Abstract
Soft tissue sarcomas (STS) are a rare group of malignancies with multiple different subtypes. Close to half of intermediate or high grade STS develop metastatic disease. Treatment of recurrent/metastatic sarcomas is quite challenging with only a few drugs showing measurable benefits. Trabectedin (ecteinascidin 743, ET-743, Yondelis) is a newly developed alkylating agent that has shown significant broad spectrum potential as a single agent second line drug alone or in combination particularly in the treatment of liposarcomas and leiomyosarcomas. Clinical benefit rates seem to favor its use especially in pretreated patients with recurrent/metastatic disease. The drug is well tolerated in general but hepatotoxicity and hematologic side effects are common. Approved in Europe, the currently ongoing Phase III trials along with the already existing clinical evidence may provide enough data for the Food and Drug Administration for an approval in the US.
Keywords: trabectedin, liposarcoma, leiomyosarcoma, metastatic sarcoma, efficacy, side effects
Introduction
Soft tissue sarcomas (STS) are a rare group of diverse tumors, mostly mesenchymal in origin, and account for approximately 1% of adult malignancies. Sarcomas can develop from multiple different tissues, with fat, vessels, nerves, bones, muscle and deep skin tissues being the most common site of origin. In 2010 there were approximately 10,520 (STS) and 2,650 (bone) new cases diagnosed in the US. This group of tumors was also responsible for 3,920 (STS) and 1,460 (bone) deaths in the same year.1 They tend to occur with increasing frequency with age, but they also represent 7%–10% of childhood malignancies. They are also one of the leading causes of death in the 14–29 year age group. The number of newly diagnosed cases has been relatively constant over the last 30 years.
STS have several different subtypes. The exact number of newly diagnosed Americans with each subtype is unknown, secondary to the difficulty differentiating these tumors from each other and other types of malignancies. The overall numbers are likely underreported. Close to half of STS patients with intermediate or high grade develop metastatic disease. The majority of these patients are being treated with chemotherapy, but the 5-year survival remains only about 50%.2,3
The importance of differentiating the over 50 subtypes of sarcomas has been increasing with the discovery of new drugs and improved knowledge of molecular mechanisms.4 Genetic abnormalities in STS have also been getting increasing attention. The association of familial neurofibromatosis, NF1 gene and malignant peripheral nerve sheet tumors has been known for a while. Similarly, familial retinoblastoma has been known to be associated with inherited mutations of retinoblastoma gene.4 Therapy for different subtypes of STS is evolving and differentiating.5
Therapy of recurrent inoperable or metastatic STS is challenging. The currently used drugs and drug combinations only have marginal proven benefit. The response rate to doxorubicin is low (10%–30%) and with ifosfamide is higher, but without survival advantage.2,3 The response rate is even lower in patients who already failed the first line agents. For leiomyosarcomas (LMS), gemcitabine with or without docetaxel is used for first or second line treatment. Dacarbazine (DTIC) also has activity in both LMS and liposarcomas (LPS). Taxanes particularly in weekly administration are used for angiosarcomas. Many of these traditionally used drugs have significant and cumulative toxicities, requiring frequent dose modifications or discontinuation of treatment.2
Trabectedin is the most extensively studied drug in patients with STS failing treatment with standard anthracycline and ifosfamide.6–8 While trabectedin does not have proven benefits in all STS’s, there is reasonable evidence from Phase I and Phase II clinical trials to prove that it is a safe and effective treatment alternative with specific efficacy in myxoid LPS/round cell sarcoma and LMS. The persistently high response rate in various studies is encouraging. In a cost-effectiveness analysis trabectedin was found to be a potentially cost effective treatment of metastatic STS patients with a previous history of treatment with standard drugs.9 The calculated cost-effectiveness of trabectedin was comparable or superior to many other cancer drugs used today. In addition, trabectedin compared to end-stage treatment was estimated to result in a 14 months of additional survival and 9 to 10 months of additional quality-adjusted survival compared to end-stage treatment alone.
