Abstract
As a group, older adults report positive affective lives. The extent to which there are subgroups of older adults whose moods are less positive, however, is unclear. The aim of the present study was to identify and characterize different subgroups of adults who exhibit distinct trajectories of mood-change across a relatively short time period. Seventy-nine young and 103 older adults continuously reported their moods while viewing emotional and neutral faces. Cluster analysis revealed four subgroups of mood-change trajectories. Both the most positive and the most negative subgroups included more older than younger adults (ps < .05), suggesting that not all older adults exhibit higher positive affect than young adults. Analyses of variance revealed that the most negative group exhibited slower processing speed, more state anxiety and neuroticism, and looked less at happy faces, than the other groups (ps < .05). The results are discussed from an adult developmental perspective, focusing on the increased variability of mood trajectories in the older adults and whether this is a reflection of adaptive functioning, or a potential harbinger of dysfunction.
Keywords: aging, mood-change, gaze, individual differences
We all strive to maximize positive affect in our lives, whether seeking out immediate pleasure or postponing such pleasure in service of achieving a long-term goal (Larsen, 2000; Tamir, 2009). Some people may be better at maximizing positive affect than others. Maintaining a certain level of positive affect is important for both mental (e.g., Gross & Munoz, 1995) and physical (Sapolsky, 2007) health. Interestingly, life satisfaction remains stable with age (Diener & Suh, 1998a) and increasing age is related to high positive affect and low negative affect when functional health limitations are controlled (Kunzmann, Little, & Smith, 2000). These findings are further qualified by new evidence that older adults report experiencing higher low arousal positive affect and lower negative affect (both low and high arousal) than young adults (Kessler & Staudinger, 2009). This phenomenon of maintained or increased affective well-being with age is surprising because older adulthood is perceived as a time of relatively greater losses than gains (Heckhausen, Dixon, & Baltes, 1989; Lineweaver & Hertzog, 1998). Together, these findings have raised interest in how older adults are able to maintain such positive affective lives given all of the losses associated with older adulthood.
Many studies have investigated whether this age-related improvement in positive affect is a result of age-related motivational shifts (e.g., socioemotional selectivity theory; Carstensen, 1995) or increased experience in maintaining a positive mood (Scheibe & Blanchard-Fields, 2009). A question less often asked in the literature is whether all older adults are exhibiting these positive affective profiles, and if not, what distinguishes older adults with positive affective profiles from those who do not exhibit such profiles? That is, another way to investigate how older adults are able to maintain positive affective lives is to compare older adults who do show high levels of positive affect to those who do not. Characteristics (such as level of cognitive and attentional functioning, personality, and emotion regulation strategy use) which differ between older adults showing more or less positive affective profiles may suggest necessary and/or sufficient characteristics for maintaining a positive affective life into old age. By identifying a distinctive profile of characteristics associated with sustained positive mood maintenance, we may be able to better understand how older adults, as a group, are able to maintain positive affective lives despite the age-related losses they are experiencing.
Before turning to our investigation of these specific factors in the present study, we first briefly review the relevant literature regarding losses in later adulthood, and current explanations for maintained subjective well-being despite these losses. We consider limitations of past approaches to studying age differences in affective experience. Finally, we describe why a person-centered combined with a variable-centered approach -- rather than only the standard variable-centered approach -- might be particularly useful in elucidating the individual characteristics that undergird age and individual differences in affective well-being.
Older Adulthood: More Losses than Gains
Older adulthood is characterized by relatively more losses than gains (Baltes, 1997; Baltes & Baltes, 1990; Heckhausen et al., 1989). For example, cognitive aging researchers have documented that memory performance, especially for episodic and working memory, declines with age (Bäckman, Small, & Wahlin, 2001). Overall, cognitive declines associated with aging have been ascribed to older adults’ slower processing speed (Salthouse, 1996), reductions in the ability to inhibit irrelevant information (Zacks, Hasher, & Li, 2000), and fewer attentional resources (Craik, 1983, 1986). In addition to these cognitive changes, visual and auditory sensory functioning exhibit age-related declines (Schneider & Pichora-Fuller, 2000), as well as physical health and functioning (e.g., Shephard, 1999). These cognitive, sensory, and physical declines are coupled with increasing social losses such as the deaths of important social partners (Lynch & George, 2002).
Maintenance of Subjective Well-Being in Later Adulthood
Despite all of the losses associated with increasing age, subjective well-being is largely maintained in older adulthood (particularly before the 8th decade; Palmore & Cleveland, 1976), a phenomenon sometimes referred to as the “paradox of well-being” (Baltes & Baltes, 1990; Brandtstädter & Greve, 1994; Diener & Suh, 1998b). How are older adults able to maintain subjective well-being levels comparable to that of young adults in the face of these losses, as evidenced in both longitudinal and cross-sectional studies (Barrick, Hutchinson, & Deckers, 1989; Gross, et al., 1997; Kunzmann et al., 2000; Stacey & Gatz, 1991)?
One focus of research in this area has examined whether there are age differences in the three components of subjective well-being, which may help to describe the nature of well-being and how it changes across the lifespan. Life satisfaction appears to remain fairly stable across the lifespan (Diener et al., 1999), although longitudinal evidence suggests a peak in life satisfaction at age 65 and a gradual decline thereafter (Mroczek & Spiro, 2005). Positive affect has been found to remain fairly stable across the lifespan (until very old age where there is a slight decline; (Stacey & Gatz, 1991) in longitudinal (Charles, Reynolds, & Gatz, 2001) and cross-sectional studies (Barrick et al., 1989), and sometimes even shows increases with age in cross-sectional studies (Kessler & Staudinger, 2009). Longitudinal and cross-sectional research suggests that negative affect decreases across the adult lifespan (Barrick et al., 1989; Charles et al., 2001; Stacey & Gatz, 1991). The presence, intensity, and frequency of positive and negative affect experienced by older adults are important given the emerging evidence that 1) older adults may have a heightened reactivity to stressors, compared to young adults (Mroczek & Almeida, 2004), and 2) positive affect may serve as an antidote that helps buffer the individual against the negative physiological effects of daily stressors (Ong, Bergeman, Bisconti, & Wallace, 2006).
Thus, older adults may need to adapt to the greater toll that stressors take on their physiology (Blanchard-Fields, 2007; Consedine, Magai, & Bonanno, 2002), perhaps by maximizing their positive emotions. But how are older adults able to achieve this adaptation? It is likely that many factors contribute to the surprisingly positive affective lives of older adults, including changes in goals, greater experience, and honed emotion regulatory skills. Each of these theoretical accounts is briefly discussed below.
One explanation offered in the literature for age-related maintenance and/or improvement of positive affect is that later adulthood is associated with a shift in goals. As adults perceive their time as more limited (e.g., with increasing age), they are motivated to prioritize emotionally gratifying experiences (Carstensen, 1995; Carstensen, Isaacowitz, & Charles, 1999). This suggests that young adults might be willing to endure unpleasant emotions in service of achieving a long-term goal, while older adults might be less likely than young to foresee achieving long-term goals in their relatively limited future, and thus less willing to endure negative emotions in the service of future achievements (Carstensen, 1995; Lang & Carstensen, 2002; Tamir, 2009). A number of studies have found that older adults, compared to young adults, do preferentially attend to (Isaacowitz, Wadlinger, Goren, & Wilson, 2006a) and remember (Charles, Mather, & Carstensen, 2003; Kennedy, Mather, & Carstensen, 2004) positive stimuli over negative stimuli, thereby exhibiting positivity effects (Carstensen & Mikels, 2005) in their information processing. Thus, older adults may be motivated to devote what resources they do have to the regulation of emotional experience. It is important to note that some studies have found that a certain level of cognitive functioning, in particular executive control, must be met in order for positivity effects to emerge (Knight, et al., 2007; Mather & Knight, 2005), although these differences are not seen under all manipulations of cognitive load (Allard & Isaacowitz, 2008). Thus, an age-related shift in goals can explain why older adults might be more motivated than young adults to maintain a positive mood, but does not fully address how they are able to do it. Older adults may be able to maximize positive affect because this shift toward maximizing positive affect becomes a chronic goal such that its activation and implementation is less and less resource-demanding over time (Bargh & Ferguson, 2000). That is, because maximizing positive affect is a chronic goal in older adulthood, older adults become so practiced at it that it no longer requires as many resources; it becomes somewhat automatized. This may also vary by individual differences in personality; for example, perhaps individuals high in neuroticism are less motivated to maximize positive affect than individuals low in neuroticism (Murray, Allen, & Trinder, 2002).
