Abstract
The vast majority of cutaneous squamous cell carcinoma (CSCC) will occur in those with fair complexion, tendency to burn, and high ultraviolet radiation (UVR) exposure. Organ transplant recipients also are an important population at great risk for CSCC. An association has been reported between oral contraceptive (OC) use, human papillomavirus virus (HPV) and cervical cancer, and there could be a similar association for CSCC. The cutaneous HPV β-E6 protein, a close cousin of the transformative E6 protein underlying anogenital cancers, has been shown to inhibit apoptosis in response to UVR damage and stimulate morphologic transformation in rodent fibroblast cell lines. Furthermore, OC use has been shown to enhance HPV transcription and may contribute to CSCC risk through this pathway.
Keywords: cutaneous squamous cell carcinoma, human papillomavirus virus, oral contraceptives
1. Introduction
Cutaneous squamous cell carcinoma (CSCC) is the second most common malignancy among Caucasians in the United States (US).1,2 Often locally aggressive and resulting in substantial morbidity, this cancer rarely spreads distally.3,4 However, while the overall 5-year metastatic rate is ≤5%, lesions of the lip and ear are particularly aggressive, with metastasis rates approaching 20%.5–7
CSCC occurs more commonly among iatrogenically immunosuppressed patients, and the potential for metastasis also is greater in this population.7–12 In immunosuppressed organ transplant recipients (OTR), lesions occur primarily in chronically sun-exposed skin,13,14 typically are histologically deeper at time of diagnosis,13 increase in incidence with duration of immunosuppressive therapy,15 and are characterized by a 4:1 reversed squamous-to-basal cell type (ie, basal cell skin cancer is the predominate type in immunocompetent patients).16 Human papillomavirus virus (HPV) DNA is detected in approximately 75% of CSCCs from OTRs, compared with 47% in non-immunosuppressed patients,17 and is thought to be a factor in the etiology of these cancers.6 Also, high metastatic rates have been reported for radiation-induced CSCCs of the face and neck18 and for CSCCs arising in patients infected with human immunodeficiency virus (HIV);19,20 rates are comparable to those of squamous cell carcinoma (SCC) of the cervical and anal tracts in immunosuppressed patients.21
CSCC is observed more frequently in Caucasians than African-Americans (AA), Asians, or Hispanics. 22 Among AA, CSCCs are commonly located on the lower extremities and have a reversed squamous cell-to-basal cell ratio resembling that of immunosuppressed OTRs.22–26 The lesions often arise from scars, burns, and ulcers and tend to be histologically invasive.24,26–29 Approximately 29% of AA with CSCC develop regional lymph node metastasis and succumb to the disease.27 CSCC is paradoxically rare in AA and Asian OTRs,15,30 possibly reflecting differences in the ethnic distribution of HLA B27 and HLA A11,31–33 the former predisposing and the latter conferring resistance to skin tumors.34
The associations of CSCC with ultraviolet radiation (UVR)35,36 and innate pigmetary factors such as fair complexion (eg, propensity to burn on initial exposure; inability to tan on repeated exposure),37–41 light-colored eyes, and red hair37,38,40–44 are well established. Chronic exposure to UVR is known to induce immunosuppression45–48 and to promote skin carcinogenesis.49–53 Sunlight does not play a significant etiologic role in CSCC among African-Americans, since most lesions present on covered areas.24,27,54–56 The effect of cumulative lifetime sun exposure on CSCC is greatly diminished after adjustment for pigmentary factors.37
The greater frequency of CSCC than basal cell skin cancer (BCSC) on the upper limbs suggests that UVR exposure alone does not fully explain risk for CSCC.57 This also is indicated by the substantially lower incidence of CSCC among Hawaiian Caucasians than Australian Caucasians.58 Other factors associated with CSCC include ionizing radiation/radon,18,59–67 certain chemical compounds,22,68,69 iatrogenic immunosuppression,11,70,71 physical trauma (burns and scars),68,72,73 human papillomavirus virus,17,74–78 higher education,42,59,79,80 and genetic predisposition (xeroderma pigmentosa, epidermoplasia verruciformis).68 Latent intervals of 40 or more years suggest that the risk of cancer in irradiated skin persists for the life of the person, and raise the possibility of long latencies for other short-term exposures in the etiology of CSCC.18
An increase in the incidence of CSCC over time has been reported in several longitudinal studies.81–87 The increase may reflect lifestyle changes such as increased voluntary exposure to UVR (eg, frequent use of tanning salons, increased outdoor recreational activity, tendency to wear less sun protective clothing, mid-winter vacations in sunny locations), increased sexual activity resulting in exposure to HPV, and/or greater life expectancy. The depletion of the ozone-layer also may be a contributing factor.88–90 A 1% percent reduction in stratospheric ozone is estimated to increase the incidence of nonmelanoma skin cancer (NMSC) between 3% and 6%.88,91
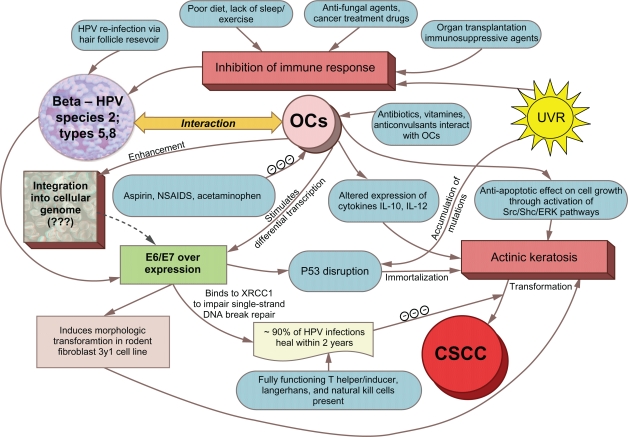
Understanding exposures that predispose individuals to CSCC is especially important since individuals with this cancer may be more likely to develop and die from certain secondary malignancies.92–116 In the paper, we briefly examine the potential role of OC use on cancer risk in general and then specifically address the plausibility of OC use as a risk factor for CSCC, as suggested by recent epidemiologic studies.42,117 Next, we summarize the supportive evidence linking HPV infection with cancer and CSCC, focusing on the transmission route for HPV. In conclusion, we examine the combine effect of OC use and HPV infection on increased CSCC risk and present a conceptual model underlying the putative association (Fig. 1). Both OC use and HPV infection are preventable exposures, and the latter is a suitable target for vaccination if implemented prior to infection.118
Figure 1.
Conceptual model depicting the association between OCs, HPV and CSCC.
Note: Risk factors for CSCC represent a complex interacting web of putative associations involving OC use, HPV infection, UVR exposure, and immunosuppression.
2. Methods
2.1. Search strategy
To identify papers on CSCC, OC use, and HPV, a comprehensive literature search was conducted using the Medline/PubMed database from inception to December 2010. We also examined the references of the papers identified electronically, and searched for unpublished studies, PhD dissertations, internal reports, and conference proceedings/abstracts. Finally, to include those studies in which OC use or HPV infection was a secondary factor, we checked the tables of every article that examined risk factors for CSCC.
2.2. Inclusion criteria
Studies were included only if the target population, outcome and exposures of interest were clearly defined and results were based on valid statistical methods with evaluation of errors and discussion of study bias. Studies in which participants were chosen at convenience were not considered. Priority was given to papers that presented original data rather than reviews or meta-analyses.
3. Association between OC Use and Cancer
Data from animal studies and tissue samples indicate that estrogen (a key ingredient in oral contraceptive pills) is genotoxic, induces carcinogenic effects, and increases the proliferation of certain cancer cells.119–121 Subcutaneous administration of oestradiol in mice increases the incidence of mammary, pituitary, uterine, cervical, vaginal, lymphoid, and testicular tumors.122 In contrast, high doses of oestradiol significantly reduce (P < 0.05) the incidence of cervical squamous cell carcinomas induced in mice with 3-methylcholanthrene (MCA).122 The use of combined OCs (co-administration of an estrogen and a progestogen) has been classified as carcinogenic to humans by the International Agency for Research on Cancer (IARC).121 However, the evidence linking OC use with cancer in humans is neither consistent nor definitive.121,123–125 For example, OC use appears to promote cancers at some sites (eg, triple-negative breast cancer126), while affording protection at other sites (eg, endometrial cancer127).
3.1. Cutaneous squamous cell carcinoma
The epidemiology of CSCC has been difficult to study because few cancer registries record this cancer on a routine basis. Furthermore, CSCC and BCSC, which are biologically and histologically distinct cancers with different clinical characteristics, often are studied under the general heading of NMSC.
An analysis of self-reported skin cancers, excluding CM, in the Oxford Family Planning Association (Oxford-FRA) contraceptive study did not observe a greater risk for these cancers among OC users (ever used, RR = 0.9, 95% CI = 0.6–1.4; recently used, RR = 0.4, 95% CI = 0.1–1.2; used in past, RR = 1.0, 95% CI = 0.6–1.6) than hospitalized referrents who had never used oral contraceptives.128 However, the findings from this study are difficult to evaluate since cases were self-reported and the comparison group consisted of hospital clinic patients whose use of oral contraceptives may not have been representative of the population from which cases were selected.
Except for the above report which included both CSCCs and BCSCs, only two papers to date specifically have studied the association between OC use and CSCC (Table 1). A large population-based case-control study117 found that OC users had a 1.6 adjusted OR for CSCC (95% CI = 1.0–2.5). ORs also were higher among women who last used OCs ≥25 years before diagnosis (OR = 2.1, 95% CI = 1.2–3.7), and within-group ORs increased with duration of use [OR for ≤ 2 years, 1.7 (95% CI = 0.9–3.5); OR for 3–6 years, 2.6 (95% CI = 1.0–6.5); OR for ≥ 7 years, 2.7 (95% CI = 0.9–8.5); Ptrend = 0.01]. Among women who had used OCs for ≥25 years, those with no variant alleles for the XPD Lys751 Gln DNA repair gene (which is responsible for repairing UVR-induced DNA lesions) had a 4.4-fold greater OR (95% CI = 1.4–13.6) for CSCC than women with ≥1 variant alleles. A similar increased risk with OC use was obseved in a nested case-control study using a large retrospective cohort of Kaiser Permanente Northern California members.42 In this study, pre-diagnostic OC use was associated with a 2.4-fold OR (95% CI = 1.2–4.8) for CSCC in univariate analyses and with borderline statistical significance in multivariable analysis (OR = 2.0, 95% CI = 0.91–4.5). The multivariable risk likely was artifactually decreased in part due to over adjustment by variables associated with OC use but not with the outcome of CSCC. Information was not collected to assess a dose-response effect. A pooled effect analysis of the two studies yielded ORpooled = 1.7 (95% CI = 1.1–2.5).179,180 Neither study adjusted for sexual behavior or presence of HPV.
Table 1.
Summary of studies on OC use and risk for CSCC.
| Author, year published, study location | Study design | No. of cases | No. of referents | Main effect risk estimates (95% CI) | Comments |
|---|---|---|---|---|---|
| Applebaum et al, 2009, New Hampshire117 | Case-referent | 261 Incident CSCC |
298 Population-based |
Ever used OR = 1.6 (1.0–2.5) Last used ≥25 yrs OR = 2.7 (0.9–8.5) |
Frequecy matched by age and sex. Results controlled for age, pigmentation, sunburns, sunbaths, and education. |
| Asgari et al, 2010, Northern Calif.42 | Case-referent (nested) | 195 CSCC |
679 HMO-based |
Used in past year Univariate OR = 2.4 (1.2–4.8) Multivariable OR = 2.0 (0.91–4.5) |
Individually matched on age at time of exam, sex, residential postal zipcode, year of health check-up. Used surrogate markers for sun exposure. |
| Vessey et al, 2000, England/Scotland126 | Cohort | 83 CSCC and BCSC |
17,032 Hospital-based |
Ever used RR = 0.9 (0.6–1.4) |
Results controlled for age (5 year groups). No measure of sun exposure collected. Self reported cases. |
3.2. Strength of the evidence
The propensity of OC use to increase risk for specific cancers in humans, including CSCC, remains uncertain due to various methodologic concerns.121,125,131,132 For example, the frequent use of analgesics among women (aspirin, nonaspirin NSAIDs, and acetaminophen) is known to be inversely associated with concentrations of estrogen (estradiol, Ptrend = 0.001; free estradiol, Ptrend = 0.01; estrone sulfate, Ptrend = 0.03; ratio of estradiol to testosterone, Ptrend = 0.04) and could have confounded the results of studies that did not account for this variable when examining the association between OC use and cancer.133 Certain antibiotics, anticonvulsants (except sodium valproate), and vitamin C similarly are known to interact with OCs.134 Furthermore, changes over time in the formulation of OCs (eg, typical estrogen doses in the 1960s were more than twice the typical doses in the 1980s and since) must be considered when evaluating OC use and cancer risk.135,136 Additional research is needed to determine whether OC use increases risk for CSCC among individuals with a specific hormone receptor profile, analogous to the increased risk for triple-negative breast cancer patients.
Although estrogen and progesterone receptors are present in normal skin,137,138 CSCCs are not thought to express significant amounts of sex hormones139 and thus OCs probably do not significantly influence CSCC risk through sex hormone pathways.140 However, OCs may operate through a non-sex hormone pathway such as p53.140 Inactivation of the p53 gene is believed to play a pivotal gatekeeper role in SCC carcinogenesis, and estrogen appears to inhibit the actions of this tumor suppressor.141 Estrogen also is believed to exert an antiapoptotic effect on cell growth through the activation of signaling pathways such as Src/Shc/ERK.138 In contrast, risk for CSCC is increased in ovariectomized Ptch1+/− and Car-S mice,142 while the incidence of CSCC is lower in women than men.41,81
The lack of a direct carcinogenic effect of OC use on CSCC does not rule out an interactive effect with another carcinogen such as HPV. Longterm OC use has been shown to enhance HPV transcription in cervical intraepithelial neoplasia and it could have a similar effect on development in CSCC.121,143 OC use also could be non-causally associated with CSCC through increased sexual activity and exposure to HPV.