Trabectedin Development and Use in Metastatic Sarcomas
Mechanism of action, metabolism and pharmacokinetic profile
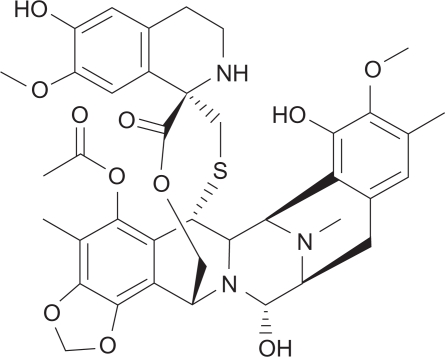
Trabectedin (ecteinascidin 743, ET-743, Yondelis) is derived from the Caribbean sea squirt Ecteinascidia turbinate (Fig. 1). Its main mode of action is as an alkylating agent against deoxyribonucleic acid (DNA). Unlike conventional alkylating agents which bind to the major groove of DNA and predominantly form crosslinks to the Guanine N7 or O6 position, trabectedin predominantly binds to the minor groove of DNA and binds to the Guanine N2 position.10 Additionally, cells deficient in transcription-coupled nucleotide excision repair, but not mismatch repair, appear much less sensitive to trabectedin than other alkylators.
Figure 1.
Structure of trabectedin: (1′R,6R,6aR,7R,13S,14S,16R)-6′,8, 14-trihydroxy-7′,9-dimethoxy-4,10,23-trimethyl-19-oxo-3′, 4′,6,7,12,13,14,16-octahydrospiro[6,16-epithiopropanooxymethano)-7,13-imino-6aH-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1′(2′H)-isoquinolin]-5-yl acetate.
Deficiencies in homologous recombination repair render the cell quite sensitive to the toxic effects of trabectedin, thus BRCA1/2 or other Fanconi gene deficiency would render the tumor cells sensitive. 11 In part, this corresponds to a direct binding interaction with DNA and Rad13 in a complex. Due to changes in DNA structure, trabectedin can inhibit the production of IL-6 and CCL2 from tumor-associated macrophages, which may also provide some of its anti-tumor effect. Also reported are more specific inhibitions of fusion proteins such as FUS-CHOP or EWS-CHOP seen in MLS and in EWS-FLI in Ewing’s. Tumor cells having p53 mutations appear more sensitive to trabectedin compared to those with wt p53.12 Preclinically, trabectedin appears additive or synergistic with doxorubicin in various sarcoma and breast lines, and with platinums in ovarian, sarcoma and other cell lines. It inhibits MDR1 transcription and thus modulates P-glycoprotein mediated resistance to other agents. Pharmacokinetics shows a mean terminal half life (T1/2) of approximately 61 hours. It is heavily protein bound, and extensively metabolized by the liver, especially P450 3A4. Systemic clearance was approximately 19% higher when dexamethasone was coadministered. The area under the curve (AUC) is somewhat higher when administered over a 24 hour infusion, although the maximum plasma concentration of the drug (Cmax) is reduced, as more drug may be delivered when adjusting for toxicity.13
Clinical studies
Trabectedin is the second marine-derived drug approved for treatment of cancer. Trabectedin has shown in vitro and in vivo activities in a variety of human malignancies: STS, breast cancer, ovarian cancer and various other solid malignancies. Although broad activity is observed across the histologic subtypes of sarcoma, activity is greatest in LPS (especially myxoid LPS) and LMS.
Single agent (phase I)
The first phase 1 studies examining the antitumor effects of trabectedin date back to early 2000. Twenty-nine pretreated for advanced STS and bone sarcoma were analyzed from a phase 1 clinical trial as well as compassionate use program.14 Every three to four-week treatment of 24 hour infusion was used in escalating doses. The most common toxicities were transaminitis, neutropenia and thrombocytopenia. Ten patients had disease stabilization, two patients had minor response and four patients had partial responses. Median duration of response was over 10 months. This study proved that trabectedin had activity in highly pretreated STS and bone sarcomas.
Another phase one study from Europe compared every-three week infusion of escalating doses of trabectedin over 1 and 3 hours.15 There were 72 adults included with a wide variety of primary tumors, including advanced or metastatic STS. The most common dose-limiting toxicities were fatigue, neutropenia and thrombocytopenia again. Hematologic and hepatic toxicities were dose dependant and not cumulative. The pharmacokinetics was linear with 3 hour infusion. Antitumor activity was evaluated in 49 patients. Most responders had a minor response (37%). Nine patients had stable disease for 6 or more cycles. The authors concluded that trabectedin can be safely administered in 1 and 3 hour iv infusions and the maximum tolerated dose (MTD) can be increased by prolonging the duration of infusion without affecting the drug’s safety profile.