One specific way in which individuals can maximize the positivity in their lives is through managing their emotions, or emotion regulation. Emotion regulation is considered any process that influences which emotion is experienced (e.g., anger vs. sadness), when it is experienced, and how we experience and express that emotion (Gross, 1998). Several studies suggest that we become more adept at regulating our emotions with age: older adults report having greater control over their emotions (Gross et al., 1997; Lawton, Kleban, Rajagopal, & Dean, 1992) and experience fewer negative emotions (Blanchard-Fields & Coats, 2008; Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Charles et al., 2001; Gross et al., 1997) than their younger counterparts, even when differential exposure to negative experiences is taken into account (Birditt, Fingerman, & Almeida, 2005).
Altogether, there are several plausible accounts for both why (shift in motivational goals) and how (greater experience) older adults overcome the losses associated with later adulthood to optimize their emotional experience. These accounts are not mutually-exclusive and some combination of such accounts is likely at play. In the present study, we used a unique approach to try to better understand the nature and ubiquity of these positive age-related effects.
Utility of a Subgroup Approach
Although many studies suggest that older adults report more positive affective profiles than their younger counterparts, most of these studies have examined this phenomenon using a limited set of paradigms, such that the heterogeneity of older adults’ affective profiles has not been investigated. Previous studies on mood and mood regulation in older adults (Barrick et al., 1989; Birditt & Fingerman, 2003; Blanchard-Fields & Coats, 2008; Carstensen et al., 2000; Charles et al., 2001; Kunzmann et al., 2000) have focused either on one report of mood in a moment or a one-time retrospective assessment of mood averaged across a year or day (even experience sampling studies do this, just over lots of moments), but a full understanding of affect requires an examination of the dynamics of affect (Larsen, Augustine, & Prizmic, 2009). For example, research on the buffering properties of positive affect highlight the need to examine the temporal dynamics of mood; individuals high in trait resilience recovered more quickly from a stressor than those low in trait resilience (Ong, Bergeman et al., 2006; Ong, Bergeman, & Boker, 2009). Timing has also been an important factor in identifying an attentional bias in trait anxious individuals for threatening material. By investigating different delays between cue and target presentation, researchers were able to determine that the bias for threatening material appears earlier, rather than later (Derryberry & Reed, 2002). Thus, emotional processes are necessarily dynamic in nature, suggesting that not only are the magnitudes of positive and negative affect important to consider, but also how these moods unfold over time. Any examination of the potential variability of affective experience across adulthood would therefore need to consider the unfolding of such experience across time.
To our knowledge, no study in the adult development literature has used a person-centered approach (Gerstorf, Smith, & Baltes, 2006; Hertzog & Nesselroade, 2003; Magnusson, 2003) to identify subgroups of mood dynamics in real time. Such an approach permits the investigation of relationships between stable and situation-specific affect, strategy use (such as gaze), cognitive functioning, attentional ability, and demographic variables as a function of mood-change subgroup. The advantage of combining a person-centered approach with the standard variable-oriented approach is that the person-centered analyses are more likely to uncover higher-order and nonlinear patterns in the data (Magnusson, 2003), -- in our case, “profiles” of mood ratings across time. Increased interindividual variability within older age groups in a number of domains, such as health (Dannefer, 1988; Morse, 1993), suggest there may also be heterogeneity in mood change profiles amongst older adults that may or may not align with those of young adults.
One way to investigate mood and how it unfolds over time is to simultaneously and continuously record mood while participants are completing a task in which they might be able to regulate their mood, so that a temporal link between mood change and behavior can be observed. In the present study, we aimed to do just that: participants’ eyes were tracked while viewing emotional images, providing a behavioral measure of gaze, or attention deployment, as one strategy to change one’s mood. At the same time, participants continuously rated their mood during the slideshow of emotional images, providing a measure of mood change. By identifying distinct subgroups (profiles) of mood change across a short time period and then examining the characteristics associated with each group (both strategy use as well as person-level variables), we were able to investigate whether certain patterns of mood change over time are associated with a specific profile of characteristics: such profiles might suggest specific barriers to, and/or pre-requisites (e.g., certain cognitive resources) for, maintaining positive affective lives. Identifying the characteristics of adults who do and who do not exhibit positive affective profiles will broaden our understanding of individuals who are the most likely to suffer in the face of stressors such as bereavement (Ong, Bergeman et al., 2006; Ong et al., 2009; Ong, Edwards, & Bergeman, 2006). On the other hand, we might also find a subgroup of older adults who are able to maintain positive moods over time, which would be consistent with the maintenance of positive affect in older adulthood.
Research Questions
The present study examined three research questions. First, we investigated whether people could be grouped according to different distinct trajectories of mood-change across a relatively short time period (~25 minutes). Past work suggests that 81% of individuals do change their moods in 20 minutes or less under conditions of mood elicitors (Eich, Ng, Macaulay, Percy, & Grebneva, 2007). Secondly, we investigated whether young and older adults would be more likely to belong to certain types of mood-change trajectory groups. Our third and final question was whether cognitive, attentional, dispositional, state, or behavioral factors could further distinguish group membership.
Hypotheses
In regard to our first research question, we expected several different trajectories of mood-change to emerge. We expected groups to differ in their initial moods due to individual differences in baseline moods, and we also expected groups to differ in their trajectory of change over time, with some groups showing a more positive trajectory and others a negative trajectory. For the second research question, we expected older adults to be more likely than young adults to belong to the group(s) that maintain the most positive mood across time, consistent with work suggesting maintenance or increases in experienced positive affect with age (Charles et al., 2001; Kessler & Staudinger, 2009), as described above. Other studies have found that older adults on average improve their moods more than young adults during lab-based emotion regulation paradigms (e.g., Mienaltowski & Blanchard-Fields, 2005; Scheibe & Blanchard-Fields, 2009; Shiota & Levenson, 2009). However, given that for many constructs the variability between individuals increases with age (Dannefer, 1988; Morse, 1993), we did not expect all older adults to exhibit this most positive pattern. Indeed, we expected older adults would not belong to a homogenous positive group, but instead older adults would belong to several distinct trajectories of mood change. Consistent with past work, however, we expected the majority of older adults to exhibit positive mood trajectories. Our third research question was more exploratory in nature. In general, we expected cognitive, attentional, personality, and behavioral characteristics to differentiate more positive and more negative mood trajectories. Specifically, we expected individuals with better cognitive and attentional abilities to be more likely to exhibit positive or neutral mood trajectories than negative mood trajectories because active emotion regulation requires cognitive and attentional resources (Knight et al., 2007; Mather & Knight, 2005). We expected personality characteristics such as neuroticism to be related to mood (Larcom & Isaacowitz, 2009). Previous work suggests that neuroticism is linked to affect across the lifespan such that individuals high in neuroticism show less of a decrease in negative affect in later adulthood (Charles et al., 2001; Griffin, Mroczek, & Spiro, 2006). Consistent with recent findings that suggest gaze can be used as a tool to regulate mood (Isaacowitz et al., 2008; Isaacowitz et al., 2009), we also expected individuals within groups that exhibited a trajectory of mood improvement, as opposed to those who do not report an improvement in mood, to be more likely to attend to the more positive faces within an emotional-neutral synthetic face pair.
Method
Participants
Eighty-six young adults (aged 18–30 years)1 and 106 community-dwelling older adults (aged 58–89 years) living in the northeastern region of the United States participated in this study. Young adults were recruited from an introductory psychology course and with flyers posted on campus. Older adults were recruited from a lifelong learning class and with advertisements. Participants received either course credit or a monetary stipend. Ten participants were excluded from analyses because part or all of their mood slider data were missing due to technical failures, leaving 79 young adults (44 women and 35 men; aged 18–30 years; M = 19.84, SD = 2.13) and 103 older adults (74 women and 29 men; aged 58–88 years; M = 72.05, SD = 6.97) for analyses. All participants spoke English fluently. Participants were highly educated; on average participants had either attended some college or were college graduates. Eighty-nine percent of the sample was White, 4% Asian, 3% Other, 1% Black, 1% Hispanic, and 2% mixed or no response. No participant scored below our cutoff criterion of 24 on the Mini-Mental State Exam (Chayer, 2002).
Materials and Measures
Gaze and mood measurement
Equipment
Face-pair stimuli were presented in random order on a 17-in. display with GazeTracker software (Eye Response Technologies, Inc., Charlottesville, VA). While viewing the 25 minute face-pair slideshow, participants continuously rated their current mood from 0 (worst) to 100 (best) in real time using a potentiometer slider (Empirisoft Corporation, New York, NY) that collected responses at a rate of once every second. We captured a mood measurement from the potentiometer slider approximately every two minutes, creating 14 mood measurement variables for each participant. Eye movements were recorded at a rate of 60 Hz with an Applied Science Laboratories (Bedford, MA) Model 504 Eye Tracker with magnetic head transmitter.