4. Human Papillomavirus Virus
4.1. Description and overview
HPV is a short sequence (eg, 7200 to 8000 base pairs of closed-circular double-stranded DNA of approximately 8 kbp), epitheliotropic, nonenveloped DNA virus that belongs to the papovaviridae family of viruses.144 Over the 30 years since the first types were isolated,145 approximately 118 papillomavirus (PV) types have been identified, and nearly 100 are known to infect humans.146 Current data support the existence of more than 200 HPV types based on the detection of subgenomic amplicons.146–148 The papilloma family of viruses is classified into 8 genera identified by Greek letters (alpha, beta, gamma, delta, kappa, mu, nu, xi), of which only two do not contain HPV types (delta, kappa).147 Genera and species containing cutaneous HPV types include alpha (species 4, 8), beta (species 1), gamma (species 1), mu (species 1, 2), and nu (species 1).147 Beta-PV types [previously referred to as epidermodysplasia verruciform (EV) types] thus far fully sequenced include 5, 8, 9, 12, 14, 15, 17, 19–25, 36–38, 47, 49, 75, 76, 80, 92, 93, and 96.149 All HPVs contain at least seven early genes (E1–E7), two late genes (L1–L2), and an upstream regulatory region that control most transcriptional events of the HPV genome.150 Evidence of an oncogenic role for papilloma virus in CSCCs was first found in a study of Shope papilloma virus, which causes skin papillomas in rabbits.151,152 Treatment of skin with a chemical co-carcinogen frequently induced transformation of rabbit papillomas into CSCC. However, animal PVs are not known to infect humans, as is the case for some other viruses like influenza, HIV-1, Ebola, or SARS.147
CSCCs mainly have been associated with beta- and gamma-PVs,75 although alpha-PVs usually linked with anogenital SCCs are found in SCCs of the hand, finger, nail, and toe.5,153–173 Except in immunosuppressed patients or individuals with the hereditary disorder EV, beta- and gamma-papillomaviruses normally are associated only with asymptomatic infections.147 Although beta- and gamma-PVs are widespread, they contain many HPV types that remain to be sequenced and formally described.147 Some novel cutaneous types such as HPV-109 have an uncertain classification because of an equal similarity in the L1 gene to different genera.174
Virtually 100% of the population is exposed to one or more HPV types in their lifetime,175,176 and most individuals will not develop skin or other cancers potentially associated with the virus. Although a broad spectrum of HPV types frequently are detected in normal skin175,177–179 and actinic keratoses (AK),180 this fact does not preclude a pathogenic role in skin cancer since the presence of the virus should precede tumor development. 17 Similarly, a large portion of the population has been exposed to the ubiquitous Epstein-Barr virus and do not develop nasopharyngeal carcinoma, even though the virus is a well established risk factor for the cancer.175 HPV is more frequently found on the forehead than on less sun exposed areas such as the thighs, possibly indicating a role for local photoimmunosuppression in the colonization of cutaneous HPV.180
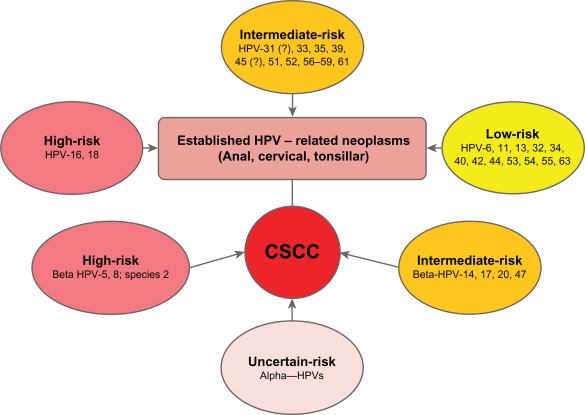
The oncogenic potential of specific HPV types has been based primarily on data from anogenital and oral cavity SCCs and their precursor lesions (Fig. 2). HPV types 16 and 18 have a high risk for malignant progression, while HPV types 31, 33, 35, 39, 45, 51, 52, 56–59, and 61 have been traditionally categorized as having intermediate risk of progression.181,182 The inclusion of HPV 31 and HPV 45 into the intermediate group likely will be readdressed in the future. Benign lesions typically harbor HPV types 6, 11, 13, 32, 34, 40, 42, 44, 53, 54, 55, and 63,181,182 although some benign types have been implicated in SCCs outside of the anogenital region (eg, oral cavity cancer183 and paranasal sinus dysplasia184). Supposedly benign non-genital warts containing HPV-1 also have been reported to manifest Bowenoid histologic features185 and they appear capable of converting to CSCC under the influence of UVR and immunosuppression.186 HPV-2, which is classically associated with palmoplantar warts, has been found in verrucous carcinoma of the foot.187 Many of the virus types detected in the anogenital tract are seen in both squamous intraepithelial lesions (carcinoma in situ) and invasive squamous cell cancers of the skin, albeit with different frequencies and risk distributions. Other HPV types are unique to the skin.78,188–190 High risk mucosal types found in CSCCs include HPV−16, −18, −51, −54, −56, −61, and −69.189,191,192 Among OTRs, low risk HPV types 6 and 11 are frequently found in benign, premalignant, and malignant cutaneous lesions.192 Mixed type HPV infections within a single lesion are commonly observed in immunosuppressed patients, but less frequently in CSCCs in the general population.78
Figure 2.
HPV types and cancer risk.
Note: In contrast to anal, cervical and tonsillar cancers, the role for alpha HPVs remain uncertain for most CSCCs.
Oncogenic HPV types are believed to promote tumor progression by expressing E6/E7 viral oncoproteins. These proteins act on cyclins E and A to stimulate cell proliferation,181 disrupt the p53-mediated cellular response to DNA damage, and induce the degradation of the retinoblastoma tumor suppressor gene product (pRB) through the proteasome.193–195 HPV-16 DNA is capable of integrating into the genome of epithelial tumor cells and altering transcription, as shown by the expression of E6 and E7 in derived cell lines.196 Immortalized human keratinocytes created in the laboratory by injecting DNA from HPV into the cells have been shown to become tumorigenic after chronic exposure to benzo(a)pyrene.197 High levels of E6/E7 protein expressed by HPV-16 stimulate apoptosis though the release of IL-1α from keratinocyte cell cultures.198
Studies of E6 in cutaneous beta HPVs indicate that this protein gene product has the potential for oncogenic transformation, though to a considerably lesser degree than high risk alpha-HPV types, and transformation probably depends on interaction with other co-carcinogens to cause mutations in the host cell genome.181 For example, the expression of the HPV-8 E6 open reading frame (ORF) is able to induce morphologic transformation in rodent fibroblasts but lacks the capability to transform NIH 3T3 cells or human keratinocytes in vitro as shown for high-risk HPV-16 and HPV-18.76,199,200 Morphologic transformation in a rat fibroblast cell line, 3Y1, also has been observed for the E6 gene of HPVs 5, 8, 47, and less potently for HPVs 14, 20, 21, and 25.201 The E6 protein consists of five regions delimited by two zinc-finger domains, the second of which is believed to be the key transforming element.201,202 Several cutaneous HPV types produce E6 proteins, which have been shown to induce apoptosis in response to UVR damage in tissue culture experiments.203–205 At the same time, a p53-independent pathway for HPV viral oncoproteins capable of inducing mutations206 and interrupting apoptosis in response to DNA damage207,208 may play a role in a subset of HPV-related cancers.209 Interestingly, E6 proteins of HPV-1, HPV-8, and HPV-16, but not HPV-6, have been shown to bind to XRCC1 (a polypeptide implicated in the repair of various DNA single-strand breaks) and impair single-strand break repair.210
HPVs reside ubiquitously, often in a latent state, in the skin and mucosal epithelia.146,181,211,212 A specific trophic affinity is seen for squamous epithelial cells at specific sites in which the complete replicative life-cycle of HPV is limited to this cell type.150,213,214 HPV infection and related neoplasms occur at greater than expected rates in individuals who are immunosuppressed,215–223 suggesting that impaired cell-mediated immunity, whether of iatrogenic origin, or related to HIV infection and/or other causes, plays an important role in the early transition from latency to the overt presentation of HPV-related disease.218 Evidence suggests that late-stage cancer invasion is not greatly influenced by immune status,224 and other factors such as genetic change may be more directly involved in the later progression from high-grade HPV-related disease to cancer.181,216–218,225,226
4.2. Route of transmission
Most studies suggest a positive association between sexual behavior (eg, young age at first intercourse, high lifetime number of sexual partners, sex with non-monogamous partners, and other sexually transmitted infections) and HPV infection in anogenital disease.211,227–237 A higher than expected concordance of HPV infection in sexual partners also suggests venereal transmission.238–242 HPV is spread in a nonhematogenous fashion (eg, infection primarily occurs at sites where the virus enters the body such as the genital tract, skin, and oral cavity) without a viremic phase, and probably is clinically significant as a consequence of ongoing sexual behaviors and persistent re-infection.219,243–246
Horizontal skin-to-skin contact in humans has been documented in several reports, however questions remain regarding whether the infection is largely transient and non-productive.143 The presence of HPV alone does not necessarily imply infection. 143 In one study, the detection of same types of HPV on the female hand and the male genitals of heterosexual couples suggested hand carriage as a pathway for HPV infection.247 Couples experiencing HPV transmission in this study were more sexually active and were more likely to use nonbarrier forms of contraception. Male self-inoculation also was observed to occur and was attributed to casual contact or masturbation. The feasibility of HPV transmission through sex related skin-to-skin contact also was demonstrated in a cross-sectional study which found that co-habiting partners of the opposite-sex shared a greater number of beta-PV types than either shared with randomly selected matched members of the population.248 Among participants in this study with at least one virus infection (26 couples), 74% of the partners and 46% of the control pairs shared at least one type (P = 0.02). In another study, transmission of beta-PV between individuals who were living in close proximity (but were not sexually intimate) was rare; suggesting that casual contact alone probably is insufficient for infection in most cases.179 Surprisingly, genital examinations of patients with digital CSCC and of their spouses have not revealed simultaneous genital SCC lesions.249 However, a history of a genital SCC successfully treated many years prior to the diagnosis of a digital SCC (both lesions revealed the same oncogenic HPV subtype) suggests that a very long delay may intervene between a genital SCC and a subsequent HPV-related digital CSCC.169,173,249
The isolation of HPV DNA in fomites on equipment after examination of patients with genital HPV infections provides further evidence that high-risk genital HPV can survive outside the mucosal environment and may even be transmitted through sex toys and massage instruments.250 Samples collected from the bedroom floor of infected individuals also have tested positive for HPV DNA.277 Further research is needed to determine whether horizontal transmission of HPV between sexual partners through intimate skin-to-skin contact (with or without penis penetration) is possible and represents a clinically meaningful source of infection.
4.3. HPV persistence and clearance
Very little is currently known about either the persistence or clearance of HPV infection in the skin. However, in the anogenital area, where the persistence of HPV infection is believed to be an important prerequisite for the development of most cervical intraepithelial neoplasias and cancers,242,243,251–253 HPV type 16 [relative risk (RR) = 0.47, 95% CI = 0.32–0.72], and to a lesser degree types 31, 33, 35, 52, 58) (RR = 0.62, 95% CI = 0.47–0.94), were observed to have a lower clearance rate than low risk types.245 This finding also has been noted in several previous studies.244,246,254–259 In one study, infection with single and multiple HPV types had similar clearance rates, eg, type-specific HPV clearance was independent of co-infection with other types.245 However, in another study, HPV persistence was associated with multiple HPV types.257 A dose-response relationship between viral load and persistence of HPV infection has been found in some244,260 but not all studies.245
Genital HPV-16 infection typically clears within 2 years if an intact immune response is present (eg, fully functioning T helper/inducer, Langerhans, and natural killer cells).233,244,245,257,261,262 In fact, less than half of HPV infections persist 6 months.245 Some studies indicate that HPV infection has a greater tendency to persist in older women which may be explained by hormonal changes and/or lifestyle differences among older women,245,261 or reactivation of latent disease in some women as they age and their immune surveillance diminishes.263 However, other studies have not observed an older age effect for HPV infection.264–266 Among women 20–29 years old, younger age was a risk factor for oncogenic HPV infection.267 In a large population-based cohort analysis, determinants of HPV-16 seropersistence included having one sexual partner during the follow-up period and former oral contraceptive use.264 A 14-fold estimated risk for cervical cancer was observed for women who had at least three positive tests for high-risk HPV than for those who had negative results.258
In constrast to genital HPV, the acquisition of cutaneous HPV appears to first occur in early infancy, posing an inherent obstacle to effective vaccine development.268,269 This also suggests other means of spread than sexual. Specific HPV types may persist in normal skin269,270 or benign skin lesions271 over long periods of time. Approximately half (48%) of healthy individuals have been found positive for the same cutaneous HPV type after 6 years of follow-up.272 Also, beta-PV type specific persistence in plucked eyebrow hairs was observed over a period of at least 6 months in 74% of the participants examined in a 2-year follow-up study of healthy adults.179 Similarly, 30% of the total beta-PV detected 7 years earlier in hair samples was found to persist at later follow-up.149 DNA from multiple beta-PV also has been detected in follow-up at 6 months or longer.179
The presence of an endogenous reservoir for HPV is believed to be an an important factor underlying the persistence of this virus. In the anogenital and oral cavities, the mucosal environment appears to serve as a receptive reservoir for HPV infection. The lack of a similar mucosal reservoir for the skin suggests that HPV may not play a role in the etiology of CSCC comparable to that in anogential or oral cancer. However, hair follicles may act as a possible reservoir for cutaneous HPV, based on the high number of samples testing positive for the virus17,273–275 and the concurrence of same type HPV-positive pairs in skin cancer biopsies and plucked eyebrow hairs.276 Also, in hair samples obtained from the head region, HPV typing has revealed the presence of multiple HPV types, including those associated with genital infection.17
4.4. Epidemiologic evidence of HPV in CSCC
In contrast to the role of high-risk HPV types in anogenital cancers, the epidemiologic data to date do not support a neccessary role for these HPV types in CSCC, given the large number of negative cases. The ubiquitous presence of cutaneous HPV in non-cancerous skin176,177,270,272,277,278 and the long-lasting HPV warts observed in transplant recipients11 further indicate that skin-borne HPV infection is not an absolute cause of CSCC. Rather, risk appears to involve an interaction between other carcinogens (eg, sunlight, immunosuppression, chemicals, hormones, and radon), race, and genetic factors. For example, a statistically significant relationship has been observed between increased time spent outdoors and detection of HPV in healthy skin.278 Similarly, HPV DNA was significantly associated with sites extensively exposed to the sun, in both lesions and healthy skin samples.279 However, one study found no convincing evidence of an association between beta- or gamma-PV seroprevalence and measures of sun sensitivity or UV exposure.280 Evidence of a supportive rather than a direct role for cutaneous HPV types in CSCC also is shown by the difficulty of obtaining positive cell lines for these HPV types, the inability of these viruses to immortalize human cells and degrade p53, and the relatively low number of viral genomes detected per cancer cell.17,181,281,282 Although integrated beta-PV has been isolated from a metastasic CSCC in an OTR, most of the evidence suggests that beta-PV is episomal in both AK and CSCC.175,283 However, integration of HPV DNA into host chromosomal DNA is not absolutely required for malignant transformation to occur as has been obseved by Sánchez-Lanier and colleagues.155 It remains to be determined whether HPV has a non passenger role in the etiology of CSCC.284 In constrast, HPV related cancers of the anogenital track meet the epidemiologic, molecular, and functional criteria of the World Health Organization (WHO) for viral carcinogenesis. 284 Three types of epidemiologic research described below provide support for the role of HPV in the etiology of CSCC.