A recently published phase I dose escalating trial also determined the safety, tolerability, pharmacokinetics of trabectedin when given as 1-hour or 3-hour iv infusion for 3 consecutive weeks every 4 weeks.16 Most treated patients had advanced STS with some patients being treated after establishing a diagnosis of breast/ovarian cancer and melanoma. Over 30 patients were treated in sequential cohorts of trabectedin on the 1 and 3 hour schedules. Neutropenia, transaminitis, fatigue and transient creatinine phosphokinase elevation limited the MTD to 0.61 mg/m2 in the 1 hour schedule and to 0.58 mg/m2 in the 3 hour schedule. These respective MTD’s were recommended for future clinical trials. Several patients had prolonged, stable disease.
Combination therapy (phase I)
Trabectedin was tested in combination trials based on in vitro studies showing synergy with doxorubicin and cisplatin in STS cells. A phase I study enrolled 41 previously treated patients in an open-label multicenter trial.17 Granulocyte colony stimulating factor (G-CSF) was added to the regimen after the first six patients were noted to have dose limiting neutropenia. Half of the patients had LPS. The maximum tolerated dose for trabectedin was 1.1 mg/m2 and for doxorubicin 60 mg/m2. Grade 3/4 treatment related toxicity was quite high at 71% combined. Median progression free survival was 9.2 months with 33 patients maintaining stable disease.
Gemcitabine is a nucleoside analog with a wide variety of treatment indications: pancreas cancer, lung cancer, breast cancer and certain sarcomas. A phase I study enrolled 15 pre-treated patients who were treated with weekly trabectedin and gemcitabine between 2003 and 2005.18 The majority of patients had sarcoma or lung cancer. The major side effect was hepatotoxicity resulting in frequent dose adjustments. Fatigue, nausea, vomiting neutropenia and anorexia were also common (27%–73%). Half of the patient maintained stable disease for 56–226 days. In conclusion the study found a potentially manageable toxicity profile with a lower dose of trabectedin compared to single agent usage.
The few studies looking at the safety of trabectedin, in combination with other drugs, found that a lower dose of trabectedin is needed in combination to maintain a manageable safety profile.
Safety profile of single agent
Demetri et al examined the safety of trabectedin in a phase II clinical trial.19 Most drug related side effects were grade 2. Only five percent of patients required hospitalization secondary to drug related side effects. Ninety-two percent of patients received trabectedin according to protocol. The most common grade 3/4 drug related side effects were fatigue, nausea and vomiting. Neutropenia and thrombocytopenia were the most common grade 3/4 hematologic side effects, but they were transient with rapid recovery. Liver related grade 3/4 toxicities were mostly limited to transient elevation of AST and ALT with bilirubin being affected uncommonly. Death attributable to the drug was rather uncommon (3%).
A non-randomized phase II study by the European Organization for the Research and Treatment of Cancer (EORTC) analyzed side effect profile of trabectedin in 99 eligible patients.20 Dose reduction was necessary at least once in 31% of patients mostly secondary to nonhematologic toxicities. Toxicities were mainly hematologic and hepatic. Grade 3 or 4 neutropenia and thrombocytopenia were the most common (53%/18%). Sixteen percent of patients experienced grade 3 to 4 anemia. Non-cumulative liver toxicities were grade 3/4 transient elevation of transaminases seen in 35%–45% of patients. Besides, 42% and 63% had elevation of bilirubin and alkaline phosphatase. The treatment related mortality was 4%. There was a correlation between liver dysfunction and the toxicities leading to death. Elevated creatinine, diarrhea and vomiting occurred in 9%–21% of treated individuals, while fatigue was a more common complaint (62%).