Emotional face stimuli
Slides of synthetic face pairs consisting of an emotional face (angry, afraid, sad, or happy) and its neutral counterpart were presented to participants while their eyes were tracked. The emotional synthetic faces were created based on Ekman and Friesen’s (1975) guidelines for each emotion (for more information on how the synthetic faces were created and validated see Isaacowitz, Wadlinger, Goren, & Wilson, 2006b; Wilson, Loffler, & Wilkinson, 2002). The face pairs were presented on a gray background. Several variables were counterbalanced to avoid order effects: the side of the screen the emotional face appeared on (left or right), the sex of the face, and the emotion of the emotional face.
Gaze measures
For the eye tracking data2, a fixation was defined as an interval when gaze was focused for 100 ms or more within 1° visual angle (Manor & Gordon, 2003). Gaze measures were calculated as ratio scores that reflect the relative fixation to the emotional face in the emotional-neutral face pairs compared to fixation to the neutral face. Thus, for each face a ratio score was calculated using this formula: (emotional − neutral)/ (emotional + neutral). These ratio scores were averaged across faces within the four emotion types to create four ratio scores: Anger, Fear, Happy, and Sad (see Table 1 for means and standard deviations of gaze ratio scores). A positive ratio score reflects greater fixations toward the emotional face, a negative ratio score reflects greater fixations toward the neutral face, and a score of zero indicates that the emotional and neutral faces received an equal number of fixations.
Table 1.
Means and Standard Deviations (in parentheses) for measures of Gaze, Cognition, Attention, Perception, and Affect for Young and Older Adults
| Young | Old | |
|---|---|---|
| Gaze Measuresa | ||
| Anger Ratio | .01 (.19) | −.01 (.32) |
| Fear Ratio | .08 (.17) | −.04 (.28) |
| Happy Ratio | .03 (.14) | −.01 (.25) |
| Sad Ratio | .01 (.18) | −.07 (.31) |
| Cognitive Functioning | ||
| Digit Span Forward | 7.61 (1.16) | 7.35 (1.19) |
| Digit Span Backward | 6.17 (1.45) | 5.67 (1.48) |
| Digit Symbolb | .48 (.07) | .52 (.10) |
| Vocabulary (number correct out of 21) | 14.30 (2.33) | 16.46 (2.53) |
| MMSEc | 29.68 (.57) | 28.77 (1.28) |
| Attentional Abilities | ||
| Alerting (ms) | 43.46 (25.61) | 24.77 (42.42) |
| Orienting (ms) | 41.01 (23.01) | 51.03 (38.36) |
| Conflict (ms) | 131.15 (51.81) | 146.85 (95.43) |
| Perceptual Functioningd | ||
| Snellen Visual Acuity | 31.71 (17.47) | 42.48 (17.89) |
| Rosenbaum Near Vision | 23.86 (4.80) | 35.53 (14.57) |
| Contrast Sensitivity | 1.52 (.13) | 1.40 (.14) |
| Affect Measures | ||
| State Anxiety | 35.14 (9.40) | 32.27 (9.85) |
| Trait Anxiety | 40.60 (10.85) | 34.52 (9.04) |
| LOTe | 4.13 (4.91) | 6.86 (5.14) |
| Neuroticism | 14.87 (3.34) | 13.59 (2.48) |
| Positive Affect | 29.16 (8.58) | 33.79 (6.45) |
| Negative Affect | 15.92 (5.21) | 14.06 (5.81) |
| CESDf | 14.75 (9.59) | 8.33 (7.48) |
Note. Sample sizes differ due to missing data: Gaze Fixation Ratios (N = 130), Cognitive Functioning (N = 179), Attentional Functioning (N = 172), Perceptual Functioning (N = 181), and Affect Measures (Ns = 173–182). Higher scores indicate better performance unless otherwise indicated.
Gaze Measures computed as ratio scores: (emotional − neutral)/ (emotional + neutral).
For the Digit Symbol, lower scores indicate better performance.
Mini-Mental State Exam, maximum score of 30 (range: 24–30).
For Snellen and Rosenbaum tests, lower scores indicate better performance.
Life Orientation Test (Scheier & Carver, 1985), Optimism Test total score.
Center for Epidemiological Studies Depression Scale (Radloff, 1977).
Measures of related constructs
See Table 1 for means and standard deviations of measures of related constructs.
Cognitive functioning
We included a battery of cognitive measures in order to investigate whether differences in cognitive functioning would impact participants’ mood ratings, especially given our interest in age effects. Short term memory was assessed with the Wechsler Adult Intelligence Scale-Revised Forward and Backward Digit Span (WAIS; Wechsler, 1981) in which participants repeat a series of numbers forward and backwards in increasingly larger sets. Processing speed was measured with the WAIS Digit Symbol Substitution (Wechsler, 1981). Crystallized intelligence was measured with the Shipley Vocabulary Test (Zachary, 1986). In addition, the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975) was administered to screen for cognitive impairment.
Attentional abilities
Attentional abilities were assessed with the Attention Network Test (ANT; Fan, McCandliss, Sommer, Raz, & Posner, 2002) which measures three different attention networks: alerting, orienting, and executive attention. In the ANT, participants indicate the direction a central arrow is pointing (left or right). Sometimes the arrow is presented above fixation, and sometimes below fixation. In addition, sometimes the arrow is presented with flankers, and sometimes without. The alerting score reflects the individual’s efficiency at alerting when a cue is present vs. absent, the orienting score reflects efficiency at orienting to a spatial cue, and the executive control score reflects the individual’s efficiency at resolving conflicts that arise due to flankers. The authors report test-retest correlations for the three networks ranging from .52 to .77 (Fan et al., 2002). Recent work has found that good attentional functioning may be related to the use of gaze for mood regulation (Isaacowitz et al., 2009).
Perceptual functioning
Because we wanted to ensure that all individuals were able to perceive the stimuli presented on the computer, participants’ perceptual functioning was assessed with three measures: the Snellen chart for visual acuity (Hetherington, 1954), the Rosenbaum Pocket Vision Screener for near vision (Rosenbaum, 1984), and the Pelli-Robson Contrast Sensitivity Chart (Pelli, Robson, & Wilkins, 1988).
Affect measures
We included measures of affect so that we could examine whether state and trait affect variables are related to mood rating changes across a short time period. Thus, participants completed five affective measures: the State-Trait Anxiety Inventory (STAI; Speilberger, 1983), the Life Orientation Test (LOT; Scheier & Carver, 1985) designed to assess dispositional optimism and pessimism, the Neuroticism Questionnaire (N-Questionnaire; Bolger & Schilling, 1991), the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988), and the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977).
Demographic variables
Participants reported their age, gender, and education level and rated their health on a 5-point Likert-type scale (1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent).
Procedure
After obtaining informed consent, participants were asked to complete the demographics form and the self-report affect measures. Next, participants were administered the cognitive and attention tests, followed by the three vision tests. Then participants were asked to self-induce into one of three randomly assigned moods: positive, neutral, or negative, using a variant of the Eich Continuous Music Technique (CMT; Eich & Metcalfe, 1989). Specifically, participants were asked to imagine hypothetical situations or think of autobiographical events that evoked the assigned mood while listening to music that matched that assigned mood. In the positive condition, participants were instructed to think about a particularly pleasant event, something very upbeat or exciting while you listen to the music. In the neutral condition, participants were instructed to think about a particularly neutral event, something that really didn’t have a significant effect on you, where you felt neither pleasant nor unpleasant, while you listen to the music. And in the negative condition, participants were instructed to imagine, in a lot of detail, something that would make you feel very unpleasant and tense or agitated, while you listen to the music. Participants continuously rated their mood on an affect grid containing valence and arousal dimensions. The mood induction was considered successful once their ratings remained within the appropriate area of the grid for at least 30 seconds.
After the mood induction, participants were seated in front of the potentiometer slider and the eye tracker. A 17-point calibration was performed to ensure accurate measurement of participants’ gaze. Participants rated their current mood from 0 (worst) to 100 (best) on the potentiometer. Participants were instructed to watch the slideshow that followed naturally, as if they were watching TV at home and to report their mood on the potentiometer throughout the presentation. The slideshow consisted of 272 face pair slides displayed for four seconds each, followed by a 500 ms crosshair slide to re-align gaze to the center of the screen. To minimize skew of results due to blinks and moments of lost tracking (from head movement, pupil obfuscation, etc.), two criteria were used to identify individual trials in which the fixation pattern indicated unreliable recording: trials with all fixations to “off” regions (no fixations on faces), or trials with < 900 ms total fixation anywhere on the slide. These trials were not included in the ratio scores. As a reminder, participants were prompted randomly throughout the slideshow to rate their current mood using the potentiometer. Immediately following the conclusion of the slideshow, participants made a final rating of their mood on the potentiometer.