4.4.1. Epidermodysplasia verruciforms (EV)
A link between beta-PVs and CSCC was first observed in patients with the rare inherited disorder EV, which is characterized by a defect in cell-mediated immunity. 285 In this condition, individuals develop persistent viral warts at a young age (eg, 4–8 years) on UVR-exposed areas of the body that progess in about 30% of cases to CSCC during middle age.178,286 EV related CSCCs test positive for HPV-5 and −8 in 90% of cases287 and for HPV-14, −17, −20, and −47 less frequently.76,287 The HPV types found in EV-related skin cancers also are detected in approximately 80% of CSCCs of OTRs,288 but CSCCs of OTRs typically have lower viral loads than the CSCCs of non-OTRs.190,284,289 Oddly, antibodies against EV-HPV are not common in the northern European population.75 EV-HPV DNA has been shown to be transcriptionally active in some CSCCs, indicating that EV-HPV may contribute directly to the pathogenesis of CSCC.70 However, to date it has not been possible to demonstrate in vitro transformation of human keratinocytes using EV-HPV or the formation of tumors in nude mice by EV-HPV-8 E6 transformed fibroblasts.78,200 Occassionally, p53-positive EV tumors have been observed in nonexposed skin, suggesting that factors other than UVR exposure may play a role in this cancer.285
4.4.2. Organ transplant recepients
A double-digit or greater increased relative risk for CSCCs among iatrogenic immunosuppressed OTRs has been observed in several studies.12,284,290–294 And risk appears to be greater for specific immunosuppressive regimens.295,296 The tumors occur on average 6 to 7 years following transplantation and typically appear on sun-exposed sites.76 Up to 70% of OTRs experience a NMSC by 20 years after transplantation, and the majority are CSCCs.290,297 Most CSCCs are characterized by multiple types of HPV infection.78 While EV-HPV is the predominant type found in this malignancy, cutaneous HPV types are found in approximately 50% of lesions, and less frequently, mucosal HPV types (<15%).78 While premalignant skin lesions and viral warts are highly prevalent in HPV infection and might indirectly suggest progression from less dysplastic forms to invasive CSCC,78,190,298,299 it should be noted that the distribution of HPV types in viral warts generally differs from that observed in precancers or CSCCs.297 Although OTRs receive better than average clinical follow-up, surveillance bias alone probably does not explain the 250 times higher incidence of CSCC among OTRs than in the general population.291
4.4.3. Association studies
In contrast to the widely accepted epidemiologic evidence linking high-risk HPVs to cervical cancer, the role of HPV in CSCC remains unclear. A retrospective study of immunocompetent participants, controlling for age, sex, and sun exposure to the head, face, neck, forearm, hands, and lower limbs observed a 32-fold greater OR (95% CI = 10–100) for CSCC among those testing positive for DNA HPV than for those testing negative.297 A 59-fold adjusted OR (95% CI = 5.4–645) for NMSC was reported for the subset of cases testing positive for high-risk HPV mucosal types (16, 31, 33, 35, and 51). An adjusted OR was not reported, but the crude OR for CSCC among those testing positive for high-risk HPV mucosal types was 31 (95% CI = 3.8–258). The prevalence of HPV infection in normal skin was low (4.7%), however, compared with a previous report,178 suggesting that ORs in this study may have been inflated. Furthermore, the percentage of mucosal types detected on the hands/fingers versus other sites was not specified in the study. HPV-16 is rarely found in non-genital, nondigital, CSCCs in healthy immunocompetent individuals.249,300 In another case-control study of immunocompetent individuals, DNA from beta-PV species 2 was found more frequently in CSCC than in adjacent healthy skin (OR = 4.0, 95% CI = 1.3–12), indicating a possible differential risk by specific beta-HPV types.301 The results of this study are difficult to interpret, however, since 1) in situ hybridization to determine viral load was not performed, and 2) tissue samples apparently were not “stripped” to reduce surface contamination.302 The significant differences also may have been attributable to chance since results were not adjusted for numerous (>45) multiple comparisons. Nonetheless, the findings were similar to those of a previous study which observed a 4.40 OR (95% CI = 1.92–10.06) for CSCC in association with beta-PV species 2.279 Neither of the above two studies addressed the possibility that the differentially greater prevalence of beta-PV infection was the result of changes to the immune system caused by the cancer.
Several association studies which tested for beta-PV antibody positivity have found an increased risk for CSCC,60,303–305 and risk was greater in the presence of a susceptible phenotype (gender, skin color, propensity to burn) or high lifetime sun exposure.304,305 No difference in HPV seropositivity has been found between BCSC patients and controls.304 The increased risk for CSCC does not necessarily imply causality since it is not possible to determine the origin of HPV infection (eg, skin, oral cavity, anogenital area) or the exact timing of infection when using antibody testing. Given the ubiquity of the virus and the relatively low number of seropositives, the majority of antibodies directed at HPV probably are generated near the time of tumor formation, suggesting reverse causality.303 Furthermore, not all infected women test positive for antibodies against HPV, due in part to the extended period necessary for the immune system to produce these proteins, or to the lack of a sustained antigenic exposure and/or a low viral load.306–308 Positive associations for CSCC have been reported when using a degenerate and nested polymerase chain reaction (PCR) technique to assess HPV positivity in normal skin309 or plucked eyebrow hairs.310 However, the concordance between specific HPV types in normal and proliferative lesions from the same individual was low.309 Overall, PCR results must be interpreted cautiously since HPV detection by this method is not necessarily equivalent to infection with HPV188 and alternatively could be a contamination or a passerby. PCR also does not provide information about the intracellular localization of viral DNA.17
Allograft recipients are especially susceptible to NMSCs and anogenital dysplasia/cancer,299 raising the question of whether HPV is a possible common risk factor in both diseases. For example, renal allograft recipients (RARs) classified with ≥4 intraepidermal carcinomas (IECs) or invasive CSCCs have been found significantly more likely to develop potentially life-threatening multiple anogenital tract neoplasms than less susceptible RARs (<4 IECs or CSCCs).211 Similarly, in the general population an increased risk for CSCC has been observed after anogenital cancer, and conversely, an increased risk of anogenital cancers has been observed following CSCC, although risk estimates varied widely by histologic category and gender.112–114,312,313 For example, a 20-fold standardized incidence ratio (SIR) for CSCC has been observed following anal cancer among women during the first year of follow-up, but not among men.112 The increased risk of non-Hodgkin’s lymphoma observed following CSCC appears to indicate that immune suppression, rather than HPV, is the mechanism underlying both cancers, since HPV is not known to be a risk factor for non-Hodgkin’s lymphoma.314 Exposure to UVR has been suggested as a common risk factor because of its effects on the immune system and the similar temporal trends and geographic patterns that exist for non-Hodgkin’s lymphoma and CSCC.97
4.5. Strength of the evidence
Proof that HPV infection is causally related to CSCC awaits definitive laboratory and epidemiologic confirmation.188,315 A consistent understanding of cutaneous HPV infection and CSCC risk is lacking largely because of methodological difficulties in HPV detection and reproducibility.309 The interpretation of results is further complicated by the so-called “hit and run” hypothesis, according to which HPV may be important only for the initiation, not the maintenance of the transformed cellular phenotype.175,288,316 Indeed, the number of beta-PV genome copies per tumor cell in AK (the precursor lesion of CSCC) is approximately 10-fold higher than in CSCC.74,175,317 This raises the question of an etiologic role for HPV even though the resulting CSCCs lack HPV viral genes or proteins.318 Nonetheless, no cutaneous “high-risk” HPV types on par with genital HPVs have been identified.279 Nor has any beta-PV type been shown to cause CSCC in experimental systems,149 although in one study 6% (9 cases) of backcrossed mice, transgenic for the HPV-8 early region under control of the keratin 14 promoter, spontaneously developed invasive SCC appearing lesions without any treatment with physical or chemical carcinogens.319,320 Furthermore, the detection of beta-PV in CSCC, in the absence of other evidence, does not prove causation. For example, beta-PV may occur as a consequence of immunosuppression, similar to other non-oncogenic opportunistic infections (eg, cytomegalovirus, herpes simplex virus, mollussum contagiosum, aspergillosis, and candidiasis).
The mechanism by which HPV causes CSCC, if that is the case, would appear to differ from that of other known HPV-related neoplasms. In the case of the cervix, anus, and tonsils, HPV tends to cause cancer in “transformation zones” where one kind of epithelium transitions within a defined boundary into another type of epithelium through a process known as metaplasia (eg, columnar epithelium into squamous epithelium).321 A comparable transformation zone does not exist for exposed skin, with the exception of the eyelids, lips, and periungual region. Furthermore, oncogenic genital HPV types are able to bind to and degrade p53 through the proteasome in contrast to cutaneous HPV types.203,322,323
5. Interactions between OCs and HPV
Much of the work on the possible effects of estrogen and OCs on HPV prevalence has focused on the inflammatory and immune regulatory properties of this hormone in the uterus.324 Response variations in these properties are thought to be important determinants of the viral persistence and progression underlying the development of SCC.325,326 Estrogen is known to cause an influx of neutrophils and macrophages, tissue edema, and proliferation of uterine epithelial cells.324 Additionally, OCs alter the expression of certain regulatory cytokines, such as IL-10 and IL-12, in the cervical mucous.325 Results reflect the down-regulation of immune markers in the cervix, corresponding to changes in reproductive hormones observed in animals during the menstrual cycle.327 Overall though, reproductive hormones appear to enhance immunity.327,328
A consistent picture has yet to emerge regarding the influence of OCs on HPV infection and the subsequent development of SCCs. In a pooled case-control study of cervical cancer, neither OC use nor increasing dose was significantly associated with HPV positivity among controls, suggesting that OCs do not have a facilitating role in HPV infection or persistence.329 Indeed, current use of OCs had a significantly protective effect on the follow-up incidence of HPV [hazard ratio (HR) = 0.49, 95% CI = 0.28–0.86].330 Similarly, in a study of atypical squamous cells of undetermined significance (ASCUS), oncogenic HPV was less common in women who were using oral contraceptives, although the difference was not statistically significant (P = 0.15).331 In a crude analysis, approximately 35% of current OC users, compared with 26% of referents, were identified with prevalent cervicovaginal HPV (OR = 1.52, 95% CI = 1.03–232); however, the difference was not statistically significant after adjustment for various demographic, sexual, and lifestyle factors.232 Similarily, positive univariable but null multivariable results were observed in an analysis of the third National Health and Nutrition Examination Survey (NHANES III).332 Three additional studies have not observed a statistically significant association between cervical HPV infection and OC use.230,231,237
In constrast, increased prevalence of HPV is seen in the lower genital tract of pregnant women.333 After adjusting for age, number of lifetime and recent sexual partners, age at first intercourse, and smoking, former OC use (OR = 1.3, 95% CI = 1.1–1.5) and current OC use (OR = 1.5, 95% CI = 1.2–1.8) were associated with HPV-16 seropositivity in a large population-based cohort of 10,000 women in Costa Rica.334 Regardless of duration, OC use [0 years, OR = 1.0; 1 year, OR = 2.2 (95% CI = 1.2–4.0); 2–3 years, OR = 2.1 (95% CI = 1.2–3.9); 4+ years, OR = 2.5 (95% CI = 1.2–5.1)] was associated with HPV infection (specific types not specified), independent of age, race, and lifetime number of male sexual partners.228 When examined by HPV type, OC use was significantly associated with increased detection of HPV-16/18 (P = 0.04).335 Inexplicably, OC use appeared to decrease the risk of low grade squamous intraepithelial lesions whether women were positive or negative for HPV infection.335 These discrepant findings, however, may be attributable to differences in study design, sampling strategies, and varying sensitivity, specificity, and accuracy of HPV detection methods.232
Supporting the role of estrogen in the malignant transformation of HPV infected cells into SCC, a laboratory study has demonstrated that estrogen stimulates the differential transcription of the E6/E7 and E1 genes of HPV-16 in SiHA cervical carcinoma cells.336 Glucocorticoids, a class of compounds resembling the steroids found in OCs,335 also have been shown to enhance the transformation of HPV-16 infected cell lines and increase the in vitro transcription and expression of the HPV-16 genome.337–339
Estrogen-induced squamous cell carcinogenesis has been demonstated in the female reproductive tract of K14-HPV-16 transgenic mice;340 and β-oestradiol has been shown to stimulate the in vivo growth of human condylomas induced with HPV in mice.336 The possibility also exists that reproductive hormones may promote the integration of HPV DNA into the host genome,341 which would be consistent with the observed increased risk for cervical cancer after prolonged use of OC.329
Conclusion
The evidence to date does not support an independent or synergistic role for OC use in the development of CSCC; nor does it fail to do so. Proof of association does not, of course, indicate a cause-and-effect relationship, as illustrated by the association between herpes genitalis and cervical carcinoma.316,342 Many questions remain to be answered. OC use may simply be a surrogate marker for sexual activity343,344 and non-causally associated with CSCC risk through increased exposure to HPV. This depends on whether HPV is a causative factor in CSCC, which remains controversial.315 On the other hand, OCs may have a contributing biologic role in the cascade of events leading up to CSCC. For example, certain beta-PV types in combination with OC use may play an initiation role, while the promotion or progression of CSCC ultimately depends on UVR exposure.61 The period between a hormonal exposure and the appearance of cancer may be lengthy, as illustrated by the development of vaginal cancer in daughters years after their mothers used diethylstilbestrol (DES) during pregnancy.345 Notably, the above initiation/promotion model does not explain the occurrence of CSCC on predominantly non sun-exposed skin, suggesting that factors other than UVR also may be important promoters in the development of this cancer. CSCCs remain a major public health concern, ranking among the most prevalent and costliest cancers in the United States and serving as an important harbinger of secondary malignancies.346–348
The incidence of nonmelanoma skin cancer (in which CSCC is an important subtype) has approximately doubled since the mid 1990’s.349 Future large-scale studies that incorporate detailed epidemiologic information (eg, OC use, sexual history, molecular markers, and exposure to UVR, chemicals and HPV) and provide analyses stratified by in situ and invasive CSCCs and tumor location are needed to help unravel the complex picture of this disease.
Acknowledgments
This manuscript was made possible by a grant from NCMHD/NIH (P20MD002289) entitled “ Teamwork in Research and Intervention to Alleviate Disparities Project (TRIAD)”. A. Toland was funded by the American Cancer Society. Elizabeth Tornquist (UNC-CH), Debra C. Wallace (UNCG), and Katherine T. Jones (ECU) offered valuable comments during the writing of this manuscript and their knowledge and insight are greatly appreciated. The author also thanks Helen Sedwick for administrative assistance and Kathleen Kihmm Connolly for graphic illustration.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material. The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Department of the Navy or US Department of Defense.
