Abstract
Activity in the primary auditory cortex (A1) is known to be essential for normal sound localization behavior, yet previous studies of the spatial sensitivity of neurons in A1 have found surprisingly broad spatial tuning. We tested the hypothesis that spatial tuning sharpens when an animal engages in an auditory task. Cats performed a task that required evaluation of the locations of sounds and another that required active listening but in which sound location was irrelevant. Some 26–44% of the units recorded in A1 showed significantly sharpened spatial tuning during the behavioral tasks compared to idle conditions, with greatest sharpening during the location-relevant task. Spatial sharpening occurred on a scale of tens of seconds and could be replicated multiple times within ~1.5-hr test sessions. Sharpening resulted primarily from increased suppression of responses to sounds at least-preferred locations. That and an observed increase in latencies suggest an important role of inhibitory mechanisms.
Introduction
In a typical auditory scene, a listener can focus auditory attention toward the location of any target. The dynamic nature of spatial listening has been demonstrated by psychophysical studies showing that: localization or identification of a target is more efficient when the target is presented at a cued or attended location1–5; prior knowledge of the location of a target enhances spatial release from masking6–8; and localization judgments can be biased by a preceding distracter9. The present study is a first attempt to explore cortical mechanisms underlying task-dependent modulation of auditory spatial sensitivity.
Lesion studies and reversible inactivation have demonstrated that activity in the primary auditory cortex (A1) is necessary for normal sound-localization behavior10–11. Also, A1 projects to the dorsal zone (DZ) and the posterior auditory field (PAF) in which reversible inactivation produces localization deficits11–13. One might speculate, therefore, that task-dependent modulation of activity in A1 contributes to dynamic aspects of spatial hearing. Single-unit studies in anesthetized animals have demonstrated quite broad spatial tuning, with most spatial receptive fields ranging from 180° to 360° in width14–16. Spatial tuning is sharper in awake animals, although most neurons still exhibit spatial receptive fields spanning more than a hemifield of space17. We tested the hypothesis that the spatial tuning of neurons in A1 is modulated according to an animal’s behavioral state and, specifically, that tuning is sharpened when an animal engages in a spatial task.
In the current study, cats were trained to perform two tasks. “Periodicity Detection” required the cat to attend to sounds in order to receive a food reward, but reward was not contingent on sound location. “Localization” required the cat to evaluate the locations of sound to distinguish targets from background probe sounds. We recorded unit activity in A1 during performance of both tasks as well as during off-task “Idle” conditions. Many neurons sharpened their spatial tuning significantly during both on-task conditions, especially during the Localization task. Several characteristics of the spatial sharpening suggest a role for inhibitory mechanisms.
Results
We recorded unit activity from A1 under Idle, Periodicity Detection, and Localization conditions. In all conditions, the cat heard a background of brief noise bursts intended to probe the spatial sensitivity of cortical units. Those probe sounds were presented at ~1.25-s intervals from varying locations in the horizontal plane. In the Idle condition, the cat refrained from the task. The Periodicity Detection and Localization conditions required the cat to initiate a sequence of trials by pressing and holding a response key. If it held the key during subsequent probe sounds and released it upon presentation of a target sound, it received a food reward. The target in the Periodicity Detection task was a 200/s click train that varied in location among trials. In that condition, the target sound was associated with reward, but its location was irrelevant to reinforcement. The target in the Localization task was a broadband noise burst, identical to the probe sounds except that it was presented from a variable elevation 40°-80° above the horizontal plane and at varying azimuths. The Localization task required the cat to evaluate the location of each sound in order to detect the change in elevation. This localization was accomplished covertly, in that the cats typically did not make orienting movements of the head or external ears towards the sounds. The physiological recordings reported here reflect responses only to the broadband probe sounds, not to the targets.
Spatial selectivity and firing patterns
Units in A1 responded to the probe sounds with a variety of temporal firing patterns and spatial sensitivities. The responses of many units also varied among task conditions. The example unit shown in Fig. 1 fired a burst of spikes after stimulus onset with little or no tonic activity following the onset burst. In the Idle condition, this unit responded to probe sounds at all locations with little discrimination among stimulus azimuths (Fig. 1a; also supplemental Fig. 1). When the animal engaged in the Periodicity Detection task, however, responses became more selective, showing suppression of responses to ipsilateral stimuli while maintaining responses to contralateral stimuli (Fig. 1b). During the Localization task, responses became even more selective, responding best to stimuli located between contralateral 10° and 50° (Fig. 1c).
Figure 1.
Task-dependant modulation of spatial sensitivity. (a) Post-Stimulus-Time-Histogram (PSTH) showing activity as a function of time (horizontal axis) and head-centered stimulus location (vertical axis) for one example unit in A1 in the right hemisphere during the Idle condition (Equivalent Rectangular Receptive Field, ERRF, width =217°). Colors indicate mean spike activity. The thin white lines at the bottom of the plots indicate the 80-ms stimulus duration. White gaps crossing the plot corresponded to the spatial bins centered at ipsi-/contra-lateral 90°, which were omitted from analysis. (b) PSTH of the same unit during the Periodicity Detection condition (ERRF width =173°). (c) PSTH of the same unit during the Localization condition (ERRF width =143°). (d) Average spike rates during the onset response [10–40ms] as functions of azimuth. Black, blue, and red lines plot rate-azimuth-function in Idle, Periodicity Detection, and Localization conditions, respectively. The data for three conditions were obtained within a single behavioral session, which lasted about 100 min. For the demonstration of the computation of the ERRF width, see Supplemental figure 1.