A non-randomized multicenter Phase II study from France examined the effects of trabectedin in 54 preterated patients with advanced STS.21 Most patients had LMS (41%). A 24-hr iv infusion every 3 weeks was used. Patients received a median of 3 cycles of trabectedin. Reversible grade 3/4 transaminitis occurred in 50% of patients and grade 3/4 neutropenia occurred in 61% of patients. The study also reported two treatment related death.
A prospective phase II study from primarily US institutions enrolled 36 pretreated patients to be treated with a 24 hour continuous infusion at a dose of 1500 ugm/m2 every three weeks.22 The predominant toxicities were neutropenia and self limited transaminitis. A total of 14% of the cycles had to be dose reduced, mostly for the above mentioned reasons. Thirty-five percent of the cycles had to be delayed for similar reasons. The frequency of Grade 3/4 hematologic toxicities were leucopenia (43%) and neutropenia (34%). Severe thrombocytopenia occurred in 17% of the patients. Severe transaminitis was seen with grade 3/4 elevations in 20%–26% (AST/ALT). Fatigue was common (69%) and nausea (15%) vomiting (6%) responded well to routine dexamethasone.
A similarly designed study but in chemo-therapy naïve patients found that dose reductions were necessary in 31% of the proposed trabectedin cycles (hematologic toxicities in 16%, alkaline phosphate elevation in 57% and bilirubin elevation in 27%). Grade 3 or 4 leukopenia occurred in 22% and 33% of the patients. Grade 3 and 4 AST and ALT elevations were also frequent (34 and 36%).23
Interestingly, in a small Italian series of 32 patients no severe adverse events were documented with a median duration of treatment of 10 months.24
Trabectedin is clinically well tolerated overall. Fatigue seems to be the most common subjective complaint. Nausea, vomiting and diarrhea are also possible, but less frequent when compared to other drugs used in the treatment of STS. Most myelosuppressive events are limited, with only a few patients developing severe neutropenia and thrombocytopenia. One of the most common laboratory abnormalities is transaminitis. This is usually self-limiting especially when treated with dose-reductions. Clinically significant liver toxicities are uncommon. There is preclinical and clinical data for the benefits of pretreatment with dexamethasone, which likely via its anti-inflammatory effects further decreases potential liver toxicities. The much less frequently reported rhabdomyolysis requires monitoring of serum creatine phosphokinase.
Dexamethasone (typically 20 mg IV) is now required as premedication for trabectedin cycles, with an observed decrease in hepatotoxicity. Whether this is simply due to an increased pharmacologic clearance of the drug and therefore a lower effective exposure, or whether there is some more liver specific mechanism is unknown.
Efficacy
Trabectedin’s efficacy as a chemotherapeutic agent has been examined in randomized multicenter phase II studies (Table 1). An open-label phase II trial randomized 270 patients with pretreated LPS or LMS after standard therapy failure.19 This trial used time to progression as a primary end-point. Patients were randomly assigned to 1.5 mg/m2 IV over 24 hours q3 week versus 0.58 mg/m2 IV over 3 hours each week 3 of 4 weeks. Cross-over after disease progression was allowed. A total of 206 progression events comprised the protocol-specified primary efficacy analysis, in which median time to progression was 3.7 months versus 2.3 months clearly favoring the q3 weeks 24 hr arm. Time to progression with trabectedin versus last cycle of prior chemotherapy was at least 33% longer for trabectedin than prior chemo therapy favoring the q3 weeks 24 hour arm (36.7% versus 31.2%). Median progression-free survival was 3.3 months versus 2.3 months and median overall survival was 13.0 months versus 11.8 months. Both of these latter analyses favored the q3 week 24-hr arm.
Table 1.
Summary of prominent phase II clinical trials with trabectedin.
| # Pts | Dose schedule | Response rate; PR + CR | Median PFS (mos) | Median OS (mos) | Death | |
|---|---|---|---|---|---|---|
| Demetri et al19 two arm-randomized trial | 136 | 3 weeks 24 hr infusion |
5.6%* | 3.3 | 13.9 | 3.1% |
| 134 | 1 week 3 hr infusion |
2.3 | 11.8 | 2.3% | ||
| Garcia-Carbonero et al**,22 | 36 | 3 weeks 24 hr infusion |
8% | 1.7 | 12.1 | None |
| Garcia-Carbonero et al23 | 35 | 3 weeks 24 hr infusion |
17% | 1.6 | 15.8 | None |
| Le Cesne et al20 | 104 | 3 weeks 24 hr infusion |
8.1% | 3.5 | 9.2 | 4% |
| Yovine et al21 | 54 | 3 weeks 24 hr infusion |
4% | 1.9 | 12.8 | 4% |
Notes:
RECIST criteria;
Chemotherapy-naïve patients only.
