Abstract
The Calibrated Automated Thrombogram (CAT), a plate-based assay that measures thrombin generation and inhibition in plasma samples, is modified to measure the procoagulant activity of phospholipid associated with plasma microparticles (MP). The assay uses a tissue factor trigger without addition of 4 µM exogenous phospholipid (PL) used in the standard CAT. Calibrated with 4:1 posphatidylcholine- phosphatidylserine (PCPS) liposomes, the assay defines a median of 40 nM procoagulant phospholipid (PPL) equivalents in plasma containing MPs from 50 normal donors, with a range from 20 – 200 nM. Like the standard CAT, the modified assay detected no difference in plasma from 36 individuals with a history of a single episode of venous thromboembolism. However the male cases had double the PPL activity, as measured by rate of thrombin generation, of females; and there was a significant correlation among cases of increased thrombin generation with age. In contrast, there were no gender disparities or age correlations among control plasmas. The findings suggest that procoagulant activity of plasma microparticles, facilitated by a simplified, one-stage plate-based assay, offer a promising avenue of investigation of mechanisms and management of venous thromboembolic disorders.
Keywords: Thrombin generation, Microparticles, Procoagulant phospholipid, Venous Thromboembolism (VTE)
Introduction
Thrombosis can occur when a vascular lesion activates the hemostasis system, or when the hemostasis system itself becomes hypersensitive to an otherwise benign vascular anomaly. This hyperresponsiveness of the system, viewed as “thrombophilia” or “propensity to thrombosis” [1] is clearly dependent upon components within blood [1,2] but is otherwise undefined [1,3]. The presymptomatic diagnosis and subsequent prediction of thrombosis after disease is established remain elusive. Elucidation of mechanisms of thrombosis, development of sensitive and specific diagnostics, and design of effective but benign therapeutics calls for better insight into the biochemistry and cell physiology of hemostasis, the pathobiology of vascular disease, and the pathophysiology of the thrombotic process itself.
The risk of recurrence after an initial VTE is the highest within six months and as high as 30%within 10 years. Standard medical practice is that all VTE patients are prescribed prophylactic anticoagulants [4,5]. Complications of prolonged anticoagulation therapy can be serious, so patients who would not require treatment are exposed to unnecessary risk [6,7]. Known risk factors for recurrence of VTE include indications of ongoing thrombosis, such as a high D-dimer level, residual thrombosis detected by ultrasound and increased thrombin generation [8–13]. Male gender has also been found to be associated with a higher risk for VTE recurrence, but a plausible mechanism is not apparent [14–17].
The presence of increased circulating procoagulant phospholipid (PPL) has been detected in a number of pre-thrombotic conditions, including myocardial infarction (MI), cancer and trauma but has not been reported for VTE [18, 19]. Triggers for venous thrombosis are not known, but the process of clot formation requires PPL and definition of thrombophilia could most certainly include increased levels of circulating procoagulant phospholipids [20]. Most PPL detected in other disease states appears to be associated with microparticles derived from platelets. PPL-positive microparticles shed from platelets are thought to result as a consequence of platelet activation and therefore an indicator of ongoing thrombosis (reviewed in [21]). Although platelets do not generally appear to be prevalent within venous thrombi [22,23], microparticles derived from circulating platelets have been shown to be very potent activators for both factor X and prothrombin [19,24]. To determine whether procoagulant phospholipids associated with microparticles are present in VTE patients, we modified an established thrombin generation assay, the calibrated automatic thrombogram (CAT) [25], to be sensitive to the PPL associated with procoagulant microparticles. Since annexin V binding has been equated to procoagulant activity, we also assayed isolated platelet microparticles by flow cytometry. We find that procoagulant phospholipid associated with microparticles is increased in VTE patients with increasing age but not with the number of annexin V-positive microparticles derived from platelets. Furthermore, significantly higher rates of thrombin generation are supported by microparticles in male compared to female VTE patients.
Methods
Patient and Control subjects
Mayo Clinic outpatients age 18 years or older with objectively-diagnosed DVT or PE (confirmed by venography, pulmonary angiography, compression venous duplex ultrasonography, ventilation/perfusion lung scan interpreted as high probability for PE, computed tomographic pulmonary angiography, magnetic resonance imaging or pathology examination of thrombus removed at surgery) who resided in the upper Midwest United States and who were referred to the Mayo Clinic Special Coagulation Laboratory or Mayo Clinic Thrombophilia Center were recruited over the study period, (10/13/2008 – 11/06/2009, 12 months), for study participation. The time between VTE and blood draw ranged from <1–33 years, with a median of 5 years. We excluded patients with VTE related to active cancer, an indwelling central venous catheter, transvenous pacemaker or other mechanical cause for thrombosis, or a lupus anticoagulant or other antiphospholipid antibodies. Controls were selected prospectively from persons undergoing outpatient general medical examinations during the same time period. VTE cases and controls were not receiving anticoagulation (heparin, warfarin) or an antithrombotic (aspirin, non-steroidal anti-inflammatory drug, thienopyridine) within the four weeks prior to blood sample collection. We collected 51 controls (27 females and 23 males) and 36 VTE patients, (15 females and 21 males) to be assayed for thrombin generation. Ages ranged from 19–81, and all were white of European ancestry. Except for one female control, no VTE cases or controls were receiving hormone therapy.
Sample Collection and Processing
Blood was collected by venipuncture using an 18 gauge winged-infusion set (Becton Dickinson, New Jersey, USA) into Vacutainer tubes containing 3.2% sodium citrate. Platelet-poor plasma (PPP) was prepared by an initial centrifugation at 1500 ×g for 10 min to remove platelets followed by a second centrifugation at 2750 ×g for 15 min. PPP for thrombin generation or microparticle analysis experiments was stored at −70 °C.
Isolation of blood microparticles for flow cytometric analysis
Frozen samples were thawed in a 37 °C water bath for 5 min, vortexed, and then centrifuged at 3,000 ×g for 15 min. The plasma sample was then centrifuged at 20,000 ×g for 30 min. Supernatants were removed, and the remaining pellets (microparticles) were reconstituted with Hanks’ solution buffered (pH 7.4) with 20 mM HEPES. Tubes containing reconstituted microparticles were vortexed and centrifuged again at 20,000 ×g for 30 min. After centrifugation, the pellets containing microparticles were reconstituted again and vortexed for 1 to 2 min before analysis. All buffers were filtered twice through a 0.2-µm membrane filter before use.
Experimental Procedures
Thrombin Generation Assay
Thrombin generation was measured with the Calibrated Automatic Thrombinogram Assay (CAT Assay, Thrombinoscope BV, Maastricht, The Netherlands) [26]. Either of two 20 µL triggers, the proscribed PPP-Reagent 5 pM (5 pM tissue factor/4 µM phospholipid, Stago, US) or diluted Innovin (Dade Behring, Newark, DE) was added to polypropylene 96-well microtiter plates (Nunc, Thermo Fischer Scientific, Rochester, NY). Then, citrated platelet poor plasma (80 µL), containing 50 µg/mL of corn trypsin inhibitor (CTI, Haematologic Technologies Inc. Essex Junction, VT) was added and the plate was warmed to 37 °C for 5 minutes in the pre-equilibrated plate-reader. Thrombin generation was initiated with pre-warmed Fluo-substrate (Stago, US) that contained calcium and buffer (Fluo-buffer, Stago, US). Thrombin progress curves were recorded continuously with a Fluoroskan FL instrument (Thermo Labsystems, Helsinki, Finland) under control of Thrombinoscope software (Thrombinoscope BV, Maastricht, The Netherlands), filtered for excitation at 390 nm and emission at 460 nm. For each plasma sample, a calibrator assay was included in which the tissue factor/PL trigger was replaced with a solution that contained a known concentration of thrombin-α2-macroglobulin complex (thrombin calibrator, Stago, US). The calibrator corrects for inner filter effects and quenching variation among individual plasmas. Each trigger and calibrator was assayed in triplicate for each plasma sample analyzed.