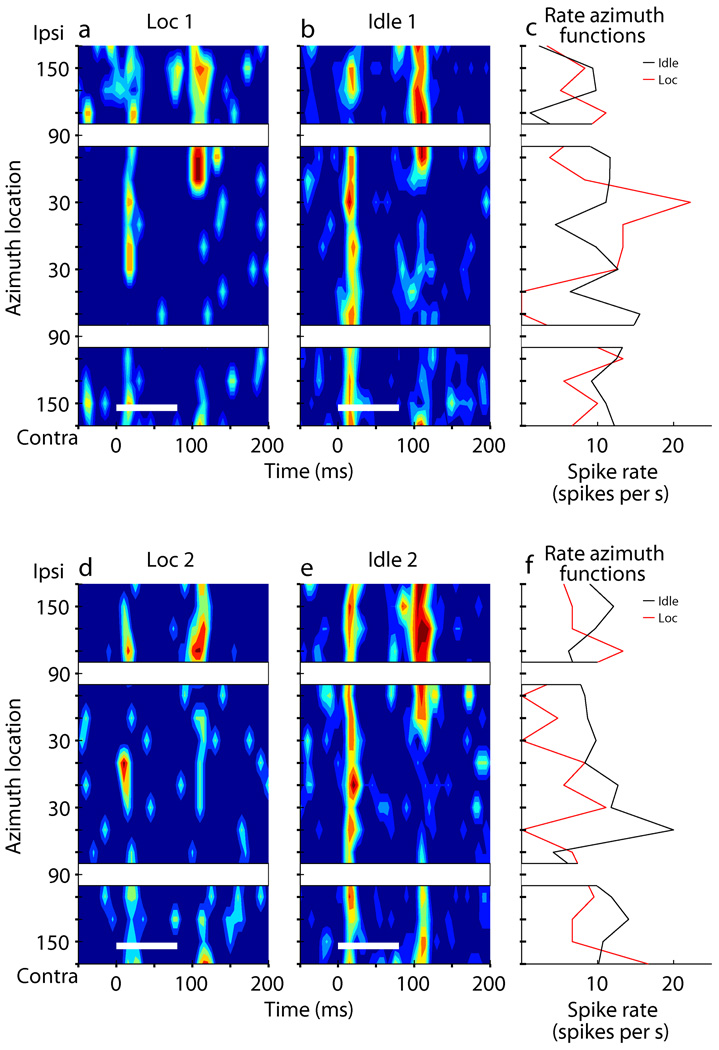
Changes in spatial selectivity among task conditions could be replicated within single ~1.5-hr recording sessions, as in the example shown in Fig. 2. Onset responses of this unit were largely restricted to frontal stimuli during the initial block of trials in the Localization condition (Fig. 2a). The tuning broadened during a subsequent Idle condition (Fig. 2b), sharpened during the second block of Localization trials (Fig. 2c), and finally broadened again during the last Idle condition (Fig. 2d). The sharpness of spatial tuning generally was similar between repeated tests of the same behavioral conditions. Forty three units were tested with two blocks of the Periodicity Detection condition or of the Localization condition within the same session, each separated by a contrasting condition. Of those 43 units, 31 showed no significant change in sharpness of tuning between the initial and repeated block (according to a Receiver-Operating-Characteristic, ROC, test described in Methods), 6 showed significant broadening, and 6 showed significant narrowing.
Figure 2.
Modulation of spatial sensitivity in sequential conditions. (a) PSTH of an example unit from the left hemisphere recorded during the first block of localization trials from the beginning of the recording (0 min) to the first 13 minutes. (b) The same unit recorded during a subsequent idle period (13 to 18 min). (c) Rate-azimuth-function of the onset responses for the first Localization (red trace) and the subsequent Idle condition (black trace). (d) A second block of localization trials (25 to 34 min). (e) A second Idle period (34 to 39 min). (f) Rate-azimuth-function of the onset responses for the second Localization (red trace) and the subsequent Idle condition (black trace). Plot conventions are the same as in Fig. 1.
About 63% of units in our sample resembled those in Figs. 1 and 2 in that they responded primarily with a burst of spikes at stimulus onset and were omnidirectional in the Idle condition. Many of those onset-only units showed on-task sharpening of spatial sensitivity like that illustrated in Figs. 1 and 2. Another 26% of units in our sample showed more complex temporal firing patterns, consisting of an onset response followed by a period of suppression followed by additional bursts of spikes. In the Idle condition, units with complex firing patterns typically showed sharper spatial tuning than did onset-only units, often with spatial tuning restricted to a hemifield. In contrast to the onset-only units, those with complex firing patterns tended to show smaller differences across behavioral conditions. We quantify the between-task modulation of spatial sensitivity in the next section.
About 8% of the units in our sample showed prominent responses that followed stimulus offset by ~15 to 20 ms. We interpreted these as “offset”, rather than “late”, responses because a similar stimulus-offset-to-response delay was observed in units tested with 80- or 150-ms stimulus durations. In these units, the ratio of offset to onset responses increased from Idle to Periodicity Detection to Localization conditions; examples of two units are shown in Fig. 3. In the Idle condition (Figs. 3a,d), these units responded strongly to the stimulus onset with or without an offset response. During the behavioral tasks, however, the onset response weakened or disappeared and was replaced by a strong offset response (Figs. 3b,c,e,f). Of the six units that showed strong offset responses, four showed this transition from onset to offset when the animal engaged in the behavioral tasks. Because of the lack of consistent excitatory responses, we excluded these six units and an additional two units that showed only suppression of spontaneous activity after stimulus onset from quantitative analysis of the task dependence of spatial tuning. The remaining ~89% of units all responded with excitation during the first 40 ms after the stimulus onset. It was the onset response of those units that typically showed the greatest task-dependent modulation, and some units showed late or offset responses for which the spatial sensitivity was qualitatively different than that of the onset response (as in Fig. 2). For those reasons, we restricted quantitative analyses of spatial tuning to onset responses.
Figure 3.
PSTH plots in three task conditions from two units recorded from the left hemisphere that showed offset-dominant responses to 150 ms stimuli. Figure a–c were PSTHs from one unit recorded across three conditions (a: Idle, b: Periodicity Detection, c: Localization) within a 105 min session. Figure d–f were PSTHs from another unit recorded across three conditions (d: Idle, e: Periodicity Detection, f: Localization) within a 120 min session. For each unit, the ratio of offset to onset responses increased from Idle to Periodicity Detection to Localization conditions. Plot conventions are the same as in Fig. 1.
Quantitative measures of spatial sharpening
We quantified the spatial tuning of units using a measure inspired by the “Equivalent Rectangular Bandwidth” introduced by Moore and Glasberg18. Here, we first computed rate-azimuth functions consisting of mean spike rates as a function of stimulus azimuth. We then computed the area under the rate-azimuth-function and re-shaped the function to form an equivalent rectangular receptive field (ERRF) having equal peak spike rate and equal area; see Supplemental Fig. 1. Generally, ERRFs were somewhat narrower than simple receptive fields, because ERRFs were influenced both by the spatial extent of tuning and by the location-related modulation of spike rates. In the Idle condition, for example, median ERRF widths and spatial tuning widths were 185° and 294°, respectively. For comparison, we previously have observed a median spatial tuning width of 340° in anesthetized conditions19; comparisons of tuning widths with our previous unanesthetized A1 study17 are difficult, because of differences in computations of spatial tuning.
