]
- 22.Hamilton JA. Nondisposable materials, chronic inflammation, and adjuvant action. J Leukoc Biol. 2003;73:702–12. doi: 10.1189/jlb.0103037. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton RF, Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44:1246–58. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakur SA, Beamer CA, Migliaccio CT, Holian A. Critical role of MARCO in crystalline silica-induced pulmonary inflammation. Toxicol Sci. 2009;108:462–71. doi: 10.1093/toxsci/kfp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–9. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 27.Willingham SB, Ting JP. NLRs and the dangers of pollution and aging. Nat Immunol. 2008;9:831–3. doi: 10.1038/ni0808-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med. 2005;11:169–73. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- 30.Davis GS, Leslie KO, Hemenway DR. Silicosis in mice: effects of dose, time, and genetic strain. J Environ Pathol Toxicol Oncol. 1998;17:81–97. [PubMed] [Google Scholar]
- 31.Granum B, Gaarder PI, Groeng E, Leikvold R, Namork E, Lovik M. Fine particles of widely different composition have an adjuvant effect on the production of allergen-specific antibodies. Toxicol Lett. 2001;118:171–81. doi: 10.1016/s0378-4274(00)00292-7. [DOI] [PubMed] [Google Scholar]
- 32.Brown JM, Archer AJ, Pfau JC, Holian A. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin Exp Immunol. 2003;131:415–21. doi: 10.1046/j.1365-2249.2003.02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JM, Pfau JC, Holian A. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal Toxicol. 2004;16:133–9. doi: 10.1080/08958370490270936. [DOI] [PubMed] [Google Scholar]
- 34.Pfau JC, Brown JM, Holian A. Silica-exposed mice generate autoantibodies to apoptotic cells. Toxicology. 2004;195:167–76. doi: 10.1016/j.tox.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Rogers NJ, Lees MJ, Gabriel L, Maniati E, Rose SJ, Potter PK, Morley BJ. A defect in Marco expression contributes to systemic lupus erythematosus development via failure to clear apoptotic cells. J Immunol. 2009;182:1982–90. doi: 10.4049/jimmunol.0801320. [DOI] [PubMed] [Google Scholar]
- 36.Pfau JC, Sentissi JJ, Weller G, Putnam EA. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environ Health Perspect. 2005;113:25–30. doi: 10.1289/ehp.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noonan CW, Pfau JC, Larson TC, Spence MR. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environ Health Perspect. 2006;114:1243–7. doi: 10.1289/ehp.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfau JC, Sentissi JJ, Li S, Calderon-Garciduenas L, Brown JM, Blake DJ. Asbestos-induced autoimmunity in C57BL/6 mice. J Immunotoxicol. 2008;5:129–37. doi: 10.1080/15476910802085756. [DOI] [PubMed] [Google Scholar]
- 39.Kettaneh A, Al Moufti O, Tiev KP, Chayet C, Toledano C, Fabre B, Fardet L, Cabane J. Occupational exposure to solvents and gender-related risk of systemic sclerosis: a metaanalysis of case-control studies. J Rheumatol. 2007;34:97–103. [PubMed] [Google Scholar]
- 40.Posada de la Paz M, Philen RM, Borda AI. Toxic oil syndrome: the perspective after 20 years. Epidemiol Rev. 2001;23:231–47. doi: 10.1093/oxfordjournals.epirev.a000804. [DOI] [PubMed] [Google Scholar]
- 41.Patterson R, Germolec D. Review article toxic oil syndrome: review of immune aspects of the disease. J Immunotoxicol. 2005;2:51–8. doi: 10.1080/15476910590960143. [DOI] [PubMed] [Google Scholar]
- 42.Bell SA, Hobbs MV, Rubin RL. Isotype-restricted hyperimmunity in a murine model of the toxic oil syndrome. J Immunol. 1992;148:3369–76. [PubMed] [Google Scholar]
- 43.Berking C, Hobbs MV, Chatelain R, Meurer M, Bell SA. Strain-dependent cytokine profile and susceptibility to oleic acid anilide in a murine model of the toxic oil syndrome. Toxicol Appl Pharmacol. 1998;148:222–8. doi: 10.1006/taap.1997.8327. [DOI] [PubMed] [Google Scholar]