Data Analysis
Raw fluorescence data were converted to thrombin activity (nM), relative to the calibrator by the Thrombinoscope software [27]. For each plasma sample and trigger combination, four parameters were derived by the software: lagtime (LT, min), endogenous thrombin potential, (area under the curve; ETP, nM-min), peak thrombin activity (Peak, nM) and time to peak (TTP, min). Rate of thrombin generation (nM/min) was calculated by: .
PPL Standard Curve
A standard curve was constructed from dilutions of synthetic phospholipid (1.3 mM) composed of 20 mol% phosphatidylserine and 80 mol% phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL, USA) [28] combined with dilute Innovin (approximately 5 pM tissue factor). Approximate TF concentration was determined by titration of diluted Innovin (Dade Behring, Newark, DE) against the PPP-Reagent 5 pM, as judged by equivalent peak height. The PC/PS-Innovin reagent was added, from zero to 4 µM PC/PS, constant Innovin, into Cryocheck control pool plasma (Precision BioLogic, Dartmouth, NS, Canada) that had been depleted of MP by centrifugation at 49,000 ×g for 10minutes, room temperature, in an Airfuge (Beckman Coulter, Brea, CA). The CAT assay was initiated with the substrate-calcium reagent and resulting Peak height (nM thrombin) was plotted versus concentration of PC/PS (µM).
Procoagulant Phospholipid Assay
To measure endogenous procoagulant phospholipid content in individual plasma samples, the CAT assay was triggered only with the diluted Innovin reagent in absentia of excess, exogenously added phospholipid. As demonstrated by the addition of purified PC/PS to MP-depleted plasma, under these conditions the extent of thrombin generation becomes dependent upon the concentration of PPL in the plasma samples. In order to define the contribution of MP to the total plasma PPL, a sample that had been depleted of MP by centrifugation at 49000 ×g for 10 minutes is also assayed with the diluted Innovin trigger. The PPL derived from microparticles is determined by subtracting the amount of thrombin generated from the MP-depleted sample from that of the non-centrifuged plasma.
Flow Cytometric Analysis of MP
Flow cytometry (FACS-Canto, BD Biosciences) was used to define microparticles by size and positive fluorescence using marker-specific antibodies and annexin V as described previously [29].
Statistical Analysis
Box and Whisker plots were constructed for each CAT parameter measured for both control and case samples. Range, median, mean, and standard deviation were calculated using Sigmaplot 8 (Systat Software, Inc. San Jose, CA). The upper and lower edge of each box within the Box and Whisker plot defines the 75th and 25th percentiles, respectively and the line within the box defines the median. The upper and lower whiskers on each box define the 95th and 5th percentile, respectively and any outliers are indicated with symbols. Quartiles, Spearman coefficients and p-values of select thrombin generation data were determined using Analyze-it software (Analyze-it Software, Ltd., Leeds, UK). Groups were compared with use of the Mann-Whitney-Wilcoxon (MWW) rank-sum test, and dependencies used the Spearman rank correlation. Linear plots of data were constructed and analyzed using Sigmaplot 8 linear regression software (Systat Software, Inc. San Jose, CA).
Results
The endogenous thrombin potential, or ETP, is the total amount of thrombin generated in plasma after addition of a defined trigger. The nominal assay for the calibrated automatic thrombogram (CAT), from which the ETP is calculated comprises 5 pM tissue factor and 4 micromolar phospholipid in citrated, platelet poor plasma, and thrombin generation after recalcification is measured continuously with a fluorogenic substrate [26]. We assayed 36 individuals with a single prior VTE, during a one year period, for their endogenous thrombin potential using the high TF and PL trigger. During this same time period, 51 plasma samples without documented VTE (controls), were also collected (Table 1).
Table 1.
Demographic characteristics of Venous Thromboembolism Case and Controls. Age of VTE case and control patients are categorized by decade and the number of male or females in each control or case group is listed along with the percent (%) within each group. Median and mean age are shown for males and females within the control or case groups.
| Variable | Controls (n = 51) | Cases (n = 36) | ||
|---|---|---|---|---|
| Sex | female | male | female | male |
| Number (%) | 27 (53) | 24 (47) | 15 (42) | 21 (58) |
| Age group | ||||
| 18–29 | 3 (11) | 1 (4) | 3 (20) | none |
| 30–39 | 3 (11) | 5 (21) | 4 (27) | 1 (5) |
| 40–49 | 7 (26) | 4 (15) | 1 (7) | 1 (5) |
| 50–59 | 4 (15) | 2 (8) | 2 (13) | 4 (19) |
| 60–69 | 7 (26) | 6 (25) | 2 (13) | 7 (33) |
| 70–79 | 3 (11) | 4 (15) | 3 (20) | 3 (14) |
| 80–89 | none | 1 (4) | none | 5 (24) |
| Median Age | 50 | 56 | 46 | 66 |
| Mean Age | 50.9±15.7 | 55.3±15.8 | 48.5±17.7 | 67.1±13.5 |
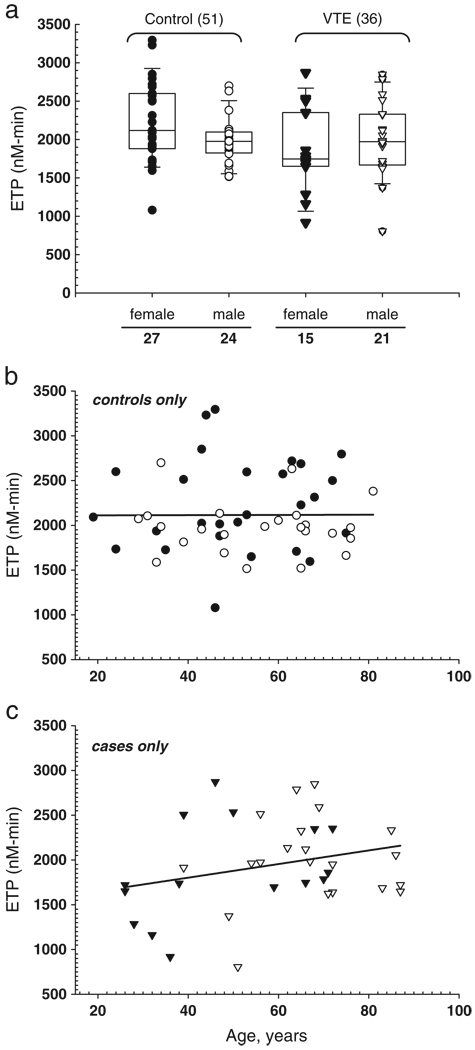
There were no significant differences in ETP between VTE cases and controls, or in males compared to females in cases or controls (Fig. 1a). Thrombin generation in the female case group showed a skewed distribution with two thirds of the samples at or below the median and one third at or above the 75th percentile. Although there was no difference in the ETP between males and females in the control group, a bimodal distribution for females was not seen. Except for male controls, the distributions showed a three-fold data range.