References
- 1.Johnson T, Rowe D, Nelson B, Swanson N. Squamous cell carcinoma of the skin (excluding lip and oral mucosa) J Am Acad Dermatol. 1992;26:467–84. doi: 10.1016/0190-9622(92)70074-p. [DOI] [PubMed] [Google Scholar]
- 2.Barksdale S, O’Connor N, Barnhill R. Prognostic factors for cutaneous squamous cell and basal cell carcinoma. Determinants of risk of recurrence, metastasis, and development of subsequent skin cancers. Surg Oncol Clin N Am. 1997;6:625–38. [PubMed] [Google Scholar]
- 3.Preston D, Stern R. Nonmelanoma cancers of the skin. N Engl J Med. 1992;327:1649–62. doi: 10.1056/NEJM199212033272307. [DOI] [PubMed] [Google Scholar]
- 4.Roth J, Granick M. Squamous cell and adnexal carcinomas of the skin. Clin Plast Surg. 1997;24:687–703. [PubMed] [Google Scholar]
- 5.Alam M, Caldwell J, Eliezri Y. Human papillomavirus-associated digital squamous cell carcinoma literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385–93. doi: 10.1067/mjd.2003.184. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg A, Ogle C, Shim E. Metastic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885–99. doi: 10.1111/j.1524-4725.2007.33190.x. [DOI] [PubMed] [Google Scholar]
- 7.Rowe D, Carroll R, Day C. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976–90. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- 8.Penn I. Tumors of the immunocompromised patient. Ann Rev Med. 1988;39:63–73. doi: 10.1146/annurev.me.39.020188.000431. [DOI] [PubMed] [Google Scholar]
- 9.Maize J. Skin cancer in immunosuppressed patients. JAMA. 1977;237:1857–58. [PubMed] [Google Scholar]
- 10.Adamson R, Obispo E, Dychter S, et al. High incidence and clinical course of aggressive skin cancer in heart transplant patients: a single-center study. Tranplant Proc. 1998;30:1124–6. doi: 10.1016/s0041-1345(98)00178-x. [DOI] [PubMed] [Google Scholar]
- 11.Euvrard S, Kanitakis J, Claudy A. Medical progress: skin cancers after organ transplantation. N Eng J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Cardella C, Haberman H. Cutaneous malignant neoplasms in patients with renal transplants. Arch Dermatol. 1986;122:1288–93. [PubMed] [Google Scholar]
- 13.Smith K, Hamza S, Skelton H. Histologic features in primary cutaneous squamous cell carcinomas in immunocompromised patients focusing on organ transplant patients. Dermatol Surg. 30:634–41. doi: 10.1111/j.1524-4725.2004.30149.x. 200; [DOI] [PubMed] [Google Scholar]
- 14.Kinlen L. Immunosuppression and cancer. IARC Sci Publ. 1992;116:237–53. [PubMed] [Google Scholar]
- 15.Webb M, Compton F, Andrews P, Koffman C. Skin tumours posttransplantation: a retrospective analysis of 28 years’ experience at a single centre. Trans Proc. 1997;29:828–30. doi: 10.1016/s0041-1345(96)00152-2. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich C, Schmook T, Sachse M, Sterry W, Stockfleth E. Comparative epidemiology and pathogenic factors for nonmelanoma skin cancer in organ transplant patients. Dermatol Surg. 2004;30:622–7. doi: 10.1111/j.1524-4725.2004.30147.x. [DOI] [PubMed] [Google Scholar]
- 17.Meyer T, Arndt R, Christophers E, Nindl I, Stockfleth E. Importance of human papillomaviruses for the development of skin cancer. Cancer Detect Prev. 2001;25:533–47. [PubMed] [Google Scholar]
- 18.Martin H, Strong E, Spiro R. Radiation-induced skin cancer of the head and neck. Cancer. 1970;25:61–71. doi: 10.1002/1097-0142(197001)25:1<61::aid-cncr2820250110>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen P, Vin-Christian K, Ming M, Berger T. Aggressive squamous cell carcinomas in persons infected with the human immunodeficiency virus. Arch Dermatol. 2002;138:758–63. doi: 10.1001/archderm.138.6.758. [DOI] [PubMed] [Google Scholar]
- 20.Overly W, Jakubek D. Multiple squamous cell carcinoma and human immunodeficiency virus infection. Ann Inter Med. 1987;106:334. doi: 10.7326/0003-4819-106-2-334_1. [DOI] [PubMed] [Google Scholar]
- 21.Palefsky J, Rubin M. The epidemiology of anal human papillomavirus and related neoplasia. Obstet Gynecol Clin N Am. 2009;36:187–200. doi: 10.1016/j.ogc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Strom S, Yamamura Y. Epidemiology of nonmelanoma skin cancer. Clin Plast Surg. 1997;24:627–36. [PubMed] [Google Scholar]
- 23.Gloster H, Brodland D. The epidemiology of skin cancer. Dermatol Surg. 1996;22:217–26. doi: 10.1111/j.1524-4725.1996.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 24.Halder R, Bang K. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397–405. [PubMed] [Google Scholar]
- 25.Halder R, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667–73. doi: 10.1002/1097-0142(19950115)75:2+<667::aid-cncr2820751409>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Isaacson C. Cancer of the skin in urban blacks of South Africa. Br J Dermatol. 1979;100:47–350. doi: 10.1111/j.1365-2133.1979.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 27.Fleming I, Barnawell J, Burlison P, Rankin J. Skin Cancer in black patients. Cancer. 1975;35:600–5. doi: 10.1002/1097-0142(197503)35:3<600::aid-cncr2820350309>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Oettle A. Skin cancer in Africa. NCI Mongr. 1963;10:197–214. [Google Scholar]
- 29.Rippey J, Schmaman A. Skin tumours of Africans. In: Marshall J, editor. Essays on Tropical Dermatology. Vol. 2. Amsterdam: Excerpta Medica; 1972. pp. 99–115. [Google Scholar]
- 30.Ishikawa N, Tanabe K, Tokumoto T, et al. Clinical study of malignancies after renal transplantation and impact of routine screening for early detection: a single center experience. Transplant Proc. 2000;32:1907–10. doi: 10.1016/s0041-1345(00)01487-1. [DOI] [PubMed] [Google Scholar]
- 31.Polednak A. Racial & Ethnic Differences in Disease. NewYork: Oxford University Press; 1989. [Google Scholar]
- 32.Lee T, Lee A, Shi W. HLA-A, -B, -DR, -DQ antigens in black North Americans. Tissue Antigens. 1991;7:9–83. doi: 10.1111/j.1399-0039.1991.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 33.Fraser P, Moore B, Stein R, et al. Complotypes in individuals of African origin: frequencies and possible extended MHC haplotypes. Immunogenetics. 1990;31:89–93. doi: 10.1007/BF00661218. [DOI] [PubMed] [Google Scholar]
- 34.Czarnecki D, Watkins F, Leahy S, et al. Skin cancers and HLA frequencies in renal transplant recipients. Dermatology. 1992;185:9–11. doi: 10.1159/000247394. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization, International Agency for Research on Cancer Solar and ultraviolet radiation. IARC Monogr Eval Carcinog Risk Hum. 1992;55:1–316. [PMC free article] [PubMed] [Google Scholar]
- 36.Young C. Solar ultraviolet radiation and skin cancer. Occup Med. 2009;59:82–8. doi: 10.1093/occmed/kqn170. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher R, Hill G, Bajdik C, et al. Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Arch Dermatol. 1995;131:164–9. [PubMed] [Google Scholar]
- 38.Aubry F, MacGibbon B. Risk factors of squamous cell carcinoma of the skin. A case-control study in the Montreal region. Cancer. 1985;55:907–11. doi: 10.1002/1097-0142(19850215)55:4<907::aid-cncr2820550433>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Giles G, Marks R, Foley P. Incidence of non-melanocytic skin cancer treated in Australia. Br Med J. 1988;296:13–7. doi: 10.1136/bmj.296.6614.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green A, Battistutta D. Incidence and determinants of skin cancer in a high-risk Australian population. Int J Cancer. 1990;46:356–61. doi: 10.1002/ijc.2910460303. [DOI] [PubMed] [Google Scholar]
- 41.English D, Armstrong B, Kricker A, Winter M, Heenan P, Randell P. Demographic characteristics, pigmentary and cutaneous risk factors for squamous cell carcinoma of the skin: a case-control study. Int J Cancer. 1998;76:628–34. doi: 10.1002/(sici)1097-0215(19980529)76:5<628::aid-ijc3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Asgari M, Efird J, Warton E, Friedman G. Potential risk factors for cutaneous squamous cell carcinoma include oral contraceptives: results of a nested case-control study. Int J Env Res Public Health. 2010;7:427–42. doi: 10.3390/ijerph7020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grodstein F, Speizer F, Hunter D. A prospective study of incident squamous cell carcinoma of the skin in the Nurses’ Health Study. J Natl Cancer Inst. 1995;87:1061–6. doi: 10.1093/jnci/87.14.1061. [DOI] [PubMed] [Google Scholar]
- 44.Kricker A, Armstrong B, English D, Heenan P. Pigmentary and cutaneous risk factors for non-melanocytic skin cnacer—a case-control study. Int J Cancer. 1991;48:650–62. doi: 10.1002/ijc.2910480504. [DOI] [PubMed] [Google Scholar]
- 45.Murphy G. Ultraviolet radiation and immunosuppression. Br J Dermatol. 2009;161:90–5. doi: 10.1111/j.1365-2133.2009.09455.x. [DOI] [PubMed] [Google Scholar]
- 46.Nishigori C, Yarosh D, Donawho C, Kripke M. The immune system in ultraviolet carcinogenesis. J Investig Dermatol Symp Proc. 1996;1:143–6. [PubMed] [Google Scholar]
- 47.Aubin F. Mechanisms involved in ultraviolet light-induced immunosuppression. Eur J Dermatol. 2003;13:515–23. [PubMed] [Google Scholar]
- 48.Beissert S, Schwarz T. Mechanisms involved in ultraviolet light-induced immunosuppression. J Investig Dermatol Symp Proc. 1999;4:61–4. doi: 10.1038/sj.jidsp.5640183. [DOI] [PubMed] [Google Scholar]
- 49.De Gruijl F. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol. 2002;15:316–20. doi: 10.1159/000064535. [DOI] [PubMed] [Google Scholar]
- 50.Findlay G. Ultra-violet light and skin cancer. Lancet. 1928;2:1070–3. doi: 10.3322/canjclin.29.3.169. [DOI] [PubMed] [Google Scholar]
- 51.Winkelmann R, Zollman P, Baldes E. Squamous cell carcinoma produced by ultraviolet light in hairless mice. J Invest Dermatol. 1963;40:217–24. [PubMed] [Google Scholar]
- 52.Kripke M. Immunology and photocarcinogenesis. New light on an old problem. J Am Acad Dermatol. 1986;14:149–55. doi: 10.1016/s0190-9622(86)70017-0. [DOI] [PubMed] [Google Scholar]
- 53.Beissert S, Schwarz T. Ultraviolet-induced immunosuppression: implications for photocarcinogenesis. Cancer Treat Res. 2009;146:109–21. doi: 10.1007/978-0-387-78574-5_10. [DOI] [PubMed] [Google Scholar]
- 54.Weaver S, Kelly A, Lopansri S. Dysplastic epidermal keratosis in a black woman. Arch Dermatol. 1981;117:800–3. [PubMed] [Google Scholar]
- 55.Amonette R, Kaplan R. Squamous-cell and basal-cell carcinomas in black patients. J Dermatol Surg. 1976;2:158–61. doi: 10.1111/j.1524-4725.1976.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 56.White J, Strudwick W, Ricketts W, Sampson C. Cancer of the skin in Negroes: A review of 31 cases. JAMA. 1961;178:845–7. doi: 10.1001/jama.1961.73040470023015b. [DOI] [PubMed] [Google Scholar]
- 57.Levi F, La Vecchia C, Te V, Mezzanotte F. Descriptive epidemiology of skin cancer in the Swiss Canton of Vaud. Int J Cancer. 1988;42:811–6. doi: 10.1002/ijc.2910420601. [DOI] [PubMed] [Google Scholar]
- 58.Stern R. The mysteries of geographic variability in nonmelanoma skin cancer incidence. Arch Dermatol. 1999;135:843–4. doi: 10.1001/archderm.135.7.843. [DOI] [PubMed] [Google Scholar]
- 59.Karagas M, Nelson H, Zens M, et al. Squamous cell and basal cell carcinoma of the skin in relation to radiation therapy and potential modification of risk by sun exposure. Epidemiology. 2007;18:776–84. doi: 10.1097/EDE.0b013e3181567ebe. [DOI] [PubMed] [Google Scholar]
- 60.Masini C, Fuchs P, Gabrielli F, et al. Evidence for the association of human papillomavirus infection and cutaneous squamous cell carcinoma in immunocompetent individuals. Arch Dermatol. 2003;139:890–4. doi: 10.1001/archderm.139.7.890. [DOI] [PubMed] [Google Scholar]
- 61.Eatough J, Henshaw D. The theoretical risk of non-melanoma skin cancer from environmental radon exposure. J Radiol Prot. 1995;15:45–51. [Google Scholar]
- 62.Rintala A. Radiation cancer. J Plast Surg Hand Surg. 1967;1:5–11. [Google Scholar]
- 63.Shore R. Overview of radiation-induced skin cancer in humans. Int J Radiat Biol. 1990;57:809–27. doi: 10.1080/09553009014550951. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Inskip P, Boice J, Li B, Zhang J, Fraumeni J. Cancer incidence among medical diagnotic x-ray workers in China, 1950–1985. Int J Cancer. 1990;45:889–95. doi: 10.1002/ijc.2910450519. [DOI] [PubMed] [Google Scholar]
- 65.Sadamori N, Mine M, Honda T. Incidence of skin cancer among Nagasaki atomic bomb survivors. J Radiat Res. 1991;32:217–25. doi: 10.1269/jrr.32.supplement2_217. [DOI] [PubMed] [Google Scholar]
- 66.Van Vloten W, Hermans J, van Daal W. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411–4. doi: 10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 67.Frieben E. Demonstration eines Cancroids des rechten Handrückens das sich nach langdauernder Einwirkung von Röntgenstrahlen entwickelt hatte. Forschr Geb Röntgenstr. 1902;6:106–11. [Google Scholar]
- 68.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–83. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 69.Gallagher R, Bajdik C, Fincham S, et al. Chemical exposures, medical history, and risk of squamous and basal cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 1996;5:419–24. [PubMed] [Google Scholar]