- 44.Cai P, Khan MF, Kaphalia BS, Ansari GA. Immunotoxic Response of Oleic Acid Anilide and its Hydrolysis Products in Female MRL (+/+) Mice. J Immunotoxicol. 2005;2:231–6. doi: 10.1080/15476910500362960. [DOI] [PubMed] [Google Scholar]
- 45.Hard GC. A search for an animal model of the Spanish toxic oil syndrome. Food Chem Toxicol. 2002;40:1551–67. doi: 10.1016/s0278-6915(02)00114-x. [DOI] [PubMed] [Google Scholar]
- 46.Hultman P, Bell LJ, Enestrom S, Pollard KM. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin Immunol Immunopathol. 1992;65:98–109. doi: 10.1016/0090-1229(92)90212-7. [DOI] [PubMed] [Google Scholar]
- 47.Hultman P, Johansson U, Turley SJ, Lindh U, Enestrom S, Pollard KM. Adverse immunological effects and autoimmunity induced by dental amalgam and alloy in mice. Faseb J. 1994;8:1183–90. doi: 10.1096/fasebj.8.14.7958626. [DOI] [PubMed] [Google Scholar]
- 48.Havarinasab S, Johansson U, Pollard KM, Hultman P. Gold causes genetically determined autoimmune and immunostimulatory responses in mice. Clin Exp Immunol. 2007;150:179–88. doi: 10.1111/j.1365-2249.2007.03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson U, Hansson-Georgiadis H, Hultman P. Murine silver-induced autoimmunity: silver shares induction of antinucleolar antibodies with mercury, but causes less activation of the immune system. Int Arch Allergy Immunol. 1997;113:432–43. doi: 10.1159/000237619. [DOI] [PubMed] [Google Scholar]
- 50.Baserga SJ, Yang XD, Steitz JA. An intact Box C sequence in the U3 snRNA is required for binding of fibrillarin, the protein common to the major family of nucleolar snRNPs. Embo J. 1991;10:2645–51. doi: 10.1002/j.1460-2075.1991.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang JM, Hildebrandt B, Luderschmidt C, Pollard KM. Human scleroderma sera contain autoantibodies to protein components specific to the U3 small nucleolar RNP complex. Arthritis Rheum. 2003;48:210–7. doi: 10.1002/art.10729. [DOI] [PubMed] [Google Scholar]
- 52.Van Eenennaam H, Vogelzangs JH, Bisschops L, Te Boome LC, Seelig HP, Renz M, De Rooij DJ, Brouwer R, Pluk H, Pruijn GJ, Van Venrooij WJ, Van Den Hoogen FH. Autoantibodies against small nucleolar ribonucleoprotein complexes and their clinical associations. Clin Exp Immunol. 2002;130:532–40. doi: 10.1046/j.1365-2249.2002.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynes MA, Fontenot AP, Lawrence DA, Rosenspire AJ, Pollard KM. Gene expression influences on metal immunomodulation. Toxicol Appl Pharmacol. 2005 doi: 10.1016/j.taap.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 54.Silva IA, Nyland JF, Gorman A, Perisse A, Ventura AM, Santos EC, Souza JM, Burek CL, Rose NR, Silbergeld EK. Mercury exposure, malaria, and serum antinuclear/antinucleolar antibodies in Amazon populations in Brazil: a cross-sectional study. Environ Health. 2004;3:11. doi: 10.1186/1476-069X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31:1928–33. [PubMed] [Google Scholar]
- 56.Abreu Velez AM, Warfvinge G, Herrera WL, Abreu Velez CE, Montoya MF, Hardy DM, Bollag WB, Hashimoto K. Detection of mercury and other undetermined materials in skin biopsies of endemic pemphigus foliaceus. Am J Dermatopathol. 2003;25:384–91. doi: 10.1097/00000372-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Van Vleet TR, Schnellmann RG. Toxic nephropathy: environmental chemicals. Semin Nephrol. 2003;23:500–8. doi: 10.1016/s0270-9295(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 58.Bates MN, Fawcett J, Garrett N, Cutress T, Kjellstrom T. Health effects of dental amalgam exposure: a retrospective cohort study. Int J Epidemiol. 2004;33:894–902. doi: 10.1093/ije/dyh164. [DOI] [PubMed] [Google Scholar]
- 59.Nylander M, Friberg L, Lind B. Mercury concentrations in the human brain and kidneys in relation to exposure from dental amalgam fillings. Swed Dent J. 1987;11:179–87. [PubMed] [Google Scholar]