Fig. 1.
Total thrombin generation potential using maximal trigger. Thrombin generation is triggered with 5 pM tissue factor and 4 µM phospholipid and integrated to derive the ETP (nM −min. a) Box and whisker plot of either control (open, males (n = 24) and filled, females (n = 27) or case, n = 36 (triangle; open, males (n = 21) and filled, females (n = 15) plasma samples as indicated, with the median represented by the line within the box, the upper boundary of the box represents the 75% of the data and the lower boundary of the box the 25%, and the whiskers (95%, upper and 5%, lower). b, c) Linear regression analysis of ETP versus age for control and case samples, respectively and symbols are as indicated for 1a.
For control and case samples, there is no correlation between increasing age and thrombin generation, in either male or females, as measured by total thrombin (ETP) generated using a trigger that contains high tissue factor and high phospholipid (Fig. 1b). However, there is a weak trend (p = 0.22) towards a positive correlation between age and ETP in the VTE case samples (Fig. 1c). We do not mean to imply that the relationship between ETP and age is linear, which would require much larger sample size to determine, but rather to illustrate the difference between control and case samples. These differences are primarily reflecting the sample number from the short duration of the study and exclusion of patients on anticoagulants.
Procoagulant phospholipid in plasma microparticles
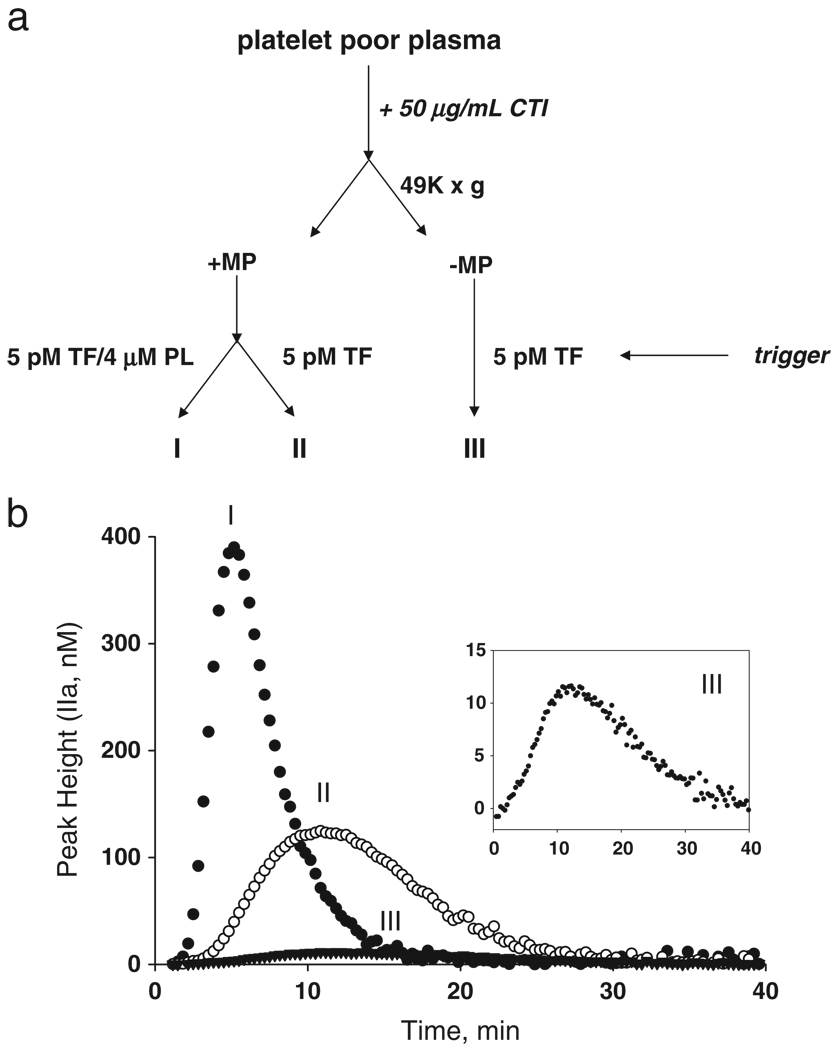
We modified the standard CAT assay to detect procoagulant phospholipid associated with microparticles in patient plasmas. The modification uses a trigger without supplemental lipid and contains the equivalent to 5 pM tissue factor. At this concentration, the PPL associated with the TF is insignificant. A pooled reference platelet poor plasma, supplemented with 50 µg/mL of corn trypsin inhibitor (CTI) to inhibit contact activation, was separated into three fractions (Fig. 2a), either containing endogenous microparticles (I and II) or depleted of microparticles by centrifugation at 49000 ×g (III). Fraction I, which contains endogenous microparticles, is assayed for maximal thrombin generation in the presence of excess phospholipid. Fraction II, which also contains endogenous microparticles, is activated with a trigger that contains 5 pM tissue factor (Innovin, Baxter, diluted 1:400), but without exogenous lipid, resulting in thrombin generation substantially lower than that of fraction I. These results imply that the microparticles endogenous to the reference plasma can support about 25% of the thrombin that is generated compared to that which is generated with the 4 µM phospholipid present in the commercial trigger (compare I and II, Fig. 2b) also containing 5 pM TF. Most of the procoagulant phospholipid in this reference plasma is contained in the microparticle fraction, as fraction III which lacks microparticles after centrifugation at 49000 ×g generates very little thrombin (Fig. 2b and inset). Mean peak thrombin was measured for each fraction on 10 individual reference plasma samples thawed and assayed on 10 different days in triplicate: 362 ± 43 nM, 96 ± 14 nM and 9 ± 2 nM, for fractions I, II, and III respectively.
Fig. 2.
Assay to measure the procoagulant phospholipid from microparticle and non-microparticle sources in plasma. Platelet poor plasma with corn trypsin inhibitor (CTI) is assayed for thrombin generation using a 5 pM tissue factor trigger with or without additional PPL before and after high-speed centrifugation to remove microparticles. a) Schematic representing the processing, fractionating, and assaying steps for thrombin generation. Three different conditions for each plasma are assayed, as indicated by I, II, or III, “(± MP)” denotes the absence or presence of microparticles. b) Representative thrombin generation profiles, plotted as thrombin concentration versus time for each condition (I, II, or III) represented in a. The inset shows enlargement of the profile generated for condition III.