Results
Mood-change Trajectory Types
In order to answer our first question regarding whether participants could be categorized into distinct groups of mood-change trajectories, we performed a cluster analysis on the mood ratings. This method allowed us to categorize participants into subgroups according to their responses on all 14 mood variables rather than on a single mood-change score. We captured a mood rating from the potentiometer device approximately every two minutes for each participant, yielding 14 mood ratings (ranging from 0 = worst to 100 = best) for each participant. These mood ratings represent the first slider rating immediately following the mood induction procedure, 12 ratings during the 23.5 minute face pair slide show, and a last slider rating immediately following the slide show. These 14 mood ratings were standardized into Z-scores and submitted to a two-step clustering procedure (Hair & Black, 2000). Not surprisingly, the 14 mood ratings were all significantly correlated (see Table 2 for intercorrelations among the 14 mood ratings). Cluster analysis is sensitive to high intercorrelations among variables (Hair & Black, 2000). Two variables which are highly correlated might exhibit undue influence on the determination of cluster group membership. However, because no two mood ratings seemed differentially highly correlated, other than what would be expected based on time proximity, we went ahead with the analysis despite significant intercorrelations among the variables to be clustered.
Table 2.
Intercorrelations Among the 14 Mood Ratings (N = 182)
| Rating | First | 2 min. | 4 min. | 6 min. | 8 min. | 10 min. | 12 min. | 14 min. | 16 min. | 18 min. | 20 min. | 22 min. | 23.5 min. | Last |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | 1.00 | |||||||||||||
| 2 min. | 0.78 | 1.00 | ||||||||||||
| 4 min. | 0.64 | 0.80 | 1.00 | |||||||||||
| 6 min. | 0.60 | 0.73 | 0.87 | 1.00 | ||||||||||
| 8 min. | 0.50 | 0.66 | 0.82 | 0.89 | 1.00 | |||||||||
| 10 min. | 0.51 | 0.66 | 0.72 | 0.82 | 0.88 | 1.00 | ||||||||
| 12 min. | 0.47 | 0.62 | 0.67 | 0.71 | 0.74 | 0.87 | 1.00 | |||||||
| 14 min. | 0.42 | 0.54 | 0.58 | 0.62 | 0.62 | 0.71 | 0.85 | 1.00 | ||||||
| 16 min. | 0.31 | 0.50 | 0.52 | 0.59 | 0.62 | 0.76 | 0.83 | 0.88 | 1.00 | |||||
| 18 min. | 0.32 | 0.47 | 0.50 | 0.53 | 0.61 | 0.67 | 0.76 | 0.83 | 0.89 | 1.00 | ||||
| 20 min. | 0.27 | 0.42 | 0.49 | 0.49 | 0.56 | 0.62 | 0.70 | 0.76 | 0.82 | 0.94 | 1.00 | |||
| 22 min. | 0.31 | 0.46 | 0.48 | 0.47 | 0.53 | 0.59 | 0.67 | 0.71 | 0.75 | 0.87 | 0.91 | 1.00 | ||
| 23.5 min. | 0.28 | 0.44 | 0.47 | 0.51 | 0.55 | 0.59 | 0.65 | 0.65 | 0.70 | 0.80 | 0.86 | 0.93 | 1.00 | |
| Last | 0.36 | 0.45 | 0.47 | 0.43 | 0.49 | 0.56 | 0.68 | 0.72 | 0.71 | 0.80 | 0.79 | 0.82 | 0.80 | 1.00 |
Note. All correlations significant, p < .05.
First, we applied a hierarchical clustering method using Ward’s (Ward, 1963) minimum-variance method with squared euclidean distances to help determine the theoretically and statistically appropriate number of clusters. We used multiple criteria to decide on the optimal number of clusters (Milligan & Cooper, 1987): specifically, we examined the clustering coefficient from the agglomeration schedule produced from the hierarchical clustering procedure in SPSS 16.0 (SPSS Inc., 2007) to identify cluster combinations that were just prior to a substantial decrease in within-cluster similarity (exhibited by a relatively large increase in the coefficient value). We also implemented the Ward’s procedure (Ward, 1963) using PROC CLUSTER in SAS 9.1 (SAS Institute, 2002–2003) to obtain the pseudo-F statistic, the pseudo-T2 statistic, and Sarle’s cubic clustering criterion (CCC; Sarle, 1983) for each cluster step. The pseudo-F statistic represents separation among all clusters at the current step and the pseudo-T2 statistic represents the dissimilarity of the two clusters most recently joined. We used a peak in the CCC (Sarle, 1983) as another indication of the ideal number of clusters (Hair & Black, 2000; Milligan & Cooper, 1987). These four decision criteria converged on either a three or a four cluster solution as the ideal number of clusters. The four cluster solution seemed most appropriate for investigating our research questions regarding variability in mood change. Given how little data there is on the heterogeneity of mood change among older adults, we chose the four cluster solution because we believed the solution that modeled greater heterogeneity would be more conceptually useful. For the second step of the two-step clustering procedure, we then submitted the mood ratings to a nonhierarchical (k-means) clustering procedure using SPSS 16.0 (SPSS Inc., 2007) to optimize cluster membership assignment.
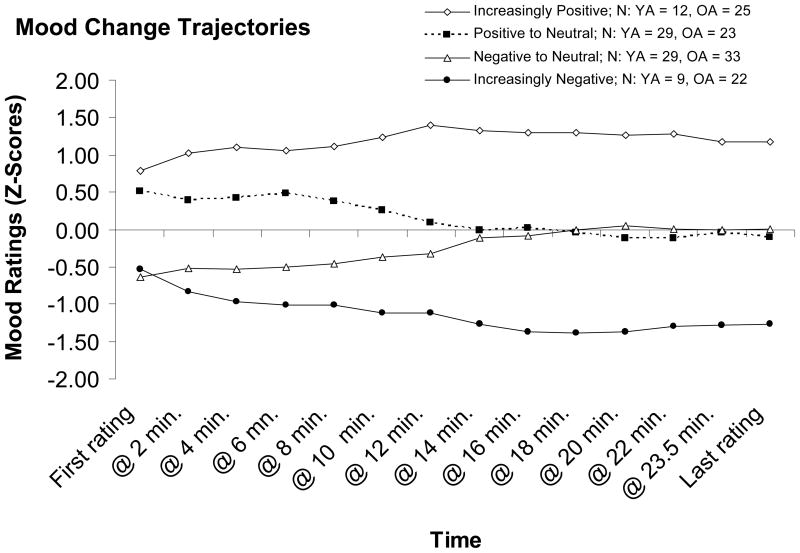
As expected, several distinct mood-change trajectories emerged from the cluster analysis (see Figure 1). The four clusters are named from the most positive subgroup (“Increasingly Positive”) to the most negative subgroup (“Increasingly Negative”). Two groups (Positive to Neutral and Negative to Neutral) exhibited a pattern of becoming more neutral over time. Participants who belong in the Negative to Neutral group reported negative initial mood ratings but gradually rated their moods more and more neutral across the mood rating period. The mood-change trajectory of the Positive to Neutral group shows the opposite pattern, with participants initially rating their moods as positive, but becoming more neutral over time. The other two groups (Increasingly Positive and Increasingly Negative) exhibited more extreme mood ratings over time. Participants in the Increasingly Positive group rated their mood as initially positive and became more positive over the mood rating period. Participants in the Increasingly Negative group showed the opposite pattern with negative initial mood ratings that became more negative over time.
Figure 1.
Mood-change trajectories across ~25 minute slide show as a function of cluster membership group.
Relationship of cluster group membership to mood induction condition
We included a mood induction in the design of the study in order to create variability in mood states before the primary tasks of interest (capturing a 25-minute interval in which none of the participants’ moods were changing would not allow a good test of mood change profiles). Moods started to vary immediately following the end of the induction: only 52% of the sample reported the same mood at the end of the induction and a few minutes later, when potentiometer mood ratings (which we focused on in the current study) started. In other words, the mood induction was not overwhelmingly successful in inducing a lasting mood, as only half of the participants were in the intended mood at the first measure of the potentiometer ratings. Nonetheless, it was important to show that mood induction condition and cluster membership were not redundant categories. Mood trajectory group membership did not entirely overlap with our mood induction conditions. That is, some participants from each of the three mood induction conditions belong to each of the four mood trajectory groups. Increasingly Positive: positive n = 13 (8 older adults), negative n = 13 (12 older adults), neutral n = 11 (5 older adults); Positive to Neutral: positive n = 25 (10 older adults), negative n = 11 (6 older adults), neutral n = 16 (7 older adults); Negative to Neutral: positive n = 12 (9 older adults), negative n = 26 (11 older adults), neutral n = 24 (13 older adults); Increasingly Negative: positive n = 8 (6 older adults), negative n = 12 (7 older adults), neutral n = 11 (9 older adults).