- 70.Purdie K, Surentheran T, Sterling J, et al. Human papillomavirus gene expression in cutaneous squamous cell carcinomas from immunosuppressed and immunocompetent individuals. J Invest Dermatol. 2005;125:98–107. doi: 10.1111/j.0022-202X.2005.23635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stockfleth E, Nindl I, Sterry W, Ulrich C, Schmook T, Meyer T. Human papillomavirus in transplant-associated skin cancers. Dermatol Surg. 2004;30:604–9. doi: 10.1111/j.1524-4725.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz R, Stoll H. Chapter 80: Squamous cell carcinoma. In: Freedberg I, Eisen A, Wolff K, Austen K, Goldsmith L, Katz S, Fitzpatrick T, editors. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York: McGraw-Hill; 1999. [Google Scholar]
- 73.Scotto J, Fears T, Kraemer K, Fraumeni J. Chapter 60: Nonmelanoma skin cancer. In: Schottenfeld D, Fraumeni J, editors. Cancer Epidemiology and Prevention. 2nd ed. New York: Oxford University Press; 1996. pp. 1313–26. [Google Scholar]
- 74.Pfister H. Chapter 8: Human papillomavirus and skin cancer. J Natl Cancer Inst Monogr. 2003;31:52–6. doi: 10.1093/oxfordjournals.jncimonographs.a003483. [DOI] [PubMed] [Google Scholar]
- 75.Sterling J. Human papillomaviruses and skin cancer. J Clin Virol. 2005;32S:S67–71. doi: 10.1016/j.jcv.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 76.De Villiers E. Human papillomavirus infections in skin cancers. Biomed & Pharmacother. 1998;52:26–33. doi: 10.1016/s0753-3322(97)86238-5. [DOI] [PubMed] [Google Scholar]
- 77.De Villiers E, Ruhland A, Šekarić P. Human papillomaviruses in non-melanoma skin cancer. Semin Cancer Biol. 1999;9:413–22. doi: 10.1006/scbi.1999.0145. [DOI] [PubMed] [Google Scholar]
- 78.Harwood C, McGregor J, Proby C, Breuer J. Human papillomavirus and the development of non-melanoma skin cancer. J Clin Pathol. 1999;52:249–53. doi: 10.1136/jcp.52.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hemminki K, Li X. Level of education and the risk of cancer in Sweden. Cancer. Epidemiol Biomarkers Prev. 2003;12:796–802. [PubMed] [Google Scholar]
- 80.Hemminki K, Zhang H, Czene K. Socioeconomic factors in cancer in Sweden. Int J Cancer. 2003;105:692–700. doi: 10.1002/ijc.11150. [DOI] [PubMed] [Google Scholar]
- 81.Glass A, Hoover R. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA. 1989;262:2097–100. [PubMed] [Google Scholar]
- 82.Gallagher R, Ma B, McLean D, et al. Trends in basal cell carcinoma, and melanoma of the skin from 1973 through 1987. J Am Acad Dermatol. 1990;23:413–21. doi: 10.1016/0190-9622(90)70234-9. [DOI] [PubMed] [Google Scholar]
- 83.Gray D, Suman V, Su W, Clay R, Harmsen W, Roenigk R. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Arch Dermatol. 1997;133:735–40. [PubMed] [Google Scholar]
- 84.Wassberg C, Thörn M, Johansson A, Bergström R, Berne B, Ringborg U. Increasing incidence rates of squamous cell carcinoma of the skin in Sweden. Acta Derm Venereol. 2001;81:268–72. doi: 10.1080/00015550152572903. [DOI] [PubMed] [Google Scholar]
- 85.Hemminki K, Zhang H, Czene K. Time trends and familial risks in squamous cell carcinoma of the skin. Arch Dermatol. 2003;139:885–9. doi: 10.1001/archderm.139.7.885. [DOI] [PubMed] [Google Scholar]
- 86.Coebergh J, Neumann H, Vrints L, van der Heijden L, Meijer W, Verhagen-Teulings M. Trends in the incidence of non-melanoma skin cancer in the SE Netherlands 1975–1988: a registry-based study. Br J Dermatol. 1991;125:353–9. doi: 10.1111/j.1365-2133.1991.tb14171.x. [DOI] [PubMed] [Google Scholar]
- 87.Staples M, Marks R, Giles G. Trends in the incidence of non-melanocytic skin cancer (NMSC) treated in Australia 1985–1995: are primary prevention programs starting to have an effect? Int J Cancer. 1998;78:144–8. doi: 10.1002/(sici)1097-0215(19981005)78:2<144::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 88.Kripke M. Impact of ozone depletion on skin cancers. J Dermatol Surg Oncol. 1988;14:853–7. doi: 10.1111/j.1524-4725.1988.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 89.Henriksen T, Dahlback A, Larsen S, Moan J. Ultraviolet-radiation and skin cancer. Effect of an ozone layer depletion. Photochem Photobiol. 1990;51:579–82. doi: 10.1111/j.1751-1097.1990.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 90.Abarca J, Casiccia C. Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: southern Chile, 1987–2000. Photodermatol Photoimmunol Photomed. 2002;18:294–302. doi: 10.1034/j.1600-0781.2002.02782.x. [DOI] [PubMed] [Google Scholar]
- 91.Amron D, Moy R. Stratospheric ozone depletion and its relationship to skin cancer. J Dermatol Surg Oncol. 1991;17:370–2. doi: 10.1111/j.1524-4725.1991.tb01713.x. [DOI] [PubMed] [Google Scholar]
- 92.Efird J, Friedman G, Habel L, Tekawa I, Nelson L. Risk of subsequent cancer following invasive or in situ squamous cell skin cancer. Ann Epidemiol. 2002;12:469–75. doi: 10.1016/s1047-2797(01)00276-9. [DOI] [PubMed] [Google Scholar]
- 93.Karagas M, Greenberg E, Mott L, Baron J, Ernster V. Occurrence of other cancers among patients with prior basal cell and squamous cell skin cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:157–61. [PubMed] [Google Scholar]
- 94.Frisch M, Melbye M. New primary cancers after squamous cell skin cancer. Am J Epidemiol. 1995;141:916–22. doi: 10.1093/oxfordjournals.aje.a117358. [DOI] [PubMed] [Google Scholar]
- 95.Kahn H, Tatham L, Patel A, Thun M, Heath C. Increased cancer mortality following a history of nonmelanoma skin cancer. JAMA. 1998;280:910–2. doi: 10.1001/jama.280.10.910. [DOI] [PubMed] [Google Scholar]
- 96.Levi R, Randimbison L, La Vecchia C, Erler G, Te V. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol. 1997;146:734–9. doi: 10.1093/oxfordjournals.aje.a009349. [DOI] [PubMed] [Google Scholar]
- 97.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. Br Med J. 1995;310:1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levi F, Randimbison L, Te V, Conconi M, La Vecchia C. Risk of prostate, breast and colorectal cancer after skin cancer diagnosis. Int J Cancer. 2008;123:2899–901. doi: 10.1002/ijc.23816. [DOI] [PubMed] [Google Scholar]
- 99.Maitra S, Gallo H, Rowland-Payne C, Robinson D, Møller H. Second primary cancers in patients with squamous cell carcinoma of the skin. Br J Cancer. 2005;92:570–1. doi: 10.1038/sj.bjc.6602306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toro J, Blake P, Björkholm M, Kristinsson S, Wang Z, Landgren O. Prior history of non-melanoma skin cancer is associated with increased mortality in patients with chronic lymphocytic leukemia. Haematologica. 2009;94:1460–4. doi: 10.3324/haematol.2008.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen J, Ruczinski I, Jorgensen T, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst. 2008;100:1215–22. doi: 10.1093/jnci/djn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosenberg C, Khandekar J, Greenland P, Rodabough R, McTiernan A. Cutaneous melanoma in postmenopausal women after nonmelanoma skin carcinoma. The Women’s Health Initiative Observational Study. Cancer. 2006;106:654–63. doi: 10.1002/cncr.21627. [DOI] [PubMed] [Google Scholar]
- 103.Rosenberg C, Greenland P, Khandekar J, Loar A, Ascensao J, Lopez A. Association of nonmelanoma skin cancer with second malignancy. The Women’s Health Initiative Observational Study. Cancer. 2004;100:130–8. doi: 10.1002/cncr.11874. [DOI] [PubMed] [Google Scholar]
- 104.Nugent Z, Demers A, Wiseman M, Mihalcioiu C, Kliewer E. Risk of second primary cancer and death following a diagnosis of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2584–90. doi: 10.1158/1055-9965.EPI-05-0379. [DOI] [PubMed] [Google Scholar]
- 105.Wassberg C, Thörn M, Yuen J, Ringborg U, Hakulinen T. Secondary primary cancers in patients with squamous cell carcinoma of the skin: a population-based study in Sweden. Int J Cancer. 1999;80:511–5. doi: 10.1002/(sici)1097-0215(19990209)80:4<511::aid-ijc5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 106.Troyanova P, Danon S, Ivanova T. Nonmelanoma skin cancers and risk of subsequent malignancies: a cancer registry-based study in Bulgaria. Neoplasma. 2002;49:81–5. [PubMed] [Google Scholar]
- 107.Hjalgrim H, Frisch M, Strom H, Glimelius B, Pedersen J, Melbye M. Nonmelanoma skin cancer may be a marker of poor prognosis in patients with non-Hodgkins lymphoma. Int J Cancer. 2000;85:639–42. doi: 10.1002/(sici)1097-0215(20000301)85:5<639::aid-ijc7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 108.Marcil I, Stern R. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524–30. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 109.Graells J. The risk and risk factors of a second non-melanoma skin cancer: a study in a mediterranean population. J Eur Acad Dermatol Venereol. 2004;18:142–7. doi: 10.1111/j.1468-3083.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- 110.Cantwell M, Murray L, Catney D, et al. Secondary primary cancers in patients with skin cancer: a population-based study in Northern Ireland. Br J Cancer. 2009;100:174–7. doi: 10.1038/sj.bjc.6604842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crocetti E, Buiatti E, Falini P, the Italian Multiple Primary Cancer Working Group Multiple primary cancer incidence in Italy. Eur J Cancer. 2001;37:2449–56. doi: 10.1016/s0959-8049(01)00314-8. [DOI] [PubMed] [Google Scholar]
- 112.Hemminki K, Jiang Y, Dong C. Second primary cancers after anogenital, skin, oral, esophageal and rectal cancers: etiological links? Int J Cancer. 2001;93:294–8. doi: 10.1002/ijc.1319. [DOI] [PubMed] [Google Scholar]
- 113.Hemminki K, Dong C. Subsequent cancers after in situ and invasive squamous cell carcinoma of the skin. Arch Dermatol. 2000;136:647–51. doi: 10.1001/archderm.136.5.647. [DOI] [PubMed] [Google Scholar]
- 114.Hemminki K, Dong C. Primary cancers following squamous cell carcinoma of the skin suggest involvement of Epstein-Barr virus. Epidemiology. 2000;11:94. doi: 10.1097/00001648-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 115.Jæger A, Gramkow A, Hjalgrim H, Melbye M, Frisch M. Bowen disease and risk of subsequent malignant neoplasms—a population-based cohort study of 1147 patients. Arch Dermatol. 1999;135:790–3. doi: 10.1001/archderm.135.7.790. [DOI] [PubMed] [Google Scholar]
- 116.Marghoob A, Slade J, Salopek T, Kopf A, Bart R, Rigel D. Basal cell and squamous cell carcinomas are important risk factors for cutaneous malignant melanoma. Screening implications. Cancer. 1995;75:707–14. doi: 10.1002/1097-0142(19950115)75:2+<707::aid-cncr2820751415>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 117.Applebaum K, Nelson H, Zens M, Stukel T, Spencer S, Karagas M. Oral contraceptives: a risk factor for squamous cell carcinoma? J Invest Dermatol. 2009;129:2760–5. doi: 10.1038/jid.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muñoz N, Kjaer S, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital disease in young women. J Natl Cancer Inst. 2010;102:325–39. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 119.Cavalieri E, Frenkel K, Liehr J, Rogan E, Roy D. Chapter 4: estrogens as endogenous genotoxic agents-DNA adducts and mutations. J Natl Cancer Inst. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 120.Cogliano V, Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, for the WHO International Agency for Research on Cancer Carcinogenicity of combined oestrogen-progestagen contraceptives and menopausal treatment. Lancet Oncol. 2005;6:552–3. doi: 10.1016/s1470-2045(05)70273-4. [DOI] [PubMed] [Google Scholar]
- 121.World Health Organization, International Agency for Research on Cancer Combined estrogen—progestogen contraceptives and combined estrogen-progestogen menopausal therapy. IARC Monogr Eval Carcinog Risk Hum. 2007;91:1–528. [PMC free article] [PubMed] [Google Scholar]
- 122.World Health Organization, International Agency for Research on Cancer Hormonal contraception and post-menopausal hormonal therapy. IARC Monogr Eval Carcinog Risk Hum. 1999;72:1–660. [Google Scholar]
- 123.Grimes D. Neoplastic effects of oral contraceptives. Int J Fertil. 1991;36:19–24. [PubMed] [Google Scholar]
- 124.Huber J, Bentz E, Ott J, Tempfer C. Non-contraceptive benefits of oral contraceptives. Expert Opin Parmacother. 2008;9:2317–25. doi: 10.1517/14656566.9.13.2317. [DOI] [PubMed] [Google Scholar]
- 125.Cibula D, Gompel A, Mueck A, et al. Hormonal contraception and risk of cancer. Hum Reprod Update. 2010;16:631–50. doi: 10.1093/humupd/dmq022. [DOI] [PubMed] [Google Scholar]
- 126.Dolle J, Daling J, White E, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–66. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dossus L, Allen N, Kaaks R, et al. Reproductive risk factors and endometrial cancer: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;127:442–51. doi: 10.1002/ijc.25050. [DOI] [PubMed] [Google Scholar]
- 128.Vessey M, Painter R, Powell J. Skin disorders in relation to oral contraception and other factors including age, social class, smoking and body mass index. Findings in a large cohort study. Br J Dermatol. 2000;143:815–20. doi: 10.1046/j.1365-2133.2000.03782.x. [DOI] [PubMed] [Google Scholar]
- 129.Cousins R. Annotated bibliography of some papers on combining significances or p-values. Dec 20, 2008. pp. 1–15. Available at http://arxiv.org/abs/0705.2209. Accessed 29 January, 2011.