Peak thrombin is directly proportional to procoagulant phospholipid
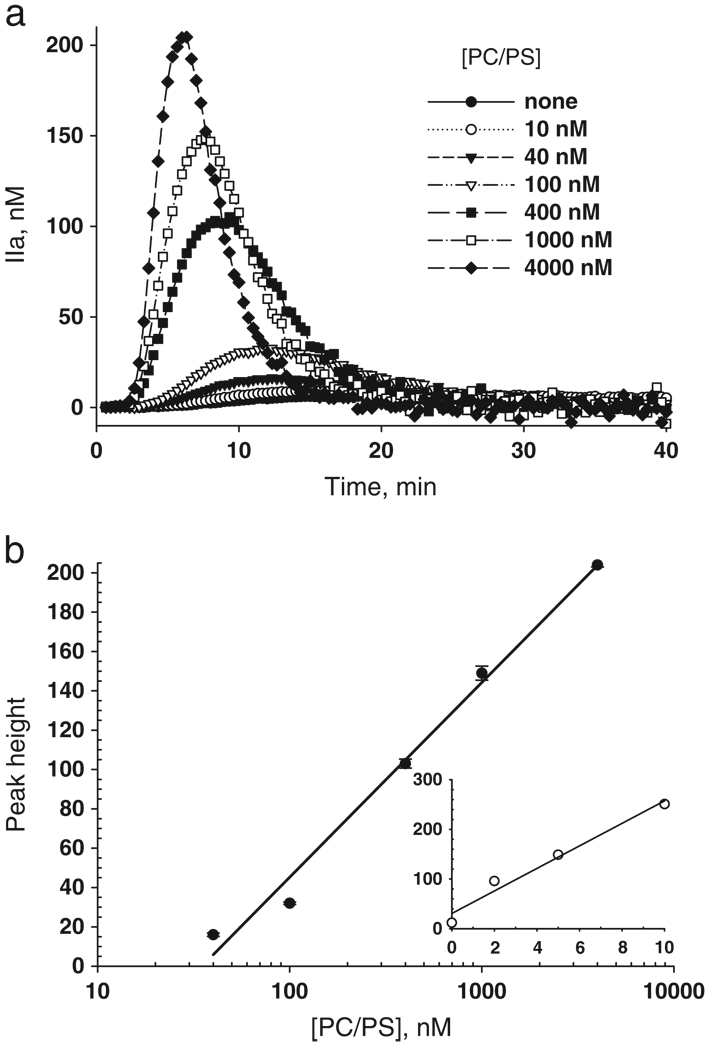
The results in Fig. 2b imply that peak thrombin is sensitive to endogenous procoagulant phospholipid. Using fraction III prepared from the reference plasma illustrated in Fig. 2, which is devoid of microparticles, we added increasing amounts of purified PC/PS vesicles to the diluted Innovin as a trigger for thrombin generation. Fig. 3a shows the thrombin generation curves plotted as thrombin versus time as the concentrations of PC/PS increase from 0–4000 nM. Plots of thrombin peak height versus log PC/PS concentration yield a straight line (r2 = 0.987) resulting in the standard curve shown in (Fig. 3b).
Fig. 3.
Peak height is proportional to procoagulant phospholipid (PPL). a) Thrombin generation after addition of PC/PS as indicated by the legend. b), Peak height of thrombin in (a) plotted versus PC/PS concentration. Each data point represents the average of three individual measurements with error bars as indicated. Inset) The microparticles were concentrated by centrifugations of repetitive plasma aliquots in a single Airfuge tube, and then resuspended by reciprocal movement through a micropipette tip. The peak height of concentrated plasma microparticles is plotted versus fold concentrated over parent plasma.
We then determined whether the relationship between peak thrombin and procoagulant phospholipid was maintained when the source of lipid was supplied by microparticles, concentrated within the reference plasma by repetitive centrifugations in the same tube at 49000 ×g. The microparticles were reconstituted at 2, 5, and 10-fold the original plasma concentrations with particle-free reference plasma (49000 ×g supernatant) and then assayed for thrombin generation using the 5 pM tissue factor trigger. The peak thrombin obtained for each fold-concentrated MP (x-axis) sample is shown as an open circle in Fig. 3b, inset. The peak height is proportional to MP concentrations as high as 10X that of the parent plasma, but recovery was less than quantitative. The peak height for fraction II-III (Fig. 2) averaged 90 nM thrombin. From the slope (linear fit) in the Fig. 3 inset of the dependence on MP concentration, the recovery of PCPS equivalent activity of the MP averaged 50%.
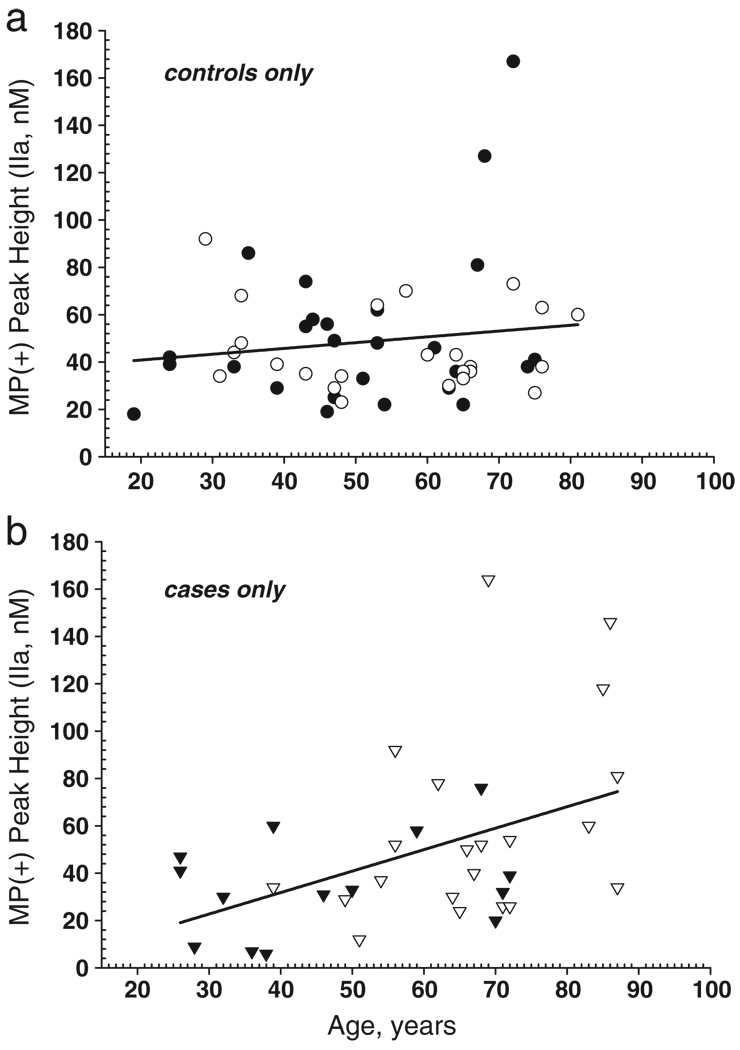
Procoagulant phospholipid (PPL) on microparticles increases with age
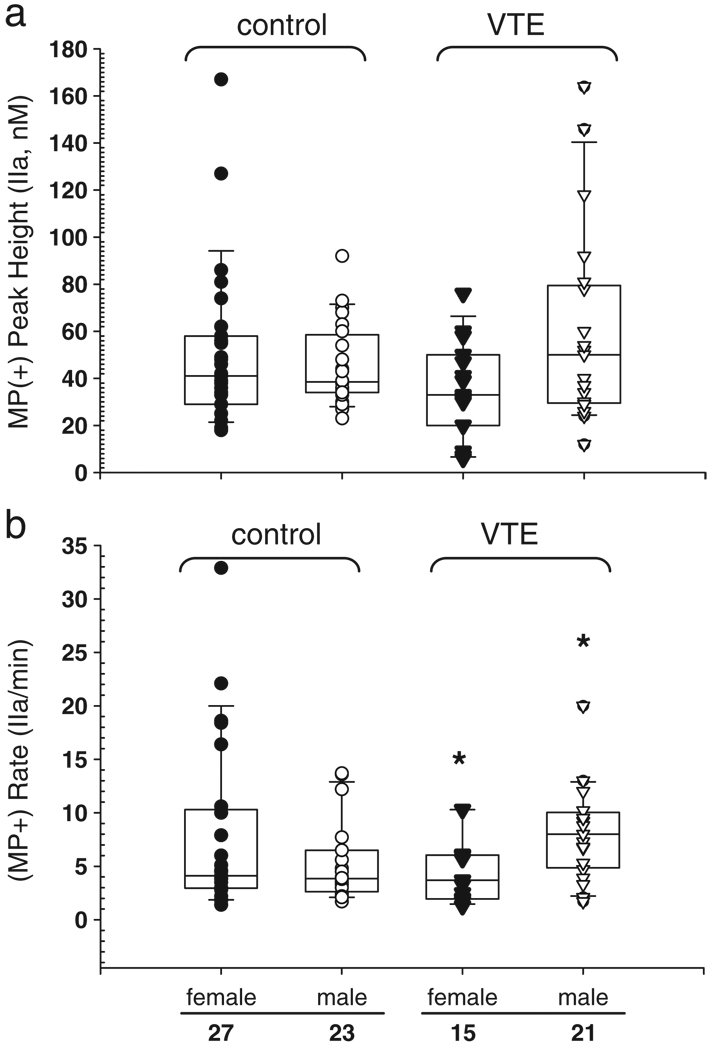
Each VTE control or case plasma fraction was assayed as described in Fig. 2. There is an increase in MP-associated thrombin generation as measured by peak height in male (median = 50) compared to female (median = 33) VTE cases (Fig. 4a). This is not seen in the control samples, where the median thrombin peak height for females (41 nM, range 18–167) is higher and has a much broader range than it does for males (38.5 nM, range 23–92). Since the CAT assay is a kinetic measurement it allows assessment of the rate of thrombin generation. There were no significant differences in the Lag time (LT, min; data not shown) between any of the groups (case versus control) within each condition (I, II, III) assayed. However, male VTE cases show more than a 2-fold increase (p = 0.007, Mann-Whitney-Wilcoxon rank sum) in the rate of thrombin generation compared to female VTE cases (Fig. 4b). Again, this difference was not seen in the control group, where females had slightly higher rates of thrombin generation compared to males. Although the median rate of thrombin generation for female cases was lower than for males, four of the fifteen female case samples had rates of thrombin at or above the 75th percentile (6 nM/min, range 6–10 nM/min).