In the idle condition, spatial tuning was relatively broad, with ERRF widths ranging from 100° to 270°. The effect of behavioral condition on tuning varied among units, with some ERRFs narrowing and others broadening. In the scatter plots in Fig. 4, ‘+’ and ‘x’ signs represent the ERRF widths of units that showed statistically significant narrowing or broadening between task conditions according to an ROC test (see Methods). On average, ERRFs narrowed significantly between Idle and Periodicity Detection conditions (median ERRF widths 185° and 176°, respectively; p <0.005). Across the population, there was no significant difference in ERRF widths between the two on-task conditions (median ERRF widths 176° and 165° for Periodicity and Localization conditions; p =0.18), although many units showed a substantial narrowing of ERRFs as indicated by the ‘+’ signs below the diagonal line. The greatest contrast in spatial tuning was between Idle and Localization conditions, with ERRF widths narrowing from medians of 185° to 165° (p <0.0005); ERRF widths in the Localization condition ranged from 41.3% to 136.3% of the widths measured in the Idle condition (median =92.7%).
Figure 4.
Comparisons of ERRF width across conditions for all units that showed excitatory responses within first 40ms after stimulus onset. (a–c) Each symbol represents one unit, with the value in horizontal and vertical axes corresponding to its ERRF width in two different conditions. The symbols lying below the diagonal line represent units for which spatial tuning sharpened (and the ERRF width narrowed) for the condition indicated on the ordinate. “o” symbols represent the units that did not show significant sharpening or broadening of ERRF widths. “+” symbols represent the units that showed significant sharpening and “x’ symbols represent the units that showed significant broadening according to the ROC test described in relation to Fig. 5. (d) Cumulative distributions of ERRF widths across three conditions. The horizontal dashed line crosses the curves at the median values. The percentages of the distributions to the left of the vertical dash line had ERRF widths narrower than a hemifield (i.e., ≤ 160°).
We evaluated the percentages of units that showed significant changes in spatial tuning as a function of task condition. That analysis employed an ROC procedure that tested for sharpening or broadening of ERRFs across conditions based on trial-by-trial-variation in spike counts of individual units (see Methods). In every 2-way comparison between task conditions (Fig. 5), more units showed significant sharpening of spatial tuning than showed broadening in the conditions that required active listening (either behavioral task versus Idle) or greater attention to sound locations (Localization versus Periodicity Detection). The contrast between the Periodicity Detection and Localization conditions (middle bar; 24% of the total sample) was especially interesting because both conditions required active listening but only the Localization task required the cat to evaluate sound locations. The units identified in that contrast included 24% of the units that showed significant sharpening in the Idle-versus-Periodicity contrast, which showed further significant sharpening in the Localization condition (6% of the total population). Additional units in this Periodicity-versus-Localization contrast showed significant sharpening only in the Localization condition. As expected, the largest percentage of units showing significant sharpening was observed in the contrast between Idle and Localization, in which 44% of units showed significantly narrower ERRFs. That group contained nearly all of the units that showed significant narrowing in the Idle-versus-Periodicity contrast plus an additional 18% of the population that showed significant narrowing only in the Idle-versus-Localization contrast.
Figure 5.
Percentage of units that showed significant sharpening or broadening of spatial tuning between condition pairs. Portions of the bars above or below 0 % line represent the percentage of units for which the ERRF width sharpened or broadened significantly for each two-way comparison. The upper portions of the middle and right bars are divided to represent units that sharpened their tuning significantly in the Periodicity-versus-Idle contrast (dark shading) or those that did not (light shading).
We have demonstrated previously in anesthetized animals that a substantial proportion of stimulus-related information can be transmitted by first-spike latencies20, although that proportion is somewhat smaller in awake conditions17. Here we investigated the influence of task condition on first-spike latencies at each unit’s preferred location. Surprisingly, median first-spike latencies averaged somewhat longer in the Localization condition compared to the Idle condition (20.1 vs. 18.8 ms, p <0.05) but were similar between the two behavioral conditions (p =0.84, Fig. 6).
Figure 6.
First spike latency for preferred locations was longer during behavioral conditions. Distributions of the trial-by-trial medians of first-spike latencies for preferred locations are plotted for three conditions. Analysis of variance (ANOVA) showed a significant effect of condition for the median first spike latency (p <0.05). Differences were significant in two-way comparison between Idle and Periodicity Detection conditions (t test, p < 0.01) and between Idle and Localization conditions (t test, p <0.01) but not between Periodicity Detection and Localization (t test, p =0.83). Each box shows the upper and lower quartile and median as horizontal lines. The plus signs indicate points beyond the quartiles.
Increased suppression by least-preferred stimuli
The sharpening of spatial sensitivity observed in the behaving conditions might have represented an enhancement of the responses to stimuli at “preferred” locations (as defined in Methods), or it might have resulted from increased suppression of the responses stimuli from “least-preferred” locations. Among the units that showed significant sharpening of spatial tuning between Localization and Idle (Fig. 5), the overall mean spike rate was significantly lower during the Localization compared to during the Idle condition (p <0.005) and also slightly lower in the Localization than in the Periodicity Detection condition (n.s., p =0.08, Fig. 7a).
Figure 7.
Spike rates decreased in the Localization condition primarily for stimuli at least-preferred locations. (a) Distributions of spike rates averaged across all stimulus locations for units that showed significant sharpening in the bootstrap analysis (Fig. 5). Mean spike rates decreased significantly across task conditions (Kruskal-Wallis test, p <0.01). (b) Mean spike rates for stimuli at preferred locations showed no significant difference between Localization and Idle conditions (Wilcoxon rank sum test, p =0.88). (c) Mean spike rates for least-preferred locations were suppressed significantly for Localization compared to Idle conditions (p <0.05).
We tested whether the reduction in responses by those units was general, or whether it reflected differential suppression of responses to stimuli at particular locations. The Idle-to-Localization contrast showed no significant differences among responses to sounds at neurons’ preferred locations (Fig. 7b; p =0.88, Localization versus Idle). In contrast, nearly every neuron showed a reduction in response to sounds at least-preferred locations (Fig. 7c; p <0.05). Spike rates elicited by sounds at intermediate locations between preferred and least-preferred locations showed intermediate amounts of modulation, depending on the spatial tuning of each unit. These results indicate that the task-dependent sharpening of spatial sensitivity acted primarily by enhanced suppression of responses to stimuli located away from units’ preferred locations.