Spearman’s rank correlation coefficient between cluster group membership and mood induction condition was not significant, ρ(181) = .13, n.s., suggesting that cluster group membership is not related to mood induction condition. Cluster group membership and mood induction condition are also not significantly related when examined separately for each age group (young adults: ρ(78) = .13 and older adults: ρ(103) = .14, ps > .15 ). These findings suggest that the cluster creation and group membership assignment was not simply a reflection of our experimental mood induction conditions, meaning that our cluster analysis was not confounded by the induction; rather, the induction simply set the stage for a context with a great deal of variability in mood change trajectories, which was important for addressing our research questions.
Age Make-Up of Groups
In order to determine whether young or older adults were more or less likely to belong to certain mood-change trajectory groups, we conducted a chi-square analysis comparing the distribution of young and older adults amongst the four groups (similar to Fiori, Smith, & Antonucci, 2007). As expected, we found that the two age groups were differentially distributed across the four mood trajectory clusters, χ2(3) = 7.94, p < .05 (see Table 3 for number of older adults in each of the mood trajectory clusters). Specifically, older adults were overrepresented in the Increasingly Positive and Increasingly Negative groups in comparison to the Positive to Neutral group, ps < .05. We expected the most positive group (Increasingly Positive) to consist of more older adults than young adults based on previous research. We did not, however, expect older adults to be overrepresented in the most negative group (Increasingly Negative) as well.
Table 3.
Mood Trajectory Group Differences by Age Group and Correlates
| Age Group (% Old) | Increasingly Positive (n = 37) | Positive to Neutral (n = 52) | Negative to Neutral (n = 62) | Increasingly Negative (n = 31) | Statistic | Follow-up Comparisonsa |
|---|---|---|---|---|---|---|
| 68 | 44 | 53 | 71 | χ2(3) = 7.94* | 1, 4 > 2* | |
| Correlates that Differ by Mood Trajectory Groupb | ||||||
| Happy Fixation Ratio | .05(.03) | .06(.02) | .02(.02) | −.03(.03) | F(3, 126) = 2.81*, η2p = .06 | 2* > 4 |
| Digit Span Forward | 7.61(.19) | 7.70(.16) | 7.03(.15) | 7.74(.21) | F(3, 175) = 4.39**, η2p = .07 | 2, 4 > 3* |
| Digit Symbol Substitution | .49(.02) | .49(.01) | .51(.01) | .54(.02) | F(3, 175) = 2.79*, η2p = .05 | 4* > 1, 2 |
| ANT Mean RT (ms)c | 749.63(24.55) | 665.92(20.75) | 702.79(19.07) | 746.17(26.52) | F(3, 168) = 3.04*, η2p = .05 | 1* > 2 |
| State Anxiety | 33.78(1.59) | 30.95(1.33) | 33.07(1.22) | 38.21(1.71) | F(3, 175) = 3.80*, η2p = .06 | 4* > 2 |
| Neuroticism | 13.40(.49) | 13.94(.42) | 14.07(.38) | 15.50(.53) | F(3, 169) = 3.05*, η2p = .05 | 4* > 1 |
Notes:
For the correlates, post-hoc comparisons of means were conducted using Tukey’s Honestly Significant Difference (HSD).
Results based on Multivariate Analyses of Variance (MANOVAs) and univariate analyses of Variance (ANOVAs). Sample sizes for the five correlate type categories differ due to missing data: Gaze Fixation Ratios (N = 130), Cognitive Functioning (N = 179), Attentional Functioning (N = 172), Perceptual Functioning (N = 181), and Affect Measures (NStateAnxiety = 179 and NNeuroticism = 173). Only those correlates which were significantly different (p < .05) by mood trajectory group are presented in the table. Reported means of correlate scores and standard errors (in parentheses) are from the estimated marginal means for the MANOVA models.
ANT Mean RT was computed for correct trials only.
p < .05;
p < .01.;
p <.001.
Because we had a fairly large range of ages in our older adult sample (31 years), we tested whether the average age of older adults in the Increasingly Negative group (M = 71.23, SE = 1.44) was older than the average age of older adults in the Increasingly Positive group (M = 74.24, SE = 1.35). A univariate analysis of variance (ANOVA) on just the older adult sample revealed that the Increasingly Negative and Increasingly Positive groups did not differ according to the average age of older adults in the group, p > .05.
Characteristics of Groups
To address our third research question, which sought to define a profile of characteristics that distinguishes mood trajectory group membership, we first conducted chi-square analyses comparing the distribution of gender, education, and self-reported health amongst the four mood trajectory groups. Because the results of these analyses failed to reach significance, these demographic variables were not included in further analyses.
Next, we conducted multivariate analyses of variance (MANOVAs) and univariate ANOVAs to identify characteristics that distinguish cluster group membership. We chose to conduct MANOVAs for groups of variables that were highly intercorrelated and represented the same construct, such as the four gaze variables, the three measures from the WAIS (Digit Span Forward, Digit Span Backward, and Digit Symbol Substitution), the three networks of the ANT, and the three perceptual measures, because MANOVA allows for the covariance structure among common variables and controls for experiment-wide error rate (Hair, Anderson, Tatham, & Black, 1998). Mood trajectory cluster membership was entered as the independent variable for each of these analyses. Univariate ANOVAs were examined to determine subgroup differences for each of the specific dependent measures following a significant effect in the MANOVA (Hair et al., 1998; Röcke & Lachman, 2008). Post-hoc analyses were conducted with Tukey’s Honestly Significant Difference (HSD) to determine which groups differed on a specific measure. Several differences between the groups emerged (see Table 3 for means, standard errors, tests of significance, and post-hoc comparisons for significant effects).
Gaze
We observed a multivariate significance for the main effect of cluster group membership on gaze variables, Wilk’s λ = .83, F(12, 383.93) = 2.30, p < .05, ηp2 = .06. Univariate ANOVAs and follow-up post-hoc tests indicated that of the four fixation ratios entered as dependent variables in the MANOVA (Anger, Fear, Happy, Sad), only the Happy Fixation Ratios differed according to cluster group membership, with the Increasingly Negative group looking less at happy faces than the Positive to Neutral group.
Cognitive functioning
The multivariate significance for the main effect of cluster group membership on the three WAIS cognition variables was significant, Wilk’s λ = .87, F(9, 421.19) = 2.70, p < .01, ηp2 = .05. Univariate ANOVAs and follow-up post-hoc comparisons indicated a significant cluster group difference for Digit Span Forward scores (a measure of short term memory), p < .01, with the Positive to Neutral and Increasingly Negative groups scoring higher than the Negative to Neutral group. In addition, there was a significant univariate effect of cluster group for the Digit Symbol Substitution Test (a measure of processing speed), p < .05. Post-hoc analyses indicated that the Increasingly Negative group had higher (slower) processing speed scores than Groups 1 and 2. The groups did not differ on vocabulary scores, p > .05.
Attentional functioning
The multivariate effect for cluster group differences in the three attention networks (alert, orient, conflict) did not reach significance, p > .05. However, a univariate ANOVA yielded a significant cluster group difference for overall reaction time (across all trial types) for correct responses in the ANT, p < .05, with the Increasingly Positive group exhibiting slower reaction times than the Positive to Neutral group.
Perceptual functioning
For the three perceptual tests (Snellen visual acuity, Pelli-Robson contrast sensitivity, and Rosenbaum near vision), the multivariate test for cluster group differences did not reach significance, p > .05.
Affect measures
Univariate ANOVAs revealed significant cluster group differences for state anxiety, F(3, 175) = 3.80, p < .05, ηp2 = .06, neuroticism, F(3, 169) = 3.05, p < .05, ηp2 = .05, and approached significance for depression, F(3, 177) = 2.63, p = .05, ηp2 = .04. Post-hoc analyses indicate that the Increasingly Negative group reported greater state anxiety than the Positive to Neutral group and greater neuroticism than the Increasingly Positive group. The Increasingly Positive group (M = 8.80, SE = 1.46) reported lower levels of depression than the Increasingly Negative group (M = 14.70, SE = 1.60), p < .05.
In sum, individuals in the most positive group (Increasingly Positive) had slower response times on average for correct responses in the ANT than individuals in the Positive to Neutral group, which is consistent with more older adults belonging to the Increasingly Positive group than the Positive to Neutral group. In addition, the most negative group (Increasingly Negative) had greater state anxiety (than the Positive to Neutral group), had better short term memory scores (than the Negative to Neutral group), slower processing speed (than the Increasingly Positive and Positive to Neutral groups), and looked less at happy faces compared to neutral faces (than the Positive to Neutral group). Finally, the Positive to Neutral group also exhibited better short term memory scores than the Negative to Neutral group.