Another EORTC study examined 99 assessable patients form eight European institutions in a phase II study.20 All 99 patients received at least one cycle of trabectedin and 30% of patients received at least six cycles of chemotherapy. Amongst the 99 treated patients there were eight partial responses, 45 patients with no change and 39 patients with progressive disease. Survival curves were generated based on an intention to treat analysis. The one year progression-free survival was 17% and overall survival was 42%. Six percent underwent surgical resection after preoperative treatment and were considered free of disease. The outcome was similar in patients with previous treatment responsive or resistant tumors. This study also analyzed response in various histologic subtypes of STS’s. Partial response or no change was seen in 56% of LMS (24/43), 61% of synovial sarcomas (11/18), 83% of malignant fibrous histiocytomas (5/6), and in 40% of LPS (4/10).
A multicenter study from French institutions reported treatment results of 52 patients who received trabectedin as a second line agent for advanced STS.21 Most patients had LMS (41%) and 24-hour continuous iv infusion every 3 weeks was used. There were two patients with partial response, four with minor response and nine with stable disease for at least 6 months. The median survival was 12.8 months with a 2-year overall survival of 30%.
A phase II pharmacokinetic study enrolling 36 previously pretreated patients with STS was performed using single agent trabectedin as a 24-hour continuous iv infusion every 3 weeks.22 Restaging was performed following every two cycles. The most frequent histological subtypes were LMS, LPS and synovial cell sarcoma with lung being the most frequent site of metastatic disease. The majority of patients (77%) had bulky disease having at least one lesion measuring 5 cm or more in longest diameter. In terms of response, three patients had major objective response; one complete and two partial. The median time to progression and overall survival were 1.7 months and 12.1 months.
Another phase II study examined the effects of trabectedin in 36 chemotherapy-naïve patients.23 The drug was administered as a 24 hr continuous infusion every 3 weeks. The predominant histologic subtypes were LMS and LPS again. The overall response rate was 17% with one complete, five partial responses. The 1-year progression-free and overall survival rates were 21% and 72%. The authors concluded the trabectedin has promising activity as a first line agent with acceptable toxicity.
A recently published French study looked at the efficacy of trabectedin in 92 patients with histologically proven, unresectable advanced, or metastatic STS treated in a single institution.25 Half of the patients were treated in phase II studies, the other half on compassionate basis. Thirty-two percent of patients had LMS or LPS, the rest of the patients had unclassified, osteosarcoma Ewing’s sarcoma or other histologic subtypes. The objective response rate was 10%. There were no complete responders but overall 47% had clinical benefit from treatment. The median progression-free survival and overall survivals were 2.2 months and 8.2 months. While initially a higher efficacy was observed in phase II studies compared to compassionate use program, no significant difference remained after adjustment for performance status. The authors concluded that patients with better performance status derive more benefit from treatment with trabectedin.
A small retrospective study from Italy examined the long-term effects of trabectedin in myxoid LPS in 32 patients.24 Trabectedin was given as a 24-h continuous infusion every 21 days with a total of 376 cycles administered (median of 12/patient). All patients had previous treatment: 15 had chemotherapy, seven had surgery, one had radiation and nine had surgery with radiation and/or chemotherapy. There were 2 complete responders, 14 patients partial response and an objective response rate of 50% was reported by the RECIST criteria. With a median follow-up of 24 months, the progression free survival was 17 months. Ten patients stopped therapy after a median of ten months secondary to absence of evident disease (seven had surgery).
Another retrospective study mostly from European institutions also examined the efficacy of trabectedin.26 Fifty-one patients with advanced pretreated myxoid LPS treated with trabectedin as a second line agent, were analyzed. With a median follow-up of 14 months two patients had complete response and 24 patients had partial response bringing the overall response rate to 51%. The median progression free survival was 14 months. Based on these encouraging results two prospective studies were initiated to assess the role of trabectedin in this subgroup of translocation related sarcoma.