Fig. 4.
The amount and rate of thrombin generation with endogenous microparticle-derived PPL is increased in male VTE cases. The trigger is 5 pM tissue factor without added PPL and the non-sedimentable activity has been subtracted, as described for Fig. 3. a) Box and whisker plots showing the peak height for VTE control or case plasmas, symbols as described in Fig. 1a. b) Rate of thrombin generation, calculated by dividing the peak height by the time to reach the peak. Single asterisk (*) indicates p-value (Mann-Whitney-Wilcoxon rank sum) of 0.007.
As noted previously, males in this study are significantly older than females. (Table 1) Although age and gender are better matched in the control group, with males being only slightly older than females, the total age range for each group spans over six decades. When procoagulant microparticles, as measured by peak thrombin, are plotted against age of individual the VTE cases, but not controls, show a significant, positive correlation (p = 0.02, Spearman rank, compare Fig. 5b to 5a). Additional statistical analysis showed that the VTE case data also fit a quadratic function (p = 0.07). This would suggest that peak thrombin is essentially flat for younger ages and increases after about age 45, and that male VTE cases tended to have more microparticle-associated procoagulant phospholipid than male controls, while female cases tended to have lower microparticle-associated procoagulant phospholipid than female controls. This alternative model recapitulates our previous findings concerning age at time of incidence of first VTE [Heit, 2001], where incidence was nearly flat for both males and females until age 45, at which point incidence rises sharply and is slightly higher (18%) in males compared to females in non-hospitalized individuals. Further analysis with a larger sample size will be required to determine whether the apparent increase in microparticle-associated procoagulant phospholipid in males detected in this study is related to the incidence difference of VTE between males and females. These findings suggest that either procoagulant microparticles increase in number, or that the total procoagulant phospholipid surface concentration of the microparticles is increased in VTE case samples.
Fig. 5.
Thrombin generation in MPs is increased with age. The linear regression lines are shown, a) Female and male controls, with symbols the same as in Fig. 1a. b) Female and male cases, with symbols the same as in Fig. 1a.
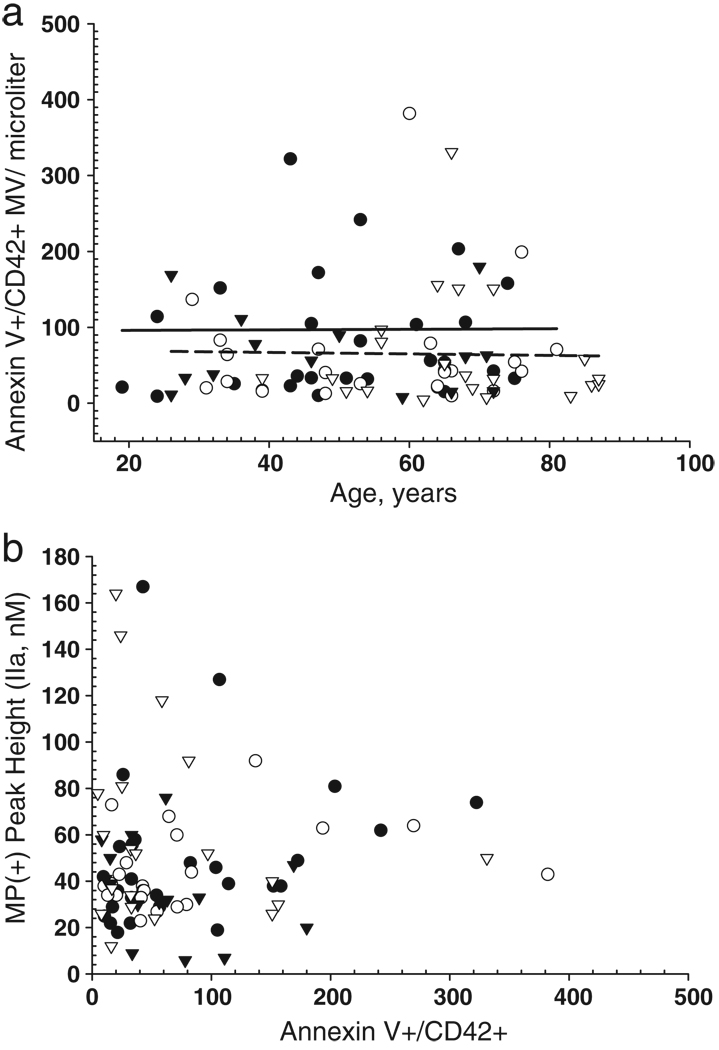
Thrombin generation by MP does not correlate to annexin V binding
Annexin V binds to exposed phosphatidylserine on either cells or microparticles and has provided quantitative measurement for procoagulant phospholipid. In parallel to measurements of thrombin generation, microparticles derived from all samples were analyzed for annexin V binding by flow cytometry, using fluoroscein-labeled annexin V. Greater than 90% of all annexin V positive microparticles were also positive for the platelet-specific marker CD42. In contrast to peak thrombin generation, there was no correlation with annexin V-positive/CD42-positive microparticle numbers with age for either control or case samples (Fig. 6a). Furthermore, in contrast to what was seen for both increasing peak height and rate of thrombin generation derived from microparticles in VTE case samples, the number of annexin V-positive/CD42-positive microparticles in case samples was lower, with a mean value of 65.2 ± 67.7, than that compared to control samples, with a mean of 97.3 ± 132.5 (t-test, p = 0.09), Fig. 6a. This finding suggest discordance between procoagulant activity and annexin V binding. This was verified by plotting annexin V-positive/ CD42-positive microparticles (number/microliter plasma) versus peak thrombin generation. There is substantially more functional PPL in VTE case samples (mean 61 ± 40, median 54, nM IIa) and controls (mean 49 ± 30 median 40, nM IIa) than can be accounted for by numbers of annexin V/ MP in VTE case samples compared to controls. These data suggest that there are procoagulant factors not detected by annexin V, presumably phospholipid in nature, that may contribute to thrombin generation, as had been previously suggested by studies using the XACT assay [30]. Alternatively, since annexin V binding requires a minimum density of phosphatidylserine for binding, thrombin generation may still occur at densities lower than that detectable by annexin V. These findings also indicate that the amount of procoagulant PPL that will trigger thrombin generation cannot be directly determined by measuring only the amount of annexin V-positive MP.