Discussion
In this study we investigated age and individual differences in mood-change profiles using a person-centered approach to complement the standard variable-oriented approach, which allowed for a richer and more complex variety of mood-change profiles than previously seen in the literature. Overall, older adults exhibited all three types of trajectories (positive, negative, and neutral), while young adults were less varied in their trajectory of mood change; most showed a pattern of shifting toward a neutral mood. Interestingly, older adults were more likely than young adults to belong to mood-change groups that exhibited the most extreme trajectories: either positive to more positive or negative to more negative. Thus, contrary to expectations based on overall age group differences in subjective well-being and positive affect (Carstensen et al., 2000; Mroczek & Almeida, 2004), some older adults were more likely than young adults to exhibit a trajectory of maintained and even increased negative mood over the course of the 25 minute slideshow.
The main factors that differentiated the two extreme groups were that the negative group (Increasingly Negative) had slower processing speed than the two more positive groups (Increasingly Positive and Positive to Neutral) and higher neuroticism than the Increasingly Positive group. Additionally, the Increasingly Negative group had more state anxiety and looked less at happy faces than the Positive to Neutral group but did not differ on those characteristics from the Increasingly Positive group. So, although members of the Increasingly Negative group were more anxious than those in the Positive to Neutral group, which could certainly usurp resources that could otherwise be devoted to mood maintenance, it does not explain the differences between the Increasingly Positive and Increasingly Negative groups. Interestingly, our measure of neuroticism did differentiate these two extreme groups, which is consistent with research suggesting that individuals high in neuroticism are less likely to repair a negative mood than those lower in neuroticism and thus tend to stay in negative moods for longer (Salovey, Mayer, Goldman, Turvey, & Palfai, 1995). In addition, individuals in the Increasingly Negative group reported higher levels of depression than those in the Increasingly Positive group, which is consistent with research that has found that less depressed individuals show greater belief in the possibility of proactively changing moods (Catanzaro & Mearns, 1990).
Unlike previous findings of a relationship between higher levels of executive control and better emotion regulation amongst older adults (Knight et al., 2007; Mather & Knight, 2005), the only cognitive variable that differentiated members of the Increasingly Positive group from the Increasingly Negative group was processing speed. One possible explanation for this relationship is that individuals with slower processing speed may be less able to engage in methods that would improve their mood. Alternatively, slow processing speed may be an overall marker of cognitive changes that constrain the ability to improve mood. The relationship could even be more direct, as faster thinking has been linked to happier thoughts (Pronin & Wegner, 2006). It may also be that processing speed undergirds higher level functioning related to the executive functioning required to maintain a positive mood. It is curious then that we did not see differences between the Increasingly Positive and Increasingly Negative groups on the measures of attentional functioning (Fan et al., 2002), which include a measure of executive control (i.e., conflict). We also found better short-term memory scores for the Positive to Neutral and Increasingly Negative groups compared with the Negative to Neutral group. This is puzzling because it is in contrast with what would be expected if cognitive resources are required for improving one’s mood: it seems the two groups who showed declines in mood at least have better short-term memory abilities than a group who improved its mood. Perhaps short-term memory is not as essential to mood maintenance as processing speed, but future research needs to address this question more directly.
Theoretically, an age-related shift toward prioritizing emotionally gratifying experiences is useful for explaining differences between age groups. Because older adults are exhibiting a variety of different patterns of mood-change in this study, it is possible that the members of the positive and negative groups differ in the degree to which they have shifted toward maximizing positive affect, or whether this is a chronically activated goal for them at all. That is, we cannot assume that all older adults are equally motivated to maximize positive affect. Past work has suggested one factor that might differ between older adults who do and do not exhibit positivity effects is the availability of cognitive and attentional resources (Isaacowitz et al., 2009; Knight et al., 2007). In the present study, members of the Positive to Neutral group looked more at happy faces and had faster processing speed than members of the Increasingly Negative group, consistent with the hypothesis that older adults with more available cognitive resources are better able to actively regulate their moods by selectively attending to positive images. Indeed, these looking preferences may have helped individuals improve their mood as individuals in the Positive to Neutral group exhibited more positive mood trajectories than did those in Increasingly Negative group.
Nevertheless, even considering the increased individual differences associated with age (Dannefer, 1988), it is still surprising that so many older adults belong to the mood-change trajectory group that is the most negative. This is in stark contrast to studies reporting overall more positive average affect for older adults than young adults (Kessler & Staudinger, 2009; Kunzmann et al., 2000). Older adults who exhibit the most negative mood trajectory do not fit with the paradox of well-being trend. Instead, these older adults (and young adults) are not maximizing positive affect in our lab-based paradigm, and whether this transfers to similar negative patterns in other situations in their daily lives remains to be seen. One possibility is that these atypical negative mood change patterns could be linked to cognitive or attentional difficulties. Although one of the advantages of this study was the real-time mood capture linked with gaze behavior over a short duration, it is unknown how long it takes individuals to regulate their moods. It may be that some of the older adults who belong to the Increasingly Negative group would change group membership to a more positive group if we extended the duration of the study. Twenty-five minutes may not be enough time for some older adults (i.e., those with slower processing speed, greater anxiety, or just transitioning into the goal state of maximizing positive affect) to transition out of negative mood states into more positive ones. Of course, it is also possible that even long-term follow-up would find these individuals still in negative states.
Limitations
There are a number of limitations to this study. Because the design of the study was cross-sectional, we cannot be sure that the age differences exhibited are due to developmental changes rather than, for example, differences between cohorts in the processing of emotions. Another limitation is the lack of differentiation in both the negative mood induction and the mood ratings. There is a growing literature which suggests that very different aging effects emerge when different discrete negative emotions are considered (e.g., anger vs. sadness; Blanchard-Fields & Coats, 2008; Kunzmann & Gruhn, 2005). We do not know from the present work whether reports of a negative mood were people feeling angry versus sad. It may be that a sad negative mood is more likely to be tolerated than an angry negative mood. Thus, some participants may have been less motivated to regulate out of their negative mood if they were sad rather than angry. Nevertheless, this study was able to differentiate groups of people who were more or less likely to improve their negative moods (whether sad or angry) over the course of 25 minutes. It is also possible that not all participants are equally motivated to regulate out of a negative mood, or to change their mood at all. This study is limited in that it did not include a measure of whether participants wanted to improve their moods. Also related to the time course issue, it is possible that 25 minutes is not an ecologically valid time frame for differentiating the globally more positive affective lives of older adults from the less positive reports generally given by young adults. In addition, it should also be noted that this was a relatively small sample size for cluster analysis (Hair & Black, 2000), thus conclusions drawn from these results are tempered until it is replicated with a larger sample size. Furthermore, although we chose to use a mood induction to insure variability in starting moods of participants, this may have unduly influenced our mood trajectory groups. Future research should use a single mood induction or use mood as a repeated measures factor to eliminate this possibility.
Conclusions and Future Directions
In this study we used a person-centered approach to capture subgroups of distinct mood change trajectories and the person and group level variables that are related to different trajectories. By employing a person-centered approach, we were able to examine the heterogeneity among subgroups of older adults in the dynamics of mood change over time. Overall, the results suggest that some older adults do experience and maintain negative moods over a short time period, even more so than young adults. There is an emerging literature identifying the conditions under which older adults experience and report negative moods and emotions (Charles & Carstensen, 2008; Grühn & Scheibe, 2008; Kunzmann & Gruhn, 2005). Future research should investigate the implications of this variability in mood change trajectories amongst older adults and whether this is a reflection of adaptive functioning or a potential indicator of dysfunction. It would also be interesting to gain a better understanding of the motivational mindset of individuals who exhibit a negative mood trajectory. It could be that an individual’s subjective age, rather than their objective age, might better differentiate mood-change trajectories (Lachman, 2004; Montepare & Lachman, 1989). Future work could also examine in more detail what characteristics distinguish the Increasingly Positive group from the Increasingly Negative group, such as functional limitations or more specific components of health. Perhaps investigating mood change among very old individuals could help to highlight such distinctions.
The characteristics that differentiate the most negative and the most positive groups suggest that they differ in their affective profiles, cognitive functioning, and their use of gaze as a regulatory tool in this paradigm. This suggests that the dynamics of mood change across a short time period may be multiply determined. Although the present study cannot address the causal direction of these relationships, it is a first glimpse into factors beyond age group and executive functioning that are related to different patterns of mood change. While these results paint a less positive portrait of affect and aging than other work, there is an upside to our findings as well. Given recent literature suggesting that positive affect may help buffer an older adults’ more vulnerable system (Blanchard-Fields, 2007; Consedine et al., 2002; Ong, Bergeman et al., 2006), the possibility that differences in multiple domains (e.g., faster processing speed, less anxiety and neuroticism, more attention to happy faces) are related to more positive mood trajectories is promising because it suggests that older adults who exhibit decline in one domain (e.g., processing speed) may still be able to maximize positive affect in their lives by capitalizing on strengths in another domain (e.g., personality traits).