Time course of modulation of spatial tuning
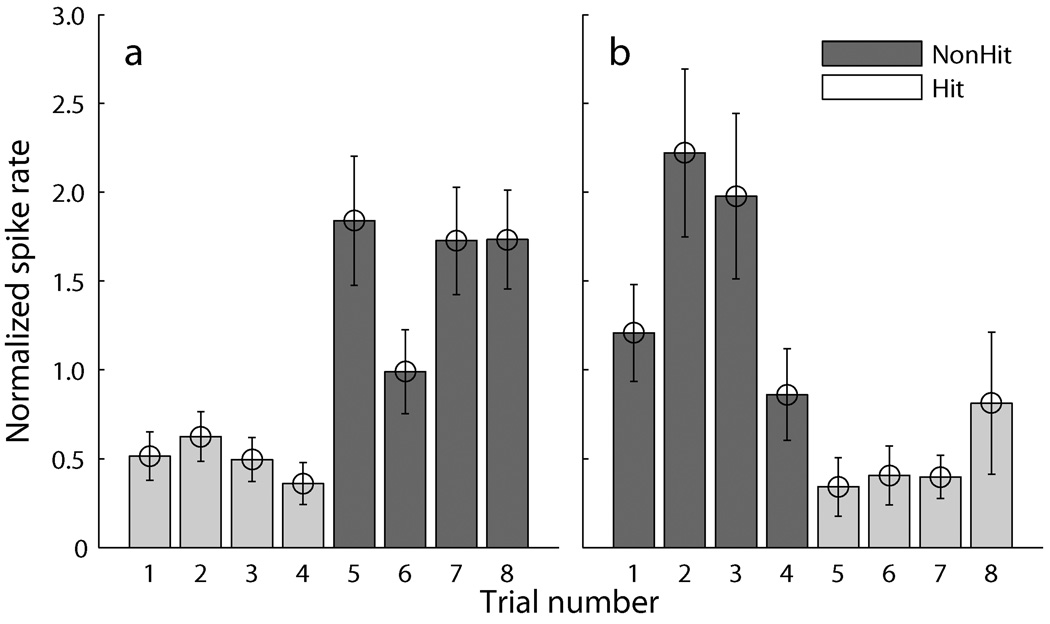
If the sharpening of spatial tuning is a result of top-down control, unit responses should change whenever an animal engages or disengages in a task. To test this hypothesis, we investigated the time course of changes in spike rates for the single location for each unit that showed the greatest task-dependent modulation. We identified blocks of trials in which that location was tested either in four consecutive hit trials followed by four consecutive non-hit trials (Fig. 8a) or vice versa (Fig. 8b). Blocks of “Hit” trials were those in which the response key was depressed and held until being released correctly upon presentation of the target. Blocks of “non-hit” trials included Idle trials (i.e., the key was not pressed) plus a small number of false alarms (the key released early), and misses (the key released late). For the cases that matched these criteria (N=51 hit to non-hit; N=35 non-hit to hit), we normalized spike rates by the mean rate across the contiguous 4 hit and 4 non-hit trials. The time between each presentation at a particular location averaged about 33 s, varying among cases because of the random order of testing of various stimulus locations. As seen in Fig. 8a, the normalized spike rate averaged about 0.5 per trial for the last four hit trials and did not differ significantly among those trials. The rate for the first non-hit trial, however, showed an immediate increase to about 1.8 per trial. The mean rate for each of the four non-hit trials was significantly higher than the mean across all the hit presentations (p <0.0001 to p <0.01, depending on trial). Essentially the reverse was seen in the transition from non-hit to hit condition (Fig. 8b). The rate for each of the first three non-hit trials was significantly higher than the mean of the later four hit presentations (p <0.001 to p <0.005). Interestingly, the rate for the last non-hit trials averaged about 0.82 (4th column; Fig. 8b), which was intermediate between the rate for the preceding three non-hit presentations and that of the following hit trials, as if these trials represented a transition period in which the animals were preparing to engage in the task. These results indicate that task-dependent changes in the spatial sensitivity of units occur on a scale no slower than some tens of seconds.
Figure 8.
Time course of the task-dependent modulation of the response at single location that showed strongest suppression when compared Localization to Idle conditions. Hit and non-hit trials are represented by light and dark bars, respectively. (a) Normalized spike rates during four consecutive hit trials (1–4) followed by the four consecutive non-hit trials (5–8). The mean normalized spike rate for each of the four non-hit trials was significantly higher than the mean of all the hit presentations (Wilcoxon rank sum test, p <0.0001 to p <0.01, depending on trial). (b) Four non-hit trials (1–4) followed by four hit trials (5–8). The normalized spike rate for each of the first three non-hit trials was significantly higher than the mean of all the hit presentations that followed (p <0.001 to p <0.005). Error bars showed the standard errors of the means.
Discussion
The results demonstrate sharpening of spatial tuning in the auditory cortex during active listening and further sharpening when the task requires localization. Many of the recorded units sharpened their spatial tuning significantly when the animal engaged in the Periodicity Detection task. We interpreted that sharpening as a generalized effect of arousal or of active listening. In a similar number of units, however, addition of a spatial component to the task resulted in significant sharpening of spatial tuning beyond that associated merely with active listening. The narrower spatial tuning during Localization compared to Periodicity Detection was not likely a result of differences in arousal or motivation, since both tasks required active listening and both required timely releases of the response key. Changes in pinna position were not likely the cause for sharpening of spatial tuning, since neurons that showed significant sharpening of ERRFs were recorded simultaneously with units that showed no significant change or even significant broadening of ERRFs. The latter argument also applies to any possible effects of eye positions or gaze directions on auditory responses; also pertinent to eye-position effects, the recordings were done in the dark. Our interpretation is that many A1 neurons responded to the spatial demands of the Localization task by sharpening their spatial tuning throughout the sound field.
Our experimental design differed from those that have sought changes in neural responses to a particular target sound21 or changes in the cortical topographical representation of such a sound22. Had we trained a cat to associate reward with a particular sound location, we think it likely that it would have adopted a posture with its head and pinnae oriented toward that location. Such a change in acoustic gaze would have increased stimulus levels at the two ears, confounding any changes that might be due to central auditory processing. In our Localization condition, the location of the reward-associated target varied among trials, and the reward location was not among the locations used to probe spatial selectivity. In that condition, we observed a global sharpening of spatial tuning that could not be attributed merely to acoustical focusing.