Fig. 6.
Annexin V/CD42-positive MP neither increases with age nor correlates to procoagulant phospholipid measured by thrombin generation. Microparticle analysis using flow cytometry was performed on isolated and washed microparticles that were co-stained for both annexin V and CD42. Thrombin generation analysis was performed in parallel on citrated platelet poor plasma processed at the same time. a) AnnexinV/CD42-double positive microparticle versus age. Control and case samples data are plotted with symbol colors defined in Fig. 6. b) The number of annexin V/CD42-positive microparticles per µL versus peak thrombin. Control and case samples data are plotted with symbols defined in Fig. 6a.
Discussion
Assays that address global hemostasis, in which both pro- and anti-coagulant processes are determinants, have a greater potential for detecting a hypercoagulable state than measurement of any single pathway. Thrombin generation assays having such global sensitivity have become revitalized by using simplified and semi-automated plate-reader-based approaches, and have become quantifiable through use of internal standardization [31–33]. These assays, which use cell-deficient plasma, allow analysis of the composite soluble factors involved in the coagulation process, including the balance of pro- and anti-coagulant factors derived from a single individual. Although cellular and vessel wall components, which also participate in the generation of thrombin in vivo, are missing, microparticles derived from these sources are still present in the plasma. Microparticles shed into circulation have been investigated both for their potential to report on prothrombotic events that have occurred at the site of activity, and for their relation to propensity for thrombosis [34]. However, existing methods for measuring the contribution of microparticles to thrombin generation involve either isolation of washed microparticle preparations and/or the use of specialized reagents, which make such assays impractical for clinical application.
We have devised a modification of the assay originally conceived and developed by Hemker et al. [35,36] to analyze the procoagulant phospholipid content of plasma and plasma-derived microparticles. Tripodi et al. [37] found that by reducing both the TF and PL concentrations from the nominal CAT, the assay becomes more sensitive to the Protein C pathway. Similar to our findings and others, increases in ETP were not significant in those with recurrent VTE compared to those without. However, when thrombomodulin was included in the assay, there were significant differences between cases with recurrent VTE in both ETP and peak height [37]. Our approach uses re-lipidated tissue factor (Innovin), a well-characterized, commercially available reagent, for triggering thrombin generation. This thromboplastin reagent is diluted to a level equivalent to 5 pM of purified tissue factor to which no exogenous phospholipid has been added. The amount of thrombin generation then is limited to the amount of procoagulant phospholipid supplied by plasma components. In this way, similar to addition of thrombomodulin which sensitizes the CAT assay to an individual's protein C pathway components, our modification sensitizes the CAT to an individual's procoagulant phospholipid.
A standard curve constructed with known amounts of purified PC/PS vesicles added to microparticle-free plasma relates PPL-equivalents to thrombin peak height. We verified a similar concentration dependence when the source of procoagulant phospholipid is supplied only by adding MP to control plasma, although recovery is reduced by incomplete suspension after the high-speed centrifugation. This finding may provide a means to validate other approaches to MP analysis. The assay entails measuring the difference between thrombin generation in platelet poor plasma with the diluted Innovin trigger and a second measurement, with the same trigger in plasma that has been centrifuged to remove microparticles (any table-top centrifuge capable of 15,000 – 20,000 ×g would suffice [38]. The difference between the two measurements is the contribution of microparticles to the procoagulant phospholipid balance of the plasma. This subtractive method eliminates the time-consuming process of isolating and washing microparticles, which also induces error by decreasing their yield. Furthermore, once isolated, microparticles lack procoagulant factors to support thrombin generation and must be assayed using yet another assay requiring additional reagents and processing. For clinical research application, the CAT measurements can be made simultaneously, in replicate, and with exactly the same reagents and assay conditions. For a standard 96-well plate, 10 individual samples can be analyzed, in triplicate, including a control.
Using the modified assay for detecting microparticle-derived PPL, median peak thrombin generation for all VTE case samples were somewhat higher than controls, but not statistically significant. After segregating the data based on gender, the difference between male and female VTE case samples was statistically significant (Mann- Whitney-Wilcoxon rank sum, p = 0.007), with males having a 2-fold increase in rate of thrombin generation relative to female VTE cases. However, this difference may be attributable to the age difference in male VTE case samples (10 to 20 years older) relative to female VTE case samples, as well as both male and female control samples. There is a significant (Spearman rank, p = 0.02) increase in peak thrombin generation associated with microparticles with increasing age of VTE case samples. Although there is a positive trend between age and MP-associated peak thrombin generation in control samples, it is not statistically significant. With the standard assay, using a 5 pM trigger with high levels of PPL (4 µM), we did not observe an increase in the total thrombin generated, as measured by the area under the curve (ETP), between control and case samples or with age.
The recent development of the XaCT (Xa clotting time) assay for measuring the procoagulant phospholipid activity in isolated microparticles has determined that the amount of thrombin generated from microparticles is about 3-times greater than can be accounted for by the phosphatidylserine content as measured by annexin V binding [16]. These findings suggest that either additional procoagulant phospholipids or other factors bound to the microparticle surface enhance thrombin generation. The XaCT assay has been used to detect procoagulant phospholipids in a variety of diseases known to have increased platelet-derived microparticles, such as cancer and in patients with multiple traumas [9]. An approach similar to ours using a modified CAT assay has been used for assay of anti-phospholipid antibodies for patients with lupus-like anticoagulants [39]. The ratio of peak height to lag time is converted to an anticoagulant activity and related to a standard curve based on the inhibition of thrombin generation by well-characterized anti-phospholipid antibodies [40].
Using a prothrombinase assay to measure procoagulant MP captured by immobilized annexin V, Ay et al. did not detect a difference between VTE case and controls [41]. However, this assay would only measure MPs that were captured by annexin V. In another study, which triggered thrombin generation with high TF/low PL ratio (71.6 pM/3.2 µM) measured with the Technothrombin assay, VTE patients with less thrombin generation had a 60% lower relative risk for recurrence than those with more thrombin generation [10]. To our knowledge, our results are the first using a novel modification of the CAT assay to directly measure PPL-dependent thrombin generation associated with microparticles including, but not limited to, those that are annexin V-positive.