- 130.Fleiss J, Gross A. Meta-analsis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44:127–39. doi: 10.1016/0895-4356(91)90261-7. [DOI] [PubMed] [Google Scholar]
- 131.Braendle W, Kuhl H, Mueck A, et al. Does hormonal contraception increase the risk for tumors? Ther Umsch. 2009;66:129–35. doi: 10.1024/0040-5930.66.2.129. [DOI] [PubMed] [Google Scholar]
- 132.Edgren R. Oral contraceptives and cancer. Int J Fertil. 1991;36:37–50. [PubMed] [Google Scholar]
- 133.Gates M, Tworoger S, Eliassen A, Missmer S, Hankinson S. Analgesic use and sex steroid hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:1033–41. doi: 10.1158/1055-9965.EPI-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller C, Murtagh J. Combined oral contraception. Aust Fam Physician. 1992;21:1787–8. [PubMed] [Google Scholar]
- 135.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23257 women with ovarian cancer from 87303 controls. Lancet. 2008;371:303–14. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 136.Satterthwaite A. A comparative study of low dosage oral contraceptives. Appl Ther. 1964;6:410–8. [PubMed] [Google Scholar]
- 137.Fraser D, Padwick M, Whitehead M, Coffer A, King R. Presence of an oestradiol receptor-related protein in the skin: changes during the normal menstrual cycle. Br J Obstet Gynaecol. 1991;98:1277–82. doi: 10.1111/j.1471-0528.1991.tb15402.x. [DOI] [PubMed] [Google Scholar]
- 138.Kousteni S, Bellido T, Plotkin L, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–30. [PubMed] [Google Scholar]
- 139.Rogers G, Flowers J, Pollack S, McCarty K. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039–43. doi: 10.1016/s0190-9622(88)70101-2. [DOI] [PubMed] [Google Scholar]
- 140.Leslie K, Espey E. Oral contraceptives and skin cancer: is there a link? Am J Clin Dermatol. 2005;6:349–55. doi: 10.2165/00128071-200506060-00002. [DOI] [PubMed] [Google Scholar]
- 141.Liu W, Konduri S, Bansal S, et al. Estrogen receptor-α binds p53 tumor suppressor protein directly and represses its function. J Biol Chem. 2006;281:9837–40. doi: 10.1074/jbc.C600001200. [DOI] [PubMed] [Google Scholar]
- 142.Mancuso M, Gallo D, Leonardi S, et al. Modulation of basal and squamous cell carcinoma by endogenous estrogen in mouse models of skin cancer. Carcinogenesis. 2009;30:340–7. doi: 10.1093/carcin/bgn243. [DOI] [PubMed] [Google Scholar]
- 143.Schmeink C, Lenselink C, Bekkers R. Use of oral contraceptives and increased risk of cervical cancer. Ned Tijdschr Geneeskd. 2008;152:1717–8. [PubMed] [Google Scholar]
- 144.Bravo I, de Sanjosé S, Gottschling M. The clinical importance of understanding the evolution of papillomaviruses. Trends Microbiol. 2010;18:432–38. doi: 10.1016/j.tim.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 145.Coggin J, zur Hausen H. Workshop on papillomaviruses and cancer. Cancer Res. 1979;39:545–6. [Google Scholar]
- 146.De Villiers E, Fauquet C, Broker T, Bernard H, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 147.Bernard H. The clinical importance of the nomenclature, evolution and taxonomy of human papillomaviruses. J Clin Virol. 2005;32S:S1–6. doi: 10.1016/j.jcv.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 148.Forslund O. Genetic diversity of cutaneous human papillomaviruses. J Gen Virol. 2007;88:2662–9. doi: 10.1099/vir.0.82911-0. [DOI] [PubMed] [Google Scholar]
- 149.Plasmeijer E, Neale R, de Koning M, et al. Persistence of betapapillomavirus infections as a risk factor for actinic keratoses, precursor to cutaneous squamous cell carcinoma. Cancer Res. 2009;69:8926–31. doi: 10.1158/0008-5472.CAN-09-1186. [DOI] [PubMed] [Google Scholar]
- 150.De Villiers E. Heterogeneity of the human papillomavirus group. J Virol. 1989;63:4898–903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beard J, Rous P. A virus-induced mamalian growth with the characters of a tumor (the Shope rabbit papilloma). II. Experimental alterations of the growth of the skin: morphological considerations: the phenomena of retrogression. J Exp Med. 1934;60:723–40. doi: 10.1084/jem.60.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rous R, Beard J. The progression to carcinoma of virus-induced rabbit papillomas (Shope) J Exp Med. 1935;62:523–48. doi: 10.1084/jem.62.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mitsuishi T, Sata T, Matsukura T, Iwasaki T, Kawashima M. The presence of mucosal human papillomavirus in Bowen’s disease of the hands. Cancer. 1997;79:1911–7. doi: 10.1002/(sici)1097-0142(19970515)79:10<1911::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 154.Forslund O, Nordin P, Hansson B. Mucosal human papillomavirus types in squamous cell carcinomas of the uterine cervix and subsequently on fingers. Br J Dermatol. 2000;142:1148–53. doi: 10.1046/j.1365-2133.2000.03540.x. [DOI] [PubMed] [Google Scholar]
- 155.Sánchez-Lanier M, Triplett C, Campion M. Possible role for human papillomavirus 16 in squamous cell carcinoma of the finger. J Med Virol. 1994;44:369–78. doi: 10.1002/jmv.1890440410. [DOI] [PubMed] [Google Scholar]
- 156.Sonnex C, Strauss J, Gray J. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex Transm Inf. 1999;75:317–9. doi: 10.1136/sti.75.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moy R, Eliezri Y, Nuovo G, Zitelli J, Bennett R, Silverstein S. Human papillomavirus type 16 DNA in periungual squamous cell carcinomas. JAMA. 1989;261:2669–73. [PubMed] [Google Scholar]
- 158.Kreuter A, Brockmeyer N, Pfister H, Altmeyer P, Wieland U, for the Competence Network HIV/AIDS Humanpapillomavirus type 26-associated periungual squamous cell carcinoma in situ in a HIV-infected patient with concomitant penile and anal intraepithelial neoplasia. J Am Acad Dermatol. 2005;53:737–9. doi: 10.1016/j.jaad.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 159.Eliezri Y, Silverstein S, Nuovo G. Occurence of human papillomavirus type 16 DNA in cutaneous squamos and basal cell neoplasms. J Am Acad Dermatol. 1990;23:836–42. doi: 10.1016/0190-9622(90)70299-w. [DOI] [PubMed] [Google Scholar]
- 160.Stone M, Noonan C, Tschen J, Bruce S. Bowen’s disease of the feet. Presence of human papillomavirus 16 DNA in tumor tissue. Arch Dermatol. 1987;123:1517–20. doi: 10.1001/archderm.123.11.1517. [DOI] [PubMed] [Google Scholar]
- 161.Forslund O, Nordin P, Andersson K, Stenquist B, Hansson B. DNA analysis indicates patient-specific human papillomavirus type 16 strains in Bowen disease on fingers and in archival samples from genital dysplasia. Br J Dermatol. 1997;136:678–82. [PubMed] [Google Scholar]
- 162.Nordin P, Stenquist B, Hansson B. Joint occurence of human papillomavirus type 16 DNA in Bowen’s disease on a finger and in dysplasia of the vulva and the uterine cervix. Br J Dermatol. 1994;131:740. doi: 10.1111/j.1365-2133.1994.tb05002.x. [DOI] [PubMed] [Google Scholar]
- 163.Kettler A, Rutledge M, Tschen J, Buffone G. Detection of human papillomavirus in nongenital Bowen’s disease by in situ DNA hybridization. Arch Dermatol. 1990;126:777–81. [PubMed] [Google Scholar]
- 164.Goshima J, Hara H, Honda A, Suzuki H, Matsukura T. Detection of human papillomavirus type 16 in Bowen’s disease of the web-space of the foot. Acta Derm Venereol. 2005;85:188–9. [PubMed] [Google Scholar]
- 165.Ashinoff R, Li J, Jacobson M, Friedman-Kien A, Geronemus R. Detection of human papillomavirus DNA in squamous cell carcinoma of the nail bed and finger determined by polymerase chain reaction. Arch Dermatol. 1991;127:1813–8. [PubMed] [Google Scholar]
- 166.Moy R, Quan M. The presence of human papillomavirus type 16 in squamous cell carcinoma of the proximal finger and reconstruction with a bilobed transposition flap. J Dermatol Surg Oncol. 1991;17:171–5. doi: 10.1111/j.1524-4725.1991.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 167.Clavel C, Huu V, Durlach A, Birembaut P, Bernard P, Derancourt C. Mucosal oncogenic human papillomaviruses and extragenital Bowen disease. Cancer. 1999;86:282–7. [PubMed] [Google Scholar]
- 168.Sau P, McMarlin S, Sperling L, Katz R. Bowen’s disease of the nail bed and periungual area. A clinicopathologic analysis of seven cases. Arch Dermatol. 1994;13:204–9. [PubMed] [Google Scholar]
- 169.Rüdlinger R, Grob R, Yu Y, Schnyder U. Human papillomavirus-35-positive bowenoid papulosis of the anogenital area and concurrent human papillomavirus-35-positive verruca with bowenoid dysplasia of the periungual area. Arch Dermatol. 1989;125:655–9. doi: 10.1001/archderm.1989.01670170069012. [DOI] [PubMed] [Google Scholar]
- 170.McGrae J, Greer C, Manos M. Multiple Bowen’s disease of the fingers associated with human papilloma virus type 16. Int J Dermatol. 1993;32:104–7. doi: 10.1111/j.1365-4362.1993.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 171.Gordon M, Palusci V. Human papillomavirus type 16 periungual carcinoma. JAMA. 1989;262:3407–8. doi: 10.1001/jama.262.24.3407. [DOI] [PubMed] [Google Scholar]
- 172.De Dobbeleer G, André J, Laporte M, Schroeder F, Peny M, Scarcériaux B, Noël J. Human papillomavirus type 6/11 and 16 in periungual squamous carcinoma in situ. Eur J Dermatol. 1993;3:12–4. [Google Scholar]
- 173.Guitart J, Bergfeld W, Tuthill R, Tubbs R, Zienowicz R, Fleegler E. Squamous cell carcinoma of the nail bed: a clinopathological study of 12 cases. Br J Dermatol. 1990;123:215–22. doi: 10.1111/j.1365-2133.1990.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 174.Ekström J, Forslund O, Dillner J. Three novel papillomaviruses (HPV 112 and HPV114) and their presence in cutaneous and mucosal samples. Virology. 2010;397:331–6. doi: 10.1016/j.virol.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 175.Feltkamp M, de Koning M, Bouwes Bavinck J, ter Schegget J. Betapapillomaviruses: innocent bystanders or causes of skin cancer. J Clin Virol. 2008;43:353–60. doi: 10.1016/j.jcv.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 176.Muller H, Bauch C. When do sexual partnerships need to be accounted for in transmission models of human papillomavirus? Int J Environ Res Public Health. 2010;7:635–50. doi: 10.3390/ijerph7020635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson B. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. 2000;74:11636–41. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Astori G, Lavergne D, Benton C, Höckmayr B, Egawa E, Garbe C, de Villiers E. Human papillomaviruses are commonly found in normal skin of immunocompetent hosts. J Invest Dermatol. 1998;110:752–5. doi: 10.1046/j.1523-1747.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- 179.De Koning M, Struijk L, Bavinck J, et al. Betapapillomaviruses frequently persist in the skin of healthy individuals. J Gen Virol. 2007;88:1489–95. doi: 10.1099/vir.0.82732-0. [DOI] [PubMed] [Google Scholar]
- 180.Pfister H, Fuchs P, Majewski S, Jablonska S, Pniewska I, Malejczyk M. High prevalence of epidermodysplasia verruciformis-associated human papillomavirus DNA in actinic keratoses of the immunocompetent populaiton. Arch Dermatol Res. 2003;295:273–9. doi: 10.1007/s00403-003-0435-2. [DOI] [PubMed] [Google Scholar]
- 181.Zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 182.McKaig R, Baric R, Olshan A. Human papillomavirus and head and neck cancer: epidemiology and molecular biology. Head Neck. 1998;20:250–65. doi: 10.1002/(sici)1097-0347(199805)20:3<250::aid-hed11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 183.Maden C, Beckmann A, Thomas D, et al. Human papillomavirus, herpes simplex viruses, and the risk of oral cancer in men. Am J Epidemiol. 1992;135:1093–102. doi: 10.1093/oxfordjournals.aje.a116209. [DOI] [PubMed] [Google Scholar]
- 184.Caruana S, Zwiebel N, Cocker R, McCormick S, Eberle R, Lazarus P. p53 alteration and human papillomavirus infection in paranasal sinus cancer. Cancer. 1997;79:1320–8. [PubMed] [Google Scholar]
- 185.Inaba Y, Egawa K, Yoshimura K, Ono T. Demonstration of human papillomavirus type 1 DNA in a wart with Bowenoid histologic changes. Am J Dermatopathol. 1993;15:172–5. doi: 10.1097/00000372-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 186.Noel J, Detremmerie O, Peny M, et al. Transformation of common warts into squamous cell carcinoma on sun-exposed areas in an immunosuppressed patient. Dermatology. 1994;189:308–11. doi: 10.1159/000246869. [DOI] [PubMed] [Google Scholar]
- 187.Noel J, Peny M, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58–61. doi: 10.1159/000247200. [DOI] [PubMed] [Google Scholar]
- 188.Burk R, Kadish A. Treasure hunt for human papillomaviruses in nonmelanoma skin cancers. J Natl Cancer Inst. 1996;88:781–2. doi: 10.1093/jnci/88.12.781. [DOI] [PubMed] [Google Scholar]
- 189.Shamanin V, zur Hausen H, Lavergne D, et al. Human papillomavirus infections in nonmelanoma skin cancers from renal transplant recipients and nonimmunosuppressed patients. J Natl Cancer Inst. 1996;88:802–11. doi: 10.1093/jnci/88.12.802. [DOI] [PubMed] [Google Scholar]
- 190.Harwood C, Spink P, Surentheran T, et al. Degenerate and nested PCR: a highly sensitive and specific method for detection of human papillomavirus infection in cutaneous warts. J Clin Microbiol. 1999;37:3545–55. doi: 10.1128/jcm.37.11.3545-3555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Soler C, Allibert P, Chardonnet Y, Cros P, Mandrand B, Thivolet J. Detection of human papillomavirus types 6, 11, 16 and 18 in mucosal and cutaneous lesions by the multiplex polymerase chain reaction. J Virol Methods. 1991;35:143–57. doi: 10.1016/0166-0934(91)90130-r. [DOI] [PubMed] [Google Scholar]
- 192.Soler C, Chardonnet Y, Allibert P, Euvrard S, Schmitt D, Mandrand B. Detection of mucosal human papillomavirus types 6/11 in cutaneous lesions from transplant recipients. J Invest Dermatol. 1993;101:286–91. doi: 10.1111/1523-1747.ep12365211. [DOI] [PubMed] [Google Scholar]
- 193.Kessis T, Slebos R, Nelson W, et al. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci U S A. 1993;90:3988–92. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Werness B, Levine A, Howley P. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 195.Scheffner M, Werness B, Huibregtse J, Levine A, Howley P. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;21:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 196.Steenbergen R, Hermsen M, Walboomers J, et al. Integrated human papillomavirus type 16 and loss of heterozygosity at 11q22 and 18q21 in an oral carcinoma and its derivative cell line. Cancer Res. 1995;55:5465–71. [PubMed] [Google Scholar]
- 197.Park N, Gujuluva C, Baek J, Cherrick H, Shin K, Min B. Combined oral carcinogenicity of HPV-16 and benzo(a)pyrene: an in vitro multistep carcinogenesis model. Oncogene. 1995;10:2145–53. [PubMed] [Google Scholar]
- 198.Iglesias M, Yen K, Gaiotti D, Hildesheim A, Stoler M, Woodworth C. Human papillomavirus type 16 E7 protein sensitizes cervical keratinocytes to apoptosis and release of interleukin-1α. Oncogene. 1998;17:1195–205. doi: 10.1038/sj.onc.1202054. [DOI] [PubMed] [Google Scholar]
- 199.Iftner T, Bierfelder S, Csapo Z, Pfister H. Involvement of human papillomavirus type 8 genes E6 and E7 in transformation and replication. J Virol. 1988;62:3655–61. doi: 10.1128/jvi.62.10.3655-3661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Woodworth C, Doniger J, DiPaolo J. Immortalization of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. J Virol. 1989;63:159–64. doi: 10.1128/jvi.63.1.159-164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kiyono T, Hiraiwa A, Ishibashi M. Differences in transforming activity and coded amino acid sequence among E6 genes of several papillomaviruses associated with epidermodysplasia verruciformis. Virology. 1992;186:28–639. doi: 10.1016/0042-6822(92)90029-o. [DOI] [PubMed] [Google Scholar]