A recent study21 demonstrated task-dependent changes in the frequency tuning of auditory cortical units. In that study, ferrets detected tones at particular target frequencies in the presence of broadband sounds. During task performance, responses to target frequencies increased and/or responses to non-target frequencies decreased. Those results were similar to ours in that neural tuning was modulated by behavioral state. In the previous study, however, the ferrets’ behavioral performance presumably was enhanced by sharpening of frequency tuning around the target frequency. An analogous change, sharpening of spatial tuning around a particular target location, would have been maladaptive for our Localization task and, indeed, was not observed. The overall sharpening of spatial tuning, regardless of neurons’ preferred locations, was an appropriate adaptation to our task, which required the cat to evaluate the locations of sounds throughout a broad range of locations. Another difference between the two studies is that the previous study demonstrated changes in frequency tuning that persisted for hours after cessation of the task, whereas we observed changes in spatial tuning within the tens-of-seconds time resolution afforded by our experimental design. The persistent effect observed previously might have resulted from the aversive conditioning paradigm that was used, in contrast to the positive reinforcement in our paradigm.
We are aware of two previous studies that addressed effects of an animal’s behavioral state on the sensitivity in auditory cortical neurons to sound locations23 or on sensitivity to interaural-phase-difference cues for sound location24. In both of those studies, spike rates tended to increase in the on-task condition. Those studies differed from ours in that the responses of interest were those to target sounds, whereas we measured spatial sensitivity to non-target probe sounds. The enhancement of excitatory responses to the target stimuli observed in these studies could coexist with our observation of global suppression at least-preferred locations. There were no indications of changes in spatial (or spatial-cue) tuning in the previous studies, but those studies tested only 5 locations or 2 phase differences, whereas we routinely probed 18 loudspeaker locations.
The on-task sharpening of spatial tuning that we observed was most evident in units that showed primarily onset responses. Units with more complex responses tended to show relatively sharp spatial tuning regardless of task condition. In unanesthetized marmosets25, cortical neurons show only onset responses to non-optimal sounds, but many respond tonically to stimuli of “optimal” spectral and temporal complexity. It might be that the onset responses in the present study reflect a non-optimum spectrotemporal quality of the Gaussian-noise-burst probe stimuli. Nevertheless, when offered only Gaussian noise bursts, our cats made accurate localization judgments, and the majority of A1 units showed onset responses to those stimuli. The sharpening of spatial tuning observed in the onset response might reflect a neural mechanism for optimizing the representation across a neuronal population to accommodate the task demands regardless of the optimality of the stimulus.
Plasticity in the functional organization of area A1 in regard to frequency representation has been demonstrated by a number of groups, generally in association with conditioned association26 or sensory discrimination22,27 paradigms. Generally, such studies have shown increases in the responses to the conditioned (i.e., target) frequencies21. It has been proposed that behaviorally relevant stimuli might activate the cholinergic or dopaminergic neurons in the basal forebrain, in addition to the sensory cortex, through a sensory cortex-to-prefrontal cortex-to-basal forebrain circuit28–29. Direct electrical stimulation of cholinergic neurons in nucleus basalis30 and dopaminergic neurons in ventral tegmental area31 of basal forebrain enhances the representation of the paired stimuli in the sensory cortex and thalamus32. It remains to be seen whether those same modulatory systems underlie the short-term task-dependent changes in cortical sensitivity that we have observed.
Several lines of evidence suggest that the sharpening of spatial tuning that we observed during on-task conditions reflects largely inhibitory mechanisms. First, the sharpened spatial tuning resulted from increased suppression of least-preferred responses during the Localization condition, compared to the Idle condition. Second, in a small number of units that showed primarily offset responses, onset responses were increasingly suppressed and offset responses strengthened during transitions from Idle to Periodicity Detection to Localization conditions. Third, the first spike latency was significantly longer during the Localization compared to the Idle condition. Inhibitory mechanisms have been proposed to play an important role for perceptual learning in sensory cortex33–34. An increase in inhibition has been reported to correlate positively with improvement of behavioral performance35–37, with increase of task difficulty38 or engagement in a behavioral task that required more global attention39. It has been suggested that attention or the effect of learning may first induce change in the prefrontal cortex, which in turn activates the GABAergic reticular nuclei in the thalamus40–41. The reticular nuclei then would send inhibitory input to medial geniculate body (MGB) to modulate its activity42–43 and further change response properties in the primary auditory cortex44–45. In the present study, we observed not only a decrease in responsiveness of the A1 units during behavioral conditions, but changes in first-spike latencies: latencies for the preferred location were delayed in the Localization condition (Fig. 6) relative to the Idle condition. Even in the Idle condition, the median of the first spike latency was longer than the latency in anesthetized A1 (17.6 ms19), which is consistent with a previous report46. These results suggest that inhibitory mechanisms, driven by top-down control, could account for task-dependent modulation of neuronal activity, either by increasing the depolarization threshold or by delaying the spiking when the animals are engaged in the behavioral tasks.
Previous electrophysiological studies of A1 and other auditory cortical areas have failed to identify a point-to-point map of auditory space15–17,19–20. Instead, we have argued that auditory space is represented in a highly distributed manner. The location of any particular sound source would be represented by the activity of populations of more-or-less panoramically sensitive neurons widely distributed within particular cortical areas and among multiple cortical areas. The task-dependent modulation of spatial sensitivity observed in the present study is consistent with that view. The present results suggest that sound-source locations are represented by dynamic cortical populations, the responses of which are modulated to optimize the representation for the demands of any particular task. We have, so far, tested only a single localization task and a single non-spatial auditory task. One might imagine that distinct modes of spatial tuning might emerge during tasks requiring discrimination of closely separated sources, compared to tasks requiring panoramic localization, compared to spatial stream-segregation tasks requiring segregation of multiple sequences of sounds. Also, one might expect to see greater modulation of responses to target sounds than to non-target probe sounds, as tested in the present study. Specific analysis of the relationship between task demands and cortical neuronal sensitivity potentially offers a fruitful means of understanding aspects of the cortical mechanisms of the dynamics of auditory spatial perception.
Methods
All animal procedures were approved by the University of Michigan Committee on Use and Care of Animals. Stimulus presentation and data acquisition utilized instruments from Tucker-Davis Technologies (Alachua, FL) and custom MATLAB software (The Mathworks, Natick, MA).