- 60.Schiraldi M, Monestier M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009;30:502–9. doi: 10.1016/j.it.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Vas J, Monestier M. Immunology of mercury. Ann N Y Acad Sci. 2008;1143:240–67. doi: 10.1196/annals.1443.022. [DOI] [PubMed] [Google Scholar]
- 62.Pollard KM, Lee DK, Casiano CA, Bluthner M, Johnston MM, Tan EM. The autoimmunity-inducing xenobiotic mercury interacts with the autoantigen fibrillarin and modifies its molecular and antigenic properties. J Immunol. 1997;158:3521–8. [PubMed] [Google Scholar]
- 63.Hultman P, Nielsen JB. The effect of toxicokinetics on murine mercury-induced autoimmunity. Environ Res. 1998;77:141–8. doi: 10.1006/enrs.1998.3833. [DOI] [PubMed] [Google Scholar]
- 64.Warfvinge K, Hansson H, Hultman P. Systemic autoimmunity due to mercury vapor exposure in genetically susceptible mice: dose-response studies. Toxicol Appl Pharmacol. 1995;132:299–309. doi: 10.1006/taap.1995.1111. [DOI] [PubMed] [Google Scholar]
- 65.Hultman P, Lindh U, Horsted-Bindslev P. Activation of the immune system and systemic immune-complex deposits in Brown Norway rats with dental amalgam restorations. J Dent Res. 1998;77:1415–25. doi: 10.1177/00220345980770060601. [DOI] [PubMed] [Google Scholar]
- 66.Pollard KM, Hultman P. Effects of mercury on the immune system. Met Ions Biol Syst. 1997;34:421–40. [PubMed] [Google Scholar]
- 67.Hultman P, Bell LJ, Enestrom S, Pollard KM. Murine susceptibility to mercury. II. autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin Immunol Immunopathol. 1993;68:9–20. doi: 10.1006/clin.1993.1088. [DOI] [PubMed] [Google Scholar]
- 68.Hultman P, Turley SJ, Enestrom S, Lindh U, Pollard KM. Murine genotype influences the specificity, magnitude and persistence of murine mercury-induced autoimmunity. J Autoimmun. 1996;9:139–49. doi: 10.1006/jaut.1996.0017. [DOI] [PubMed] [Google Scholar]
- 69.Hultman P, Enestrom S, Pollard KM, Tan EM. Anti-fibrillarin autoantibodies in mercury-treated mice. Clin Exp Immunol. 1989;78:470–7. [PMC free article] [PubMed] [Google Scholar]
- 70.Yang JM, Baserga SJ, Turley SJ, Pollard KM. Fibrillarin and other snoRNP proteins are targets of autoantibodies in xenobiotic-induced autoimmunity. Clin Immunol. 2001;101:38–50. doi: 10.1006/clim.2001.5099. [DOI] [PubMed] [Google Scholar]
- 71.Arnett FC, Reveille JD, Goldstein R, Pollard KM, Leaird K, Smith EA, Leroy EC, Fritzler MJ. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum. 1996;39:1151–60. doi: 10.1002/art.1780390712. [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi K, Turley SJ, Tan EM, Pollard KM. Analysis of the autoantibody response to fibrillarin in human disease and murine models of autoimmunity. J Immunol. 1995;154:961–71. [PubMed] [Google Scholar]
- 73.Pollard KM, Pearson DL, Bluthner M, Tan EM. Proteolytic cleavage of a self-antigen following xenobiotic-induced cell death produces a fragment with novel immunogenic properties. J Immunol. 2000;165:2263–70. doi: 10.4049/jimmunol.165.4.2263. [DOI] [PubMed] [Google Scholar]
- 74.Johansson U, Sander B, Hultman P. Effects of the murine genotype on T cell activation and cytokine production in murine mercury-induced autoimmunity. J Autoimmun. 1997;10:347–55. doi: 10.1006/jaut.1997.0149. [DOI] [PubMed] [Google Scholar]
- 75.Pollard KM, Pearson DL, Hultman P, Deane TN, Lindh U, Kono DH. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus- prone bxsb mice. Environ Health Perspect. 2001;109:27–33. doi: 10.1289/ehp.0110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pollard KM, Arnush M, Hultman P, Kono DH. Costimulation requirements of induced murine systemic autoimmune disease. J Immunol. 2004;173:5880–7. doi: 10.4049/jimmunol.173.9.5880. [DOI] [PubMed] [Google Scholar]
- 77.Hultman P, Johansson U, Dagnaes-Hansen F. Murine mercury-induced autoimmunity: the role of T-helper cells. J Autoimmun. 1995;8:809–23. doi: 10.1016/s0896-8411(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 78.van Vliet E, Uhrberg M, Stein C, Gleichmann E. MHC control of IL-4-dependent enhancement of B cell Ia expression and Ig class switching in mice treated with mercuric chloride. Int Arch Allergy Immunol. 1993;101:392–401. doi: 10.1159/000236482. [DOI] [PubMed] [Google Scholar]