A European multi-institutional study assessed the effects of trabectedin treatment in patients with advanced gastrointestinal stromal tumors (GIST). Twenty-eight patients with a median performance status of 0 were enrolled. No objective response was seen. One-third of the patients had stable disease. Trabectedin appears to be an ineffective drug for GIST.27
Between 2005 and 2009, 1404 patients with advanced STS were enrolled in an Expanded Access Program using trabectedin at 1.5 mg/m2/24 h infusion q3 weeks.28 LMS (35%) and LPS (15%) were the most common subgroups. Most had prior treatment with anthracycline and ifosfamide (median 3 lines of therapy). More than 6 cycles were given to 30%, and median dose intensity was 1.3 mg/m2/cycle. Most common reasons for dose adjustments included dose delay for neutropenia, and dose decrease for transaminitis or increased alkaline phosphatase. Severe elevation of CPK was less common. Of 504 evaluable patients, 8% had complete or partial response, 33% had stable disease, and progression occurred in 45% of patients.
Patient preference
In phase I studies trabectedin showed cytotoxic potential in patients with advanced STS, breast cancer, ovarian cancer and other types of solid tumors. Trials have reported its use as a single agent or in conjunction with another agent, such as doxorubicin, docetaxel, gemcitobine, carboplatin. The currently available preclinical and clinical evidence suggests, that trabectedin is best used as a second line agent in metastatic/recurrent LPS and LMS with favorable response rates compared to traditionally used drugs.
Since trabectedin has consistently shown hepatoxicity and hematologic side effects in various studies, patients with alcohol abuse, underlying liver disease/elevated bilirubin or hematologic abnormalities may not be the best candidates for this drug. The pharmacokinetics of trabectedin have not been studied in patients with hepatic impairment. On the other hand, renal impairment does not seem to be a contraindication to its use in patients with underlying mild to moderate renal disease.6 There is also some published evidence for its use in elderly patients with compromised performance status without significant additional risks.6 On the other hand, a retrospective review from France found 0–1 performance status patients benefiting the most form treatment with trabectedin.25
There is some data for trabectedin being used in the neoadjuvant setting in locally advanced, nonmetastatic patients with favorable response rates compared to standard chemotherapeutic agents traditionally used in the same setting.29
Place in therapy: Food and Drug Administration (FDA) and European Status
A phase III trial of pegylated liposomal doxorubicin (PLD) with or without trabectedin was performed in 672 women with recurrent ovarian cancer.30 The combination was superior for median progression free survival (7.3 vs. 5.8 months, HR 0.79, P = 0.017), for response rate (27.6% vs. 18.8%, P = 0.008), but not for overall survival (20.5 vs. 19.4 months, HR 0.95, P = 0.15). Platinum-sensitive patients appeared to derive greater benefit. The combination had more neutropenia, transient transaminitis, but less hand foot syndrome (HFS) and mucositis. This trial led to European accelerated approval in cisplatin-sensitive ovarian cancer.
Trabectedin is approved in the European Union and some other countries as a second-line treatment for advanced STS based on the clinical benefit rates documented in phase II trials. The FDA, however, recently disapproved accelerated approval, because of hepatotoxicity and a lack of documented survival advantage. With phase III studies on the way, the current evidence for its efficacy may provide enough data for the FDA for an approval in the treatment of STS in previously pretreated patients.
Current randomized trials of trabectedin in sarcomas are listed in the Table 2. Each has a unique design. The Spanish trial (phase II) is examining the value of adding trabectedin to doxorubicin in first line metastatic treatment. The EORTC is testing the comparison of doxorubicin versus trabectedin in the same population. Two trials sponsored by Pharma Mar (Tres Cantos, Madrid, Spain) include a doxorubicin-containing regimen versus trabectedin in translocation-related sarcomas and a comparison to dacarbazine in LMS and LPS. This latter study, the design of which is still under negotiation at the FDA, will look at OS as the primary endpoint and therefore no crossover to trabectedin will be allowed.
Table 2.