Stimulus generation
Sounds were presented under free-field conditions in a sound-attenuating chamber that was lined with 2-inches Pinta Sonex foam to suppress sound reflection. Small loudspeakers were positioned on a horizontal hoop, 1.2 m in radius, in 20° increments of azimuth. A vertical arc, 1.1 m in radius, held speakers in 20° increments of elevation. The vertical arc could be rotated in azimuth from contralateral 50° to ipsilateral 50°. The loudspeakers were calibrated to equalize their levels and to flatten their broadband spectra 47. Independent Gaussian noise bursts were 80 or 150 ms in duration with abrupt onsets and offsets. Click trains consisted of 80- or 150-ms trains of 10-µs impulses at a rate of 200/s. Sound levels were 50 dB SPL (for 66 units) or 30 dB SPL (for 4 units).
Behavioral tasks and training
Data were obtained from four spayed female and one neutered male purpose-bred domestic cats that had clean external ears and no obvious hearing deficits. The cat sat or stood on a small platform centered within the arrays of loudspeakers. A harness retained the cat on the platform, but permitted it to move its head and limbs freely. Cats were monitored by video-camera from outside the chamber. A feeder mounted on a pneumatic cylinder was raised to provide behavioral reinforcement (liquefied canned cat food) and was lowered during data collection.
Each cat learned two behavioral tasks: Periodicity Detection and Localization. In all conditions, noise-burst probe stimuli were presented continuously at intervals of 1.25 s, jittered by 0.2 s, varying in azimuth from trial to trial. The noise bursts probed the spatial sensitivity of neurons. The two tasks differed in the nature of the targets. The Periodicity Detection target was a click train, a distinct “buzz”, which was presented from randomly varying azimuths and elevations. The Localization target was a Gaussian noise burst, identical to the probe stimuli except for its location. The Localization targets were presented from varying elevations 40° to 80° above the horizontal plane with azimuths that varied daily within a range of contralateral 50° to ipsilateral 50° azimuth; trial-by-trial differences in the cat’s head position introduced additional variance in the proximal acoustic cues associated with the targets. Probe stimuli also were presented during Idle periods, which were defined by an absence of key-pressing and occurred interspersed with periods of task performance or near the end of a session when the cat was satiated. Frequent movements of the head and body indicated that the cats were awake during Idle periods.
Behavioral sessions lasted ~1.5-hr and were conducted once or twice daily for each cat. All the behavioral sessions were conducted in the dark. The cat began each block of trials by pressing the response key with a forepaw to start a “hold” period, during which probe stimuli were presented. Hold periods were varied randomly in duration from 10–20 s and terminated with presentation of a target sound. If the key was released within 1.5 s after the target onset, the trial was scored as a “hit”, the feeder was raised, and the cat was rewarded. Early release was scored as a “false alarm” and triggered a 2-s timeout period. Late (or no) release was scored as a “miss”, and no food was delivered. The next block of trials began immediately if the key was still depressed or when the cat pressed the key again.
Training lasted 4 to 10 months, depending on the animal. Three cats were first trained in the Periodicity Detection task followed by the Localization task, and the other two cats started with Localization followed by Periodicity Detection. Criteria for completed training were median hit rates of ≥ 80% for the Periodicity Detection task or ≥ 70% for the Localization task. The reaction time for the behavioral responses averaged 0.63 second (STD: 0.19 s) across five animals. After reaching criteria for one task, each cat practiced that task daily for two weeks in order to consolidate its behavior and then began training in the second task. Once both tasks were learned and consolidated, each cat was trained to switch between the two tasks within single sessions. Within each session, each block of trials of a particular task was signaled by presentation of five of the targets from that task. After each cat learned to switch tasks in this way, it usually could perform one or more blocks of each task within each session. The order of the behavioral tasks for each daily behavioral session was determined pseudo-randomly.
Surgery
After training, a skull fixture and recording electrodes were implanted under aseptic conditions in an approved surgical suite. The skull fixture provided points of attachment for the recording head stage and for the electromagnetic sensor that tracked the head orientation. Recording electrodes were silicon-substrate multi-site chronic probes from NeuroNexus (Ann Arbor, MI). Each probe had 16 recording sites located along a single shank, spaced in 100- or 150-µm intervals. Two to four probes were placed in A1 or other cortical areas in each surgical procedure. Responses were attributed to cortical area A1 on the basis of cortical landmarks, frequency tuning, and spike latency. The number of sites with responsive units in area A1 ranged from 1 to 16 per probe (median =6). After placing the electrodes, the dura opening was covered with calcium alginate48, and the craniotomy was filled with SILASTIC, a silicone elastomer (World Precision Instruments, Sarasota, FL) and sealed with dental acrylic. After one week of recovery, cats began performing daily behavioral sessions with physiological recordings for a period of several weeks to several months. After sufficient data were obtained from each set of probes, or after the quality of recording deteriorated, probes were removed and new probes were implanted under similar aseptic surgical procedures. Each animal received a total of 3 to 10 sets of probes. Two cats received probe placements in both right and left hemispheres, whereas penetrations were restricted to the right hemisphere in the other three cats. Across the 5 animals, usable single- or multi-unit activity was recorded at a total of 70 A1 sites on 15 of the 16-site probes. Characteristic frequencies of units often were ambiguous, in contrast to the sharp frequency tuning usually encountered under anesthetized conditions, but those characteristic frequencies that could be measured ranged from 2 to 22 kHz.
Physiological recording
Behavioral conditions during physiological recording were identical to those during training, except that a headstage and a head-tracker receiver were mounted on the skull fixture. The neural waveform at each probe site was amplified, digitized, and stored on the computer hard disk for later analysis.
The possible influence of pinna movements on neural spatial sensitivity was a concern for this study. Video monitoring of the cats, however, indicated that pinna movements were minimal during recording sessions, consistent with our previous observations17. Moreover, significant sharpening, broadening, and/or no change in spatial sensitivity could be recorded from a set of units recorded simultaneously, which could not easily be attributed to a change in pinna position.
Data analysis
Extracellular action potentials (“spikes”) were identified offline from the stored neural waveforms using custom software based on principal component analysis of spike shape19. We encountered well-isolated single units and, more often, spikes from multiple unresolved units. We observed no systematic differences between single units and multi-unit recordings and therefore referred to both as “unit activity”, consistent with our previous reports and those of others. Unit activity was recorded at a total of 70 sites. Spike times were expressed relative to the sound onset at the loudspeaker; therefore, latencies include 3.5 ms of acoustic travel time. Unit activity at each site typically varied day to day, suggesting that the probe was moving relative to the brain or that the electrode-neural interface was changing. For that reason, we compared task-dependent characteristics of unit responses only within single ~1.5-hr recording sessions, during which spike shapes and spike count statistics tended to be stable.