- 79.Reardon CL, Lucas DO. Heavy-metal mitogenesis: thymocyte activation by Zn++ requires 2-mercaptoethanol and lipopolysaccharide as cofactors. Immunobiology. 1987;174:233–43. doi: 10.1016/S0171-2985(87)80042-6. [DOI] [PubMed] [Google Scholar]
- 80.Reardon CL, Lucas DO. Heavy-metal mitogenesis: Zn++ and Hg++ induce cellular cytotoxicity and interferon production in murine T lymphocytes. Immunobiology. 1987;175:455–69. doi: 10.1016/S0171-2985(87)80073-6. [DOI] [PubMed] [Google Scholar]
- 81.Pollard KM, Landberg GP. The in vitro proliferation of murine lymphocytes to mercuric chloride is restricted to mature T cells and is interleukin 1 dependent. Int Immunopharmacol. 2001;1:581–93. doi: 10.1016/s1567-5769(00)00034-5. [DOI] [PubMed] [Google Scholar]
- 82.Jiang Y, Moller G. In vitro effects of HgCl2 on murine lymphocytes. I. Preferable activation of CD4+ T cells in a responder strain. J Immunol. 1995;154:3138–46. [PubMed] [Google Scholar]
- 83.Hu H, Moller G, Abedi-Valugerdi M. Major histocompatibility complex class II antigens are required for both cytokine production and proliferation induced by mercuric chloride in vitro. J Autoimmun. 1997;10:441–6. doi: 10.1006/jaut.1997.9997. [DOI] [PubMed] [Google Scholar]
- 84.Layland LE, Wulferink M, Dierkes S, Gleichmann E. Drug-induced autoantibody formation in mice: triggering by primed CD4+CD25- T cells, prevention by primed CD4+CD25+ T cells. Eur J Immunol. 2004;34:36–46. doi: 10.1002/eji.200324406. [DOI] [PubMed] [Google Scholar]
- 85.Whitekus MJ, Santini RP, Rosenspire AJ, McCabe MJ., Jr Protection against CD95-mediated apoptosis by inorganic mercury in Jurkat T cells. J Immunol. 1999;162:7162–70. [PubMed] [Google Scholar]
- 86.McCabe MJ, Jr, Whitekus MJ, Hyun J, Eckles KG, McCollum G, Rosenspire AJ. Inorganic mercury attenuates CD95-mediated apoptosis by interfering with formation of the death inducing signaling complex. Toxicol Appl Pharmacol. 2003;190:146–56. doi: 10.1016/s0041-008x(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 87.McCabe MJ, Jr, Eckles KG, Langdon M, Clarkson TW, Whitekus MJ, Rosenspire AJ. Attenuation of CD95-induced apoptosis by inorganic mercury: caspase-3 is not a direct target of low levels of Hg2+ Toxicol Lett. 2005;155:161–70. doi: 10.1016/j.toxlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 88.Ziemba SE, McCabe MJ, Jr, Rosenspire AJ. Inorganic mercury dissociates preassembled Fas/CD95 receptor oligomers in T lymphocytes. Toxicol Appl Pharmacol. 2005;206:334–42. doi: 10.1016/j.taap.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 89.Ziemba SE, Menard SL, McCabe MJ, Jr, Rosenspire AJ. T-cell receptor signaling is mediated by transient Lck activity, which is inhibited by inorganic mercury. Faseb J. 2009;23:1663–71. doi: 10.1096/fj.08-117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mattingly RR, Felczak A, Chen CC, McCabe MJ, Jr, Rosenspire AJ. Low concentrations of inorganic mercury inhibit Ras activation during T cell receptor-mediated signal transduction. Toxicol Appl Pharmacol. 2001;176:162–8. doi: 10.1006/taap.2001.9272. [DOI] [PubMed] [Google Scholar]
- 91.Ziemba SE, Mattingly RR, McCabe MJ, Jr, Rosenspire AJ. Inorganic mercury inhibits the activation of LAT in T-cell receptor-mediated signal transduction. Toxicol Sci. 2006;89:145–53. doi: 10.1093/toxsci/kfj029. [DOI] [PubMed] [Google Scholar]
- 92.Kono DH, Park MS, Szydlik A, Haraldsson KM, Kuan JD, Pearson DL, Hultman P, Pollard KM. Resistance to xenobiotic-induced autoimmunity maps to chromosome 1. J Immunol. 2001;167:2396–403. doi: 10.4049/jimmunol.167.4.2396. [DOI] [PubMed] [Google Scholar]
- 93.Vyse TJ, Kotzin BL. Genetic susceptibility to systemic lupus erythematosus. Annu Rev Immunol. 1998;16:261–92. doi: 10.1146/annurev.immunol.16.1.261. [DOI] [PubMed] [Google Scholar]