Consistent with published results using the X-act assay, we also find more thrombogenic activity on microparticles than can be accounted for by measuring the amount of surface annexin V binding. We show that microparticles derived from VTE case samples generate more thrombin per number of annexin V-positive microparticles than controls. It has been shown that annexin V binding significantly underestimates the amount of procoagulant phospholipid compared to that measured with lactadherin [42–44]. Furthermore, the amount of PPL, as measured by thrombin generation, increases with increasing age of the individual VTE patient. In contrast, there is no correlation between age and annexin V positive microparticles, in either VTE case or control samples. It has been widely recognized that initial VTE incidence increases with age; however, among patients with an initial unprovoked VTE, a correlation between a later recurrence and the age of first VTE has not been shown [45]. Interestingly, a 2-fold increase in recurrence has been detected in males compared to females, and we find a comparable increase in the rate of thrombin generation in male VTE cases compared to female VTE cases. However, our male VTE cases are also significantly older than our female VTE cases, complicating the interpretation of our findings. One limitation of our current study is the lack of younger male and older female VTE case samples due to the small sample size collected during only one year. Therefore, it is possible that the increase in procoagulant microparticles seen in older VTE case samples may also be dependent on the sex of the individual. Even with this limitation, we regard this to be the first report correlating increased functional procoagulant microparticles with increased age of patient at time of diagnosis of VTE. Whether this correlation might be exploited in tailoring anticoagulant therapy for prevention of VTE recurrence remains to be tested.
Many procoagulant factors increase with age, including fibrinogen, factors V, VII, VIII, IX, XIII, without a compensatory increase in anticoagulant factors, e.g. antithrombin, which is decreased in males with age, in theory disturbing the hemostatic balance towards prothrombotic [46]. However, this prothrombotic profile does not always result in thrombosis, as evidenced by thrombosis-free centenarians [47]. These findings suggest that additional components of the hemostatic system need to be assessed in order to gain a clearer picture of the entire hemostatic balance. For example, it has been shown recently that the protein C activation pathway is reduced with increasing age despite an increase in the actual protein C concentration [47].
In conclusion, we have developed a simple method based on the CAT assay [26] to measure procoagulant phospholipid associated with plasma microparticles. The technique does not require microparticle isolation or additional reagents or procedures, making it amenable for future use within the clinical laboratory setting. Furthermore, we determine that procoagulant microparticles increase with the age of an individual that has experienced a single VTE. Although our sample numbers are small in this observational study, given the findings of several retrospective studies that conclude that increased thrombin generation is associated with recurrence of VTE, we propose that this assay might prove to be a valuable tool in addressing the basis of VTE and responses to prophylactic anticoagulation. We find similar to van Dreden et al. [16] that there is more procoagulant phospholipid associated with microparticles than can be measured by annexin V binding, which suggests that alternative assays may be needed for accurately measuring the full procoagulant capacity of microparticles.
Acknowledgments
This work was supported by grants HL83141 (Heit, J.A. and Owen, W.G.) and HL90639 (Heit, J.A. and Owen, W.G.) from the National Heart Lung and Blood Institute.
Footnotes
Conflict of interest statement
None.
References
- 1.Karnicki K, Owen WG, Miller RS, McBane RD. Factors contributing to an individual propensity for arterial thrombosis. Arterioscler Thromb Vasc Biol. 2002;22:1495–1499. doi: 10.1161/01.atv.0000029968.34056.94. [DOI] [PubMed] [Google Scholar]
- 2.Bogaty P, Robitaille NM, Solymoss S, Boyer L, Auger D, Labbe L, et al. Atherogenic, hemostatic, and other potential risk markers in subjects with previous isolated myocardial infarction compared with long-standing uncomplicated stable angina. Am Heart J. 1998;136:884–893. doi: 10.1016/s0002-8703(98)70135-8. [DOI] [PubMed] [Google Scholar]
- 3.Bogaty P, Poirier P, Simard S, Boyer L, Solymoss S, Dagenais GR. Biological Profiles in Subjects With Recurrent Acute Coronary Events Compared With Subjects With Long-Standing Stable Angina. Circulation. 2001;103:3062–3068. doi: 10.1161/01.cir.103.25.3062. [DOI] [PubMed] [Google Scholar]
- 4.Eichinger S, Kyrle PA. Duration of anticoagulation after initial idiopathic venous thrombosis - the swinging pendulum: Risk assessment to predict recurrence. J Thromb Haemost. 2009;7 Suppl 1:291–295. doi: 10.1111/j.1538-7836.2009.03383.x. [DOI] [PubMed] [Google Scholar]
- 5.Baglin T. What happens after venous thromboembolism? J Thromb Haemost. 2009;7 Suppl 1:287–290. doi: 10.1111/j.1538-7836.2009.03409.x. [DOI] [PubMed] [Google Scholar]
- 6.Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102:268–278. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 7.Bounameaux H, Perrier A. Duration of Anticoagulation Therapy for Venous Thromboembolism. Hematol Am Soc Hematol Educ Program. 2008:252–258. doi: 10.1182/asheducation-2008.1.252. [DOI] [PubMed] [Google Scholar]
- 8.Kearon C. Balancing risks and benefits of extended anticoagulant therapy for idiopathic venous thrombosis. J Thromb Haemost. 2009;7 Suppl 1:296–300. doi: 10.1111/j.1538-7836.2009.03388.x. [DOI] [PubMed] [Google Scholar]
- 9.Besser M, Luddington CRBaglin, Baglin, van Hylckama Vlieg A, Baglin T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J Thromb Haemost. 2008;6:1720–1725. doi: 10.1111/j.1538-7836.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 10.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. J Am Med Assoc. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 11.Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Peak thrombin generation and subsequent venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) study. J Thromb Haemost. 2009;7:1639–1648. doi: 10.1111/j.1538-7836.2009.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijer K, Schulman S. The absence of ‘minor‘ risk factors for recurrent venous thromboembolism: a systematic review of negative predictive values and negative likelihood ratios. J Thromb Haemost. 2009;7:1619–1628. doi: 10.1111/j.1538-7836.2009.03557.x. [DOI] [PubMed] [Google Scholar]
- 13.Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of Recurrent Venous Thromboembolism by Endogenous Thrombin Potential and D-Dimer. Clin Chem. 2008;54:2042–2048. doi: 10.1373/clinchem.2008.112243. [DOI] [PubMed] [Google Scholar]
- 14.Zhu T, Martinez I, Emmerich J. Venous Thromboembolism: Risk Factors for Recurrence. Arterioscler Thromb Vasc Biol. 2009;29:298–310. doi: 10.1161/ATVBAHA.108.182428. [DOI] [PubMed] [Google Scholar]
- 15.McRae S, Tran H, Schulman S, Ginsberg J, Kearon C. Effect of patient's sex on risk of recurrent venous thromboembolism: a meta-analysis. Lancet. 2006;368:371–378. doi: 10.1016/S0140-6736(06)69110-1. [DOI] [PubMed] [Google Scholar]