The cat’s head was free to move, resulting in a variable alignment of the head relative to the fixed loudspeakers. The location and orientation of the head at the time of each stimulus onset was given by an electromagnetic head tracking system (Polhemus Fastrak, Colchester, VT). Offline, each stimulus location was expressed in head-centered coordinates based on the relative positions of the stimulus and the head. Head-centered stimulus azimuths were quantized into 18 20°-wide bins, centered at contralateral170 to ipsilateral 170° with 20° intervals. Very few stimuli fell into the bins centered at contra/ipsilateral 90° because the nearest loudspeakers fell precisely on the edges of those bins and because the cats seldom held their heads precisely horizontal. For that reason, we omitted from analysis the bins at contra/ipsilateral 90°, leaving 16 bins of head-centered azimuth.
The stimulus-location-dependent responses of units are represented by two-dimensional post-stimulus-time histograms, as in Figs. 1, 2 and 3, which show mean spike rate as a function of peri-stimulus time; plots were smoothed in the time domain with a 3-point Hanning window. White gaps crossing the plots correspond to the bins centered at contra- and ipsi-lateral 90°, which were excluded from analysis. Spatial sensitivity of units was quantified by rate-azimuth functions, which plotted mean spike counts within the first 40 ms after stimulus onset as a function of azimuth. Rate-azimuth functions for Periodicity and Localization conditions were compiled only from responses to probe stimuli on “hit” trials in which the response key was depressed and held until the cat correctly released the key in response to presentation of a target. Early or late releases of the key were scored as “false alarms” or “misses”, respectively. Note that comparison of spike counts elicited by a particular stimulus during hits or misses was not meaningful because a hit, miss, or false alarm was defined by the behavioral response to a target presented on a later trial. Rate-azimuth functions in the Idle condition were compiled during periods in which the key was not pressed. For some units, the rate-azimuth-functions for one condition were combined from more than one block of trials of the same condition during the same experimental session. The number of trials used to compute spike rates varied among stimulus locations and behavioral conditions from 3 to 105 trials (average 28.1). The Equivalent Rectangular Receptive Field (ERRF) of each unit was computed by computing the area under the rate-azimuth function and reshaping to form a rectangle of equivalent peak rate and area (see Supplementary Fig. 1).
We used a bootstrap procedure49 to estimate the trial-by-trial variation in ERRF widths of individual units. Each bootstrapped ERRF width was computed from a rate-azimuth function formed from the mean of a random sample of spike rates at each azimuth, sampled with replacement. The bootstrap sample size for each unit was the mean number of trials across locations for that unit. Comparisons of spatial selectivity between two task conditions were made by forming a Receiver Operating Characteristic (ROC) curve50 based on 1000 such computations for each condition. The area under the ROC curve yielded the proportion of trials in which spatial selectivity was sharper (or broader) in a particular task condition. A proportion of 0.76 (corresponding to a discrimination index of 1.0) was used as the criterion indicating that an individual unit showed significant sharpening or broadening.
For the purpose of comparing spike rates and first-spike latencies among behavioral conditions, we first smoothed rate-azimuth functions in each condition with a three-point Hanning window. Next, average rate-azimuth functions were formed by the means of functions for Idle, Periodicity, and Localization conditions. Finally, the “preferred” and “least-preferred” locations were given by the maxima and minima, respectively, of the average rate-azimuth functions. For each unit, the same preferred and least-preferred locations were used across all three behavioral conditions.
Supplementary Material
Acknowledgements
We thank I. Harrington, E. Macpherson, J. Wiler, E. Hand, G. Rising, D. Vailliencourt, C. Ellinger and Z. Onsan for technical support. We thank A. Kirby, and R. Adrian for helpful comments on the manuscript. This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01 DC-000420, and P30 DC-05188 (to J. Schacht).
Footnotes
Author Contributions
C.-C. L. and J.C.M. designed the experiments. C.-C. L. conducted the experiments. C.-C. L. collected and analyzed the data. C.-C. L. and J.C.M. co-wrote the manuscript.
References
- 1.Mondor TA, Zatorre RJ. Shifting and focusing auditory spatial attention. J Exp Psychol Hum Percept Perform. 1995;21:387–409. doi: 10.1037//0096-1523.21.2.387. [DOI] [PubMed] [Google Scholar]
- 2.Spence CJ, Driver J. Covert spatial orienting in audition - exogenous and endogenous mechanisms. J Exp Psychol Human. 1994;20:555–574. [Google Scholar]
- 3.McDonald JJ, Ward LM. Spatial relevance determines facilitatory and inhibitory effects of auditory covert spatial orienting. J Exp Psychol Human. 1999;25:1234–1252. [Google Scholar]
- 4.Roberts KL, Summerfield AQ, Hall DA. Covert auditory spatial orienting: an evaluation of the spatial relevance hypothesis. J Exp Psychol Hum Percept Perform. 2009;35:1178–1191. doi: 10.1037/a0014249. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes G. Auditory attention and the representation of spatial information. Percept Psychophys. 1987;42:1–14. doi: 10.3758/bf03211508. [DOI] [PubMed] [Google Scholar]
- 6.Kidd G, Jr, Arbogast TL, Mason CR, Gallun FJ. The advantage of knowing where to listen. J Acoust Soc Am. 2005;118:3804–3815. doi: 10.1121/1.2109187. [DOI] [PubMed] [Google Scholar]
- 7.Best V, Ozmeral EJ, Kopco N, Shinn-Cunningham BG. Object continuity enhances selective auditory attention. Proc Natl Acad Sci U S A. 2008;105:13174–13178. doi: 10.1073/pnas.0803718105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen K, Alais D, Carlile S. Speech intelligibility reduces over distance from an attended location: evidence for an auditory spatial gradient of attention. Atten Percept Psychophys. 2009;71:164–173. doi: 10.3758/APP.71.1.164. [DOI] [PubMed] [Google Scholar]