- 94.Tsao BP, Wallace DJ. Genetics of systemic lupus erythematosus. Curr Opin Rheumatol. 1997;9:377–9. doi: 10.1097/00002281-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Moser KL, Gray-McGuire C, Kelly J, Asundi N, Yu H, Bruner GR, Mange M, Hogue R, Neas BR, Harley JB. Confirmation of genetic linkage between human systemic lupus erythematosus and chromosome 1q41. Arthritis Rheum. 1999;42:1902–7. doi: 10.1002/1529-0131(199909)42:9<1902::AID-ANR16>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 96.Johanneson B, Lima G, von Salome J, Alarcon-Segovia D, Alarcon-Riquelme ME. A major susceptibility locus for systemic lupus erythemathosus maps to chromosome 1q31. Am J Hum Genet. 2002;71:1060–71. doi: 10.1086/344289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pollard KM, Hultman P, Kono DH. Immunology and genetics of induced systemic autoimmunity. Autoimmun Rev. 2005;4:282–8. doi: 10.1016/j.autrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Pollard KM, Hultman P, Arnush M, Hildebrandt B, Kono DH. Immunology and genetics of xenobiotic-induced autoimmunity. In: Conrad K, Bachmann MP, Chan EK, Fritzler MJ, Humbel RL, Sack U, Shoenfeld Y, editors. From animal models to human genetics: research in the induction and pathogenicity of autoantibodies. Vol. 4. Pabst: Lengerich; 2004. pp. 130–144. [Google Scholar]
- 99.Kono DH, Balomenos D, Pearson DL, Park MS, Hildebrandt B, Hultman P, Pollard KM. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN- gamma and not Th1/Th2 imbalance. J Immunol. 1998;161:234–40. [PubMed] [Google Scholar]
- 100.Cauvi DM, Cauvi G, Pollard KM. Reduced expression of decay-accelerating factor 1 on CD4+ T cells in murine systemic autoimmune disease. Arthritis Rheum. 2007;56:1934–44. doi: 10.1002/art.22639. [DOI] [PubMed] [Google Scholar]
- 101.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD, Song WC. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201:567–77. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miwa T, Maldonado MA, Zhou L, Yamada K, Gilkeson GS, Eisenberg RA, Song WC. Decay-Accelerating Factor Ameliorates Systemic Autoimmune Disease in MRL/lpr Mice via Both Complement-Dependent and -Independent Mechanisms. Am J Pathol. 2007;170:1258–66. doi: 10.2353/ajpath.2007.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haggqvist B, Hultman P. Murine metal-induced systemic autoimmunity: baseline and stimulated cytokine mRNA expression in genetically susceptible and resistant strains. Clin Exp Immunol. 2001;126:157–64. doi: 10.1046/j.1365-2249.2001.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol. 1997;17:5328–37. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Suk K, Chang I, Kim YH, Kim S, Kim JY, Kim H, Lee MS. Interferon gamma (IFNgamma ) and tumor necrosis factor alpha synergism in ME-180 cervical cancer cell apoptosis and necrosis. IFNgamma inhibits cytoprotective NF-kappa B through STAT1/IRF-1 pathways. J Biol Chem. 2001;276:13153–9. doi: 10.1074/jbc.M007646200. [DOI] [PubMed] [Google Scholar]
- 107.Pollard KM, Pearson DL, Hultman P, Hildebrandt B, Kono DH. Lupus-prone mice as models to study xenobiotic-induced acceleration of systemic autoimmunity. Environ Health Perspect. 1999;107(Suppl 5):729–35. doi: 10.1289/ehp.99107s5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burek CL, Rose NR. Autoimmune thyroiditis and ROS. Autoimmun Rev. 2008;7:530–7. doi: 10.1016/j.autrev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 109.Bournaud C, Orgiazzi JJ. Iodine excess and thyroid autoimmunity. J Endocrinol Invest. 2003;26:49–56. [PubMed] [Google Scholar]
- 110.Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun. 2009;32:231–9. doi: 10.1016/j.jaut.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vladutiu AO. Experimental autoimmune thyroiditis in mice chronically treated from birth with anti-IgM antibodies. Cell Immunol. 1989;121:49–59. doi: 10.1016/0008-8749(89)90004-x. [DOI] [PubMed] [Google Scholar]