Acknowledgments
This research was supported by National Institute of Health Grants: R01AG-026323 awarded to Derek M. Isaacowitz & T32AG-00204. We wish to acknowledge Deborah Goren and Hugh Wilson for creating the synthetic faces used in the study.
Footnotes
This study was part of a larger study, portions of which have been published elsewhere ((Isaacowitz et al., 2008; Isaacowitz et al., 2009; Larcom & Isaacowitz, 2009). Previous results reported from this sample restricted the age range for young adults to an upper limit of 25 years (Isaacowitz et al., 2008). In the present study, we included the 30-year-old in our analyses. We also ran the analyses excluding the 30-year-old and our cluster creations did not differ from that with the 30-year-old nor did any participant’s cluster group membership change. Thus, we report here analyses including the 30-year-old.
Data from 52 participants were excluded from the eye tracking analyses because 1) they were not trackable: eye movements could not be calibrated (e.g., due to droopy eyelids), they were not successfully tracked for at least 25% of the trials (68 trials), or 2) one of their fixation ratio scores was more than 3 SD away from the group means. After excluding these 52 participants from any eye-tracking analyses, a total of 130 participants’ eye tracking data remained (74 young adults, 56 older adults). Comparison of trackable participants to nontrackable participants within each age group revealed that older nontrackable participants had worse visual acuity (Snellen) than trackable participants, and nontrackable young adults had slower response times in the attention network test (ANT) than trackable young adults, ps < .05. No other cognitive, affective, or demographic measures significantly differed between trackable and nontrackable participants within the two age groups.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/dev
Contributor Information
Jennifer Tehan Stanley, Psychology Department and Volen National Center for Complex Systems, Brandeis University.
Derek M. Isaacowitz, Psychology Department and Volen National Center for Complex Systems, Brandeis University
References
- Allard ES, Isaacowitz DM. Are preferences in emotional processing affected by distraction? Examining the age-related positivity effect in visual fixation within a dual-task paradigm. Aging, Neuropsychology, & Cognition. 2008;15:725–743. doi: 10.1080/13825580802348562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Wahlin Å. Aging and memory: Cognitive and biological perspectives. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5. San Diego, CA: Academic Press; 2001. pp. 349–377. [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037/0003-066X.52.4.366. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM. Psychological perspectives on successful aging: The model of selective optimization with compensation. In: Baltes PB, Baltes MM, editors. Successful aging: Perspectives from the behavioral sciences. New York, NY: Cambridge University Press; 1990. pp. 1–34. [Google Scholar]
- Bargh JA, Ferguson MJ. Beyond behaviorism: On the automaticity of higher mental processes. Psychological Bulletin. 2000;126:925–945. doi: 10.1037/0033-2909.126.6.925. [DOI] [PubMed] [Google Scholar]
- Barrick AL, Hutchinson RL, Deckers LH. Age effects on positive and negative emotions. Journal of Social Behavior & Personality. 1989;4:421–429. [Google Scholar]
- Birditt KS, Fingerman KL. Age and gender differences in adults’ descriptions of emotional reactions to interpersonal problems. Journal of Gerontology. 2003;58B:237–245. doi: 10.1093/geronb/58.4.p237. [DOI] [PubMed] [Google Scholar]
- Birditt KS, Fingerman KL, Almeida DM. Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging. 2005;20:330–340. doi: 10.1037/0882-7974.20.2.330. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F. Everyday problem solving and emotion: An adult developmental perspective. Current Directions in Psychological Science. 2007;16:26–31. doi: 10.1111/j.1467-8721.2007.00469.x. [DOI] [Google Scholar]
- Blanchard-Fields F, Coats AH. The experience of anger and sadness in everyday problems impacts age differences in emotion regulation. Developmental Psychology. 2008;44:1547–1556. doi: 10.1037/a0013915. [DOI] [PubMed] [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: The role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59:355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Brandtstädter J, Greve W. The aging self: Stabilizing and protective processes. Developmental Review. 1994;14:52–80. doi: 10.1006/drev.1994.1003. [DOI] [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science. 1995;4:151–156. doi: 10.1111/1467-8721.ep11512261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. doi: 10.1111/j.0963-7214.2005.00348.x. [DOI] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. doi: 10.1037/0022-3514.79.4.644. [DOI] [PubMed] [Google Scholar]
- Catanzaro SJ, Mearns J. Measuring generalized expectancies for negative mood regulation: Initial scale development and implications. Journal of Personality Assessment. 1990;54:546–563. doi: 10.1080/00223891.1990.9674019. [DOI] [PubMed] [Google Scholar]
- Charles ST, Carstensen LL. Unpleasant situations elicit different emotional responses in younger and older adults. Psychology and Aging. 2008;23:495–504. doi: 10.1037/a0013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, Gatz M. Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology. 2001;80:136–151. doi: 10.1037/0022-3514.80.1.136. [DOI] [PubMed] [Google Scholar]
- Chayer C. The neurologic examination: Brief mental status. The Journal of Geriatric Care. 2002;1:265–267. [Google Scholar]
- Consedine NS, Magai C, Bonanno GA. Moderators of the emotion inhibition-health relationship: A review and research agenda. Review of General Psychology. 2002;6:204–228. doi: 10.1037/1089-2680.6.2.204. [DOI] [Google Scholar]
- Craik FIM. On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society of London. 1983;B302:341–359. [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Flix F, Hagendorf H, editors. Human memory and cognitive capabilities, mechanisms and performances. Amsterdam: Elsevier; 1986. pp. 409–422. [Google Scholar]
- Dannefer D. What’s in a name?: An account of the neglect of variability in the study of aging. In: Birren JE, Bengston VL, editors. Emergent Theories of Aging. New York, NY: Springer Publishing Co; 1988. pp. 356–384. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037/0021-843X.111.2.225. [DOI] [PubMed] [Google Scholar]
- Diener E, Suh EM, Lucas RE, Smith HL. Subjective well-being: Three decades of progress. Psychological Bulletin. 1999;125:276–302. doi: 10.1037/0033-2909.125.2.276. [DOI] [Google Scholar]
- Diener E, Suh ME. Subjective well-being and age: An international analysis. In: Schaie KW, Lawton MP, editors. Annual Review of Gerontology and Geriatrics: Focus on Emotion and Adult Development. Vol. 17. New York, NY: Springer Publishing Co; 1998a. pp. 304–324. [Google Scholar]
- Diener E, Suh ME. Subjective well-being and age: An international analysis. In: Schaie KW, Lawton MP, editors. Annual Review of Gerontology and Geriatrics, Vol. 17: Focus on emotion and adult development. Vol. 17. New York, NY: Springer Publishing Co; 1998b. pp. 304–324. [Google Scholar]
- Eich E, Metcalfe J. Mood dependent memory for internal versus external events. Journal of Experimental Psychology: Learning, Memory and Cognition. 1989;15:443–455. doi: 10.1037/0278-7393.15.3.443. [DOI] [Google Scholar]
- Eich E, Ng JTW, Macaulay D, Percy AD, Grebneva I. Combining music with thought to change mood. In: Coan JA, BAllen JJ, editors. Handbook of emotion elicitation and assessment. Oxford: Oxford University Press; 2007. pp. 124–136. [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face: A guide to recognizing emotions from facial cues. Englewood Cliffs, NJ: Prentice Hall; 1975. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fiori KL, Smith J, Antonucci TC. Social network types among older adults: A multidimensional approach. Journal of Gerontology: Psychological Sciences. 2007;62B:P322–P333. doi: 10.1093/geronb/62.6.p322. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive states of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Smith J, Baltes PB. A systematic-wholistic approach to differential aging: Longitudinal findings from the Berlin Aging Study. Psychology and Aging. 2006;21:645–663. doi: 10.1037/0882-7974.21.4.645. [DOI] [PubMed] [Google Scholar]
- Griffin PW, Mroczek DK, Spiro A. Variability in affective change among aging men: Longitudinal findings from the VA Normative Aging Study. Journal of Research in Personality. 2006;40:942–965. doi: 10.1016/j.jrp.2005.09.011. [DOI] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–299. doi: 10.1037/1089-2680.2.3.271. [DOI] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Gotestam Skorpen C, Hsu AYC. Emotion and aging: Experience, Expression, and Control. Psychology and Aging. 1997;12:590–599. doi: 10.1037/0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Muñoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–164. [Google Scholar]
- Grühn D, Scheibe S. Age-related differences in valence and arousal ratings of pictures from the International Affective Picture System (IAPS): Do ratings become more extreme with age? Behavior Research Methods. 2008;40:512–521. doi: 10.3758/BRM.40.2.512. [DOI] [PubMed] [Google Scholar]