- 9.Kopco N, Best V, Shinn-Cunningham BG. Sound localization with a preceding distractor. J Acoust Soc Am. 2007;121:420–432. doi: 10.1121/1.2390677. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol. 1984;52:819–847. doi: 10.1152/jn.1984.52.5.819. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol. 2004;92:1625–1643. doi: 10.1152/jn.01205.2003. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra S, Stecker GC, Middlebrooks JC, Lomber SG. Sound localization deficits during reversible deactivation of primary auditory cortex and/or the dorsal zone. J Neurophysiol. 2008;99:1628–1642. doi: 10.1152/jn.01228.2007. [DOI] [PubMed] [Google Scholar]
- 13.Lee CC, Winer JA. Connections of cat auditory cortex: III. Corticocortical system. J Comp Neurol. 2008;507:1920–1943. doi: 10.1002/cne.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middlebrooks JC, Pettigrew JD. Functional classes of neurons in primary auditory cortex of the cat distinguished by sensitivity to sound location. J Neurosci. 1981;1:107–120. doi: 10.1523/JNEUROSCI.01-01-00107.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imig TJ, Irons WA, Samson FR. Single-unit selectivity to azimuthal direction and sound pressure level of noise bursts in cat high-frequency primary auditory cortex. J Neurophysiol. 1990;63:1448–1466. doi: 10.1152/jn.1990.63.6.1448. [DOI] [PubMed] [Google Scholar]
- 16.Middlebrooks JC, Clock AE, Xu L, Green DM. A panoramic code for sound location by cortical neurons. Science. 1994;264:842–844. doi: 10.1126/science.8171339. [DOI] [PubMed] [Google Scholar]
- 17.Mickey BJ, Middlebrooks JC. Representation of auditory space by cortical neurons in awake cats. J Neurosci. 2003;23:8649–8663. doi: 10.1523/JNEUROSCI.23-25-08649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore BC, Glasberg BR. Suggested formulae for calculating auditory-filter bandwidths and excitation patterns. J Acoust Soc Am. 1983;74:750–753. doi: 10.1121/1.389861. [DOI] [PubMed] [Google Scholar]
- 19.Stecker GC, Harrington IA, Macpherson EA, Middlebrooks JC. Spatial sensitivity in the dorsal zone (area DZ) of cat auditory cortex. J Neurophysiol. 2005;94:1267–1280. doi: 10.1152/jn.00104.2005. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa S, Middlebrooks JC. Cortical representation of auditory space: information-bearing features of spike patterns. J Neurophysiol. 2002;87:1749–1762. doi: 10.1152/jn.00491.2001. [DOI] [PubMed] [Google Scholar]
- 21.Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- 22.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson DA, Hienz RD, Goldstein MH., Jr Single-unit activity in the auditory cortex of monkeys actively localizing sound sources: spatial tuning and behavioral dependency. Brain Res. 1981;219:249–267. doi: 10.1016/0006-8993(81)90290-0. [DOI] [PubMed] [Google Scholar]
- 24.Scott BH, Malone BJ, Semple MN. Effect of behavioral context on representation of a spatial cue in core auditory cortex of awake macaques. J Neurosci. 2007;27:6489–6499. doi: 10.1523/JNEUROSCI.0016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- 26.Diamond DM, Weinberger NM. Role of context in the expression of learning-induced plasticity of single neurons in auditory cortex. Behav Neurosci. 1989;103:471–494. doi: 10.1037//0735-7044.103.3.471. [DOI] [PubMed] [Google Scholar]
- 27.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmusson DD, Smith SA, Semba K. Inactivation of prefrontal cortex abolishes cortical acetylcholine release evoked by sensory or sensory pathway stimulation in the rat. Neuroscience. 2007;149:232–241. doi: 10.1016/j.neuroscience.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 29.Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 30.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 31.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Yan J. Corticothalamic feedback for sound-specific plasticity of auditory thalamic neurons elicited by tones paired with basal forebrain stimulation. Cereb Cortex. 2008;18:1521–1528. doi: 10.1093/cercor/bhm188. [DOI] [PubMed] [Google Scholar]
- 33.Ghose GM. Learning in mammalian sensory cortex. Curr Opin Neurobiol. 2004;14:513–518. doi: 10.1016/j.conb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Ohl FW, Scheich H. Learning-induced plasticity in animal and human auditory cortex. Curr Opin Neurobiol. 2005;15:470–477. doi: 10.1016/j.conb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Witte RS, Kipke DR. Enhanced contrast sensitivity in auditory cortex as cats learn to discriminate sound frequencies. Brain Res Cogn Brain Res. 2005;23:171–184. doi: 10.1016/j.cogbrainres.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Brechmann A, Scheich H. Hemispheric shifts of sound representation in auditory cortex with conceptual listening. Cereb Cortex. 2005;15:578–587. doi: 10.1093/cercor/bhh159. [DOI] [PubMed] [Google Scholar]
- 37.Cansino S, Williamson SJ. Neuromagnetic fields reveal cortical plasticity when learning an auditory discrimination task. Brain Res. 1997;764:53–66. doi: 10.1016/s0006-8993(97)00321-1. [DOI] [PubMed] [Google Scholar]
- 38.Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–654. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura A, Imbe H, Donishi T, Tamai Y. Axonal projections of single auditory neurons in the thalamic reticular nucleus: implications for tonotopy-related gating function and cross-modal modulation. Eur J Neurosci. 2007;26:3524–3535. doi: 10.1111/j.1460-9568.2007.05925.x. [DOI] [PubMed] [Google Scholar]
- 43.Cotillon-Williams N, Huetz C, Hennevin E, Edeline JM. Tonotopic control of auditory thalamus frequency tuning by reticular thalamic neurons. J Neurophysiol. 2008;99:1137–1151. doi: 10.1152/jn.01159.2007. [DOI] [PubMed] [Google Scholar]
- 44.Verbny YI, Erdelyi F, Szabo G, Banks MI. Properties of a population of GABAergic cells in murine auditory cortex weakly excited by thalamic stimulation. J Neurophysiol. 2006;96:3194–3208. doi: 10.1152/jn.00484.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X, Suga N. Specific and nonspecific plasticity of the primary auditory cortex elicited by thalamic auditory neurons. J Neurosci. 2009;29:4888–4896. doi: 10.1523/JNEUROSCI.0167-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci. 2007;27:6091–6102. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou B, Green DM, Middlebrooks JC. Characterization of external ear impulse responses using Golay codes. J Acoust Soc Am. 1992;92:1169–1171. doi: 10.1121/1.404045. [DOI] [PubMed] [Google Scholar]
- 48.Nunamaker EA, Purcell EK, Kipke DR. In vivo stability and biocompatibility of implanted calcium alginate disks. J Biomed Mater Res A. 2007;83:1128–1137. doi: 10.1002/jbm.a.31275. [DOI] [PubMed] [Google Scholar]
- 49.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 50.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. p. 455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.