- 112.Stafford EA, Rose NR. Newer insights into the pathogenesis of experimental autoimmune thyroiditis. Int Rev Immunol. 2000;19:501–33. doi: 10.3109/08830180009088510. [DOI] [PubMed] [Google Scholar]
- 113.Vladutiu AO, Rose NR. Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science. 1971;174:1137–9. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 114.Tang H, Sharp GC, Peterson KP, Braley-Mullen H. IFN-gamma-deficient mice develop severe granulomatous experimental autoimmune thyroiditis with eosinophil infiltration in thyroids. J Immunol. 1998;160:5105–12. [PubMed] [Google Scholar]
- 115.Alimi E, Huang S, Brazillet MP, Charreire J. Experimental autoimmune thyroiditis (EAT) in mice lacking the IFN-gamma receptor gene. Eur J Immunol. 1998;28:201–8. doi: 10.1002/(SICI)1521-4141(199801)28:01<201::AID-IMMU201>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 116.Jin Z, Mori K, Fujimori K, Hoshikawa S, Tani J, Satoh J, Ito S, Satomi S, Yoshida K. Experimental autoimmune thyroiditis in nonobese diabetic mice lacking interferon regulatory factor-1. Clin Immunol. 2004;113:187–92. doi: 10.1016/j.clim.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 117.Vladutiu AO, Sulkowski E. Inhibition of murine autoimmune thyroiditis by interferon. Biomedicine. 1980;33:173–4. [PubMed] [Google Scholar]
- 118.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81:287–92. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 119.Hutchings PR, Verma S, Phillips JM, Harach SZ, Howlett S, Cooke A. Both CD4(+) T cells and CD8(+) T cells are required for iodine accelerated thyroiditis in NOD mice. Cell Immunol. 1999;192:113–21. doi: 10.1006/cimm.1998.1446. [DOI] [PubMed] [Google Scholar]
- 120.Braley-Mullen H, Yu S. Early requirement for B cells for development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2000;165:7262–9. doi: 10.4049/jimmunol.165.12.7262. [DOI] [PubMed] [Google Scholar]
- 121.Yu S, Sharp GC, Braley-Mullen H. Dual roles for IFN-gamma, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2002;169:3999–4007. doi: 10.4049/jimmunol.169.7.3999. [DOI] [PubMed] [Google Scholar]
- 122.Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. CD4+CD25+ naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J Autoimmun. 2007;29:195–202. doi: 10.1016/j.jaut.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 123.Fang Y, Yu S, Braley-Mullen H. Contrasting roles of IFN-gamma in murine models of autoimmune thyroid diseases. Thyroid. 2007;17:989–94. doi: 10.1089/thy.2007.0261. [DOI] [PubMed] [Google Scholar]
- 124.Obermayer-Straub P, Strassburg CP, Manns MP. Autoimmune hepatitis. J Hepatol. 2000;32:181–97. doi: 10.1016/s0168-8278(00)80425-0. [DOI] [PubMed] [Google Scholar]
- 125.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leung PS, Park O, Tsuneyama K, Kurth MJ, Lam KS, Ansari AA, Coppel RL, Gershwin ME. Induction of primary biliary cirrhosis in guinea pigs following chemical xenobiotic immunization. J Immunol. 2007;179:2651–7. doi: 10.4049/jimmunol.179.4.2651. [DOI] [PubMed] [Google Scholar]
- 127.Wakabayashi K, Yoshida K, Leung PS, Moritoki Y, Yang GX, Tsuneyama K, Lian ZX, Hibi T, Ansari AA, Wicker LS, Ridgway WM, Coppel RL, Mackay IR, Gershwin ME. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577–86. doi: 10.1111/j.1365-2249.2008.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, Yoshida K, Yang GX, Hibi T, Ansari AA, Ridgway WM, Coppel RL, Mackay IR, Gershwin ME. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–40. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30:415–20. doi: 10.1016/j.it.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 130.Oertelt S, Ridgway WM, Ansari AA, Coppel RL, Gershwin ME. Murine models of primary biliary cirrhosis: Comparisons and contrasts. Hepatol Res. 2007;37(Suppl 3):S365–9. doi: 10.1111/j.1872-034X.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 131.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30:455–64. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cruickshank B, Thomas MJ. Mineral oil (follicular) lipidosis: II. Histologic studies of spleen, liver, lymph nodes, and bone marrow. Hum Pathol. 1984;15:731–7. doi: 10.1016/s0046-8177(84)80163-x. [DOI] [PubMed] [Google Scholar]