- Hair JF, Anderson RE, Tatham RL, Black WC. Multivariate Data Analysis. 5. Delhi, India: Pearson Education; 1998. Multivariate analysis of variance; pp. 326–386. [Google Scholar]
- Hair JF, Black WC. Cluster analysis. In: Grimm LG, Yarnold PR, editors. Reading and understanding more multivariate statistics. Washington, DC: American Psychological Association; 2000. pp. 147–205. [Google Scholar]
- Heckhausen J, Dixon RA, Baltes PB. Gains and losses in development throughout adulthood as perceived by different adult age groups. Developmental Psychology. 1989;25:109–121. doi: 10.1037/0012-1649.25.1.109. [DOI] [Google Scholar]
- Hertzog C, Nesselroade JR. Assessing psychological change in adulthood: An overview of methodological issues. Psychology and Aging. 2003;18:639–657. doi: 10.1037/0882-7974.18.4.639. [DOI] [PubMed] [Google Scholar]
- Hetherington R. The Snellen Chart as a test of visual acuity. Psychologische Forschung. 1954;24:349–357. doi: 10.1007/BF00422033. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson H. Looking while unhappy: Mood congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19:848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Neupert SD. Use of gaze for real-time mood regulation: Effects of age and attentional functioning. Psychology and Aging. 2009;24:989–994. doi: 10.1037/a0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006a;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging. 2006b;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological Science. 2004;15:208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Kessler EM, Staudinger UM. Affective experience in adulthood and old age: The role of affective arousal and perceived affect regulation. Psychology and Aging. 2009;24:349–362. doi: 10.1037/a0015352. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Gruhn D. Age differences in emotional reactivity: The sample case of sadness. Psychology and Aging. 2005;20:47–59. doi: 10.1037/0882-7974.20.1.47. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Little TD, Smith J. Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychology and Aging. 2000;15:511–526. doi: 10.1037/0882-7974.15.3.511. [DOI] [PubMed] [Google Scholar]
- Lachman ME. Development in midlife. Annual Review of Psychology. 2004;55:305–331. doi: 10.1146/annurev.psych.55.090902.141521. [DOI] [PubMed] [Google Scholar]
- Lang FR, Carstensen LL. Time counts: Future time perspective, goals, and social relationships. Psychology and Aging. 2002;17:125–139. doi: 10.1037/0882-7974.17.1.125. [DOI] [PubMed] [Google Scholar]
- Larcom MJ, Isaacowitz DM. Rapid emotion regulation after mood induction: Age and individual differences. Journal of Gerontology: Psychological Sciences. 2009;64B:733–741. doi: 10.1093/geronb/gbp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RJ. Toward a science of mood regulation. Psychological Inquiry. 2000;11:130–143. [Google Scholar]
- Larsen RJ, Augustine AA, Prizmic Z. A process approach to emotion and personality: Using time as a facet of data. Cognition & Emotion. 2009;23:1407–1426. doi: 10.1080/02699930902851302. [DOI] [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. Dimensions of affective experience in three age groups. Psychology and Aging. 1992;7:171–184. doi: 10.1037/0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Lineweaver TT, Hertzog C. Adults’ efficacy and control beliefs regarding memory and aging: Separating general from personal beliefs. Aging, Neuropsychology, & Cognition. 1998;5:264–296. doi: 10.1076/anec.5.4.264.771. [DOI] [Google Scholar]
- Lynch SM, George LK. Interlocking trajectories of loss-related events and depressive symptoms among elders. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2002;57B:117–125. doi: 10.1093/geronb/57.2.s117. [DOI] [PubMed] [Google Scholar]
- Magnusson D. The person approach: Concepts, measurement models, and research strategy. New Directions for Child & Adolescent Development. 2003;2003:3–23. doi: 10.1002/cd.79. [DOI] [PubMed] [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free-viewing visuocognitive tasks. Journal of Neuroscience Methods. 2003;128:85–93. doi: 10.1016/S0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mienaltowski A, Blanchard-Fields F. The differential effects of mood on age differences in the correspondence bias. Psychology and Aging. 2005;20:589–600. doi: 10.1037/0882-7974.20.4.589. [DOI] [PubMed] [Google Scholar]
- Milligan GW, Cooper MC. Methodology review: Clustering methods. Applied Psychological Measurement. 1987;11:329–354. doi: 10.1177/014662168701100401. [DOI] [Google Scholar]
- Montepare JM, Lachman ME. “You’re only as old as you feel”: Self-perceptions of age, fears of aging, and life satisfaction from adolescence to old age. Psychology and Aging. 1989;4:73–78. doi: 10.1037/0882-7974.4.1.73. [DOI] [PubMed] [Google Scholar]
- Morse CK. Does variability increase with age? An archival study of cognitive measures. Psychology and Aging. 1993;8:156–164. doi: 10.1037/0882-7974.8.2.156. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–378. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A. Change in life satisfaction during adulthood: Findings from the Veterans Affairs Normative Aging Study. Journal of Personality and Social Psychology. 2005;88:189–202. doi: 10.1037/0022-3514.88.1.189. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. Longitudinal investigation of mood variability and the ffm: Neuroticism predicts variability in extended states of positive and negative affect. Personality & Individual Differences. 2002;33:1217–1228. [Google Scholar]
- Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. Journal of Personality and Social Psychology. 2006;91:730–749. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- Ong AD, Bergeman CS, Boker SM. Resilience comes of age: Defining feature in later adulthood. Journal of Personality. 2009;77:1777–1804. doi: 10.1111/j.1467-6494.2009.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Edwards LM, Bergeman CS. Hope as a source of resilience in later adulthood. Personality and Individual Differences. 2006;41:1263–1273. doi: 10.1016/j.paid.2006.03.028. [DOI] [Google Scholar]
- Palmore E, Cleveland W. Aging, terminal decline, and terminal drop. Journal of Gerontology. 1976;31:76–81. doi: 10.1093/geronj/31.1.76. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Sciences. 1988;2:187–199. [Google Scholar]
- Pronin E, Wegner DM. Manic thinking: Independent effects of thought speed and thought control on mood. Psychological Science. 2006;17:807–813. doi: 10.1111/j.1467-9280.2006.01786.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Röcke C, Lachman ME. Perceived trajectories of life satisfaction across past, present, and future: Profiles and correlates of subjective change in young, middle-aged, and older adults. Psychology and Aging. 2008;23:833–847. doi: 10.1037/a0013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JG. The biggest reward for my invention isn’t money. Medical Economics. 1984;61:152–163. [Google Scholar]
- Salovey P, Mayer JD, Goldman SL, Turvey C, Palfai TP. Emotional attention, clarity, and repair: Exploring emotional intelligence using the Trait Meta-Mood Scale. In: Pennebaker JW, editor. Emotion, Disclosure, & Health. Washington, D.C: American Psychological Association; 1995. pp. 125–154. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, stress-related disease, and emotional regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. pp. 606–615. [Google Scholar]
- Sarle WS. Cubic clustering criterion. Cary, NC: SAS Institute; 1983. [Google Scholar]
- Scheibe S, Blanchard-Fields F. Effects of regulating emotions on cognitive performance: What is costly for young adults is not so costly for older adults. Psychology and Aging. 2009;24:217–223. doi: 10.1037/a0013807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037/0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 2000. pp. 155–219. [Google Scholar]
- Shephard RJ. Age and physical work capacity. Experimental Aging Research. 1999;25:331–343. doi: 10.1080/036107399243788. [DOI] [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speilberger CD. Manual for the state-trait anxiety inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stacey CA, Gatz M. Cross-sectional age differences and longitudinal change on the Bradburn Affect Balance Scale. Journal of Gerontology. 1991;46:76–78. doi: 10.1093/geronj/46.2.p76. [DOI] [PubMed] [Google Scholar]
- Tamir M. What do people want to feel and why?: Pleasure and utility in emotion regulation. Current Directions in Psychological Science. 2009;18:101–105. doi: 10.1111/j.1467-8721.2009.01617.x. [DOI] [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association. 1963;58:236–244. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Revised. New York: Psychological Corp; 1981. [Google Scholar]
- Wilson HR, Loffler G, Wilkinson F. Synthetic faces, face cubes, and the geometry of face space. Vision Research. 2002;42:2909–2923. doi: 10.1016/s0042-6989(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Zachary R. Shipley Institute of Living Scale, Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 2000. pp. 293–357. [Google Scholar]