Current randomized trials of trabectedin in sarcomas.*
| Phase | Arms | Setting | Sponsor | NCT # | Endpoint |
|---|---|---|---|---|---|
| II | Doxorubicin ± trabectedin | 1st line STS | Spain | 01104298 | PFS |
| II/III | Doxorubicin vs. trabectedin | 1st line STS | EORTC | 01189253 | PFS |
| III | Doxorubicin ± IFF vs. trabectedin | TRS** | J and J | 00796120 | PFS |
| III | DTIC vs. trabectedin | LMS/LPS | J and J | Pending | OS |
Conclusions
Various trabectedin regimens demonstrated significant antitumor activity in patients with LPS or LMS after failure of treatment with currently available approved (anthracyclines and ifosfamides) and nonapproved drugs (gemcitabine, docetaxel). In addition, the overall survival rates of over 10 months with trabectedin therapy are also significantly better than the published results of 6 months with standard therapy for advanced/metastatic STS. In general, trabectedin is well tolerated with comparable toxicity profile to standard treatments. Transient, noncumulative hepatic toxicity is usually self-limiting and can be pretreated with steroids. Trabectedin can be safely used in patients with STS especially in LPS and LMS with improved progression free and overall survival. Trabectedin is one the most promising cytotoxic agents tested in the last two decades. This drug is becoming an important therapeutic option for patients who exhausted anthracycline/ifosfamide treatments with progression of their disease. Trabectedin also has the potential for becoming a front-line treatment for patients with advanced/metastatic STS.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.American Cancer Society. 2010. www.cancer.org. Cancer Facts and Figures. [Google Scholar]
- 2.Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;13:1–15. doi: 10.1155/2010/506182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueredo A, Bramwell VHC, Bell R, et al. Adjuvant chemotherapy following complete resection of soft tissue sarcoma in adults: a clinical practice guideline. Sarcoma. 2002;5:5–18. doi: 10.1080/13577140220127512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd R, Lunec J. Molecular pathology and potential therapeutic targets in soft-tissue sarcoma. Expert Rev Anticancer Ther. 2008;8(6):939–48. doi: 10.1586/14737140.8.6.939. [DOI] [PubMed] [Google Scholar]
- 5.Ganjoo KN. New developments in targeted therapy for soft tissue sarcoma. Curr Oncol Rep. 2010;12:261–5. doi: 10.1007/s11912-010-0107-2. [DOI] [PubMed] [Google Scholar]
- 6.Carter NJ, Keam SJ. Trabectedin, a review of its use in soft tissue sarcoma and ovarian cancer. Drugs. 2010;70(3):355–76. doi: 10.2165/11202860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Thornton KA. Trabectedin: the evidence for its place in therapy in the treatment of soft tissue sarcoma. Core Evidence. 2009;4:191–8. doi: 10.2147/ce.s5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casali PG, Sanfilippo R, D’Incalci M. Trabectedin therapy for sarcomas. Curr Opin Oncol. 2010;22:342–6. doi: 10.1097/CCO.0b013e32833aaac1. [DOI] [PubMed] [Google Scholar]
- 9.Soini EJO, Garcia San Andres B, Joensuu T, et al. Trabectedin in the treatment of metastatic soft tissue sarcoma: cost-effectiveness, cost-utility and value of information. Ann Oncol. 2011;22(1):215–23. doi: 10.1093/annonc/mdq339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther. 2010;9:2157–63. doi: 10.1158/1535-7163.MCT-10-0263. [DOI] [PubMed] [Google Scholar]
- 11.Soares DG, Escargueil AE, Poindessous V, et al. Replication and homologous recombination repair regulate DNA double-strand break formation by the antitumor alkylator ecteinascidin 743. PNAS. 2007;104:13062–7. doi: 10.1073/pnas.0609877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moneo V, Serelde BG, Fominaya J, et al. Extreme sensitivity to Yondelis (Trabectedin, ET-743) in low passaged sarcoma cell lines correlates with mutated p53. J Cell Biochem. 2007;100:339–48. doi: 10.1002/jcb.21073. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Ruixo JJ, Zannikos P, Hirankarn S, et al. Population pharmacokinetic meta-analysis of trabectedin (ET-743, Yondelis) in cancer patients. Clin Pharmakokinet. 2007;46:867–84. doi: 10.2165/00003088-200746100-00005. [DOI] [PubMed] [Google Scholar]