- 133.Satoh M, Kuroda Y, Yoshida H, Behney KM, Mizutani A, Akaogi J, Nacionales DC, Lorenson TD, Rosenbauer RJ, Reeves WH. Induction of lupus autoantibodies by adjuvants. J Autoimmun. 2003;21:1–9. doi: 10.1016/s0896-8411(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 134.Yoshida H, Satoh M, Behney KM, Lee CG, Richards HB, Shaheen VM, Yang JQ, Singh RR, Reeves WH. Effect of an exogenous trigger on the pathogenesis of lupus in (NZB x NZW)F1 mice. Arthritis Rheum. 2002;46:2235–44. doi: 10.1002/art.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–90. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–83. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16:801–7. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 138.Satoh M, Weintraub JP, Yoshida H, Shaheen VM, Richards HB, Shaw M, Reeves WH. Fas and Fas ligand mutations inhibit autoantibody production in pristane-induced lupus. J Immunol. 2000;165:1036–43. doi: 10.4049/jimmunol.165.2.1036. [DOI] [PubMed] [Google Scholar]
- 139.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–42. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 141.Young AL, Giesy JP, Jones PD, Newton M. Environmental fate and bioavailability of Agent Orange and its associated dioxin during the Vietnam War. Environ Sci Pollut Res Int. 2004;11:359–70. doi: 10.1007/BF02979652. [DOI] [PubMed] [Google Scholar]
- 142.Jennings AM, Wild G, Ward JD, Ward AM. Immunological abnormalities 17 years after accidental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Br J Ind Med. 1988;45:701–4. doi: 10.1136/oem.45.10.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim HA, Kim EM, Park YC, Yu JY, Hong SK, Jeon SH, Park KL, Hur SJ, Heo Y. Immunotoxicological effects of Agent Orange exposure to the Vietnam War Korean veterans. Ind Health. 2003;41:158–66. doi: 10.2486/indhealth.41.158. [DOI] [PubMed] [Google Scholar]
- 144.Tonn T, Esser C, Schneider EM, Steinmann-Steiner-Haldenstatt W, Gleichmann E. Persistence of decreased T-helper cell function in industrial workers 20 years after exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ Health Perspect. 1996;104:422–6. doi: 10.1289/ehp.96104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baccarelli A, Mocarelli P, Patterson DG, Jr, Bonzini M, Pesatori AC, Caporaso N, Landi MT. Immunologic effects of dioxin: new results from Seveso and comparison with other studies. Environ Health Perspect. 2002;110:1169–73. doi: 10.1289/ehp.021101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Signorini S, Gerthoux PM, Dassi C, Cazzaniga M, Brambilla P, Vincoli N, Mocarelli P. Environmental exposure to dioxin: the Seveso experience. Andrologia. 2000;32:263–70. doi: 10.1046/j.1439-0272.2000.00394.x. [DOI] [PubMed] [Google Scholar]
- 147.Wolfe WH, Michalek JE, Miner JC, Rahe A, Silva J, Thomas WF, Grubbs WD, Lustik MB, Karrison TG, Roegner RH, et al. Health status of Air Force veterans occupationally exposed to herbicides in Vietnam. I. Physical health. Jama. 1990;264:1824–31. [PubMed] [Google Scholar]
- 148.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4184–8. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 149.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 150.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends in Immunology. doi: 10.1016/j.it.2009.06.005. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 151.Ishimaru N, Takagi A, Kohashi M, Yamada A, Arakaki R, Kanno J, Hayashi Y. Neonatal exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin causes autoimmunity due to the disruption of T cell tolerance. J Immunol. 2009;182:6576–86. doi: 10.4049/jimmunol.0802289. [DOI] [PubMed] [Google Scholar]