- 202.Thomas M, Pim D, Banks L. The role of E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 203.Jackson S, Storey A. E6 proteins from diverse cutaneous HPV types inhibit apoptosis in response to UV damage. Oncogene. 2000;19:592–8. doi: 10.1038/sj.onc.1203339. [DOI] [PubMed] [Google Scholar]
- 204.Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of BAK in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065–73. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Underbrink M, Howie H, Bedard K, Koop J, Galloway D. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol. 2008;82:10408–17. doi: 10.1128/JVI.00902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim H, Guo W, Park N. HPV-16 E6 oncoprotein induces mutations via p53-dependent and –independent pathways. Oncol Rep. 2000;7:707–12. [PubMed] [Google Scholar]
- 207.Magal S, Jackman A, Pei X, Schlegel R, Sherman L. Induction of apoptosis in human keratinocytes containing mutated p53 alleles and its inhibition by both E6 and E7 oncoproteins. Int J Cancer. 1998;75:96–104. doi: 10.1002/(sici)1097-0215(19980105)75:1<96::aid-ijc15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 208.Song S, Gulliver G, Lambert P. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci U S A. 1998;95:2290–5. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shah K. Do human papillomavirus infections cause oral cancer? J Natl Cancer Inst. 1998;90:1585–6. doi: 10.1093/jnci/90.21.1585. [DOI] [PubMed] [Google Scholar]
- 210.Iftner T, Elbel M, Schopp B, Hiller T, Loizou J, Caldecott K, Stubenrauch F. Interference of papillomavirus E6 protein with single-strand break repair by interaction with XRCC1. EMBOJ. 2002;21:4741–8. doi: 10.1093/emboj/cdf443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schiffman M, Kjaer S. Chapter 2: Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Mongr. 2003;31:14–9. doi: 10.1093/oxfordjournals.jncimonographs.a003476. [DOI] [PubMed] [Google Scholar]
- 212.Tay S, Ho T, Lim-Tan S. Is genital human papillomavirus infection always sexually transmitted? Aust N Z J Obstet Gynaecol. 1990;30:240–2. doi: 10.1111/j.1479-828x.1990.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 213.Howley P, Münger K. Human papillomaviruses and squamous cell carcinomas. In: Parsonnet J, editor. Microbes and Malignancy: Infection as a Cause of Human Cancers. New York: Oxford University Press; 1999. pp. 157–79. [Google Scholar]
- 214.Arends M, Wyllie A, Bird C. Papillomaviruses and human cancer. Human Pathol. 1990;21:686–98. doi: 10.1016/0046-8177(90)90027-3. [DOI] [PubMed] [Google Scholar]
- 215.Holly E, Ralston M, Darragh T, Greenblatt R, Jay N, Palefsky J. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843–9. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 216.Palefsky J, Holly E, Ralston M, Da Costa M, Greenblatt R. Prevalence and risk factors for anal and human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–91. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 217.Palefsky J. Anal squamous intraepithelial lesions in human immunodeficiency virus-positive men and women. Semin Oncol. 2000;27:471–9. [PubMed] [Google Scholar]
- 218.Palefsky J, Holly E. Chapter 6: Immunosuppression and co-infection with HIV. J Natl Cancer Inst Monogr. 2003;31:41–6. doi: 10.1093/oxfordjournals.jncimonographs.a003481. [DOI] [PubMed] [Google Scholar]
- 219.Gillison M, Shah K. Chapter 9: Role of mucosal human papillomavirus in nongenital cancers. J Natl Cancer Inst Monogr. 2003;31:57–65. doi: 10.1093/oxfordjournals.jncimonographs.a003484. [DOI] [PubMed] [Google Scholar]
- 220.Fairley C, Chen S, Tabrizi S, et al. Prevalence of HPV DNA in cervical specimens in women with renal transplants: a comparison with dialysis-dependent patients and patients with renal impairment. Nephrol Dial Transplant. 1994;9:416–20. [PubMed] [Google Scholar]
- 221.Moscicki A, Ellenberg J, Vermund S, et al. Prevalence of risks for cervical human papillomavirus infection and squamous intraepithelial lesions in adolescent girls: impact of infection with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2000;154:127–34. doi: 10.1001/archpedi.154.2.127. [DOI] [PubMed] [Google Scholar]
- 222.Jamieson D, Duerr A, Burk R, et al. HIV Epidemiology Research Study (HERS) Group. Characterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infection. Am J Obstet Gynecol. 2002;186:21–7. doi: 10.1067/mob.2002.119776. [DOI] [PubMed] [Google Scholar]
- 223.Sun X, Kuhn L, Ellerbrock T, Chiasson M, Bush T, Wright T. Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337:1343–9. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 224.Frisch M, Biggar R, Goedert J. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–10. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 225.Palefsky J. Anal squamous intraepithelial lesions: relation to HIV and human papillomavirus infection. J Acquir Immune Defic Syndr. 1999;21:S42–8. [PubMed] [Google Scholar]
- 226.Melbye M, Palefsky J, Gonzales J, et al. Immune status as a determinant of human papillomavirus detection and its association with anal epithelial abnormalities. Int J Cancer. 1990;46:203–6. doi: 10.1002/ijc.2910460210. [DOI] [PubMed] [Google Scholar]
- 227.World Health Organization, International Agency for Research on Cancer Human papillomavirus. IARC Monogr Eval Carcinog Risk Hum. 2007;90:1–670. [Google Scholar]
- 228.Ley C, Bauer H, Reingold A, et al. Determinants of genital human papillomavirus infectin in young women. J Natl Cancer Inst. 1991;83:997–1003. doi: 10.1093/jnci/83.14.997. [DOI] [PubMed] [Google Scholar]
- 229.World Health Organization, International Agency for Research on Cancer Human papillomavirus. IARC Monogr Eval Carcinog Risk Hum. 1995;64:1–409. [Google Scholar]
- 230.Davidson M, Schnitzer P, Bulkow L, et al. The prevalence of cervical infection with human papillomaviruses and cervical dysplasia in Alaska native women. J Infect Dis. 1994;169:792–800. doi: 10.1093/infdis/169.4.792. [DOI] [PubMed] [Google Scholar]
- 231.Karlsson R, Jonsson M, Edlund K, et al. Lifetime number of partners as the only independent risk factor for human papillomavirus infection: a population-based study. Sex Transm Dis. 1995;22:119–27. doi: 10.1097/00007435-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 232.Burk R, Ho G, Beardsley L, Lempa M, Peters M, Bierman R. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis. 1996;174:679–89. doi: 10.1093/infdis/174.4.679. [DOI] [PubMed] [Google Scholar]
- 233.Schneider A. Pathogenesis of genital HPV infection. Genitourin Med. 1993;69:165–73. doi: 10.1136/sti.69.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dillner J, Kallings I, Brihmer C, et al. Seropositivities to human papillomavirus types 16, 18, or 33 capsids and to Chlamydia trachomatis are markers of sexual behaviour. J Infect Dis. 1996;173:1394–8. doi: 10.1093/infdis/173.6.1394. [DOI] [PubMed] [Google Scholar]
- 235.Winer R, Lee S, Hughes J, Adam D, Kiviat N, Koutsky L. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 236.Hildesheim A, Gravitt P, Schiffman M, et al. Determinants of genital human papillomavirus infection in low-income women in Washington, DC. Sex Transm Dis. 1993;20:279–85. doi: 10.1097/00007435-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 237.Wheeler C, Parmenter C, Hunt W, et al. Determinants of genital human papillomavirus infection among cytologically normal women attending the University of New Mexico student health center. Sex Transm Dis. 1993;20:286–89. doi: 10.1097/00007435-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 238.Bleeker M, Hogewoning C, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin Infect Dis. 2005;41:612–20. doi: 10.1086/431978. [DOI] [PubMed] [Google Scholar]
- 239.Baken L, Koutsky L, Kuypers J, et al. Genital human papillomavirus infection among male and female sex partners: prevalence and type-specific concordance. J Infect Dis. 1995;171:429–32. doi: 10.1093/infdis/171.2.429. [DOI] [PubMed] [Google Scholar]
- 240.Kyo S, Inoue M, Koyama M, Fujita M, Tanizawa O, Hakura A. Detection of high-risk papillomavirus in the cervix and semen of sex partners. J Infect Dis. 1994;170:682–5. doi: 10.1093/infdis/170.3.682. [DOI] [PubMed] [Google Scholar]
- 241.Strand A, Rylander E, Wilander E, Zehbe I. HPV infection in male partners of women with squamous intraepithelial neoplasia and/or high-risk HPV. Acta Derm Venereol. 1995;75:312–6. doi: 10.2340/0001555575312316. [DOI] [PubMed] [Google Scholar]
- 242.Hippeläinen M, Yliskoski M, Syrjänen S, et al. Low concordance of genital human papillomavirus (HPV) lesions and viral types in HPV-infected women and their male sexual partners. Sex Transm Dis. 1994;21:76–82. doi: 10.1097/00007435-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 243.Gerberding J. Prevention of Genital Human Papillomavirus Infection; Centers for Disease Control, Report to Congress; January 2004; pp. 1–35. [Google Scholar]
- 244.Ho G, Burk R, Klein S, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 245.Molano M, Van den Brule A, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–94. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 246.Schlecht N, Platt R, Duarte-Franco E, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95:1336–43. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 247.Hernandez B, Wilkens L, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emer Infect Dis. 2008;14:888–94. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Plasmeijer E, Green A, de Koning M, et al. Transmission of betapapillomaviruses between domestic partners in an Australian community. J Clin Virol. 2010;47:216–8. doi: 10.1016/j.jcv.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 249.Moy R, Eliezri Y. Significance of human papillomavirus-induced squamous cell carcinoma to dermatologists. Arch Dermatol. 1994;130:235–8. [PubMed] [Google Scholar]
- 250.Ferenczy A, Bergeron C, Richart R. Human papillomavirus DNA in fomites on objects used for the management of patients with genital human papillomavirus infections. Obstet Gynecol. 1989;74:950–4. [PubMed] [Google Scholar]
- 251.Kjaer S, van den Brule A, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. Br Med J. 2002;325:572–8. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nobbenhuis M, Walboomers J, Helmerhorst T, et al. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354:20–5. doi: 10.1016/S0140-6736(98)12490-X. [DOI] [PubMed] [Google Scholar]
- 253.Remmink A, Walboomers J, Helmerhorst T, et al. The presence of persistent high-risk HPV genotypes in dysplastic cervical lesions is associated with progressive disease: natural history up to 36 months. Int J Cancer. 1995;61:306–11. doi: 10.1002/ijc.2910610305. [DOI] [PubMed] [Google Scholar]
- 254.Liaw K, Hildesheim A, Burk R, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 255.Brisson J, Bairati I, Morin C, et al. Determinants of persistent detection of human papillomavirus DNA in the uterine cervix. J Infect Dis. 1996;173:794–9. doi: 10.1093/infdis/173.4.794. [DOI] [PubMed] [Google Scholar]
- 256.Woodman C, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–6. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 257.Ho G, Bierman R, Beardsley L, Chang C, Burk R. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 258.Moscicki A, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 259.Elfgren K, Kalantari M, Moberger B, Hagmar B, Dillner J. A population-based five-year follow-up study of cervical human papillomavirus infection. Am J Obstet Gynecol. 2000;183:561–7. doi: 10.1067/mob.2000.106749. [DOI] [PubMed] [Google Scholar]
- 260.Van Duin M, Snijders P, Schrijnemakers H, et al. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int J Cancer. 2002;98:590–5. doi: 10.1002/ijc.10232. [DOI] [PubMed] [Google Scholar]
- 261.Hildeheim A, Schiffman M, Gravitt P, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–40. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 262.Tyring S. Human papillomavirus infections: epidemiology, pathogenesis, and host immune response. J Am Acad Dermatol. 2000;43:S18–26. doi: 10.1067/mjd.2000.107807. [DOI] [PubMed] [Google Scholar]
- 263.Sellors J, Karwajtys T, Kaczorowski J, et al. Prevalence of infection with carcinogenic human papillomavirus among older women. CMAJ. 2002;167:871–3. [PMC free article] [PubMed] [Google Scholar]
- 264.Wang S, Schiffman M, Herrero R, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10,000 women in Costa Rica. Br J Cancer. 2004;91:1269–74. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Jacobs M, Walboomers J, Snijders P, et al. Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer. 2000;87:221–7. [PubMed] [Google Scholar]
- 266.Molano M, Posso H, Weiderpass E, et al. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer. 2002;87:324–33. doi: 10.1038/sj.bjc.6600442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kjaer S, van den Brule A, Bock J, et al. Determinants for genital human papillomavirus (HPV) infection in 1000 randomly chosen young Danish women with normal Pap smear: are there different risk profiles for oncogenic and nononcogenic HPV types? Cancer Epidemiol Biomarkers Prev. 1997;6:799–805. [PubMed] [Google Scholar]
- 268.Antonsson A, Erfurt C, Hazard K, et al. Prevalence and type spectrum of human papillomaviruses in healthy skin samples collected in three continents. J Gen Virol. 2003;84:1881–6. doi: 10.1099/vir.0.18836-0. [DOI] [PubMed] [Google Scholar]
- 269.Hsu J, Chen A, Keleher A, McMillan N, Antonsson A. Shared and persistent asymptomatic cutaneous human papillomavirus infections in healthy skin. J Med Virol. 2009;81:1444–9. doi: 10.1002/jmv.21529. [DOI] [PubMed] [Google Scholar]
- 270.Weissenborn S, de Koning M, Wieland U, Quint W, Pfister H. Intrafamilial transmmission of family-specific spectra of cutaneous betapapillomaviruses. J Virol. 2009;83:811–6. doi: 10.1128/JVI.01338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Berkhout R, Bouwes Bavinck J, ter Schegget J. Persistence of human papillomavirus DNA in benign and (pre)malignant skin lesions from renal transplant recipients. J Clin Microbiol. 2000;38:2087–96. doi: 10.1128/jcm.38.6.2087-2096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hazard K, Karlsson A, Andersson K, Ekberg H, Dillner J, Forslund O. Cutaneous human papillomaviruses persist on healthy skin. J Invest Dermatol. 2007;127:116–9. doi: 10.1038/sj.jid.5700570. [DOI] [PubMed] [Google Scholar]
- 273.Boxman I, Berkhout R, Mulder L, et al. Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J Invest Dermatol. 1997;108:712–5. doi: 10.1111/1523-1747.ep12292090. [DOI] [PubMed] [Google Scholar]
- 274.Boxman I, Hogewoning A, Mulder L, Bouwes Bavinck J, ter Schegget J. Detection of human papillomavirus types 6 and 11 in pubic and perianal hair from patients with genital warts. J Clin Microbiol. 1999;37:2270–3. doi: 10.1128/jcm.37.7.2270-2273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wolf P, Seidl H, Bäck B, et al. Increased prevalence of human papillomavirus in hairs plucked from patients with psoriasis treated with psoralen-UV-A. Arch Dermatol. 2004;140:317–24. doi: 10.1001/archderm.140.3.317. [DOI] [PubMed] [Google Scholar]
- 276.Boxman I, Russell A, Mulder L, Bouwes Bavinck J, ter Schegget J, Green A. Case-control study in a subtropical Australian population to assess the relation between non-melanoma skin cancer and epidermodysplasia verruciformis human papillomavirus DNA in plucked eyebrow hairs.The Nambour Skin Cancer Prevention Study Group. Int J Cancer. 2000;86:118–21. doi: 10.1002/(sici)1097-0215(20000401)86:1<118::aid-ijc18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 277.Weissenborn S, Höpfl R, Weber F, Smola H, Pfister H, Fuchs P. High prevalence of a variety of epidermodysplasia verruciformis-associated human papillomaviruses in psoriatic skin of patients treated or not treated with PUVA. J Invest Dermatol. 1999;113:122–6. doi: 10.1046/j.1523-1747.1999.00641.x. [DOI] [PubMed] [Google Scholar]