- 14.Delaloge S, Yovine A, Taamma A. Ecteinascidin-743: a marine-derived compound in advanced, pretreated sarcoma patients-preliminary evidence of activity. J Clin Oncol. 2001;19(5):1248–55. doi: 10.1200/JCO.2001.19.5.1248. [DOI] [PubMed] [Google Scholar]
- 15.Twelves C, Hoekman K, Bowman A, et al. Phase 1 and pharmacokinetic study of Yondelis (ecteinascidin-743; ET-743) administered as an infusion over 1 h or 3 h every 21 days in patients with solid tumours. Eur J Cancer. 2003;39:1842–51. doi: 10.1016/s0959-8049(03)00458-1. [DOI] [PubMed] [Google Scholar]
- 16.Forouzesh B, Hidalgo M, Chu Q, et al. Phase 1 and pharmacokinetic study of trabectedin as a 1- or 3-hour infusion weekly in patients with advanced solid malignancies. Clin Cancer Res. 2009;15(10):3591–9. doi: 10.1158/1078-0432.CCR-08-2889. [DOI] [PubMed] [Google Scholar]
- 17.Baly JY, Mehren M, Samuels BL, et al. Phase 1 combination study of trabectedin and doxorubicin in patients with soft-tissue sarcoma. Clin Cancer Res. 2008;14(20):6656–62. doi: 10.1158/1078-0432.CCR-08-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messersmith WA, Jimeno A, Ettinger D, et al. Phase 1 trial of weekly trabectedin (ET-743) and gemcitabine in patients with advanced solid tumors. Caner Chemother Pharmacol. 2008;63:181–8. doi: 10.1007/s00280-008-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demetri GD, Chawla SP, Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase 2 study of two different schedules. J Clin Oncol. 2009;27(25):4188–96. doi: 10.1200/JCO.2008.21.0088. [DOI] [PubMed] [Google Scholar]
- 20.Le Cesne A, Blay JY, Judson I, et al. Phase 2 study of ET-743 in advanced soft tissue sarcomas: a European Organization for Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23(3):576–84. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 21.Yovine A, Riofrio M, Blay JY, et al. Phase 2 study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–9. doi: 10.1200/JCO.2004.05.210. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol. 2004;22(8):1480–90. doi: 10.1200/JCO.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Carbonero R, Supko JG, Maki RG, et al. Ectenascidin-743 (ET-743) for chemotherapy-naïve patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol. 2005;23(24):5484–92. doi: 10.1200/JCO.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Grosso F, Sanfilippo R, Virdis E, et al. Trabectedin in myxoid liposarcomas (MLS): a long-term analysis of a single-institution series. Ann of Oncol. 2009;20:1439–44. doi: 10.1093/annonc/mdp004. [DOI] [PubMed] [Google Scholar]
- 25.Fayette J, Boyle H, Chabaud S, et al. Efficacy of trabectedin for advanced sarcomas in clinical trials versus compassionate use programs: analysis of 92 patients treated in a single institution. Anti-Cancer Drugs. 2010;21(1):113–9. doi: 10.1097/CAD.0b013e328333057b. [DOI] [PubMed] [Google Scholar]
- 26.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-732) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 27.Blay JY, Le Cesne A, Verweij J, et al. A phase II study of ET-743/trabectedin (Yondelis) for patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2004;40:1327–31. doi: 10.1016/j.ejca.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Samuels BL, Tap WD, Patel S, et al. Trabectedin (TR) as single agent for advanced soft tissue sarcomas (STS) failing standard of care: interim analysis of 1,400 patients in an expanded access program study. Proc ASCO. 2010;28:704s. (A-10027) [Google Scholar]
- 29.Gronchi A, Palmerini E, Demetri G, et al. A phase II clinical trial of neoadjuvant trabectedin in patients with non metastatic advanced myxoid/round cell liposarcoma (MRCL) Eur J Cancer Suppl. 2009;7(2):590. [Google Scholar]
- 30.Monk BJ, Herzog TJ, Kaye SB, et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28:3107–14. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]