- 152.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 153.Cai P, Konig R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, Ansari GA. Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol Appl Pharmacol. 2008;228:68–75. doi: 10.1016/j.taap.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Blossom SJ, Doss JC, Gilbert KM. Chronic exposure to a trichloroethylene metabolite in autoimmune-prone MRL+/+ mice promotes immune modulation and alopecia. Toxicol Sci. 2007;95:401–11. doi: 10.1093/toxsci/kfl149. [DOI] [PubMed] [Google Scholar]
- 155.Gilbert KM, Pumford NR, Blossom SJ. Environmental Contaminant Trichloroethylene Promotes Autoimmune Disease and Inhibits T-cell Apoptosis in MRL(+/+) Mice. J Immunotoxicol. 2006;3:263–7. doi: 10.1080/15476910601023578. [DOI] [PubMed] [Google Scholar]
- 156.Blossom SJ, Doss JC. Trichloroethylene Alters Central and Peripheral Immune Function in Autoimmune-Prone MRL(+/+) Mice Following Continuous Developmental and Early Life Exposure. J Immunotoxicol. 2007;4:129–41. doi: 10.1080/15476910701337035. [DOI] [PubMed] [Google Scholar]
- 157.Cooper GS, Makris SL, Nietert PJ, Jinot J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect. 2009;117:696–702. doi: 10.1289/ehp.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parks CG, Cooper GS. Occupational exposures and risk of systemic lupus erythematosus. Autoimmunity. 2005;38:497–506. doi: 10.1080/08916930500285493. [DOI] [PubMed] [Google Scholar]
- 159.Michielsen CC, van Loveren H, Vos JG. The role of the immune system in hexachlorobenzene-induced toxicity. Environ Health Perspect. 1999;107(Suppl 5):783–92. doi: 10.1289/ehp.99107s5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cripps DJ, Peters HA, Gocmen A, Dogramici I. Porphyria turcica due to hexachlorobenzene: a 20 to 30 year follow-up study on 204 patients. Br J Dermatol. 1984;111:413–22. doi: 10.1111/j.1365-2133.1984.tb06603.x. [DOI] [PubMed] [Google Scholar]
- 161.Daniel V, Huber W, Bauer K, Suesal C, Conradt C, Opelz G. Associations of blood levels of PCB, HCHS, and HCB with numbers of lymphocyte subpopulations, in vitro lymphocyte response, plasma cytokine levels, and immunoglobulin autoantibodies. Environ Health Perspect. 2001;109:173–8. doi: 10.1289/ehp.01109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ezendam J, Vos JG, Pieters R. Research articles mechanisms of hexachlorobenzene-induced adverse immune effects in brown norway rats. J Immunotoxicol. 2005;1:167–75. doi: 10.1080/15476910490907026. [DOI] [PubMed] [Google Scholar]
- 163.Yurino H, Ishikawa S, Sato T, Akadegawa K, Ito T, Ueha S, Inadera H, Matsushima K. Endocrine disruptors (environmental estrogens) enhance autoantibody production by B1 cells. Toxicol Sci. 2004;81:139–47. doi: 10.1093/toxsci/kfh179. [DOI] [PubMed] [Google Scholar]
- 164.Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J, Roberts SM. Acceleration of autoimmunity by organochlorine pesticides in (NZB x NZW)F1 mice. Environ Health Perspect. 2005;113:323–8. doi: 10.1289/ehp.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leffel EK, Wolf C, Poklis A, White KL., Jr Drinking water exposure to cadmium, an environmental contaminant, results in the exacerbation of autoimmune disease in the murine model. Toxicology. 2003;188:233–50. doi: 10.1016/s0300-483x(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 166.Hudson CA, Cao L, Kasten-Jolly J, Kirkwood JN, Lawrence DA. Susceptibility of lupus-prone NZM mouse strains to lead exacerbation of systemic lupus erythematosus symptoms. J Toxicol Environ Health A. 2003;66:895–918. doi: 10.1080/15287390306456. [DOI] [PubMed] [Google Scholar]
- 167.Pumford NR, Halmes NC. Protein targets of xenobiotic reactive intermediates. Annu Rev Pharmacol Toxicol. 1997;37:91–117. doi: 10.1146/annurev.pharmtox.37.1.91. [DOI] [PubMed] [Google Scholar]
- 168.Kono DH, Haraldsson MK, Lawson BR, Pollard KM, Koh YT, Du X, Arnold CN, Baccala R, Silverman GJ, Beutler BA, Theofilopoulos AN. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106:12061–6. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]