- 16.Kyrle PA, Minar E, Bialonczyk C, Hirschl M, Weltermann A, Eichinger S. The Risk of Recurrent Venous Thromboembolism in Men and Women. N Engl J Med. 2004;350:2558–2563. doi: 10.1056/NEJMoa032959. [DOI] [PubMed] [Google Scholar]
- 17.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123 Suppl 4:S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 18.van Dreden P. Plasma thrombomodulin activity, tissue factor activity and high levels of circulating procoagulant phospholipid as prognostic factors for acute myocardial infarction. Blood Coagul Fibrinolysis. 2009;20:635–641. doi: 10.1097/MBC.0b013e32832e05dd. [DOI] [PubMed] [Google Scholar]
- 19.van Dreden P. Clinical evaluation of a new functional test for detection of plasma procoagulant phospholipids. Blood Coagul Fibrinolysis. 2009;20:494–502. doi: 10.1097/MBC.0b013e32832c5e51. [DOI] [PubMed] [Google Scholar]
- 20.Barton PG, Yin ET, Wessler S. Reactions of activated factor X-phosphatide mixtures in vitro and in vivo. J Lipid Res. 1970;11:87–95. [PubMed] [Google Scholar]
- 21.Morel O, Morel N, Freyssinet J-M, Toti F. Platelet microvesicles and vascular cells interactions: A checkpoint between the haemostatic and thrombotic responses. Platelets. 2008;19:9–23. doi: 10.1080/09537100701817232. [DOI] [PubMed] [Google Scholar]
- 22.Piazza G, Goldhaber SZ. Venous Thromboembolism and Atherothrombosis An Integrated Approach. Circulation. 2010;121:2146–2150. doi: 10.1161/CIRCULATIONAHA.110.951236. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Hsu M-Y, Steinbacher TE, Monticello TM, Schumacher WA. Quantification of platelet composition in experimental venous thrombosis by real-time polymerase chain reaction. Thromb Res. 2007;119:593–600. doi: 10.1016/j.thromres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Ederhy S, Di Angelantonio E, Mallat Z, Hugel B, Janower S, Meuleman C, et al. Levels of Circulating Procoagulant Microparticles in Nonvalvular Atrial Fibrillation. Am J Cardiol. 2007;100:989–994. doi: 10.1016/j.amjcard.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 25.Hemker HC, Al Dieri R, Béguin S. Thrombin generation assays: accruing clinical relevance. Curr Opin Hematol. 2004;11:170–175. doi: 10.1097/01.moh.0000130314.33410.d7. [DOI] [PubMed] [Google Scholar]
- 26.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 27.Hemker HC, Giesen PLA, Ramjee M, Wagenvoord R, Béguin The S. Thrombogram: Monitoring Thrombin Generation in Platelet Rich Plasma. Thromb Haemost. 2000;83:589–591. [PubMed] [Google Scholar]
- 28.Gitel SN, Owen WG, Esmon CT, Jackson CM. A Polypeptide Region of Bovine Prothrombin Specific for Binding to Phospholipids. Proc Natl Acad Sci USA. 1973;70:1344–1348. doi: 10.1073/pnas.70.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayachandran M, Litwiller RD, Owen WG, Heit JA, Behrenbeck T, Mulvagh SL, et al. Characterization of blood borne microparticles as markers of premature coronary calcification in newly menopausal women. Am J Physiol Heart Circ Physiol. 2008;295:H931–H938. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor DE, Exner T, Ma DDF, Joseph JE. Detection of the procoagulant activity of microvesicle associated phosphatidylserine using XACT. Blood Coagul Fibrinolysis. 2009;20:558–564. doi: 10.1097/MBC.0b013e32832ee915. [DOI] [PubMed] [Google Scholar]
- 31.Rijkers DTS, Wielders SJH, Be´guin S, Hemker HC. Prevention of the Influence of Fibrin and β2-Macroglobulin in the Continuous Measurement of the Thrombin Potential: Implications for an Endpoint Determination of the Optical Density. Thromb Res. 1998;89:161–169. doi: 10.1016/s0049-3848(97)00312-5. [DOI] [PubMed] [Google Scholar]
- 32.Chandler WL, Roshal M. Optimization of Plasma Fluorogenic Thrombin-Generation Assays. Am J Clin Pathol. 2009;132:169–179. doi: 10.1309/AJCP6AY4HTRAAJFQ. [DOI] [PubMed] [Google Scholar]
- 33.Varadi K, Turecek PL, Schwarz HP. Thrombin generation assay and other universal tests for monitoring haemophilia therapy. Haemophilia. 2004;10 Suppl 2:17–21. doi: 10.1111/j.1365-2516.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 34.Morel O, Toti F, Morel N, Freyssinet JM. Microparticles in endothelial cell and vascular homeostasis: are they really noxious? Haematologica. 2009;94:313–317. doi: 10.3324/haematol.2008.003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smed E, Wagenvoord R, et al. The Calibrated Automated Thrombogram (CAT): a universal routine test for hyper and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–253. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 36.Gerotziafas GT, Depasse F, Busson J, Leflem L, Elalamy I, Samama MM. Towards a standardization of thrombin generation assessment: The influence of tissue factor, platelets and phospholipids concentration on the normal values of Thrombogram-Thrombinoscope assay. Thromb J. 2005;3:16. doi: 10.1186/1477-9560-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tripodi A, Legnani C, Chantarangkul V, Cosmi B, Palareti G, Mannucci PM. High thrombin generation measured in the presence of thrombomodulin is associated with an increased risk of recurrent venous thromboembolism. J Thromb Haemost. 2008;6:1327–1333. doi: 10.1111/j.1538-7836.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 38.Dignat-George F, Freyssinet J-M, Key NS. Centrifugation is a crucial step impacting microparticle measurement. Platelets. 2009;20:225–226. doi: 10.1080/09537100902795500. [DOI] [PubMed] [Google Scholar]
- 39.Devreese K, Peerlinck K, Arnout J, Hoylaerts MF. Laboratory detection of the antiphospholipid syndrome via calibrated automated thrombography. Thromb Haemost. 2009;101:185–196. [PubMed] [Google Scholar]
- 40.Devreese K, Peerlinck K, Hoylaerts MF. Thrombotic risk assessment in the antiphospholipid syndrome requires more than the quantification of lupus anticoagulants. Blood. 2010;115:870–878. doi: 10.1182/blood-2009-09-244426. [DOI] [PubMed] [Google Scholar]
- 41.Ay C, Freyssinet J-M, Sailer T, Vormittag R, Pabinger I. Circulating procoagulant microparticles in patients with venous thromboembolism. Thromb Res. 2009;123:724–726. doi: 10.1016/j.thromres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Dasgupta SK, Guchhait P, Thiagarajan P. Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5. Transl Res. 2006;148:19–25. doi: 10.1016/j.lab.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Shi J, Pipe SW, Rasmussen JT, Heegard CW, Gilbert GE. Lactadherin blocks thrombosis and hemostasis in vivo: correlation with platelet phosphatidylserine exposure. J Thromb Haemost. 2008;6:1167–1174. doi: 10.1111/j.1538-7836.2008.03010.x. [DOI] [PubMed] [Google Scholar]
- 44.Albanyan A-M, Murphy MF, Rasmussen JT, Heegaard CW, Harrison P. Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: a comparison study with annexin V. Transfusion. 2009;49:99–107. doi: 10.1111/j.1537-2995.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 45.Eischer L, Eichinger S, Kyrle PA. Age at First Venous Thromboembolism and Risk of Recurrence: A Prospective Cohort Study. Medicine. 2009;88:366–370. doi: 10.1097/MD.0b013e3181c29e31. [DOI] [PubMed] [Google Scholar]
- 46.Bucciarelli P, Mannucci PM. The hemostatic system through aging and menopause. Climateric. 2009;12 S1:47–51. doi: 10.1080/13697130903006365. [DOI] [PubMed] [Google Scholar]
- 47.Mari D, Mannucci PM, Coppola R, Bottasso B, Bauer KA, Rosenberg RD. Hypercoagulability in Centenarians: The Paradox of Successful Aging. Blood. 1995;85:3144–3149. [PubMed] [Google Scholar]