- 278.Chen A, McMillan N, Antonsson A. Human papillomavirus type spectrum in normal skin of individuals with or without a history of frequent sun exposure. J Gen Virol. 2008;89:2891–7. doi: 10.1099/vir.0.2008/003665-0. [DOI] [PubMed] [Google Scholar]
- 279.Forslund O, Iftner T, Andersson K, et al. Cutaneous human papillomaviruses found in sun-exposed skin: beta-papillomavirus species 2 predominates in squamous cell carcinoma. J Infect Dis. 2007;196:876–83. doi: 10.1086/521031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Waterboer T, Neale R, Michael K, et al. Antibody responses to 26 skin human papillomavirus types in the Netherlands, Italy and Australia. J Gen Virol. 2009;90:1986–98. doi: 10.1099/vir.0.010637-0. [DOI] [PubMed] [Google Scholar]
- 281.Barbosa M, Vass W, Lowy D, Schiller J. In vitro biological activities of E6 and E7 genes vary among human papillomaviruses of different oncogenic potential. J Virol. 1991;65:292–8. doi: 10.1128/jvi.65.1.292-298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Storey A, Osborn K, Crawford L. Co-transformation by human papillomavirus types 6 and 11. J Gen Virol. 1990;71:165–71. doi: 10.1099/0022-1317-71-1-165. [DOI] [PubMed] [Google Scholar]
- 283.Ostrow R, Bender M, Niimura M, et al. Human papillomavirus DNA in cutaneous primary and metastasized squamous cell carcinomas from patients with epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1982;79:1634–8. doi: 10.1073/pnas.79.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Harwood C, Surentheran T, McGregor J, et al. Human papillomavirus infectin and non-melanoma skin cancer immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–97. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 285.Majewski S, Jablonska S. Do epidermodysplasia verruciformis human papillomaviruses contribute to malignant and benign epidermal proliferations? Arch Dermatol. 2002;138:649–54. doi: 10.1001/archderm.138.5.649. [DOI] [PubMed] [Google Scholar]
- 286.Tommasino M, Pawlita M. Epidermodysplasia verruciformis (EV) human papillomavirus types: co-factors in skin carcinogenesis beyond EV? Papillomavirus Report. 2004;15:337–8. [Google Scholar]
- 287.Orth G. Epidermodysplasia verruciformis. In: Salzman N, Howley P, editors. The Papovaviridae: The Papillomaviruses. Vol. 2. New York: Plenum Publishing Corp; 1987. pp. 199–243. [Google Scholar]
- 288.De Jong-Tieben L, Berkhout R, Smits H, et al. High frequency of detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in biopsies from malignant and premalignant skin lesions from renal transplant recipients. J Invest Dermatol. 1995;105:367–71. doi: 10.1111/1523-1747.ep12320803. [DOI] [PubMed] [Google Scholar]
- 289.Surentheran T, Harwood C, Spink P, et al. Detection and typing of human papillomaviruses in mucosal and cutaneous biopies from immunosuppressed and immunocompetent patients and patients with epidermodysplasia verruciformis: a unified diagnostic approach. J. Clin Path. 1998;51:606–10. doi: 10.1136/jcp.51.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Birkeland S, Storm H, Lamm L, et al. Cancer risk after renal transplantation in the nordic countries, 1964–1986. Int J Cancer. 1995;60:183–9. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 291.Hartevelt M, Bavinck J, Kootte A, Vermeer B, Vanderbroucke J. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–9. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 292.Hardie I, Strong R, Hartley L, Woodruff P, Clunie G. Skin cancer in Caucasian renal allograft recipients living in a subtropical climate. Surgery. 1980;87:177–83. [PubMed] [Google Scholar]
- 293.Walder B, Robertson M, Jeremy D. Skin cancer and immunosuppression. Lancet. 1971;21:1282–3. doi: 10.1016/s0140-6736(71)90602-7. [DOI] [PubMed] [Google Scholar]
- 294.Kinlen L, Sheil A, Peto J, Doll R. Collaborative United Kingdom-Australasian study of cancer in patients treated with immunosuppressive drugs. Br Med. 1979;2:1461–6. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wu X, Nguyen B, Dziunycz P, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–72. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Salgo R, Grossman J, Schöfer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-) malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10:1385–93. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 297.Iftner A, Klug S, Garbe C, et al. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer Res. 2003;63:7515–9. [PubMed] [Google Scholar]
- 298.De Villiers E, Lavergne D, McLaren K, Benton E. Prevailing papillomavirus types in non-melanoma carcinomas of the skin in renal allograft recipients. Int J Cancer. 1997;73:356–61. doi: 10.1002/(sici)1097-0215(19971104)73:3<356::aid-ijc9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 299.Blessing K, McLaren K, Benton E, et al. Histopathology of skin lesions in renal allograft recipients—an assessment of viral features and dysplasia. Histopathology. 1989;14:129–39. doi: 10.1111/j.1365-2559.1989.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 300.Goldberg L, Griego R. Absence of human papillomavirus in squamous cell carcinomas of nongenital skin from immunocompromised renal transplant patients: a comment. Arch Dermatol. 1995;131:107–8. doi: 10.1001/archderm.131.1.107. [DOI] [PubMed] [Google Scholar]
- 301.Asgari M, Kiviat N, Critchlow C, et al. Detection of human papillomavirus DNA in cutaneous squamous cell carcinoma among immunocompetent individuals. J Invest Dermatol. 2008;128:1409–17. doi: 10.1038/sj.jid.5701227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Forslund O, Lindelöf B, Hradil E, et al. High prevalence of cutaneous human papillomavirus DNA on the top of skin tumors but not in “stripped” biopsies from the same tumors. J Invest Dermatol. 2004;123:388–94. doi: 10.1111/j.0022-202X.2004.23205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Feltkamp M, Broer R, di Summa F, et al. Seroreactivity to epidermodysplasia verruciformis-related human papillomavirus types is associated with nonmelanoma skin cancer. Cancer Res. 2003;63:2695–700. [PubMed] [Google Scholar]
- 304.Karagas M, Nelson H, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389–95. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- 305.Hall L, Struijk L, Neale R, Feltkamp M. Re: human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:1425–6. doi: 10.1093/jnci/djj379. [DOI] [PubMed] [Google Scholar]
- 306.Baseman J, Koutsky L. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32S:S16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 307.Ho G, Studentsov Y, Bierman R, Burk R. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev. 2004;13:110–6. doi: 10.1158/1055-9965.epi-03-0191. [DOI] [PubMed] [Google Scholar]
- 308.Carter J, Koutsky L, Wipf G, et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996;174:927–36. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 309.Harwood C, Surentheran T, Sasieni P, et al. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br J Dermatol. 2004;150:949–57. doi: 10.1111/j.1365-2133.2004.05847.x. [DOI] [PubMed] [Google Scholar]
- 310.Struijk L, Bouwes Bavinck J, Wanningen P, et al. Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J Invest Dermatol. 2003;121:1531–5. doi: 10.1046/j.1523-1747.2003.12632.x. [DOI] [PubMed] [Google Scholar]
- 311.Arends M, Benton E, McLaren K, Stark L, Hunter J, Bird C. Renal allograft recipients with high susceptibility to cutaneous malignancy have an increased prevalence of human papillomavirus DNA in skin tumours and a greater risk of anogenital malignancy. Br J Cancer. 1997;75:722–8. doi: 10.1038/bjc.1997.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Levi F, Randimbison L, Te V, La Vecchia C. Nonmelanomatous skin cancer following cervical, vaginal, and vulvar neoplasms: etiologic association. J Natl Cancer Inst. 1998;90:1570–1. doi: 10.1093/jnci/90.20.1570. [DOI] [PubMed] [Google Scholar]
- 313.Hemminki K, Dong C, Vaittinen P. Secondary primary cancer after in situ and invasive cervical cancer. Epidemiology. 2000;11:457–61. doi: 10.1097/00001648-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 314.Hemminki K, Jiang Y, Steineck G. Skin cancer and non-Hodgkin’s lymphoma as second malignancies: markers of impaired immune function? Eur J Cancer. 2003;39:223–9. doi: 10.1016/s0959-8049(02)00595-6. [DOI] [PubMed] [Google Scholar]
- 315.Majewski S, Jablonska S. Current views on the role of human papillomaviruses in cutaneous oncogenesis. Int J Dermatol. 2006;45:192–6. doi: 10.1111/j.1365-4632.2006.02758.x. [DOI] [PubMed] [Google Scholar]
- 316.Skinner G. Transformation of primary hamster embryo fibroblasts by type 2 simplex virus: evidence for a “hit and run” mechanism. Br J Exp Path. 1976;57:361–76. [PMC free article] [PubMed] [Google Scholar]
- 317.Weissenborn S, Nindl I, Purdie K, et al. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol. 2005;125:93–7. doi: 10.1111/j.0022-202X.2005.23733.x. [DOI] [PubMed] [Google Scholar]
- 318.Nevels M, Täuber B, Spruss T, Wolf H, Dobner T. “Hit-and Run” transformation by adenovirus oncogenes. J Virol. 2001;75:3089–94. doi: 10.1128/JVI.75.7.3089-3094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bouwes Bavinck J, Plasmeijer E, Feltkamp M. ß-Pappillomavirus infection and skin cancer. J Infect Dis. 2008;128:1355–8. doi: 10.1038/jid.2008.123. [DOI] [PubMed] [Google Scholar]
- 320.Schaper I, Marcuzzi G, Weissenborn S, et al. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005;65:1394–400. doi: 10.1158/0008-5472.CAN-04-3263. [DOI] [PubMed] [Google Scholar]
- 321.Moscicki A, Schiffman M, Kjaer S, Villa L. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24:S3/42–51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 322.Steger G, Pfister H. In vitro expressed HPV 8 E6 protein does not bind p53. Arch. Virol. 1992;125:355–60. doi: 10.1007/BF01309654. [DOI] [PubMed] [Google Scholar]
- 323.Tommasino M, Accardi R, Caldeira S, Dong W, et al. The role of TP53 in cervical carcinogenesis. Hum Mutat. 2003;21:307–12. doi: 10.1002/humu.10178. [DOI] [PubMed] [Google Scholar]
- 324.Tibbetts T, Conneely O, O’Malley B. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod. 1999;60:1158–65. doi: 10.1095/biolreprod60.5.1158. [DOI] [PubMed] [Google Scholar]
- 325.Gravitt P, Hildesheim A, Herrero R, et al. Correlates of IL-10 and IL-12 concentrations in cervical secretions. J Clin Immunol. 2003;23:175–83. doi: 10.1023/a:1023305827971. [DOI] [PubMed] [Google Scholar]
- 326.Clerici M, Shearer G, Clerici E. Cytokine dysregulation in invasive cervical carcinoma and other human neoplasias: time to consider the TH1/TH2 paradigm. J Natl Cancer Inst. 1998;90:261–3. doi: 10.1093/jnci/90.4.261. [DOI] [PubMed] [Google Scholar]
- 327.Franklin R, Kutteh W. Characterization of immunoglobulins and cytokines in human cervical mucus: influence of exogenous and endogenous hormones. J Reprod Immunol. 1999;42:93–106. doi: 10.1016/s0165-0378(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 328.Wira C, Sandoe C. Specific IgA and IgG antibodies in the secretions of the female reproductive tract: effects of immunization and estradiol on expression of this response in vivo. J Immunol. 1987;138:4159–64. [PubMed] [Google Scholar]
- 329.Moreno V, Bosch F, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–192. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 330.Moscicki A, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 331.Boardman L, Stanko C, Weitzen S, Sung C. Atypical squamous cells of undetermined significance: human papillomavirus testing in adolescents. Obstet Gynecol. 2005;105:741–6. doi: 10.1097/01.AOG.0000157126.12678.a6. [DOI] [PubMed] [Google Scholar]
- 332.Stone K, Karem K, Sternberg M, et al. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis. 2002;186:1396–402. doi: 10.1086/344354. [DOI] [PubMed] [Google Scholar]
- 333.Schneider A, Hotz M, Gissmann L. Increased prevalence of human papillomaviruses in the lower genital tract of pregnant women. Int J Cancer. 1987;40:198–201. doi: 10.1002/ijc.2910400212. [DOI] [PubMed] [Google Scholar]
- 334.Wang S, Schiffman M, Shields T, et al. Seroprevalence of human papillomavirus-16, −18, −31, and −45 in a population-based cohort of 10000 women in Costa Rica. Br J Cancer. 2003;89:1248–54. doi: 10.1038/sj.bjc.6601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Negrini B, Schiffman M, Kurman R, et al. Oral contraceptive use, human papillomavirus infection, and risk of early cytological abnormalities of the cervix. Cancer Res. 1990;50:4670–5. [PubMed] [Google Scholar]
- 336.Mitrani-Rosenbaum S, Tsviel R, Tur-Kaspa R. Oestrogen stimulates differential transcription fo human papillomavirus type 16 in Si Ha cervical carcinoma cells. J Gen Virol. 1989;70:2227–32. doi: 10.1099/0022-1317-70-8-2227. [DOI] [PubMed] [Google Scholar]
- 337.Gloss B, Bernard H, Seedorf K, Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBOJ. 1987;6:3735–43. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Pater M, Hughes G, Hyslop D, Nakshatri H, Pater A. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature. 1988;335:832–5. doi: 10.1038/335832a0. [DOI] [PubMed] [Google Scholar]
- 339.Chan W, Klock G, Bernard H. Progesterone and glucocorticoid response elements occur in the long control regions of several human papillomaviruses involved in anogenital neoplasia. J Virol. 1989;63:3261–9. doi: 10.1128/jvi.63.8.3261-3269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Arbeit J, Howley P, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci. 1996;93:2930–5. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Castellsagué X, Muñoz N. Chapter3: cofactors in human papillomavirus carcinogenesis-role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–8. [PubMed] [Google Scholar]
- 342.Naib A, Nahmias A, Josey W. Cytology and histopathology of cervical herpes simplex infection. Cancer. 1966;19:1026–31. doi: 10.1002/1097-0142(196607)19:7<1026::aid-cncr2820190718>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 343.Garris L, Steckler A, McIntire J. The relationship between oral contraceptives and adolescent sexual behavior. J Sex Res. 1976;12:135–46. doi: 10.1080/00224497609550930. [DOI] [PubMed] [Google Scholar]
- 344.Abma J, Martinez G, Mosher W, Dawson B. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat. 2004;23:1–48. [PubMed] [Google Scholar]
- 345.Herbst A, Ulfelder H, Poskanzer D. Adenocarcinoma of the vagina: association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–81. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 346.Chen J, Fleischer A, Smith E, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. 2001;27:1035–8. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 347.Housman T, Feldman S, Williford P, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–9. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 348.Wheless L, Black J, Alberg A. Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2010;19:1686–95. doi: 10.1158/1055-9965.EPI-10-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Rogers H, Weinstock M, Harris A, et al. Incidence estimates of nonmelanoma skin in the United States, 2006. Arch Dermatology. 2010;146:283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]